Abstract
Biochar is the carbon-rich product of the pyrolysis of biomass under oxygen-limited conditions, and it has received increasing attention due to its multiple functions in the fields of climate change mitigation, sustainable agriculture, environmental control, and novel materials. To design a “smart” biochar for environmentally sustainable applications, one must understand recent advances in biochar molecular structures and explore potential applications to generalize upon structure–application relationships. In this review, multiple and multilevel structures of biochars are interpreted based on their elemental compositions, phase components, surface properties, and molecular structures. Applications such as carbon fixators, fertilizers, sorbents, and carbon-based materials are highlighted based on the biochar multilevel structures as well as their structure-application relationships. Further studies are suggested for more detailed biochar structural analysis and separation and for the combination of macroscopic and microscopic information to develop a higher-level biochar structural design for selective applications.
1. INTRODUCTION
Biochar is a solid, carbon-rich product obtained from the thermal treatment of biomass pyrolyzed under an oxygen-limited atmosphere.1 The massive production of agricultural waste in the world provides an abundant source for biochar preparation. It was estimated that both China and the USA have ~1.4 billion tons of annual agricultural biomass waste production, which has the potential to produce ~420 Mton biochar per year.2,3 Other countries, such as Zimbabwe4 and Ghana,5 can produce 3.5 and 7.2 Mton of biochar annually, respectively. Increasing attention is being devoted to biochar applications in the fields of agriculture, climate change, environmental remediation, and materials science. Biochar, originating 2000 years ago in the Amazon Basin as terra preta black soil, is a long-lasting additive producing rich agricultural soil.6,7 In 2006, Johannes Lehmann commented that biochar could lock-up carbon from the atmosphere in the soil to mitigate climate change.8,9 Later on, the sorption behaviors of biochar toward different organic pollutants and heavy metals were investigated for environmental remediation in 200810 and 2009,11 respectively. Recently, biochars were developed into functional materials due to their cheap and sustainable properties.12 The diverse functionalities of biochar suggest multipurpose applications, but they require deeper understanding for smart biochar design.
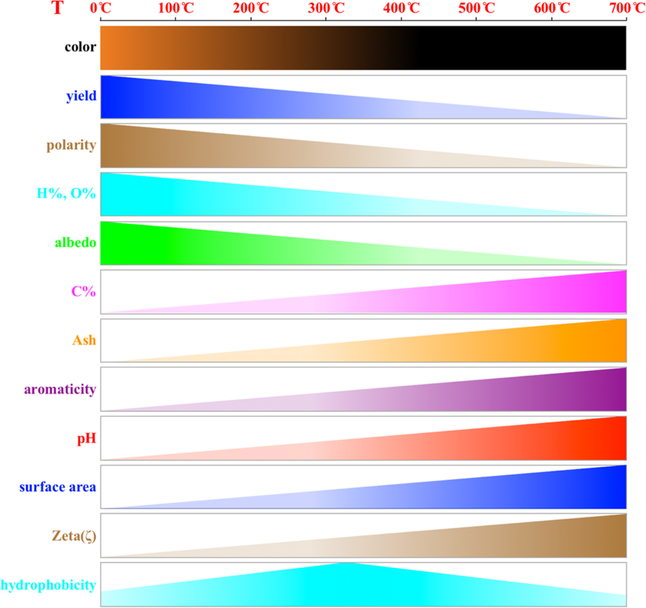
Many different biomass materials can be utilized to produce biochars for different purposes. The challenge is how to choose the right precursors and preparation procedures to obtain biochars for specific purposes. The preparation methods for biochars could be classified as pyrolysis, gasification, hydrothermal carbonization, and flash carbonization.13 Compared to biomass, which is a living, colorful, and water-rich material with a low surface area, biochar is a black carbon-rich product with a high surface area and no cellular structure. The transformation of biomass during pyrolysis alters the structure in different ways and further influences the properties of biochar. Typical properties vary with the pyrolysis temperature and are listed in Figure 1. With increasing pyrolysis temperatures (100~700 °C), the biomass color changes from its original color to black, and the product yield (typically 91%–14% for pine needle-derived biochars10), polarity, hydrogen content (H%) (typically 6.2–1.3% for pine needle-derived biochars10), oxygen content (O%) (typically 42–11% for pine needle-derived biochars10), and albedo of the biochars decreased. In contrast, the carbon content (C%) (typically 50%–86% for pine needle-derived biochars10), ash percentage (typically 13%–49% for rice straw-derived biochars14), aromaticity, pH, surface area (typically 0.65–490 m2 g−1 for pine needle-derived biochars10), and zeta potential (ζ) increased with increasing pyrolysis temperature (100–700 °C). The hydrophobicity of biochar increases and then decreases with pyrolysis temperature, as recent studies show that aromatic surfaces are intrinsically mildly hydrophilic, but adsorbed hydrocarbon contaminants provide the hydrophobicity of the aromatic surfaces in the midtemperature range.15 The diversity of biochar with regards to its properties, structures, applications, and mechanisms inevitably requires a clarification of the relationships among variables, especially for structure-application relationships, which are indispensable and of fundamental concern.
Figure 1.
Biochar property variations with increased pyrolysis temperatures.
Several reviews summarized the applications of biochar.16,17 For example, researchers reviewed the sorption properties of biochars toward both organic and inorganic contaminants.18,19 Applications for climate13 and agriculture20 were also reviewed. In 2015, Liu et al. suggested that biochars be considered as functional carbon platform materials considering their lost-cost and sustainability, thus increasing the applications of biochar into the field of novel materials.12 These reviews made great strides to summarize and characterize biochar applications, which will greatly promote research. However, the body of literature regarding the structure of biochar, especially with regards to biochar applications, is inadequate. Despite considerable research and reviews on biochar, a critical review on the structure and application relationships of biochar is urgently needed to understand and develop these novel materials.
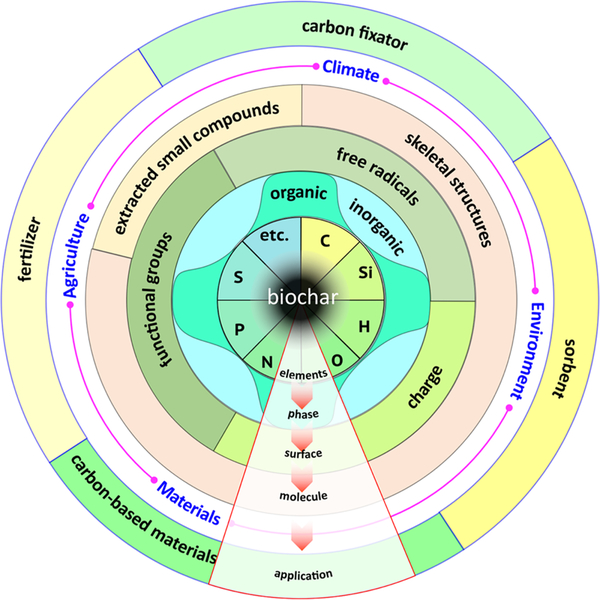
A large number of precursor biomass and different preparation methods offers selective utilization opportunities for biochar, despite the enormous uncertainty and heterogeneity of biochar structures. To investigate the aromatic structure of biochar, many researchers suggested graphene and/or graphite as an ideal model.1,21,22 Nonetheless, the biomass will go through aromatization and graphitization processes at high pyrolysis temperatures (≥500 °C), but the heterogeneous nature and structures of middle pyrolysis temperature-derived biochar are not fully comprehended. Additionally, besides the major element “carbon”, many other elements exist in biochar, which affects the corresponding role, function, and application of the material. The elements form different phases within biochar, which provides unique physical properties. The surface chemistry of biochar becomes critical because the interfaces between biochar and the aqueous phase provides important sites for sorption and reaction, including surface functional groups, surface radicals and surface charge. Heterogeneous biochar can be viewed as an aggregation of multiple molecules, containing both skeletal and small extracted molecules (Figure 2). To date, a few studies have focused on biochar structures, but a systematic summary detailing the structures of biochars is still lacking. In 2017, a quaternary structural model of biochars was proposed from the macroscopic to microscopic level for high pyrolysis temperature-derived biochars.21 In this review, we will interpret the multiple and multilevel structures of biochars from their elements, to phases, to surfaces, and to molecular structures (Figure 2). With the knowledge of biochar structures, their relationships to applications are further discussed in four different areas: agriculture, fertilizers; climate, carbon sequestration; environment, sorbents; and materials, functionalization. We summarize the advances in biochar structures and applications and begin to establish structure–application relationships with the overall goal to design smart biochars for environmentally sustainable applications.
Figure 2.
Heterogeneous structures and potential applications of biochar from a consideration of the elemental composition, phase, surface chemistry, and molecular structure.
2. MULTIPLE AND MULTILEVEL STRUCTURES OF BIOCHARS
2.1. Elements.
The elements present are the fundamental building blocks of biochars. During the pyrolysis of biomass, elements within the biochar experience different physicochemical processes and form different species and products, thus contributing to the diversity of biochars. The species and functions of these elements are listed in Table 1. These elements are part of global geochemical processing, and they have different mass percentages and functions within biochars. Among these elements, C, H, O, and N are the most common elements and generally contribute to the major structures of biochar; Si, Fe, P, and S show a wide range of mass percentages in specific biochars, whereas some elements (such as Si, N, P, S, and Fe) are nutrient elements for specific plants. In global geochemistry cycles, some elements (C, H, O, S, and N) are bidirectional in their cycling from the atmosphere to the earth, whereas other elements (P,and Si) are unidirectional due to the lack of atmospheric species. The multiple roles of multiple elements endow biochar with unique structures and functions. Thus, in this section, the chemical state, species, functions, applications, and geochemistry cycling involved with biochars are discussed.
Table 1.
Species, Functions, and Applications of Selected Elements within Biochars
| Elements | Species | Functions |
|---|---|---|
| c |  |
a) Key element within biochars; |
| b) Develop functional groups by linking to other elements; | ||
| c) Inorganic carbon contributes to the alkalinity and buffering properties of biochars;23 | ||
| d) Carbon sequestration.9 | ||
| Si | Inorganic: Silicon acid, polymeric Si, crystal Si. | a) Nutrient element; |
| b) Co-precipitator with heavy metals, 24 | ||
| c) Major element of biochar inorganic phases;14 | ||
| d) Protect the organic phase.14 | ||
| H | 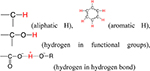 |
a) For association/ dissociation; |
| b) To form the hydrogen bond;25 | ||
| c) H/C atomic ratio is the aromaticity index of biochars.22 | ||
| O | 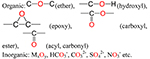 |
a) To form the functional groups; |
| b) Complexation with metal ions;26 | ||
| c) O/C atomic ratio is an index of the degree of aging of biochars.27 | ||
| N | 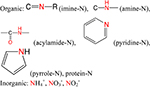 |
a) Nutrient element; |
| b) Improve the thermal stability; | ||
| c) Active sites for reaction and modification; | ||
| d) Nitrogen fixation.28 | ||
| P | 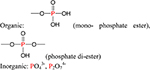 |
a) Nutrient element; |
| b) Precipitator with heavy metals.11 | ||
| S | a) Increase the solubility of carbon materials;29 | |
| b) Solid acid catalyst;30 | ||
| c) Nutrient element. | ||
| Fe | Fe2O3, Fe3O4, FeO, α-Fe, Fe3C, FeO(OH), Zero valent iron, other metal complex | a) Provide magnetism;31 |
| b) Increase the sorption ability for anions;31 | ||
| c) Promote graphitizing during pyrolysis;32 | ||
| d) Catalysis/activator for organic pollutant degradation.33 | ||
| Mg | MgO, MgCO3, other metal complex | a) Increase anions sorption;34 |
| b) CO2 capture;35 | ||
| c) Nutrient element. | ||
| Mn | MnOx, Mn(OH)x, other metal complex | a) Increase heavy metal sorption;36 |
| b) Increase capacity. | ||
| Ni | Ni(OH)2, NIO, Ni2O3, other metal complex | a) H2 evolution; |
| b) Catalysis the formation of syngas during pyrolysis.37 | ||
Carbon.
Carbon is the most important and the most abundant element in biochars. The carbon species within biochars can be classified as carbonate and bicarbonate in the inorganic phase and aliphatic carbon, aromatic carbon and functionalized carbon in the organic phase (Table 1). It is quite difficult to precisely differentiate these carbon species. During the pyrolysis process, the inorganic carbon may transform from hydrated carbonate to bicarbonate, carbonate or even be lost in the form of CO2, depending on the type of inorganic crystal.38 As for the organic carbon, the carbon transformed from cellulose/hemicellulose/lignin in the biomass becomes aliphatic carbon in the middle pyrolysis temperature-derived biochars, and further also results in aromatic carbon at high pyrolysis temperatures after dewatering, cracking, and aromatization reactions.39 These transitions were confirmed by Fourier transform infrared spectroscopy (FT-IR),10,40 Raman spectroscopy,41 cross-polarization magic angle spinning (CPMAS), solid 13C nuclear magnetic resonance spectroscopy (NMR),42 and other characterizations. More recently developed technologies were applied to gather more chemical information about the carbon species in biochar, such as two-dimensional 13C NMR.43,44 These characterization methods can distinguish between atomic and alkyl carbons; however, it is still difficult to differentiate the functionalization of the carbon. The chemical species of carbon is critical for applications in agriculture, ecosystems, and carbon sequestration because the chemical species determine the stability and reactivity of the carbon in biochar.
Silicon.
Although not all of the biomass or agricultural waste that is pyrolyzed into biochars contains silicon, silicon-rich biomass such as rice straw, rice husk, and corn straw are vital feedstocks for biochar production and essential plants for food production.14,45 In fact, silicon is a required nutrient for silicophilic plants (such as rice, maize, wheat, and barley),46 protecting these plants from insects, heavy metals, infestation, light, and drought.47–49 Moreover, our previous studies found that the silicon in biochars contributed to the sorption and retention of heavy metals (such as aluminum26,50 and cadmium24) in aqueous systems. The dissolution of silicon from biochars to form monosilicic acid in water25 was found to be important in the biochar sorption of metals.
Our research group first investigated silicon transformation in rice straw derived biochars under different pyrolysis temperatures.14 In the biomass, silicon existed as phytoliths in the form of silicic acid and polymeric silicon that can easily dissolve.45 During charring, the silicon becomes dehydrated and polymerized, and upon further pyrolysis, and the polymeric silicon was partially crystallized. Rice straw biochars pyrolized at high temperature display higher silicon dissolution than low pyrolysis temperature-derived biochars. Additionally, Si-rich biochars show much higher silicon dissolution tendencies than their corresponding ash or soil, which contain large amounts of silicon in the form of silicate.14,51 Because many arable lands in the world are suffering from silicon depletion, the Si-rich biochars with high silicon dissolution capacities may provide a slow-release Si-fertilizer for soil.
Silicon represents a major fraction of the inorganic phase in Sirich biochars (composing ~38% of the mass in rice straw ash),14 and it seems to be separated from the organic matter in biochars. However, according to the results of scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS), a mutual protection exists between carbon and silicon under different pyrolysis temperatures. The carbon layer protects the silicon from dissolution under low pyrolysis temperatures, and the silicon layer protects the carbon from loss under high pyrolysis temperatures.14 Our study further indicated that the silicon may promote recalcitrance and stability in biochars.52
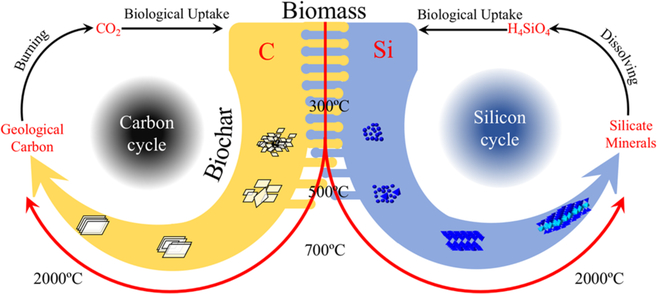
Figure 3 provides a schematic of the coupling cycles of carbon and silicon in biochar and the terrestrial ecosystem. In the environment, the Si-rich biomass takes-up CO2 from the atmosphere and silicic acid from the soil solution. However, with increased charring temperature, the interlaced C−Si content starts to separate and form corresponding crystals, which are stable over long periods. With burning and dissolution over a long period of time, the carbon and silicate minerals release CO2 and silicic acid which becomes available to plants, thus completing the geochemical cycle. This type of C−Si coupling illustrates the vital role that biochars play in the geochemical cycling of these essential nutrient elements. It also indicates the need for more research for a better understanding of the role of biochar in global carbon and silicon balances as well as the important agricultural function of silicon in biochar.
