Summary
Background
Lung disease is a common comorbidity in people with HIV/AIDS, independent of smoking status. The effects of marijuana smoking on risk of lung disease in HIV-infected individuals are unclear.
Methods
In this prospective cohort study, we quantified lung disease risk among men enrolled in the Multicenter AIDS Cohort Study (MACS), a long-term observational cohort of HIV-infected and uninfected men who have sex with men. Eligible participants were aged ≥ 30 years with self-reported marijuana and tobacco smoking data from biannual study visits between 1996 and 2014. Pulmonary diagnoses were obtained from self-report and medical records. Analyses were performed using Cox models and Generalized Estimating Equations adjusted for tobacco smoking, CD4 T cell count, and other risk factors.
Findings
1630 incident pulmonary diagnoses were reported among 1352 HIV-seropositive and 1352 HIV-seronegative eligible participants matched for race and baseline age (53,794 total person-visits, median follow-up 10.5 years). 27% of HIV-infected participants reported daily or weekly marijuana smoking for one or more years in follow-up, compared to 18% of uninfected participants (median 4·0 and 4·5 years daily/weekly use, respectively). HIV-infected participants had an increased likelihood of infectious or non-infectious pulmonary diagnoses compared to uninfected participants (33·2% vs. 21·5%, and 20·6% vs. 17·2%, respectively). Among HIV-infected participants, recent marijuana smoking was associated with increased risk of infectious pulmonary diagnoses and chronic bronchitis independent of tobacco smoking and other risk factors for lung disease (hazard ratio [95% confidence interval] 1·43 [1·09–1·86], and 1·54 [1·11–2·13], respectively); these risks were additive in participants smoking both substances. There was no association between marijuana smoking and pulmonary diagnoses in HIV-uninfected participants.
Interpretation
In this longitudinal study, long-term marijuana smoking was associated with lung disease independent of tobacco smoking and other risk factors in HIV-infected individuals. These findings could be used to reduce modifiable risks of lung disease in high-risk populations.
Funding
U.S. National Institutes of Health.
Research in Context
Evidence Before the Study
We searched the PubMed database for original research articles using the following MeSH terms: “HIV”, and “marijuana” or “cannabis” or “smoking”, and “pulmonary disease” or “lung disease” or “bronchitis” or “COPD” or “emphysema” or “pneumonia”. Among 504 articles published between January 1, 1996, and August 15, 2018 (the date of the final search), 17 articles included the terms “marijuana” or “cannabis”. There were no previous observational cohort studies that systematically assessed the association between marijuana use and lung disease in HIV-infected individuals. In previous primary studies and systematic reviews of marijuana smoking in healthy individuals, including the U.S. National Academy of Sciences report in 2017, regular marijuana smoking was associated with respiratory symptoms including cough and phlegm production, but its association with other respiratory disease was unclear. Factors contributing to contradictory findings among previous studies likely include adequately controlling for tobacco smoking, small sample sizes, and limited availability of longitudinal data.
Added Value of This Study
The Multicenter AIDS Cohort Study (MACS) is a large cohort of HIV-infected and uninfected men who have sex with men in the U.S. Since 1984, the MACS has collected longitudinal medical and health outcome, substance use, biological specimen, and behavioral data for over 7,000 participants. By studying 1,352 HIV-infected and 1,352 uninfected individuals contributing over 53,000 person-visits between 1996 and 2014, we identified 363 HIV-infected and 244 uninfected participants reporting one or more years of daily or weekly marijuana smoking (median 4.0 and 4.5 years daily/weekly smoking, respectively). In HIV-infected participants, daily or weekly marijuana smoking was associated with increased risk of infectious pulmonary diagnoses and chronic bronchitis after adjusting for tobacco smoking and other risk factors. There was no association between marijuana smoking and risk of either infectious diagnoses or chronic bronchitis in uninfected individuals with similar demographic characteristics. To our knowledge, this is the first large-scale observational study investigating marijuana smoking and lung disease in HIV-infected individuals, and among the largest studies in healthy individuals.
Implications of All the Available Evidence
Our study finds that long-term marijuana smoking is a risk factor for lung disease in people infected with HIV, independent of tobacco smoking, CD4 T-cell count, and other factors. In contrast, we found no association between marijuana smoking and lung disease in HIV-uninfected people with similar demographic characteristics. The effect on lung disease risk is additive in HIV-infected individuals smoking both marijuana and tobacco. These findings suggest that healthcare providers should consider marijuana smoking as a modifiable risk factor for lung disease in people infected with HIV. They also highlight the need for research on the relative risks and benefits of non-smoked versus smoked forms of marijuana for medicinal and other purposes.
Alt-text: Unlabelled Box
1. Introduction
Lung disease remains a common comorbidity in persons living with HIV, despite the widespread use of combination antiretroviral therapy (cART) that has substantially reduced morbidity and mortality related to opportunistic lung infections [1]. Previous studies in the U.S. have reported higher incidence of both infectious and non-infectious lung diseases in HIV-infected (HIV+) compared to uninfected (HIV−) populations [1], [2], [3]. This increased prevalence is explained, in part, by more tobacco smoking among HIV+ individuals [4], while HIV disease-related factors including unsuppressed viral load and low CD4 T cell count may also contribute to higher rates of lung disease [5], [6], [7]. The high prevalence of non-infectious obstructive lung disease is expected to continue to increase among HIV+ individuals in the U.S. and globally [1], [8], yet the potential contributions of other risk factors remain poorly defined.
Smoked cannabis is a potential risk factor for lung disease, as it contains many of the same toxic constituents present in tobacco smoke [9]. In the U.S., the proportion of HIV+ individuals who frequently smoke marijuana is higher than in the general population [10], [11], [12] and has increased in recent years [12]. Previous studies of HIV-uninfected populations reported an association between long-term marijuana smoking and increased respiratory symptoms [13], [14], [15], chronic bronchitis [15], and chronic obstructive pulmonary disease (COPD) and emphysema [13], while other studies reported no significant association between marijuana smoking and these diagnoses or other measures of lung health [15], [16], [17]. Among HIV-infected individuals, few data exist regarding the association between marijuana smoking and respiratory burden, despite high prevalence of lung disease in HIV-infected populations and its associated mortality and morbidity [1], [3].
The aims of this study were to investigate the effects of marijuana smoking on infectious and non-infectious pulmonary diagnoses in HIV-infected individuals in the combination antiretroviral therapy (cART) era, and to compare its effects in HIV-infected vs. uninfected individuals with similar demographic characteristics using data from a large prospective cohort of men who have sex with men (MSM).
2. Methods
2.1. Study Design and Participants
The MACS is an ongoing prospective study of HIV-infected and -uninfected MSM (https://statepi.jhsph.edu/macs/macs.html) [18], [19]. A total of 7343 participants have been recruited from four U.S. urban centers (Los Angeles, Chicago, Pittsburgh, and Baltimore/Washington D.C.) in four recruitment waves: 1984–1985, 1987–1991, 2001–2003, and 2010-present. Participants undergo standardized interviews detailing substance use, behavioral characteristics, medical treatments or conditions, physical examinations, and collection of biological specimens at biannual study visits. All participants provided written informed consent, and the study protocols were approved by each site's institutional review board (Northwestern University (Evanston, IL), University of California, Los Angeles (Los Angeles, CA), the University of Pittsburgh (Pittsburgh, PA), and the Johns Hopkins Bloomberg School of Public Health (Baltimore, MD)).
