Abstract
Post-traumatic stress disorder (PTSD) is characterized by intrusions, re-experiencing, avoidance and hyperarousal. These symptoms might be linked to dysfunction in core neurocognitive networks subserving self-referential mental processing (default mode network, DMN), detection of salient stimuli (salience network, SN) and cognitive dysfunction (central executive network, CEN). Resting state studies in adolescent PTSD are scarce and findings are inconsistent, probably due to differences in patient symptom severity. Resting state brain activity was measured in 14 adolescents with severe PTSD and 24 age-matched controls. Seed-based connectivity analyses were used to examine connectivity between the DMN and the whole brain, including regions from other networks (SN and CEN). The relationships of network properties with symptom dimensions (severity, anxiety and depression) and episodic memory were also examined. Analyses revealed decreased within-DMN connectivity (between PCC and occipital cortex) in patients compared to controls. Furthermore, within-DMN connectivity (between PCC and hippocampus) correlated negatively with symptom dimensions (severity and anxiety), while increased connectivity (DMN-SN and DMN-CEN) correlated positively with episodic memory measures. These abnormal network properties found in adolescent PTSD corroborate those previously reported in adult PTSD. Decreased within-DMN connectivity and disrupted DMN-SN and DMN-CEN coupling could form the basis for intrusive trauma recollection and impaired episodic autobiographical recall in PTSD.
Keywords: Default mode network, Episodic memory, Functional connectivity, Post-traumatic stress disorder
Highlights
-
•
Adolescent PTSD is linked to dysfunction in core neurocognitive networks.
-
•
Results show decreased within-DMN connectivity in patients compared to controls.
-
•
Within-DMN connectivity correlates negatively with severity and anxiety.
-
•
Increased DMN-SN connectivity correlates positively with episodic memory.
-
•
Disrupted connectivity may form the basis for intrusive trauma recollection in PTSD.
1. Introduction
Post-traumatic stress disorder (PTSD) is a psychiatric disorder characterized by intrusions or re-experiencing, avoidance, hyperarousal and negative alterations in cognition and mood (Diagnostic and Statistical Manual of Mental Disorders, DSM-5, 2013). These symptoms have been linked to deficits in episodic memory, attentional control, executive functions and working memory (for reviews, Scott et al., 2015; Kavanaugh et al., 2017). Neuroimaging studies have shown that these cognitive processes are subserved by different brain regions, including the medial prefrontal cortex (mPFC), amygdala, hippocampus and insula (Patel et al., 2012). Menon (2011) proposes a triple network model that synthesizes findings into a common framework to understand dysfunction in core neurocognitive networks across multiple disorders, including PTSD. The default mode network (DMN) is anchored in the posterior cingulate cortex (PCC), mPFC, hippocampus and angular gyrus. Its role comprises different aspects of self-referential mental processing (e.g., episodic memory, autobiographical memory, social cognitive processes). The salience network (SN), anchored in the fronto-insular cortex and dorsal anterior cingulate cortex, is involved in detecting, integrating and filtering relevant interoceptive, automatic and emotional information. The central executive network (CEN), anchored in the dorsolateral PFC, lateral posterior parietal cortex and cerebellum (Habas et al., 2009), has a role in high level cognitive function, including planning, decision-making and working memory. At rest, the DMN is typically active, while the SN and CEN are quiescent. A powerful way to examine intrinsic connections between large brain networks is resting state connectivity (Fox and Raichle, 2007).
Resting state connectivity studies in adult PTSD are crucial to pinpoint interactions between regions at rest without the confounds of cognitive tasks during the scanning procedure that may be biased to elicit brain activity per se (e.g., hippocampus, amygdala, insula) and/or provoke PTSD symptoms. Abnormalities in functional connectivity within the DMN were reported in adult PTSD, compared to controls, whatever the neuroimaging modality used, either magnetoencephalographic (MEG; Dunkley et al., 2014, Dunkley et al., 2015; Badura-Brack et al., 2017; Huang et al., 2014), electroencephalographic (EEG; Imperatori et al., 2014; Lee et al., 2014) or functional magnetic resonance imaging studies (fMRI; Bluhm et al., 2009; Daniels et al., 2010; Lanius et al., 2010; Zhang et al., 2015; Sripada et al., 2012). The latter found reduced within-DMN connectivity and increased connectivity between DMN and SN regions in patients compared to controls (Sripada et al., 2012). The authors suggested a disruption of equilibrium between large-scale networks in PTSD subserving internally-focused thought versus salience detection. Alternatively, Reuveni et al. (2016) identified similar functional connectivity patterns in the DMN of PTSD and controls, but functional connectivity parameters were more strongly correlated with clinical measures (severity, anxiety and depression) in PTSD patients. Correlations between PTSD symptom severity and functional connectivity patterns have also been reported elsewhere (fMRI: Cisler et al., 2013; Lanius et al., 2010; Qin et al., 2012; Sripada et al., 2012; MEG: Dunkley et al., 2014; EEG: Lee et al., 2014). Within the DMN, functional connectivity of the hippocampus, a region that is particularly sensitive to chronic stress (Gould, 2007), was found to negatively (Sripada et al., 2012; Birn et al., 2014; Herringa et al., 2013) or positively (Dunkley et al., 2014) correlate with symptom severity, depending on the imaging modality, fMRI or MEG respectively, or on the nature of the trauma, military (Sripada et al., 2012; Birn et al., 2014; Dunkley et al., 2014) or interpersonal trauma (e.g., childhood abuse; Herringa et al., 2013; see Hugues et al., 2011, for a review). We, and others, have previously shown that the hippocampus presents both structural and functional abnormalities in adult (Bremner et al., 2003) and adolescent abuse-related PTSD (Dégeilh et al., 2017; Postel et al., 2019). Symptoms in adolescent PTSD largely reflect those present in adults, with deficits in attentional control (Guillery-Girard et al., 2013), autobiographical memory (Crane et al., 2014; Ogle et al., 2013) and fear extinction (Keding and Herringa, 2015). What happens at the brain level? Findings from resting state studies in adolescent PTSD are inconsistent, some results bearing similarity to those found in adult PTSD, with increased cross-network connectivity (Suo et al., 2015) and reduced within-DMN connectivity in maltreated youth (Herringa et al., 2013). However, other studies show the opposite pattern, with increased within-DMN connectivity and decreased cross-network connectivity in adolescent PTSD (Patriat et al., 2016). Adolescence is a transitional period before entering into adulthood. Chai et al. (2014) revealed that connectivity between the DMN and task-positive networks (SN and CEN) develops with age going from positive in childhood to negative connectivity in adolescents and young adults.
Discrepancies between studies may be explained by the patient group characteristics, either focusing on maltreated youth (non PTSD; Herringa et al., 2013) or on patients with short PTSD duration, distant trauma onset and low severity (Suo et al., 2015; Patriat et al., 2016). In this study, we aimed to investigate this discrepancy in the literature on adolescent PTSD by assessing functional connectivity between brain networks at rest in a group of adolescents with severe PTSD. We included only patients meeting full diagnosis of PTSD and mainly characterized by interpersonal trauma, indicating high symptom severity. Seed-based connectivity analyses were used to examine DMN connectivity with the whole brain, including regions of the SN and CEN. We also examined the relationships of network properties with symptom dimensions (severity, anxiety and depression) and episodic memory, altered in PTSD (Ogle et al., 2013; for review, Zlomuzica et al., 2014). Symptoms in PTSD adolescents bearing similarity to those present in adults, we hypothesized similar network alterations, with decreased within-DMN connectivity and increased connectivity between DMN and SN or CEN regions in patients compared to controls.
2. Materials and methods
2.1. Participants
Fifteen adolescents with PTSD aged 13 to 18 years old, were recruited through the University Hospitals of Caen, Rennes and Rouen (France). Data of one patient were not exploitable because she slept during the resting state fMRI scan. PTSD adolescents received no psychotropic medication during the previous week and were free from other mental disorders including major depression. All presented a chronic PTSD for at least 6 months. Twenty-four typically developing adolescents with no history of trauma were chosen to match the patient group in terms of age and IQ. Healthy participants were recruited by prospecting in several junior high schools of the region (Normandy, France).
Altogether, 14 PTSD patients (12 females) and 24 controls (13 females) were included in the analyses (see Table 1 for descriptive statistics). All were right-handed and French native speakers. None of them reported any prior or current neurological or learning disabilities, head trauma, and MRI contraindications. The study was approved by the local Ethics Committee (CPP Nord Ouest III). All adolescents and their parents signed informed consent after a comprehensive description of the study. Compared to our previous study on self-reference processing (Dégeilh et al., 2017), we increased our sample sizes by scanning five additional patients and 14 new controls.
Table 1.
Demographic, psychopathological and neuropsychological measures, depicting means and standard deviations (S.D.) for patients (N = 14, 12 females) and controls (N = 24, 13 females) at a p significant threshold of p < .05 (bold font indicates a significant difference between groups).
| PTSD |
Controls |
t-test |
||||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | t value | p | |
| Population characteristics | ||||||
| Age (months) | 188.71 | 18.16 | 191.92 | 20.63 | 0.48 | 0.633 |
| Age (years) | 15.73 | 1.51 | 15.99 | 1.720 | 0.541 | 0.592 |
| IQa | 98.92 | 18.66 | 107.83 | 19.43 | 1.33 | 0.192 |
| PTSD duration (months) | 27 | 24.54 | – | – | – | – |
| Age of onset (years) | 13.36 | 1.78 | – | – | – | – |
| Index trauma (n) | Sexual abuse (11) | – | – | – | – | |
| Accident (1) | ||||||
| Loss of loved one (1) | ||||||
| Witness of suicide (1) | ||||||
| Psychopathological scores | ||||||
| IES-R | 51.0 | 18.90 | 12.96 | 8.85 | 8.45 | <0.001 |
| R-CMAS | 18.21 | 6.75 | 8.79 | 5.27 | 4.79 | <0.001 |
| CDI | 18.93 | 9.40 | 8.83 | 4.66 | 4.44 | <0.001 |
| Neuropsychological scores | ||||||
| Episodic Memory (CMSa) | ||||||
| Stories subtest | ||||||
| Immediate recall | 43.46 | 13.27 | 53.63 | 14.13 | 2.13 | 0.040 |
| Delayed recall | 39.36 | 14.30 | 50.21 | 14.38 | 2.19 | 0.035 |
| Family Scenes subtest | ||||||
| Immediate recall | 44.62 | 3.36 | 44.04 | 3.48 | −0.48 | 0.631 |
| Delayed recall | 44.69 | 3.73 | 43.96 | 3.64 | −0.58 | 0.535 |
Abbreviations: CMS = Children's Memory Scale (Cohen, 1997).
Missing data of one patient.
2.2. Assessment
A board-certified child and adolescent psychiatrist interviewed and screened all participants, incorporating both caregiver and youth reports. Psychiatric diagnoses and trauma exposure were assessed using the Structured Clinical Interview-Clinician Version (SCID-CV; First et al., 2002; Lobbestael et al., 2011). PTSD severity was additionally examined with the French version of the Impact of the Event Scale-Revised (IES-R; Brunet et al., 2003; Weiss and Marmar, 1997). Major depression was categorically screened using the SCID-CV (First et al., 2002; Lobbestael et al., 2011) and dimensionally measured with the French version of the Children Depression Inventory (CDI; Dugas and Bouvard, 1996; Kovacs, 1981). Anxiety was scored with the French version of the Revised-Children's Manifest Anxiety Scale (R-CMAS; Reynolds and Richmond, 1997; Reynolds et al., 1999). Of note, clinical assessments for this study have been previously described (Dégeilh et al., 2017).
Episodic memory deficits being consistently reported in PTSD (for review, Zlomuzica et al., 2014), neuropsychological assessments of episodic memory were also conducted. The French version of the Children's Memory Scale (CMS; Cohen, 1997) was used to evaluate verbal episodic memory with the Stories subtest and visual episodic memory with the Family Scenes subtest. Immediate and delayed recall scores were obtained for both subtests.
2.3. Neuroimaging data acquisition
All participants were scanned with a Philips Achieva 3.0 T MRI scanner at the Cyceron Center (Caen, France). High-resolution T1-weighted anatomical volumes were acquired using a three-dimensional fast-field echo sequence (3D-T1-FFE sagittal; repetition time = 20 ms, echo time = 4.6 ms, flip angle = 10°, 180 slices, slice thickness = 1 mm, field of view = 256 × 256 mm2, in-plane resolution = 1 × 1 mm2).
Resting state functional volumes were obtained using an interleaved 2DT2* SENSE EPI sequence designed to reduce geometric distortions, with parallel imaging, short echo time, and small voxels (2D-T2∗-FFE-EPI axial, SENSE = 2; repetition time = 2382 ms, echo time = 30 ms, flip angle = 80°, 42 slices, slice thickness = 2.8 mm, field of view = 224 × 224 mm2, in-plane resolution = 2.8 × 2.8 mm2, 280 volumes). Resting state duration was 11.26 min. Participants were equipped with earplugs, their head was stabilized with foam pads to minimize head motion, and the scanner room's light was turned off. During this acquisition, which was the last one in the MRI scanning session, participants were asked to relax, lie still in the scanner, and keep their eyes closed, without falling asleep. A subsequent debriefing questionnaire allowed us to ensure that the participants had no difficulty staying awake throughout the duration of the resting state fMRI scan and that nothing particular had disturbed their attention during the scanning.
2.4. Neuroimaging data preprocessing
Individual datasets were first checked for artifacts through the application of the TSDiffana routines (http://imaging.mrc-cbu.cam.ac.uk/imaging/DataDiagnostics). For each 38 individual datasets, we verified that head motion did not exceed 3 mm (translation) and 1.5° (rotation). Data were then processed as described in Mevel et al. (2013). Briefly, the first 6 volumes were discarded because of saturation effects. The EPI volumes were corrected for slice timing and realigned to the first volume. Data were then spatially normalized using a technique designed to reduce geometric distortion effects (Villain et al., 2010; Mevel et al., 2013). This procedure includes for each individual i) a coregistration of the mean EPI volume, non-EPI T2*, T2, and T1 volumes; ii) a warping of the mean EPI volume to match the non-EPI T2* volume; iii) a segmentation of the T1 volume using voxel-based morphometry; iv) a normalization of the coregistered T1, EPI, and non-EPI T2* volumes using the parameters obtained from the T1 segmentation; v) a 4 mm full-width at half-maximum (FWHM) smooth of the EPI volumes and vii) and temporal bandpass filtering (0.01–0.08 Hz). Finally, a binary mask was created from the group segmented mean grey matter T1 volume in conjunction with the mean non EPI-T2* volume in the MNI space (including only voxels with values > 0.25 in both mean volumes; Villain et al., 2010).
2.5. Statistical analyses
2.5.1. Behavioral analyses
To compare patients and controls on the psychopathological and neuropsychological measures, unpaired two-sample t-tests were performed using Statistica (Statsoft, Tulsa, USA).
2.5.2. Seed-based connectivity analyses
Using the GIFT toolbox (http://mialab.mrn.org/software/gift/index.html), whole-brain resting state fMRI analyses were performed on both groups using a seed-based approach. DMN connectivity was calculated using 4-mm radius spheres located in the bilateral PCC. The coordinates were obtained from previous studies of the DMN in PTSD: Patriat et al. (2016) in the right PCC (MNI coordinates [2 –52 26]) and Miller et al. (2017) in the left PCC (MNI coordinates [−8 –56 26]). The PCC seeds were chosen because they represent hubs of the DMN. Seeds based on these coordinates were created using the MarsBar toolbox (Brett et al., 2002) and the 38 individual mean time courses were extracted for each seed. For all participants and each seed of interest, correlations were assessed between the mean time course in the seed and the time course of each grey matter voxel. To remove potential sources of spurious variance, the time courses from white matter, cerebrospinal fluid, their derivatives, and the six movements parameters generated from realignment of head motion were introduced as covariates. Head motion parameters were also examined across groups (Framewise Displacement rate, FWD, and Root Mean Square, RMS; Power et al., 2012, Power et al., 2014; van Dijk et al., 2012; Siegel et al., 2014). Groups did not significantly differ in head motion (FWD: t(36) = 0.4431, p = .66; RMN: t(36) = 1.2726, p = .21). A Fisher's z transform, as well as a 6.9 mm FWHM smooth, were then applied to the resulting individual connectivity maps. Z-score images from the individual functional connectivity analyses were then entered into a two-sample t-test implemented in SPM12, with covariates including age and sex. Family wise error (FWE) corrected p < .05 significance level was used, with a cluster extent threshold of 10 voxels.
2.5.3. Correlations with neuropsychological and psychopathological scores
The relationships between connectivity measures with neuropsychological and psychopathological scores were examined within the PTSD group, adjusting for age and sex. Neuropsychological measures (episodic memory, both immediate and delayed recalls, as measured with the Stories and Family Scenes CMS subtests) and psychopathological scores (symptom severity (IES-R), anxiety (R-CMAS), depression (CDI)) were added as regressors in separate whole brain analyses of connectivity between seed regions (bilateral PCC) and the rest of the brain, adjusting for age and sex. Because of the targeted, exploratory nature of the correlation analyses, a statistical threshold of p < .001 uncorrected was used, with a corrected cluster extent threshold of 80 voxels. This combination of activation thresholds was determined using the 3DClustSim program (Ward, 2000) to correspond to a false positive rate of p < .05, corrected for multiple comparisons.
In addition, a hypothesis-driven regions of interest (ROI) analysis of the hippocampal region (p < .05 corrected for multiple comparisons, small volume correction or SVC) was conducted to specifically examine the correlation between PCC-hippocampal connectivity with neuropsychological and psychopathological scores, based on a priori hypotheses regarding the implication of the hippocampus in the pathophysiology of PTSD (Etkin and Wager, 2007; Lanius et al., 2010) and its specific role in episodic autobiographical memory retrieval which is impaired in PTSD (Crane et al., 2014; Ogle et al., 2013). Right and left hippocampal ROIs were obtained from the Automated Anatomical Labeling (AAL) toolbox (Tzourio-Mazoyer et al., 2002) and used as masks for the SVC analyses. All reported voxel coordinates correspond to standardized MNI space.
3. Results
3.1. Participant characteristics
Participant characteristics are summarized in Table 1. There were no significant differences between groups for age, IQ and the Family Scenes subtest of the CMS. For psychopathological measures, PTSD patients exhibited significantly higher levels of symptom severity (IES-R scale: t = 8.45, p < .001), anxiety (R-CMAS anxiety scale: t = 4.79, p < .001) and depression (CDI score: t = 4.44, p < .001) compared to controls. For neuropsychological measures, PTSD patients had significantly lower performances for both immediate (t = 2.13, p < .040) and delayed recall (t = 2.19, p = .035) on the Stories subtest of the CMS compared to controls.
3.2. fMRI results
3.2.1. Seed-based connectivity analyses
Results of the seed-based connectivity analyses for the DMN seeds (right and left PCC) are presented in Table 2 and Fig. 1. DMN seeds in both groups separately showed significant connectivity with other DMN regions (PCC, precuneus, mPFC, angular and middle temporal gyri, hippocampus and parahippocampal gyrus) and with CEN regions (cerebellum 9 and crus 1). Direct comparisons showed no differences using the FWE stringent threshold. By lowering the threshold at p < .001 uncorrected, another DMN region (left middle occipital gyrus) showed decreased connectivity with both the right and left PCC seeds in patients compared to controls.
Table 2.
Results of the seed-based connectivity analyses depicting the interactions between bilateral PCC and the rest of the brain, with age and sex as covariates, at p < .05 FWE corrected, k > 10. L: left; R: right.
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Side | x | y | z | k | t value |
| R PCC connectivity | ||||||
| PTSD | ||||||
| PCC | R | 4 | −50 | 26 | 3131 | 36.76 |
| Precuneus | R | 4 | −60 | 34 | 17.93 | |
| L | −4 | −62 | 26 | 17.17 | ||
| mPFC | L | −6 | 54 | −12 | 1763 | 12.10 |
| R | 2 | 58 | −12 | 11.87 | ||
| Superior frontal gyrus | L | −18 | 42 | 44 | 28 | 7.56 |
| R | 20 | 38 | 42 | 28 | 6.97 | |
| Superior medial frontal gyrus | L | −6 | 50 | 48 | 11 | 6.80 |
| ACC | L | −4 | 34 | 6 | 10 | 7.00 |
| Angular gyrus | L | −48 | −66 | 36 | 521 | 11.23 |
| R | 48 | −62 | 40 | 82 | 7.84 | |
| Middle temporal gyrus | R | 62 | −8 | −18 | 178 | 10.09 |
| L | −58 | 0 | −22 | 64 | 7.70 | |
| Temporal pole | R | −36 | 14 | −36 | 11 | 7.16 |
| Parahippocampal gyrus | L | −22 | −22 | −20 | 111 | 8.33 |
| R | 28 | −20 | −20 | 45 | 7.71 | |
| Cerebellum 9 | R | 8 | −50 | −42 | 532 | 7.91 |
| Cerebellum crus 1 | L | −24 | −84 | −30 | 20 | 7.44 |
| R | 32 | −78 | −34 | 57 | 7.35 | |
| Controls | ||||||
| PCC | R | 4 | −50 | 26 | 3975 | 52.13 |
| Precuneus | L | −4 | −62 | 26 | 22.06 | |
| mPFC | R | 2 | 64 | −12 | 3127 | 14.32 |
| L | −6 | 54 | −10 | 14.26 | ||
| Angular gyrus | L | −48 | −66 | 38 | 1246 | 14.29 |
| R | 50 | −62 | 36 | 736 | 11.45 | |
| Middle temporal gyrus | R | 64 | −6 | −16 | 941 | 13.18 |
| L | −62 | −14 | −18 | 765 | 11.44 | |
| Parahippocampal gyrus | L | −22 | −36 | −14 | 44 | 8.28 |
| R | 26 | −18 | −20 | 24 | 6.96 | |
| Hippocampus | L | −22 | −18 | −20 | 75 | 7.94 |
| Cerebellum 9 | R | 6 | −52 | −42 | 12 | 7.14 |
| Cerebellum crus 1 | R | 28 | −78 | −32 | 792 | 8.19 |
| L | −28 | −84 | −32 | 39 | 7.23 | |
| PTSD > Controls | – | – | – | – | – | – |
| Controls > PTSD | ||||||
| Middle occipital gyrus | L | −34 | −96 | 0 | 70 | 5.08⁎ |
| L PCC connectivity | ||||||
| PTSD | ||||||
| Precuneus | L | −6 | −54 | 26 | 3100 | 31.98 |
| R | 6 | −56 | 26 | 15.35 | ||
| mPFC | L | −6 | 52 | −12 | 876 | 11.58 |
| R | 0 | 48 | 4 | 9.86 | ||
| Angular gyrus | L | −46 | −66 | 38 | 230 | 8.94 |
| R | 46 | −62 | 40 | 47 | 7.52 | |
| Middle temporal gyrus | R | 66 | −8 | −12 | 19 | 6.73 |
| Parahippocampal gyrus | L | −24 | −26 | −16 | 58 | 8.56 |
| Controls | ||||||
| Precuneus | L | −6 | −54 | 26 | 4127 | 42.97 |
| R | 6 | −56 | 26 | 24.32 | ||
| PCC | R | 6 | −48 | 24 | 21.03 | |
| mPFC | L | −2 | 60 | −6 | 2452 | 14.89 |
| R | 6 | 56 | −6 | 12.57 | ||
| L | −8 | 48 | 50 | 65 | 8.20 | |
| Angular gyrus | L | −42 | −66 | 36 | 1168 | 13.66 |
| R | 46 | −62 | 38 | 630 | 10.08 | |
| Middle temporal gyrus | L | −62 | −14 | −18 | 351 | 11.09 |
| R | 64 | −4 | −18 | 515 | 10.91 | |
| Temporal pole | L | −48 | 10 | −34 | 41 | 7.57 |
| Parahippocampal gyrus | L | −20 | 36 | −12 | 35 | 7.70 |
| Hippocampus | L | −22 | −22 | −18 | 55 | 7.98 |
| PTSD > Controls | – | – | – | – | – | – |
| Controls > PTSD | ||||||
| Middle occipital gyrus | L | −38 | −68 | 32 | 54 | 4.27⁎ |
Abbreviations: ACC: anterior cingulate cortex k: number of voxels; mPFC: medial prefrontal cortex; PCC: posterior cingulate cortex.
p < .001 uncorrected.
Fig. 1.
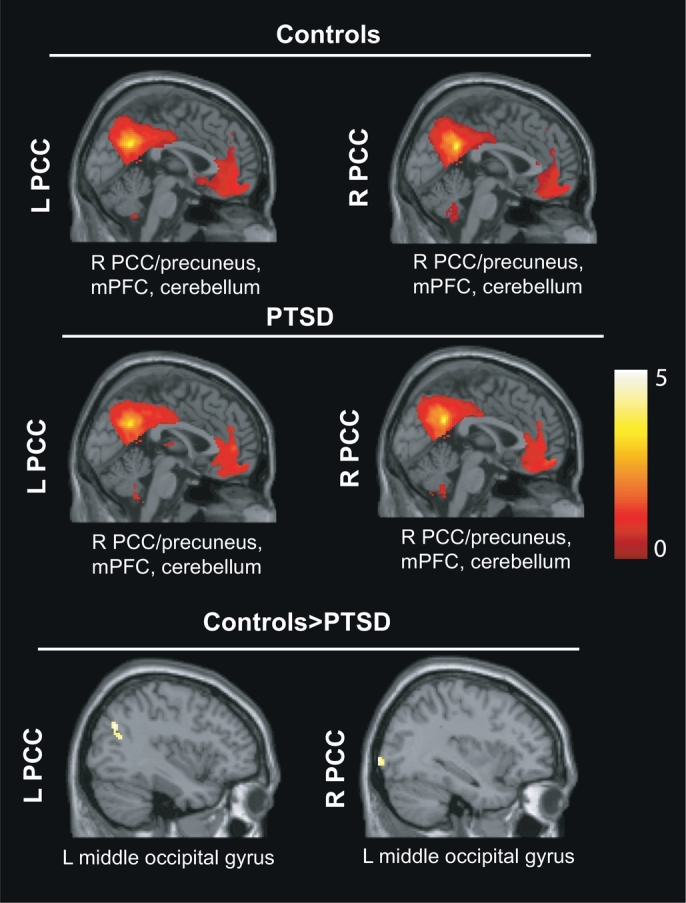
Seed-based connectivity analyses between the right and left PCC and the whole brain in PTSD compared to controls, controlling for age and sex, at p < .05 FWE corrected, k >10 voxels. R = right; L = left.
3.2.2. Correlations with neuropsychological and psychopathological scores
Relationships between connectivity measures and neuropsychological and psychopathological scores are presented in Table 3 and Fig. 2. For the right PCC seed, connectivity between the DMN (PCC) and SN regions (insula, anterior cingulate cortex or ACC) was positively correlated with episodic memory performances of the Stories subtest, both for immediate and delayed recalls, and for immediate recall on the Family Scenes subtest. Additionally, for both PCC seeds, connectivity between the DMN (PCC) and CEN regions (middle frontal gyrus) was positively correlated with the Family Scenes subtest, for immediate recall. Conversely, connectivity between the DMN (PCC) and a CEN region (cerebellum crus I) was negatively correlated with performances on the Family Scenes subtest, for immediate and delayed recalls. No significant correlations appeared for the psychopathological scores. Direct comparisons with the control group can be found in Supplemental Table 1 (see Supplemental Material). Main results from these comparisons revealed stronger positive correlations between PCC/occipital cortex connectivity with episodic memory and with psychopathological (anxiety, depression) scores in controls than patients (see Supplemental Table 1).
Table 3.
Results of the correlations between PCC connectivity and neuropsychological (Stories and Family Scenes subtests) and psychopathological scores (severity, anxiety, depression) within the PTSD group, adjusting for age and sex, at p < .001 uncorrected, cluster-level corrected (p < .05, k > 80 voxels). L: left; R: right.
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Side | x | y | z | k | t value | |
| R PCC connectivity | ||||||
| Stories immediate recall | ||||||
| Positive correlations | ||||||
| Insula | L | −42 | 14 | −2 | 161 | 5.15 |
| L | −32 | 12 | 4 | 5.04 | ||
| L | −34 | 4 | 2 | 4.24 | ||
| ACC | R | 4 | 22 | 28 | 105 | 4.80 |
| L | −12 | 22 | 26 | 3.81 | ||
| Stories delayed recall | ||||||
| Positive correlations | ||||||
| Insula | L | −44 | 14 | −2 | 198 | 5.36 |
| L | −32 | 12 | 4 | 5.33 | ||
| L | −34 | 4 | 2 | 4.47 | ||
| ACC | R | 4 | 22 | 28 | 122 | 4.49 |
| L | −4 | 22 | 30 | 4.39 | ||
| R | 2 | 16 | 44 | 4.32 | ||
| Family scenes immediate recall | ||||||
| Positive correlations | ||||||
| Insula | R | 28 | 22 | −12 | 148 | 5.10 |
| R | 30 | 24 | −2 | 5.08 | ||
| R | 36 | 22 | 10 | 4.17 | ||
| ACC | R | 10 | 38 | 14 | 127 | 4.89 |
| R | 0 | 40 | 10 | 4.10 | ||
| R | 4 | 30 | 22 | 3.71 | ||
| Middle frontal gyrus | R | 28 | 46 | 14 | 138 | 4.90 |
| R | 40 | 44 | 14 | 4.70 | ||
| R | 36 | 56 | 24 | 4.30 | ||
| Negative correlations | ||||||
| Cerebellum crus 1 | R | 22 | −84 | −26 | 82 | 4.46 |
| R | 4 | −84 | −30 | 4.21 | ||
| R | 12 | −86 | −30 | 4.02 | ||
| Family scenes delayed recall | ||||||
| Negative correlations | ||||||
| Cerebellum crus 1 | R | 22 | −90 | −24 | 107 | 4.90 |
| R | 22 | −82 | −26 | 4.89 | ||
| R | 4 | −84 | −30 | 4.46 | ||
| Severity | – | – | – | – | – | – |
| Anxiety | – | – | – | – | – | – |
| Depression | – | – | – | – | – | – |
| L PCC connectivity | ||||||
| Stories immediate recall | – | – | – | – | – | – |
| Stories delayed recall | – | – | – | – | – | – |
| Family scenes immediate recall | ||||||
| Positive correlations | ||||||
| Middle frontal gyrus | R | 34 | 56 | 24 | 237 | 5.51 |
| R | 38 | 44 | 10 | 4.58 | ||
| R | 28 | 46 | 12 | 4.23 | ||
| Inferior frontal gyrus | R | 52 | 26 | 6 | 256 | 5.02 |
| R | 50 | 18 | 6 | 4.64 | ||
| R | 54 | 22 | −4 | 4.60 | ||
| Family scenes delayed recall | – | – | – | – | – | – |
| Severity | – | – | – | – | – | – |
| Anxiety | – | – | – | – | – | – |
| Depression | – | – | – | – | – | – |
Abbreviations: ACC: anterior cingulate cortex; PCC: posterior cingulate cortex; k: number of voxels.
Fig. 2.
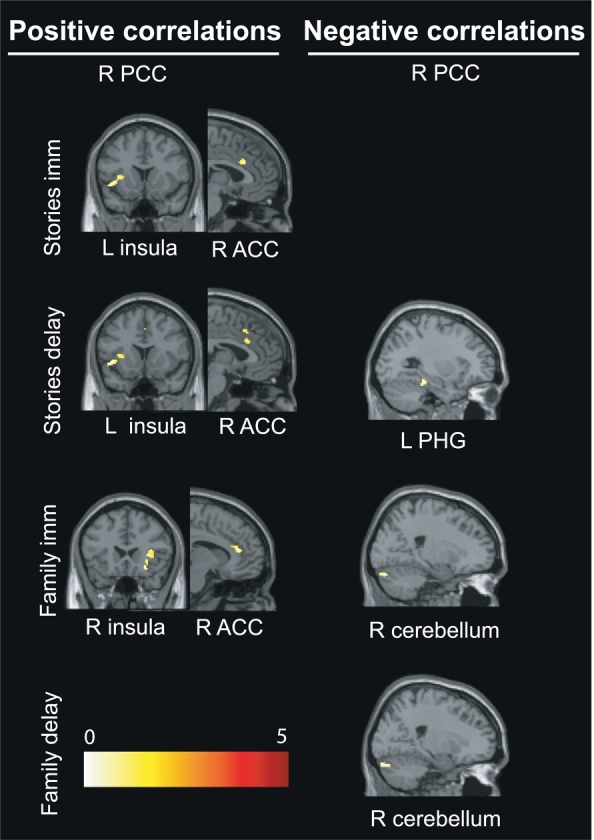
Results of the correlations between PCC connectivity and episodic memory scores (Stories and Family Scenes subtests of the Children's Memory Scale, CMS) within the PTSD group, adjusting for age and sex, at p < .001 uncorrected, cluster-level corrected (p < .05, k > 80 voxels). R = right; L = left. For representational purposes, only connectivity with the right PCC seed is shown.
Abbreviations: family delay: family scenes delayed recall; family imm: family scenes immediate recall; stories delay: stories delayed recall; stories imm: stories immediate recall; PCC: posterior cingulate cortex; PHG: parahippocampal gyrus.
Results of the SVC approach centered on the hippocampus are presented in Table 4 and Fig. 3, Fig. 4. Within-DMN connectivity between the right PCC seed and bilateral hippocampi was negatively correlated with the Stories subtest (immediate and delayed recalls), right PCC and left hippocampus with the Family Scenes subtest (immediate recall), symptom severity (IES-R) and anxiety (R-CMAS), and, finally, the right PCC and right hippocampus with the Family Scenes subtest (delayed recall). Within-DMN connectivity between the left PCC seed and left hippocampus was negatively correlated with Stories (delayed recall), Family Scenes (immediate and delayed recalls), symptom severity (IES-R) and anxiety (R-CMAS). No significant correlations were found with depression (CDI). Direct comparisons with the control group can be found in Supplemental Table 2 (see Supplemental Material).
Table 4.
Results of the small volume correction (SVC) analysis centered on the hippocampus (p < .05 corrected for multiple comparisons) depicting negative correlations between PCC connectivity with neuropsychological (Stories and Family Scenes subtests) and psychopathological scores (severity, anxiety, depression) within the PTSD group, adjusting for age and sex. L: left; R: right.
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Side | x | y | z | t value | ||
| R PCC connectivity | ||||||
| Stories immediate recall | HPC ant | R | 28 | −20 | −10 | 5.30 |
| HPC ant | L | −12 | −4 | −12 | 4.61 | |
| Stories delayed recall | HPC ant | R | 28 | −20 | −10 | 5.94 |
| HPC post | L | −16 | −36 | 0 | 3.74 | |
| Family scenes immediate recall | HPC ant | L | −34 | −12 | −22 | 3.72 |
| Family scenes delayed recall | HPC ant | R | 28 | −18 | −12 | 3.55 |
| Severity | HPC ant | L | −20 | −2 | −26 | 4.79 |
| Anxiety | HPC ant | L | −18 | 0 | −26 | 4.06 |
| Depression | – | – | – | – | – | – |
| L PCC connectivity | ||||||
| Stories immediate recall | – | – | – | – | – | – |
| Stories delayed recall | HPC ant | L | −36 | −10 | −22 | 3.80 |
| Family scenes immediate recall | HPC ant | L | −34 | −10 | −22 | 3.56 |
| Family scenes delayed recall | HPC ant | L | −14 | −4 | −16 | 3.69 |
| Severity | HPC ant | L | −18 | −2 | −28 | 4.64 |
| Anxiety | HPC ant | L | −18 | −2 | −28 | 4.42 |
| Depression | – | – | – | – | – | – |
Abbreviations: CDI: Children Depression Inventory; HPC ant: anterior hippocampus; HPC post: posterior hippocampus; IES-R: Impact of the Event Scale-Revised; PCC: posterior cingulate cortex; R-CMAS: Revised-Children's Manifest Anxiety Scale.
Fig. 3.
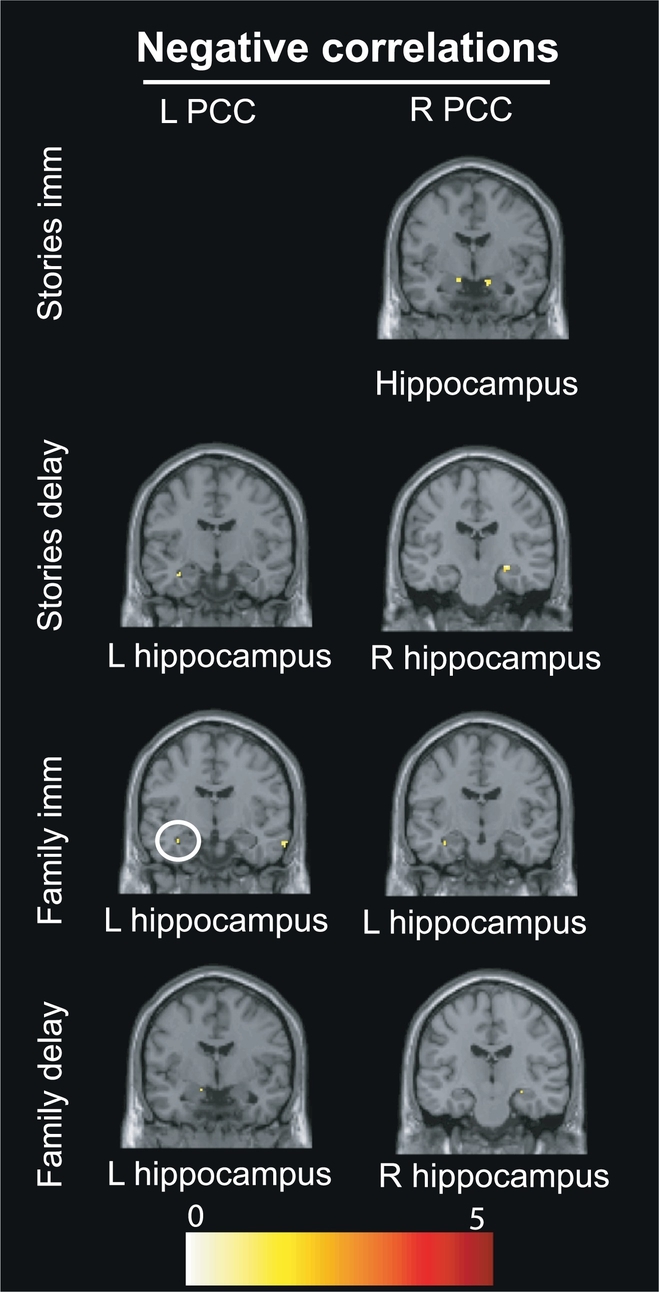
Results of the small volume correction (SVC) analysis centered on the hippocampus depicting the correlations between PCC-hippocampal connectivity and episodic memory scores (Stories and Family Scenes subtests) within the PTSD group, adjusting for age and sex. R = right; L = left.
Abbreviations: Family delay: Family Scenes immediate recall; Family imm: Family Scenes immediate recall; PCC: posterior cingulate cortex; Stories delay: Stories delayed recall; Stories imm: Stories immediate recall.
Fig. 4.
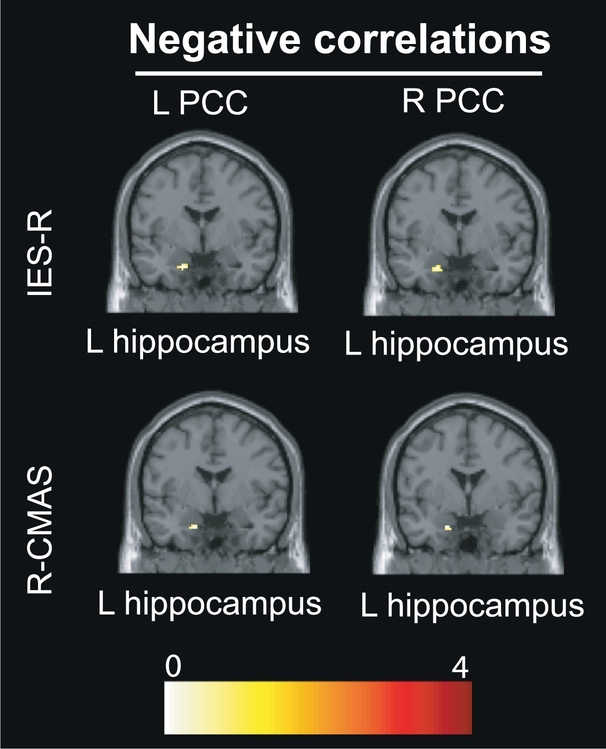
Results of the small volume correction (SVC) analysis centered on the hippocampus depicting the correlations between PCC-hippocampal connectivity and psychopathological scores (IES-R, R-CMAS, CDI) within the PTSD group, adjusting for age and sex. R = right; L = left.
Abbreviations: CDI: Children Depression Inventory; IES-R: Impact of the Event Scale-Revised; PCC: posterior cingulate cortex; R-CMAS: Revised-Children's Manifest Anxiety Scale.
4. Discussion
In this study, we examined connectivity between the DMN and the whole brain in PTSD adolescents compared to healthy controls and determined if pathological scores (i.e., symptom severity, anxiety, depression and episodic memory) correlated positively or negatively with these patterns. Analyses revealed within-DMN connectivity and connectivity between the DMN and CEN regions in both groups separately. Differences between groups appeared by lowering the threshold and showed decreased within-DMN connectivity in PTSD patients compared to controls. Correlational analyses revealed that changes in functional connectivity were related to pathological patterns in patients: within-DMN connectivity between the PCC and hippocampus correlated negatively with symptom dimensions (severity and anxiety), while increased connectivity correlated positively (DMN-SN and DMN-CEN) or negatively (DMN-CEN) with episodic memory measures. We will discuss the three main findings and confront them to the existing adolescent and adult literature.
4.1. Within-DMN connectivity
In both the PTSD and control groups, we found that both PCC seeds were functionally connected with a number of regions previously identified as part of the DMN, including other regions in the PCC, precuneus, mPFC, angular gyri, middle temporal gyri and medial temporal lobe. These results confirm prior findings in adult PTSD which showed that DMN seeds, such as the PCC, were functionally connected with other DMN areas in controls and in PTSD patients at rest (Bluhm et al., 2009; Reuveni et al., 2016; Badura-Brack et al., 2017). By lowering the threshold at p < .001 uncorrected, direct statistical comparison showed that PTSD patients exhibited weakened within network DMN connectivity between the (right and left) PCC seeds and another DMN area (middle occipital gyrus) in PTSD compared to controls. Although this result was obtained at a lower threshold, it is nonetheless consistent with prior resting state fMRI (Mueller-Pfeiffer et al., 2013) or MEG (Badura-Brack et al., 2017; Dunkley et al., 2015) studies reporting abnormal connectivity with the visual cortex in PTSD. Moreover, correlation analyses revealed stronger positive correlations between PCC/occipital cortex connectivity with episodic memory and with psychopathological scores in controls than patients. Decreased connectivity of the PCC with occipital areas, both known for their role in visual mental imagery (Brewin et al., 2010), may be related to distorted images, dysfunctional autobiographical memory retrieval (e.g., gist memory) or flashbacks. Distortions in trauma memory may lead to inadequate contextualization of the memory trace in time, place and relative to other autobiographical memories (Ehlers and Clark, 2000). Weakened DMN functional integration during resting state (Bluhm et al., 2009; Sripada et al., 2012; Lui et al., 2009; Miller et al., 2017) or alterations in DMN function more generally (Daniels et al., 2010; Wu et al., 2011) have been previously shown in adult PTSD and Patel et al. (2012) suggested that increased suppression of DMN regions may serve as a reliable neural marker of reduced cognitive flexibility in PTSD. Our results in adolescent PTSD resonate with these findings, but must be replicated at a more stringent threshold to be considered solid.
Additional analyses revealed a negative correlation between PCC-hippocampal connectivity and symptom dimensions (severity and anxiety): higher pathological scores corresponded to less within-DMN connectivity between the bilateral PCC and left hippocampus. These findings mirror prior research showing that less DMN connectivity to the hippocampus was associated with higher scores on the Clinician Administered PTSD Scale (CAPS) in adult PTSD (Sripada et al., 2012; Birn et al., 2014), although other studies showed the opposite pattern with increased hippocampal connectivity associated with greater severity (fMRI: Shin et al., 2004; Osuch et al., 2001; MEG: Dunkley et al., 2014). Hughes and Shin (2011) reviewed these inconsistencies and suggested that “the direction of hippocampal functional abnormalities depends on the type of tasks and analyses employed.” Our findings in adolescent PTSD nonetheless confirm those in adult PTSD suggesting that the reduced cohesiveness of DMN may be associated with greater symptom severity in PTSD (see also Miller et al., 2017).
A negative correlation also emerged between PCC-hippocampal connectivity and episodic memory scores (both for the Stories and Family Scenes subtests): the better patients' performances were on either episodic memory test, the more DMN-DMN connectivity decreased. The fact that greater episodic memory performances was associated with lower connectivity of the PCC with the anterior hippocampus, known for its role in autobiographical memory retrieval (Viard et al., 2007, Viard et al., 2012), may contribute to the intrusive nature of trauma recollection in PTSD (Patel et al., 2012). An fMRI study in adult PTSD showed that reduced hippocampal activity was associated with high arousal symptoms on the CAPS (Hayes et al., 2011). Altered activity in hippocampus during encoding of traumatic memories may contribute to the development and maintenance of the disorder. These results support neurobiological theories which propose that stress may be responsible for inhibiting hippocampal activity (Rauch et al., 2006).
4.2. DMN connectivity with SN regions
A positive correlation was detected between PCC connectivity and SN regions (insula and ACC) with episodic memory performances for both tests (Stories and Family Scenes subtests). The insula is implicated in disparate cognitive and affective functions, including interoceptive awareness, emotional and empathetic responses (Menon and Uddin, 2010). As part of the SN, it biases attention towards emotionally salient stimuli or internal mental events (Menon, 2011). The insula has been frequently involved in the pathophysiology of PTSD (see meta-analysis, Etkin and Wager, 2007) and activation studies have shown increased activity in areas of the SN (insula, ACC), suggesting they may underlie disruption in conflict monitoring, autonomic regulation and reward processing (Patel et al., 2012). At rest, the SN is typically anti-correlated with the DMN in healthy subjects and likely supports the switching between large-scale networks (Menon and Uddin, 2010). In PTSD however, our findings show that the lower patients' performances were on either episodic memory test, the more DMN-SN connectivity decreased, suggesting a disruption of equilibrium between these networks as shown in adult PTSD (Sripada et al., 2012). This imbalance may help to explain sustained hypervigilance and hyperarousal in PTSD patients (Sripada et al., 2012). This positive correlation also suggests that the higher patients' performances were on either episodic memory test, the more DMN-SN connectivity increased. This connectivity increase was efficient in normalizing performances for the Family Scenes subtest (patients' performances were not different from controls), but was inefficient for the Stories subtest (patients' performances were significantly lower than controls). It is also plausible that behavioral differences may result from the nature of the task: the Family Scenes subtest, in which participants are shown family scenes close to real-life experiences, may appear more self-referential than the Stories subtest in which a factual story is presented (e.g., hot air balloon story or buffalos story).
4.3. DMN connectivity with CEN regions
Findings also revealed, in both groups, functional connectivity between both PCC seeds and a region identified as part of the central executive network (CEN), the cerebellum crus I (Stoodley and Schmahmann, 2005; Habas et al., 2009). In healthy adults, DMN and CEN are anti-correlated, meaning that these networks are competing and switching during external versus internal processing of stimuli (Fox et al., 2009; Whitfield-Gabrieli and Ford, 2012; Chai et al., 2012). Activation studies have shown increases in CEN and SN regions during stimulus-driven cognitive processes, while DMN activation decreases (Menon, 2011). In our PTSD adolescents, seed-based analyses revealed a negative correlation between connectivity of the right PCC (DMN) and cerebellum crus I (CEN) with episodic memory performances on the Family Scenes subtest (both immediate and delayed recalls). Behaviorally, patients had high performances on the Family Scenes subtest which did not significantly differ from controls. Hence, high episodic memory scores corresponded to lower DMN-CEN connectivity between the PCC seed and cerebellum crus I. The cerebellum is not frequently recruited in episodic memory tasks (unlike, for example, PFC or hippocampus) and decreasing connectivity with this region could serve to “facilitate” performances on the episodic memory tests. This decrease in DMN-CEN connectivity could be an effective way to normalize episodic memory performances in our patients.
Results also showed a positive correlation between both PCC seeds and the middle frontal gyrus (in another terminology also referred to as the dorsolateral PFC, also part of the CEN) - and additional inferior frontal gyrus (or ventrolateral PFC) for the left PCC seed - with episodic memory performances on the Family Scenes subtest (immediate recall). Behaviorally, patients did not significantly differ from controls on this subtest, suggesting that high episodic memory scores corresponded to high DMN-CEN connectivity between the bilateral PCC and lateral PFC. Episodic memory tasks, such as the Family Scenes subtest, may rely more on prefrontal regions, than the cerebellar area (see above). Hence, this DMN-CEN connectivity could be a compensatory mechanism which was effective in normalizing patients' performances. To sum, the DMN-CEN connectivity showed both positive (PCC-dorsolateral PFC) and negative (PCC-cerebellum) correlations with episodic memory scores in patients. The imbalance between DMN and CEN connectivity patterns may be explained by a “contamination” of autobiographical remembering while performing the episodic memory tasks in PTSD, i.e. autobiographical remembering interferes with the episodic memory tasks. Of interest, Bluhm et al. (2009) reported a positive correlation between DMN connectivity with the dorsolateral PFC and frequency of dissociative experiences in adult PTSD. They suggest that dissociative experiences may involve alterations in the relation between the DMN and CEN, which may relate to difficulties switching between DMN and CEN.
Overall, our results confirm findings of resting state studies in adult PTSD, but are partly contrary to a recent adolescent PTSD study (Patriat et al., 2016) using a similar method (seed-based connectivity analyses). These authors found increased within-DMN connectivity, as found in healthy adults, but inconsistent with adult PTSD studies. These discrepancies between our results and those of Patriat et al. (2016) can be explained by the characteristics of our patient groups, in terms of origin, severity of trauma and percentage of full PTSD diagnosis. First, the origin of trauma in our sample was sexual abuse in the majority of cases (11/14 = 78%) compared to Patriat's sample (13/29 = 44%). Interpersonal trauma has been identified as the most severe type of childhood trauma compared to others (e.g., accident, death of a loved one, witness of suicide) (see McGloin and Widom, 2001; Fischer et al., 2016; Hagenaars et al., 2011). Second, using comparable tests (IESR in our study; PTSD-RI in Patriat et al., 2016), trauma severity was highest in our sample (mean severity score: 51, cut-off > 37) compared to Patriat's patients (mean severity score: 47, cut-off > 38). Third, with regard to DSM-5, 2013 criteria, 76% of patients met full diagnosis of PTSD in Patriat et al. (2016) versus 100% for patients in our study, indicating higher symptom severity in our sample. Hence, patient characteristics (origin, severity and percentage of full PTSD diagnosis) are clinically meaningful differences in trauma severity and indicate that our patients had a more severe PTSD compared to Patriat's group of patients, which may help to explain why our results mirror findings in adult PTSD, while Patriat's results bear similarities with healthy adults.
5. Limitations
Limitations of the current study should be mentioned. First, our sample size for the patient group was relatively small (N = 14) which has the risk that the study is under-powered. Our results should thus be confirmed in future studies with greater cohorts. Second, our sample was predominantly female and consisted of sexually-abused adolescents. This is characteristic of most resting state studies in adolescent PTSD in which more females than males are included (Patriat et al., 2016; Aghajani et al., 2016; Thomason et al., 2015; Suo et al., 2015). It is unknown whether the findings would generalize to males. We have re-analyzed the whole dataset including females only and results remain broadly the same (data not shown), suggesting that gender does not have an effect on the present results. Indeed, it has been previously shown that sex differences do not have major effects on DMN connectivity in healthy controls (Bluhm et al., 2009). Nevertheless, to account for this imbalance between groups, sex was added as nuisance variable in all analyses to control the gender effect. Third, to test if the age of participants could have a differential effect on each group, we performed two different analyses: setting age as a covariate of interest and performing mediation analyses. Both analyses showed that age does not have a significant effect on our results, neither on the patient nor on the control group (data not shown). Finally, the direct group comparisons and correlation analyses used an uncorrected height threshold (p < .001) associated to a corrected extent threshold (p < .05), commonly used in resting state studies on adolescent PTSD (Patriat et al., 2016; Aghajani et al., 2016; Nooner et al., 2013). It will be important for future studies to validate these findings with other statistical methods.
6. Conclusions
In summary, we report functional connectivity alterations selective to the PCC subsystem of the DMN in adolescent PTSD. Specifically, PTSD showed reduced within-DMN functional connectivity between the PCC and occipital cortex compared to controls and connectivity between the PCC and hippocampus was associated to increased trauma severity and anxiety. In addition, we found disruptions in functional connectivity between the DMN (PCC), SN (insula, ACC) and CEN (lateral PFC, cerebellum) associated to episodic memory deficits. Our results corroborate abnormal network properties previously reported in PTSD adults. Hence, both in adolescent and adult populations, PTSD pathophysiology is associated to a disruption in within-DMN connectivity, subserving internally-focused thought, and connectivity to other areas subserving salience detection (SN) and executive control (CEN). The association between decreased within-DMN connectivity and disrupted DMN-SN and DMN-CEN coupling could form the basis for intrusive trauma recollection and impaired episodic autobiographical recall in PTSD. This disrupted DMN connectivity observed in both adults and adolescents could thus represent a hallmark of PTSD leading to disrupted episodic memory and PTSD symptomatology.
Acknowledgments
This study was supported by the University Hospital of Caen, the Caen School District and the Mutuelle Générale de l'Education Nationale (insurance company). We would like to thank C. Lebouleux, M.H. Noël and M.C. Onfroy for their help in data acquisition, F. Dégeilh, S. Egret and F. Mézenge for assistance in testing participants and P. Gagnepain, M. Naveau and B. Landeau for programming skills. We are also thankful to the adolescents and institutions that took part in our research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101731.
Appendix A. Supplementary data
Supplementary material
References
- Aghajani M., Veer I.M., van Hoof M.J., Rombouts S.A., van der Wee N.J., Vermeiren R.R. Abnormal functional architecture of amygdala-centered networks in adolescent posttraumatic stress disorder. Hum. Brain Mapp. 2016;37:1120–1135. doi: 10.1002/hbm.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura-Brack A.S., Heinrichs-Graham E., McDermott T.J., Becker K.M., Ryan T.J., Khanna M.M., Wilson T.W. Resting-state neurophysiological abnormalities in posttraumatic stress disorder: a magnetoencephalography study. Front. Hum. Neurosci. 2017;11:205. doi: 10.3389/fnhum.2017.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Patriat R., Phillips M.L., Germain A., Herringa R.J. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress. Anxiety. 2014;31:880–892. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.A., Frewen P.A., Stevens T.K., Boksman K., Neufeld R.W., Théberge J., Lanius R.A. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatry Neurosci. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Vythilingam M., Vermetten E., Southwick S.M., McGlashan T., Nazeer A., Khan S., Vaccarino L.V., Soufer R., Garg P.K., Ng C.K., Staib L.H., Duncan J.S., Charney D.S. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatr. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(2) [Google Scholar]
- Brewin C.R., Gregory J.D., Lipton M., Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol. Rev. 2010;117:210–232. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A., St-Hilaire A., Jehel L., King S. Validation of a French version of the impact of event scale-revised. Can. J. Psychiatr. 2003;48:56–61. doi: 10.1177/070674370304800111. [DOI] [PubMed] [Google Scholar]
- Chai X.J., Castanon A.N., Ongur D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Ofen N., Gabrieli J.D., Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cogn. Neurosci. 2014;26:501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Scott Steele J., Smitherman S., Lenow J.K., Kilts C.D. Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: a network-level analysis among adolescent girls. Psychiatry Res. 2013;214:238–246. doi: 10.1016/j.pscychresns.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. The Psychological Corporation, Texas; San Antonio: 1997. Children's Memory Scale. [Google Scholar]
- Crane C., Heron J., Gunnell D., Lewis G., Evans J., Williams J.M. Childhood traumatic events and adolescent overgeneral autobiographical memory: findings in a U.K. cohort. J. Behav. Ther. Exp. Psychiatry. 2014;45:330–338. doi: 10.1016/j.jbtep.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels J.K., McFarlane A.C., Bluhm R.L., Moores K.A., Clark C.R., Shaw M.E., Williamson P.C., Densmore M., Lanius R.A. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J. Psychiatry Neurosci. 2010;35:258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dégeilh F., Viard A., Guénolé F., Gaubert M., Egler P.J., Egret S., Gerardin P., Baleyte J.M., Eustache F., Dayan J., Guillery-Girard B. Functional brain alterations during self-reference processing in adolescents with sexual abuse-related post-traumatic stress disorder: a preliminary report. Neurocase. 2017;23:52–59. doi: 10.1080/13554794.2017.1290807. [DOI] [PubMed] [Google Scholar]
- DSM-5 . American Psychiatric Publishing Incorporated; Washington DC: 2013. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Dugas M., Bouvard M. French version of the children depression inventory. In: Guelfi J.D., editor. L'évaluation clinique standardisée en psychiatrie. Pierre Fabre, Boulogne. 1996. pp. 543–548. [Google Scholar]
- Dunkley B.T., Doesburg S.M., Sedge P.A., Grodecki R.J., Shek P.N., Pang E.W., Taylor M.J. Resting-state hippocampal connectivity correlates with symptom severity in post-traumatic stress disorder. Neuroimage Clin. 2014;5:377–384. doi: 10.1016/j.nicl.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley B.T., Doesburg S.M., Jetly R., Sedge P.A., Pang E.W., Taylor M.J. Characterising intra- and inter-intrinsic network synchrony in combat-related post-traumatic stress disorder. Psychiatry Res. 2015;234:172–181. doi: 10.1016/j.pscychresns.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Ehlers A., Clark D.M. A cognitive model of posttraumatic stress disorder. Behav. Res. Ther. 2000;38:319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J. New York State Psychiatric Institute, Biometrics Research; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I DSM-IV Disorders, Research Version, Patient Edition (SCID-I/P) [Google Scholar]
- Fischer S., Dölitzsch C., Schmeck K., Fegert J.M. Interpersonal trauma and associated psychopathology in girls and boys living in residential care. Child Youth Serv. Rev. 2016;67:203–211. [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. Structural plasticity. In: Andersen P., Morris R., Amaral D., Bliss T., O'Keefe J., editors. The Hippocampus Book. Oxford University Press; New York: 2007. pp. 321–341. [Google Scholar]
- Guillery-Girard B., Clochon P., Giffard B., Viard A., Egler P.J., Baleyte J.M., Eustache F., Dayan J. “Disorganized in time”: impact of bottom-up and top-down negative emotion generation on memory formation among healthy and traumatized adolescents. J. Physiol. Paris. 2013;107:247–254. doi: 10.1016/j.jphysparis.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V., Greicius M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars M.A., Fisch I., van Minnen A. The effect of trauma onset and frequency on PTSD-associated symptoms. J. Affect. Disord. 2011;132:192–199. doi: 10.1016/j.jad.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Hayes J.P., LaBar K.S., McCarthy G., Selgrade E., Nasser J., Dolcos F., VISN 6 Mid-Atlantic MIRECC Workgroup, Morey R.A. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J. Psychiatr. Res. 2011;45:660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.A., Stodola D.E., Davidson R.J., Essex M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. U. S. A. 2013;110:19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.X., Yurgil K.A., Robb A., Angeles A., Diwakar M., Risbrough V.B., Nichols S.L., McLay R., Theilmann R.J., Song T., Huang C.W., Lee R.R., Baker D.G. Voxel-wise resting-state MEG source magnitude imaging study reveals neurocircuitry abnormality in active-duty service members and veterans with PTSD. Neuroimage Clin. 2014;5:408–419. doi: 10.1016/j.nicl.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K.C., Shin L.M. Functional neuroimaging studies of post-traumatic stress disorder. Expert. Rev. Neurother. 2011;11(2):275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperatori C., Farina B., Quintiliani M.I., Onofri A., Castelli Gattinara P., Lepore M., Gnoni V., Mazzucchi E., Contardi A., Della Marca G. Aberrant EEG functional connectivity and EEG power spectra in resting state post-traumatic stress disorder: a sLORETA study. Biol. Psychol. 2014;102:10–17. doi: 10.1016/j.biopsycho.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Kavanaugh B.C., Dupont-Frechette J.A., Jerskey B.A., Holler K.A. Neurocognitive deficits in children and adolescents following maltreatment: neurodevelopmental consequences and neuropsychological implications of traumatic stress. Appl. Neuropsychol. Child. 2017;6:64–78. doi: 10.1080/21622965.2015.1079712. [DOI] [PubMed] [Google Scholar]
- Keding T.J., Herringa R.J. Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology. 2015;40:537–545. doi: 10.1038/npp.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica. 1981;46:305–315. [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R.L., Coupland N.J., Hegadoren K.M., Rowe B., Théberge J., Neufeld R.W., Williamson P.C., Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr. Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Yoon S., Kim J.I., Jin S.H., Chung C.K. Functional connectivity of resting state EEG and symptom severity in patients with post-traumatic stress disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;51:51–57. doi: 10.1016/j.pnpbp.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Lobbestael J., Leurgans M., Arntz A. Inter-rater reliability of the structured clinical interview for DSM-IV axis I disorders (SCID I) and axis II disorders (SCID II) Clin. Psychol. Psychother. 2011;18:75–79. doi: 10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- Lui S., Huang X., Chen L., Tang H., Zhang T., Li X., Kuang W., Chan R.C., Mechelli A., Sweeney J.A., Gong Q. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proc. Natl. Acad. Sci. U. S. A. 2009;106:15412–15417. doi: 10.1073/pnas.0812751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGloin J.M., Widom C.S. Resilience among abused and neglected children grown up. Dev. Psychopathol. 2001;13:1021–1038. doi: 10.1017/s095457940100414x. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevel K., Landeau B., Fouquet M., La Joie R., Villain N., Mézenge F., Perrotin A., Eustache F., Desgranges B., Chételat G. Age effect on the default mode network, inner thoughts, and cognitive abilities. Neurobiol. Aging. 2013;34:1292–1301. doi: 10.1016/j.neurobiolaging.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Miller D.R., Hayes S.M., Hayes J.P., Spielberg J.M., Lafleche G., Verfaellie M. Default mode network subsystems are differentially disrupted in posttraumatic stress disorder. Biol. Psychiatry. 2017;2:363–371. doi: 10.1016/j.bpsc.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Pfeiffer C., Schick M., Schulte-Vels T., O'Gorman R., Michels L., Martin-Soelch C., Blair J.R., Rufer M., Schnyder U., Zeffiro T., Hasler G. Atypical visual processing in posttraumatic stress disorder. Neuroimage Clin. 2013;3:531–538. doi: 10.1016/j.nicl.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner K.B., Mennes M., Brown S., Castellanos F.X., Leventhal B., Milham M.P., Colcombe S.J. Relationship of trauma symptoms to amygdala-based functional brain changes in adolescents. J. Trauma. Stress. 2013;26:784–787. doi: 10.1002/jts.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle C.M., Block S.D., Harris L.S., Goodman G.S., Pineda A., Timmer S., Urquiza A., Saywitz K.J. Autobiographical memory specificity in child sexual abuse victims. Dev. Psychopathol. 2013;25:321–332. doi: 10.1017/S0954579412001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuch E.A., Benson B., Geraci M., Podell D., Herscovitch P., McCann U.D., Post R.M. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol. Psychiatry. 2001;50(4):246–253. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- Patel R., Spreng R.N., Shin L.M., Girard T.A. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Patriat R., Birn R.M., Keding T.J., Herringa R.J. Default-mode network abnormalities in pediatric posttraumatic stress disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55:319–327. doi: 10.1016/j.jaac.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel C., Viard A., André C., Guénolé F., de Flores R., Baleyte J.M., Gerardin P., Eustache F., Dayan J., Guillery-Girard B. Hippocampal subfields alterations in adolescents with post-traumatic stress disorder. Hum. Brain Mapp. 2019;40:1244–1252. doi: 10.1002/hbm.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L.D., Wang Z., Sun Y.W., Wan J.Q., Su S.S., Zhou Y., Xu J.R. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Brain Res. 2012;1484:50–56. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Shin L.M., Phelps E.A. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol. Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Reuveni I., Bonne O., Giesser R., Shragai T., Lazarovits G., Isserles M., Schreiber S., Bick A.S., Levin N. Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum. Brain Mapp. 2016;37:589–599. doi: 10.1002/hbm.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C.R., Richmond B.O. What I think and feel: a revised measure of children's manifest anxiety. J. Abnorm. Child Psychol. 1997;25:15–20. doi: 10.1023/a:1025751206600. [DOI] [PubMed] [Google Scholar]
- Reynolds C.R., Richmond B.O., Castro D. Les Éditions du Centre de Psychologie Appliquée; Paris: 1999. Echelle Révisée d'Anxiété Manifeste pour Enfants (R-CMAS) [Google Scholar]
- Scott J.C., Matt G.E., Wrocklage K.M., Crnich C., Jordan J., Southwick S.M., Krystal J.H., Schweinsburg B.C. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull. 2015;141:105–140. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Shin P.S., Heckers S., Krangel T.S., Macklin M.L., Orr S.P., Lasko N., Segal E., Makris N., Richert K., Levering J., Schacter D.L., Alpert N.M., Fischman A.J., Pitman R.K., Rauch S.L. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14(3):292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- Siegel J.S., Power J.D., Dubis J.W., Vogel A.C., Church J.A., Schlaggar B.L., Petersen S.E. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 2014;35:1981–1996. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2005;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Suo X., Lei D., Li K., Chen F., Li F., Li L., Huang X., Lui S., Li L., Kemp G.J., Gong Q. Disrupted brain network topology in pediatric posttraumatic stress disorder: a resting-state fMRI study. Hum. Brain Mapp. 2015;36:3677–3686. doi: 10.1002/hbm.22871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Marusak H.A., Tocco M.A., Vila A.M., McGarragle O., Rosenberg D.R. Altered amygdala connectivity in urban youth exposed to trauma. Soc. Cogn. Affect. Neurosci. 2015;10:1460–1468. doi: 10.1093/scan/nsv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A., Piolino P., Desgranges B., Chételat G., Lebreton K., Landeau B., Young A., de La Sayette V., Eustache F. Hippocampal activation for autobiographical memories over the entire lifetime in healthy aged subjects: an fMRI study. Cereb. Cortex. 2007;17:2453–2567. doi: 10.1093/cercor/bhl153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A., Desgranges B., Eustache F., Piolino P. Factors affecting medial temporal lobe engagement for past and future episodic events: an ALE meta-analysis of neuroimaging studies. Brain Cogn. 2012;80:111–125. doi: 10.1016/j.bandc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Villain N., Landeau B., Groussard M., Mevel K., Fouquet M., Dayan J., Eustache F., Desgranges B., Chételat G. A simple way to improve anatomical mapping of functional brain imaging. J. Neuroimaging. 2010;20:324–333. doi: 10.1111/j.1552-6569.2010.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. AFNI 3dDeconvolve Documentation. Medical College of Wisconsin; 2000. Simultaneous inference for fMRI data. [Google Scholar]
- Weiss D.S., Marmar C.R. In: Assessing Psychological Trauma and PTSD: A practitioner's Handbook. Wilson J.P., Keane T.M., editors. Guilford Press; New York City: 1997. pp. 399–411. The impact of event scale-revised. [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Wu R.Z., Zhang J.R., Qiu C.J., Meng Y.J., Zhu H.R., Gong Q.Y., Huang X.Q., Zhang W. Study on resting state default mode network in patients with posttraumatic stress disorder after the earthquake. Sichuan Da Xue Xue Bao Yi Xue Ban. 2011;42:397–400. [PubMed] [Google Scholar]
- Zhang Y., Liu F., Chen H., Li M., Duan X., Xie B., Chen H. Intranetwork and internetwork functional connectivity alterations in post-traumatic stress disorder. J. Affect. Disord. 2015;187:114–121. doi: 10.1016/j.jad.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Zlomuzica A., Dere D., Machulska A., Adolph D., Dere E., Margraf J. Episodic memories in anxiety disorders: clinical implications. Front. Behav. Neurosci. 2014;8:131. doi: 10.3389/fnbeh.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


