Abstract
Objective
Progressive supranuclear palsy (PSP) is an atypical parkinsonian syndrome characterized by vertical gaze palsy and postural instability. Midbrain atrophy is suggested as a hallmark, but it has not been validated systematically in whole-brain imaging.
Methods
We conducted whole-brain meta-analyses identifying disease-related atrophy in structural MRI. Eighteen studies were identified (N = 315 PSP, 393 controls) and separated into gray or white matter analyses (15/12). All patients were diagnosed according to the National Institute of Neurological Disorders and Stroke and the Society for PSP (NINDS-SPSP criteria, Litvan et al. (1996a)), which are now considered as PSP-Richardson syndrome (Höglinger et al., 2017). With overlay analyses, we double-validated two meta-analytical algorithms: anatomical likelihood estimation and seed-based D mapping. Additionally, we conducted region-of-interest effect size meta-analyses on radiological biomarkers and subtraction analyses differentiating PSP from Parkinson's disease.
Results
Whole brain meta-analyses revealed consistent gray matter atrophy in bilateral thalamus, anterior insulae, midbrain, and left caudate nucleus. White matter alterations were consistently detected in bilateral superior/middle cerebellar pedunculi, cerebral pedunculi, and midbrain atrophy. Region-of-interest meta-analyses demonstrated that midbrain metrics generally perform very well in distinguishing PSP from other parkinsonian syndromes with strong effect sizes. Subtraction analyses identified the midbrain as differentiating between PSP and Parkinson's disease.
Conclusions
Our meta-analyses identify gray matter atrophy of the midbrain and white matter atrophy of the cerebral/cerebellar pedunculi and midbrain as characteristic for PSP. Results support the incorporation of structural MRI data, and particularly these structures, into the revised PSP diagnostic criteria.
Keywords: Imaging biomarker, Cerebral pedunculi, Cerebellar pedunculi, Meta-analysis, Midbrain, Progressive supranuclear palsy, Anatomical likelihood estimation, Seed-based D mapping
Highlights
-
•
Whole-brain gray matter meta-analyses show atrophy in the midbrain in PSP.
-
•
Whole-brain white matter atrophy in the cerebral/cerebellar pedunculi & midbrain
-
•
Region-of-interest effect size meta-analyses confirm strong pathology in midbrain.
-
•
Results support inclusion of structural MRI data into diagnostic criteria for PSP.
1. Introduction
Progressive supranuclear palsy (PSP) is a gradually progressive, atypical parkinsonian syndrome characterized by vertical gaze palsy and prominent postural instability with backward falls from disease onset on (Litvan et al., 1996a). Commonly applied diagnostic criteria were proposed by the National Institute of Neurological Disorders and Stroke and the Society for PSP (NINDS-SPSP criteria) in 1996 (Litvan et al., 1996a, Litvan et al., 1996b). To fulfill the diagnostic criteria for possible PSP, patients' age of onset should be >40 years. Additionally, postural instability leading to falls, accompanied by slower vertical saccades, or vertical gaze palsy alone is required for diagnosis. Probable PSP is diagnosed, when a patient shows all of the aforementioned features. Certainty of the underlying tauopathy can only be confirmed by histopathology - the remaining gold standard for diagnosis (Litvan et al., 1996b). Despite advances in research, the evaluation of atypical parkinsonian syndromes has remained primarily dependent on clinical diagnostics. Disease-specific, brain imaging, biomarkers have been introduced to increase diagnostic validity of neurodegenerative diseases, for example, in frontotemporal lobar degeneration and primary progressive aphasia (Bisenius et al., 2016; Gorno-Tempini et al., 2011; Rascovsky et al., 2011; Schroeter et al., 2014). In terms of potential biomarkers, previous PSP studies have focused on the local boundary shift integral, longitudinal diffusion changes, or measurements of the midbrain/cerebellum (Kato et al., 2003; Longoni et al., 2011; Oba et al., 2005; Paviour et al., 2006; Slowinski et al., 2008; Zhang et al., 2016). The aforementioned metrics of midbrain and cerebellum were mainly investigated in small cohorts. During the analysis phase of the present study, the diagnostic criteria for PSP were updated in an attempt to increase diagnostic confidence by including neuroimaging measures as supportive and exclusion criteria, highlighting the timeliness of our report. Clinical diagnostic criteria were further improved by including more detailed diagnosis and acknowledging the several phenotypes of PSP. The amended criteria propose that former diagnosis according to Litvan et al. (1996a) relates to the phenotype of PSP-Richardson syndrome. Indeed, diagnostic criteria by Litvan et al. (1996a) have been shown to be 95–100% specific, validated by pathological examination (Höglinger et al., 2017). In the new criteria, in particular the use of midbrain atrophy or hypometabolism and/or postsynaptic, striatal, dopaminergic degeneration (Höglinger et al., 2017) has been suggested. Whitwell et al. (2017) published a qualitative review of radiological biomarkers, supporting midbrain and cerebellar pedunculi as specific to PSP even in the early phases of disease. However, it is important to prove the reliability of those disease patterns in quantitative whole-brain analyses.
We investigated the neural correlates of PSP to validate pathognomonic signs and further identify possible imaging biomarkers with systematic and quantitative meta-analyses; a thorough and powerful data-driven approach (Bisenius et al., 2016; Schroeter et al., 2014). The meta-analysis focused on whole-brain voxel-based morphometry (VBM) studies that applied structural magnetic resonance imaging (MRI) in PSP. By including only whole-brain studies, we prevented possible vicious circles if only expected brain regions are examined. We investigated disease-associated atrophy separately in gray and white matter in PSP compared with controls. Two widely acknowledged meta-analytical approaches, anatomical likelihood estimation (ALE) (Eickhoff et al., 2012) and anisotropic effect size seed-based D mapping (SDM) (Radua and Mataix-Cols, 2009; Radua et al., 2014), were applied and double-validated against each other. Overlay analyses were used to confirm the findings of the individual approaches, by assessing the degree of overlap. This provided reliable results and enabled us to identify a robust, disease-characteristic imaging biomarker. To investigate disease-specificity we run subtraction analyses to compare PSP with related diseases, i.e. Parkinson's disease (Albrecht et al., 2018). We hypothesized atrophy in PSP of white matter in the midbrain and gray matter in the midbrain, thalamus, and insulae (Shao et al., 2014; Shi et al., 2013; Yu et al., 2015). Additionally we performed a supportive region-of-interest effect size meta-analysis on the disease-specific imaging marker studies identified by (Whitwell et al., 2017).
2. Materials and methods
2.1. General study selection
The meta-analysis was conducted according to the PRISMA guidelines (www.prisma-statement.org) and Müller et al. (2018). Two authors (FA, SB) independently searched the PubMed database with the following search strategy: (“progressive supranuclear palsy” OR “Steele-Richardson-Olszewski syndrome”) AND (voxel* OR gray matter OR VBM). Studies were included if they: (1) were peer-reviewed, (2) used established diagnostic criteria, (3) were original work, (4) made comparisons with age-matched healthy controls, (5) reported results normalized to a stereotactic space (Talairach or the Montreal Neurological Institute's (MNI)), and (6) took a whole-brain approach. Region-of-interest analyses, small volume corrected peaks, and case studies were discarded to prevent any regional a priori assumptions. As we did not find enough [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET) or diffusion tensor imaging (DTI) studies fulfilling our criteria, we limited analysis to structural MRI, investigating gray and/or white matter atrophy. The literature search was performed between February and May 2016, reviewing all studies regardless of their publication date. We contacted corresponding authors to request local maxima not reported in their original publications and obtained data (Burciu et al., Premi et al., and Hughes et al.). The corresponding author of Burciu et al. confirmed by email that they used the NINDS-SPS criteria for diagnosis.