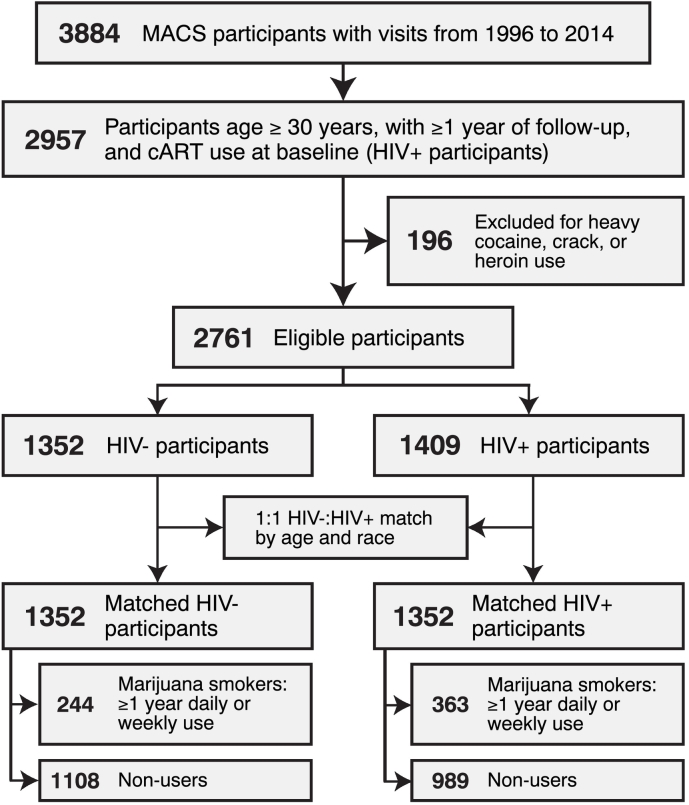
Eligible participants were 2704 MSM from the MACS (P25 release of the public use data), age ≥ 30, with a minimum of one year of follow-up between 1996 and 2014, and self-reported marijuana and tobacco smoking exposure data. Exclusion criteria included daily or weekly heroin or cocaine use for one or more years during follow-up. For HIV+ participants (n = 1352), baseline was indexed to first visit with reported cART use. HIV− participants (n = 1352) were individually matched to HIV+ participants by baseline age and race (white vs. non-white) using the “optimal” method (minimized average absolute distances across all matched pairs) with the R MatchIt propensity score matching package (v. 3.0.1). Following matching, mean difference balances for age and race between HIV+ and HIV− participants were improved by 16·8% and 30·1%, respectively.
2.2. Procedures and Definitions
Participants reported the frequency of marijuana use (daily, weekly, monthly, less than monthly, or no use) since their last biannual study visit; smoking was the most likely predominant mode of exposure. Tobacco use history (current, former, never) and quantity of cigarette packs currently smoked (none, > 0–< 1/2 packs/day, 1/2–< 1 packs/day, 1–< 2 packs/day, 2 or more packs/day) were also reported. In longitudinal analyses, current (daily or weekly vs. monthly smoking or less) was evaluated as a time-varying categorical variable. Time-varying marijuana smoking in the prior two-year period was calculated as a moving average after approximating continuous from categorical values as follows: daily = 20 days/month, weekly = 4·36 days/month, monthly = 1 day/month, less than monthly = 0·33 days/month, not at all = 0 days/month [20]. A continuous variable for prior two-year average tobacco smoking was similarly calculated using the following conversion: none = 0 packs/day, > 0–< 1/2 packs/day = 0·125 packs/day, ½–< 1 packs/day = 0·75 packs/day, 1–< 2 packs/day = 1·5 packs/day, 2 or more packs/day = 2 packs/day.
Incident pulmonary diagnoses were obtained from self-report, and abstracted medical record, cancer, and death registry data as described [18], [21], [22]. Participants were asked if, since their most recent visit, they were newly diagnosed with viral pneumonia, bacterial pneumonia, other pneumonia, or tuberculosis, or if newly diagnosed with or experienced recurring chronic bronchitis. These data were merged with additional variables provided as International Classification of Disease Codes, version 9 (ICD-9) or version 10 as follows: influenza or viral pneumonia (480, 487, ICD-10 J14), bacterial pneumonia (481–482), other pneumonia (031, 114, 117, 483–486, ICD-10 A31 and J18), acute bronchitis (466), tuberculosis (011, 018), chronic obstructive pulmonary disease (COPD) or emphysema (492, 496, ICD-10 J43–J44), pulmonary hypertension (416, ICD-10 I27), other non-infectious diagnoses (pulmonary fibrosis (515), pulmonary pneumopathy (516)), and other lung disease, not otherwise-specified (518–519). Lung cancers were determined from cancer registry linkage data (International Classification of Diseases for Oncology, third edition (ICD-O-3) site codes 34.0–34.9), death registry data (ICD-10 C34), and self-report (162). Pneumocystis pneumonia diagnoses were obtained from medical record and CDC AIDS diagnosis data. Chronic bronchitis was defined as the first of multiple bronchitis diagnoses or ICD codes 490–491.
HIV serostatus, and CD4 + T lymphocyte count and viral load for HIV+ participants, were obtained as previously described [10], [18]. Education level and race at study entry, ART use, and alcohol use were obtained from self-report. Missing time-varying data were imputed by carrying forward values from nearest available previous visit, and by multiple imputation in validation analyses using predictive mean matching with the R mice package (version 3.3.0).
2.3. Statistical Analysis
Cross-sectional analyses of baseline characteristics and prevalence of pulmonary diagnoses were stratified by HIV serostatus and marijuana smoking, and HIV serostatus, marijuana, and tobacco smoking, respectively. Marijuana smokers were defined as participants reporting ≥ 1 year of daily or weekly use in follow-up; tobacco smokers were participants reporting any tobacco smoking in follow-up.
Cox proportional hazard models were used to assess the association between marijuana smoking and first incident infectious pulmonary diagnosis, and chronic bronchitis, which comprised the majority of non-infectious diagnoses. Participants were followed until their first incident diagnosis, loss to follow-up, death, or end of study in 2014, whichever came first. For each outcome, models were fit separately for HIV+ and HIV− participants using two time-varying variables for marijuana and tobacco smoking: Model 1, current marijuana smoking (daily or weekly vs. monthly or less) and prior two-year average tobacco smoking (≥ 1/2 packs/day or > 0–< 1/2 pack/day vs. 0 packs/day, which included former and never smokers), and Model 2, prior two-year average marijuana smoking (days smoked/month, continuous variable) and prior two-year average tobacco smoking (packs/day, continuous variable). Models were adjusted by time-invariant baseline education status, race, and baseline calendar period (2001–2014 vs. 1996–2000) to adjust for potential differences in participant composition between MACS recruitment periods. Models for HIV+ participants were also adjusted by time-varying CD4 T cell count, and by time-varying self-reported ART use (binary categorical covariate) in models of infectious diagnoses; 65 participants with no CD4 data during follow-up were omitted from these analyses. Age was used as the time variable in all models (i.e., the analysis data was left-truncated to age at first study visit and right-censored to end of follow-up for each participant). Continuous variables summarizing marijuana and tobacco smoking were right-skewed and therefore square-root transformed and reported as back-transformed estimates in their original scale. Additional analyses were performed with models for HIV+ participants using: a) visits restricted to CD4 count ≥ 200 cells/μl, b) time-varying cumulative exposures of marijuana and tobacco smoking in the prior ten years (mean days marijuana smoking/month and tobacco pack-years), and c) visits restricted to HIV viral load < 400 copies/ml. Cumulative hazard curves estimating risk differences by marijuana and tobacco smoking were constructed by holding other model covariates at their mean values.
Generalized estimating equation (GEE) logistic regression models were used to assess the association between marijuana smoking and infectious pulmonary diagnoses while including repeated measures and potentially multiple diagnoses within the same individual. To minimize the potential for duplicate reports of a single diagnosis, only the first of identical diagnoses at consecutive visits were included. Exposure and adjustment variables were the same as for Cox models, except time-varying, continuous duration in study, and baseline age were included. All analyses were performed with R (version 3.4).
2.4. Role of the Funding Source
The study funders had no role in study design, data collection, analysis, or interpretation, or writing of this report. The corresponding author had full access to all study data and final responsibility for the decision to submit for publication.
