Abstract
Background
A population-based estimate of risk of second primary malignancy (SPM) in patients with cholangiocarcinoma (CCA) is still lacking.
Objectives
To investigate the overall and site-specific risk of SPM in patients with CCA. To identify risk factors of SPM and further evaluate the impact of SPM on overall survival (OS) and disease specific survival (DSS) in patients with CCA.
Methods
Patients with histologically diagnosed CCA between 1973 and 2015 were identified from the Surveillance, Epidemiology and End Results database. Standardized incidence ratio (SIR) was calculated. Risk factors for SPM and CCA survival were evaluated by logistic, Cox, and nomogram methods.
Results
We found that the overall risk of SPM in patients with CCA was significantly higher than that in the general population (SIR =1.27, 95% CI =1.03–1.55, P<0.05). The risk of SPM was significantly increased at specific sites, including transverse colon, intrahepatic bile duct, other biliary, and thyroid. A significant increase in overall risk was characterized in the subgroups of patients aged ≤29, patients aged 30–59 years, females, whites, and patients with latency ≤11 months (63.41, 2.45, 1.4, 1.3, and 2.6-fold, respectively). In patients with CCA, not having undergone surgery for the first primary malignancy (vs having undergone surgery for the first primary malignancy; HR =0.269; 95% CI =0.211–0.342; P<0.001) was associated with significantly decreased risk of SPM. Patients with SPM had better OS and DSS than those without SPM (Log rank P<0.001). Absence of SPM was an independent risk factor for poorer OS and DSS.
Conclusion
Although the risk of SPM in patients with CCA was significantly increased, the presence of SPM did not shorten OS and DSS of patients with CCA, possibly due to the relatively poorer survival of patients with CCA.
Keywords: cholangiocarcinoma, second primary malignancy, multiple primaries-standardized incidence ratio, SEER, nomogram
Introduction
Cholangiocarcinoma (CCA), which arises in the bile duct, is the second most common primary hepatobiliary malignancy.1,2 According to the anatomic site, CCA is classified as intrahepatic, perihilar, and distal.3 The incidence of CCA is increasing, especially intrahepatic CCA. Analyses of Surveillance, Epidemiology and End Results (SEER) data from 1973–2012 reported that the incidence of intrahepatic CCA in the US increased from 0.44–1.18 cases per 100,000, representing an annual percentage (APC) of 2.30%; this trend has accelerated during the past decade to an APC of 4.36%. The incidence of extrahepatic CCA has increased modestly from 0.95–1.02 cases per 100,000 during the 40-year period (APC, 0.14%).4 Although CCA is usually considered as highly malignant with poor prognosis, survival has improved due to advances in early diagnosis and therapeutic approaches in the past few years. Five-year survival rates following radical surgery in patients with intrahepatic CCA, perihilar CCA, and distal CCA are in the range of 17%–48%, 22%–60%, and 27%–62%, respectively.5–7 Several studies have shown improved survival of patients with CCA after locoregional therapies (including ablation, arterially directed therapies, and external beam radiotherapy) and gemcitabine-based systemic therapy.8–12 As the number of cancer survivors increases, it is important to evaluate the risk of second primary malignancy (SPM) in CCA survivors, which can provide insight into post-treatment surveillance of patients with CCA. Previous studies have shown significantly increased risk of SPM after treatment of primary cancer in various solid tumors, including hepatocellular carcinoma, the most frequent primary hepatic malignancy.13–17 However, evaluation of the risk of developing SPM in patients with CCA is still lacking. Our study aimed to investigate the overall and site-specific risk of SPM in patients with CCA and further evaluate the impact of SPM on overall survival (OS) and disease specific survival (DSS) in patients with CCA.
Established risk factors for CCA development include primary sclerosing cholangitis, hepatobiliary parasites, hepatolithiasis, Caroli disease, choledochal cysts and Thorotrast. Other possible risk factors are hepatitis B virus or hepatitis C virus infection, diabetes mellitus, obesity, alcohol use (>80 g/day), and tobacco.18 However, risk factors for development of SPM in patients with CCA are unknown. In the current study, we also aimed to identify risk factors of SPM in patients with CCA. These risk factors could help us to intensify surveillance of high-risk patients.
The SEER database includes information on cancer incidence, treatment, and survival for ~30% of the US population.19 Using data from the SEER database, we conducted this study to examine whether patients diagnosed with CCA have an increased risk of SPM compared with patients without an initial CCA diagnosis. Additionally, we conducted this study to identify risk factors for developing SPM and to determine the impact of SPM on patient survival.
Methods
Patient selection
The dataset ‘incidence-SEER 9 Regs Research Data, Nov 2017 Sub (1973–2015)< Katrina/Rita population adjustment>’ was used for analysis of multiple primaries-standardized incidence ratio (MP-SIR). Case listing was based on dataset ‘incidence-SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2017 Sub (1973–2015 varying)’. Patients were selected based on the following inclusion criteria: 1) diagnostic confirmation by positive histology; 2) histology indicates CCA; and 3) CCA that presents as the first of two or more primaries. Patients in whom the diagnosis of CCA was made at autopsy or on the death certificate were excluded.
Definition
According to the SEER definition of multiple primary tumors, the following criteria were used in our study: 1) tumors with ICD-O-3 histology codes that are different at the first, second or third number are multiple primaries; 2) one tumor characterized as “adenocarcinoma, NOS” and another as a specific adenocarcinoma is regarded as a single tumor; 3) an invasive tumor following an in situ tumor >60 days after diagnosis is a multiple primary; 4) tumors with ICD-O-3 topography codes that are different at the second and/or third characters are multiple primaries; 5) tumors diagnosed >1 year apart are multiple primaries. Latency was defined as the interval from the diagnosis of CCA to that of SPM. Standardized incidence ratio (SIR), an indicator of SPM risk, was calculated by dividing the observed number of SPM cases by the expected number based on general population rates.
Statistical analyses
Data from the SEER database were retrieved using the SEER*Stat version 8.3.5 (http://seer.cancer.gov/seerstat/). A two-tailed Student’s t-test or a two-way ANOVA was used to compare the mean values for continuous variables, while a chi-squared test was applied to categorical variables. Multivariable logistic regression was used to determine the effect of individual factors on the presence of SPM. Survival estimates were obtained using the Kaplan–Meier method. Multivariable Cox regression was performed to identify covariates associated with increased all-cause mortality. A P-value <0.05 was considered significant. Statistical analysis was performed using the PASW Statistics 23 software program (Release 23.0, IBM Corporation, Armonk, NY, USA) with the exception of the nomogram, which was performed in R (version 3.5.1; R Foundation).
Results
Risk of developing SPM in patients with CCA
The overall risk of developing SPM in patients with CCA was significantly higher than that in the general population (SIR 1.27, 95% CI 1.03–1.55, P<0.05). Specific sites where the risk of SPM was significantly increased included transverse colon, intrahepatic bile duct, other biliary sites, and thyroid. The risk of SPM in other sites did not change significantly in patients with CCA (Table 1).
Table 1.