Figure 3.
Carbon and silicon cycles along with the carbon−silicon coupling relationship in biochar and the terrestrial ecosystem.
Hydrogen.
There are three species of hydrogen that exist in biochars: aliphatic hydrogen, aromatic hydrogen, and active functionalized hydrogen (Table 1). For typical biochar, the hydrogen weight percentage in organic matter decreases from ~6% at low pyrolysis temperatures to ~1% at high pyrolysis temperatures.10 The low hydrogen content makes it more difficult to characterize biochars by FT-IR or H NMR. Therefore, the speciation of hydrogen within biochar is qualitatively assessed by elemental analysis and characterizing other elements, such as carbon or oxygen. Because the H/C atomic ratio for aliphatic organic matter in biochars is approximately 2.0, and the H/C atomic ratio of aromatic organic matter decreases with larger fused aromatic clusters, the H/C atomic ratio is widely regarded as an aromaticity index for biochars.22 Compared to the stable aliphatic and aromatic hydrogen in biochars, more uncertainty exists regarding the active functionalized hydrogen. Active hydrogen atoms in functional groups can be associated/dissociated under proper pH conditions, and this is easily probed by a pH meter. The association/dissociation interaction may lead to formation of intra-and intermolecular H-bonds, causing different polarities between the bulk biochar and its surface.53 Hydrogen bonds (D−H···:A) are generated between the biochar surface and ionizable molecules by sharing protons.54 Therefore, hydrogen acts as an important building block for biochar structures, and it plays a key bridging role in biochar sorption toward ionizable molecules.
Oxygen.
In the inorganic phase of biochars, oxygen mainly exists in the form of metallic oxides, hydroxides, or inorganic clusters such as carbonate, bicarbonate, and sulfate (Table 1). Inorganic oxygen is generally stable and contributes to the alkalinity of biochars. In the organic phase of biochars, oxygen atoms are mostly attached to carbon atoms, forming different types of functional groups, including hydroxyl, epoxy, carboxyl, acyl, carbonyl, ether, ester, and sulfonic groups (Table 1). Because the oxidation process in natural environments regularly induces oxygen into the organic structures of biochars, the O/C atomic ratio is thus widely considered to be an indicator of the degree of aging/oxidization undergone by biochars.55–58 This oxidation induces the formation of carboxyl groups, and it could somehow benefit the sorption of aluminum59 and lead.60 The release of CO2 from carboxyl groups may be an important reaction in the labile fraction of biochars, although the structural framework to which the carboxyl groups were attached might be considered to be stable carbon. More details are discussed in the section on surface functional groups because oxygen-containing groups contribute significantly to biochar functional groups.
Nitrogen.
In biomass, nitrogen mainly contributes to the peptide bond in protein. Normally, biochar feedstocks such as plants and wood are nitrogen-poor materials due to their rather low protein content, whereas materials such as grass or leaves contain relatively higher protein contents and are nitrogen-rich materials. For example, casein, grass, and wood-derived biochars were reported as containing ~15%, ~7%, and 0.3−1 wt % nitrogen, respectively.10,61 Although many researchers have studied nitrogen-rich biochars, only a few focused on the nitrogen speciation within biochars. Theoretically, the structures related to nitrogen contain pyrrole, pyridine, amine, imine, acylamide, nitroso, and nitro groups (Table 1). Reports indicate that the total nitrogen content within biochars increases and then slightly decreases with increasing pyrolysis temperature.10,61,62 Knicker et al. identified a model biochar and suggested that the N forms are mainly pyrrole-N, amide-N, and pyridine-N.63 During pyrolysis, peptide-N bonds were transformed to N-heteroar-omatic carbon compounds, whereas some amide-N within biochars decreased with increasing pyrolysis temperature.61 Pietrzak et al.64,65 studied the reaction of ammonia/urea with coal and found that the nitrogen was introduced at 500−700 °C in the form of pyridinic-N and pyrrolic-N as well as imine-N, amine-N, and amide-N. For the pyridinic-N and pyrrolic-N, many studies on carbon materials (such as graphene66) were conducted with nitrogen-doped carbon materials to modify the electronic band structures, and they acted as the active sites for reaction. This may explain the observations that nitrogen-rich biochars show greater heat resistance but less chemical-oxidation stability than nitrogen-poor biochars.61 After application into soil, the fate of extractable N becomes of research interest. By applying different extraction methods, different species of extractable N, including that from ammonia, amino sugars, amino acids, and total hydrolyzable forms, were found to decrease with pyrolysis temperature.67
In fact, there is uncertainty whether the extracted N comes from organic matter or inorganic matter in biochar. Nitro- and nitrito-N were also found in biochars derived from agricultural biomass (rice husk), forestry waste (sawdust), and wetland plants (Acorus calamus) at 650 °C.68 However, the nitrogen content of biochars is usually too low to be considered a nitrogen fertilizer alone. The biochars with high C/N ratios have limited N availability, and additional nitrogen fertilizer might be needed to improve microbial and plant growth in soils.69–71 For example, it was reported that 5% biochar combined with mineral fertilizer (containing 0.0134% N) shows remarkably higher promotion of oat growth than biochar alone.72
The nitrogen dynamics of biochars applied as additives in soil systems are of considerable interest, including the effects on nitrogen-sorption, nitrogen-fixation, nitrification, and N2O greenhouse gas (GHG) emissions. Multiple studies indicate that biochars show a high affinity for the sorption of ammonium nitrogen (NH4+) by cation-exchange, but no influence on the removal of nitrate-N.73–77 Ammonia sorbed onto biochars is reported to be bioavailable,78 and thus biochars are regarded as a nutrient-retention additive to maintain the nitrogen in soil.73,79 It is a soil “conditioner,” which improves the tilth, water holding capacity, and nutrient holding capacity of soil.1 Additionally, it stabilizes the carbon and nitrogen, thus reducing the emissions of GHGs (CO2 and N2O). Biochars were reported to increase the biological nitrogen fixation proportion of common beans from 50% without biochar to 72% with 90 g kg−1 biochar added,28 and stimulated both the nitrification and denitrification processes with a result of reducing N2O emissions.80 The reduction order of N2O emissions by giant reed-derived biochars additives in soils differed with pyrolysis temperatures, following the order BC200 ≈ BC600 > BC500 ≈ BC300 ≈ BC350 > BC400 (BC refers to biochar, the suffix number represents the preparation pyrolysis temperature of biochar).81 The “electron shuttle” of biochar may facilitate the transfer of electrons to denitrifying microorganisms as well as the “liming effect” of biochars, which promote the reduction of N2O to N2, leading to a 10−90% decrease in N2O emissions in 14 different agricultural soils.82 The carbon and nitrogen cycles are affected in three ways by biochar. (1) During biochar processing, carbon becomes more recalcitrant and nitrogen becomes less available, thus stabilizing the biochar product.83 (2) Microbial and plant growth in soils with biochar additives are limited by the low nitrogen content, and less microbial growth stabilizes and reduces carbon mineralization.84 (3) The sorption of NH4+ onto the carbon components of biochars improves the nitrogen retention in soil systems.
Phosphorus.
Unlike carbon, hydrogen, oxygen, and nitrogen, which are lost during the pyrolysis process, phosphorus does not volatilize at temperatures below 700 °C,16 leading to an enhancement of phosphorus under typical pyrolysis temperatures. Ngo et al.85 reported an overwhelming dominance of inorganic phosphorus compared to organic phosphorus in bamboo-derived biochar produced at 500−600 °C. Considering the fact that the phosphorus in biomass is mostly organic (such as monophosphate and phosphate diesters62) (Table 1), the phosphorus species transformation during pyrolysis is dramatic and discussed by Uchimiya and Hiradate.86 Using 31P NMR analysis, they proposed that the phytate is converted to inorganic phosphorus from manure and plant-derived biochars at 350 °C.86 Regarding inorganic phosphorus, the pyrophosphate (P2O74−) in plant material was persistent in biochars pyrolyzed at temperatures up to 650 °C and the orthophosphate (PO43−) became the sole P-species for manure biochars at pyrolysis temperatures higher than 500 °C.86
Similar to silicon, phosphorus is an important nutrient for plant and microbe growth. The geochemical cycle of phosphorus is also unidirectional from land to sea because there is no gaseous phosphorus species, and it is difficult to transport ocean phosphorus back to the land ecosystem except via the slow crustal plate movement of subduction. Thus, making a slow releasing P-additive in the form of biochar was proposed to retain more phosphorus in soil.87 The effects of the pH and other anions and cations on the release of phosphorus were further investigated.88 These results report that high pH soils greatly hinder the release of phosphorus.88 However, other anions could enhance the orthophosphate (PO43−) release due to competing ion exchange reactions.89 Competing cations will decrease the release of P due to the formation of precipitates.89 Furthermore, phosphate in biochars could immobilize some heavy metals by precipitation, such as β-Pb9(PO4)6 precipitates11or insoluble hydroxypyromorphite Pb5(PO4)3(OH).90 In summary, phosphorus in biomass was transformed from organic P to inorganic P via carbonization and can then serve as a nutrient for the soil system or as a sorption site for heavy metals.
Other Elements.
In addition to the major elements mentioned above, there are other trace elements that could exist in biochar. Under most circumstances, these elements constitute only a small fraction of biochars (typically with the concentration level of mg kg−128). Dopants may also be added to provide special chemical properties to biochars. Nutrient elements such as Na, K, Ca, Mg, and Mn88,91–96 constitute important fractions of biochars, and other elements designed to functionalize biochars may be added. For example, ~20% Mg (in the form of MgO) was loaded onto biochars and increased CO2 capture from ~0.1 to ~5 mol CO2 per kg biochar.35 By inducing sulfuric acid groups onto biochars, a solid acid catalyst can be formed for transesterification,97,98 hydrolysis reactions,30,99 and biodiesel production.100,101 Sulfonation is known to increase the solubility of carbon materials in aqueous systems.29 Early in 2011, our research group31 reported on novel magnetic biochars prepared by the coprecipitation of Fe3+/Fe2+ onto orange peels followed by pyrolysis. These magnetic biochars (with ~13 wt % Fe) show efficient sorption performance toward organic pollutants and almost thrice the sorption capacity toward phosphate compared to nonmagnetic biochars pyrolyzed at 700 °C. Magnetic biochars can be easily separated from wastewater and thus have great potential for environmental and agricultural applications. Manganese oxide-modified biochars have been reported to benefit the sorption of arsenate, lead, and copper.102,103 Moreover, some elements have a great impact on shaping the biochar structure, such as Zn as a pore-maker and Fe for its promotion of graphitization.32 In addition, some trace metals (such as Cu and Al) show promise for producing favorable structural properties in biochars, but they must be evaluated for their toxicity prior to being adopted as soil additives.104,105 There are many other elements that could be bonded to biochars, but we must first understand their toxicity, speciation within biochars during pyrolysis, and influence on biochar carbon stability and structure.
2.2. The Phases.
Organic Phase.
The bulk of biochar was formed via forming massive chemical bonds between the mentioned elements. Previous researchers, without knowing the exact structure, considered biochar to be a solid phase. Furthermore, this solid biochar could be subdivided into various different phases. Our knowledge of biochar phases continues to evolve over time (Figure 4). Besides classifying biochars into organic and inorganic phases, we may also consider their aliphatic and aromatic chemical nature.106–108 In terms of their sorption mechanisms, we can differentiate biochar into an organic-partitioning and a surface adsorption phase, which comprises the total sorption of organic molecules.10,109–111 Furthermore, on the basis of solubility, biochar can be classified into a dissolved phase and an undissolved phase;25,112–114 and in view of carbon stability, a labile phase can be defined in which the carbon fraction can be mineralized under natural soil conditions, while the stable phase remains recalcitrant.115–118 With increasing pyrolysis temperature, biochar undergoes dehydration, disruption of the organic components, and successive aromatization. The aliphatic labile portion and dissolved-phase fraction decrease, whereas the aromatic stable portion and undissolved-phase increase. It was reported that the labile carbon percentage of sugar cane-derived biochars decreased from 1.08% at a 350 °C pyrolysis temperature to 0.29% at a 550 °C pyrolysis temperature.118 Additionally, the dissolved carbon percentage decreased from ~14% in 100 °C-derived dairy manure biochar to ~0.2% in 700 °C-derived dairy manure biochar at a pH of ~12.25
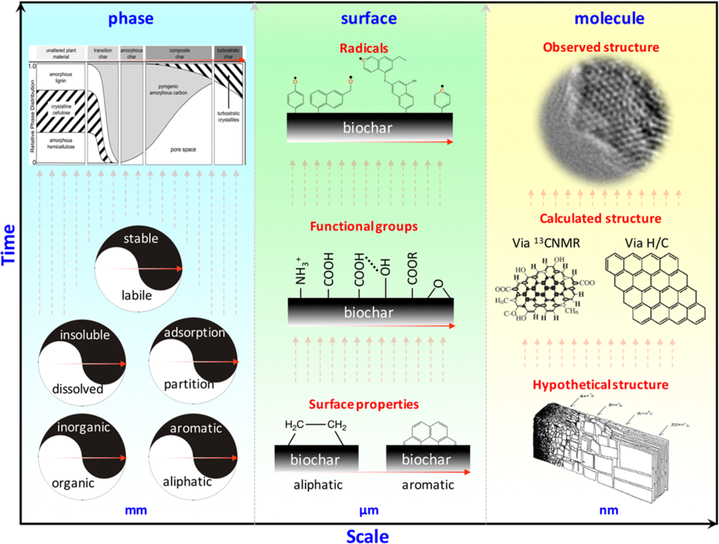
Figure 4.
Knowledge evolution concerning the phases, surface chemistry, and molecular structure of biochars as a function of the inverse biochar scale (size) and research time. Inner figures were cited from refs 1, 21, 22, 40, 119. Copyright 2010, 2016, 2017 American Chemical Society.
Recently, four different chemical phases and physical states were proposed: (1) a transition phase with the preserved crystalline character of the precursors; (2) an amorphous phase with cracked molecules and aromatic polycondensations; (3) a composite phase with poorly ordered graphene stacks; and (4) a turbostratic phase with disordered graphitic crystallites.40 The aliphatic phase, labile phase, partition phase, and dissolved phase are similar but not the same as each other. The aromatic phase, stable phase, adsorption phase, and undissolved phase are also similar but not the same as each other. For example, unlike the other phases where the pH has little effect, the dissolved carbon phase is strongly affected by the pH.25 Part of the dissolved carbon phase can be oxidized to CO2, indicating that the dissolved phase partially contributes to the labile phase.113 The labile carbon fraction is related but not the same as the dissolved carbon fraction.117 A portion of the biochar aromatic phase represents the labile form in biochars but may not be persistent.108 Even though there are various definitions and research on the organic phase of biochar, more investigations are needed, especially regarding the separation methods, structure−function relationships, biological effects, and environmental fate of these different phases including their nanoformed particles.
Inorganic Phase.
Because of the existence of minerals in biomass, biochars are actually a mixture of interlaced inorganic−organic structures. These inorganic phases interact with and influence the organic phases in biochars and thus impact their properties, making the understanding of mineral phase transformations during pyrolysis of great importance.
