Abstract
The RhlR quorum sensing (QS) receptor in the pathogen Pseudomonas aeruginosa plays a prominent role in infection, and both antagonism and agonism of RhlR have been shown to negatively regulate important virulence phenotypes. Non-native lactone ligands are known to modulate RhlR activity, but their utility as chemical probes is relatively limited due to hydrolytic instability. Herein, we report our design and biological evaluation of a suite of hybrid AHL analogs with structures merging (1) features of reported lead RhlR ligands and (2) head groups with improved hydrolytic stabilities. The most promising compounds identified were N-acyl L-homocysteine thiolactones, which displayed enhanced stabilities relative to lactones. Moreover, they were highly selective for RhlR over another key QS receptor in P. aeruginosa, LasR. These compounds are amongst the most potent RhlR modulators known and represent robust chemical tools to dissect the complex roles of RhlR in the P. aeruginosa QS circuitry.
GRAPHICAL ABSTRACT
INTRODUCTION
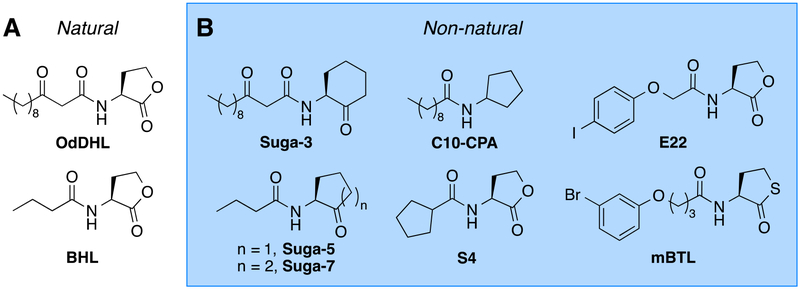
Quorum sensing (QS) is a chemical signaling pathway that certain bacteria use to assess their local population densities and coordinate group behavior once a threshold cell number is achieved (1). Gram-negative bacteria typically use N-acyl L-homoserine lactones (AHLs) as their QS signals, which are produced by LuxI-type synthases and sensed by cytoplasmic LuxR-type transcription factors (2). Upon ligand binding, LuxR-type receptors most commonly dimerize, bind to DNA, and regulate QS-associated genes. The opportunistic pathogen Pseudomonas aeruginosa utilizes a relatively complex QS system to regulate a host of virulence factors at high cell density. Two LuxI-type synthases, LasI and RhlI, produce N-(3-oxo-dodecanoyl) HL (OdDHL) and N-butyryl HL (BHL), respectively (Figure 1A) (3). These two signaling molecules are recognized by their cognate LuxR-type receptors, LasR and RhlR. OdDHL is also recognized by a third LuxR-type receptor, QscR, which has been found to both negatively regulate LasR and activate its own unique regulon of P. aeruginosa (4). LasR is generally considered to be at the top of the P. aeruginosa QS receptor hierarchy, as it regulates genes associated with other QS circuits (3). Due to this prominent role, LasR has been a primary target over the past ~15 years for the design of small molecule antagonists to block QS and reduce virulence in P. aeruginosa (5–10). However, there is increasing evidence that targeting RhlR with small molecule tools could be advantageous.
Figure 1:
Selected native AHLs and reported RhlR ligands. (A) Natural AHLs [OdDHL and BHL] and (B) lead non-natural modulators of RhlR [Suga-3, Suga-5, and Suga-7, Suga and coworkers (15); C10-CPA, Kato and coworkers (16); S4 and E22, Blackwell and coworkers (13); mBTL, Bassler and coworkers (11)].