3. Results
A total of 2704 participants met eligibility criteria for this study (n = 1352 HIV+, n = 1352 HIV−), contributing a total of 53,794 person-visits (Fig. 1). Participants were predominantly white, with > 12 years of education, median age 44 years at baseline (interquartile range [IQR] 39–50), and median follow-up of 10·5 years (IQR 5·5–13·0). Participants reporting daily or weekly marijuana smoking for ≥ 1 year during follow-up comprised 27% of HIV+ participants (median 4·0 years daily/weekly use [IQR 1·5–8·0]), and 18% of HIV − participants (median 4·5 years daily/weekly use [IQR 2·0–10·5]) (Table 1). Marijuana smokers were slightly younger at baseline than non-smokers, and more likely to smoke ≥ 1/2 packs/day of tobacco on average during the study period among both HIV+ and HIV− participants (25·6% vs. 18·1%, and 24·6% vs. 11·4%, respectively). Heavy or binge alcohol use during follow-up was also more common among marijuana smokers. Among HIV+ participants, CD4 T cell count, HIV viral load, or ART use did not differ between marijuana smokers and non-smokers.
Fig. 1.
Cohort selection flowchart.
Table 1.
Characteristics of the study cohort by HIV status and marijuana smoking.
| HIV+ |
HIV− |
|||||
|---|---|---|---|---|---|---|
| All participants |
Non-smokers |
Marijuana smokersa |
All participants |
Non-smokers |
Marijuana smokersa |
|
| (n = 1352) | (n = 989) | (n = 363) | (n = 1352) | (n = 1108) | (n = 244) | |
| Age (median [IQR]) | 43·0 [38·0–48·5] | 43·0 [38·5–49·0] | 42·0 [37·5–47·5] | 45·0 [39·0–51·0] | 45·0 [40·0–52·0] | 43·0 [38·0–47·0] |
| Years of follow-up (median [IQR]) | 9·50 [4·0–13·5] | 9·0 [4·0–13·0] | 10·5 [5·5–15·5] | 11·5 [7·0–13·0] | 11·5 [7·0–13·0] | 11·8 [8·0–13·0] |
| Race | ||||||
| White | 887 (65·6) | 638 (64·5) | 249 (68·6) | 1009 (74·6) | 836 (75·5) | 173 (70·9) |
| Black | 289 (21·4) | 209 (21·1) | 80 (22·0) | 230 (17·0) | 182 (16·4) | 48 (19·7) |
| Otherb | 176 (13·0) | 142 (14·4) | 34 (9·4) | 113 (8·4) | 90 (8·1) | 23 (9·4) |
| Education ≤ 12 years | 284 (21·0) | 222 (22·4) | 62 (17·1) | 186 (13·8) | 145 (13·1) | 41 (16·8) |
| Marijuana smoking – ≥ 1 years daily or weekly use in follow-up | 363 (26·8) | – | 363 (100·0) | 244 (18·0) | – | 244 (100·0) |
| Marijuana smoking – years daily or weekly use (median [IQR]) | – | – | 4·0 [1·5–8·0] | – | – | 4·5 [2·0–10·5] |
| Marijuana smoking – mean days use/year in follow-up (median [IQR]) | – | – | 66·7 [26·1–231·5] | – | – | 67 [24·6–195·9] |
| Tobacco smoking at baseline | ||||||
| Never | 465 (34·4) | 380 (38·4) | 85 (23·4) | 424 (39·6) | 380 (43·6) | 44 (22·1) |
| Former | 401 (29·7) | 287 (29·0) | 114 (31·4) | 316 (29·5) | 251 (28·8) | 65 (32·7) |
| < 1/2 packs/day | 179 (13·2) | 118 (11·9) | 61 (16·8) | 128 (12·0) | 99 (11·4) | 29 (14·6) |
| ≥ 1/2 packs/day | 307 (22·7) | 204 (20·6) | 103 (28·4) | 203 (19·0) | 142 (16·3) | 61 (30·7) |
| Tobacco smoking, mean packs/day in follow-up | ||||||
| None | 749 (55·4) | 599 (60·6) | 150 (41·3) | 872 (64·5) | 761 (68·7) | 111 (45·5) |
| > 0–<1/2 | 331 (24·5) | 211 (21·3) | 120 (33·1) | 294 (21·7) | 221 (19·9) | 73 (29·9) |
| ≥ 1/2 | 272 (20·1) | 179 (18·1) | 93 (25·6) | 186 (13·8) | 126 (11·4) | 60 (24·6) |
| Heavy or binge alcohol usec | 304 (22·5) | 201 (20·3) | 103 (28·4) | 346 (25·6) | 255 (23·0) | 91 (37·3) |
| CD4 count (cells/μl) (median [IQR]) | 422 [252–599] | 432 [260–603] | 396 [238–592] | 924 [731–1144] | 915 [724–1134] | 970 [777–1171] |
| CD4 count (cells/μl) | ||||||
| 350 + | 769 (60·9) | 571 (62·4) | 198 (57·1) | – | – | – |
| 200–349 | 258 (20·4) | 176 (19·2) | 82 (23·6) | – | – | – |
| < 200 | 235 (18·6) | 168 (18·4) | 67 (19·3) | – | – | – |
| CD4 nadir in follow-up (cells/μl) (median [IQR]) | 295 [171–446] | 303 [172–451] | 279 [164–435] | – | – | – |
| HIV viral load ≥ 400 copies/ml | 496 (39·7) | 350 (38·7) | 146 (42·3) | – | – | – |
| Antiretroviral use at 75% of follow-up visits | 1220 (90·2) | 888 (89·8) | 332 (91·5) | – | – | – |
| Antiretroviral use at 95% of follow-up visits | 876 (64·8) | 647 (65·4) | 229 (63·1) | – | – | – |
Data are n (%) at baseline unless otherwise indicated.
≥ 1 years daily or weekly smoking in follow-up.
American Indian or Alaskan Native, Asian or Pacific Islander, Hispanic, other race.
Heaviest reported usage at ≥ 1 year in follow-up: heavy, > 14 drinks/week, binge, ≥ 5 drinks/occasion at least monthly.
Marijuana smokers were more likely to report one or more infectious or non-infectious pulmonary diagnosis compared to non-smokers during follow-up among HIV+ participants (41·0% vs. 30·0%, and 24·8% vs. 19·0%, respectively) (Table 2), while there was no association between marijuana smoking and either diagnosis among HIV− participants (24·2% vs. 20·9%, and 14·8% vs. 17·7%, respectively). Given the high prevalence of tobacco smoking among participants, frequencies of diagnoses were determined after stratifying participants by marijuana (≥ 1 year daily or weekly vs. monthly or less) and tobacco (any vs. none) smoking during follow-up. The proportion of HIV+ participants with one or more infectious pulmonary diagnosis was elevated among marijuana smokers and participants reporting any tobacco smoking in follow-up. Influenza and viral pneumonia and other pneumonias were among diagnoses occurring more frequently in marijuana smokers: other pneumonias were reported in 22% and 23·9% of participants smoking marijuana only, or both marijuana and tobacco, respectively, compared to non-smokers (16%). Non-infectious pulmonary diagnoses were more common in both marijuana and tobacco smokers. Among HIV− participants, infectious diagnoses were more prevalent in individuals smoking both marijuana and tobacco, and non-infectious diagnoses were elevated among tobacco but not marijuana smokers. The majority (60%) of diagnoses were assessed from ICD coding data, while chronic bronchitis diagnoses were predominantly assessed from self-report (Supplementary Table 1).
Table 2.