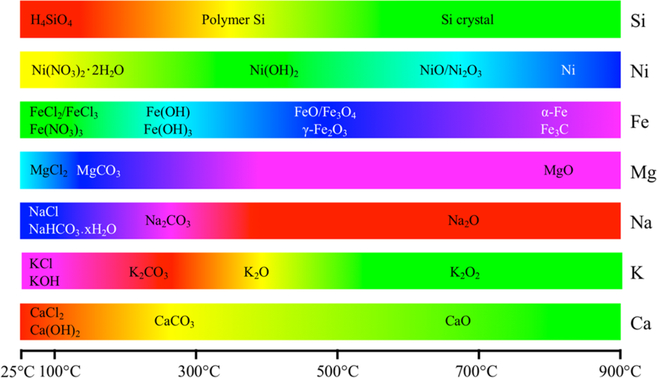
Figure 5 summarizes the possible phase transformations of several typical mineral components generated during biomass pyrolysis. Possible reactions that could occur in the inorganic phase during pyrolysis include dehydration, dechlorination, polymerization, decarboxylation, crystallization, and reduction. For example, the silicic acid within the rice straw will polymerize to form polymer silicon and further crystallize to quartz with increasing pyrolysis temperatures.14 Commonly, with increasing pyrolysis temperatures under limited oxygen, the inorganic matter starts as chloritized, hydroxylated, or carbonated metals and then transitions to metallic oxides, some of which can be reduced to pure metals because of the strong reducing atmosphere during pyrolysis (Figure 5). By taking advantage of this transformation process during biochar preparation, researchers are able to create magnetic,31 zerovalent Fe,33 and zerovalent Cu coated biochars.120 Under higher pyrolysis temperatures, calcium carbide (CaC2), an important starting material for the production of many chemicals, could be manufactured by mixing fine biochars with CaO.121 Figure 5 gives the mineral speciation of several elements in biochar. Studies on the inorganic phase of biochars are relatively easier to define than the organic phase due to the higher atomic sensitivity of metal atoms for characterization. Still, there are many other metals, species and mixed crystals that remain unknown and require further research.
Figure 5.
Possible phase and chemical transformations of several mineral components during biomass pyrolysis under limited oxygen. For each inorganic element, the color illustrates the corresponding transition from one state to another. Cited from the corresponding references: Si,14 Ni,37 Fe,37,122,123 and Mg.34,35
The functions of inorganic matter in biochars include but not limited to the following: providing magnetism;31 catalyzing the decomposition of organic pollutants and metals-involved catalysis reactions;33,37,120,124–127 and providing pore-forming agents,128–130 carbon sequestration agents,35,131,132 slow-release nutrients,87,88,133–136 heavy metal coprecipitators,11,103,137–144 and heavy metal stabilizers.145 Magnetism emanates from the loading of γ-Fe2O331 and the catalysis is from active zerovalent iron or transition metals,33,37,120,124–127 and pore-forming agents include KOH, ZnCl2, and others.128–130 The effect of carbon fixation depends on the mineral type. A five year laboratory experiment indicated that 0.5%−8.9% of biochar carbon was mineralized, and manure-derived biochars mineralize faster than plant-derived biochars,131 whereas copyrolysis with kaolin, calcite (CaCO3), or calcium dihydrogen phosphate (Ca-(H2PO4)2) decreases 0.3%−38% loss of carbon from biochars during tests with potassium dichromate oxidation.132
By converting the easy-to-dissolve metallic elements to their difficult-to-dissolve species, heavy metals can be stabilized within biochars.145 In summary, mineral species within the biochar are transformed by the pyrolysis temperature and affect the biochar structure and applications in various ways. However, more knowledge is needed on the dissolution behavior, toxicity, carbon stability, nanoeffects, and possible catalytic ability of inorganic nanoparticles.
2.3. The Surfaces.
The surface of biochar is the main interface where many chemical and biological reactions and interactions occur. Knowledge of the biochar surface chemistry has evolved over time (Figure 4). After categorizing the nature of the biochar surface (aliphatic or aromatic), the functional groups on biochar were elucidated.25 Following that came the discovery of free radicals on the biochar surface.146 In this section, the surface functional groups, charge, and free radicals are discussed.
Surface Functional Groups.
Characterizing the surface functional groups of biochars can explain their surface chemistry and reactions. Besides aliphatic and aromatic groups, surfaces may include hydroxyl, epoxy, carboxyl, acyl, carbonyl, ether, ester, amido, sulfonic, and azyl groups. Characterization methods have been applied to analyze these functional groups, such as solid-state 13C NMR,147 FT-IR,148 X-ray photoelectron spectroscopy (XPS),149 and C-1s near-edge X-ray absorption fine structure spectroscopy (NEXAFS).149–153 Although there are many different types of functional groups and advanced technology for the analysis of functional groups, the precise characterization to distinguish all functional groups was still difficult.
To simplify this process, some studies have focused on surface phenolic−OH, carboxyl, carbonyl, and ester groups as functional groups on biochars.101,154 These are among the most abundant functional groups at the surface of biochars.149,154 Another reason is that these functional groups can associate/dissociate with protons in the environment, thus leading to hydrogen bondinduced sorption,59,143,155–157 biochar alkalis,23 pH buffering,158 hydrophilicity/hydrophobicity, surface charge alterations, a cation exchange capacity (CEC),158 and capacitance improvement.159 Consequently, defining the dissociation constants (pKa) and the contents of these functional groups become vital to understanding the surface properties of biochars. Boehm titration methods have been widely applied to measure the carboxylic acid fraction, lactonic group fraction, and phenolic functional groups for organic materials by NaHCO3, Na2CO3, and NaOH, respectively.160–162 This method offers an easy way to measure different functional groups without knowing their pKa values.
Recently, we proposed an integrated method combining FT-IR and a modified acid/base consuming model to quantitatively identify the chemical states, dissociation constants (pKa), and contents of surface functional groups within dairy manure-derived biochars.25 This model offers a method to quantify the contents of different functional groups, but it still requires further investigation to improve the detection accuracy and parameter-fitting results due to the complexity of biochars.
Surface functional groups vary greatly with pyrolysis temperature. The original lignocellulose H-bond networks in biomass were removed, and free hydroxyls were transformed into carboxyls during the pyrolysis process.163 FT-IR indicated that oxygen-containing groups decreased markedly with increasing pyrolysis temperatures.10,164 Two-dimensional (2D) 13C NMR data showed that carbonization resulted in a dehydroxylation/dehydrogenation and aromatization process, along with cleavage of O-alkylate and aromatic O−C−O groups as well as the formation of aromatic C−O groups.43
The functional groups on biochar not only served as sorption sites for metal cations26,50,155,157,165 and ionizable organic pollutants166,167 but also provided a perfect and cheap active site for the surface chemistry modification of biochar, including oxidation,60 amination,168 and sulfonation.98 For example, the oxidation of biochar can induce more carboxyl groups after soil aging149 or treatment with hydrogen peroxide.60 The amino-modification of biochar enhanced the sorption of copper ions.168 Additionally, sulfonation increased the stearic acid esterification speed of pine pellet biochar.98 Other studies have reported the introduction of sulfonic acid as benefiting the solubility of carbon materials.29 As a vital part of the biochar surface, functional groups are important interfacial sites, and more studies are required to quantify them, their contents, their chemical states, and their corresponding applications.
Surface Charge.
We are lacking sufficient knowledge of the surface charge on biochars. The surface charge makes biochar attractive or repulsive to other charged molecules24,169 and bacteria,170 forming an electrical double layered structure. Thus, biochars display colloidal properties similar to the properties of soil. The origin of the surface charge on biochars is believed to be its aliphatic/aromatic surface and the dissociation of functional groups, such as carboxyl moieties.163 Therefore, the surface charge of biochars is highly correlated to the pH in the aqueous phase. It remains negative over the natural pH range (pH = 4−12), but in strongly acidic systems, the surfaces of biochars are positively charged. Once the pH increases above a pH of 4, the surfaces of biochars become more negatively charged.171 Nonetheless, electrostatic interactions impact the transport of ions, nanoparticles, and biochar particles.172 This influence is strongly regulated by the solution pH.
Surface Free Radicals.
In 2014, Liao et al. published a comprehensive description of the biochar’s surface free radicals of biochars.173 They reported abundant and quite persistent free radicals within biochars derived from corn stalks, rice and wheat straws as observed by electron paramagnetic resonance (EPR).173 The radical species transitioned from oxygen-centered radicals to carbon-centered and oxygen-centered combined radicals with increasing pyrolysis temperatures.173 Free radicals can be generated during the charring process.173–175 Free radical transitions were monitored via in situ EPR observations.173 Aromatic carbon atoms played an important role in stabilizing the surface free radicals due to their electron-rich aromatic surface at high pyrolysis temperatures.
Although it is difficult to know the specific structures of all free radicals, phenoxyl and semiquinone radicals were typically assumed to be the oxygen-centered radicals.173 Strong ·OH radicals in the aqueous phase were induced by free radicals in biochars. The free radicals in biochars may be a major reason for the inhibition of the germination of plant seeds, root and shoot growth retardation, and damage to the plasma membrane.173 Other studies indicated that abundant free radicals on the biochar surface could react to activate hydrogen peroxide,146 persulfate176 and benefit the degradation of organic contaminants, such as 2-chlorobiphenyl,146 diethyl phthalate177 and polychlorinated biphenyls.176 Free radicals within biochars may be the reason for the electron accepting capacity in high temperature pyrolysisderived biochars, as measured by mediated electrochemical analysis.178 Surface radicals on biochars are essential reactive sites, and more attention to their persistence, toxicity, pollutant degradation ability, and environmental impact should be paid.
2.4. Molecular Structures.
Extracted Small Compounds.
As one part of biochar molecules, the extracted small compounds have received much attention due to their high mobility and possible toxicity. It was reported that the released fraction from two softwood pellets-derived biochars with high volatile organic compounds (VOCs) almost completely inhibited 94% and 100% of cress seed germination under 5% biochar treatment.179 After excluding the effects of salt and water stress, researchers believed that the mobile organic compounds were responsible for the adverse effects on germination.180 However, it is still unclear which compounds are responsible, although phenolic compounds are the leading suspect.180 Additionally, full identification remains a big challenge due to their low concentration on biochars, lack of viable extraction methods, and theoretical limitation for interpreting mass spectral results. Even if these compounds could be identified, quantification becomes difficult due to the lack of standards and low concentrations. In that case, using a similar compound as a standard or applying relative contents calculated from the peak area could be compromise methods.181,182
The extraction of small molecules from different biochars is reported in Table S1.183–190 Regarding content analysis of the compounds, the depolymerization and C3-side chain shortening occurred at approximately 200−300 °C. The demethoxylation of syringyl and gualacyl lignins appeared at 300−400 °C; dehydroxylation and demethoxylation to remove methoxyl groups appeared at 350−400 °C; complete dehydroxylation of lignin and other biopolymers occurred at 400−500 °C; condensation into aromatic clusters occurred at 300−500 °C; and the partial removal of alkyl bridges between aromatic moieties occurred at 450−500 °C.182 The related results showed that short carbon chain aldehydes, furans and ketones were the major adsorbed VOCs on biochars pyrolyzed below 350 °C, whereas adsorbed aromatic compounds and longer carbon chain hydrocarbons dominated in biochars produced under 350 °C.
Regardless of the impact of the pyrolysis temperature, gasified biochars contained higher polycyclic aromatic hydrocarbons (PAHs) and more bioavailable PAHs than produced by fast pyrolysis, which were generally less than biochars derived at slow pyrolysis temperatures.185 Normally, the total PAH content within a biochar was ~60 mg kg−1, which also depends on the type of precursors. For example, biochars produced from rice husks contained 64 mg total PAHs/kg, whereas wood biochar produced 9.5 mg kg−1 PAH.189 Hydrothermally carbonized plant materials displayed much higher VOC contents (0.2−1.6 wt %), including benzenic, phenolic and furanic volatiles along with other aldehydes and ketones.183 Mostly, the total PAHs refers to the 16 USEPA PAHs, which are considered toxic and persistent. They generally decrease with increasing pyrolysis time and temperature,185 and some researchers suggested that the solvent-extractable PAH concentrations in biochars reach a peak concentration at a pyrolysis temperature between 400 and 500 °C.187,191 However, some researchers argued that the total PAHs extracted from biochars are quite low in most cases, and they are below the existing environmental quality standards (maximum acceptable concentration of 8 mg kg−1 for Norway).184,185
The conversion of sewage sludge containing noticeable PAHs to biochar could greatly reduce the PAH content,190 and the addition of biochar could adsorb the dissolved PAHs from sewage sludge.192 Thermal drying at 100, 200, and 300 °C can greatly reduce the PAH concentrations of biochar within 24 h at the levels of 33.8−88.1%, >93.3%, and >97%, respectively.188 In addition, blending with low VOC biochar (containing 90% low VOC biochar) was effective in reducing the toxicity of small compounds by at least 50%, whereas simple open-air storage was insufficient.179 In short, the extraction of small compounds comprised only a small part of the total biochar mass (generally <5%), whereas a large fraction of the small molecules in biochar remain unknown. The mobility, toxicity, concentration, and bioavailability are the main questions regarding these small compounds extracted from biochars. Whether biochar is a source or sink for small extracted compounds and the role of these compounds in the formation of surface radicals remain unclear.
Skeletal Structures and Models of Biochars.
The small compounds extracted from biochars differ depending on various precursors (Table S1) and pyrolysis temperatures. However, the small extracted molecules comprise only a small part of biochar mass, less than one-hundredth. Thus, a large amount of biochar mass remains nonextractable and unknown. Theoretically, the unextracted fraction of biochars should contain larger molecular weight compounds and stronger chemical bonds than the extracted compounds and are thus difficult to detect. As it contains heterogeneous structures, biochar is postulated to contain multiple molecules with different molecular weights following the Gaussian distribution law,21 similar to the molecular weight distribution in polymers. Therefore, the research focus of biochar structures should consider both the entirety of the structure and small-scale features. Studying the extracted small compounds will benefit the understanding of reactions and properties at the micro- and nanoscales. Meanwhile, an integrated and skeletal structural view will help to elucidate the heterogeneous structures of biochars and will assist in the modification, engineering, and application of biochars for solving new problems, especially as we begin to see their potential as integrated global materials.
To investigate the skeletal structures of biochars, solid 13C NMR, Raman spectroscopy, and the H/C atomic ratio were applied. Understanding biochar skeletal structures evolved from a hypothesis to a calculated structure to an actual observation (Figure 4). According to the ideal development of biochar structures proposed by Downie et al.,1 the skeletal carbon within biochars experienced amorphous aromatic carbon, conjugated aromatic carbon, and graphitic carbon successively with increasing pyrolysis temperatures.1 Later on, Brewer et al. characterized the structures of biochars from fast pyrolysis and gasification, and they proposed aromatic cluster models of these biochars calculated by quantitative 13C NMR.119 The skeletal structures of the chars in Terra Preta soils were analyzed by quantitative 13C NMR spectroscopy and were shown to have ~6 fused aromatic rings with abundant COO− groups. Recently, we proposed a simplified method to calculate the fused aromatic clusters of biochars derived from different pyrolysis temperatures by the H/C atomic ratios according to the rectangle-like polycyclic aromatic ring model.22 The aromatic cluster structures of biochars derived at pyrolysis temperatures of 500 and 700 °C were further calculated as owning 5 × 5 and 10 × 10 rectangle-like polycyclic aromatic structures with the aromatic cluster sizes of 1.25 nm × 1.25 and 2.5 nm × 2.5 nm, respectively.22 In addition, the H/C atomic ratio could also act as a smart linkage to build a quantitative relationship based on the pyrolysis temperatures, aromatic clusters, and sorption properties of biochars, as summarized from published and unpublished data from worldwide laboratories with diverse precursor materials.22 Afterward, an observation of clear aromatic clusters within biochars was reported by applying high-resolution transmission electron microscopy.21
The Stone−Wales transformation was also first reported in biochar,21 which is possibly the reason why fullerenes can be found in the natural environment.193 These studies provide an integrated and skeletal structure in view of aromatic clusters especially attributed to biochars produced under high pyrolysis temperatures. Although alkyl moieties may coexist with the aromatic clusters in the high pyrolysis temperatures-derived biochars,147 the hypothesized and observed aromatic structures of biochars provide a simplified and integrated structural model by considering biochar as a whole. These models do not deny the existence of other small compounds though, either internal to biochar or on the surface. We recognize that further studies are still needed to improve the structure to account for more varied observations. In addition, challenges remain to understand typical skeletal structures for biochars derived at middle pyrolysis temperatures.
3. BIOCHAR APPLICATIONS
3.1. Carbon Sequestration in Soil.
In the field of climate, the most important application of biochar is to capture the carbon and store it in biochar amended soils, which makes the stability of biochar essential for the control of climate change and for implementing negative GHG emissions. Measuring the carbon stability of biochars is key to evaluating carbon fixation.