2.2. Statistical analyses
2.2.1. Whole-brain ALE & SDM meta-analyses
To obtain robust and reliable neural correlates of PSP, we applied two separate meta-analysis methods, enabling confirmation of the results of each individual technique. Our methods included the widely used approaches of ALE and SDM (Eickhoff et al., 2012; Radua and Mataix-Cols, 2009). For in depth details and differences between the methods see Table e-1. In brief, we first applied SDM (v4.31) (Radua and Mataix-Cols, 2009; Radua et al., 2014). SDM involves rebuilding effect size and variance maps for each study using t-scores and the extracted local maxima (i.e. points of difference between patients and controls). Each map consists of voxels with assigned values depending on their proximity to reported maxima coordinates. Voxels close to reported coordinates have higher values. If voxels lie in the proximity of more than one coordinate, the values are summed. Effect sizes from peaks close to each other are calculated by a weighted average. From these study-specific effect size and variance maps, SDM calculates a mean meta-analytical map, taking between study variability into account. The between study variability is defined as the weighted inverse of the variance of the difference of the studies. The resulting map is tested, voxel-wise, against a null distribution of the meta-analytical values. Analyses were performed on 500 permutations with an anisotropic kernel of 1.0. Results are reported and displayed at p < 0.001, uncorrected for multiple comparisons, because SDM does not correct the brain mask for the number of statistical tests by default. However, to correct the resulting values for multiple comparisons, probabilities were put into the web-converter of SDM (false discovery rate (FDR) correction) (Hochberg and Benjamini, 1990) and are reported in the Supplement (Table e-3). Note that this only provides a peak-based FDR correction for multiple comparisons. To examine the robustness of the main meta-analytical output, jack-knife analyses were carried out by removing one study at a time and repeating the analysis. Clusters present in >90% of iterations are marked as such in Table e-3.
Secondly, we ran meta-analyses using ALE (GingerALE 2.3.6) (2012). This technique transforms the study-specific extracted peaks, of difference between patients and controls, into Gaussian probability distributions surrounding the coordinates. Coordinates reported in the Talairach stereotactic space were transformed into the stereotactic MNI space using the Lancaster transform implemented in GingerALE (Lancaster et al., 2007). The estimation of the width of these Gaussian probability distributions is adapted for each study using the number of included subjects based on empirical estimates of between-subject and between-template variability. The resulting ALE maps are then combined, across studies, and tested against the null hypothesis of a random spatial distribution between the modeled maps. We first analyzed the data at p < 0.001, uncorrected for multiple comparisons, to ensure comparability with SDM. Thereafter, we corrected the ALE results with FDR, as was done with SDM (Figure e-2) (Laird et al., 2005). FDR is the only method implemented in ALE and SDM, but they use different algorithms.
Thirdly, separate conjunction analyses were performed for gray and white matter by superimposing the results of SDM and ALE, revealing the most consistent atrophic regions. To overcome the potential bias the different FDR algorithms may introduce, we used the results at p < 0.001 uncorrected.
Finally, to investigate disease-specificity of the results, we ran subtraction analyses comparing the present results with the findings of our recently published meta-analysis on Parkinson's disease (For further information please refer to Albrecht et al., 2018). We used the results at p < 0.001 uncorrected from the present analysis and the white and gray matter MRI cohort of the Parkinson's disease meta-analysis (cohort with Parkinson's disease only). Data were analyzed in GingerALE with 10.000 randomizations at p < 0.001 uncorrected. Again, we corrected the ALE results applying FDR afterwards. Results are shown for the contrasts of PSP - Parkinson's disease for gray and white matter.
To reveal differences in age, gender, disease duration, Mini-Mental-State Examination (MMSE), and the motor score of the Unified Parkinson's Disease Rating Scale (UPDRS-III) between the gray and white matter cohorts, we performed Pearson's χ2 and unpaired Student's t-tests in R with a significance threshold at p < 0.05.
2.2.2. Region-of-interest effect size meta-analyses
As supportive analyses, we performed an effect size meta-analysis on the biomarker studies provided by the systematic review of (Whitwell et al., 2017). To include recently published studies, two authors searched the Pubmed database applying the same search tags as Whitwell and colleagues (October 2017–March 2018). Analyses were performed on studies reporting three metrics: midbrain area, midbrain-pons area ratio, and the MR Parkinsonism Index (MRPI), a midbrain to pons and middle to superior cerebellar pedunculus ratio. Data were analyzed with metafor in R (Viechtbauer, 2010) and normalized by calculating the standardized mean difference (Hedges' g), with 95% confidence intervals as an effect size estimate. A random-effects model was applied to account for both within- and between-study variance. Between-study heterogeneity was assessed using Q-statistics and I2 estimate.
2.3. Potential sources of bias and error
To avoid biasing the results toward specific brain areas, only quantitative and automated whole-brain studies were included for the whole-brain meta-analyses; region-of-interest studies were excluded. To eliminate age as a confounding variable, we only included comparisons with age-matched controls. Studies comparing PSP with other neurodegenerative diseases were further excluded. In line with the PRISMA recommendations, two investigators performed the literature search independently and discussed discrepancies (FA, SB). SDM and ALE algorithms are able to balance analyses by taking the sample size of each included study into account, thereby equalizing the contributions of each study. It may be the case that only studies finding atrophy in PSP patients were published and others finding null results were not, leading to a publication bias. While we cannot rule this out completely it does seem unlikely as the effect sizes in the published studies tend to be large and the proportion of patients showing quite severe atrophy has been extremely high.
2.4. Data availability statement
Data and analysis methods are shared at request for purposes of replicating procedures and results.
3. Results
3.1. Whole-brain meta-analyses
3.1.1. Study characteristics
A total of 18, gray and white matter, MRI studies, with 315 patients and 393 healthy controls, were included (Table 1, Figure e-1). Some studies reported both measures, leading to the inclusion of 12 white matter and 15 gray matter patient cohorts consisting of 257 and 210 patients respectively. Unpaired Student's t-tests indicated no significant difference between the mean values of the white and gray matter samples concerning age (t = 0.28,p = 0.78), disease duration (t = −0.04,p = 0.97), and severity of clinical symptoms using MMSE scores (t = −0.84,p = 0.41), and UPDRS-III scores (t = −0.02,p = 0.99). Furthermore, Pearson's χ2 test showed no significant differences in gender distribution (χ2 = 0.41,p = 0.52). There were also no significant differences in standard deviations between the samples regarding age (t = −0.83,p = 0.42), disease duration (t = −0.44,p = 0.67), and severity of clinical symptoms using MMSE scores (t = −0.26,p = 0.80), and UPDRS-III scores (t = −0.35,p = 0.74).
Table 1.
Studies included in the whole-brain meta-analyses identifying the neural correlates of progressive supranuclear palsy with structural magnetic resonance imaging.
| Study | PSP (N) | HC (N) | Age (years) | Gender (male/ female) | Disease Duration (years) | MMSE | UPDRS-III | Notes | Criteria |
|---|---|---|---|---|---|---|---|---|---|
| Gray matter studies | |||||||||
| Boxer et al., 2006 | 15 | 80 | 70.9 ± 6.9 | 9/6 | 4.8 ± 1.7 | 24.0 ± 3.2 | NR | a,b | Litvan |
| Brenneis et al., 2004 | 12 | 12 | 67.5 ± 6.6 | NR | 2.7 ± 0.9 | NR | 38.9 ± 10.9 | a,b | Litvan |
| Burciu et al., 2015 | 20 | 20 | 67.8 ± 7.1 | 10/10 | 2.6 ± NR | NR | 39.0 ± 14.5 | a,b,* | Litvan |
| Cordato et al., 2005 | 21 | 23 | 70.3 ± 6.4 | 14/7 | 4.0 ± 2.8 | 25.4 ± 3.2 | 23.1 ± 10.1 | a,b | Litvan |
| Ghosh et al., 2012 | 23 | 22 | 71.1 ± 8.6 | 14/9 | 2.5 | NR | 33.8 ± 15.7 | a,b,* | Litvan |
| Giordano et al., 2013 | 15 | 15 | 68.9 ± 1.2 | 8/7 | 3.2 ± 1.3 | 21.2 ± 1.2 | 38.3 ± 4.0 | a,b,* | Litvan |
| Kamiya et al., 2013 | 16 | 21 | 71.4 ± 6.0 | 10/6 | NR | NR | NR | b | Litvan |
| Lagarde, 2015 | 21 | 18 | 65.5 ± 6.5 | 8/13 | 4.4 ± 1.7 | 25.8 ± 2.7 | NR | a,b* | Litvan |
| Lehericy et al., 2010 | 10 | 9 | 66.9 ± 6.4 | 6/4 | 4.3 ± 1.0 | 27.0 | 30.0 | a,b | Litvan |
| Padovani et al., 2006 | 14 | 14 | 73.0 ± 5.6 | 7/7 | 3.1 ± 1.0 | 25.8 ± 2.7 | 22.1 ± 8.9 | a,b | Litvan |
| Piattella et al., 2015 | 16 | 16 | 68.1 ± 5.9 | 9/7 | 3.1 ± NR | 24.3 ± 3.9 | 27.0 ± 3.9 | a,b,* | Litvan |
| Premi et al., 2016 | 32 | 32 | 73.5 ± 6.9 | 17/15 | 6.9 ± 3.5 | 24.9 ± 3.9 | NR | b | Litvan |
| Sandhya et al., 2014 | 10 | 8 | NR | 9/1 | NR | NR | NR | a,b,* | Litvan |
| Takahashi et al., 2011 | 16 | 20 | 64.6 ± 6.4 | 11/5 | NR | 21.0 ± 4.4 | NR | b | Litvan |
| Whitwell et al., 2013 | 16 | 20 | 72.1 ± 4.6 | 8/8 | 4.1 ± NR | 25.8 ± 2.7 | 52.9 ± 12.6 | b,* | Litvan |
| Total GM | ∑257 | ∑334 | 69.4 ± 2.8 | 140/105 | 3.9 ± 1.2 | 24.5 ± 2.0 | 33.9 ± 9.7 | ||
| White matter studies | |||||||||
| Boxer et al., 2006 | 15 | 80 | 70.9 ± 6.9 | 9/6 | 4.8 ± 1.7 | 24.0 ± 3.2 | NR | a,b | Litvan |
| Brenneis et al., 2004 | 12 | 12 | 67.5 ± 6.6 | NR | 2.7 ± 0.9 | NR | 38.9 ± 10.9 | a,b | Litvan |
| Burciu et al., 2015 | 20 | 20 | 67.8 ± 7.1 | 10/10 | 2.6 ± NR | NR | 39.0 ± 14.5 | a,b,* | Litvan |
| Cordato et al., 2005 | 21 | 23 | 70.3 ± 6.4 | 14/7 | 4.0 ± 2.8 | 25.4 ± 3.2 | 23.1 ± 10.1 | a,b | Litvan |
| Ghosh et al., 2012 | 23 | 22 | 71.1 ± 8.6 | 14/9 | 2.5 | NR | 38.3 ± 4.0 | a,b,* | Litvan |
| Hughes et al., 2014 | 13 | 15 | 68.0 ± 6.8 | 9/4 | 4.3 ± 3.1 | 26.9 ± 2.9 | 29.2 ± 14.3 | b,* | Litvan |
| Lagarde, 2013 | 19 | 18 | 65.9 ± 6.5 | 7/12 | 4.5 ± 1.8 | 25.5 ± 2.7 | NR | a,b,* | Litvan |
| Lehericy et al., 2010 | 10 | 9 | 66.9 ± 6.4 | 6/4 | 4.3 ± 1.0 | 27.0 | 30,0 | a,b | Litvan |
| Price, 2004 | 12 | 12 | 65.3 ± 5.8 | 7/5 | 4.8 ± 1.7 | 27.0 ± 3.3 | 20.4 ± 8.7 | a,b | Litvan |
| Sakurai et al., 2015 | 33 | 32 | 78.0 ± 6.0 | 20/13 | 4.8 ± 2.6 | NR | NR | Litvan | |
| Takahashi et al., 2011 | 16 | 20 | 64.6 ± 6.4 | 11/5 | NR | 21.0 ± 4.4 | NR | b | Litvan |
| Whitwell et al., 2017 | 16 | 20 | 72.1 ± 4.6 | 8/8 | 4.1 ± NR | 25.8 ± 2.7 | 52.9 ± 12.6 | b,* | Litvan |
| Total WM | ∑210 | ∑265 | 69.0 ± 3.7 | 115/99 | 4.1 ± 0.8 | 25.3 ± 2.0 | 34.0 ± 10.5 | ||
| Total GM + WM | ∑315 | ∑393 | 69.2 ± 3.2 | 176/127 | 4.0 ± 1.0 | 24.9 ± 2.0 | 33.9 ± 9.7 | ||
Gender, age, disease duration, MMSE and UPDRS scores are specified for patients (mean ± standard deviation). All MRI studies used 1.5 T (except for * = 3 T). Disease duration mean scores were calculated without Gosh et al. because they reported data as median. Abbreviations: GM gray matter, HC healthy controls, MMSE mini-mental state examination, N number of subjects, NR not reported, PSP progressive supranuclear palsy, UDPRS-III motor score of unified Parkinson's disease rating scale, WM white matter. a corrected for multiple comparisons, b modulated
All included studies reported decreased gray/white matter volume or density. There were no reports of gray/white increases found in the literature. Moreover, all studies used exactly the same clinical diagnostic criteria (Litvan et al., 1996a), avoiding a potential vicious circle as no imaging biomarker was included. Out of 315 patients, the majority was diagnosed with PSP-Richardson syndrome according to the new criteria by Höglinger et al. (2017). Note that a maximum of 19 patients (Burciu et al., 2015) may have been diagnosed with PSP-parkinsonism additionally, although they fulfill the Litvan criteria for PSP. Hence, in the following, we refer the naming of our cohort to PSP as proposed by the Litvan criteria.
3.1.2. Seed-based D mapping analysis
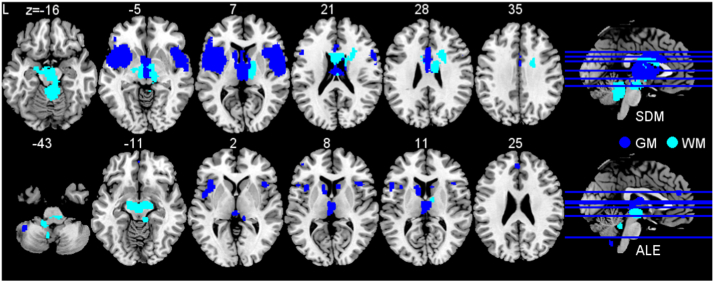
The upper part of Fig. 1 and Table e-2 illustrate the consolidated neural correlates of PSP identified by SDM meta-analysis. The analysis across structural MRI studies revealed regional gray matter atrophy convergence bilaterally in the inferior and middle frontal and superior temporal gyri, adjacent to the insulae, in the putamen, caudate nuclei, midcingulate cortex, thalamus, and midbrain. White matter convergence occurred bilaterally in the corpus callosum, thalamus, cerebral and superior cerebellar pedunculi, and right middle cerebellar pedunculus.
Fig. 1.
Brain regions consistently associated with progressive supranuclear palsy. Analysis of gray matter (GM, dark blue) included 257 patients contrasted to 334 healthy subjects. White matter analysis (WM, light blue) included 210 patients contrasted to 265 healthy subjects. Upper image shows effect size estimates (seed-based D mapping, SDM) and bottom image anatomical likelihood estimate (ALE) meta-analyses. Abbreviation: L left. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1.3. Anatomical likelihood analysis
The bottom part of Fig. 1 and Table e-3 display the results of the ALE meta-analysis in PSP. The analysis revealed gray matter atrophy convergence in the paracingulate and anterior cingulate gyri, insulae, inferior frontal gyrus, superior parietal lobule, thalamus, caudate nuclei, and cerebellum. White matter regions consistently found were the midbrain, pyramid, pons, thalamus, and cerebral and superior cerebellar pedunculi. FDR correction of the ALE meta-analysis confirmed involvement of the anterior insula and thalamus for gray matter and midbrain for white matter (see Supplement, Figure e-2).
3.1.4. Overlap analysis
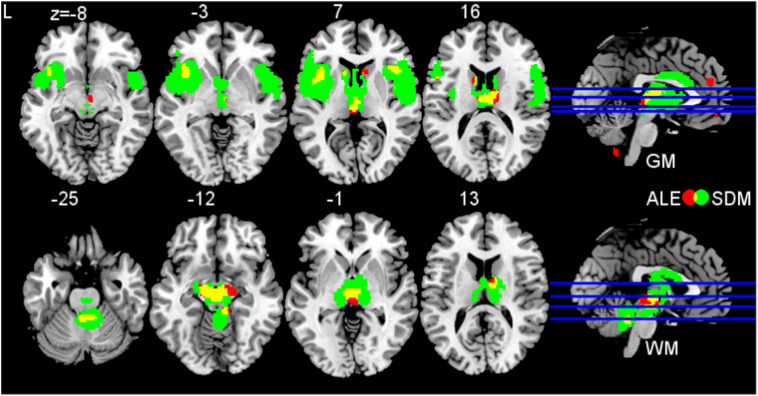
Finally, we performed an overlap analysis by superimposing the results of the two separate approaches meta-analyzing PSP (Fig. 2). The results for gray matter revealed that clusters in the bilateral thalamus, bilateral anterior insulae, midbrain, and left caudate nucleus were consistently identified as affected by PSP. The results for white matter analysis revealed consistent converged atrophy bilaterally in the superior and middle cerebellar pedunculi, cerebral pedunculi, midbrain, and right thalamus.
Fig. 2.
Overlap analysis. Overlay analysis for impaired brain regions in progressive supranuclear palsy as revealed by anatomical likelihood estimate (ALE) and effect size estimates (seed-based D mapping, SDM) meta-analyses. Results of ALE are shown in red. Results of SDM are shown in green. Yellow clusters indicate overlap of both analyses (ALE and SDM). Upper image displays gray matter (GM) atrophy, bottom image displays white matter (WM) atrophy. Abbreviation: L left. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1.5. Subtraction analysis
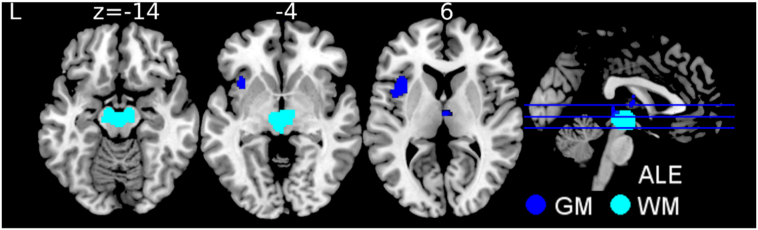
We performed a subtraction analysis to directly compare disease-specificity of the aforementioned results by integrating white and gray matter atrophy of PSP and Parkinson's disease patients. The Parkinson's disease cohort consist of 809 patients from 29 gray matter studies and 75 patients from four white matter studies (Fig. 3, Table e-5). Gray matter atrophy differed between PSP and Parkinson's disease in the thalamus bilaterally as well as the left insula and claustrum, both surviving multiple comparisons correction. Concerning white matter, subtracting Parkinson's disease from PSP revealed the midbrain. Note that the white matter analysis did not survive FDR correction, which may have been due to underpowered analysis.
Fig. 3.
Brain regions consistently associated with progressive supranuclear palsy in comparison to Parkinson's disease. Analysis of gray matter (GM, dark blue) included 257 patients with PSP contrasted to 809 patients with PD. White matter analysis (WM, light blue) included 210 patients with PSP contrasted to 75 patients with PD. Image shows subtraction anatomical likelihood estimate (ALE) meta-analyses. Abbreviation: L left, PD Parkinson's disease, PSP progressive supranuclear palsy. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Region-of-interest effect size meta-analyses
3.2.1. Study characteristics
Our literature search identified one study (Kim et al., 2017) that we included additionally to the studies already summarized by (Whitwell et al., 2017) in their systematic review. Study details and demographics are specified in Table-e6 in the Supplement. In total, 15 studies, including 246 patients and 173 healthy controls reported midbrain metrics in three neurodegenerative diseases: PSP (15 cohorts, age = 69.8 ± 3.6 years, disease duration = 3.3 ± 0.8 years), Parkinson's disease (14 cohorts, age = 66.6 ± 6.7, disease duration = 6.3 ± 2.4), and multiple system atrophy (5 cohorts, age = 64.7 ± 4.3, disease duration = 5.6 ± 1.6). The cohort of PSP patients in the region-of-interest effect size meta-analyses did not differ in age (t = 0.50, p = 0.62) or gender distribution (χ2 = 0.77, p = 0.38) compared to the PSP cohort of the whole-brain meta-analyses. Note that disease duration (t = −2.10, p = 0.04) was slightly higher in the PSP cohort of the whole-brain meta-analyses.
3.2.2. Effect size meta-analyses
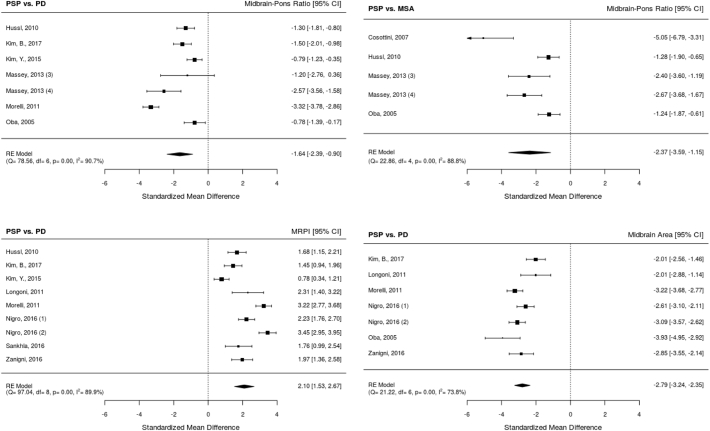
All random-effects models revealed significant results, highlighting that midbrain metrics generally perform very well in distinguishing PSP from multiple system atrophy and Parkinson's disease with strong effect sizes (Fig. 4). Note that inter-study heterogeneity was overall quite large. Midbrain area measurements yielded smallest between-study heterogeneity in our study as well as largest effect size (I2 = 73.8%,Hedges' g = −2.79). The highest between-study heterogeneity but still high effect size was observed in midbrain-pons area ratio studies (I2 = 90.7%,Hedges' g = −1.64). Notably, even multiple system atrophy and PSP were distinguished by using midbrain-pons ratio with a high effect size (Hedges' g = −2.37). MRPI also yielded a large effect size but a high between-study heterogeneity (I2 = 89.9%,Hedges' g = 2.10) in distinguishing PSP from Parkinson's disease. These results emphasize the importance of our work in validating and replicating these patterns in whole brain with quantitative statistical methods.
Fig. 4.
Effect size (Hedges' g) meta-analyses of radiological biomarkers of progressive supranuclear palsy (PSP) based on Whitwell et al. (2017) and additional literature search. (1) Measurement using 1.5 Tesla, (2) measurement using 3 Tesla, (3) pathologically confirmed, and (4) clinically diagnosed groups. Abbreviation: CI confidence interval, df degrees of freedom, RE random effects, PD Parkinson's disease, MSA multiple system atrophy, MRPI Magnetic Resonance Parkinsonism Index.
4. Discussion
To our knowledge, we report the largest most powerful data-driven whole-brain meta-analyses of the neural correlates of PSP to date. The analyses included 315 patients and 393 healthy controls. To help ensure high validity and specificity, all included patients were diagnosed by the same clinical diagnostic criteria, by Litvan et al. (1996a). In the aforementioned criteria imaging biomarkers are not implemented, which avoids a biased and circular design. According to the new PSP criteria (Höglinger et al., 2017), these patients would be referred to as PSP-Richardson syndrome. Note, however, that time criteria are different between both diagnostic systems, that our cohort might have included a minority of subjects with PSP-Parkinsonism additionally to PSP-Richardson syndrome (see Results, 3.1.1), and that one has to be generally very careful in translating old into new clinical criteria see Bisenius et al. (2016 for a related discussion on primary progressive aphasia).
Including only whole-brain studies in our meta-analysis guaranteed a data-driven approach. Notably, the overlap analysis between the two meta-analytical methods revealed gray matter atrophy in four regions in PSP: the bilateral thalamus, bilateral anterior insulae, midbrain, and left caudate nucleus. Concerning white matter atrophy, an overlap of the two methods was observed in the bilateral superior/middle cerebral and cerebellar pedunculi, midbrain, and right thalamus in PSP. Subtraction analysis differentiating PSP from Parkinson's disease confirmed the midbrain as specific for PSP.
Although previous meta-analyses of gray and white matter atrophy have been conducted for PSP, studies investigated either gray or white matter alterations alone and relied on a single meta-analytical algorithm (Shao et al., 2014; Shi et al., 2013; Yang et al., 2014; Yu et al., 2015). Remarkably, these meta-analyses may have been biased by systematic errors introduced by flawed multiple comparison correction analysis tools, as has been stated recently by (Eickhoff et al., 2016) for former GingerALE software versions. Despite using distributions that contained errors in the FDR calculation, the results are still quite consistent with our own (Yang et al., 2014; Yu et al., 2015).
The present meta-analysis improves on previous studies in several ways. First, we used a novel approach, combining and double-validating two independent meta-analytical techniques to increase validity of our biomarker findings. Second, we included the largest cohort of PSP patients studied to date. Third, we investigated changes in gray and white matter with exactly the same methods to enable comparability of results. Finally, our meta-analyses support region-specific atrophy as an imaging biomarker for PSP – a move toward the inclusion of MRI in the diagnostic workflow for PSP.
4.1. Validating pathognomonic imaging markers for PSP with meta-analyses
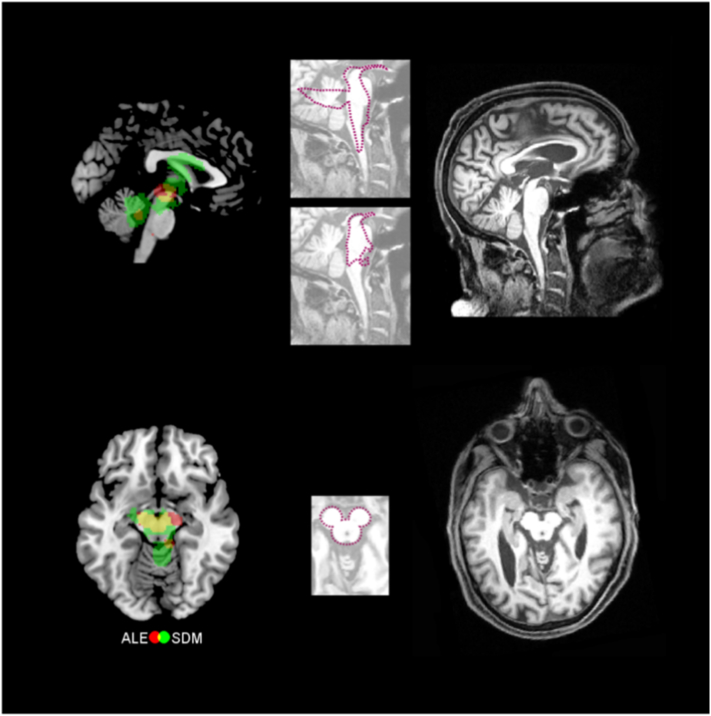
Thinning of regions of the midbrain has been suggested as a pathognomonic marker of PSP; previously identified in small cohorts by precisely measuring the diameters of the pons, midbrain, and superior/middle cerebellar pedunculi (Kato et al., 2003; Longoni et al., 2011; Oba et al., 2005; Slowinski et al., 2008). A recent multi-centric volumetric and manual morphometry study has endorsed this view, demonstrating that the midsagittal area and the anterior-posterior diameter of the midbrain distinguishes between PSP and other parkinsonian syndromes (Moller et al., 2017). Previous findings describe three different pathognomonic signs, typically found in PSP patients. First, the ‘hummingbird sign’ reflects a thinning of the anterior part of the midbrain tegmentum (Kato et al., 2003). The second characteristic feature called the ‘penguin silhouette sign’ relates to an atrophy in the shapes of midbrain tegmentum and pons (Oba et al., 2005). The hummingbird and the penguin silhouette signs are apparent in mid-sagittal MRI scans. Another feature, the ‘Mickey Mouse sign’, describes selective atrophy of the midbrain tegmentum, with relative preservation of the tectum and cerebral pedunculi (Schott, 2007). Axial MRI scans of PSP patients reveal this sign. We were able to lend support to these pathognomonic markers with a large cohort (Fig. 5) by revealing white matter loss in the midbrain (specifically the tectum and parts of the tegmentum such as cerebellar pedunculi, red nucleus, and substantia nigra). The Mickey Mouse sign seems less specific, according to our data, because atrophy spread to the cerebral pedunculi (Fig. 5). Due to the algorithm, assumptions concerning the exact neuroanatomical substrates of meta-analyses should be interpreted with caution. Nevertheless, our confidence is increased by the fact that both meta-analytical algorithms across whole-bran studies revealed midbrain atrophy. By replicating previously observed atrophy patterns in a large meta-analytical study, using multiple approaches, we provide further evidence supporting the use of these signs as a diagnostic tool for PSP.
Fig. 5.
Meta-analyses confirm proposed pathognomonic imaging markers of progressive supranuclear palsy (PSP). Left column shows meta-analytic results (green seed-based D mapping – SDM – analysis, red anatomical likelihood estimation analysis – ALE –, and yellow overlay). Right column depicts individual examples of corresponding structural magnetic resonance images of individual PSP patients with midbrain atrophy. Images in the middle are the silhouettes of a penguin, a hummingbird, and Mickey Mouse projected onto these patients' brain images. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4.2. Atrophy in the midbrain and cerebral/cerebellar pedunculi is disease-characteristic for PSP as compared to other neurodegenerative diseases
Through the conjunctive overlay of the two meta-analytic approaches, we identified gray matter atrophy in PSP patients in the midbrain, thalamus, insulae, and caudate nuclei compared to controls. White matter atrophy was found in the midbrain, thalamus, cerebral pedunculus and superior/middle cerebellar pedunculi. To assess the potential disease-specificity of these patterns we compared our findings to other whole-brain meta-analyses of neurodegenerative diseases qualitatively (Table 2) and quantitatively (Table e-5, Fig. 4). A direct statistical comparison of PSP with another neurodegenerative disease, i.e. Parkinson's disease (Albrecht et al., 2018), revealed that PSP differs in white matter midbrain atrophy. This highlights again the importance of the midbrain in PSP, especially in distinguishing closely related diseases. Gray matter was significantly impaired in PSP compared to Parkinson's disease in the thalamus and insula. Note that in qualitative comparisons, gray matter loss in the insulae, caudate nuclei, and thalamus has been recently reported in several other neurodegenerative diseases. Hence, these patterns are neither specific to PSP nor other neurodegenerative diseases.
Table 2.
Meta-analytical evidence for biomarker specificity of regional atrophy in progressive supranuclear palsy as measured with structural magnetic resonance imaging.
| Anatomical region | ||||||
|---|---|---|---|---|---|---|
| Neurodegenerative disease | Study | Thalamus | Midbrain | Insula | Cerebellar & cerebral pedunculi | Caudate nucleus |
| Gray matter | ||||||
| Parkinson's disease | (Shao et al., 2014; Shao et al., 2015, Albrecht et al., 2018) | − | − | − | − | − |
| Atypical Parkinson's syndromes | ||||||
| Corticobasal degeneration & syndrome | (Yu et al., 2015; Albrecht et al., 2017) | + | − | − | − | + |
| Progressive supranuclear palsy | (Present study, Shao et al., 2014; Yu et al., 2015; Shi et al., 2013) | + | + | + | − | + |
| Multiple system atrophy | (Shao et al., 2015) | + | + (Red nucleus) | + | − | − |
| Lewy body dementia | (Zhong et al., 2014) | − | − | + | − | − |
| Alzheimer's disease | (Schroeter and Neumann, 2011; Schroeter et al., 2009; Yang et al., 2012a) | + | − | + | − | − |
| Behavioral variant frontotemporal dementia | (Schroeter et al., 2014; Schroeter and Neumann, 2011; Schroeter et al., 2008; Schroeter et al., 2007; Pan et al., 2012) | − | − | + | − | + |
| Primary progressive aphasias | ||||||
| Nonfluent/ agrammatic variant (progressive non-fluent aphasia) | (Bisenius et al., 2016; Schroeter and Neumann, 2011; Schroeter et al., 2007) | − | − | + | − | − |
| Semantic variant (semantic dementia) | (Bisenius et al., 2016; Schroeter and Neumann, 2011; Schroeter et al., 2007; Yang et al., 2012b) | − | − | − | − | − |
| Logopenic variant (logopenic aphasia) | (Bisenius et al., 2016) | − | − | − | − | − |
| White matter | ||||||
| Parkinson's disease | (Albrecht et al., 2018) | − | − | − | − | + |
| Atypical Parkinson's syndromes | ||||||
| Progressive supranuclear palsy | (Present study, Yang et al., 2014) | + | + | − | + | − |
| Alzheimer's disease | (Yin et al., 2015; Li et al., 2012a) | − | − | − | − | − |
Meta-analyses were conducted by calculating either anatomical likelihood estimates (ALE) or effect-size signed differential mapping (SDM) in the gray or white matter.
As also illustrated in Table 2 white matter atrophy in the midbrain has not been observed in other neurodegenerative diseases and thus is suggested as a pathognomonic signature of PSP. PSP shows also severe gray matter atrophy distributed over the whole midbrain indicating disease-relatedness in this comparison. Although gray matter atrophy was detected in ALE meta-analyses in the red nucleus in multiple system atrophy (Shao et al., 2015), it was restricted to this brain region. Moreover, white matter atrophy in the cerebral or superior/middle cerebellar pedunculi seems to be characteristic for PSP as we did not find any other evidence in the literature. Missing effects in the cerebral and cerebellar pedunculi in our meta-analytical subtraction analysis might be related to the small number of white matter studies investigating Parkinson's disease. Although, one might assume cerebral pedunculi atrophy in amyotrophic lateral sclerosis/motor neuron disease, a recent meta-analysis of DTI data rejects such an assumption (Li et al., 2012b). White matter atrophy in the thalamus/adjacent white matter structures seem to be characteristic for PSP as compared to other meta-analyses of Parkinson's disease and Alzheimer's disease (Albrecht et al., 2018; Li et al., 2012a; Yin et al., 2015). As studies on white matter are rare, greatly limiting meta-analytical assessment, disease-specificity should be interpreted with care. Nonetheless, our meta-analyses suggest that gray matter loss in the midbrain and white matter atrophy in the midbrain, cerebral pedunculus, superior/middle cerebellar pedunculi, and thalamus may serve as a disease-characteristic signature for PSP, quantitatively and qualitatively compared to related diagnoses. Further meta-analyses might apply the same methods and parameters to compare several diseases and assess disease-specificity quantitatively by comparing disease patterns with subtraction analyses.
During the course of the present investigation the diagnostic criteria were revised by the Movement Disorder Society-endorsed, P.S.P. Study Group (Höglinger et al., 2017). In this context, (Whitwell et al., 2017) reviewed radiological biomarkers. Our argumentation is in line with their qualitative review. The researchers identified the MRPI, a midbrain to pons and middle to superior cerebellar pedunculus ratio, measured from structural MRI scans, as a supportive criterion even for early clinical diagnosis. Our region-of-interest effect size analyses based on Whitwell et al.'s review demonstrate large effect sizes for distinguishing PSP from Parkinson's disease and multiple system atrophy. Our dual-approach meta-analytic assessment with a large PSP sample is consistent with these recent developments, supporting the use of these particular regions in diagnosing PSP.
It is clearly necessary to validate the applicability of these disease-related MRI biomarkers for individual diagnosis in single patients before being transferable to clinical routine, for example by support vector machine classification for single-patient neurodegenerative disease discrimination (Bisenius et al., 2017; Dukart et al., 2013; Meyer et al., 2017). In an atlas-based MRI volumetry study, high classification accuracy for PSP versus other parkinsonian syndromes (86%) was indeed driven by atrophy in the midbrain and cerebellar pedunculi (Huppertz et al., 2016). Another MRI study suggests that disease-characteristic regions extracted from meta-analyses can outperform whole-brain approaches in identifying PSP by increasing classification accuracy from 80% to 85% (Mueller et al., 2017). Furthermore, combining structural MRI with DTI in support vector machine classification can increase classification results to 100% accurate (Cherubini et al., 2014). One may conclude that our meta-analytically extracted disease-specific atrophy pattern is ready for application as an imaging biomarker for PSP.
4.3. Study limitations
Our study identified consistent findings of atrophy in PSP, but we recognize some limitations. MRI studies on autopsy-proven cases of PSP are extremely rare (Table 1), hence, we could not validate clinical syndromes with histopathology (Albrecht et al., 2017). Furthermore, we could not disentangle the clinical syndromes seen in PSP as too few studies reported those data. Our meta-analyses had to be limited to MRI studies, because insufficient number of studies applied FDG-PET or DTI. We recommend including other imaging modalities and longitudinal data in the future (Bisenius et al., 2016; Schroeter et al., 2014; Schroeter and Neumann, 2011). The accuracy of the disease-specific patterns needs further validation in multi-centric, independent patient cohorts, which provide clinical imaging data, surrogate markers for histopathology from serum/cerebrospinal fluid or post-mortem examination. Researchers have already applied this successfully in Alzheimer's disease (Klöppel et al., 2008). Due to the lack of combined white matter and gray matter meta-analyses, direct statistical investigation of the results was only possible between PSP and Parkinson's disease. Note that SDM results are only peak-based FDR corrected for multiple comparisons, which should be preferably replaced by cluster- or voxel-based corrections in the future like it is already available for GingerALE.
4.4. Conclusion
We conducted a comprehensive, systematic, and quantitative meta-analysis investigating the neural correlates of PSP. Patients were all diagnosed applying the same diagnostic criteria of Litvan et al. (1996a), which is now referred to as PSP-Richardson syndrome according to Höglinger et al. (2017). By combining two commonly used meta-analytical algorithms, we identified consistent results validating well-known pathognomonic signs in whole-brain imaging to support the diagnosis of PSP. Results suggest gray matter atrophy in the midbrain and white matter atrophy in the cerebral pedunculus, superior/middle cerebellar pedunculi, and midbrain as imaging biomarkers for diagnosis of PSP ante-mortem. Further, subtraction analyses of meta-analyses of related neurodegenerative diseases could strengthen the disease-specificity of midbrain atrophy by quantitatively comparing atrophy patterns. Taken together, our results are completely consistent with the recent move toward the inclusion of MRI data in the diagnostic workflow for PSP.
Conflict of interest
The authors declare that they have no conflict of interest. All financial disclosures are mentioned in the Acknowledgements.
Acknowledgments
Acknowledgements
This study has been supported by the Parkinson's Disease Foundation (PDF-IRG-1307), the Michael J Fox Foundation (MJFF-11362), the German Federal Ministry of Education and Research (BMBF; FKZ 01GI1007A; German FTLD consortium), the German Research Foundation (DFG, SCHR 774/5-1), the National Institutes of Health (NIH, R01-NS89757), and the International Max Planck Research School (IMPRS) NeuroCom by the Max Planck Society. Jane Neumann is supported by the Federal Ministry of Education and Research Germany (BMBF, FKZ: 01EO1001), and the German Research Foundation (DFG-SFB1052).
Authors' roles
-
1.
Research project:
-
A.
Conception: F. Albrecht, M. Schroeter.
-
B.
Organization: F. Albrecht, M. Schroeter
-
C.
Execution: F. Albrecht, S. Bisenius, J. Neumann, M. Schroeter
-
2.Statistical Analysis:
-
A.Design: F. Albrecht, J. Neumann, M. Schroeter.
-
B.Execution: F. Albrecht, S. Bisenius.
-
C.Review and Critique: F. Albrecht, J. Neumann, J. Whitwell, M. Schroeter
-
A.
-
3.Manuscript Preparation:
-
A.Writing of the first draft: F. Albrecht.
-
B.Review and Critique: F. Albrecht, S. Bisenius, J. Neumann, J. Whitwell, M. Schroeter.
-
A.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101722.
Contributor Information
Franziska Albrecht, Email: falbrecht@cbs.mpg.de.
Sandrine Bisenius, Email: bisenius@cbs.mpg.de.
Jane Neumann, Email: neumann@cbs.mpg.de.
Jennifer Whitwell, Email: Whitwell.Jennifer@mayo.edu.
Matthias L. Schroeter, Email: schroet@cbs.mpg.de.
Appendix A. Supplementary data
Supplementary material
References
- Albrecht F., Bisenius S., Morales Schaack R., Neumann J., Schroeter M.L. 2017. Disentangling the Neural Correlates of Corticobasal Syndrome and Corticobasal Degeneration with Systematic and Quantitative ALE Meta-Analyses. (npj Parkinson's Disease 3, 12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht F., Ballarini T., Neumann J., Schroeter M.L. FDG-PET hypometabolism is more sensitive than MRI atrophy in Parkinson's disease: a whole-brain multimodal imaging meta-analysis. NeuroImage Clin. 2018 doi: 10.1016/j.nicl.2018.11.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisenius S., Neumann J., Schroeter M.L. Validating new diagnostic imaging criteria for primary progressive aphasia via anatomical likelihood estimation meta-analyses. Eur. J. Neurol. 2016;23:704–712. doi: 10.1111/ene.12902. [DOI] [PubMed] [Google Scholar]
- Bisenius S., Mueller K., Diehl-Schmid J., Fassbender K., Grimmer T., Jessen F., Kassubek J., Kornhuber J., Landwehrmeyer B., Ludolph A., Schneider A., Anderl-Straub S., Stuke K., Danek A., Otto M., Schroeter M.L., group F.T.s. Predicting primary progressive aphasias with support vector machine approaches in structural MRI data. Neuroimage Clin. 2017;14:334–343. doi: 10.1016/j.nicl.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer A.L., Geschwind M.D., Belfor N. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch. Neurol. 2006;63(1):81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Brenneis C., Seppi K., Schocke M., Benke T., Wenning G.K., Poewe W. Voxel based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry. 2004;75(2):246–249. [PMC free article] [PubMed] [Google Scholar]
- Burciu R.G., Ofori E., Shukla P., Planetta P.J., Snyder A.F., Li H., Hass C.J., Okun M.S., McFarland N.R., Vaillancourt D.E. Distinct patterns of brain activity in progressive supranuclear palsy and Parkinson's disease. Mov. Disord. 2015;30:1248–1258. doi: 10.1002/mds.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A., Morelli M., Nistico R., Salsone M., Arabia G., Vasta R., Augimeri A., Caligiuri M.E., Quattrone A. 2014. Magnetic Resonance Support Vector Machine Discriminates between Parkinson Disease and Progressive Supranuclear Palsy. [DOI] [PubMed] [Google Scholar]
- Cordato N.J., Duggins A.J., Halliday G.M., Morris J.G., Pantelis C. Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain. 2005;128:1259–1266. doi: 10.1093/brain/awh508. Pt 6. [DOI] [PubMed] [Google Scholar]
- Dukart J., Mueller K., Barthel H., Villringer A., Sabri O., Schroeter M.L., Alzheimer's Disease Neuroimaging, I Meta-analysis based SVM classification enables accurate detection of Alzheimer's disease across different clinical centers using FDG-PET and MRI. Psychiatry Res. 2013;212:230–236. doi: 10.1016/j.pscychresns.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Bzdok D., Laird A.R., Kurth F., Fox P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Fox P.M., Lancaster J.L., Fox P.T. Implementation errors in the GingerALE software: description and recommendations. Hum. Brain Mapp. 2016;38(1):7–11. doi: 10.1002/hbm.23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B.C., Calder A.J., Peers P.V. Social cognitive deficits and their neural correlates in progressive supranuclear palsy. Brain. 2012;135:2089–2102. doi: 10.1093/brain/aws128. Pt 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A., Tessitore A., Corbo D. Clinical and cognitive correlations of regional gray matter atrophy in progressive supranuclear palsy. Parkinsonism Relat. Disord. 2013;19(6):590–594. doi: 10.1016/j.parkreldis.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Ogar J.M., Rohrer J.D., Black S., Boeve B.F., Manes F., Dronkers N.F., Vandenberghe R., Rascovsky K., Patterson K., Miller B.L., Knopman D.S., Hodges J.R., Mesulam M.M., Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y., Benjamini Y. More powerful procedures for multiple significance testing. Stat. Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Höglinger G.U., Respondek G., Stamelou M., Kurz C., Josephs K.A., Lang A.E., Mollenhauer B., Muller U., Nilsson C., Whitwell J.L., Arzberger T., Englund E., Gelpi E., Giese A., Irwin D.J., Meissner W.G., Pantelyat A., Rajput A., van Swieten J.C., Troakes C., Antonini A., Bhatia K.P., Bordelon Y., Compta Y., Corvol J.C., Colosimo C., Dickson D.W., Dodel R., Ferguson L., Grossman M., Kassubek J., Krismer F., Levin J., Lorenzl S., Morris H.R., Nestor P., Oertel W.H., Poewe W., Rabinovici G., Rowe J.B., Schellenberg G.D., Seppi K., van Eimeren T., Wenning G.K., Boxer A.L., Golbe L.I., Litvan I., Movement Disorder Society-endorsed, P.S.P.S.G Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov. Disord. 2017;32:853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L.E., Rowe J.B., Ghosh B.C., Carlyon R.P., Plack C.J., Gockel H.E. The binaural masking level difference: cortical correlates persist despite severe brain stem atrophy in progressive supranuclear palsy. J. Neurophysiol. 2014;112(12):3086–3094. doi: 10.1152/jn.00062.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz H.J., Moller L., Sudmeyer M., Hilker R., Hattingen E., Egger K., Amtage F., Respondek G., Stamelou M., Schnitzler A., Pinkhardt E.H., Oertel W.H., Knake S., Kassubek J., Hoglinger G.U. Differentiation of neurodegenerative parkinsonian syndromes by volumetric magnetic resonance imaging analysis and support vector machine classification. Mov. Disord. 2016;31:1506–1517. doi: 10.1002/mds.26715. [DOI] [PubMed] [Google Scholar]
- Kamiya K., Sato N., Ota M. Diffusion tensor tract-specific analysis of the uncinate fasciculus in patients with progressive supranuclear palsy. J. Neuroradiol. 2013;40(2):121–129. doi: 10.1016/j.neurad.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Kato N., Arai K., Hattori T. Study of the rostral midbrain atrophy in progressive supranuclear palsy. J. Neurol. Sci. 2003;210:57–60. doi: 10.1016/s0022-510x(03)00014-5. [DOI] [PubMed] [Google Scholar]
- Kim B.C., Choi S.-M., Choi K.-H., Nam T.-S., Kim J.-T., Lee S.-H., Park M.-S., Yoon W. MRI measurements of brainstem structures in patients with vascular parkinsonism, progressive supranuclear palsy, and Parkinson's disease. Neurol. Sci. 2017;38:627–633. doi: 10.1007/s10072-017-2812-1. [DOI] [PubMed] [Google Scholar]
- Klöppel S., Stonnington C.M., Chu C., Draganski B., Scahill R.I., Rohrer J.D., Fox N.C., Jack C.R., Jr., Ashburner J., Frackowiak R.S. Automatic classification of MR scans in Alzheimer's disease. Brain. 2008;131:681–689. doi: 10.1093/brain/awm319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A.R., Fox P.M., Price C.J., Glahn D.C., Uecker A.M., Lancaster J.L., Turkeltaub P.E., Kochunov P., Fox P.T. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutierrez D., Martinez M., Salinas F., Evans A., Zilles K., Mazziotta J.C., Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S., Hartmann A., Lannuzel A. Magnetic resonance imaging lesion pattern in Guadeloupean parkinsonism is distinct from progressive supranuclear palsy. Brain. 2010;133:2410–2425. doi: 10.1093/brain/awq162. Pt 8. [DOI] [PubMed] [Google Scholar]
- Li J., Pan P., Huang R., Shang H. A meta-analysis of voxel-based morphometry studies of white matter volume alterations in Alzheimer's disease. Neurosci. Biobehav. Rev. 2012;36:757–763. doi: 10.1016/j.neubiorev.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Li J., Pan P., Song W., Huang R., Chen K., Shang H. A meta-analysis of diffusion tensor imaging studies in amyotrophic lateral sclerosis. Neurobiol. Aging. 2012;33:1833–1838. doi: 10.1016/j.neurobiolaging.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Litvan I., Agid Y., Calne D., Campbell G., Dubois B., Duvoisin R.C., Goetz C.G., Golbe L.I., Grafman J., Growdon J.H., Hallett M., Jankovic J., Quinn N.P., Tolosa E., Zee D.S. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Litvan I., Hauw J.J., Bartko J.J., Lantos P.L., Daniel S.E., Horoupian D.S., McKee A., Dickson D., Bancher C., Tabaton M., Jellinger K., Anderson D.W. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J. Neuropathol. Exp. Neurol. 1996;55:97–105. doi: 10.1097/00005072-199601000-00010. [DOI] [PubMed] [Google Scholar]
- Longoni G., Agosta F., Kostic V.S., Stojkovic T., Pagani E., Stosic-Opincal T., Filippi M. MRI measurements of brainstem structures in patients with Richardson's syndrome, progressive supranuclear palsy-parkinsonism, and Parkinson's disease. Mov. Disord. 2011;26:247–255. doi: 10.1002/mds.23293. [DOI] [PubMed] [Google Scholar]
- Meyer S., Mueller K., Stuke K., Bisenius S., Diehl-Schmid J., Jessen F., Kassubek J., Kornhuber J., Ludolph A.C., Prudlo J., Schneider A., Schuemberg K., Yakushev I., Otto M., Schroeter M.L., Group, F.T.S Predicting behavioral variant frontotemporal dementia with pattern classification in multi-center structural MRI data. Neuroimage Clin. 2017;14:656–662. doi: 10.1016/j.nicl.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller L., Kassubek J., Sudmeyer M., Hilker R., Hattingen E., Egger K., Amtage F., Pinkhardt E.H., Respondek G., Stamelou M., Moller F., Schnitzler A., Oertel W.H., Knake S., Huppertz H.J., Hoglinger G.U. Manual MRI morphometry in Parkinsonian syndromes. Mov. Disord. 2017;32:778–782. doi: 10.1002/mds.26921. [DOI] [PubMed] [Google Scholar]
- Mueller K., Jech R., Bonnet C., Tintera J., Hanuska J., Moller H.E., Fassbender K., Ludolph A., Kassubek J., Otto M., Ruzicka E., Schroeter M.L., Group, F.T.S Disease-specific regions outperform whole-brain approaches in identifying progressive Supranuclear palsy: a multicentric MRI study. Front. Neurosci. 2017;11:100. doi: 10.3389/fnins.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V.I., Cieslik E.C., Laird A.R., Fox P.T., Radua J., Mataix-Cols D., Tench C.R., Yarkoni T., Nichols T.E., Turkeltaub P.E., Wager T.D., Eickhoff S.B. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2018;84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba H., Yagishita A., Terada H., Barkovich A., Kutomi K., Yamauchi T., Furui S., Shimizu T., Uchigata M., Matsumura K. New and reliable MRI diagnosis for progressive supranuclear palsy. Neurology. 2005;64:2050–2055. doi: 10.1212/01.WNL.0000165960.04422.D0. [DOI] [PubMed] [Google Scholar]
- Padovani A., Borroni B., Brambati S.M. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry. 2006;77(4):457–463. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P.L., Song W., Yang J., Huang R., Chen K., Gong Q.Y.…Shang H.F. Gray matter atrophy in behavioral variant frontotemporal dementia: a meta-analysis of voxel-based morphometry studies. Dement. Geriatr. Cogn. Disord. 2012;33(2-3):141–148. doi: 10.1159/000338176. [DOI] [PubMed] [Google Scholar]
- Paviour D.C., Price S.L., Jahanshahi M., Lees A.J., Fox N.C. Longitudinal MRI in progressive supranuclear palsy and multiple system atrophy: rates and regions of atrophy. Brain. 2006;129:1040–1049. doi: 10.1093/brain/awl021. [DOI] [PubMed] [Google Scholar]
- Piattella M.C., Upadhyay N., Bologna M. Neuroimaging evidence of gray and white matter damage and clinical correlates in progressive supranuclear palsy. J. Neurol. 2015;262(8):1850–1858. doi: 10.1007/s00415-015-7779-3. [DOI] [PubMed] [Google Scholar]
- Premi E., Gualeni V., Costa P. Looking for measures of disease severity in the frontotemporal dementia continuum. J. Alzheimers Dis. 2016;52(4):1227–1235. doi: 10.3233/JAD-160178. [DOI] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Radua J., Rubia K., Canales-Rodriguez E.J., Pomarol-Clotet E., Fusar-Poli P., Mataix-Cols D. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U., Hillis A.E., Josephs K.A., Boeve B.F., Kertesz A., Seeley W.W., Rankin K.P., Johnson J.K., Gorno-Tempini M.L., Rosen H., Prioleau-Latham C.E., Lee A., Kipps C.M., Lillo P., Piguet O., Rohrer J.D., Rossor M.N., Warren J.D., Fox N.C., Galasko D., Salmon D.P., Black S.E., Mesulam M., Weintraub S., Dickerson B.C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T.W., Manes F., Grafman J., Cappa S.F., Freedman M., Grossman M., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K., Imabayashi E., Tokumaru A.M. The feasibility of white matter volume reduction analysis using SPM8 plus DARTEL for the diagnosis of patients with clinically diagnosed corticobasal syndrome and Richardson's syndrome. Neuroimage Clin. 2015;7:605–610. doi: 10.1016/j.nicl.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhya M., Saini J., Pasha S.A., Yadav R., Pal P.K. A voxel based comparative analysis using magnetization transfer imaging and T1-weighted magnetic resonance imaging in progressive supranuclear palsy. Ann. Indian Acad. Neurol. 2014;17(2):193–198. doi: 10.4103/0972-2327.132626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott J.M. A neurological MRI menagerie. Pract. Neurol. 2007;7:186–190. doi: 10.1136/jnnp.2007.120261. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Neumann J. Combined imaging markers dissociate Alzheimer's disease and Frontotemporal lobar degeneration - an ALE meta-analysis. Front. Aging Neurosci. 2011;3:10. doi: 10.3389/fnagi.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Raczka K., Neumann J., Von Cramon D.Y. Towards a nosology for frontotemporal lobar degenerations—a meta-analysis involving 267 subjects. Neuroimage. 2007;36(3):497–510. doi: 10.1016/j.neuroimage.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Raczka K., Neumann J., Von Cramon D.Y. Neural networks in frontotemporal dementia—a meta-analysis. Neurobiol. Aging. 2008;29(3):418–426. doi: 10.1016/j.neurobiolaging.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Stein T., Maslowski N., Neumann J. Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage. 2009;47(4):1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Laird A.R., Chwiesko C., Deuschl C., Schneider E., Bzdok D., Eickhoff S.B., Neumann J. Conceptualizing neuropsychiatric diseases with multimodal data-driven meta-analyses - the case of behavioral variant frontotemporal dementia. Cortex. 2014;57:22–37. doi: 10.1016/j.cortex.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao N., Yang J., Li J., Shang H.F. Voxelwise meta-analysis of gray matter anomalies in progressive supranuclear palsy and Parkinson's disease using anatomic likelihood estimation. Front. Hum. Neurosci. 2014;8:63. doi: 10.3389/fnhum.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao N., Yang J., Shang H. Voxelwise meta-analysis of gray matter anomalies in Parkinson variant of multiple system atrophy and Parkinson's disease using anatomic likelihood estimation. Neurosci. Lett. 2015;587:79–86. doi: 10.1016/j.neulet.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Shi H.C., Zhong J.G., Pan P.L., Xiao P.R., Shen Y., Wu L.J., Li H.L., Song Y.Y., He G.X., Li H.Y. Gray matter atrophy in progressive supranuclear palsy: meta-analysis of voxel-based morphometry studies. Neurol. Sci. 2013;34:1049–1055. doi: 10.1007/s10072-013-1406-9. [DOI] [PubMed] [Google Scholar]
- Slowinski J., Imamura A., Uitti R.J., Pooley R.A., Strongosky A.J., Dickson D.W., Broderick D.F., Wszolek Z.K. MR imaging of brainstem atrophy in progressive supranuclear palsy. J. Neurol. 2008;255:37–44. doi: 10.1007/s00415-007-0656-y. [DOI] [PubMed] [Google Scholar]
- Takahashi R., Ishii K., Kakigi T., Yokoyama K., Mori E., Murakami T. Brain alterations and mini-mental state examination in patients with progressive supranuclear palsy: voxel-based investigations using f-fluorodeoxyglucose positron emission tomography and magnetic resonance imaging. Dement. Geriatr. Cogn. Dis. Extra. 2011;1(1):381–392. doi: 10.1159/000333368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:1–48. [Google Scholar]
- Whitwell J.L., Duffy J.R., Strand E.A. Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. Eur. J. Neurol. 2013;20(4):629–637. doi: 10.1111/ene.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Hoglinger G.U., Antonini A., Bordelon Y., Boxer A.L., Colosimo C., van Eimeren T., Golbe L.I., Kassubek J., Kurz C., Litvan I., Pantelyat A., Rabinovici G., Respondek G., Rominger A., Rowe J.B., Stamelou M., Josephs K.A., Movement Disorder Society-endorsed, P.S.P.S.G Radiological Biomarkers for Diagnosis in PSP: Where are we and where do we need to be? Mov. Disord. 2017;32(7):955–971. doi: 10.1002/mds.27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Pan P., Song W., Huang R., Li J., Chen K.…Shang H. Voxelwise meta-analysis of gray matter anomalies in Alzheimer's disease and mild cognitive impairment using anatomic likelihood estimation. J. Neurol. Sci. 2012;316(1-2):21–29. doi: 10.1016/j.jns.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Yang J., Pan P., Song W., Shang H.F. Quantitative meta-analysis of gray matter abnormalities in semantic dementia. J. Alzheimers Dis. 2012;31(4):827–833. doi: 10.3233/JAD-2012-120736. [DOI] [PubMed] [Google Scholar]
- Yang J., Shao N., Li J., Shang H. Voxelwise meta-analysis of white matter abnormalities in progressive supranuclear palsy. Neurol. Sci. 2014;35:7–14. doi: 10.1007/s10072-013-1512-8. [DOI] [PubMed] [Google Scholar]
- Yin R.H., Tan L., Liu Y., Wang W.Y., Wang H.F., Jiang T., Radua J., Zhang Y., Gao J., Canu E., Migliaccio R., Filippi M., Gorno-Tempini M.L., Yu J.T. Multimodal voxel-based meta-analysis of white matter abnormalities in Alzheimer's disease. J. Alzheimers Dis. 2015;47:495–507. doi: 10.3233/JAD-150139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Barron D.S., Tantiwongkosi B., Fox P. Patterns of gray matter atrophy in atypical parkinsonism syndromes: a VBM meta-analysis. Brain Behav. 2015;5 doi: 10.1002/brb3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Walter R., Ng P., Luong P.N., Dutt S., Heuer H., Rojas-Rodriguez J.C., Tsai R., Litvan I., Dickerson B.C., Tartaglia M.C., Rabinovici G., Miller B.L., Rosen H.J., Schuff N., Boxer A.L. Progression of microstructural degeneration in progressive Supranuclear palsy and Corticobasal syndrome: a longitudinal diffusion tensor imaging study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Pan P., Dai Z., Shi H. Voxelwise meta-analysis of gray matter abnormalities in dementia with Lewy bodies. Eur. J. Radiol. 2014;83(10):1870–1874. doi: 10.1016/j.ejrad.2014.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data and analysis methods are shared at request for purposes of replicating procedures and results.