Incident pulmonary diagnoses by HIV status and marijuana or tobacco smoking.
| HIV+ |
HIV− |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Marijuana and tobacco smoking exposurea | All participants | MJ− TS>− | MJ− TS+ | MJ+ TS− | MJ+ TS+ | All participants | MJ− TS− | MJ− TS+ | MJ+ TS− | MJ+ TS+ |
| n participants | 1352 | 599 | 390 | 150 | 213 | 1352 | 761 | 347 | 111 | 133 |
| No diagnosis | 779 (57·6) | 381 (63·6) | 220 (56·4) | 74 (49·3) | 104 (48·8) | 939 (69·5) | 546 (71·7) | 230 (66·3) | 78 (70·3) | 85 (63·9) |
| Infectious diagnoses | ||||||||||
| Influenza or viral pneumonia | 189 (14·0) | 76 (12·7) | 48 (12·3) | 28 (18·7) | 37 (17·4) | 159 (11·8) | 91 (12·0) | 33 (9·5) | 12 (10·8) | 23 (17·3) |
| Bacterial pneumonia | 122 (9·0) | 47 (7·8) | 36 (9·2) | 17 (11·3) | 22 (10·3) | 33 (2·4) | 16 (2·1) | 13 (3·7) | 3 (2·7) | 1 (0·8) |
| Other pneumonia | 261 (19·3) | 96 (16·0) | 81 (20·8) | 33 (22·0) | 51 (23·9) | 130 (9·6) | 66 (8·7) | 44 (12·7) | 7 (6·3) | 13 (9·8) |
| Other opportunistic infectionsb | 51 (3·8) | 14 (2·3) | 16 (4·1) | 8 (5·3) | 13 (6·1) | 6 (0·4) | 0 (0·0) | 3 (0·9) | 2 (1·8) | 1 (0·8) |
| Any infectious diagnosis | 449 (33·2) | 170 (28·4) | 130 (33·3) | 62 (41·3) | 87 (40·8) | 291 (21·5) | 153 (20·1) | 79 (22·8) | 24 (21·6) | 35 (26·3) |
| Non-infectious diagnoses | ||||||||||
| Chronic bronchitis | 238 (17·6) | 84 (14·0) | 73 (18·7) | 34 (22·7) | 47 (22·1) | 192 (14·2) | 101 (13·3) | 64 (18·4) | 11 (9·9) | 16 (12·0) |
| COPD or emphysema | 28 (2·1) | 5 (0·8) | 16 (4·1) | 2 (1·3) | 5 (2·3) | 35 (2·6) | 8 (1·1) | 19 (5·5) | 0 (0·0) | 8 (6·0) |
| Lung cancerc | 10 (0·7) | 1 (0·2) | 8 (2·1) | 0 (0·0) | 1 (0·5) | 7 (0·5) | 1 (0·1) | 4 (1·2) | 2 (1·8) | 0 (0·0) |
| Other non-infectious diagnosesd | 37 (2·7) | 15 (2·5) | 12 (3·1) | 6 (4·0) | 4 (1·9) | 29 (2·1) | 19 (2·5) | 7 (2·0) | 1 (0·9) | 2 (1·5) |
| Any non-infectious diagnoses | 278 (20·6) | 96 (16·0) | 92 (23·6) | 40 (26·7) | 50 (23·5) | 232 (17·2) | 118 (15·5) | 78 (22·5) | 14 (12·6) | 22 (16·5) |
Data are n (%) of participants with one or more indicated diagnosis.
Exposure during follow-up; MJ+, ≥ 1 year daily or weekly marijuana smoking; MJ−, < 1 year daily or weekly marijuana smoking; TS−, 0 tobacco smoking; TS+, > 0 mean packs/day tobacco smoking. The median years of daily or weekly smoking among MJ+ participants were 4·0 and 4·5 years for HIV+ and HIV− participants, respectively.
Pneumocystis pneumonia or tuberculosis.
Among the two lung cancer diagnoses in HIV− MJ+ participants, one case reported a history of tobacco smoking prior to study entry, the other was diagnosed with a pulmonary sarcoma (a rare form of lung cancer not linked to smoking).
Pulmonary hypertension, alveolar pneumonopathy, pulmonary fibrosis, bronchiectasis, other lung disease (not otherwise specified).
In multivariable Cox regression models, current daily or weekly marijuana smoking was associated with increased risk of first incident infectious pulmonary diagnosis in HIV + participants (adjusted hazard ratio [HR] 1·34 [95% confidence interval (CI) 1·06–1·71], p = 0·016; Supplementary Table 2). When modeled as a continuous variable, each 10 days/month increase in marijuana smoking in the prior two-year period was associated with a 6% increased risk of infectious pulmonary diagnosis (HR 1·06 [95% CI 1·00–1·11]; p = 0·041). CD4 T cell count < 200 vs. ≥ 350 cells/μl was most strongly associated with infectious pulmonary diagnosis risk in both models (HR 3·37 [95% CI 2·58–4·41], p < 0·0001; and HR 3·38 [95% CI 2·59–4·41], p < 0·0001), and therefore, additional models were fit restricted to 17,529 person-visits with CD4 T cell count ≥ 200 cells/μl of 19,009 total person-visits. Both current daily or weekly and prior two-year average marijuana smoking remained associated with increased risk of infectious pulmonary diagnoses in these adjusted models (HR 1·43 [95% CI 1·09–1·86], p = 0·0085; and HR 1·10 [95% CI 1·04–1·17], p = 0·012; Table 3). Smoking ≥ 1/2 vs. 0 packs of tobacco/day was strongly associated with increased risk of infectious pulmonary diagnoses (HR 1·48 [95% CI 1·14–1·93], p = 0·0037), while low CD4 count remained associated with a higher risk of infectious pulmonary diagnoses. Cumulative estimates of marijuana and tobacco smoking in the prior ten years remained associated with infectious pulmonary diagnoses in additional models adjusted for the same factors (HR 1·11 [1·04–1·19], p = 0·016, per ten days marijuana smoked/month increase, and HR 1·13 [1·03–1·22], p = 0·021, per ten pack-year increase, data not shown). Additionally, the association between daily or weekly marijuana smoking and infectious pulmonary diagnoses remained significant in models restricted to 14,666 person-visits with suppressed HIV viral load (HR 1·41 [1·03–1·91], p = 0·029, data not shown). There were no associations between marijuana smoking and infectious pulmonary diagnoses in HIV − participants, while tobacco smoking showed a strong association (Table 3).
Table 3.
Factors associated with risk of infectious pulmonary diagnoses in univariable and multivariable Cox regression analysis.
| HIV+ |
HIV− |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Model 1 – current marijuana smoking |
Model 2 – prior two-year average marijuana smoking |
Univariate analysis |
Model 1 – current marijuana smoking |
Model 2 – prior two-year average marijuana smoking |
|||||||
| HR (95% CI) |
p Value |
HR (95% CI) |
p Value |
HR (95% CI) |
p Value |
HR (95% CI) |
p Value |
HR (95% CI) |
p Value |
HR (95% CI) |
p Value |
|
| Marijuana smoking - current | ||||||||||||
| Daily or weekly | 1·52 (1·18–1·98) |
0·0014 | 1·43 (1·09–1·86) |
0·0085 | – | 0·92 (0·64–1·33) |
0·65 | 0·82 (0·57–1·20) |
0·31 | – | ||
| Monthly or less | 1·00 (Ref.) | 1·00 (Ref.) | – | 1·00 (Ref.) | 1·00 (Ref.) | – | ||||||
| Marijuana smoking - prior two-year average (per 10 days use/month increase, continuous variable) | 1·16 (1·10–1·23) |
0·0013 | – | 1·10 (1·04–1·17) |
0·012 | 1·01 (0·89–1·15) |
0·56 | – | 1·05 (0·91–1·20) |
0·26 | ||
| Tobacco smoking - prior two-year average | ||||||||||||
| ≥ 1/2 packs/day | 1·54 (1·19–2·00) |
0·0010 | 1·48 (1·14–1·93) |
0·0037 | – | 1·75 (1·29–2·37) |
0·0003 | 1·85 (1·35–2·54) |
0·0001 | – | ||
| > 0–<1/2 packs/day | 0·97 (0·68–1·36) |
0·85 | 0·98 (0·68–1·39) |
0·89 | – | 1·17 (0·82–1·68) |
0·38 | 1·23 (0·85–1·76) |
0·27 | – | ||
| 0 packs/daya | 1·00 (Ref.) | 1·00 (Ref.) | – | 1·00 (Ref.) | 1·00 (Ref.) | – | ||||||
| Tobacco smoking - prior two-year average (per pack/day increase, continuous variable) | 1·14 (1·07–1·21) |
0·0042 | – | 1·10 (1·03–1·17) |
0·018 | 1·25 (1·15–1·35) |
0·0009 | – | 1·32 (1·21–1·43) |
0·0003 | ||
| Baseline calendar year | ||||||||||||
| 2001–2014 | 0·68 (0·54–0·86) |
0·0011 | 0·69 (0·53–0·90) |
0·0051 | 0·70 (0·54–0·90) |
0·0059 | 0·99 (0·78–1·27) |
0·96 | 1·00 (0·78–1·30) |
0·98 | 1·00 (0·78–1·30) |
0·97 |
| 1996–2000 | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | ||||||
| CD4 count (cells/μL) | ||||||||||||
| 200–349 | 1·52 (1·16–2·00) |
0·0025 | 1·43 (1·08–1·88) |
0·011 | 1·43 (1·09–1·89) |
0·011 | – | – | – | |||
| 350–499 | 1·40 (1·08–1·81) |
0·011 | 1·35 (1·05–1·75) |
0·021 | 1·35 (1·05–1·75) |
0·021 | – | – | – | |||
| 500 + | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | – | – | – | ||||||
| ART use | ||||||||||||
| Yesb | 0·73 (0·47–1·13) |
0·15 | 0·79 (0·51–1·23) |
0·30 | 0·79 (0·51–1·22) |
0·29 | – | – | – | |||
| No | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | – | – | – | ||||||
Hazard ratios (HR) for first incident infectious pulmonary diagnosis (influenza or viral pneumonia, bacterial pneumonia, other pneumonia, acute bronchitis, Pneumocystis pneumonia, tuberculosis). All models were adjusted by race and education. All covariates were time-varying except baseline calendar year, race, and education; age was used as the time variable. Continuous variables for prior two-year average marijuana and tobacco smoking were square-root transformed to reduce skewness and reported as back-transformed estimates in their original scale. HIV+ models evaluated 331 events in 1196 participants with 17,529 person-visits with CD4 ≥ 200 cells/μl. HIV− models evaluated 291 diagnoses in 1350 participants with 24,232 person-visits.