Five different evaluation methods have been proposed to measure carbon stability in biochars including the following: the H/C atomic ratio method194 as well as physical,195 chemical,194,196 biological,197 and thermodynamic methods.198 The H/C atomic ratio is considered to be an index of the aromaticity of biochars due to hydrogenation and dehydrogenation processes,22 and the O/C atomic ratio refers to the oxidative degree of biochars due to oxidation or reduction processes.27,55,56,58,199 Elemental ratios offer an easy first-approximation to evaluate the integral properties and carbon stability of biochar. Nevertheless, it is worth noting that the elemental analysis may not be accurate when other elements (such as S, P, Cl,...) are ignored, and this inaccuracy becomes even larger when oxygen is determined indirectly by weight difference.22 Physical processes generally refer to the disintegration process due to frost, temperature, and moisture changes; salt weathering; root stress; or mechanical stress through tillage.200 They are not widely used195 because of the low carbon loss and because no standard physical method is available for carbon stability evaluations. Several studies propose physical methods to imitate natural weathering processes by repeated wetting/drying and freezing/thawing.57,201 Biological carbon stability is highly related to the types of biochar, microbes and soils,197,202 making the results somewhat variable and uncertain. It is conducted by incubating biochars with soils for many days.57,201
Chemical evaluation is the most popular method to measure carbon stability, which can be differentiated into the H2O2 and the dichromate method. In the H2O2 method, 0.1 g of biochars is treated with 7 mL of 5% H2O2 at 80 °C for 48 h, and the stable carbon is reported as the percentage of the initial 0.1 g biochar that remains after the reaction.194 Borrowed from the national environmental protection standard of China related to soil organic matter (SOM), Chen et al.196 proposed a dichromate method to test biochar carbon stability, described as 0.15 g of biochar added to 55 mL of 0.1 M K2Cr2O7 and 11 M H2SO4 and heated at 135 °C for 30 min. The carbon remaining is regarded as stable carbon. Other modified dichromate methods have also been reported.201,203 As for thermal carbon stability, in 2012, Harvey et al.198 reported an index, a ratio of the temperatures of biochar to graphite, where 50% of the weight of the organic matter was lost (ash-correction was needed) under thermogravimetric analysis using a 10 mL min−1 air flow.
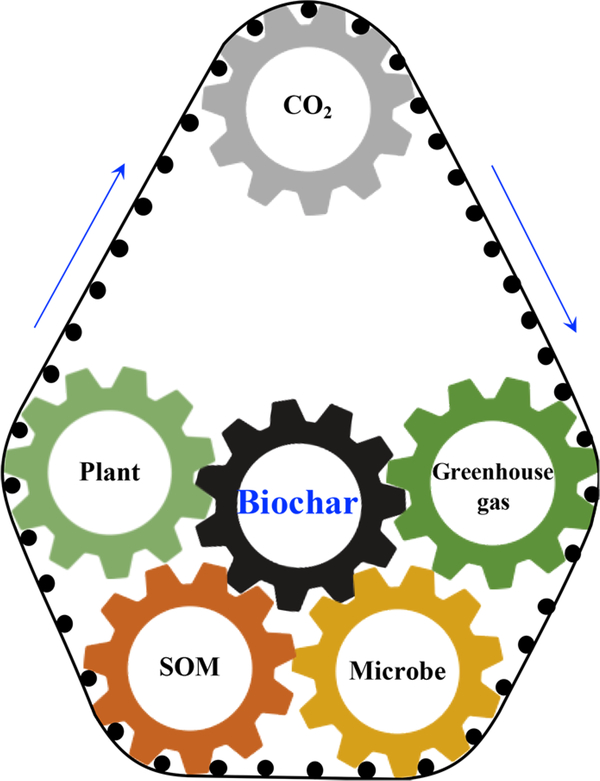
Of course, the application of biochar for carbon sequestration is not only a matter of stabilizing the carbon within the biochar itself but also the mass of carbon that the biochar could remove from the atmosphere. For example, the uptake of CO2 was reported as 73.55 mg g−1 at 25 °C for biochar produced from sugar cane bagasse at 600 °C.204 Regardless, the impact on the soil carbon sink should be evaluated after biochar is added into the soil. The clay in the soil could benefit carbon stability in biochars due to the protection of carbon from microbial decomposition.205 Carbon mineralization of biochars can be influenced by the type of soil.70,206 Variables include the growth of plant carbon and vegetation, SOM, microbial carbon in the soil, GHG, and atmospheric carbon dioxide concentrations (Figure 6). The addition of biochars to soils affects carbon sinks and sources, but mostly, the biochar acts as a carbon sink for CO2 from the atmosphere, which is stored in the soil. In turn, the biochar may cause plants and microbes to grow better and store more carbon, increasing the SOM, and CO2 in the soil.
Figure 6.
Factors affecting biochar stability as well as net carbon sequestration and, in turn, positive biochar effects on plant vegetation and soil properties.
Regarding plant carbon, it is reported that the addition of 4% straw-derived biochar to soil increased ryegrass production by 68% compared to the control.207 Although many short-term positive effects of biochars on plant growth were reported,208,209 there were few statistically significant positive results in long-term experiments.210 However, statistically, a meta-analysis composed of 177 individual studies indicated an average increase in crop productivity of approximately 10% with the addition of biochar.17 Another recent meta-analysis of 371 independent studies from 114 published papers revealed a similar positive effect of biochar addition on crop productivity.96 As for the effect of biochar on SOM mineralization, contrary effects (such as stimulating (known as the “positive priming effect”),211,212 suppressing (known as the “negative priming effect”),118,213,214 and no effect215,216) were reported.217,218 A meta-analysis of 650 data points from 18 studies revealed an average positive priming effect of 0.3 mg C g−1 soil on the SOM, and this priming effect decreased with time.217
The labile fraction may also trigger the activity of soil microorganisms in the short term,217 and a higher dissolved organic carbon flux was observed in one study.219 Over the long-term, the positive priming effect will decrease or even turn into a negative priming effect due to physical protection mechanisms by adsorbing organic matter onto the biochar surface or into microaggregates.217,220,221 In fact, much of the variation of the SOM with the addition of biochar can be explained by the activities of the soil microbial community.222 The impact of biochar on soil microbes depends on the precursors of the biochar and soil type. Steinbeiss et al. stated that the yeast-derived biochar can promote fungal microorganisms, whereas glucose-derived biochar can be utilized by Gram-negative bacteria in soil.223 The application of biochar increased the bacterial biomass in flinty clay loam soil, especially the abundance of Gram-negative bacteria and Actinobacteria.84 Meanwhile, for coarse textured soils, the addition of biochar decreased the microbial community.224
Other functions of biochars are providing nutrients or acting as conductive carbon materials due to their aromatic structures, which enhances the metabolism of microorganisms.225–227 Moreover, biochar can also be the habitat for immobilized bacteria to enhance the bioremediation of PAH-contaminated soil.228 GHGs in soils may be affected by the addition of biochar. These can be divided into CO2 and non-CO2 gases, which usually refer to CH4 and N2O. As a GHG, CH4 is 23 times and N2O is several hundred times more potent than CO2.229 Biochar was reported to reduce GHGs emission by approximately 37−91.2% in most cases.229–234 On the other hand, a few studies reported no obvious reduction235 or even a 100% increase in CO2 emissions from soils as a result of the addition of biochar.236
The weathering process can greatly weaken the function of fresh biochars in reducing GHG emissions.237 According to real-time quantitative polymerase chain reaction (qPCR), the mechanism of the CH4 emission reductions in the presence of biochar in Chinese paddy soils was not the inhibition of methanogenic archaeal growth but rather the 3−7 times increase in the abundance of methanotrophic proteobacterial as well as a decrease in the ratio of methanogenic to methanotrophic abundances (from 36.2% to 4.0%−5.3%).238
Rather than focusing on the impact of biochars to a single carbon sink, full-scale assessments, such as life cycle assessments of biochar on soil carbon systems, are needed. It was reported that the addition of biochar to soils has a carbon abatement potential,239 and the biochars obtained through slow pyrolysis show the best carbon abatement performance.240 However, after analyzing the energetic, economic, and climate change potential, researchers are still concerned that biochar only delivers climate change mitigation benefits, whereas the economic viability still remains unclear, and the need for proper waste management shows a greater potential for economic profitability.241
The relationships between the structures and carbon stability of biochars could be interpreted from their elements, phases, surfaces, and molecules. The species and content of carbon are certainly two of the most important factors that determine biochar carbon fixation. As for the phases, the loading of the inorganic phase has a great effect on biochar carbon stability.108 It has been reported that some special structures between the organic and inorganic phases (such as the core−shell C−Si phase structure) may influence the carbon stability of biochars,14,52 suggesting that the precursor structures can be an important way to improve biochar carbon sequestration. The surface charge and surface free radicals of biochars prevented microbes from attaching and mineralizing on the carbon structure, thus making biochars less bioavailable and enhancing carbon stability. The loss of small extracted molecules and the oxidation of skeletal carbon molecules are vital ways to lose carbon. Molecular computer calculations can be utilized to distinguish the labile functionalized carbon type. In summary, to evaluate the effect of the addition of biochar to soils on climate change, many studies have included stability assessment methods, analyzed the impact of biochar toward other GHGs, and evaluated the life cycle of biochar in carbon fixation. However, assessing different biochar structures and their effects on the life cycle assessment are still needed to engineer and design the best biochar.
3.2. Fertilizer for Soil.
In the field of agriculture, one of the key applications for biochars is the use of biochar as a fertilizer for plant growth. Unlike traditional fertilizers that could possibly cause serious eutrophication,242 biochar can be used as a slow-releasing nutrient material, which has been receiving increasing attention. Two major mechanisms endow biochar with the slow-releasing property: unique biochar structures and the sorption−desorption process. For unique biochar structures, the porous network within biochars hinders the release of the nutrients. Unique connections (including the chemical bonding and physical wrapping) of nutrient elements to the carbon materials also leads to slow nutrient release. For example, the silicon dissolution in biochars was reported to protect the carbon structure due to a silicon−carbon wrapping effect.14 The strong sorption behavior of biochar makes it a perfect naturally formed soil additive to concentrate nutrients in soil and thus allow for slow desorption into the aqueous phase for plant uptake. Many researchers are now taking advantage of this biochar sorption ability to recycle phosphate and other nutrient elements and to hold them in the soil longer.87 With this property of the slow-release of fertilizers from biochars, the design of biochar for specific nutrient release is vital for agricultural applications, either by loading nutrient species onto biochars or by choosing the right precursors. Multiple precursors offer the possibility for different choices. For example, rice straw is known for its high silicon content;14 soybean-derived biochar contains a high nitrogen percentage; eggshell-derived biochar has a large amount of calcium;104 and manure-derived biochar is rich in mineral content. These various attributes in different precursors allow biochars to be selectively chosen as specific elemental fertilizers. Moreover, the elemental species and structures within biochars have a great effect on biochar fertilizer release. It was reported that silicic acid is much easier to release than crystal silicon within biochars,14 and nitrogen becomes less available under increasing pyrolysis temperatures resulting from a transformation of NH4+ to heterocyclic-N.243 Because the recent literature has focused on biochar function as a fertilizer, more information is needed about the release of other nutrients, such as Si, Na, Mg, and some rare elements. Future challenges remain regarding the control of the precise release rate of nutrients by designing proper structures of biochars and by coupling different nutrient elements.
3.3. Sorbent for Removing Pollutants.
In the environmental remediation field, sorption is an essential interfacial process that controls the catalysis, transformation, sorption/desorption, fate, and bioavailability of pollutants. Thus, this review seeks to summarize and develop structure-sorption relationships for various biochars and pollutants. Typical pollutants include nonpolar organic pollutants, polar organic pollutants, ionizable organic pollutants, inorganic ions, heavy metals, and gases. The sorption of these pollutants is greatly affected by the biochar structure including the elements, phases, surfaces and molecules. Strong interactions between two elements can be applied for selective pollutant removal, such as the S−Hg bond.244 The sorption of ionizable pollutants and cationic heavy metals may lead to a hydrogen proton release or adsorption, possibly resulting from H-bonding and/or ionexchange sorption mechanisms.167
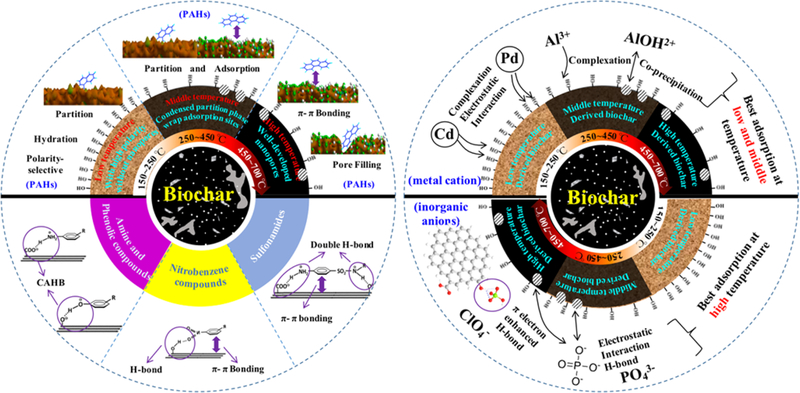
Phase effects on the sorption of charcoal have been studied for a long time. As shown in Figure 7, we found that the sorption mechanisms of aromatic contaminants by biochars under different pyrolysis temperatures evolved from the dominance of a partitioning behavior at low temperature to an adsorption dominance at high pyrolysis temperatures.10 The adsorption mechanism of biochar transited from polar-selective to pore filling character with increasing pyrolysis temperatures.10 This transitional sorption mechanism from partitioning to adsorption induced the sorption rate transition from a fast to a slow process and finally back to fast with increasing pyrolysis temperature.245 This structural transition of biochars further influenced bisolute sorption and the thermodynamic behavior of biochars.246 In addition, the washing process with water or the deashing process increased the sorption by biochars because of their enhanced hydrophobic sorption sites.53 Furthermore, the existence of dissolved organic matter will block the micropores and hinder the sorption of biochars.247
Figure 7.
Sorption mechanisms of organic (left) and inorganic pollutants (right) to biochars prepared under different pyrolytic temperatures. PAHs: polycyclic aromatic hydrocarbons. CAHB: charge-assisted H-bond, including negative charge-assisted H-bond (−) CAHB and positive charge-assisted H-bond (+) CAHB.
New phases may be generated in biochars when heavy metals are adsorbed due to a coprecipitation mechanism. For the most-studied heavy metals, a coprecipitation sorption mechanism was widely reported between the metal ions and biochar inorganic phases. Biochar inorganic phases are characterized as containing CO32−, PO43−, SiO32−, OH−, silicon particles,26 γ-Fe2O3 particles,248 and MnOx particles,36 while also forming precipitates such as β-Pb9(PO4)6,11 Pb3(CO3)2(OH)2,11 5PbO·P2O5· SiO2,143 Cu3(CO3)2(OH)2,249 KAlSi3O,26 CdCO3, Cd3P2, Cd3(PO4)2, and K4CdCl6.250
Surface functional groups are responsible for the surface effect on the sorption of biochars. The dissociation/association of biochar surface functional groups and the ionizable organic pollutants control the H-bond interactions. Many researchers reported a pH-dependent sorption behavior of ionizable organic pollutants.166,251–254 For the sorption of sulfamethazine onto charcoal, the sorption mechanism was proposed to be a π+−π donor−acceptor interaction at low pH, and a negative charge-assisted H-bond at high pH ((−)CAHB, Figure 7).166 A quantitative study on the H-bond interactions is still needed to evaluate the contribution of H-bonding with pKa considerations from both biochar functional groups and ionizable pollutants.
As for inorganic pollutants, complexation, electrostatic effects, and coprecipitation are called upon to describe the interactions between metal ions and the surface of biochars (Figure 7). In a sorption study of rice straw-derived biochars toward Pb2+, low pyrolysis temperature biochars exhibited a higher sorption ability,255,256 which may be due to abundant oxygen-containing groups and uncrystallized silicon.157 The highest adsorption for metal cations occurred for low and middle pyrolysis temperature-derived biochars (Figure 7). As for other anionic inorganic pollutants, such as F−, ClO4−, PO43−, Cr(III), and Cr(VI), they generally exhibited much lower sorption onto biochars due to electrostatic repulsion interactions. Besides electrostatic interactions, H-bonding to oxygen-containing functional groups within biochars could be a dominant mechanism for ClO4− adsorption, and this hydrogen bonding could be enhanced by the aromatic and hydrophobic surfaces of high pyrolysis temperature-derived biochars,171 leading to the best sorption toward inorganic anions occurring for high pyrolysis temperature-derived biochars (Figure 7).