Site-specific risk of developing SPM in patients with cholangiocarcinoma
| Site of second malignancy | Observed | Expected | SIR | 95% CI |
|---|---|---|---|---|
|
| ||||
| All sites | 96 | 75.66 | 1.27a | 1.03–1.55 |
| All solid tumors | 78 | 67.27 | 1.16 | 0.92–1.45 |
| Oral cavity and pharynx | 2 | 1.8 | 1.11 | 0.13–4.02 |
| Digestive system | 26 | 15.12 | 1.72a | 1.12–2.52 |
| Respiratory system | 15 | 11.98 | 1.25 | 0.7–2.07 |
| Bones and joints | 0 | 0.07 | 0 | 0–53.61 |
| Soft tissue including heart | 0 | 0.39 | 0 | 0–9.4 |
| Skin excluding basal and squamous | 1 | 3.21 | 0.31 | 0.01–1.74 |
| Breast | 9 | 9.36 | 0.96 | 0.44–1.83 |
| Female genital system | 2 | 3.70 | 0.54 | 0.07–1.95 |
| Male genital system | 11 | 13.23 | 0.83 | 0.41–1.49 |
| Urinary system | 8 | 6.52 | 1.23 | 0.53–2.42 |
| Eye and orbit | 0 | 0.11 | 0 | 0–32.46 |
| Brain and other nervous system | 0 | 0.77 | 0 | 0–4.78 |
| Endocrine system | 5 | 1.07 | 4.69a,b | 1.52–10.94 |
| Lymphoma | 6 | 3.33 | 1.8 | 0.66–3.92 |
| Leukemia | 3 | 2.11 | 1.42 | 0.29–4.16 |
| Mesothelioma | 0 | 0.2 | 0 | 0–18.88 |
| Kaposi sarcoma | 0 | 0.06 | 0 | 0–61.22 |
| Miscellaneous | 5 | 1.5 | 3.33a | 1.08–7.77 |
Note:
P<0.05; risk of SPM in transverse colon (SIR 5.42, 95% CI 1.12–15.84) and intrahepatic bile duct and other biliary (SIR 11.97, 95% CI 4.39–26.06) significantly increased;
risk of SPM in thyroid (SIR 5.05, 95% CI 1.64–11.79) significantly increased.
Abbreviations: SIR, standardized incidence ratio; SPM, second primary malignancy.
Impact of age at diagnosis, sex, race, and latency on the risk of SPM in patients with CCA
Since age at diagnosis, sex, race, and latency might be significant determinants of SPM development, we further evaluated the effect of these factors on the risk of developing SPM in patients with CCA. As shown in Table 2, patients aged ≤29 and patients aged 30–59 years had an increased overall risk of developing SPM (63.41 and 2.45-fold, respectively). A significant increase in overall risk was also characterized in the subgroups of females, whites, and patients with latency ≤11 months (1.4, 1.3, and 2.6-fold, respectively). Patients aged ≤29 years had significantly increased site-specific risk of developing SPM in descending colon (SIR 34,994.55, 95% CI 885.99–194,977.16). Specific sites where the risk of developing SPM was significantly increased in patients with CCA aged 30–59 years included hypopharynx, hepatic flexure, intrahepatic bile duct, and thyroid. Significantly increased site-specific risk of developing SPM in intrahepatic and extrahepatic bile ducts and other biliary sites, all lymphatic and hematopoietic diseases were evident in females and whites. Additionally, the risk of SPM in transverse colon was also found to be significantly increased in whites. Patients with latency ≤11 months carried significantly increased site-specific risk of SPM in esophagus, small intestine, descending colon, intrahepatic bile duct, other biliary sites, breast, prostate, kidney, and thyroid as well as non-Hodgkin lymphoma.
Table 2.
Effect of age at diagnosis, sex, race, and latency on the overall risk of developing SPM in patients with cholangiocarcinoma
| Parameters | Observed | Expected | SIR | 95% CI |
|---|---|---|---|---|
|
| ||||
| Age at diagnosis, | ||||
| years | ||||
| ≤29 | 1 | 0.02 | 63.41a | 1.61–353.32 |
| 30–59 | 24 | 9.81 | 2.45a,b | 1.57–3.64 |
| ≥60 | 71 | 65.84 | 1.08 | 0.84–1.36 |
| Sex | ||||
| Male | 51 | 43.45 | 1.17 | 0.87–1.54 |
| Female | 45 | 32.21 | 1.4a,c | 1.02–1.87 |
| Race | ||||
| White | 80 | 61.32 | 1.3a,d | 1.03–1.62 |
| Black | 4 | 5.68 | 0.7 | 0.19–1.8 |
| Other | 12 | 8.56 | 1.4 | 0.72–2.45 |
| Latency | ||||
| ≤11 months | 99 | 38.03 | 2.6a,e | 2.12–3.17 |
| 12–35 months | 31 | 23.48 | 1.32 | 0.9–1.87 |
| 36–59 months | 15 | 9.16 | 1.64 | 0.92–2.7 |
| 60–119 months | 8 | 9.83 | 0.81 | 0.35–1.6 |
| ≥120 months | 9 | 5.5 | 1.64 | 0.75–3.11 |
Notes:
P<0.05; risk of SPM in descending colon (SIR 34,994.55, 95% CI 885.99– 194,977.16) significantly increased;
risk of SPM in hypopharynx (SIR 47.08, 95% CI 1.19–262.31), hepatic flexure (SIR 46.68, 95% CI 1.18–260.11), intrahepatic bile duct (SIR 57.81, 95% CI 1.46–322.1), and thyroid (SIR 15, 95% CI 4.87–35.01) significantly increased;
risk of SPM in intrahepatic and extrahepatic bile ducts and other biliary sites (SIR 9.06, 95% CI 1.1–32.74) and all lymphatic and hematopoietic diseases (SIR 2.67, 95% CI 1.06–5.41) significantly increased;
risk of SPM in transverse colon (SIR 6.84, 95% CI 1.41–20), intrahepatic and extrahepatic bile ducts, and other biliary sites (SIR 11.13, 95% CI 3.03–28.5), thyroid (SIR 6.45, 95% CI 2.09–15.05) and all lymphatic and hematopoietic diseases (SIR 5.44, 95% CI 1.14–2.2) significantly increased;
risk of SPM in esophagus (SIR 9.38, 95% CI 2.56–24.03), small intestine (SIR 11.91, 95% CI 1.44–43.02), descending colon (SIR 11.22, 95% CI 1.36–40.55), intrahepatic bile duct (SIR 23.82, 95% CI 2.88–86.03), other biliary sites (SIR 18.68, 95% CI 3.85–54.59), breast (SIR 2.16, 95% CI 1.04–3.98), prostate (SIR 2.36, 95% CI 1.35–3.83), kidney (SIR 9.67, 95% CI 4.64–17.78), thyroid (SIR 6.15, 95% CI 1.27–17.96) and non-Hodgkin lymphoma (SIR 6.15, 95% CI 1.27–17.96) significantly increased.
Abbreviations: SIR, standardized incidence ratio; SPM, second primary malignancy.