Reference category includes former tobacco smokers.
Includes person-visits with HIV viral load < 400 copies/ml where ART data missing.
The association between marijuana smoking and infectious pulmonary diagnoses remained significant in GEE logistic regression models using longitudinal data, which allowed for multiple diagnoses within the same individual (Table 4). A total of 516 diagnoses were reported for 421 of 1287 total participants at 22,694 person-visits with ≥ 200 CD4 T cells/μl. Both daily or weekly current and prior two-year average marijuana smoking were associated with increased infectious pulmonary diagnosis risk in adjusted models (odds ratio (OR) 1·32 [95% CI 1·05–1·75], p = 0·035; and OR 1·10 [95% CI 1·04–1·16], p = 0·012, respectively). Similar to the Cox models, 200–349 vs. ≥ 500 CD4 T cells/μl and ≥ 1/2 vs. 0 packs/day tobacco smoking in the prior two years was more strongly associated with increased risk compared to marijuana smoking (Table 4). There was no association between marijuana smoking and infectious pulmonary diagnoses in HIV − individuals.
Table 4.
Factors associated with risk of one or more infectious pulmonary diagnoses among HIV+ subjects in generalized estimating equation models, adjusted for intraparticipant correlation.
| HIV+ |
HIV− |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Model 1 – current marijuana smoking |
Model 2 – prior two-year average marijuana smoking |
Univariate analysis |
Model 1 – current marijuana smoking |
Model 2 – prior two-year average marijuana smoking |
|||||||
| OR (95% CI) |
p Value |
OR (95% CI) |
p Value |
OR (95% CI) |
p Value |
OR (95% CI) |
p Value |
OR (95% CI) |
p Value |
OR (95% CI) |
p Value |
|
| Marijuana smoking – current | ||||||||||||
| Daily or weekly | 1·36 (1·05–1·75) |
0·019 | 1·32 (1·02–1·70) |
0·035 | – | 0·80 (0·55–1·14) |
0·22 | 0·71 (0·49–1·03) |
0·071 | – | ||
| Monthly or less | 1·00 (Ref.) | 1·00 (Ref.) | – | 1·00 (Ref.) | 1·00 (Ref.) | – | ||||||
| Marijuana smoking - prior two-year average (per 10 days use/month increase, continuous variable) | 1·12 (1·06–1·18) |
0·0042 | – | 1·10 (1·04–1·16) |
0·012 | 1·02 (0·90–1·17) |
0·42 | – | 1·09 (0·93–1·27) |
0·15 | ||
| Tobacco smoking - prior 2-year average | ||||||||||||
| ≥ 1/2 packs/day | 1·56 (1·21–2·02) |
0·0007 | 1·47 (1·13–1·90) |
0·0036 | – | 1·92 (1·39–2·66) |
< 0·0001 | 1·93 (1·34–2·77) |
< 0·0001 | – | ||
| > 0–<1/2 packs/day | 1·02 (0·74–1·40) |
0·90 | 1·01 (0·73–1·40) |
0·94 | – | 1·33 (0·95–1·87) |
0·10 | 1·34 (0·95–1·89) |
0·099 | – | ||
| 0 packs/daya | 1·00 (Ref.) | 1·00 (Ref.) | – | 1·00 (Ref.) | 1·00 (Ref.) | – | ||||||
| Tobacco smoking - prior two-year average (per pack/day increase, continuous variable) | 1·16 (1·09–1·23) |
0·0024 | – | 1·09 (1·03–1·16) |
0·017 | 1·41 (1·29–1·55) |
0·0002 | – | 1·40 (1·25–1·57) |
0·0007 | ||
| Duration in study (years) | 0·96 (0·94–0·98) |
< 0·0001 | 0·96 (0·94–0·98) |
< 0·001 | 0·96 (0·94–0·98) |
< 0·001 | 0·96 (0·93–0·99) |
0·0032 | 0·96 (0·94–0·99) |
0·015 | 0·96 (0·94–0·99) |
0·014 |
| Baseline age (per 5 year increase) | 1·09 (1·00–1·19) |
0·053 | 1·10 (1·01–1·20) |
0·021 | 1·11 (1·02–1·21) |
0·017 | 0·99 (0·92–1·07) |
0·88 | 1·00 (0·92–1·08) |
0·99 | 0·99 (0·92–1·08) |
0·89 |
| Baseline calendar year | ||||||||||||
| 2001–2014 | 0·76 (0·60–0·95) |
0·016 | 0·63 (0·48–0·82) |
0·0006 | 0·63 (0·49–0·82) |
0·0006 | 1·05 (0·82–1·35) |
0·71 | 0·95 (0·71–1·26) |
0·71 | 0·95 (0·72–1·27) |
0·74 |
| 1996–2000 | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | ||||||
| CD4 count (cells/μL) | ||||||||||||
| 200–349 | 1·62 (1·25–2·11) |
0·0003 | 1·40 (1·07–1·82) |
0·013 | 1·40 (1·07–1·82) |
0·013 | – | – | – | |||
| 350–499 | 1·44 (1·13–1·83) |
0·0033 | 1·34 (1·05–1·70) |
0·017 | 1·34 (1·05–1·70) |
0·017 | – | – | – | |||
| 500 + | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | – | – | – | ||||||
| ART use | ||||||||||||
| Yesb | 0·68 (0·46–1·00) |
0·048 | 0·74 (0·50–1·10) |
0·141 | 0·74 (0·50–1·10) |
0·14 | – | – | – | |||
| No | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | – | – | – | ||||||
Odds ratios (OR) for incident infectious pulmonary diagnoses (influenza or viral pneumonia, bacterial pneumonia, other pneumonia, acute bronchitis, Pneumocystis pneumonia, tuberculosis). All models were adjusted by race and education. All covariates were time-varying except baseline calendar year, baseline age, race, and education; duration in study was used as the time variable. Continuous variables for prior two-year average marijuana and tobacco smoking were square-root transformed to reduce skewness and reported as back-transformed estimates in their original scale. HIV+ models evaluated 516 events in 1196 participants with 22,694 person-visits with CD4 ≥ 200 cells/μl. HIV− models evaluated 399 diagnoses in 1350 participants at 28,478 person-visits.
Reference category includes former tobacco smokers.
Includes person-visits with HIV viral load < 400 copies/ml where ART data missing.