Regarding molecular effects, only a few researchers have reached this far. In 2016, a structure−sorption relationship between biochar and naphthalene/phenanthrene was proposed after considering the assumed aromatic cluster structures and summarizing dozens of published or unpublished data.22 This is probably a very important step to build a structure−sorption relationship between biochar molecular structures and various pollutants. Most studies on sorption mechanisms remains at the empirical/observational level. However, understanding at the molecular level is needed to distinguish the contributions of various sorption mechanisms and different fractions of biochars.
3.4. Carbon-Based Materials.
Much previous traditional carbon-based material research was focused on activated carbon. The difference between biochars and activated carbon include the following: (1) biochars usually contains mineral constituents, whereas activated carbon does not, and biochar can be considered to be a robust product, while activated carbon was a refined product; (2) the preparation temperature for biochars (<700 °C) is generally lower than that of activated carbon (>800 °C), and biochar preparation does not need an activation process, whereas the preparation of activated carbon does need chemical activation; (3) biochar shows a much lower surface area than activated carbon; and (4) the types of precursors for biochar preparation are much wider than those for activated carbon, making biochar much cheaper than activated carbon. Because of the cheapness and the wide elements of biochars, biochar is more suitable for carbon fixation and as a soil fertilizer. Although activated carbon shows a higher sorption ability than biochar, a soil remediation project could hardly afford activated carbon as a sorbent, in addition to the fact that other organic matters in the soil may block the pores of activated carbon and thus decrease its sorption ability. Meanwhile, activated carbon is still preferable for rigorous situations such as medicine purification.
Recently, because of the cheap biomass sources, biochars are increasingly considered as carbon-based materials for economically viable and sustainable applications. Chemical modification of biochars and microbial attachment for bioaugmentation are receiving increased attention. Chemical modifications could be designed based on the multilevel structures of biochars. Other elements such as MgO have been loaded onto biochars for phosphate retention34,87 and CO2 capture.35 The additions of magnetic Fe was first reported in 2011 to enhance the sorption performance and to benefit the solid−liquid phase separation of biochars.31 By inducing sulfuric acid groups onto biochars as functional groups, a biochar-based solid acid catalyst was built for transesterification,97,98 hydrolysis reactions,30,99 and biodiesel production.100,101 Liu et al. has summarized five major directions for biochar modification, including activation, amination, oxidation, sulfonation, and recombination with supported nanostructures.12 In short, the purpose behind the modification of biochars was to add other properties, such as increasing their sorption ability and attaching nanoparticles, or by loading the catalyst and taking advantage of the aromatic platform on biochar, its functional groups, and/or high surface area.12,257 Modifications have enriched the applications of biochars, but evaluation of their practicality and stability are lacking. Attached chemicals and nanoparticles may be lost, and feasible applications require more research. Homogeneous carbon structures and special three-dimensional structural designs may be needed to achieve better performance for carbon-based material applications.
Another important application of biochars as carbon-based materials is for microbial growth. By taking advantage of the sorption ability of biochar, for example, the bioremediation ability of Pseudomonas putida bacterial strains was enhanced to degrade PAHs by 24%−38% once immobilized onto biochars compared to biomass.228 The electron shuttle role that biochars can play may benefit Shewanella for reduction reactions.227 Microbial growth on biochars actually combines the merits of both biochars and microbes, where biochars can adsorb but not degrade the pollutants, whereas microbes can then access the contaminant and degrade them. Interactions between biochars and microbes are achieving increasing attention in the agricultural field. In addition, due to the conductive properties of high pyrolysis temperature-derived biochar, biochar could also be used for energy applications as a carbon-based material, such as microbial fuel cells,258 supercapacitors,159 electrocoagulation,259 and other systems.
4. OUTLOOK OF BIOCHAR STRUCTURES AND APPLICATIONS
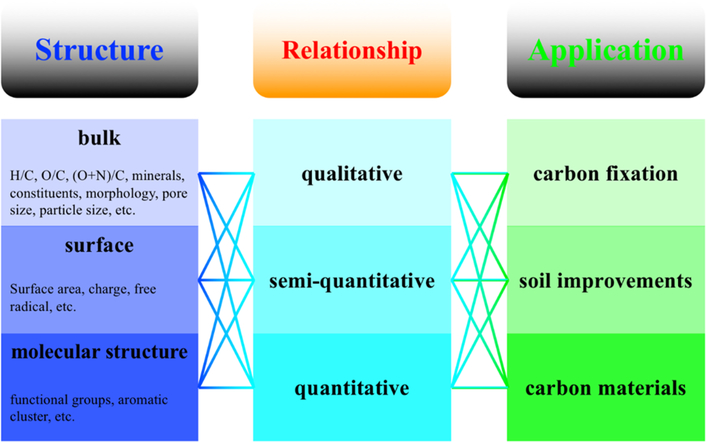
Extensive research on biochars continues because of their low cost and versatile applications. Multiple biochar sources and multilevel structures as well as their diverse applications have created many opportunities. We summarize several aspects of biochar structures and applications in Figure 8. Similar to the multilevel discussion in this review, the studies on biochar structures should evolve from the bulk, to the surface and finally to the molecular scale. Applications for biochar include carbon fixation, soil improvement, and novel carbon-based materials as soil additives including fertilizers, sorbents, and the bioaugmentation of microbes. Regarding the biochar structure, a deeper understanding is expected on the functions, mobility, toxicity, concentration, bioavailability, and environmental fate of phases, surface radicals, and molecules. Furthermore, the development of standard methods will be needed, including but not limited to the following: the quantification and identification of different species, phases, surfaces, and molecules of varying molecular weights in biochars, standard extraction procedures, standard methods for the exfoliation of biochars, and standard comparison methods.
Figure 8.
Structure−application relationships of biochars.
For applications, the deeper our understanding of the structure−application relationships, the better we can choose and design biochars. As shown in Figure 8, the relationships between the structure and application of biochars are gradually improving from qualitative to semiquantitative and finally to quantitative relationships. Although some of these relationships have been previously explored, much research remains related to the structure−application relationships, especially for molecular structural relationships. Phenomena have been reported where one property impacts an application behavior, but seldom do we know the underlying mechanism, and we can never predict a quantitative relationship. On the basis of the interpretation of biochar structures in this review, we recommend that studies on biochars should combine at both the macroscopic and the microscopic levels, while distinguishing both causes and effects. For example, when considering the fate of biochars in soil systems, research should report the release of total elements (including carbon), and the effects on biochar-nanoparticles, the dissolved biochar phase, extracted molecules, and the skeletal biochar structure. When considering biochar toxicity, the persistent free radicals, trace heavy metals, small extracted molecules, and main skeletal molecules of biochars should be investigated. More attention should be paid to linking the structures of biochar with its applications based on fundamental underlying mechanisms. Precise and quantitative predictions of different biochars with various structures for various applications are needed. We anticipate the intelligent design of biochars to achieve the greatest agricultural and environmental benefits possible.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by the National Natural Science Foundations of China (Grant 21621005, 21537005, and 21425730), and the National Basic Research Program of China (Grant 2014CB441106), and the National Key Technology Support Program of China (Grant 2015BAC02B01). JLS funding for this research was provided by the U.S. National Institute of Environmental Health Science (NIEHS), Iowa Superfund Research Program Grant No. P42ES013661, and the Center for Global and Regional Environmental Research, University of Iowa.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.7b06487.
REFERENCES
- (1).Lehmann J; Joseph S Biochar for Environmental Management: Science and Technology; Earthscan: Sterling, VA, 2009. [Google Scholar]
- (2).Shi Y China’s resources of biomass feedstock. Eng. Sci 2011, 13 (2), 16–23. [Google Scholar]
- (3).Meng J; Chen W Biochar in China: Status quo of research and trend of industrial development. J. Shenyang Agric. Univ. (Social Sciences Edition) 2013, 15 (1), 1–5. [Google Scholar]
- (4).Gwenzi W; Chaukura N; Mukome FND; Machado S; Nyamasoka B Biochar production and applications in sub-Saharan Africa: Opportunities, constraints, risks and uncertainties. J. Environ. Manage 2015, 150, 250–261. [DOI] [PubMed] [Google Scholar]
- (5).Akolgo GA; Essandoh EO; Gyamfi S; Atta-Darkwa T; Kumi EN; Maia CMBD The potential of a dual purpose improved cookstove for low income earners in Ghana - Improved cooking methods and biochar production. Renewable Sustainable Energy Rev. 2018, 82, 369–379. [Google Scholar]
- (6).Marris E Putting the carbon back: Black is the new green. Nature 2006, 442 (7103), 624–626. [DOI] [PubMed] [Google Scholar]
- (7).De Gisi S; Petta L; Wendland C History and technology of Terra Preta sanitation. Sustainability 2014, 6 (3), 1328–1345. [Google Scholar]
- (8).Lehmann J A handful of carbon. Nature 2007, 447 (7141), 143–144. [DOI] [PubMed] [Google Scholar]
- (9).Woolf D; Amonette JE; Street-Perrott FA; Lehmann J; Joseph S Sustainable biochar to mitigate global climate change. Nat. Commun 2010, 1, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Chen B; Zhou D; Zhu L Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol 2008, 42 (14), 5137–5143. [DOI] [PubMed] [Google Scholar]
- (11).Cao X; Ma L; Gao B; Harris W Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol 2009, 43 (9), 3285–3291. [DOI] [PubMed] [Google Scholar]
- (12).Liu W; Jiang H; Yu H Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev 2015, 115 (22), 12251–12285. [DOI] [PubMed] [Google Scholar]
- (13).Meyer S; Glaser B; Quicker P Technical, economical, and climate-related aspects of biochar production technologies: A literature review. Environ. Sci. Technol 2011, 45 (22), 9473–9483. [DOI] [PubMed] [Google Scholar]
- (14).Xiao X; Chen B; Zhu L Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures. Environ. Sci. Technol 2014, 48 (6), 3411–3419. [DOI] [PubMed] [Google Scholar]
- (15).Kozbial A; Zhou F; Li Z; Liu H; Li L Are graphitic surfaces hydrophobic? Acc. Chem. Res 2016, 49 (12), 2765–2773. [DOI] [PubMed] [Google Scholar]
- (16).Atkinson CJ; Fitzgerald JD; Hipps NA Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337 (1–2), 1–18. [Google Scholar]
- (17).Jeffery S; Verheijen FGA; van der Velde M; Bastos AC A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric., Ecosyst. Environ 2011, 144 (1), 175–187. [Google Scholar]
- (18).Ahmad M; Rajapaksha AU; Lim JE; Zhang M; Bolan N; Mohan D; Vithanage M; Lee SS; Ok YS Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [DOI] [PubMed] [Google Scholar]
- (19).Inyang MI; Gao B; Yao Y; Xue YW; Zimmerman A; Mosa A; Pullammanappallil P; Ok YS; Cao XD A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol 2016, 46 (4), 406–433. [Google Scholar]
- (20).Devi P; Saroha AK Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ 2017, 578, 16–33. [DOI] [PubMed] [Google Scholar]
- (21).Xiao X; Chen B A direct observation of the fine aromatic clusters and molecular structures of biochars. Environ. Sci. Technol 2017, 51 (10), 5473–5482. [DOI] [PubMed] [Google Scholar]
- (22).Xiao X; Chen Z; Chen B H/C atomic ratio as a smart linkage between pyrolytic temperatures, aromatic clusters and sorption properties of biochars derived from diverse precursory materials. Sci. Rep 2016, 6, 22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Yuan J; Xu R; Zhang H The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol 2011, 102 (3), 3488–3497. [DOI] [PubMed] [Google Scholar]
- (24).Xu Y; Chen B Organic carbon and inorganic silicon speciation in rice-bran-derived biochars affect its capacity to adsorb cadmium in solution. J. Soils Sediments 2015, 15 (1), 60–70. [Google Scholar]
- (25).Chen Z; Xiao X; Chen B; Zhu L Quantification of chemical states, dissociation constants and contents of oxygen-containing groups on the surface of biochars produced at different temperatures. Environ. Sci. Technol 2015, 49 (1), 309–317. [DOI] [PubMed] [Google Scholar]
- (26).Qian L; Chen B Dual role of biochars as adsorbents for aluminum: The effects of oxygen-containing organic components and the scattering of silicate particles. Environ. Sci. Technol 2013, 47 (15), 8759–8768. [DOI] [PubMed] [Google Scholar]
- (27).Spokas KA Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manage. 2010, 1 (2), 289–303. [Google Scholar]
- (28).Rondon MA; Lehmann J; Ramirez J; Hurtado M Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43 (6), 699–708. [Google Scholar]
- (29).Zhao G; Jiang L; He Y; Li J; Dong H; Wang X; Hu W Sulfonated graphene for persistent aromatic pollutant management. Adv. Mater 2011, 23 (34), 3959–3963. [DOI] [PubMed] [Google Scholar]
- (30).Ormsby R; Kastner JR; Miller J Hemicellulose hydrolysis using solid acid catalysts generated from biochar. Catal. Today 2012, 190 (1), 89–97. [Google Scholar]
- (31).Chen B; Chen Z; Lv S A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol 2011, 102 (2), 716–723. [DOI] [PubMed] [Google Scholar]
- (32).Xiao X; Chen B; Zhu L; Schnoor JL Sugar cane-converted graphene-like material for the superhigh adsorption of organic pollutants from water via coassembly mechanisms. Environ. Sci. Technol 2017, 51 (21), 12644–12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Yan J; Han L; Gao W; Xue S; Chen M Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol 2015, 175, 269–274. [DOI] [PubMed] [Google Scholar]
- (34).Zhang M; Gao B; Yao Y; Xue Y; Inyang M Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J 2012, 210, 26–32. [Google Scholar]
- (35).Liu W; Jiang H; Tian K; Ding Y; Yu H Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ. Sci. Technol 2013, 47 (16), 9397–9403. [DOI] [PubMed] [Google Scholar]
- (36).Wang H; Gao B; Wang S; Fang J; Xue Y; Yang K Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol 2015, 197, 356–362. [DOI] [PubMed] [Google Scholar]