Our laboratory has recently shown that small-molecule activation and inhibition of RhlR can alter the expression levels of several different and important virulence factors in P. aeruginosa; for example, when RhlR is activated, pyocyanin production is reduced (7). In turn, when RhlR is inhibited, rhamnolipid production is decreased. Bassler and co-workers have shown that partial agonism of RhlR can reduce P. aeruginosa virulence in a C. elegans infection model (11), and very recently, that RhlR can also control certain virulence phenotypes via a yet to be identified ligand unique from BHL (12). To date, the most potent reported RhlR modulators contain homoserine lactone headgroups (i.e., agonist S4 and antagonist E22, Figure 1A). We reported these two compounds in a comprehensive analysis of our non-native AHL libraries for RhlR modulators in 2015 (13). However, the hydrolytic instability of these ligands’ lactone head groups is a drawback to their use as chemical probes, especially as P. aeruginosa culture media is observed to become more alkaline over time (14). Synthetic ligands for RhlR with enhanced stabilities over S4 and E22, whilst maintaining their potencies, would be of significant utility to study QS pathways in P. aeruginosa.
In general, RhlR has seen far less scrutiny as a target for non-native ligand design relative to LasR in P. aeruginosa, largely due to its perceived smaller role in QS (see above (5, 6)). Interestingly, beyond our recent forays into the development of RhlR modulators (13, 17), most prior studies on synthetic RhlR ligands have actually involved AHL analogs with non-lactone headgroups. In 2003, Suga and co-workers investigated both BHL and OdDHL analogs that contained heterocyclic replacements for the lactone head group yet retained the native 4- or 12-carbon tail groups. The authors found that BHL variants with cyclopentanone and cyclohexanone head groups showed agonistic activity towards RhlR (Suga-5, Suga-7; Figure 1B (15)). Surprisingly, a 12-carbon OdDHL mimic with a cyclohexanone head group proved to be the most potent RhlR antagonist in this study (Suga-3; Figure 1B), suggesting the utility of longer tail groups in inhibiting RhlR. Later, Kato and co-workers found that a 10-carbon AHL analog with cyclopentyl head group (C10-CPA, Figure 1B) inhibits P. aeruginosa QS through the antagonism of both RhlR and LasR (16). More recently, Bassler and co-workers reported that a meta-bromo aryl homocysteine thiolactone (i.e., mBTL; Figure 1B) was a RhlR partial agonist (11). Homocysteine thiolactones have been examined in AHL analogs previously (11, 18–22), but except for mBTL, have not been explored as modulators of RhlR. Together, these prior studies indicated that RhlR can accommodate non-lactone head groups (assuming these close AHL mimetics target the BHL-binding site) and that further research into such compound scaffolds could be fruitful for new ligand design.
Herein, we report our design and biological evaluation of a set of hybrid AHL analogs with structures merging (1) features of the most promising reported RhlR ligands and (2) head groups with improved hydrolytic stabilities. These studies revealed, to our knowledge, the most potent non-native RhlR agonist to be reported, along with a highly potent RhlR antagonist. Notably, these two compounds both contain homocysteine thiolactones, a head group that shows improved hydrolytic stability relative to homoserine lactone, and are selective for RhlR over the other key LuxR-type receptor in P. aeruginosa, LasR.