Cox proportional hazard models of non-infectious pulmonary diagnoses were limited to chronic bronchitis, given these diagnoses comprised the majority of events. Current daily or weekly marijuana smoking was associated with increased risk of chronic bronchitis among HIV+ participants, while there was a marginal association between marijuana smoking in the prior two years and chronic bronchitis in these models (HR 1·54 [95% CI 1·11–1·23], p = 0·0093; and HR 1·09 [95% CI 0·99, 1·21], p = 0·067, respectively; Table 5). CD4 T cell count (< 200 vs. 350 + cells/μl) and tobacco smoking (≥ 1/2 vs. 0 packs/day) in the prior two years were more strongly associated with chronic bronchitis risk compared to marijuana smoking (HR 1·84 [95% CI 1·25–2·67], p = 0·0015; and HR 1·68 [95% CI 1·23–2·29], p = 0·0009, respectively). Cumulative estimates of marijuana and tobacco smoking in the prior ten years showed similar associations with chronic bronchitis in additional models adjusted for the same factors (HR 1·07 [0·94–1·22], p = 0·151, per ten days marijuana smoked/month increase, and HR 1·19 [1·06–1·33], p = 0·014, per ten pack-year increase, data not shown). There were no associations between marijuana smoking and chronic bronchitis in HIV− participants, although tobacco smoking was strongly associated with this outcome (Table 5).
Table 5.
Factors associated with risk of chronic bronchitis in univariable and multivariable Cox regression analysis.
| HIV+ |
HIV− |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Model 1 – current marijuana smoking |
Model 2 – prior two-year average marijuana smoking |
Univariate analysis |
Model 1 – current marijuana smoking |
Model 2 – prior two-year average marijuana smoking |
|||||||
| HR (95% CI) |
p Value |
HR (95% CI) |
p Value |
HR (95% CI) |
p Value |
HR (95% CI) |
p Value |
HR (95% CI) |
p Value |
HR (95% CI) |
p Value |
|
| Marijuana smoking - current | ||||||||||||
| Daily or weekly | 1·52 (1·11–2·06) |
0·0080 | 1·54 (1·11–2·13) |
0·0093 | – | 0·80 (0·51–1·28) |
0·36 | 0·66 (0·41–1·07) |
0·090 | – | ||
| Monthly or less | 1·00 (Ref.) | 1·00 (Ref.) | – | 1·00 (Ref.) | 1·00 (Ref.) | – | ||||||
| Marijuana smoking - prior two-year average (per 10 days use/month increase, continuous variable) | 1·10 (1·01–1·19) |
0·040 | – | 1·09 (0·99–1·21) |
0·067 | 0·82 (0·56–1·20) |
0·30 | – | 0·70 (0·47–1·05) |
0·083 | ||
| Tobacco smoking - prior two-year average | ||||||||||||
| ≥ 1/2 packs/day | 1·74 (1·28–2·35) |
0·0003 | 1·68 (1·23–2·29) |
0·0009 | – | 2·02 (1·41–2·88) |
0·0001 | 2·29 (1·58–3·31) |
< 0·0001 | – | ||
| > 0–<1/2 packs/day | 1·13 (0·77–1·65) |
0·54 | 1·25 (0·84–1·85) |
0·27 | – | 1·12 (0·72–1·74) |
0·62 | 1·25 (0·80–1·96) |
0·33 | – | ||
| 0 packs/daya | 1·00 (Ref.) | 1·00 (Ref.) | – | 1·00 (Ref.) | 1·00 (Ref.) | – | ||||||
| Tobacco smoking - prior two-year average (per pack/day increase, continuous variable) | 1·42 (1·31–1·53) |
< 0·0001 | – | 1·35 (1·25–1·46) |
0·0001 | 1·72 (1·55–1·91) |
< 0·0001 | – | 2·04 (1·83–2·27) |
< 0·0001 | ||
| Baseline calendar year | ||||||||||||
| 2001–2014 | 0·59 (0·45–0·79) |
0·0003 | 0·72 (0·53–0·99) |
0·042 | 0·72 (0·52–0·98) |
0·039 | 0·63 (0·45–0·87) |
0·0052 | 0·64 (0·45–0·90) |
0·0097 | 0·64 (0·46–0·90) |
0·010 |
| 1996–2000 | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | ||||||
| CD4 count (cells/μL) | ||||||||||||
| < 200 | 1·95 (1·35–2·83) |
0·0003 | 1·84 (1·26–2·67) |
0·0015 | 1·83 (1·26–2·66) |
0·0015 | – | – | – | |||
| 200–349 | 1·38 (0·99–1·93) |
0·058 | 1·30 (0·93–1·81) |
0·13 | 1·29 (0·93–1·81) |
0·13 | – | – | – | |||
| 350 + | 1·00 (Ref.) | 1·00 (Ref.) | 1·00 (Ref.) | – | – | – | ||||||
Hazard ratios (HR) for first incident chronic bronchitis report. All models were adjusted by race and education. All covariates were time-varying except baseline calendar year, race, and education; age was used as the time variable. Continuous variables for prior two-year average marijuana and tobacco smoking were square-root transformed to reduce skewness and reported as back-transformed estimates in their original scale. To adjust for limited follow-up among HIV+ heavy marijuana smokers with education ≤ 12 years, multivariable models included an interaction between marijuana use and education (Model 1: ß = − 1·24, p = 0·048; Model 2: ß = − 0·88, p = 0·080). HIV+ models evaluated 232 events in 1287 participants with 24,232 person-visits. HIV− models evaluated 192 diagnoses in 1350 participants with 24,996 person-visits.
Reference category includes former tobacco smokers.
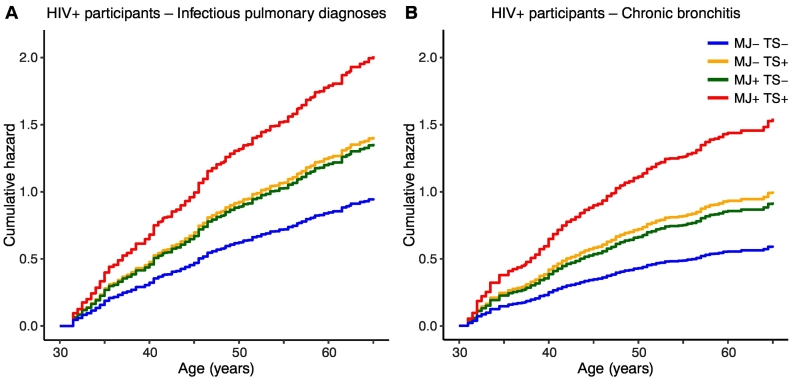
There were no significant interactions between marijuana and tobacco smoking in any multivariable model tested for HIV+ participants, indicating independent effects of these factors, and no significant interactions between marijuana smoking and time in GEE analyses of repeated infectious pulmonary events, indicating no evidence for changing risk level over the study duration. Imputation of missing observations by last value carried forward was validated with Cox model estimates pooled from ten datasets using predictive mean matching multiple imputation, which demonstrated similar associations between daily or weekly marijuana smoking and infectious pulmonary diagnoses (HR 1·44 [95% CI 1·07–1·92], p = 0·014) and chronic bronchitis (HR 1·62 [95% CI 1·14–2·28], p = 0·006) for HIV+ participants (data not shown). Cumulative hazard curves from multivariable Cox models (Table 3, HIV+ model 1, and Table 5, HIV+ model 1) are shown in Fig. 2, which predicts highest hazard rates for both infectious pulmonary diagnoses and chronic bronchitis among HIV+ participants smoking both marijuana and tobacco.
Fig. 2.
Cumulative hazards of infectious pulmonary and chronic bronchitis by marijuana and tobacco smoking in HIV+ participants. Estimated cumulative hazard curves from Cox regression models of (A) infectious pulmonary diagnoses (Table 3, HIV+ Model 1), and (B) chronic bronchitis (Table 5, HIV+ Model 1). Estimates were obtained holding other model covariates at their mean values; curves were right-truncated at age 65, corresponding to 10% of participants at risk remaining. MJ+ denotes daily or weekly current marijuana smoking, MJ−, monthly smoking or less, TS+, ≥ 1/2 pack/day tobacco smoking, prior two-year average, TS−, 0 packs/day tobacco smoking, prior two-year average.