Clinicopathologic characteristics of patients
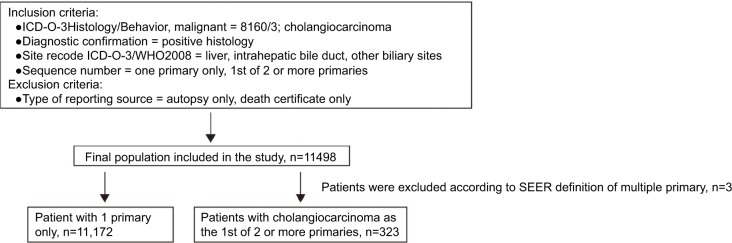
From the case listing section of SEER database, we identified 11,495 patients with CCA, including 11,172 patients with only one primary and 323 patients with CCA as the first of two or more primaries. The detailed patient selection procedure is displayed in Figure 1. Clinicopathologic characteristics of patients with CCA as the lone primary and patients with CCA as the first of two or more primaries are summarized in Table 3. In the group of patients with CCA and SPM, 92.88% (300/323) of patients developed two primaries, and 7.12% (23/323) of patients developed at least three primaries. Mean latency for patients with CCA to develop SPM was 23.73 months. Of SPMs, 19.2% (62/323) were diagnosed at distant stage. Additionally, there were significant differences in sex, grade of tumor tissues, SEER histological stage, American Joint Committee on Cancer (AJCC) sixth stage, tumor size, and surgery at the first primary site between the two subgroups. To validate whether the difference was caused by the difference in sample size between the two subgroups, we randomly chose 323 patients from the group of patients with only one primary, then compared the clinicopathologic characteristics in the two subgroups, which were now matched in number of cases. This analysis revealed similar results (Table 4).
Figure 1.
Flowchart of patient selection.
Abbreviation: SEER, Surveillance, Epidemiology and End Results.
Table 3.
Clinicopathological characteristics of patients with cholangiocarcinoma as the lone primary and those with cholangiocarcinoma as the first of two or more primaries
| One primary, n=11,172 | First of two or more primaries, n=323 | P-value | |
|---|---|---|---|
|
| |||
| Age at diagnosis, years | 64.96±12.64 | 64.73±12.25 | 0.743 |
| Sex, n (%) | 0.048 | ||
| Male | 5,769 (51.64) | 185 (57.28) | |
| Female | 5,403 (48.36) | 138 (42.72) | |
| Race, n (%) | 0.546 | ||
| White | 8,751 (78.33) | 262 (81.11) | |
| Black | 901 (8.06) | 20 (6.19) | |
| Other | 1,510 (13.52) | 41 (12.69) | |
| Unknown | 10 (0.09) | 0 (0) | |
| Marital status, n (%) | 0.87 | ||
| Married | 6,651 (59.53) | 197 (60.99) | |
| Unmarried | 4,132 (36.99) | 115 (35.6) | |
| Unknown | 389 (3.48) | 11 (3.41) | |
| Location, n (%) | 0.278 | ||
| Intrahepatic | 7,775 (69.59) | 222 (68.73) | |
| Perihilar | 2,362 (21.14) | 79 (24.46) | |
| Distal | 516 (4.62) | 12 (3.72) | |
| Other | 519 (4.65) | 10 (3.1) | |
| Grade, n (%) | <0.001 | ||
| Grade I, well differentiated | 605 (5.42) | 29 (8.98) | |
| Grade II, moderately differentiated | 2,322 (20.78) | 99 (30.65) | |
| Grade III, poorly differentiated | 2,153 (19.27) | 59 (18.27) | |
| Grade IV, undifferentiated | 85 (0.76) | 1 (0.31) | |
| Unknown | 6,007 (53.77) | 135 (41.8) | |
| SEER histological stage, n (%) | <0.001 | ||
| Localized | 2,320 (20.77) | 105 (32.51) | |
| Regional | 3,432 (30.72) | 107 (33.13) | |
| Distant | 4,422 (39.58) | 75 (23.22) | |
| Unknown | 998 (8.93) | 36 (11.15) | |
| AJCC sixth stage, n (%) | <0.001 | ||
| I | 1,137 (10.18) | 50 (15.48) | |
| II | 485 (4.34) | 31 (9.6) | |
| III | 2,098 (18.78) | 58 (17.96) | |
| IV | 3,280 (29.36) | 49 (15.17) | |
| Unknown | 4,172 (37.34) | 135 (41.8) | |
| Tumor size, n (%) | <0.001 | ||
| <5 cm | 2,009 (17.98) | 90 (27.86) | |
| 5–10 cm | 1,916 (17.15) | 52 (16.56) | |
| >10 cm | 750 (6.71) | 13 (3.99) | |
| Unknown | 6,497 (58.15) | 168 (52.01) | |
| Surgery for first primary site, n (%) | <0.001 | ||
| Yes | 2,502 (22.40) | 157 (48.61) | |
| No | 7,122 (63.75) | 120 (37.15) | |
| Unknown | 1,548 (13.86) | 46 (14.24) | |
| Latency | – | 23.73±40.83 | |
| Number of primaries, n (%) | |||
| 2 primaries | – | 300 (92.88) | |
| 3 primaries | – | 18 (5.57) | |
| 4 primaries | – | 4 (1.24) | |
| 5 primaries | – | 1 (0.31) | |
| SEER histological stage of SPM, n (%) | |||
| Distant | – | 62 (19.2) | |
| No distant | – | 184 (56.97) | |
| Unknown | – | 77 (23.84) | |
Abbreviations: AJCC, American Joint Committee on Cancer; SEER, Surveillance, Epidemiology and End Results; SPM, second primary malignancy.
Table 4.
Clinicopathological characteristics of patients with cholangiocarcinoma as the only primary and patients with cholangiocarcinoma as the first of two or more primaries (matched number of cases)
| Parameters | One primary, n=323 | First of two or more primaries, n=323 | P-value |
|---|---|---|---|
|
| |||
| Age at diagnosis, years (mean ± SD) | 64.76±12.47 | 64.73±12.25 | 0.972 |
| Sex, n (%) | 0.027 | ||
| Male | 156 (48.3) | 185 (57.28) | |
| Female | 167 (51.7) | 138 (42.72) | |
| Race, n (%) | 0.114 | ||
| White | 241 (74.61) | 262 (81.11) | |
| Black | 31 (9.6) | 20 (6.19) | |
| Other | 51 (15.79) | 41 (12.69) | |
| Marital status, n (%) | 0.697 | ||
| Married | 188 (58.20) | 197 (60.99) | |
| Unmarried | 121 (37.46) | 115 (35.6) | |
| Unknown | 14 (4.33) | 11 (3.41) | |
| Location, n (%) | 0.440 | ||
| Intrahepatic | 236 (73.07) | 222 (68.73) | |
| Perihilar | 62 (19.20) | 79 (24.46) | |
| Distal | 13 (4.02) | 12 (3.72) | |
| Others | 12 (3.72) | 10 (3.1) | |
| Grade, n (%) | 0.001 | ||
| Grade I, well differentiated | 17 (5.26) | 29 (8.98) | |
| Grade II, moderately differentiated | 65 (20.12) | 99 (30.65) | |
| Grade III, poorly differentiated | 58 (17.96) | 59 (18.27) | |
| Grade IV, undifferentiated | 3 (0.93) | 1 (0.31) | |
| Unknown | 180 (55.73) | 135 (41.8) | |
| SEER histological stage, n (%) | <0.001 | ||
| Localized | 63 (19.50) | 105 (32.51) | |
| Regional | 122 (37.77) | 107 (33.13) | |
| Distant | 118 (36.53) | 75 (23.22) | |
| Unknown | 20 (6.19) | 36 (11.15) | |
| AJCC sixth stage, n (%) | <0.001 | ||
| I | 32 (9.91) | 50 (15.48) | |
| II | 6 (1.86) | 31 (9.6) | |
| III | 70 (21.67) | 58 (17.96) | |
| IV | 91 (28.17) | 49 (15.17) | |
| Unknown | 124 (38.39) | 135 (41.8) | |
| Tumor size, n (%) | 0.007 | ||
| <5 cm | 54 (16.72) | 90 (27.86) | |
| 5–10 cm | 61 (18.89) | 52 (16.56) | |
| >10 cm | 19 (5.88) | 13 (3.99) | |
| Unknown | 189 (58.51) | 168 (52.01) | |
| Surgery for first primary site, n (%) | <0.001 | ||
| Yes | 66 (20.43) | 157 (48.61) | |
| No | 213 (65.94) | 120 (37.15) | |
| Unknown | 44 (13.62) | 46 (14.24) | |
Abbreviations: AJCC, American Joint Committee on Cancer; SEER, Surveillance, Epidemiology and End Results.
Risk factors of developing SPM in patients with CCA
To determine risk factors of developing SPM in patients with CCA, we further performed multivariable logistic regression. As shown in Table 5, not having undergone surgery for the first primary (vs having undergone surgery for the first primary; HR, 0.269; 95% CI, 0.211–0.342; P<0.001) was associated with significantly decreased risk of developing SPM. Factors including age, sex, race, marital status, tumor location, grade, SEER histological stage, AJCC sixth stage, and tumor size were not able to predict the development of SPM.
Table 5.
Multivariable logistic regression for the presence of multiple primary malignancies after cholangiocarcinoma diagnosis
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
| ||||
| Age at diagnosis, years | ||||
| ≤29 | 1 [Reference] | |||
| 30–59 | 0.621 (0.192–2.01) | 0.427 | / | / |
| ≥60 | 0.654 (0.204–2.095) | 0.475 | / | / |
| Sex | ||||
| Male | 1 [Reference] | |||
| Female | 0.796 (0.637–0.996) | 0.046 | / | / |
| Race | ||||
| White | 1 [Reference] | |||
| Black | 0.741 (0.468–1.174) | 0.202 | / | / |
| Other | 0.907 (0.650–1.266) | 0.566 | / | / |
| Unknown | 0 | 0.999 | / | / |
| Marital status | ||||
| Married | 1 [Reference] | |||
| Unmarried | 0.94 (0.744–1.186) | 0.601 | / | / |
| Unknown | 0.955 (0.516–1.767) | 0.883 | / | / |
| Location | ||||
| Intrahepatic | 1 [Reference] | |||
| Perihilar | 1.171 (0.902–1.521) | 0.235 | / | / |
| Distal | 0.814 (0.453–1.466) | 0.494 | / | / |
| Other | 0.675 (0.356–1.280) | 0.228 | / | / |
| Grade | ||||
| Grade I, well differentiated | 1 [Reference] | |||
| Grade II, moderately differentiated | 0.889 (0.582–1.358) | 0.588 | / | / |
| Grade III, poorly differentiated | 0.572 (0.363–0.9) | 0.016 | / | / |
| Grade IV, undifferentiated | 0.245 (0.033–1.825) | 0.17 | / | / |
| Unknown | 0.469 (0.311–0.706) | <0.001 | / | / |
| SEER histological stage | ||||
| Localized | 1 [Reference] | |||
| Regional | 0.689 (0.524–0.906) | 0.008 | / | / |
| Distant | 0.375 (0.277–0.506) | <0.001 | / | / |
| Unknown | 0.797 (0.542–1.172) | 0.249 | / | / |
| AJCC sixth stage | ||||
| I | 1 [Reference] | |||
| II | 1.453 (0.917–2.304) | 0.111 | / | / |
| III | 0.629 (0.428–0.924) | 0.018 | / | / |
| IV | 0.34 (0.228–0.507) | <0.001 | / | / |
| Unknown | 0.736 (0.528–1.025) | 0.069 | / | / |
| Tumor size | ||||
| <5 cm | 1 [Reference] | |||
| 5–10 cm | 0.606 (0.428–0.857) | 0.005 | / | / |
| >10 cm | 0.387 (0.215–0.696) | 0.002 | / | / |
| Unknown | 0.577 (0.445–0.749) | <0.001 | / | / |
| Surgery for first primary site | ||||
| Yes | 1 [Reference] | 1 [Reference] | ||
| No | 0.269 (0.211–0.342) | <0.001 | 0.269 (0.211–0.342) | <0.001 |
| Unknown | 0.474 (0.339–0.662) | <0.001 | 0.474 (0.339–0.662) | <0.001 |
Abbreviations: AJCC, American Joint Committee on Cancer; SEER, Surveillance, Epidemiology and End Results.
Prognostic impact of SPM on patients with CCA
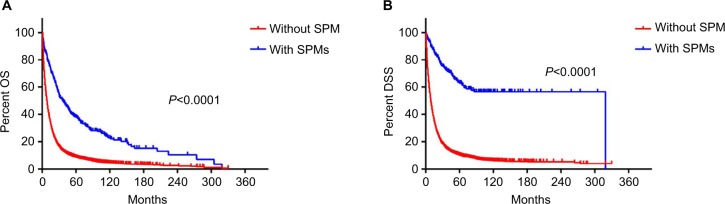
Having determined the incidence and site-specific risk of SPM, we next sought to investigate the effect of SPM on prognosis in patients with CCA. OS was better in patients with SPM than in patients without SPM (Log rank =189.618, P<0.001) (Figure 2A). Median OS and the corresponding 95% CI were 31 (23.773–38.227) months and 6 (5.741–6.259) months in patients with CCA with and without SPM, respectively. DSS was better in patients with SPM than in patients without SPM (Log rank =325.423, P<0.001) (Figure 2B). Median DSS and the corresponding 95% CI were 319 (not available) months and 7 (6.706–7.294) months in patients with CCA with and without SPM, respectively.
Figure 2.
OS and DSS in patients with cholangiocarcinoma with or without SPM.
Note: (A) OS and (B) DSS in patients with cholangiocarcinoma with or without SPM.
Abbreviations: DSS, disease specific survival; OS, overall survival; SPM, second primary malignancy.
We further performed multivariate Cox regression analysis to identify variables that might influence overall mortality and disease specific mortality of patients with CCA. As shown in Table 6, absence of SPM was an independent factor for poorer OS (HR, 2.009; 95% CI, 1.76–2.294; P<0.001). Variables that were significantly associated with increased overall mortality were age ≥60 years, black race, unmarried status, poorly differentiated/undifferentiated cancer tissues, regional/distant disease, AJCC III stage, tumor size >10 cm, and not having undergone surgery for the first primary. Female sex (vs male; HR, 0.837; 95% CI, 0.803–0.872; P<0.001) was significantly associated with reduced overall mortality. As shown in Table 7, absence of SPM was an independent factor for poorer DSS (HR, 4.011; 95% CI, 3.299–4.876; P<0.001). Variables that were significantly associated with increased disease-specific mortality were age ≥60 years, black race, unmarried status, distal CCA, moderately/poorly differentiated/undifferentiated cancer tissues, regional/distant disease, AJCC II/III/IV stage, tumor size >10 cm, and not having undergone surgery for the first primary. Female sex (vs male; HR, 0.854; 95% CI, 0.818–0.891; P<0.001) was significantly associated with reduced disease-specific mortality.
Table 6.
Multivariable Cox regression for OS among patients diagnosed with cholangiocarcinoma
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
| ||||
| Age at diagnosis, years | ||||
| ≤29 | 1 [Reference] | 1 [Reference] | ||
| 30–59 | 1.177 (0.911–1.520) | 0.212 | / | / |
| ≥60 | 1.550 (1.202–1.999) | 0.001 | 1.718 (1.331–2.219) | <0.001 |
| Sex | ||||
| Male | 1 [Reference] | 1 [Reference] | ||
| Female | 0.915 (0.88–0.952) | <0.001 | 0.837 (0.803–0.872) | <0.001 |
| Race | ||||
| White | 1 [Reference] | 1 [Reference] | ||
| Black | 1.107 (1.029–1.19) | 0.007 | 1.124 (1.044–1.21) | 0.002 |
| Other | 0.918 (0.866–0.974) | 0.005 | 0.967 (0.912–1.026) | 0.269 |
| Unknown | 0.595 (0.267–1.326) | 0.204 | / | / |
| Marital status | ||||
| Married | 1 [Reference] | 1 [Reference] | ||
| Unmarried | 1.227 (1.177–1.279) | <0.001 | 1.187 (1.137–1.239) | <0.001 |
| Unknown | 1.005 (0.898–1.125) | 0.931 | / | / |
| Location | ||||
| Intrahepatic | 1 [Reference] | |||
| Perihilar | 0.933 (0.889–0.979) | 0.005 | / | / |
| Distal | 0.759 (0.684–0.842) | <0.001 | / | / |
| Other | 1.324 (1.207–1.454) | <0.001 | / | / |
| Grade | ||||
| Grade I, well differentiated | 1 [Reference] | 1 [Reference] | ||
| Grade II, moderately differentiated | 1.078 (0.976–1.19) | 0.139 | / | / |
| Grade III, poorly differentiated | 1.545 (1.399–1.707) | <0.001 | 1.486 (1.344–1.642) | <0.001 |
| Grade IV, undifferentiated | 2.232 (1.76–2.831) | <0.001 | 1.697 (1.337–2.153) | <0.001 |
| Unknown | 1.964 (1.79–2.154) | <0.001 | 1.334 (1.215–1.466) | <0.001 |
| SEER histological stage | ||||
| Localized | 1 [Reference] | 1 [Reference] | ||
| Regional | 1.255 (1.184–1.332) | <0.001 | 1.118 (1.043–1.199) | 0.002 |
| Distant | 2.349 (2.219–2.487) | <0.001 | 1.68 (1.549–1.823) | <0.001 |
| Unknown | 1.895 (1.752–2.05) | <0.001 | 1.116 (1.022–1.22) | 0.015 |
| AJCC sixth stage | ||||
| I | 1 [Reference] | 1 [Reference] | ||
| II | 0.902 (0.791–1.028) | 0.123 | / | / |
| III | 1.454 (1.334–1.585) | <0.001 | 1.264 (1.142–1.399) | <0.001 |
| IV | 2.754 (2.54–2.985) | <0.001 | 1.107 (0.99–1.237) | 0.074 |
| Unknown | 2.317 (2.144–2.504) | <0.001 | 1.331 (1.206–1.469) | <0.001 |
| Tumor size | ||||
| <5 cm | 1 [Reference] | 1 [Reference] | ||
| 5–10 cm | 1.288 (1.198–1.385) | <0.001 | 1.045 (0.97–1.125) | 0.248 |
| >10 cm | 1.622 (1.476–1.783) | <0.001 | 1.178 (1.069–1.297) | 0.001 |
| Unknown | 2.029 (1.915–2.149) | <0.001 | 1.215 (1.134–1.301) | <0.001 |
| Surgery for first primary site | ||||
| Yes | 1 [Reference] | 1 [Reference] | ||
| No | 3.202 (3.03–3.383) | <0.001 | 2.435 (2.285–2.595) | <0.001 |
| Unknown | 3.23 (3.015–3.461) | <0.001 | 2.347 (2.162–2.549) | <0.001 |
| Number of primary malignancies | ||||
| Multiple primary malignancies | 1 [Reference] | 1 [Reference] | ||
| One primary malignancy | 2.382 (2.089–2.717) | <0.001 | 2.009 (1.76–2.294) | <0.001 |
Abbreviations: AJCC, American Joint Committee on Cancer; OS, overall survival; SEER, Surveillance, Epidemiology and End Results.
Table 7.
Multivariable Cox regression for DSS of patients diagnosed with cholangiocarcinoma
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
| ||||
| Age at diagnosis, years | ||||
| ≤29 | 1 [Reference] | 1 [Reference] | ||
| 30–59 | 1.137 (0.875–1.478) | 0.338 | / | / |
| ≥60 | 1.452 (1.119–1.885) | 0.005 | 1.603 (1.233–2.083) | <0.001 |
| Sex | ||||
| Male | 1 [Reference] | 1 [Reference] | ||
| Female | 0.936 (0.898–0.976) | 0.002 | 0.854 (0.818–0.891) | <0.001 |
| Race | ||||
| White | 1 [Reference] | 1 [Reference] | ||
| Black | 1.105 (1.024–1.193) | 0.01 | 1.122 (1.038–1.211) | 0.004 |
| Other | 0.912 (0.857–0.97) | 0.003 | 0.963 (0.906–1.025) | 0.239 |
| Unknown | 0.649 (0.291–1.444) | 0.289 | / | / |
| Marital status | ||||
| Married | 1 [Reference] | 1 [Reference] | ||
| Unmarried | 1.216 (1.165–1.27) | <0.001 | 1.173 (1.121–1.226) | <0.001 |
| Unknown | 0.954 (0.846–1.076) | 0.443 | / | / |
| Location | ||||
| Intrahepatic | 1 [Reference] | |||
| Perihilar | 0.917 (0.872–0.965) | 0.001 | 0.948 (0.898–1.002) | 0.058 |
| Distal | 0.708 (0.634–0.792) | <0.001 | 0.85 (0.758–0.954) | 0.006 |
| Other | 1.328 (1.206–1.463) | <0.001 | 1.031 (0.933–1.139) | 0.549 |
| Grade | ||||
| Grade I, well differentiated | 1 [Reference] | 1 [Reference] | ||
| Grade II, moderately differentiated | 1.125 (1.012–1.251) | 0.029 | 1.218 (1.095–1.354) | <0.001 |
| Grade III, poorly differentiated | 1.656 (1.489–1.84) | <0.001 | 1.587 (1.427–1.766) | <0.001 |
| Grade IV, undifferentiated | 2.233 (1.732–2.88) | <0.001 | 1.662 (1.288–2.145) | <0.001 |
| Unknown | 2.065 (1.87–2.28) | <0.001 | 1.386 (1.253–1.532) | <0.001 |
| SEER histological stage | ||||
| Localized | 1 [Reference] | 1 [Reference] | ||
| Regional | 1.279 (1.202–1.361) | <0.001 | 1.129 (1.049–1.216) | 0.001 |
| Distant | 2.441 (2.3–2.592) | <0.001 | 1.701 (1.562–1.852) | <0.001 |
| Unknown | 1.888 (1.737–2.052) | <0.001 | 1.097 (0.999–1.205) | 0.054 |
| AJCC sixth stage | ||||
| I | 1 [Reference] | 1 [Reference] | ||
| II | 0.887 (0.771–1.022) | 0.097 | / | / |
| III | 1.511 (1.38–1.655) | <0.001 | 1.313 (1.179–1.462) | <0.001 |
| IV | 2.924 (2.684–3.185) | <0.001 | 1.153 (1.026–1.296) | 0.017 |
| Unknown | 2.427 (2.235–2.635) | <0.001 | 1.384 (1.247–1.536) | <0.001 |
| Tumor size | ||||
| <5 cm | 1 [Reference] | 1 [Reference] | ||
| 5–10 cm | 1.343 (1.245–1.449) | <0.001 | 1.042 (0.962–1.128) | 0.312 |
| >10 cm | 1.707 (1.548–1.883) | <0.001 | 1.172 (1.058–1.298) | 0.002 |
| Unknown | 2.101 (1.977–2.233) | <0.001 | 1.222 (1.137–1.314) | <0.001 |
| Surgery for first primary site | ||||
| Yes | 1 [Reference] | 1 [Reference] | ||
| No | 3.366 (3.176–3.568) | <0.001 | 2.478 (2.317–2.651) | <0.001 |
| Unknown | 3.387 (3.149–3.643) | <0.001 | 2.365 (2.167–2.582) | <0.001 |
| Number of primary malignancies | ||||
| Multiple primary malignancies | 1 [Reference] | 1 [Reference] | ||
| One primary malignancy | 4.82 (3.967–5.857) | <0.001 | 4.011 (3.299–4.876) | <0.001 |
Abbreviations: AJCC, American Joint Committee on Cancer; DSS, disease specific survival; SEER, Surveillance, Epidemiology and End Results.
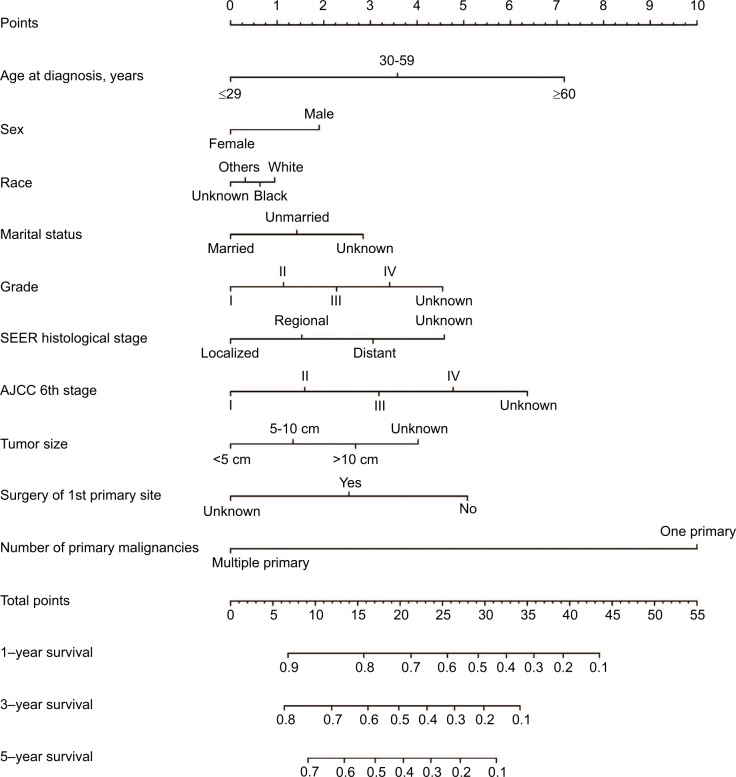
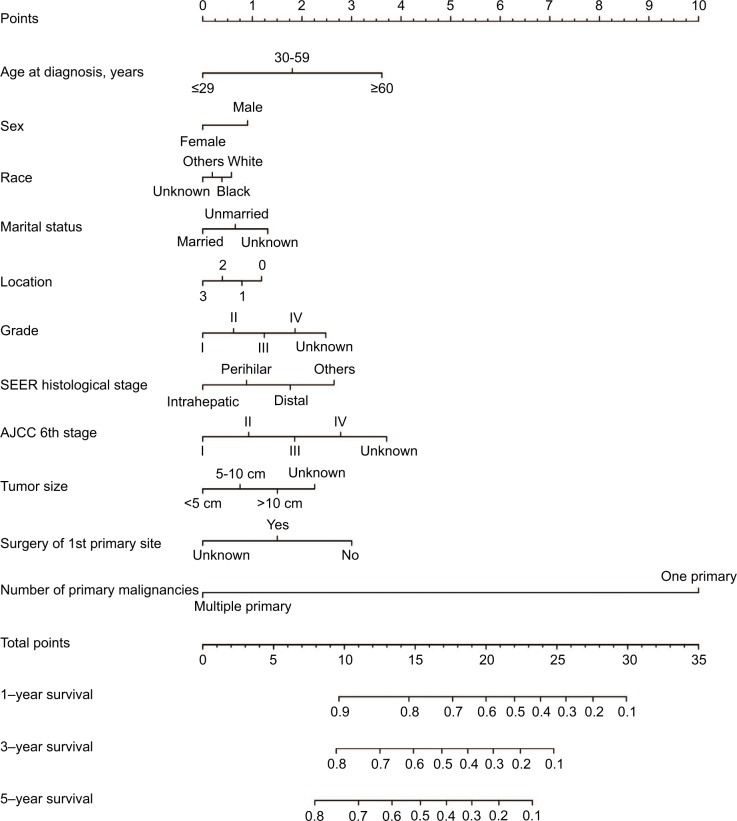
Prognostic nomogram for OS and DSS
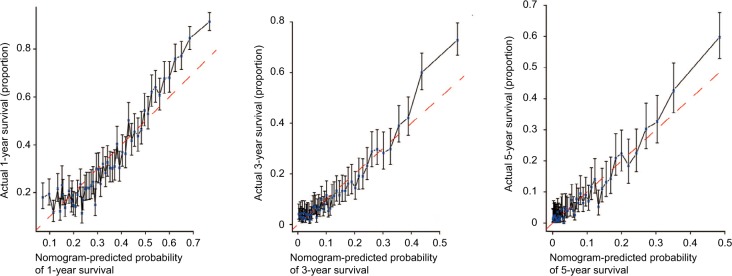
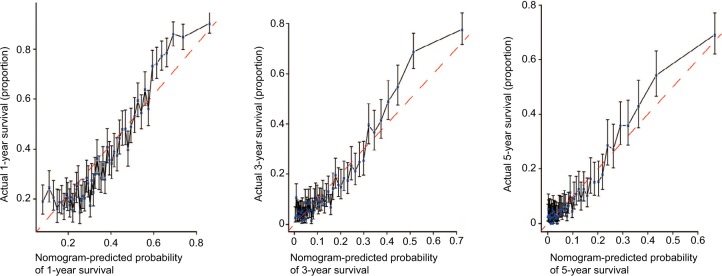
The prognostic nomogram that integrated all significant independent factors for OS in patients with CCA is shown in Figure 3. The C-index for OS prediction was 0.657. The calibration plot for the probability of survival at 1 year, 3 years, and 5 years after diagnosis showed an agreement between the prediction by nomogram and actual observation (Figure 4). The prognostic nomogram that integrated all significant independent factors for DSS in patients with CCA is shown in Figure 5. The C-index for DSS prediction was 0.661. The calibration plot for the probability of survival at 1 year, 3 years, and 5 years after diagnosis showed an agreement between the prediction by nomogram and actual observation (Figure 6).