- (37).Shen Y; Zhao P; Shao Q; Ma D; Takahashi F; Yoshikawa K In-situ catalytic conversion of tar using rice husk char-supported nickeliron catalysts for biomass pyrolysis/gasification. Appl. Catal., B 2014, 152, 140–151. [Google Scholar]
- (38).Wang T; Camps-Arbestain M; Hedley M; Singh BP; Calvelo-Pereira R; Wang CY Determination of carbonate-C in biochars. Soil Res. 2014, 52 (5), 495–504. [Google Scholar]
- (39).Xu Y; Chen B Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol 2013, 146, 485–493. [DOI] [PubMed] [Google Scholar]
- (40).Keiluweit M; Nico PS; Johnson MG; Kleber M Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol 2010, 44 (4), 1247–1253. [DOI] [PubMed] [Google Scholar]
- (41).Chia CH; Gong B; Joseph SD; Marjo CE; Munroe P; Rich AM Imaging of mineral-enriched biochar by FTIR, Raman and SEM-EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar]
- (42).Jindo K; Mizumoto H; Sawada Y; Sanchez-Monedero MA; Sonoki T Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11 (23), 6613–6621. [Google Scholar]
- (43).Li X; Shen Q; Zhang D; Mei X; Ran W; Xu Y; Yu G Functional groups determine biochar properties (pH and EC) as studied by two-dimensional C-13 NMR correlation spectroscopy. PLoS One 2013, 8 (6), No. e65949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Le Brech Y; Delmotte L; Raya J; Brosse N; Gadiou R; Dufour A High resolution solid state 2D NMR analysis of biomass and biochar. Anal. Chem 2015, 87 (2), 843–847. [DOI] [PubMed] [Google Scholar]
- (45).Ma J; Takahashi E Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier Science: Amsterdam, 2002. [Google Scholar]
- (46).Savant NK; Datnoff LE; Snyder GH Depletion of plantavailable silicon in soils: A possible cause of declining rice yields. Commun. Soil Sci. Plant Anal 1997, 28 (13–14), 1245–1252. [Google Scholar]
- (47).Detmann KC; Araujo WL; Martins SCV; Sanglard LMVP; Reis JV; Detmann E; Rodrigues FA; Nunes-Nesi A; Fernie AR; DaMatta FM Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 2012, 196 (3), 752–762. [DOI] [PubMed] [Google Scholar]
- (48).Epstein E Silicon: its manifold roles in plants. Ann. Appl. Biol 2009, 155 (2), 155–160. [Google Scholar]
- (49).Keeping MG; Reynolds OL Silicon in agriculture: New insights, new significance and growing application. Ann. Appl. Biol 2009, 155 (2), 153–154. [Google Scholar]
- (50).Qian L; Chen B; Hu D Effective alleviation of aluminum phytotoxicity by manure-derived biochar. Environ. Sci. Technol 2013, 47 (6), 2737–2745. [DOI] [PubMed] [Google Scholar]
- (51).Dai G; Duan M; Wang Z; Zhao Y; Zhu G; Zhou J Study on characteristics of available silicon content in soils in Shaanxi province. J. Soil Water Conserv 2004, 18 (5), 51–53. [Google Scholar]
- (52).Guo J; Chen B Insights on the molecular mechanism for the recalcitrance of biochars: interactive effects of carbon and silicon components. Environ. Sci. Technol 2014, 48 (16), 9103–9112. [DOI] [PubMed] [Google Scholar]
- (53).Sun K; Kang M; Zhang Z; Jin J; Wang Z; Pan Z; Xu D; Wu F; Xing B Impact of deashing rreatment on biochar structural properties and potential sorption mechanisms of phenanthrene. Environ. Sci. Technol 2013, 47 (20), 11473–11481. [DOI] [PubMed] [Google Scholar]
- (54).Gilli P; Gilli G Hydrogen bond models and theories: The dual hydrogen bond model and its consequences. J. Mol. Struct 2010, 972 (1–3), 2–10. [Google Scholar]
- (55).Zimmerman AR Abiotic and microbial oxidation of laboratoryproduced black carbon (biochar). Environ. Sci. Technol 2010, 44 (4), 1295–1301. [DOI] [PubMed] [Google Scholar]
- (56).Nguyen BT; Lehmann J; Kinyangi J; Smernik R; Riha SJ; Engelhard MH Long-term black carbon dynamics in cultivated soil. Biogeochemistry 2008, 89 (3), 295–308. [Google Scholar]
- (57).Hale SE; Hanley K; Lehmann J; Zimmerman AR; Cornelissen G Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar. Environ. Sci. Technol 2011, 45 (24), 10445–10453. [DOI] [PubMed] [Google Scholar]
- (58).Nguyen BT; Lehmann J Black carbon decomposition under varying water regimes. Org. Geochem 2009, 40 (8), 846–853. [Google Scholar]
- (59).Qian L; Chen B Interactions of aluminum with biochars and oxidized biochars: Implications for the biochar aging process. J. Agric. Food Chem 2014, 62 (2), 373–380. [DOI] [PubMed] [Google Scholar]
- (60).Xue Y; Gao B; Yao Y; Inyang M; Zhang M; Zimmerman AR; Ro KS Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J 2012, 200, 673–680. [Google Scholar]
- (61).Knicker H ″Black nitrogen″ - An important fraction in determining the recalcitrance of charcoal. Org. Geochem 2010, 41 (9), 947–950. [Google Scholar]
- (62).Cantrell KB; Hunt PG; Uchimiya M; Novak JM; Ro KS Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol 2012, 107, 419–428. [DOI] [PubMed] [Google Scholar]
- (63).Knicker H; Hilscher A; Gonzalez-Vila FJ; Almendros G A new conceptual model for the structural properties of char produced during vegetation fires. Org. Geochem 2008, 39 (8), 935–939. [Google Scholar]
- (64).Pietrzak R; Wachowska H; Nowicki P Preparation of nitrogen-enriched activated carbons from brown coal. Energy Fuels 2006, 20 (3), 1275–1280. [Google Scholar]
- (65).Pietrzak R; Jurewicz K; Nowicki P; Babel K; Wachowska H Nitrogen-enriched bituminous coal-based active carbons as materials for supercapacitors. Fuel 2010, 89 (11), 3457–3467. [Google Scholar]
- (66).Guo B; Liu Q; Chen E; Zhu H; Fang L; Gong J Controllable N-doping of graphene. Nano Lett. 2010, 10 (12), 4975–4980. [DOI] [PubMed] [Google Scholar]
- (67).Wang T; Arbestain MC; Hedley M; Bishop P Chemical and bioassay characterisation of nitrogen availability in biochar produced from dairy manure and biosolids. Org. Geochem. 2012, 51, 45–54. [Google Scholar]
- (68).Wang Y; Jing X; Li L; Liu W; Tong Z; Jiang H Biotoxicity evaluations of three typical biochars using a simulated system of fast pyrolytic biochar extracts on organisms of three kingdoms. ACS Sustainable Chem. Eng. 2017, 5 (1), 481–488. [Google Scholar]
- (69).Prommer J; Wanek W; Hofhansl F; Trojan D; Offre P; Urich T; Schleper C; Sassmann S; Kitzler B; Soja G; HoodNowotny RC Biochar decelerates soil organic nitrogen cycling but stimulates soil nitrification in a temperate arable field trial. PLoS One 2014, 9 (1), No. e86388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Haefele SM; Konboon Y; Wongboon W; Amarante S; Maarifat AA; Pfeiffer EM; Knoblauch C Effects and fate of biochar from rice residues in rice-based systems. Field. Crop. Res 2011, 121 (3), 430–440. [Google Scholar]
- (71).Lehmann J; Pereira da Silva J Jr.; Steiner C; Nehls T; Zech W; Glaser B Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 2003, 249 (2), 343–357. [Google Scholar]
- (72.) Schulz H; Glaser B Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J. Plant Nutr. Soil Sci 2012, 175 (3), 410–422. [Google Scholar]
- (73).Ding Y; Liu Y-X; Wu W-X; Shi D-Z; Yang M; Zhong Z-K Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water, Air, Soil Pollut. 2010, 213 (1–4), 47–55. [Google Scholar]
- (74).Gai X; Wang H; Liu J; Zhai L; Liu S; Ren T; Liu H Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS One 2014, 9 (12), No. e113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Hale SE; Alling V; Martinsen V; Mulder J; Breedveld GD; Cornelissen G The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91 (11), 1612–1619. [DOI] [PubMed] [Google Scholar]
- (76).Hollister CC; Bisogni JJ; Lehmann J Ammonium, nitrate, and phosphate sorption to and solute leaching from biochars prepared from corn stover (Zea mays L.) and oak wood (Quercus spp.). J. Environ. Qual 2013, 42 (1), 137–144. [DOI] [PubMed] [Google Scholar]
- (77).Yao Y; Gao B; Zhang M; Inyang M; Zimmerman AR Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89 (11), 1467–1471. [DOI] [PubMed] [Google Scholar]
- (78).Taghizadeh-Toosi A; Clough TJ; Sherlock RR; Condron LM Biochar adsorbed ammonia is bioavailable. Plant Soil 2012, 350 (1–2), 57–69. [Google Scholar]
- (79).Spokas KA; Novak JM; Venterea RT Biochar’s role as an alternative N-fertilizer: ammonia capture. Plant Soil 2012, 350 (1–2), 35–42. [Google Scholar]
- (80).Xu H; Wang X; Li H; Yao H; Su J; Zhu Y Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol 2014, 48 (16), 9391–9399. [DOI] [PubMed] [Google Scholar]
- (81.) Wang Z; Zheng H; Luo Y; Deng X; Herbert S; Xing B Characterization and influence of biochars on nitrous oxide emission from agricultural soil. Environ. Pollut 2013, 174, 289–296. [DOI] [PubMed] [Google Scholar]
- (82).Luz Cayuela M; Sanchez-Monedero MA; Roig A; Hanley K; Enders A; Lehmann J Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep 2013, 3, No. 1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Soong JL; Cotrufo MF Annual burning of a tallgrass prairie inhibits C and N cycling in soil, increasing recalcitrant pyrogenic organic matter storage while reducing N availability. Global Change Biol. 2015, 21 (6), 2321–2333. [DOI] [PubMed] [Google Scholar]
- (84).Prayogo C; Jones JE; Baeyens J; Bending GD Impact of biochar on mineralisation of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol. Fertil. Soils 2014, 50 (4), 695–702. [Google Scholar]
- (85).Ngo P-T; Rumpel C; Ngo Q-A; Alexis M; Vargas GV; Gil M. d. l. L. M.; Dang D-K; Jouquet P Biological and chemical reactivity and phosphorus forms of buffalo manure compost, vermicompost and their mixture with biochar. Bioresour. Technol 2013, 148, 401–407. [DOI] [PubMed] [Google Scholar]
- (86).Uchimiya M; Hiradate S Pyrolysis temperature-dependent changes in dissolved phosphorus speciation of plant and manure biochars. J. Agric. Food Chem 2014, 62 (8), 1802–1809. [DOI] [PubMed] [Google Scholar]
- (87).Yao Y; Gao B; Chen J; Yang L Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol 2013, 47 (15), 8700–8708. [DOI] [PubMed] [Google Scholar]
- (88).Silber A; Levkovitch I; Graber ER pH-dependent mineral release and surface properties of cornstraw biochar: Agronomic implications. Environ. Sci. Technol 2010, 44 (24), 9318–9323. [DOI] [PubMed] [Google Scholar]
- (89).Qian T; Zhang X; Hu J; Jiang H Effects of environmental conditions on the release of phosphorus from biochar. Chemosphere 2013, 93 (9), 2069–2075. [DOI] [PubMed] [Google Scholar]
- (90).Cao X; Ma L; Liang Y; Gao B; Harris W Simultaneous immobilization of lead and atrazine in contaminated soils using dairymanure biochar. Environ. Sci. Technol 2011, 45 (11), 4884–4889. [DOI] [PubMed] [Google Scholar]
- (91).Novak JM; Busscher WJ; Laird DL; Ahmedna M; Watts DW; Niandou MAS Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174 (2), 105–112. [Google Scholar]
- (92).Rajkovich S; Enders A; Hanley K; Hyland C; Zimmerman AR; Lehmann J Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48 (3), 271–284. [Google Scholar]
- (93).Major J; Rondon M; Molina D; Riha S; Lehmann J Nutrient leaching in a Colombian savanna Oxisol amended with biochar. J. Environ. Qual 2012, 41 (4), 1076–1086. [DOI] [PubMed] [Google Scholar]
- (94).Bird MI; Wurster CM; de Paula Silva PH; Bass AM; de Nys R Algal biochar - production and properties. Bioresour. Technol 2011, 102 (2), 1886–1891. [DOI] [PubMed] [Google Scholar]
- (95).Xu G; Wei LL; Sun JN; Shao HB; Chang SX What is more important for enhancing nutrient bioavailability with biochar application into a sandy soil: Direct or indirect mechanism? Ecol. Eng 2013, 52, 119–124. [Google Scholar]
- (96).Biederman LA; Harpole WS Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5 (2), 202–214. [Google Scholar]
- (97).Yu JT; Dehkhoda AM; Ellis N Development of biocharbased catalyst for transesterification of canola oil. Energy Fuels 2011, 25, 337–344. [Google Scholar]
- (98).Kastner JR; Miller J; Geller DP; Locklin J; Keith LH; Johnson T Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal. Today 2012, 190 (1), 122–132. [Google Scholar]
- (99).Shen S; Cai B; Wang C; Li H; Dai G; Qin H Preparation of a novel carbon-based solid acid from cocarbonized starch and polyvinyl chloride for cellulose hydrolysis. Appl. Catal., A 2014, 473, 70–74. [Google Scholar]
- (100).Dehkhoda AM; West AH; Ellis N Biochar based solid acid catalyst for biodiesel production. Appl. Catal., A 2010, 382 (2), 197–204. [Google Scholar]
- (101).Rao BVSK; Mouli KC; Rambabu N; Dalai AK; Prasad RBN Carbon-based solid acid catalyst from de-oiled canola meal for biodiesel production. Catal. Commun 2011, 14 (1), 20–26. [Google Scholar]
- (102.) Wang S; Gao B; Li Y; Mosa A; Zimmerman AR; Ma LQ; Harris WG; Migliaccio KW Manganese oxide-modified biochars: Preparation, characterization, and sorption of arsenate and lead. Bioresour. Technol 2015, 181, 13–17. [DOI] [PubMed] [Google Scholar]
- (103).Song Z; Lian F; Yu Z; Zhu L; Xing B; Qiu W Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem. Eng. J 2014, 242, 36–42. [Google Scholar]
- (104).Zhao L; Cao X; Wang Q; Yang F; Xu S Mineral constituents profile of biochar derived from diversified waste biomasses: implications for agricultural applications. J. Environ. Qual 2013, 42 (2), 545–552. [DOI] [PubMed] [Google Scholar]
- (105).Lentz RD; Ippolito JA Biochar and manure affect calcareous soil and corn silage nutrient concentrations and uptake. J. Environ. Qual 2012, 41 (4), 1033–1043. [DOI] [PubMed] [Google Scholar]
- (106).Cimo G; Kucerik J; Berns AE; Schaumann GE; Alonzo G; Conte P Effect of heating time and temperature on the chemical characteristics of biochar from poultry manure. J. Agric. Food Chem 2014, 62 (8), 1912–1918. [DOI] [PubMed] [Google Scholar]
- (107).Sun K; Jin J; Keiluweit M; Kleber M; Wang Z; Pan Z; Xing B Polar and aliphatic domains regulate sorption of phthalic acid esters (PAEs) to biochars. Bioresour. Technol 2012, 118, 120–127. [DOI] [PubMed] [Google Scholar]
- (108).McBeath AV; Wurster CM; Bird MI Influence of feedstock properties and pyrolysis conditions on biochar carbon stability as determined by hydrogen pyrolysis. Biomass Bioenergy 2015, 73, 155–173. [Google Scholar]
- (109).Chen Z; Chen B; Zhou D Composition and sorption properties of rice-straw derived biochars. Acta Scientiae Circumstantiae 2013, 33 (1), 9–19. [Google Scholar]
- (110).Chen B; Chen Z Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 2009, 76 (1), 127–133. [DOI] [PubMed] [Google Scholar]
- (111).Huang W; Chen B Interaction mechanisms of organic contaminants with burned straw ash charcoal. J. Environ. Sci 2010, 22 (10), 1586–1594. [DOI] [PubMed] [Google Scholar]
- (112).Dittmar T; Paeng J; Gihring TM; Suryaputra IGNA; Huettel M Discharge of dissolved black carbon from a fire-affected intertidal system. Limnol. Oceanogr 2012, 57 (4), 1171–1181. [Google Scholar]
- (113).Dong X; Ma LQ; Gress J; Harris W; Li Y Enhanced Cr(VI) reduction and As(III) oxidation in ice phase: Important role of dissolved organic matter from biochar. J. Hazard. Mater 2014, 267, 62–70. [DOI] [PubMed] [Google Scholar]
- (114).Jamieson T; Sager E; Gueguen C Characterization of biochar-derived dissolved organic matter using UV-visible absorption and excitation-emission fluorescence spectroscopies. Chemosphere 2014, 103, 197–204. [DOI] [PubMed] [Google Scholar]
- (115).Keith A; Singh B; Singh BP Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ. Sci. Technol 2011, 45 (22), 9611–9618. [DOI] [PubMed] [Google Scholar]
- (116).Calvelo Pereira R; Kaal J; Camps Arbestain M; Pardo Lorenzo R; Aitkenhead W; Hedley M; Macias F; Hindmarsh J; Macia-Agullo JA Contribution to characterisation of biochar to estimate the labile fraction of carbon. Org. Geochem 2011, 42 (11), 1331–1342. [Google Scholar]
- (117).Al-Wabel MI; Al-Omran A; El-Naggar AH; Nadeem M; Usman ARA Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol 2013, 131, 374–379. [DOI] [PubMed] [Google Scholar]
- (118).Cross A; Sohi SP The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem 2011, 43 (10), 2127–2134. [Google Scholar]
- (119).Brewer CE; Schmidt-Rohr K; Satrio JA; Brown RC Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustainable Energy 2009, 28 (3), 386–396. [Google Scholar]
- (120).Liu W; Tian K; Jiang H; Zhang X; Ding H; Yu H Selectively improving the bio-oil quality by catalytic fast pyrolysis of heavy-metal-polluted biomass: Take copper (Cu) as an example. Environ. Sci. Technol 2012, 46 (14), 7849–7856. [DOI] [PubMed] [Google Scholar]
- (121).Li G; Liu Q; Liu Z; Zhang ZC; Li C; Wu W Production of calcium carbide from fine biochars. Angew. Chem., Int. Ed 2010, 49 (45), 8480–8483. [DOI] [PubMed] [Google Scholar]
- (122).Yan Q; Wan C; Liu J; Gao J; Yu F; Zhang J; Cai Z Iron nanoparticles in situ encapsulated in biochar-based carbon as an effective catalyst for the conversion of biomass-derived syngas to liquid hydrocarbons. Green Chem. 2013, 15 (6), 1631–1640. [Google Scholar]
- (123).Sun L; Tian C; Li M; Meng X; Wang L; Wang R; Yin J; Fu H From coconut shell to porous graphene-like nanosheets for highpower supercapacitors. J. Mater. Chem. A 2013, 1 (21), 6462–6470. [Google Scholar]
- (124).Zhou Y; Gao B; Zimmerman AR; Chen H; Zhang M; Cao X Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour. Technol 2014, 152, 538–542. [DOI] [PubMed] [Google Scholar]
- (125).Zhu X; Liu Y; Qian F; Zhou C; Zhang S; Chen J Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour. Technol 2014, 154, 209–214. [DOI] [PubMed] [Google Scholar]
- (126).Cheah S; Gaston KR; Parent YO; Jarvis MW; Vinzant TB; Smith KM; Thornburg NE; Nimlos MR; Magrini-Bair KA Nickel cerium olivine catalyst for catalytic gasification of biomass. Appl. Catal., B 2013, 134, 34–45. [Google Scholar]
- (127).Lahijani P; Zainal ZA; Mohamed AR; Mohammadi M CO2 gasification reactivity of biomass char: Catalytic influence of alkali, alkaline earth and transition metal salts. Bioresour. Technol 2013, 144, 288–295. [DOI] [PubMed] [Google Scholar]
- (128).Azargohar R; Dalai AK Steam and KOH activation of biochar: Experimental and modeling studies. Microporous Mesoporous Mater. 2008, 110 (2–3), 413–421. [Google Scholar]
- (129).Azargohar R; Dalai AK Biochar as a precursor of activated carbon. Appl. Biochem. Biotechnol 2006, 131 (1–3), 762–773. [DOI] [PubMed] [Google Scholar]
- (130).Angin D; Altintig E; Kose TE Influence of process parameters on the surface and chemical properties of activated carbon obtained from biochar by chemical activation. Bioresour. Technol 2013, 148, 542–549. [DOI] [PubMed] [Google Scholar]
- (131).Singh BP; Cowie AL; Smernik RJ Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ. Sci. Technol 2012, 46 (21), 11770–11778. [DOI] [PubMed] [Google Scholar]
- (132).Li F; Cao X; Zhao L; Wang J; Ding Z Effects of mineral additives on biochar formation: Carbon retention, stability, and properties. Environ. Sci. Technol. 2014, 48 (19), 11211–11217. [DOI] [PubMed] [Google Scholar]
- (133).Wang T; Camps-Arbestain M; Hedley M; Bishop P Predicting phosphorus bioavailability from high-ash biochars. Plant Soil 2012, 357 (1–2), 173–187. [Google Scholar]
- (134).Tsai W; Liu S; Chen H; Chang Y; Tsai Y Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 2012, 89 (2), 198–203. [DOI] [PubMed] [Google Scholar]
- (135.) Wu H; Yip K; Kong Z; Li C-Z; Liu D; Yu Y; Gao X Removal and recycling of inherent inorganic nutrient species in Mallee biomass and derived biochars by water leaching. Ind. Eng. Chem. Res 2011, 50 (21), 12143–12151. [Google Scholar]
- (136).Yao FX; Arbestain MC; Virgel S; Blanco F; Arostegui J; Macia-Agullo JA; Macias F Simulated geochemical weathering of a mineral ash-rich biochar in a modified Soxhlet reactor. Chemosphere 2010, 80 (7), 724–732. [DOI] [PubMed] [Google Scholar]
- (137).Xu X; Cao X; Zhao L Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92 (8), 955–961. [DOI] [PubMed] [Google Scholar]
- (138).Uchimiya M; Klasson KT; Wartelle LH; Lima IM Influence of soil properties on heavy metal sequestration by biochar amendment: 1. Copper sorption isotherms and the release of cations. Chemosphere 2011, 82 (10), 1431–1437. [DOI] [PubMed] [Google Scholar]
- (139).Agrafioti E; Bouras G; Kalderis D; Diamadopoulos E Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar]
- (140).Ahmad M; Lee SS; Lim JE; Lee S-E; Cho JS; Moon DH; Hashimoto Y; Ok YS Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 2014, 95, 433–441. [DOI] [PubMed] [Google Scholar]
- (141).Inyang M; Gao B; Ding W; Pullammanappallil P; Zimmerman AR; Cao X Enhanced lead sorption by biochar derived from anaerobically digested sugarcane bagasse. Sep. Sci. Technol 2011, 46 (12), 1950–1956. [Google Scholar]
- (142).Inyang M; Gao B; Yao Y; Xue Y; Zimmerman AR; Pullammanappallil P; Cao X Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol 2012, 110, 50–56. [DOI] [PubMed] [Google Scholar]
- (143).Lu H; Zhang W; Yang Y; Huang X; Wang S; Qiu R Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46 (3), 854–862. [DOI] [PubMed] [Google Scholar]
- (144).Park JH; Choppala GK; Bolan N; Chung JW; Chuasavathi T Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348 (1–2), 439–451. [Google Scholar]
- (145).Debela F; Thring RW; Arocena JM Immobilization of heavy metals by co-pyrolysis of contaminated soil with woody biomass. Water, Air, Soil Pollut 2012, 223 (3), 1161–1170. [Google Scholar]
- (146).Fang G; Gao J; Liu C; Dionysiou DD; Wang Y; Zhou D Key role of persistent free radicals in hydrogen peroxide activation by biochar: implications to organic contaminant degradation. Environ. Sci. Technol 2014, 48 (3), 1902–1910. [DOI] [PubMed] [Google Scholar]
- (147).Cao X; Ro KS; Chappell M; Li Y; Mao J Chemical structures of swine-manure chars produced under different carbonization conditions investigated by advanced solid-state C-13 nuclear magnetic resonance (NMR) spectroscopy. Energy Fuels 2011, 25, 388–397. [Google Scholar]
- (148).Uchimiya M; Orlov A; Ramakrishnan G; Sistani K In situ and ex situ spectroscopic monitoring of biochar’s surface functional groups. J. Anal. Appl. Pyrolysis 2013, 102, 53–59. [Google Scholar]
- (149).Singh B; Fang Y; Cowie BCC; Thomsen L NEXAFS and XPS characterisation of carbon functional groups of fresh and aged biochars. Org. Geochem. 2014, 77, 1–10. [Google Scholar]
- (150).Cody GD; Ade H; Wirick S; Mitchell GD; Davis A Determination of chemical-structural changes in vitrinite accompanying luminescence alteration using C-NEXAFS analysis. Org. Geochem. 1998, 28 (7–8), 441–455. [Google Scholar]
- (151).Francis JT; Hitchcock AP Inner-shell spectroscopy of pbenzoquinone, hydroquinone, and phenol: Distinguishing quinoid and benzenoid structures. J. Phys. Chem 1992, 96 (16), 6598–6610. [Google Scholar]
- (152).Heymann K; Lehmann J; Solomon D; Schmidt MWI; Regier TC 1s K-edge near edge X-ray absorption fine structure (NEXAFS) spectroscopy for characterizing functional group chemistry of black carbon. Org. Geochem 2011, 42 (9), 1055–1064. [Google Scholar]
- (153).Schumacher M; Christl I; Scheinost AC; Jacobsen C; Kretzschmar R Chemical heterogeneity of organic soil colloids investigated by scanning transmission X-ray microscopy and C-1s NEXAFS microspectroscopy. Environ. Sci. Technol. 2005, 39 (23), 9094–9100. [DOI] [PubMed] [Google Scholar]
- (154).Mao JD; Johnson RL; Lehmann J; Olk DC; Neves EG; Thompson ML; Schmidt-Rohr K Abundant and stable char residues in soils: Implications for soil fertility and carbon sequestration. Environ. Sci. Technol 2012, 46 (17), 9571–9576. [DOI] [PubMed] [Google Scholar]
- (155).Uchimiya M; Chang S; Klasson KT Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater 2011, 190 (1–3), 432–441. [DOI] [PubMed] [Google Scholar]
- (156).Dong X; Ma LQ; Zhu Y; Li Y; Gu B Mechanistic investigation of mercury sorption by Brazilian pepper biochars of different pyrolytic temperatures based on X-ray photoelectron spectroscopy and flow calorimetry. Environ. Sci. Technol 2013, 47 (21), 12156–12164. [DOI] [PubMed] [Google Scholar]
- (157).Uchimiya M; Bannon DI; Wartelle LH Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J. Agric. Food Chem 2012, 60 (7), 1798–1809. [DOI] [PubMed] [Google Scholar]
- (158).Xu R; Zhao A; Yuan J; Jiang J pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J. Soils Sediments 2012, 12 (4), 494–502. [Google Scholar]
- (159).Jiang J; Zhang L; Wang X; Holm N; Rajagopalan K; Chen F; Ma S Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes. Electrochim. Acta 2013, 113, 481–489. [Google Scholar]
- (160).Boehm HP; Heck W; Sappok R; Diehl E Surface oxides of carbon. Angew. Chem., Int. Ed. Engl 1964, 3 (10), 669–677. [Google Scholar]
- (161).Mukherjee A; Zimmerman AR; Harris W Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163 (3–4), 247–255. [Google Scholar]
- (162).Shaaban A; Se S-M; Dimin MF; Juoi JM; Husin MHM; Mitan NMM Influence of heating temperature and holding time on biochars derived from rubber wood sawdust via slow pyrolysis. J. Anal. Appl. Pyrolysis 2014, 107, 31–39. [Google Scholar]
- (163).Harvey OR; Herbert BE; Kuo L-J; Louchouarn P Generalized two-dimensional perturbation correlation infrared spectroscopy reveals mechanisms for the development of surface charge and recalcitrance in plant-derived biochars. Environ. Sci. Technol 2012, 46 (19), 10641–10650. [DOI] [PubMed] [Google Scholar]
- (164).Song Y; Tahmasebi A; Yu J Co-pyrolysis of pine sawdust and lignite in a thermogravimetric analyzer and a fixed-bed reactor. Bioresour. Technol 2014, 174, 204–211. [DOI] [PubMed] [Google Scholar]
- (165).Uchimiya M; Wartelle LH; Klasson KT; Fortier CA; Lima IM Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J. Agric. Food Chem 2011, 59 (6), 2501–2510. [DOI] [PubMed] [Google Scholar]
- (166).Teixido M; Pignatello JJ; Beltran JL; Granados M; Peccia J Speciation of the ionizable antibiotic sulfamethazine on black carbon (Biochar). Environ. Sci. Technol 2011, 45 (23), 10020–10027. [DOI] [PubMed] [Google Scholar]
- (167).Ni J; Pignatello JJ; Xing B Adsorption of aromatic carboxylate ions to black carbon (biochar) is accompanied by proton exchange with water. Environ. Sci. Technol 2011, 45 (21), 9240–9248. [DOI] [PubMed] [Google Scholar]
- (168.) Yang G; Jiang H Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res. 2014, 48, 396–405. [DOI] [PubMed] [Google Scholar]
- (169).Qian L; Chen M; Chen B Competitive adsorption of cadmium and aluminum onto fresh and oxidized biochars during aging processes. J. Soils Sediments 2015, 15 (5), 1130–1138. [Google Scholar]
- (170).Abit SM; Bolster CH; Cai P; Walker SL Influence of feedstock and pyrolysis temperature of biochar amendments on transport of Escherichia coli in saturated and unsaturated soil. Environ. Sci. Technol 2012, 46 (15), 8097–8105. [DOI] [PubMed] [Google Scholar]
- (171).Fang Q; Chen B; Lin Y; Guan Y Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ. Sci. Technol 2014, 48 (1), 279–288. [DOI] [PubMed] [Google Scholar]
- (172).Wang D; Zhang W; Hao X; Zhou D Transport of biochar particles in saturated granular media: effects of pyrolysis temperature and particle size. Environ. Sci. Technol 2013, 47 (2), 821–828. [DOI] [PubMed] [Google Scholar]
- (173).Liao S; Pan B; Li H; Zhang D; Xing B Detecting free radicals in biochars and determining their ability to inhibit the germination and growth of corn, wheat and rice seedlings. Environ. Sci. Technol 2014, 48 (15), 8581–8587. [DOI] [PubMed] [Google Scholar]
- (174).Bohm H; Jander H; Tanke D PAH growth and soot formation in the pyrolysis of acetylene and benzene at high temperatures and pressures: Modeling and experiment. Symp. (Int.) Combust., [Proc.] 1998, 27, 1605–1612. [Google Scholar]
- (175).Qiu N; Li H; Jin Z; Zhu Y Temperature and time effect on the concentrations of free radicals in coal: Evidence from laboratory pyrolysis experiments. Int. J. Coal Geol 2007, 69 (3), 220–228. [Google Scholar]
- (176).Fang G; Liu C; Gao J; Dionysiou DD; Zhou D Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ. Sci. Technol 2015, 49 (9), 5645–5653. [DOI] [PubMed] [Google Scholar]
- (177).Fang G; Zhu C; Dionysiou DD; Gao J; Zhou D Mechanism of hydroxyl radical generation from biochar suspensions: Implications to diethyl phthalate degradation. Bioresour. Technol 2015, 176, 210–217. [DOI] [PubMed] [Google Scholar]
- (178).Kluepfel L; Keiluweit M; Kleber M; Sander M Redox properties of plant biomass-derived black carbon (biochar). Environ. Sci. Technol 2014, 48 (10), 5601–5611. [DOI] [PubMed] [Google Scholar]
- (179).Buss W; Masek O Mobile organic compounds in biochar - A potential source of contamination - Phytotoxic effects on cress seed (Lepidium sativum) germination. J. Environ. Manage 2014, 137, 111–119. [DOI] [PubMed] [Google Scholar]
- (180).Oleszczuk P; Josko I; Kusmierz M Biochar properties regarding to contaminants content and ecotoxicological assessment. J. Hazard. Mater 2013, 260, 375–382. [DOI] [PubMed] [Google Scholar]
- (181).Spokas KA; Novak JM; Stewart CE; Cantrell KB; Uchimiya M; DuSaire MG; Ro KS Qualitative analysis of volatile organic compounds on biochar. Chemosphere 2011, 85 (5), 869–882. [DOI] [PubMed] [Google Scholar]
- (182).Kaal J; Martinez Cortizas A; Reyes O; Solino M Molecular characterization of Ulex europaeus biochar obtained from laboratory heat treatment experiments - A pyrolysis-GC/MS study. J. Anal. Appl. Pyrolysis 2012, 95, 205–212. [Google Scholar]
- (183).Becker R; Dorgerloh U; Helmis M; Mumme J; Diakite M; Nehls I Hydrothermally carbonized plant materials: Patterns of volatile organic compounds detected by gas chromatography. Bioresour. Technol 2013, 130, 621–628. [DOI] [PubMed] [Google Scholar]
- (184).Freddo A; Cai C; Reid BJ Environmental contextualisation of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ. Pollut 2012, 171, 18–24. [DOI] [PubMed] [Google Scholar]
- (185).Hale SE; Lehmann J; Rutherford D; Zimmerman AR; Bachmann RT; Shitumbanuma V; O’Toole A; Sundqvist KL; Arp HPH; Cornelissen G Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol 2012, 46 (5), 2830–2838. [DOI] [PubMed] [Google Scholar]
- (186).Hilber I; Blum F; Leifeld J; Schmidt H-P; Bucheli TD Quantitative determination of PAHs in biochar: A prerequisite to ensure its quality and safe application. J. Agric. Food Chem 2012, 60 (12), 3042–3050. [DOI] [PubMed] [Google Scholar]
- (187).Keiluweit M; Kleber M; Sparrow MA; Simoneit BRT; Prahl FG Solvent-extractable polycyclic aromatic hydrocarbons in biochar: Influence of pyrolysis temperature and feedstock. Environ. Sci. Technol 2012, 46 (17), 9333–9341. [DOI] [PubMed] [Google Scholar]
- (188).Koltowski M; Oleszczuk P Toxicity of biochars after polycyclic aromatic hydrocarbons removal by thermal treatment. Ecol. Eng 2015, 75, 79–85. [Google Scholar]
- (189).Quilliam RS; Rangecroft S; Emmett BA; DeLuca TH; Jones DL Is biochar a source or sink for polycyclic aromatic hydrocarbon (PAH) compounds in agricultural soils? GCB Bioenergy 2013, 5 (2), 96–103. [Google Scholar]
- (190).Zielinska A; Oleszczuk P The conversion of sewage sludge into biochar reduces polycyclic aromatic hydrocarbon content and ecotoxicity but increases trace metal content. Biomass Bioenergy 2015, 75, 235–244. [Google Scholar]
- (191).Kloss S; Zehetner F; Dellantonio A; Hamid R; Ottner F; Liedtke V; Schwanninger M; Gerzabek MH; Soja G Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual 2012, 41 (4), 990–1000. [DOI] [PubMed] [Google Scholar]
- (192).Oleszczuk P; Zielinska A; Cornelissen G Stabilization of sewage sludge by different biochars towards reducing freely dissolved polycyclic aromatic hydrocarbons (PAHs) content. Bioresour. Technol 2014, 156, 139–145. [DOI] [PubMed] [Google Scholar]
- (193).Becker L; Bada JL; Winans RE; Hunt JE; Bunch TE; French BM Fullerenes in the 1.85-billion-year-old sudbury impact structure. Science 1994, 265 (5172), 642–645. [DOI] [PubMed] [Google Scholar]
- (194).Crombie K; Masek O; Sohi SP; Brownsort P; Cross A The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy 2013, 5 (2), 122–131. [Google Scholar]
- (195).Spokas KA; Novak JM; Masiello CA; Johnson MG; Colosky EC; Ippolito JA; Trigo C Physical disintegration of biochar: An overlooked process. Environ. Sci. Technol. Lett 2014, 1 (8), 326–332. [Google Scholar]
- (196).Chen D; Yu X; Song C; Pang X; Huang J; Li Y Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresour. Technol 2016, 218, 1303–1306. [DOI] [PubMed] [Google Scholar]
- (197).Fang Y; Singh B; Singh BP Effect of temperature on biochar priming effects and its stability in soils. Soil Biol. Biochem 2015, 80, 136–145. [Google Scholar]
- (198).Harvey OR; Kuo L-J; Zimmerman AR; Louchouarn P; Amonette JE; Herbert BE An index-based approach to assessing recalcitrance and soil carbon sequestration potential of engineered black carbons (Biochars). Environ. Sci. Technol 2012, 46 (3), 1415–1421. [DOI] [PubMed] [Google Scholar]
- (199).Kim S; Kramer RW; Hatcher PG Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram. Anal. Chem 2003, 75 (20), 5336–5344. [DOI] [PubMed] [Google Scholar]
- (200).Lehmann J; Joseph S Biochar for Environmental Management: Science, Technology and Implementation; Taylor & Francis: Boca Raton, FL, 2015. [Google Scholar]
- (201).Naisse C; Girardin C; Lefevre R; Pozzi A; Maas R; Stark A; Rumpel C Effect of physical weathering on the carbon sequestration potential of biochars and hydrochars in soil. GCB Bioenergy 2015, 7 (3), 488–496. [Google Scholar]
- (202).Zimmermann M; Bird MI; Wurster C; Saiz G; Goodrick I; Barta J; Capek P; Santruckova H; Smernik R Rapid degradation of pyrogenic carbon. Global Change Biol. 2012, 18 (11), 3306–3316. [Google Scholar]
- (203).Naisse C; Alexis M; Plante A; Wiedner K; Glaser B; Pozzi A; Carcaillet C; Criscuoli I; Rumpel C Can biochar and hydrochar stability be assessed with chemical methods? Org. Geochem. 2013, 60, 40–44. [Google Scholar]
- (204).Creamer AE; Gao B; Zhang M Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J 2014, 249, 174–179. [Google Scholar]
- (205).Bolan NS; Kunhikrishnan A; Choppala GK; Thangarajan R; Chung JW Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci. Total Environ. 2012, 424, 264–270. [DOI] [PubMed] [Google Scholar]
- (206).Qayyum MF; Steffens D; Reisenauer HP; Schubert S Kinetics of carbon mineralization of biochars compared with wheat straw in three soils. J. Environ. Qual 2012, 41 (4), 1210–1220. [DOI] [PubMed] [Google Scholar]
- (207).Hua L; Zhang C; Ma H; Yu W Environmental benefits of biochar made by agricultural straw when applied to soil. Ecol. Environ. Sci 2010, 19 (10), 2489–2492. [Google Scholar]
- (208).Schulz H; Dunst G; Glaser B Positive effects of composted biochar on plant growth and soil fertility. Agron. Sustainable Dev 2013, 33 (4), 817–827. [Google Scholar]
- (209).Liu J; Schulz H; Brandl S; Miehtke H; Huwe B; Glaser B Short-term effect of biochar and compost on soil fertility and water status of a Dystric Cambisol in NE Germany under field conditions. J. Plant Nutr. Soil Sci 2012, 175 (5), 698–707. [Google Scholar]
- (210).Jones DL; Rousk J; Edwards-Jones G; DeLuca TH; Murphy DV Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem 2012, 45, 113–124. [Google Scholar]
- (211.) Wardle DA; Nilsson M-C; Zackrisson O Fire-derived charcoal causes loss of forest humus. Science 2008, 320 (5876), 629–629. [DOI] [PubMed] [Google Scholar]
- (212).Luo Y; Durenkamp M; De Nobili M; Lin Q; Brookes PC Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol. Biochem 2011, 43 (11), 2304–2314. [Google Scholar]
- (213).Kuzyakov Y; Subbotina I; Chen H; Bogomolova I; Xu X Black carbon decomposition and incorporation into soil microbial biomass estimated by C-14 labeling. Soil Biol. Biochem 2009, 41 (2), 210–219. [Google Scholar]
- (214).Lu W; Ding W; Zhang J; Li Y; Luo J; Bolan N; Xie Z Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: A negative priming effect. Soil Biol. Biochem 2014, 76, 12–21. [Google Scholar]
- (215).Santos F; Torn MS; Bird JA Biological degradation of pyrogenic organic matter in temperate forest soils. Soil Biol. Biochem 2012, 51, 115–124. [Google Scholar]
- (216).Abiven S; Andreoli R Charcoal does not change the decomposition rate of mixed litters in a mineral cambisol: a controlled conditions study. Biol. Fertil. Soils 2011, 47 (1), 111–114. [Google Scholar]
- (217).Maestrini B; Nannipieri P; Abiven S A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy 2015, 7 (4), 577–590. [Google Scholar]
- (218).Zimmerman AR; Gao B; Ahn M-Y Positive and negative carbon mineralization priming effects among a variety of biocharamended soils. Soil Biol. Biochem 2011, 43 (6), 1169–1179. [Google Scholar]
- (219).Bell MJ; Worrall F Charcoal addition to soils in NE England: A carbon sink with environmental co-benefits? Sci. Total Environ 2011, 409 (9), 1704–1714. [DOI] [PubMed] [Google Scholar]
- (220).Singh BP; Cowie AL Long-term influence of biochar on native organic carbon mineralisation in a low-carbon clayey soil. Sci. Rep 2015, 4, No. 3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Kuang C; Jiang C; Li Z; Hu F Effects of biochar amendments on soil organic carbon mineralization and microbial biomass in red paddy soils. Soils 2012, 44 (4), 570–575. [Google Scholar]
- (222).Ronsse F; van Hecke S; Dickinson D; Prins W Production and characterization of slow pyrolysis biochar: influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5 (2), 104–115. [Google Scholar]
- (223).Steinbeiss S; Gleixner G; Antonietti M Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem 2009, 41 (6), 1301–1310. [Google Scholar]
- (224).Dempster DN; Gleeson DB; Solaiman ZM; Jones DL; Murphy DV Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 2012, 354 (1–2), 311–324. [Google Scholar]
- (225).Zhao Z; Zhang Y; Woodard TL; Nevin KP; Lovley DR Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol 2015, 191, 140–145. [DOI] [PubMed] [Google Scholar]
- (226).Chen S; Rotaru A-E; Shrestha PM; Malvankar NS; Liu F; Fan W; Nevin KP; Lovley DR Promoting interspecies electron transfer with biochar. Sci. Rep 2015, 4, No. 5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (227).Kappler A; Wuestner ML; Ruecker A; Harter J; Halama M; Behrens S Biochar as an electron shuttle between bacteria and Fe(III) minerals. Environ. Sci. Technol. Lett 2014, 1 (8), 339–344. [Google Scholar]
- (228).Chen B; Yuan M; Qian L Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J. Soils Sediments 2012, 12 (9), 1350–1359. [Google Scholar]
- (229.) Renner R Rethinking biochar. Environ. Sci. Technol. 2007, 41 (17), 5932–5933. [DOI] [PubMed] [Google Scholar]
- (230).Wang J; Zhang M; Xiong Z; Liu P; Pan G Effects of biochar addition on N2O and CO2 emissions from two paddy soils. Biol. Fertil. Soils 2011, 47 (8), 887–896. [Google Scholar]
- (231).Wang J; Pan X; Liu Y; Zhang X; Xiong Z Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant Soil 2012, 360 (1–2), 287–298. [Google Scholar]
- (232).Dong D; Yang M; Wang C; Wang H; Li Y; Luo J; Wu W Responses of methane emissions and rice yield to applications of biochar and straw in a paddy field. J. Soils Sediments 2013, 13 (8), 1450–1460. [Google Scholar]
- (233.) Sarkhot DV; Berhe AA; Ghezzehei TA Impact of biochar enriched with dairy manure effluent on carbon and nitrogen dynamics. J. Environ. Qual 2012, 41 (4), 1107–1114. [DOI] [PubMed] [Google Scholar]
- (234).Liu Y; Yang M; Wu Y; Wang H; Chen Y; Wu W Reducing CH4 and CO2 emissions from waterlogged paddy soil with biochar. J. Soils Sediments 2011, 11 (6), 930–939. [Google Scholar]
- (235).Scheer C; Grace PR; Rowlings DW; Kimber S; Van Zwieten L Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant Soil 2011, 345 (1–2), 47–58. [Google Scholar]
- (236).Troy SM; Lawlor PG; O’ Flynn CJ; Healy MG Impact of biochar addition to soil on greenhouse gas emissions following pig manure application. Soil Biol. Biochem 2013, 60, 173–181. [Google Scholar]
- (237).Spokas KA Impact of biochar field aging on laboratory greenhouse gas production potentials. GCB Bioenergy 2013, 5 (2), 165–176. [Google Scholar]
- (238).Feng Y; Xu Y; Yu Y; Xie Z; Lin X Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Soil Biol. Biochem 2012, 46, 80–88. [Google Scholar]
- (239).Peters JF; Iribarren D; Dufour J Biomass pyrolysis for biochar or energy applications? A life cycle assessment. Environ. Sci. Technol 2015, 49 (8), 5195–5202. [DOI] [PubMed] [Google Scholar]
- (240).Ibarrola R; Shackley S; Hammond J Pyrolysis biochar systems for recovering biodegradable materials: A life cycle carbon assessment. Waste Manage. 2012, 32 (5), 859–868. [DOI] [PubMed] [Google Scholar]
- (241).Roberts KG; Gloy BA; Joseph S; Scott NR; Lehmann J Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environ. Sci. Technol 2010, 44 (2), 827–833. [DOI] [PubMed] [Google Scholar]
- (242).Conley DJ; Paerl HW; Howarth RW; Boesch DF; Seitzinger SP; Havens KE; Lancelot C; Likens GE Ecology controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323 (5917), 1014–1015. [DOI] [PubMed] [Google Scholar]
- (243).Zheng H; Wang Z; Deng X; Zhao J; Luo Y; Novak J; Herbert S; Xing B Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol 2013, 130, 463–471. [DOI] [PubMed] [Google Scholar]
- (244).Liu P; Ptacek CJ; Blowes DW; Landis RC Mechanisms of mercury removal by biochars produced from different feedstocks determined using X-ray absorption spectroscopy. J. Hazard. Mater 2016, 308, 233–242. [DOI] [PubMed] [Google Scholar]
- (245).Chen Z; Chen B; Chiou CT Fast and slow rates of naphthalene sorption to biochars produced at different temperatures. Environ. Sci. Technol 2012, 46 (20), 11104–11111. [DOI] [PubMed] [Google Scholar]
- (246).Chen Z; Chen B; Zhou D; Chen W Bisolute sorption and thermodynamic behavior of organic pollutants to biomass-derived biochars at two pyrolytic temperatures. Environ. Sci. Technol 2012, 46 (22), 12476–12483. [DOI] [PubMed] [Google Scholar]
- (247).Zhang W; Sun H; Wang L Influence of the interactions between black carbon and soil constituents on the sorption of pyrene. Soil Sediment Contam. 2013, 22 (4), 469–482. [Google Scholar]
- (248).Zhang M; Gao B; Varnoosfaderani S; Hebard A; Yao Y; Inyang M Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour. Technol 2013, 130, 457–462. [DOI] [PubMed] [Google Scholar]
- (249).Ippolito JA; Strawn DG; Scheckel KG; Novak JM; Ahmedna M; Niandou MAS Macroscopic and molecular investigations of copper sorption by a steam-activated biochar. J. Environ. Qual 2012, 41 (4), 1150–1156. [DOI] [PubMed] [Google Scholar]
- (250).Zhang F; Wang X; Yin D; Peng B; Tan C; Liu Y; Tan X; Wu S Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J. Environ. Manage 2015, 153, 68–73. [DOI] [PubMed] [Google Scholar]
- (251).Xu R; Xiao S; Yuan J; Zhao A Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues. Bioresour. Technol 2011, 102 (22), 10293–10298. [DOI] [PubMed] [Google Scholar]
- (252).Yang Y; Lin X; Wei B; Zhao Y; Wang J Evaluation of adsorption potential of bamboo biochar for metal-complex dye: equilibrium, kinetics and artificial neural network modeling. Int. J. Environ. Sci. Technol 2014, 11 (4), 1093–1100. [Google Scholar]
- (253).Essandoh M; Kunwar B; Pittman CU Jr.; Mohan D; Mlsna T Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem. Eng. J 2015, 265, 219–227. [Google Scholar]
- (254).Xie M; Chen W; Xu Z; Zheng S; Zhu D Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ. Pollut 2014, 186, 187–194. [DOI] [PubMed] [Google Scholar]
- (255).Chen Z; Fang Y; Xu Y; Chen B Adsorption of Pb~(2+) by rice straw derived-biochar and its influential factors. Acta Scientiae Circumstantiae 2012, 32 (4), 769–776. [Google Scholar]
- (256).An Z; Hou Y; Cai C; Xue X Lead(II) adsorption characteristics on different biochars derived from rice straw. Environ. Chem 2011, 30 (11), 1851–1857. [Google Scholar]
- (257).Liu J; Chen X; Wang Y; Strathmann TJ; Werth CJ Mechanism and mitigation of the decomposition of an oxorhenium complex-based heterogeneous catalyst for perchlorate reduction in water. Environ. Sci. Technol 2015, 49 (21), 12932–12940. [DOI] [PubMed] [Google Scholar]
- (258).Huggins T; Wang HM; Kearns J; Jenkins P; Ren ZJ Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol 2014, 157, 114–119. [DOI] [PubMed] [Google Scholar]
- (259).Lobo FL; Wang H; Huggins T; Rosenblum J; Linden KG; Ren ZJ Low-energy hydraulic fracturing wastewater treatment via AC powered electrocoagulation with biochar. J. Hazard. Mater 2016, 309, 180–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.