4. Discussion
In this prospective study of 2704 MSM contributing over 53,000 person-visits, marijuana smoking was associated with increased risk of both infectious and non-infectious pulmonary diagnoses among 1352 HIV-infected participants independent of tobacco smoking, CD4 count, ART adherence, and demographic factors. To our knowledge, this study is the largest investigation of smoked marijuana and pulmonary diagnoses in HIV-infected individuals to date. In multivariable Cox models, current daily or weekly marijuana smoking and increased average marijuana smoking over the prior two years were associated with elevated risk of infectious pulmonary diagnoses in HIV+ participants; these results were confirmed in GEE logistic regression models allowing for repeated diagnoses within the same individual. Current marijuana smoking was also associated with increased risk of chronic bronchitis in multivariable Cox models, while increased average marijuana smoking in the prior two years was marginally associated with these outcomes (p = 0·067). Marijuana and tobacco smoking were independent risk factors in Cox models, and the predicted additive risk of smoking both substances was markedly higher than smoking either substance alone. In contrast, there were no significant associations between marijuana use and infectious pulmonary diagnoses or chronic bronchitis in multivariable models of 1352 HIV − participants adjusted by tobacco smoking, age, race, education, and calendar period. These findings confirm the known association between HIV infection and increased prevalence of pulmonary disease [1], [2], [3], and provide evidence that HIV-infected individuals may be more vulnerable to marijuana's effects on lung disease compared to uninfected participants with similar exposures.
In contrast to the well-studied effects of tobacco smoking, the effects of marijuana smoking on lung function and health in the general population remain unclear, though multiple studies reported an increased prevalence of chronic respiratory symptoms (wheeze, cough, sputum) in frequent marijuana smokers [13], [15], [23]. Spirometric forced expiratory volume (FEV1), forced vital capacity (FVC), and their ratio (FEV1/FVC) are measures of airflow obstruction; FEV1/FVC is reduced in tobacco smokers compared to non-smokers, while studies of marijuana smokers have reported conflicting results [14], [16], [17]. Likewise, a cross-sectional study of pulmonary function among HIV-infected recreational drug users in the U.S. reported no association between marijuana smoking and airflow obstruction (FEV1/FVC) or radiographic emphysema [24]. In the U.S., marijuana remains an illegal Schedule 1 substance nationally, and is not regulated by the Food and Drug Administration. Marijuana contains substantial variation in the quantity of psychoactive delta-9 tetrahydrocannabinol (THC) and other combustible components, complicating systematic comparisons based on observational studies. While marijuana and tobacco smoke contain many of the same toxic constituents, previous studies reported higher concentrations of hydrogen cyanide, ammonia, and polycyclic aromatic hydrocarbons in marijuana smoke [9], and higher concentrations of blood carboxyhemoglobin concentrations, greater depth of inhalation, and greater puff volume in marijuana compared to tobacco smokers [25].
Pulmonary disease risk may be increased in HIV-infected vs. uninfected individuals due to HIV-specific factors including lung immune cell depletion and dysfunction [7], persistent immune cell activation [26], systemic inflammation [26], respiratory microbiome alterations [27], and oxidative stress [5], or a combination of these effects with modifiable risk factors including tobacco smoking. Previous studies linked marijuana smoke with alveolar macrophage dysfunction in both humans and mouse models [28], [29], and a potential additive risk of marijuana smoking and HIV disease may explain the increased prevalence of infectious pulmonary diagnoses in our adjusted analyses. The association between HIV infection, low CD4 T cell count, and increased risk of infectious and non-infectious pulmonary disease has been previously reported [5], [26], and was the strongest predictor of infectious pulmonary diagnoses in HIV+ participants in our study.
Low CD4 T cell count (< 200 cells/μl) was the dominant predictor of infectious pulmonary diagnoses in multivariable analyses, yet the association between marijuana smoking and infectious pulmonary diagnoses remained significant in adjusted analyses restricted to visits with CD4 ≥ 200 cells/μl, and sensitivity analyses with no CD4 restriction. Tobacco smoking, reported during follow-up for 44% of HIV+ participants, was a stronger risk factor for chronic bronchitis in HIV+ participants compared to marijuana smoking, while for infectious pulmonary diagnoses in HIV+ participants, the risk associated with ≥ 1/2 pack/day tobacco smoking was comparable to that of daily or weekly marijuana smoking (HR 1·48 [95% CI 1·14–1·93], and HR 1·43 [95% CI 1·09–1·86], respectively).
Strengths of this study included the large sample size, large number of diagnoses reported, and substantial length of follow-up (median 10·5 years). Men at risk for HIV are recruited by the MACS from four U.S. urban sites, and thus HIV-infected participants share similar demographic and lifestyle characteristics with uninfected participants. HIV+ and HIV− participants reported substantial daily or weekly marijuana smoking (median 4·0 and 4·5 years, respectively), which allowed assessments of both current and average exposures during follow-up. The sample size allowed for stricter control of tobacco smoking with large numbers of participants in stratified analyses (150 and 111 HIV+ and HIV− participants with ≥ 1 year of marijuana smoking and no tobacco smoking, respectively), which may in part explain the lack of association between marijuana smoking and pulmonary disease among HIV− individuals found here compared to previous studies. Injection drug use is a possible risk factor for lung disease among HIV-infected persons, and polydrug use is common in HIV-infected individuals [11], [21], and we therefore excluded heavy cocaine and heroin users from our analysis to reduce the potential for competing risks from multiple inhaled or injected substances.
Limitations of this study include those inherent to longitudinal prospective cohort studies, including the potential for findings to be specific to MSM populations recruited by the MACS, and for nonrandom dropout and ascertainment biases. Measures of marijuana intake were limited to self-report during follow-up, with limited detail regarding exposure prior to MACS enrollment or marijuana potency, source, or quantity. These concerns are mitigated in part by the use of three separate measures of marijuana smoking (current, two-year prior average, and average smoking in follow-up), all of which were associated with infectious pulmonary diagnoses and chronic bronchitis in models of HIV-infected participants. Furthermore, the proportion of daily or weekly HIV+ marijuana smokers here (27%) is comparable to reports from other U.S. cohorts of HIV-infected individuals [11]. The use of self-reported pulmonary symptoms and diagnoses is an additional potential source of bias, and possible under-representation of specific non-infectious diagnoses in the MACS, particularly COPD and pulmonary hypertension, has been reported [3]. Most diagnoses were not assessed from both self-report and ICD-coded sources. This limitation was mitigated in part because most diagnoses were obtained from ICD code data, which consists of more specific diagnosis classifications (72% of the total in both HIV+ and HIV− participants, excluding chronic bronchitis). Potential ambiguities regarding terminology for diagnoses, and discordance between highly prevalent pulmonary symptoms and low rate of pulmonary diagnostic testing among HIV-infected persons has been noted in other clinical settings [1], and is mitigated here in part by use of composite diagnoses. The number of diagnoses reported for several categories, including tuberculosis, lung cancers, and Pneumocystis pneumonia, was small and lacked statistical power to assess an adjusted association with marijuana or tobacco smoking.
In summary, we found a significant association between long-term marijuana smoking and risk of infectious lung disease and chronic bronchitis in HIV-infected men on ART, independent of tobacco smoking and other risk factors (Table 3, Table 4, Table 5). In contrast, we detected no association between marijuana smoking and lung disease among HIV-uninfected men while controlling for tobacco smoking and other demographic characteristics. These findings suggest that marijuana smoking is a modifiable risk factor that healthcare providers should consider when seeking to prevent or treat lung disease in people infected with HIV, particularly those with other known risk factors including heavy tobacco smoking, and low CD4 T cell count or advanced HIV disease. Given increasing trends of regular marijuana smoking among HIV-infected people and other high-risk populations in the U.S. and other developed and developing countries, more studies are needed to evaluate potential merits of non-smoked rather than smoked forms of marijuana for medicinal and other purposes.