RESULTS AND DISCUSSION
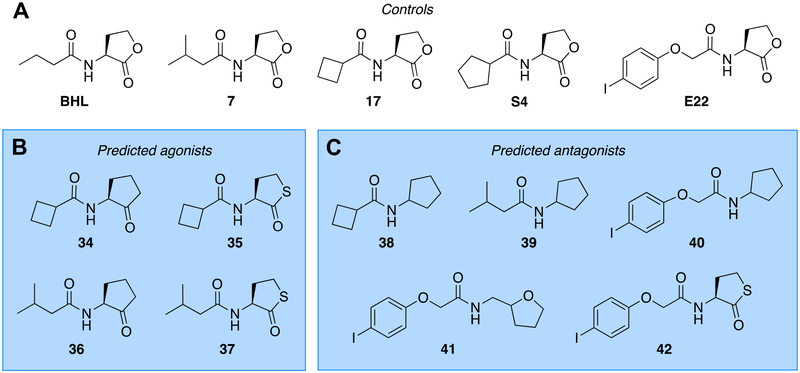
Active compounds uncovered in our recent BHL structure-activity relationship (SAR) study (17) and previously published RhlR leads (Figure 1B), as well as structural motifs with enhanced hydrolytic stability, motivated our selection of head and tail groups for new ligand design. Our SAR study suggested that both cyclopentanone and homocysteine thiolactone BHL analogs were capable of RhlR agonism, consistent with the work of the Suga and Bassler labs, respectively (11, 15). In addition, we found that RhlR well tolerates additional bulk at or near the α-position of the acyl tail, as exemplified by cyclopentyl HL S4, isovaleryl HL 7, and cyclobutyl HL 17 (Figure 2A), resulting in agonists exceeding the potency of BHL (17). We reasoned that combining these structural features could yield new RhlR agonists, and tested this hypothesis by uniting the cyclopentanone and homocysteine thiolactone head groups with either isovaleryl or cyclobutanoyl tails to give compounds 34–37 (Figure 2B). Building on the prior work of Kato (16) and with an eye toward the development of new RhlR antagonists, we coupled the cyclopentyl head group with the isovaleryl or cyclobutanoyl tails to yield derivatives 38 and 39 (Figure 2C). Also with a view toward RhlR antagonism, we combined the cyclopentyl, tetrahydrofurfuryl, and homocysteine thiolactone head groups with the 4-iodo aryl tail from our potent RhlR antagonist E22 (Figure 2A (7, 13)) to provide compounds 40–42 (Figure 2C). These hybrid compounds were synthesized using standard amide coupling chemistry in modest to good yields (40–80%) and purified to >95% prior to biological testing (41 generated as a racemic mixture; see Methods).
Figure 2.
Chemical structures of AHLs and analogs thereof examined in this study. (A) Control compounds for comparison to new ligands. New analogs blending (B) agonist head/tail groups for predicted RhlR agonist generation or (C) agonist head/tail groups and antagonist head/tail groups for predicted RhlR antagonist generation. Compound numbering originates from our previous study (17).
The compounds were evaluated for their ability to either agonize or antagonize RhlR using an Escherichia coli strain harboring a RhlR expression plasmid and a reporter plasmid that allowed for straightforward read-out of RhlR activity (Table S1; see Methods). Simultaneously, we also screened the compounds in an analogous E. coli reporter system for LasR to investigate their selectivity for RhlR over LasR (Table S2). In the RhlR agonism screen, compounds 34–37 proved highly active at 10 μM and 1 mM, displaying greater than 50% activation at 10 μM. In the RhlR antagonism screen, compounds 38 and 41 were modest antagonists, while compound 42 was found to inhibit RhlR more than any other compound in this study at both 10 μM (28% inhibition) and 1 mM (74% inhibition). Notably, all of the compounds were largely inactive in the LasR assays as either agonists or antagonists, highlighting the selectivity of these hybrid ligand classes for RhlR modulation over LasR. The four lead hybrid RhlR agonists (34–37) and three lead hybrid RhlR antagonists (38, 41, and 42) identified in these primary screens were submitted to dose-response analyses in the E. coli RhlR reporter to determine their potencies. The native RhlR ligand, BHL, along with four parent compounds from our previous studies (7, 17, S4, and E22; Figure 2A (13, 17)) were included as controls to better assess relative compound potency and maximal activity (i.e., efficacy). The resulting EC50 and IC50 values for the compounds, along with their associated efficacies, are listed in Table 1.
Table 1:
EC50 and IC50 values and efficacy data for AHL analogs in the E. coli and P. aeruginosa RhlR reporter strains.a Data for control compounds shaded in grey.
| Compound | EC50 (μM) | 95% CI (μM)b | Max. RhlR Activation (%) | EC50 (μM) | 95% CI (μM)b | Max. RhlR Activation (%) |
| 34 | 5.94 | 4.19 – 8.41 | 93 | 7.35 | 5.26 – 10.3 | 96 |
| 35 | 1.72 | 1.34 – 2.21 | 110 | 1.65 | 1.24 – 2.21 | 90 |
| 36 | 7.58 | 5.80 – 9.90 | 100 | 11.24 | 7.41 – 17.1 | 96 |
| 37 | 0.463 | 0.336 – 0.640 | 93 | 2.58 | 1.86 – 3.56 | 91 |
| BHL | 8.95 | 5.86 – 13.7 | 100 | 8.08 | 6.09 – 10.7 | 100 |
| 7 | 1.02 | 0.67 – 1.55 | 110 | 1.42 | 1.08 – 1.86 | 94 |
| 17 | 1.78 | 1.37 – 2.31 | 100 | 1.41 | 1.14 – 1.74 | 96 |
| S4 | 1.58 | 1.32 – 1.90 | 100 | 1.22 | 1.03 – 1.45 | 110 |
| IC50 (μM) | 95% CI (μM)b | Max. RhlR Inhibition (%) | IC50 (μM) | 95% CI (μM)b | Max. RhlR Inhibition (%) | |
| 38 | 26.7 | 10.1 – 71.0 | 32 | – | – | – |
| 41 | >100 | – | 56 | – | – | – |
| 42 | 19.6 | 14.3 – 26.9 | 81 | 31.4 | 19.6 – 50.4 | 85 |
| E22 | 17.3 | 12.1 – 24.6 | 74 | 23.9 | 16.6 – 31.6 | 96 |
See Methods for assay details. For full dose response curves, see Figures S1–S4.
CI = confidence interval.
Hybrid compounds 34–37 proved either equipotent (34 and 36) or more potent (35 and 37) agonists than the native RhlR ligand, BHL (Table 1). The homocysteine thiolactone derivatives were the most potent overall, with cyclobutanoyl derivative 35 equipotent to its parent lactone compound 17, and more notably, isovaryl homocysteine thiolactone 37 displaying two-fold greater potency over its lactone variant 7 and our previous lead agonist S4. Thiolactone 37, with an EC50 of 463 nM in the E. coli reporter, represented the most potent RhlR agonist identified in this study.
In terms of RhlR antagonism, a homocysteine thiolactone derivative again was the most potent (aryl thiolactone 42), showing potency comparable to its parent aryl lactone E22 in the E. coli reporter (Table 1). This result is interesting, as a previous study with a pair of aryl lactone and thiolactone analogs in LasR were found to display opposite activities (i.e., antagonist and agonist), respectively. Mutagenesis and computational studies in LasR implicated a hydrogen bond between the homoserine lactone (or homocysteine thiolactone) carbonyl and a conserved Trp residue in the LasR ligand-binding site (Trp 60) to be important for tuning compound activity (23). RhlR contains an analogous Trp residue (Trp 68). Our results showing that both homocysteine thiolactone 42 and its lactone analog E22 are strong RhlR antagonists suggest that this Trp hypothesis may not be accurate for RhlR, at least with this aryl ligand scaffold. Of the other two RhlR antagonists submitted to dose-response analyses, cyclopentyl derivative 38 proved the next most active, with a potency only slightly lower than thiolactone 42, albeit with a significantly lower inhibition efficacy (32% vs. 81%, Table 1).
We next sought to determine if the activity profiles for the most potent compounds in the E. coli reporter would be maintained in RhlR’s native background, P. aeruginosa. Active efflux, along with the presence of acylases and reduced overall permeability, has been shown to decrease the activity of AHLs in P. aeruginosa relative to E. coli (24). Agonists 34–37 and antagonist 42 were submitted to analogous dose-response assays in a P. aeruginosa RhlR reporter strain (see Methods). Compounds 34–36 maintained their strong potency profiles between the two different reporters (Table 1), while compound 37 demonstrated a ~5-fold lower potency in P. aeruginosa relative to E. coli. Still, the homocysteine lactone analogs 35 and 37 were the most potent agonists in P. aeruginosa (EC50 values of 1.65 and 2.58 μM, respectively), further underscoring the utility of this head group for potent RhlR agonism. This trend was continued for RhlR antagonism, with homocysteine lactone 42 maintaining its strong potency and efficacy in P. aeruginosa (with 85% maximum inhibition, Table 1) and marking this compound as one of the most potent antagonists of RhlR reported to date.
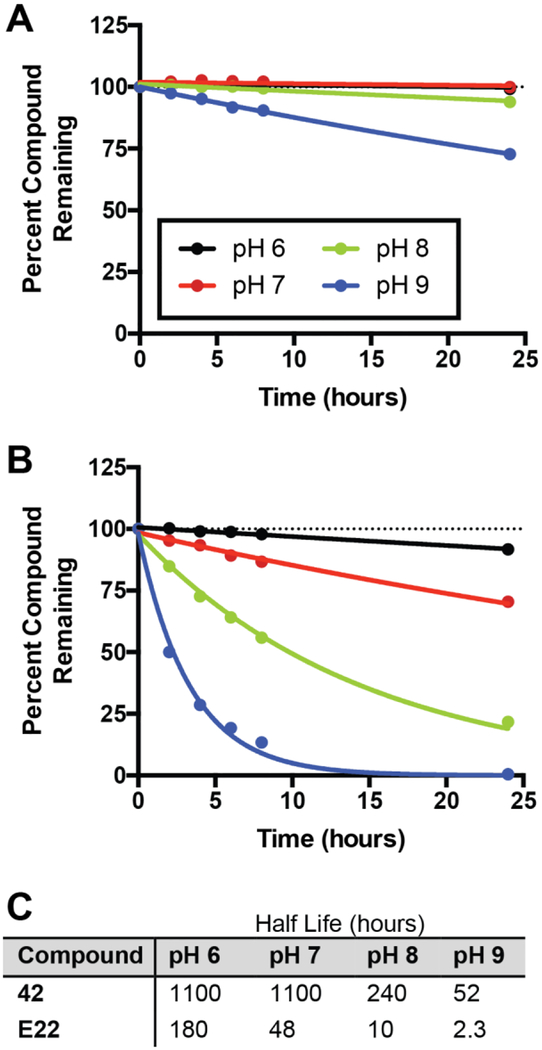
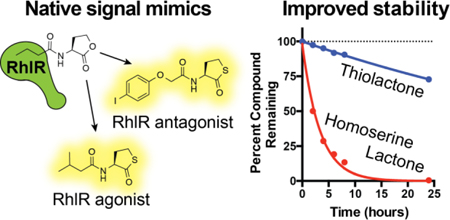
We were intrigued that both our lead RhlR agonist (35) and our lead RhlR antagonist (42) in P. aeruginosa were homocysteine thiolactone derivatives, a trend that corroborated the strong activity reported by the Bassler lab for the thiolactone mBTL (11). We reasoned that these alternate headgroups could alter the hydrolytic stabilities of these derivatives relative to AHLs. Indeed, in a 2011 study we showed that certain homocysteine thiolactone derivatives have increased hydrolytic stabilities relative to AHLs in Luria-Bertani medium as monitored via a biosensor assay (21). To evaluate their stability in a more direct and quantitative assay, we elected to monitor the stability of homocysteine thiolactone 42 relative to its homoserine lactone homolog E22 over time and at varying pH values (6–9) using HPLC and MS (see Methods). Interestingly, the homocysteine thiolactone displayed remarkable stability in this assay, with half-lives ranging from approximately 6 to 23 times longer than the half-lives of the homoserine lactone (Figure 3). The differences in half-lives for 42 vs. E22 grew dramatically larger at pH ≥ 7 (e.g., 240 h vs. 10 h at pH 8, respectively).
Figure 3.
Compound degradation data. Degradation profiles at varying pH values for (A) homocysteine thiolactone 42 and (B) homoserine lactone E22 and resulting half-lives (C) as measured via HPLC. MS data reported in Table S3.
The results of these stability studies for 42 and E22 do not align with earlier reports supporting the thermodynamic favorability of alkyl thioester hydrolysis (25). However, thioesters are known to have slow rates of hydrolysis, and published rate constants have typically been for electronically activated thioesters (e.g., trifluorothioacetate (26)). In the compounds tested here, homocysteine thiolactone ring size may also play a crucial role in the observed hydrolysis rates. Previous studies comparing homocysteine thiolactones and homoserine lactones in water/acetone mixtures showed that homoserine lactones hydrolyze at a two-fold faster rate (27). The resulting γ-mercapto acids from homocysteine thiolactone hydrolysis also readily recyclize upon acid exposure, while thiolactones with larger ring sizes are far less likely to recyclize (28). The HPLC/MS data for 42 and E22 (Figure 3) support these past reports on the kinetics of homocysteine thiolactone hydrolysis. We note that the P. aeruginosa reporter assay used in this study was six hours in length and the final pH did not exceed 7.6, suggesting hydrolysis has a negligible effect on the activities of 42 and E22 in these experiments. Nevertheless, for assays performed over more extended periods of time (> 10 h), and in view of the increasing alkalinity of P. aeruginosa culture media over time (14) and the observed preference of certain bacterial lactonases for homoserine lactones over homocysteine thiolactones (18), we believe that our homocysteine thiolactone RhlR modulators (35, 37, and 42) should constitute physically robust probes for the study of P. aeruginosa QS in a variety of biologically relevant environments.
SUMMARY
Both antagonism and agonism of the RhlR receptor have been shown to negatively regulate important virulence phenotypes in P. aeruginosa. While prior chemical efforts have delivered synthetic ligands for RhlR, the most potent of these compounds are all lactone based and suffer from relatively low hydrolytic stability. We designed a suite of new compounds that integrated the structures of these lead compounds with alternate head groups, and evaluated them in cell-based reporter assays for RhlR activity. The most promising compounds identified contain homocysteine thiolactone head groups (35, 37, and 42), and this motif showed improved hydrolytic stability relative to the homoserine lactone group. These new ligands were highly selective for RhlR over another key QS receptor in P. aeruginosa, LasR, and are active in the P. aeruginosa background. Homocysteine thiolactones 35, 37, and 42 represent some of the most potent RhlR modulators known and constitute new tools to investigate the role of RhlR in QS regulation. Furthermore, they underscore the potential utility of the thiolactone motif for the design of synthetic ligands for other LuxR-type receptors.
METHODS
Chemistry
AHLs and AHL analogs were synthesized and purified using our previously reported procedures (17, 29). See SI for details of instrumentation and full characterization data for new compounds.
Bacteriology
Bacteria were cultured in Luria-Bertani medium (LB) at 37 °C. Absorbance measurements were performed in 96-well microtiter plates and path length-corrected using a Biotek Synergy 2 plate reader running Gen 5 software (version 1.05). Bacterial growth was assessed by measuring absorbance at 600 nm (OD600).
Bacterial strains and assay protocols
The bacterial reporter strains used for this study were (i) E. coli strain JLD271 (ΔsdiA) harboring the RhlR expression plasmid pJN105R2 and the rhlI-lacZ transcriptional fusion reporter pSC11-rhlI*, (ii) E. coli strain JLD271 (ΔsdiA) harboring the LasR expression plasmid pJN105L and the lasI-lacZ transcriptional fusion reporter pSC11, and (iii) P. aeruginosa strain PAO-JP2 (ΔlasIrhlI) harboring the rhlI-gfp transcriptional fusion reporter prhlI-LVAgfp. Miller assays and GFP fluorescence assays were performed in these reporters as previously described (17, 30).
Homocysteine thiolactone/homoserine lactone stability studies
Stability studies were performed as reported previously (30) with some minor modifications (see SI for method, MS data, and RP-HPLC traces).
Supplementary Material
ACKNOWLEDGMENT
Financial support for this project was provided by the NIH (R01 GM109403). M.E.B. was funded in part by the NSF through the UW–Madison Materials Research Science and Engineering Center (DMR-1121288). NMR facilities were supported by the NSF (CHE-0342998) and a gift from P. Bender. MS facilities were supported by the NSF (CHE-9974839).
Footnotes
Electronic supplementary information (ESI) available
General chemical information, details of instrumentation and analytical methods, compound characterization data, and supplementary assay data.
REFERENCES
- 1.Whiteley M, Diggle SP, and Greenberg EP (2017) Progress in and promise of bacterial quorum sensing research, Nature 551, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papenfort K, and Bassler BL (2016) Quorum sensing signal-response systems in Gram-negative bacteria, Nat. Rev. Microbiol 14, 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster M, and Greenberg EP (2006) A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa, Int. J. Med. Microbiol 296, 73–81. [DOI] [PubMed] [Google Scholar]
- 4.Ding FM, Oinuma K-I, Smalley NE, Schaefer AL, Hamwy O, Greenberg EP, and Dandekar AA (2018) The Pseudomonas aeruginosa Orphan Quorum Sensing Signal Receptor QscR Regulates Global Quorum Sensing Gene Expression by Activating a Single Linked Operon, Mbio 9, e01274–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welsh MA, and Blackwell HE (2016) Chemical probes of quorum sensing: from compound development to biological discovery, FEMS Microbiol. Rev 40, 774–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galloway WRJD, Hodgkinson JT, Bowden SD, Welch M, and Spring DR (2011) Quorum sensing in Gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways, Chem. Rev 111, 28–67. [DOI] [PubMed] [Google Scholar]
- 7.Welsh MA, Eibergen NR, Moore JD, and Blackwell HE (2015) Small Molecule Disruption of Quorum Sensing Cross-Regulation in Pseudomonas aeruginosa Causes Major and Unexpected Alterations to Virulence Phenotypes, J. Am. Chem. Soc 137, 1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amara N, Gregor R, Rayo J, Dandela R, Daniel E, Liubin N, Willems HME, Ben-Zvi A, Krom BP, and Meijler MM (2016) Fine-Tuning Covalent Inhibition of Bacterial Quorum Sensing, ChemBioChem 17, 825–835. [DOI] [PubMed] [Google Scholar]
- 9.Amara N, Mashiach R, Amar D, Krief P, Spieser S. a. H., Bottomley MJ, Aharoni A, and Meijler MM (2009) Covalent inhibition of bacterial quorum sensing, J. Am. Chem. Soc 131, 10610–10619. [DOI] [PubMed] [Google Scholar]
- 10.Galloway WRJD, Hodgkinson JT, Bowden S, Welch M, and Spring DR (2012) Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria, Trends Microbiol 20, 449–458. [DOI] [PubMed] [Google Scholar]
- 11.O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, and Bassler BL (2013) A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation, Proc. Natl. Acad. Sci. U. S. A 110, 17981–17986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee S, Moustafa DA, Stergioula V, Smith CD, Goldberg JB, and Bassler BL (2018) The PqsE and RhlR proteins are an autoinducer synthase-receptor pair that control virulence and biofilm development in Pseudomonas aeruginosa, Proc. Natl. Acad. Sci. U. S. A 115, E9411–E9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eibergen NR, Moore JD, Mattmann ME, and Blackwell HE (2015) Potent and Selective Modulation of the RhlR Quorum Sensing Receptor by Using Non-native Ligands: An Emerging Target for Virulence Control in Pseudomonas aeruginosa, ChemBioChem 16, 2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yates EA, Philipp B, Buckley C, Atkinson S, Chhabra SR, Sockett RE, Goldner M, Dessaux Y, Cámara M, Smith H, and Williams P (2002) N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa, Infect. Immun 70, 5635–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith KM, Bu Y, and Suga H (2003) Induction and Inhibition of Pseudomonas aeruginosa Quorum Sensing by Synthetic Autoinducer Analogs, Chem. Biol 10, 81–89. [DOI] [PubMed] [Google Scholar]
- 16.Ishida T, Ikeda T, Takiguchi N, Kuroda A, Ohtake H, and Kato J (2007) Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides, Appl. Environ. Microbiol 73, 3183–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boursier ME, Moore JD, Heitman KN, Shepardson-Fungairino SP, Combs JB, Koenig LC, Shin D, Brown EC, Nagarajan R, and Blackwell HE (2018) Structure−Function Analyses of the N-Butanoyl L-Homoserine Lactone Quorum-Sensing Signal Define Features Critical to Activity in RhlR, ACS Chem. Biol 13, 2655–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssens JCA, Metzger K, Daniels R, Ptacek D, Verhoeven T, Habel LW, Vanderleyden J, De Vos DE, and De Keersmaecker SCJ (2007) Synthesis of N-Acyl Homoserine Lactone Analogues Reveals Strong Activators of SdiA, the Salmonella enterica Serovar Typhimurium LuxR Homologue, Appl. Environ. Microbiol 73, 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer AL, Hanzelka BL, Eberhard A, and Greenberg EP (1996) Quorum sensing in Vibrio fischeri: Probing autoinducer-LuxR interactions with autoinducer analogs, J. Bacteriol 178, 2897–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chhabra SR, Stead P, Bainton NJ, Salmond GPC, Stewart GSAB, Williams P, and Bycroft BW (1993) Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-L-homoserine lactone, J. Antibiot 46, 441–454. [DOI] [PubMed] [Google Scholar]
- 21.McInnis CE, and Blackwell HE (2011) Thiolactone modulators of quorum sensing revealed through library design and screening, Bioorg. Med. Chem. Lett 19, 4820–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Styles MJ, and Blackwell HE (2018) Non-native autoinducer analogs capable of modulating the SdiA quorum sensing receptor in Salmonella enterica serovar Typhimurium, Beilstein J. Org. Chem 14, 2651–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerdt JP, McInnis CE, Schell TL, Rossi FM, and Blackwell HE (2014) Mutational Analysis of the Quorum-Sensing Receptor LasR Reveals Interactions that Govern Activation and Inhibition by Nonlactone Ligands, Chem. Biol 21, 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore JD, Gerdt JP, Eibergen NR, and Blackwell HE (2014) Active efflux influences the potency of quorum sensing inhibitors in Pseudomonas aeruginosa, ChemBioChem 15, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jencks WP, Cordes S, and Carriuolo J (1960) The Free Energy of Thiol Ester Hydrolysis, J. Biol. Chem 235, 3608–3614. [PubMed] [Google Scholar]
- 26.Barnett R, and Jencks WP (1969) The Rates of Hydrolysis of Two Thiol Esters in Water, J. Org. Chem 34, 38–40. [Google Scholar]
- 27.Stevens CM, and Tarbell DS (1954) The Kinetics Of Basic Hydrolysis Of Some γ-Lactones And γ-Thiolactones In Aqueous Acetone, J. Org. Chem 19, 1996–2003. [Google Scholar]
- 28.Lin’kova MG, Kuleshova ND, and Knunyants IL (1964) Thiolactones, Russ. Chem. Rev 33, 493–507. [Google Scholar]
- 29.Morkunas B, Galloway WR, Wright M, Ibbeson BM, Hodgkinson JT, O’Connell KM, Bartolucci N, Della Valle M, Welch M, and Spring DR (2012) Inhibition of the production of the Pseudomonas aeruginosa virulence factor pyocyanin in wild-type cells by quorum sensing autoinducer-mimics, Org. Biomol. Chem 10, 8452–8464. [DOI] [PubMed] [Google Scholar]
- 30.O’Reilly MC, and Blackwell HE (2016) Structure-Based Design and Biological Evaluation of Triphenyl Scaffold-Based Hybrid Compounds as Hydrolytically Stable Modulators of a LuxR-Type Quorum Sensing Receptor, ACS Infect. Dis 2, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.