Figure 3.
OS nomogram.
Abbreviations: AJCC, American Joint Committee on Cancer; OS, overall survival; SEER, Surveillance, Epidemiology and End Results.
Figure 4.
Calibration curve for predicting patient OS at 1, 3, and 5 years.
Abbreviation: OS, overall survival.
Figure 5.
DSS nomogram.
Abbreviations: AJCC, American Joint Committee on Cancer; DSS, disease specific survival; SEER, Surveillance, Epidemiology and End Results.
Figure 6.
Calibration curve for predicting patient DSS at 1, 3, and 5 years.
Abbreviation: DSS, disease specific survival.
Discussion
Improvement in diagnostic and therapeutic strategies for cancer has led to an increase in cancer survivors. Approximately 18 million cancer survivors are expected by 2022.20 It is important to figure out the long-term impact of cancer and its treatment in the population of cancer survivors. SPM is one of the long-term complications of cancer. Identifying the exact risks of SPM following primary cancer will contribute to development of evidence-based guidelines for surveillance of cancer survivors.
To the best of our knowledge, the current study is the first to report the risk of SPM in CCA survivors. Our study showed that the overall risk of SPM in patients with CCA was significantly higher than that in the general population. The site-specific risk of SPM in colon was significantly increased, especially in whites, patients aged ≤29, patients aged 30–59 years, and patients with latency ≤11 months. In view of our results, colon cancer screening is suggested in these specific groups of patients. There are two possible explanations for the correlation between CCA and colon cancer. Inflammatory bowel disease, a major risk factor for colon cancer, was reported as a risk factor for CCA.21,22 Additionally, CCA and colon cancer had shared genetic mutations, including FGFR2 and HER-2.23–26
Previous studies reported that patients were at increased risk of developing thyroid cancer after several cancers, including Hodgkin lymphoma,27 gastric cancer,28 kidney cancer,29 and prostate cancer.30 Our study found that the risk of thyroid cancer also increased in CCA survivors, especially in whites, patients aged 30–59 years, and patients with latency ≤11 months. Some shared common etiological factors could be responsible for the association between CCA and thyroid cancer. Dietary iodide deficiency and intracellular iodide deficiency caused by mislocalization of sodium/iodine symporter (NIS) were shown to play a role in the carcinogenesis of thyroid and CCA.31,32 The influences of sex hormones and obesity on the development of CCA and thyroid cancer were also reported.33–35 However, the causal relationship underlying the association is not well understood and needs further study.
A significantly elevated risk of cancer in the bile duct was also evident in our study, especially in whites, females, patients aged 30–59 years, and patients with latency ≤11 months. The main concern is whether these bile duct cancers are metastases or recurrences from the primary malignancy. International Agency for Research on Cancer (IARC) and SEER rules are two widely used sets of rules for coding multiple primary cancers. Different rules used to collect and consolidate multiple primary cancers would result in differences in incidence rates and trends.36,37 SEER rules were used by cancer registries throughout North America. Weir et al reported that, compared to SEER multiple primary coding rules, IARC rules reported fewer multiple primary cancers.38 As stated in the Methods section, we applied SEER definitions, which were developed to enumerate primary cancers including differentiating a new primary cancer from a distant metastasis or a recurrent cancer, to identify patients with multiple primary cancers.39 Since there is still no golden rule for defining multiple primary cancers, we should still be concerned whether these bile duct cancers are metastases or recurrences from the primary malignancy.
Surgery is currently the best available potentially curative treatment in patients with CCA who present with early stage disease.40 We found that patients with CCA with regional and distant disease had a decreased risk of developing SPM in comparison with patients with localized disease. Additionally, having undergone surgery for CCA was a favorable factor for SPM development and better OS. We assumed that this is a result of relatively long-term follow-up of patients with relatively early stage disease and who underwent surgery. Furthermore, we found that the presence of SPM was an independent factor for OS and DSS prediction. OS and DSS were better in patients with SPM than patients without SPM. The most plausible explanation might be the relatively poorer survival of patients with CCA.
Compared with previously published studies, our study has the following strengths. First, it was the first study reporting the risk of developing SPM in patients with CCA. Using a standard method, population-based data were collected from high-quality registries that covered ~28% of the US population. The study covered a large number of patients with CCA across 42 years (1973–2015), forming a highly generalizable dataset that is likely more reflective of the population experience. Second, it provided strong evidence for the development of guidelines for surveillance of CCA survivors. Our analysis revealed that 19.2% (62/323) of SPM were diagnosed at distant stage. The rate was underestimated due to 23.84% of SPM being unstaged at the initial diagnosis. The result indicated a need for enhanced surveillance of patients with CCA at high risk of SPM, which would enable the early diagnosis of SPM. We analyzed the risk of developing SPM in subgroups of patients of different age, sex, race, and latency, which provided detailed information regarding patients’ prognosis and how to focus clinical follow-up in each subgroup. Third, our study indicated an association between CCA and the most common SPM, a finding which would improve our understanding of the biological behaviors of these malignancies and direct further exploration of the association.
Our study should be considered in the context of its limitations. First, the SEER database does not collect information on factors such as habits (smoking, alcohol use), comorbidities (diabetes, hepatitis, obesity), exposure to carcinogens, family history, or chemotherapy used, all of which would affect the comprehensive analysis of risk factors for SPM development. Second, there is a potential for underestimation of SPM risk in the case of patient migration out of the SEER geographic registry. Third, the main limitation of the study was that we did not collect detailed treatment information for these SPMs.
Conclusion
In conclusion, we observed that patients who survived CCA were at a significantly higher overall risk of developing SPM. Specific sites where the risk of SPM was significantly increased included colon, thyroid, intrahepatic bile duct, and other biliary sites. Additionally, the risk of developing specific SPM varied by age, sex, race, and latency. Development of SPM was a favorable predictor of OS and DSS in patients with CCA. The results of our study suggested that the increased risk of SPM did not shorten OS and DSS of patients with CCA, possibly due to the relatively poorer survival of patients with CCA.
Data sharing statement
The data accessed from the SEER database are freely available.
Acknowledgments
The authors acknowledge the efforts of the SEER Program cancer registries in creating the SEER database.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45(6):856–867. doi: 10.1016/j.jhep.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel T. Cholangiocarcinoma–controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8(4):189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21(5):594–599. doi: 10.1634/theoncologist.2015-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribero D, Pinna AD, Guglielmi A, et al. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg. 2012;147(12):1107–1113. doi: 10.1001/archsurg.2012.1962. [DOI] [PubMed] [Google Scholar]
- 6.Akamatsu N, Sugawara Y, Hashimoto D. Surgical strategy for bile duct cancer: advances and current limitations. World J Clin Oncol. 2011;2(2):94–107. doi: 10.5306/wjco.v2.i2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruzzenente A, Conci S, Valdegamberi A, Pedrazzani C, Guglielmi A. Role of surgery in the treatment of intrahepatic cholangiocarcinoma. Eur Rev Med Pharmacol Sci. 2015;19(15):2892–2900. [PubMed] [Google Scholar]
- 8.Carrafiello G, Laganà D, Cotta E, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33(4):835–839. doi: 10.1007/s00270-010-9849-3. [DOI] [PubMed] [Google Scholar]
- 9.Kiefer MV, Albert M, McNally M, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2011;117(7):1498–1505. doi: 10.1002/cncr.25625. [DOI] [PubMed] [Google Scholar]
- 10.Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36(2):440–448. doi: 10.1007/s00270-012-0463-4. [DOI] [PubMed] [Google Scholar]
- 11.Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30(16):1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- 12.Kim TH, Han SS, Park SJ, et al. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys. 2011;81(5):e853–e859. doi: 10.1016/j.ijrobp.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Rubino C, de Vathaire F, Dottorini ME, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89(9):1638–1644. doi: 10.1038/sj.bjc.6601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah BK, Budhathoki N. Second Primary malignancy in Anal carcinoma – a US population-based study. Anticancer Res. 2015;35(7):4131–4134. [PubMed] [Google Scholar]
- 15.Raymond JS, Hogue CJ. Multiple primary tumours in women following breast cancer, 1973-2000. Br J Cancer. 2006;94(11):1745–1750. doi: 10.1038/sj.bjc.6603172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah BK, Kandel P, Khanal A. Second primary malignancies in hepatocellular cancer - A US Population-based Study. Anticancer Res. 2016;36(7):3511–3514. [PubMed] [Google Scholar]
- 17.Davis EJ, Beebe-Dimmer JL, Yee CL, Cooney KA. Risk of second primary tumors in men diagnosed with prostate cancer: a population-based cohort study. Cancer. 2014;120(17):2735–2741. doi: 10.1002/cncr.28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blechacz B. Cholangiocarcinoma: current knowledge and new developments. Gut Liver. 2017;11(1):13–26. doi: 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cancer Institute Surveillance, Epidemiology, and End Results Program [homepage on the Internet] SEER program research data 1973–2013 National Cancer Institute DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016 based on the November 2015 submission. [Accessed August 15, 2016]. Available from: http://www.seercancer.
- 20.Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer survivorship Resource Center: developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147–150. doi: 10.3322/caac.21183. [DOI] [PubMed] [Google Scholar]
- 21.Din S, Wong K, Mueller MF, et al. Mutational analysis identifies therapeutic biomarkers in inflammatory bowel disease-associated colorectal cancers. Clin Cancer Res. 2018;24(20):5133–5142. doi: 10.1158/1078-0432.CCR-17-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huai JP, Ding J, Ye XH, Chen YP. Inflammatory bowel disease and risk of cholangiocarcinoma: evidence from a meta-analysis of population-based studies. Asian Pac J Cancer Prev. 2014;15(8):3477–3482. doi: 10.7314/apjcp.2014.15.8.3477. [DOI] [PubMed] [Google Scholar]
- 23.Carter JH, Cottrell CE, McNulty SN, et al. FGFR2 amplification in colorec-tal adenocarcinoma. Cold Spring Harb Mol Case Stud. 2017;3(6):a001495. doi: 10.1101/mcs.a001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholan-giocarcinoma. Hepatology. 2014;59(4):1427–1434. doi: 10.1002/hep.26890. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Yoo TW, Park DI, et al. Gene amplification and protein overexpression of HER-2/neu in human extrahepatic cholangiocarcinoma as detected by chromogenic in situ hybridization and immunohistochem-istry: its prognostic implication in node-positive patients. Ann Oncol. 2007;18(5):892–897. doi: 10.1093/annonc/mdm006. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Zheng J, Xu B, Ling J, Qiu W, Wang Y. Simvastatin inhibits tumor angiogenesis in HER2-overexpressing human colorectal cancer. Biomed Pharmacother. 2017;85:418–424. doi: 10.1016/j.biopha.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 27.Chowdhry AK, Fung C, Chowdhry VK, et al. A population-based study of prognosis and survival in patients with second primary thyroid cancer after Hodgkin lymphoma. Leuk Lymphoma. 2018;59(5):1180–1187. doi: 10.1080/10428194.2017.1369063. [DOI] [PubMed] [Google Scholar]
- 28.Morais S, Antunes L, Bento MJ, Lunet N. Risk of second primary cancers among patients with a first primary gastric cancer: a population-based study in North Portugal. Cancer Epidemiol. 2017;50(Pt A):85–91. doi: 10.1016/j.canep.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Rahman O. Risk of subsequent primary kidney cancer after another malignancy: a population-based study. Clin Genitourin Cancer. 2017;15(5):e747–e754. doi: 10.1016/j.clgc.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Bezak E, Takam R, Yeoh E, Marcu LG. The risk of second primary cancers due to peripheral photon and neutron doses received during prostate cancer external beam radiation therapy. Phys Med. 2017;42:253–258. doi: 10.1016/j.ejmp.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Nettore IC, Colao A, Macchia PE. Nutritional and environmental factors in thyroid carcinogenesis. Int J Environ Res Public Health. 2018;15(8) doi: 10.3390/ijerph15081735. E1735:1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B, Hervé J, Bioulac-Sage P, et al. Sodium iodide symporter is expressed at the preneoplastic stages of liver carcinogenesis and in human cholangiocarcinoma. Gastroenterology. 2007;132(4):1495–1503. doi: 10.1053/j.gastro.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 33.Mancino A, Mancino MG, Glaser SS, et al. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis. 2009;41(2):156–163. doi: 10.1016/j.dld.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong L, Lu J, Zhao B, Wang W, Zhao Y. Review of the possible association between thyroid and breast carcinoma. World J Surg Oncol. 2018;16(1):130. doi: 10.1186/s12957-018-1436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li JS, Han TJ, Jing N, et al. Obesity and the risk of cholangiocarcinoma: a meta-analysis. Tumour Biol. 2014;35(7):6831–6838. doi: 10.1007/s13277-014-1939-4. [DOI] [PubMed] [Google Scholar]
- 36.Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer. 2014;14:272. doi: 10.1186/1471-2407-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filali K, Hédelin G, Schaffer P, et al. Multiple primary cancers and estimation of the incidence rates and trends. Eur J Cancer. 1996;32A(4):683–690. doi: 10.1016/0959-8049(95)00621-4. [DOI] [PubMed] [Google Scholar]
- 38.Weir HK, Johnson CJ, Ward KC, Coleman MP. The effect of multiple primary rules on cancer incidence rates and trends. Cancer Causes Control. 2016;27(3):377–390. doi: 10.1007/s10552-016-0714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Cancer Institute Surveillance, Epidemiology, and End Results Program [homepage on the Internet] Multiple primary and histology coding rules. NIH; 2007. [Accessed April 9, 2015]. [updated August 24, 2012 Available from: http://seer.cancer.gov/tools/mphrules/ [Google Scholar]
- 40.Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18(3):651–658. doi: 10.1245/s10434-010-1325-4. [DOI] [PubMed] [Google Scholar]