Contributors
DL, HU, DG designed the study and analysis plan. DL, SW, and DG contributed to data collection. DL performed the statistical analyses with guidance from HU and DG. DL and DG wrote the first draft of the report. DL, HU, SW, and DG contributed to interpretation of analysis results, and critical review and writing of the Article. All authors have approved the final version of the report.
Declaration of Interests
We declare no competing interests.
Acknowledgements/Funding
This study was supported by NIH grants to D.G. (R01 DA030985 and DA046203) and in part by NIH funding to the Northwestern University Clinical Research Unit of the MACS (U01 - AI35039, with additional co-funding from National Cancer Institute (NCI) and National Institute on Drug Abuse (NIDA)). The MACS is funded by the National Institute of Allergy and Infectious Diseases (NIAID; U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, and UM1-AI35043), with additional co-funding from the National Cancer Institute (NCI), National Institute on Drug Abuse (NIDA), and National Institute of Mental Health (NIMH) at the National Institutes of Health (NIH). MACS data collection is also supported by UL1-TR000424 (JHU CTSA). The study funders had no role in study design, data collection, analysis, or interpretation, or writing of this report.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.01.003.
Appendix A. Supplementary Data
Supplementary tables
References
- 1.Fitzpatrick M.E., Kunisaki K.M., Morris A. Pulmonary disease in HIV-infected adults in the era of antiretroviral therapy. AIDS. 2018;32(3):277–292. doi: 10.1097/QAD.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crothers K., Huang L., Goulet J.L., Goetz M.B., Brown S.T., Rodriguez-Barradas M.C. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gingo M.R., Balasubramani G.K., Kingsley L., Rinaldo C.R., Jr., Alden C.B., Detels R. The impact of HAART on the respiratory complications of HIV infection: longitudinal trends in the MACS and WIHS cohorts. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collini P., Morris A. Maintaining lung health with longstanding HIV. Curr Opin Infect Dis. 2016;29(1):31–38. doi: 10.1097/QCO.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Depp T.B., McGinnis K.A., Kraemer K., Akgun K.M., Edelman E.J., Fiellin D.A. Risk factors associated with acute exacerbation of chronic obstructive pulmonary disease in HIV-infected and uninfected patients. AIDS. 2016;30(3):455–463. doi: 10.1097/QAD.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leader J.K., Crothers K., Huang L., King M.A., Morris A., Thompson B.W. Risk factors associated with quantitative evidence of lung emphysema and fibrosis in an HIV-infected cohort. J Acquir Immune Defic Syndr. 2016;71(4):420–427. doi: 10.1097/QAI.0000000000000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popescu I., Drummond M.B., Gama L., Lambert A., Hoji A., Coon T. HIV suppression restores the lung mucosal CD4 + T-cell viral immune response and resolves CD8 + T-cell alveolitis in patients at risk for HIV-associated chronic obstructive pulmonary disease. J Infect Dis. 2016;214(10):1520–1530. doi: 10.1093/infdis/jiw422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triplette M., Crothers K., Attia E.F. Non-infectious pulmonary diseases and HIV. Curr HIV/AIDS Rep. 2016;13(3):140–148. doi: 10.1007/s11904-016-0313-0. [DOI] [PubMed] [Google Scholar]
- 9.Moir D., Rickert W.S., Levasseur G., Larose Y., Maertens R., White P. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21(2):494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz D.R., Dutta A., Mukerji S.S., Holman A., Uno H., Gabuzda D. Marijuana use impacts midlife cardiovascular events in HIV-infected men. Clin Infect Dis. 2017;65(4):626–635. doi: 10.1093/cid/cix391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mimiaga M.J., Reisner S.L., Grasso C., Crane H.M., Safren S.A., Kitahata M.M. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am J Public Health. 2013:1457–1467. doi: 10.2105/AJPH.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okafor C.N., Cook R.L., Chen X., Surkan P.J., Becker J.T., Shoptaw S. AIDS Behav: Springer US; 2016. Trajectories of marijuana use among HIV-seropositive and HIV-seronegative MSM in the multicenter AIDS cohort study (MACS), 1984–2013; pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinasek M.P., McGrogan J.B., Maysonet A. A systematic review of the respiratory effects of inhalational marijuana. Respir Care. 2016;61(11):1543–1551. doi: 10.4187/respcare.04846. [DOI] [PubMed] [Google Scholar]
- 14.Morris M.A., Jacobson S.R., Kinney G.L., Tashkin D.P., Woodruff P.G., Hoffman E.A. Marijuana use associations with pulmonary symptoms and function in tobacco smokers enrolled in the Subpopulations and Intermediate Outcome Measures In Copd Study (SPIROMICS) Chronic Obstr Pulm Dis. 2018;5(1):46–56. doi: 10.15326/jcopdf.5.1.2017.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Academies of Sciences E, and Medicine . The National Academies Press; Washington, DC: 2017. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. [PubMed] [Google Scholar]
- 16.Kempker J.A., Honig E.G., Martin G.S. The effects of marijuana exposure on expiratory airflow. A study of adults who participated in the U.S. National Health and Nutrition Examination Study. Ann Am Thorac Soc. 2015;12(2):135–141. doi: 10.1513/AnnalsATS.201407-333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pletcher M.J., Vittinghoff E., Kalhan R., Richman J., Safford M., Sidney S. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012;307(2):173–181. doi: 10.1001/jama.2011.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detels R., Jacobson L., Margolick J., Martinez-Maza O., Munoz A., Phair J. The multicenter AIDS Cohort Study, 1983 to. Public Health. 2012;126(3):196–198. doi: 10.1016/j.puhe.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaslow R.A., Ostrow D.G., Detels R., Phair J.P., Polk B.F., Rinaldo C.R., Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 20.Shoptaw S., Stall R., Bordon J., Kao U., Cox C., Li X. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. Int J STD AIDS. 2012;23(8):576–580. doi: 10.1258/ijsa.2012.011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutta A., Uno H., Holman A., Lorenz D.R., Wolinsky S.M., Gabuzda D. Long-term nitrite inhalant exposure and cancer risk in MSM. AIDS. 2017;31(8):1169–1180. doi: 10.1097/QAD.0000000000001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hessol N.A., Kalinowski A., Benning L., Mullen J., Young M., Palella F. Mortality among participants in the multicenter AIDS cohort study and the women's interagency HIV study. Clin Infect Dis. 2007;44(2):287–294. doi: 10.1086/510488. [DOI] [PubMed] [Google Scholar]
- 23.Tashkin D.P. Marijuana and Lung Disease. Chest. 2018;154(3):653–663. doi: 10.1016/j.chest.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Simonetti J.A., Gingo M.R., Kingsley L., Kessinger C., Lucht L., Balasubramani G. Pulmonary function in HIV-infected recreational drug users in the era of anti-retroviral therapy. J AIDS Clin Res. 2014;5(11) doi: 10.4172/2155-6113.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu T.C., Tashkin D.P., Djahed B., Rose J.E. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med. 1988;318(6):347–351. doi: 10.1056/NEJM198802113180603. [DOI] [PubMed] [Google Scholar]
- 26.Fitzpatrick M.E., Nouraie M., Gingo M.R., Camp D., Kessinger C.J., Sincebaugh J.B. Novel relationships of markers of monocyte activation and endothelial dysfunction with pulmonary dysfunction in HIV-infected persons. AIDS. 2016;30(9):1327–1339. doi: 10.1097/QAD.0000000000001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawani M.B., Morris A. The respiratory microbiome of HIV-infected individuals. Expert Rev Anti-Infect Ther. 2016;14(8):719–729. doi: 10.1080/14787210.2016.1206469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldwin G.C., Tashkin D.P., Buckley D.M., Park A.N., Dubinett S.M., Roth M.D. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med. 1997;156(5):1606–1613. doi: 10.1164/ajrccm.156.5.9704146. [DOI] [PubMed] [Google Scholar]
- 29.Shay A.H., Choi R., Whittaker K., Salehi K., Kitchen C.M., Tashkin D.P. Impairment of antimicrobial activity and nitric oxide production in alveolar macrophages from smokers of marijuana and cocaine. J Infect Dis. 2003;187(4):700–704. doi: 10.1086/368370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables