Abstract
Introduction
Gene for gastrokine 1 (GKN1) was identified as one of the most significant in gastric cancer and indicated as a potential therapeutic target.
Aim
The aim was a review of literature reports concerning the role and diagnostic potential of GKN1 in gastric cancer.
Materials and methods
PubMED database was searched for sources using the following keywords: gastrokine 1/GKN1/AMP-18 and gastric cancer, Helicobacter pylori, aspirin, nonsteroidal anti-inflammatory drugs. Preference was given to the sources which were published within the past 10 years.
Conclusion
GKN1 is a stomach-specific protein, and its role consists of maintaining mucosal integrity as well as the replenishment of the surface lumen epithelial cells layer. The evaluation of GKN1 expression seems to be a useful indicator of the presence of neoplastic or inflammatory lesions in the gastric mucosa. GKN1 expression is decreased in gastric tumor tissues and derived cell lines and its upregulation in cell lines of gastric cancer induces cells apoptosis. The mechanism by which GKN1 is inactivated in gastric cancer cells is still not fully understood. The future diagnostic capabilities of gastric cancer concern the assessment of serum GKN1 concentration by means of ELISA method. Serum GKN1 concentration is not related to patients’ sex. Moreover, the measurement of GKN1 concentration is possible only after the incubation of samples at 70°C for 10 minutes. Nevertheless, the aspect of quantitative serum GKN1 evaluation is new in the context of available literature and requires further studies.
Keywords: gastrokine 1/GKN1, gastric cancer, Helicobacter pylori
Introduction
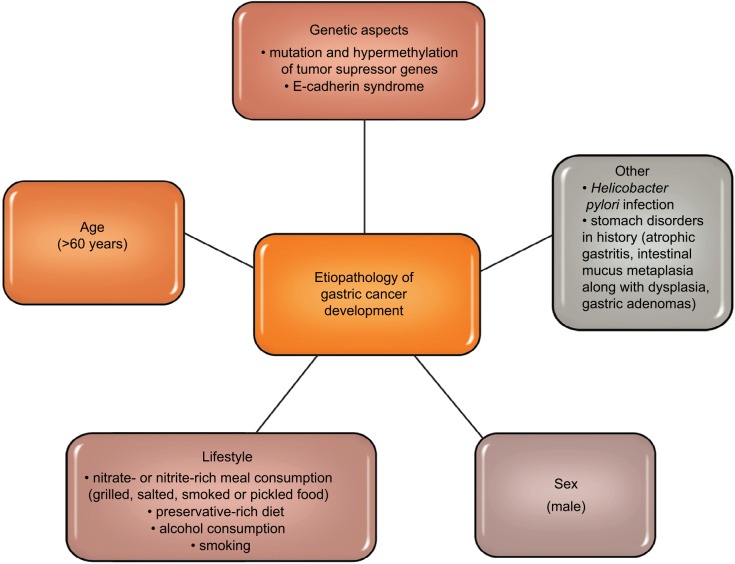
Gastric cancer is one of the most commonly diagnosed malignancies of the digestive tract.1,2 Literature data show that in the last few decades, both the number of new cases and deaths from gastric cancer have been decreasing.3,4 Figure 1 shows etiopathological factors of gastric cancer development (Figure 1).5–11 In about 80% of cases of gastric cancer, the early stage of the disease is asymptomatic, and complaints such as an early feeling of fullness during a meal, nausea or epigastric pain are unspecific and often associated with peptic ulcer disease or other gastrointestinal diseases. Therefore, gastric cancer is diagnosed in advanced stages, which is inevitably combined with ineffective treatment and poor prognosis.12,13
Figure 1.
The criterion for the diagnosis of gastric cancer is the finding of tumor cells in the histopathological examination of mucosal sections taken during endoscopic examination (gastroscopy). The gastroscopy is supplemented by a radiological examination of the upper gastrointestinal tract. Laboratory tests that may suggest a neoplastic process are not very specific (decreased serum total protein and albumin in the proteinogram and a positive stool test for the blood). Iron deficiency anemia usually occurs in the advanced stages of the disease. Cancer markers such as: carbohydrate antigen (CA) 72, carcinoembryonic antigen (CEA), and CA 19-9 are also useful in diagnosis and monitoring of gastric cancer. However, with a diagnostic specificity of 95%, their diagnostic sensitivity for gastric cancer is 35%–50%, 25%–35% and 30%–40%, respectively, which encourages the search for new, more sensitive tests.14,15
The literature pays attention to the recently discovered protein – gastrokine 1 (GKN1) with a molecular mass of 18 kDa, which has been isolated from cells of the gastric mucosa of healthy people, as well as other species of mammals (including mice, rats, pigs and cows).16–19 The gene for GKN1, along with genes for secreted phosphoprotein 1 (SPP1), sulfatase 1 (SULF1), thrombospondin 2 (THBS2), ATPase H+/K+ transporting beta subunit (ATP4B), gastric intrinsic factor (GIF), was identified as the most significant in gastric cancer and, moreover, indicated as potential target for improving the diagnosis and clinical effects in patients with gastric cancer.2 Therefore, the aim of the current paper is a review of literature reports concerning the role and diagnostic potential of GKN1 in gastric cancer.
Search strategy
We searched PubMED database for sources using the following keywords: gastrokine 1/GKN1/AMP-18 and gastric cancer, Helicobacter pylori, aspirin, nonsteroidal anti-inflammatory drugs. Preference was given to the sources which were published within the past 10 years.
Gastrokine 1 (GKN1)
In earlier reports, GKN1 was reported as AMP-18 (antrum mucosal protein-18), CA11, foveolin or TFIZ2.17,19,20 The GKN1 coding gene (CA11, accession number: BK0017373) is located on the 2p13 chromosome and consists of six exons. Many studies have shown that GKN1 occurs inside cytoplasmic granules, suggesting that it is rather a secretory protein than an integral membrane protein.17,19,21,22 Martin et al17 to localize this protein in the antral part of the stomach, studied the pigs’ gastric mucosal membrane by using the following methods: immunohistochemistry, immunoblotting and northern blot. It was found that the newly discovered protein has tissue specificity, therefore in November 2003 the Human Gene Nomenclature Committee gave it its official name – gastrokine 1.23,24
Studies conducted in recent years have shown that gastrointestinal mucosa cells secrete not only GKN1, but also other gastrokines: 2 (GKN2/GDDR/TFIZ1/blottin) and 3 (GKN3). GKN2 is indicated as a gastric epithelial-secreted protein, however human GKN3 persists only as a non-expressed pseudogene (Table 1).5,20–22,25–36 These proteins also have a role in maintaining cell integrity, and their expression in the gastric mucosa also decreases in inflammation and gastric cancer.21,29,35 Gastrokines show similarity in 46% of their amino acid sequences, and the characteristic linking these proteins is the presence in their structure of the BRICHOS domain consisting of ~100 amino acids and associated with the occurrence of gastric cancer.37–39
Table 1.
GKN2 and GKN3 in gastric cancer.
| Gastrokine 2 (GKN2) | |
|---|---|
| Alternative names: GDDR/TFIZI/Blottin | |
| Sánchez- Pulido et al25 | • Proposed GKN2 function in folding and intracellular transport or secretion |
| Du et al26 | • GKN2 mRNA is located in gastric mucosa, and only expressed in gastric tissue |
| • GKN2 encoded protein including a trans-membrane peptide homolog to GKN1 | |
| Wesley et al27 | • GKN2 forms a heterodimer with the trefoil factor family (TFF) protein 1 via an intermolecular disulfide bond between cysteine residues in the carboxy-terminus of TFF1 and in the BRICHOS domain of GKN2 |
| Otto et al22 | • GKN2 may modulate the integrity of the mucus barrier and its mode of action may be enhanced in a wound situation, perhaps by influencing mucus viscosity |
| Baus-Loncar et al21 | • The regulation of GKN2 parallels that of TFF genes, indicating that together they may play a role in the homeostasis of the gastrointestinal tract |
| Kouznetsova et al28 | • The TFF1-GKN2 heterodimer was not associated with the mucin fraction, whereas TFF2 did associate with mucins |
| Moss et al20 | • GKN2 mRNA was downregulated in distal gastric cancer |
| • GKN2 may be an independent marker of prognosis | |
| Zhang et al29 | • GKN2 is expressed on the surface area of nontumoral mucosa |
| • GKN2 expression is weak in deeper glands of stomach | |
| • In deeper zones of mucosa GKN2 expression coexisted with beta-catenin nuclear accumulation, which may indicate some interactions between them | |
| Chu et al30 | • GKN2 is expressed in human gastric epithelial cells and significantly downregulated in gastric cancer cells |
| • GKN2 gastric cancer growth inhibition is a TFF1-dependent | |
| Kim et al31 | • GKN2 may inhibit GKN1 activity |
| • GKN2 expression positively correlated with GKN1 expression | |
| Dai et al32 | • GKN2 expression was decreased or absent in gastric cancer cell lines, gastric intestinal metaplasia and tumor tissues |
| • GKN2 overexpression inhibited the proliferation, migration, and invasion of gastric cancer cells and arrested the cell cycle at the G1-S transition phase | |
| • Overexpression of GKN2 and TFF2 together showed the same inhibitory effect as overexpression of GKN2 alone, which may indicate that TFF2 may not structurally or functionally interact with GKN2 | |
| Shi et al5 | • GKN2 overexpression in gastric cancer cells upregulated Fas expression, but it did not significantly influence the expression of Bcl-2 and Bax, which indicate the extrinsic pathways of apoptosis activation |
| Menheniott et al33 | • In vivo GKN2 loss in chronic inflammation induced by H. pylori infection or genetically induced hyperactivation of oncogenic gp130/STAT3 signaling plays a role in gastric cancer progression |
| • Gkn2–/– mice lacked spontaneous tumor growth or significant stomach epithelial hyperplasia, which may indicate that isolated GKN2 loss is inadequate for cell-autonomous transformation | |
| • Dual overexpression of GKN2 and GKN1 may significantly decrease gastric tumor growth in vivo | |
| Ouyang et al34 | • GKN2 overexpression significantly inhibits the JAK2/STAT3 signal pathway to further upregulate Bax, and downregulate Bcl-2, Cyclin D1, MMP2 and MMP9, which cause reduced gastric cancer proliferation and invasiveness, increased apoptosis and cell cycle arrest in the G1 phase |
| Gastrokine 3 (GKN3) | |
| Menheniott et al35 | • GKN3 encodes a secreted protein, suggesting an extracellular function |
| • Mouse GKN3 is overexpressed in atrophic gastritis associated with H. pylori infection | |
| • Human GKN3 persists only as a nonexpressed pseudogene | |
| • GKN3 and TFF2 protein are unlikely to form heterodimer | |
| Geahlen et al36 | • GKN3 is a stomach specific protein expressed in a gastric mucous in mice and pigs |
| • GKN3 protein was not detect in normal gastric tissue of patients, about half of which were heterozygous for the A/G allele | |
| • The transcripts for GKN3 gene in humans were not detected, no matter the genotype, which may indicate its pseudogenicity | |
Abbreviation: H. pylori, Helicobacter pylori.
GKN1 and gastric cancer
In vitro studies have shown that both GKN118,23,24,40,41 and GKN229 occur in the gastric mucosa of healthy individuals. On the other hand, in the gastric mucosa of gastric cancer patients compared to subjects with superficial gastritis, a decreased GKN1 protein and mRNA GKN1 expression is noted.42,43 GKN1 was also absent in patients with intestinal metaplasia.43 Perhaps the reason for reduced expression or absence of GKN1 in these diseases is damage to the gastric mucosa.
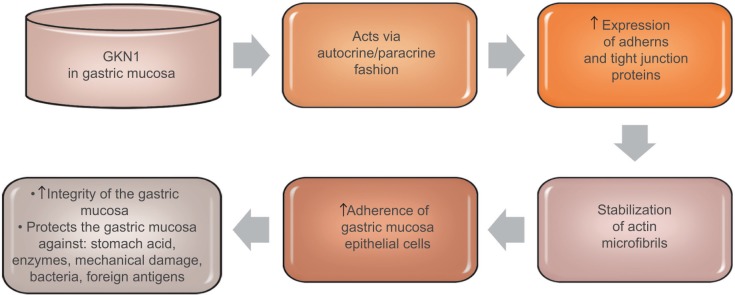
GKN1 acts on cells in an autocrine or paracrine fashion.18 The studies of Oien et al24 showed the expression of GKN1 in the gastric mucosa of both the surface epithelium and gastric ulcer. However, no GKN1 expression was found in the deeper parts of the gastric glands. The results of previous studies suggest that GKN1 is a gastric specific mitogenic protein and most likely plays a protective and reparative role in the gastric mucosa.23,24,44 GKN1 maintains the integrity of the gastric mucosa, protects it against the action of stomach acid and enzymes as well as mechanical damage, bacteria or foreign antigens.17,19,44 Walsh-Reitez et al45 conducted studies in mice that were administered dextran sulfate sodium to damage the intestinal mucosa and then injected with GKN1. It was found that under the influence of GKN1 the immunoreactivity of occludens proteins increased, forming tight connections responsible for the adherence of epithelial cells in the colon mucosa. In addition, during the exposure of tested epithelial cells to monochloramine, it was shown that GKN1 protects them from loss of integrity by increasing the accumulation of intercellular proteins (zonula occludens-1, claudin 5, E-cadherin) and stabilization of actin microfibrils.45 These findings were further confirmed by studies of Rippa et al,46 which showed that in normal gastric tissue GKN1 is co-localized with actin and GKN1 overexpression in gastric cancer cells leads to increased expression of adherens and tight junction proteins, which indicates this protein as a main factor helpful in actin stabilization. Figure 2 schematically explains GKN1 role in gastric mucosa.
Figure 2.
GKN1 role in gastric mucosa.
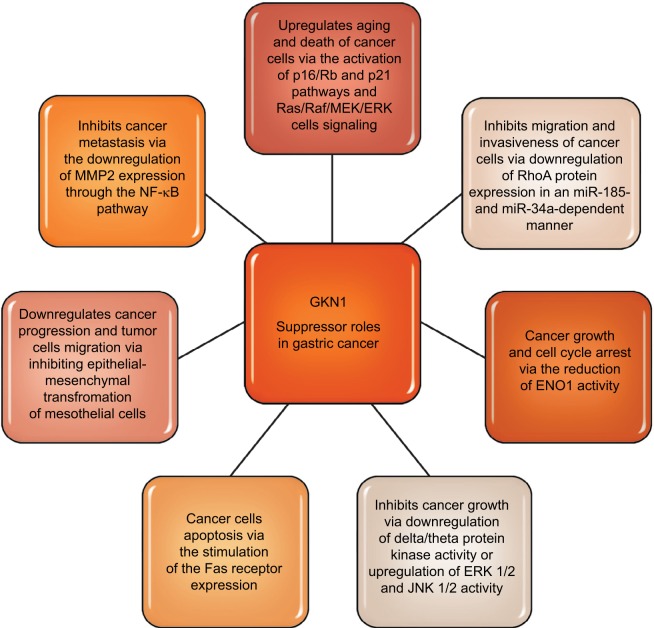
Toback et al47 in the in vitro studies demonstrated the possibility of using GKN1 to stimulate and accelerate the regeneration of damaged epithelium. Some authors19,37,41,48,49 attribute GKN1 as a possible suppressor function in stomach cancer. Yoon et al19 have shown that GKN1 limits the formation of the MKN-28 gastric cancer cell line colony. GKN1 causes the biological aging of gastric cancer cells leading to their death by activating the p16/Rb and p21 pathways and Ras/Raf/MEK/ERK cell signaling.18 Xing et al49 showed that GKN1 inhibits gastric cancer cell invasion via downregulation of MMP2 expression through the NF-κB pathway, which shows that it plays a role in inhibition of cancer metastasis and indicates GKN1 as a novel potential therapeutic target. It is also suggested that GKN1 is able to inhibit migration and invasiveness of gastric cancer cells via downregulation of RhoA protein expression in a miR-185- and miR-34a-dependent manner. Yoon et al50 found that RhoA GKN1 expression and GKN1 mRNA were increased in gastric cancer tissues and related to TNM stage. Also, Yan et al40 in an in vitro study attempted to explain how GKN1 inhibits the growth of gastric cancer cells. The authors analyzed the effect of GKN1 on the SGC-7901 gastric cancer cell line lacking expression of the tested protein. GKN1 has been shown to inhibit tumor cell growth and reduce the number of cell colonies by stopping the G2/M cell cycle instead of inducing apoptosis.40 On the other hand, Yoon et al19 found that in the AGS gastric cancer cell line transfected with GKN1 both inhibiting the proliferation of these cells and activation of the apoptosis process were induced. In the group of patients with gastric cancer, the lack of or decrease in expression of GKN1 gene was detected by Western blot analysis. In addition, it was found that the number of DNA copies and transcripts of GKN1 mRNA were statistically significantly reduced in this study group.19
It is suggested that inactivation of GKN1 gene may play an important role in the development of some cases of gastric cancer. GKN1 also regulates protein kinase activity, including inhibition of delta/theta protein kinase activity, or increases ERK 1/2 and JNK 1/2 activity, which has inhibitory effect on the growth of gastric cancer cells. In the studies of Yan et al,40 it was found that GKN1 affects the activity of 74 proteins, including α-enolase (ENO1) and cathepsin D.ENO1 is an enzyme involved in the metabolism of glycogen, in the presence of magnesium ions, reversibly converts the 2-phosphoglycerate to phosphoenolpyruvate at the phos-photriese stage.51 In addition, ENO1 exhibits a number of nonenzymatic activities, eg, as a surface protein of epithelial cells, endothelium, fibroblasts, cancer cells or some hematopoietic cells, it acts as a plasminogen receptor, and as a ligand binding cholesterol esters causes the accumulation of cholesterol esters in cells (affects the process of atherosclerosis, multiple sclerosis and encephalopathy). The increase in ENO1 expression has been demonstrated also in small-cell lung carcinoma, breast cancer, head and neck cancer.40,52 During proliferation, tumor cells have a high demand for ATP, therefore the rate of aerobic glycolysis is elevated and increased expression of enzymes in the glycolysis pathway is observed. ENO1 present in the cytosol of lung cancer cells by association with cytoskeleton proteins facilitated the migration of these cells. Conversely, ENO1 presented on the surface of non-small-lung carcinoma cells caused a local increase in plasminogen density, which accelerated the degradation of fibrin and extracellular matrix proteins, also affecting invasion of cancer cells and metastasis.51,52 It was shown that in gastric cancer cells the reduction of ENO1 activity and overexpression of GKN1 caused growth and cell cycle arrest. In contrast, overexpression of ENO1 blocked the inhibitory effect of GKN1 on the growth of tumor cells and the arrest of the cell cycle.40 Therefore it was suggested that the “down regulation” phenomenon of ENO1 may play an important role in inducing inhibition of gastric cancer cell growth. Rippa et al38 found that the increased expression of GKN1 in the cell lines (AGS and MKN-28) of gastric cancer stimulated the expression of the Fas receptor which induced programmed death of these cells. The results of research by Rippa et al38 suggest that GKN1 may be a significant protein modulating apoptotic signal and performs the reparative role of damaged tissues in early stages of neoplastic transformation.
Excessive expression of GKN1 may also inhibit epithelial-mesenchymal transformation of mesothelial cells (epithelial-mesenchymal transition [EMT]) by inactivation of the PI3K/Akt pathway and reduction of ROS.19 In addition, GKN1 expression is accompanied by increased expression of E-cadherin and reduction of cytoplasmic and nuclear expression of β-catenins, fibronectin, vimentin and cyclin D1. As a result EMT mesothelial cells receive a phenotype of fibroblastic cells expressing α-actin of smooth muscle and the ability to migrate. These data suggest that the GKN1 gene, in sporadic cases of gastric cancer, can play an important role in its progression by inhibiting EMT and tumor cell migration. Studies by Zhang et al29 have shown a large accumulation of β-catenin in the nucleus of gastric cancer cells. In addition, nuclear expression of β-catenin occurred in normal cells. The authors suggest that in this case it may be a paraneoplastic element. The conclusion is that the accumulation of β-catenin in the nuclei of normal cells of the mucous membrane may be an alarm signal that there is a probability of neoplastic changes in neighboring cells.29 Some literature data indicate that during H. pylori infection due to the action of cytotoxin-associated gene A (CagA) and proinflammatory cytokines, nuclear accumulation of β-catenin and disturbance of the β-catenin dependent signaling pathway may also occur.53 These results partially explain why colonization of the gastric mucosa by H. pylori increases the risk of cancer development. Figure 3 summarizes suggested GKN1suppressor roles in gastric cancer.
Figure 3.
GKN1 suppressor roles in gastric cancer.
The mechanism by which gastrokine 1 is inactivated in gastric cancer cells is still not fully understood and obtained results are controversial.19,54,55 Yoon et al19 did not detect GKN1 gene mutation in gastric cancer, whereas the hypermethylation of GKN1 promoter was found only in two cases. Conversely, Lu et al54 revealed that GKN1 and GKN2 genes are transcriptionally silenced by methylation of DNA. They also found that the Epstein-Barr nuclear antigen 1 (EBNA1) binds to different promoters of the GKN1 and GKN2 genes in gastric cancer cells. The authors suggest that EBNA1 leads to the complex transcriptional and epigenetic deregulation of these tumor suppressor genes in Epstein–Barr virus positive gastric cancer.54 Altieri et al55 tested how GKN1 histonic modification may lead to its downregulation. The authors, for this purpose, immunoprecipitated from human normal and cancerous gastric tissues chromatin for the trimethylation of histone 3 at lysine 9 (H3K9triMe) and its specific histone-lysine N-methyltransferase (SUV39H1). They found that epigenetic mechanisms responsible for GKN1 silencing in gastric cancer samples were related to high H3K9triMe levels and SUV39H1 recruitment to the GKN1 promoter. GKN1 mRNA levels were markedly increased after the inhibition of histone deacetylases with trichostatin A.55
GKN1 and H. pylori infection
H. pylori is a Gram-negative, spiral shaped bacterium commonly found in the human population. In 1994, the International Agency for Research on Cancer at the WHO indicated H. pylori as a class I carcinogen, whose participation in the formation of gastric cancer has been proven. Colonization of the gastric mucosa by H. pylori leads to a local inflammatory reaction with the production of ROS and increases the concentration of nitric oxide, which can affect the DNA of these cells and cause mutations resulting in the development and progression of gastric cancer.44 It has been observed that H. pylori infection leads to the phenomenon of downregulation and lack of expression of GKN1.24,44,56,57 The number of GKN1 mRNA copies in patients with chronic gastritis infected with H. pylori was statistically significantly lower compared to the group of patients without infection.44 In addition, the analysis of Western blots of gastric mucosa biopsies in patients with gastric cancer and in patients with premalignant disease did not show gene expression for GKN1,24 and in the reverse transcriptase PCR (RT-PCR), statistically significantly lower GKN1 mRNA levels were found in patients with H. pylori infection.19 Activation of proinflammatory cytokines such as TNF-α, IL-1β, IL-6 and IFN-γ occurring in the course of H. pylori infection may also inhibit the expression of GKN2 in gastrointestinal mucosa.21 Moss et al20 evaluated the expression of GKN1 and GKN2 mRNA and the expression of these proteins in biopsies of normal gastric mucosa cells and tumor-altered biopsies. They showed statistically significantly lower expression of GKN1 and GKN2 in gastric adenocarcinoma, especially in the scattered cancer subtype compared to the control group. The loss of GKN1 and GKN2 expression was an unfavorable prognostic marker in surgically treated patients because it correlated with a shorter survival time.20
On the molecular level, H. pylori CagA directly injected into gastric epithelial cells drives morphology changes of these cells, leads to their apoptosis, increases cell proliferation and motility, and thus promotes gastric carcinogenesis. Additionally, CagA induces activation of NF-κB and PI3K/Akt signaling pathways and EMT-related proteins.58 The role of GKN1 relies on inhibition CagA injection into gastric epithelial cells, reduction of ROS via a positive regulatory effect on antioxidant enzymes production, stimulation the negative cell cycle regulators expression (p53, p16 and p21), and induction of miR-185 expression which inhibits genomic DNA epigenetic modification. Moreover, both on human and mice gastric cells and mucosal tissues, it was shown that H. pylori CagA decreases gene copy number for GKN1 and GKN1 expression. Interestingly, increased GKN1 expression decreased the carcinogenic effects of H. pylori CagA through binding to CagA, which strongly indicates gastrokine 1 as a potential therapeutic target.58
GKN1 and acetylsalicylic acid (ASA)
ASA has anti-inflammatory, analgesic and antipyretic properties, and long-term administration also has anticoagulant activity. ASA is a cyclooxygenase inhibitor which inhibits prostaglandin production and contributes to the reduction of mucus secretion that protects the gastric mucosa. Therefore, about 25% of patients receiving ASA suffer damage to the gastrointestinal mucosa in the form of stomach or intestinal erosions or ulcers.
The aim of the study of Martin et al23 was to show whether receiving low doses of ASA affects the expression of GKN1 in antral and corpus mucosa. The study group consisted of healthy volunteers without H. pylori infection who were taking ASA at a dose of 100 mg/day for 7 days. GKN1 baseline was similarly expressed in both antral and corpus mucosa and it was not changed after 3 days of ASA administration. After 7 days GKN1 expression level was significantly reduced in gastric antrum, whereas in gastric corpus GKN1 transcript levels were slightly elevated. Mild gastritis and mucosal erosions were limited only to gastric antrum and correlated with GKN1 expression.23 In an animal model study, intra-gastric administration of indomethacin showed a decrease in GKN1 concentration.47 The authors suggest that GKN1 is involved in maintaining the integrity of gastric mucosa cells and stimulates repair mechanisms after its damage. Indeed, exogenous GKN1 applied in vitro to gastrointestinal cells stimulated their reconstruction.47
Circulating GKN1 evaluation – diagnostic potential
Currently carbohydrate antigen (CA) 19-9, carcinoembryonic antigen (CEA), and CA 72-4 are used for gastric cancer: diagnosis, monitoring of recurrence, distal metastasis presence, chemotherapy evaluation and prognosis. However, the interpretation of CEA, CA 19-9 and CA 72-4 evaluation should be performed carefully, especially when the tumor biomarker concentration is borderline or only slightly above the reference value. Moreover, the above-mentioned tumor biomarkers are not useful in diagnosis and screening of early gastric cancer. Elevated concentrations of CEA, CA 19-9 and CA 72-4 were observed also in other types of tumors as well as conditions not related to malignancy.59 It is also highlighted that the combined testing of CEA, CA 19-9 and CA 72-4 is superior compared to single biomarker testing, as it significantly increases their diagnostic usefulness, which is briefly summarized in Table 2.60–69 Because of these limitations scientists are still looking for an ideal gastric cancer tumor biomarker.
Table 2.
Combined testing results of tumor markers in gastric cancer.
| Authors | Patients | Tumor markers | Conclusions |
|---|---|---|---|
| Xu et al60 | GC (n=50) Benign GC (n=50) Healthy control (n=50) |
CEA, CA 19-9, CA 72-4, CA 242 | The tumor incidence for combined CEA, CA 19-9, CA 72-4, CA 242 testing was higher than that of single tumor marker testing |
| Ning et al61 | GC (n=169) Healthy control (n=75) |
CEA, CA 19-9, CA 72-4, TK1 | The highest AUC (0.895) for single tumor biomarker revealed TK1 The combined TK1, CA 19-9, CA 72-4 and CEA testing showed higher AUC (0.953) than for each tumor marker tested separately |
| Feng et al62 | Early GC (n=587) | CEA, CA19-9, AFP, CA 125 | The positive rates of CEA, CA 19-9, AFP and CA 125 were relatively low: 4.3%, 4.8%, 1.5% and 1.9%, respectively The positive rate of combined CEA, CA 19-9, AFP and CA 125 testing equaled 10.4% |
| Chen et al63 | GC (n=87) Healthy control (n=40) |
CEA, CA 19-9, CA 72-4, CA 15-3, CA 125 | The combined CEA, CA 72-4, CA 19-9 and CA 125 testing had a higher positive rate (detection rate 60.9%) and diagnostic value for GC than the single tumor markers evaluation |
| Virgilio et al64 | GC (n=38) non-GC (n=41) |
CEA, CA 19-9, CA 72-4, CA 50 | Gastric lavage cytopathology correlated significantly with advancement of tumor invasion depth and the presence of local lymph node metastases Gastric lavage CEA, CA 19-9, CA 72-4, or CA 50 concentration did not achieve a statistically significant correlation with cancer staging |
| Liang et al65 | GC (n=2,288) Healthy control (n=1,869) |
CEA, CA 19-9, CA 72-4 | The sensitivity of CEA, CA 19-9 and CA 72-4 in the GC diagnosis was 20.1%, 21.4% and 27.6%, respectively and increased to 48.2% when tumor markers were used in combination |
| Rehena et al66 | Endoscopically suspected GC (71) |
CEA, CA 72-4 | CA 72-4 sensitivity (48.3%) was higher compared to CEA (31%) in diagnosis of gastric cancer, therefore CA 72-4 is recommended for use in conjunction with other diagnostic tests (eg, endoscopy) |
| Yu et al67 | GC (n=216) | CEA, CA 19-9, CA 72-4 | The combined positive rate of CEA, CA 19–9 and CA 72–4 was significantly higher compared with the individual CEA, CA 19–9 and CA 72–4 positive rates (44.91% vs 22.69%, 18.98% and 22.69%, respectively) |
| Yin et al68 | GC (n=45) GBD (n=40) Healthy control (n=30) |
CEA, CA 19-9, CA 72-4, TSGF | The AUCs for single detection of CEA, CA 72-4, CA 19-9 and TSGF in the diagnosis of GC equaled 0.833, 0.805, 0.810 and 0.839, respectively The AUC for combined testing of tumor markers revealed 0.913 |
| Yang et al69 | GC (n=106) GBD (n=149) Healthy control (n=171) |
CEA, CA19-9, CA 72-4, CA 125 | The sensitivities of CA72-4, CEA, CA 125 and CA 19-9 at the recommended cut-off level for all patients were 33.0%, 25.5%, 31.1% and 38.7%, respectively When all tumor markers were used in combination the sensitivity increased to 66.0% |
Abbreviations: AFP, alpha fetoprotein; AUC, area under receiver operating characteristics curve; CA 125, cancer antigen 125; CA 15-3, cancer antigen 15-3; CA 19-9, cancer antigen 19-9; CA 242, cancer antigen 242; CA 50, cancer antigen 50; CA 72-4, cancer antigen 72-4; CEA, carcinoembryonic antigen; GBD, benign gastric diseases; GC, gastric cancer; TK1, an enzyme involved in the regulation of the mammalian cell cycle; TSGF, tumor-specific growth factor.
In the available literature there are only two research papers evaluating quantitatively circulating concentration of GKN1 protein or GKN1 mRNA in individuals with gastric cancer.70,71 For example Villano et al70 did not confirm the utility of serum GKN1 mRNA evaluation in gastric cancer patients compared to serum samples from healthy individuals. However, Yoon et al71 did indicate the potential clinical application of serum GKN1 protein evaluation by means of ELISA in gastric cancer. The authors showed that GKN1 concentration was significantly lower in gastric cancer subjects compared to healthy individuals, and moreover to the hepatocellular carcinoma as well as colorectal cancer patients.71
The relation between the sensitivity and specificity of the test is illustrated using a receiver operating characteristic (ROC) curve, which is generated to calculate the area under the ROC curve (AUC). The ROC curve is a line graph that plots the probability of true positive results – or the sensitivity of the test – against the probability of false positive results for a range of different cut-off points.72 In the study of Yoon et al71 AUCs in differentiating gastric cancer patients from healthy subjects, hepatocellular carcinoma and colorectal cancer patients were: 1.00, 0.99 and 0.99, respectively. Moreover, Yoon et al71 found that GKN1 serum concentration was also useful for the assessment of gastric cancer cell invasion, as protein concentration was significantly higher in early gastric cancer (EGC) compared to advanced gastric cancer. EGC patients, based on the GKN1 concentration in the serum, additionally could be distinguished from: healthy subjects (AUC =1.00), atrophic gastritis patients (AUC =1.00), and individuals with atrophy and intestinal metaplasia (AUC =0.98). Interestingly, authors did not find differences between males and females for serum GKN1 concentration. It should be noted that the measurement of GKN1 concentration was possible only after the incubation of samples at 70°C for 10 minutes; under nonheating conditions Yoon et al71 did not detect GKN1 in human sera. Nevertheless the aspect of utility of GKN1 serum quantitative evaluation requires further studies.
Conclusion
Gastric cancer is one of the most common lethal cancers worldwide. GKN1 is a protein specific for stomach, and its role consists of maintaining mucosal integrity as well as the replenishment of the surface lumen epithelial cells layer. The evaluation of GKN1 expression seems to be a useful indicator of the presence of neoplastic or inflammatory lesions in the gastric mucosa. Because GKN1 expression is decreased in gastric tumor tissues and derived cell lines and its upregulation in cell lines of gastric cancer induces apoptosis, a suppressor role of this protein is suggested in tumor progression. However, the mechanism by which GKN1 is inactivated in gastric cancer cells is still not fully understood and obtained results are controversial. In the available literature few studies evaluate quantitatively circulating concentration of GKN1 protein or GKN1 mRNA in gastric cancer individuals. The future diagnostic capabilities of gastric cancer concern the assessment of serum GKN1 concentration by means of ELISA. Serum GKN1 concentration did not differ depending on patients’ sex. Interestingly, the measurement of GKN1 concentration is possible only after the incubation of samples at 70°C for 10 minutes. Nevertheless, the aspect of quantitative serum GKN1 evaluation is new in the context of available literature and requires further studies.
Acknowledgments
We are grateful to Martin Lenkiewicz, MSc, for his language assistance. The research did not receive any specific grant from funding agencies in the public, commercial or not for-profit sectors.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Fei HJ, Chen SC, Zhang JY, et al. Identification of significant biomarkers and pathways associated with gastric carcinogenesis by whole genome-wide expression profiling analysis. Int J Oncol. 2018;52(3):955–966. doi: 10.3892/ijo.2018.4243. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Karim-Kos HE, Coebergh JW, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European cancer observatory. Eur J Cancer. 2015;51(9):1164–1187. doi: 10.1016/j.ejca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Park JY, Forman D, Greenberg ER, Herrero R. Helicobacter pylori eradication in the prevention of gastric cancer: are more trials needed? Curr Oncol Rep. 2013;15(6):517–525. doi: 10.1007/s11912-013-0341-5. [DOI] [PubMed] [Google Scholar]
- 5.Shi LS, Wang H, Wang F, Feng M, Wang M, Guan WX. Effects of gastrokine-2 expression on gastric cancer cell apoptosis by activation of extrinsic apoptotic pathways. Mol Med Rep. 2014;10(6):2898–2904. doi: 10.3892/mmr.2014.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida T, Ono H, Kuchiba A, Saeki N, Sakamoto H. Genome-wide germline analyses on cancer susceptibility and GeMDBJ database: gastric cancer as an example. Cancer Sci. 2010;101(7):1582–1589. doi: 10.1111/j.1349-7006.2010.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirvish SS. Gastric cancer and salivary nitrate and nitrite. Nature. 1985;315(6019):461–462. doi: 10.1038/315461c0. [DOI] [PubMed] [Google Scholar]
- 8.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 9.Lo SS, Wu CW, Hsieh MC, Kuo HS, Lui WY, P’Eng FK. Relationship between age and clinical characteristics of patients with gastric cancer. J Gastroenterol Hepatol. 1996;11(6):511–514. doi: 10.1111/j.1440-1746.1996.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 10.Hwang H, Dwyer J, Diet RRM. Helicobacter pylori infection. Food preservation and gastric cancer risk: are there new roles for preventative factors? Nutr Rev. 1994;52(3):75–83. doi: 10.1111/j.1753-4887.1994.tb01394.x. [DOI] [PubMed] [Google Scholar]
- 11.Roberts-Thomson IC, Butler WJ. Polymorphism and gastric cancer. J Gastroenterol Hepatol. 2005;20(5):793–794. doi: 10.1111/j.1440-1746.2005.03938.x. [DOI] [PubMed] [Google Scholar]
- 12.D’Elia L, Galletti F, Strazzullo P. Dietary salt intake and risk of gastric cancer. Cancer Treat Res. 2014;159:83–95. doi: 10.1007/978-3-642-38007-5_6. [DOI] [PubMed] [Google Scholar]
- 13.Shu L, Wang XQ, Wang SF, et al. Dietary patterns and stomach cancer: a meta-analysis. Nutr Cancer. 2013;65(8):1105–1115. doi: 10.1080/01635581.2013.828086. [DOI] [PubMed] [Google Scholar]
- 14.Jiexian J, Xiaoqin X, Lili D, et al. Clinical assessment and prognostic evaluation of tumor markers in patients with gastric cancer. Int J Biol Markers. 2013;28(2):192–200. doi: 10.5301/jbm.5000023. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Yang Y, Lu M, Shen L. Predictive value of serum CEA, CA19-9 and CA72.4 in early diagnosis of recurrence after radical resection of gastric cancer. Hepatogastroenterology. 2011;58(112):2166–2170. doi: 10.5754/hge11753. [DOI] [PubMed] [Google Scholar]
- 16.Hnia K, Notarnicola C, de Santa Barbara P, et al. Biochemical properties of gastrokine-1 purified from chicken gizzard smooth muscle. PLoS One. 2008;3(12):e3854. doi: 10.1371/journal.pone.0003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin TE, Powell CT, Wang Z, et al. A novel mitogenic protein that is highly expressed in cells of the gastric antrum mucosa. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G332–G343. doi: 10.1152/ajpgi.00453.2002. [DOI] [PubMed] [Google Scholar]
- 18.Xing R, Li W, Cui J, et al. Gastrokine 1 induces senescence through p16/Rb pathway activation in gastric cancer cells. Gut. 2012;61(1):43–52. doi: 10.1136/gut.2010.230623. [DOI] [PubMed] [Google Scholar]
- 19.Yoon JH, Song JH, Zhang C, et al. Inactivation of the gastrokine 1 gene in gastric adenomas and carcinomas. J Pathol. 2011;223(5):618–625. doi: 10.1002/path.2838. [DOI] [PubMed] [Google Scholar]
- 20.Moss SF, Lee JW, Sabo E, et al. Decreased expression of gastrokine 1 and the trefoil factor interacting protein TFIZ1/GKN2 in gastric cancer: influence of tumor histology and relationship to prognosis. Clin Cancer Res. 2008;14(13):4161–4167. doi: 10.1158/1078-0432.CCR-07-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baus-Loncar M, Lubka M, Pusch CM, Otto WR, Poulsom R, Blin N. Cytokine regulation of the trefoil factor family binding protein GKN2 (GDDR/TFIZ1/blottin) in human gastrointestinal epithelial cells. Cell Physiol Biochem. 2007;20(1–4):193–204. doi: 10.1159/000104166. [DOI] [PubMed] [Google Scholar]
- 22.Otto WR, Patel K, McKinnell I, et al. Identification of blottin: a novel gastric trefoil factor family-2 binding protein. Proteomics. 2006;6(15):4235–4245. doi: 10.1002/pmic.200500911. [DOI] [PubMed] [Google Scholar]
- 23.Martin G, Wex T, Treiber G, Malfertheiner P, Nardone G. Low-dose aspirin reduces the gene expression of gastrokine-1 in the antral mucosa of healthy subjects. Alimentary Pharmacol Therapeutics. 2008;28(6):782–788. doi: 10.1111/j.1365-2036.2008.03793.x. [DOI] [PubMed] [Google Scholar]
- 24.Oien KA, McGregor F, Butler S, et al. Gastrokine 1 is abundantly and specifically expressed in superficial gastric epithelium, down-regulated in gastric carcinoma, and shows high evolutionary conservation. J Pathol. 2004;203(3):789–797. doi: 10.1002/path.1583. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Pulido L, Devos D, Valencia A. BRICHOS: a conserved domain in proteins associated with dementia, respiratory distress and cancer. Trends Biochem Sci. 2002;27(7):329–332. doi: 10.1016/s0968-0004(02)02134-5. [DOI] [PubMed] [Google Scholar]
- 26.Du JJ, Dou KF, Peng SY, et al. [Study on novel gene GDDR related to gastric cancer] Zhonghua Wai Ke Za Zhi. 2005;43(1):10–13. Chinese. [PubMed] [Google Scholar]
- 27.Westley BR, Griffin SM, May FE. Interaction between TFF1, a gastric tumor suppressor trefoil protein, and TFIZ1, a brichos domain-containing protein with homology to SP-C. Biochemistry. 2005;44(22):7967–7975. doi: 10.1021/bi047287n. [DOI] [PubMed] [Google Scholar]
- 28.Kouznetsova I, Laubinger W, Kalbacher H, et al. Biosynthesis of gastrokine-2 in the human gastric mucosa: restricted spatial expression along the antral gland axis and differential interaction with TFF1, TFF2 and mucins. Cell Physiol Biochem. 2007;20(6):899–908. doi: 10.1159/000110450. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F, Tang JM, Wang L, et al. Detection of β-catenin, gastrokine-2 and embryonic stem cell expressed Ras in gastric cancers. Int J Clin Exp Pathol. 2010;3(8):782–791. [PMC free article] [PubMed] [Google Scholar]
- 30.Chu G, Qi S, Yang G, Dou K, Du J, Lu Z. Gastrointestinal tract specific gene GDDR inhibits the progression of gastric cancer in a TFF1 dependent manner. Mol Cell Biochem. 2012;359(1–2):369–374. doi: 10.1007/s11010-011-1030-z. [DOI] [PubMed] [Google Scholar]
- 31.Kim O, Yoon JH, Choi WS, et al. GKN2 contributes to the homeostasis of gastric mucosa by inhibiting GKN1 activity. J Cell Physiol. 2014;229(6):762–771. doi: 10.1002/jcp.24496. [DOI] [PubMed] [Google Scholar]
- 32.Dai J, Zhang N, Wang J, Chen M, Chen J. Gastrokine-2 is downregulated in gastric cancer and its restoration suppresses gastric tumorigenesis and cancer metastasis. Tumor Biol. 2014;35(5):4199–4207. doi: 10.1007/s13277-013-1550-0. [DOI] [PubMed] [Google Scholar]
- 33.Menheniott TR, O’Connor L, Chionh YT, et al. Loss of gastrokine-2 drives premalignant gastric inflammation and tumor progression. J Clin Invest. 2016;126(4):1383–1400. doi: 10.1172/JCI82655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang J, Pan X, Lin H, Hu Z, Xiao P, Hu H. GKN2 increases aoptosis, reduces the proliferation and invasion ability of gastric cancer cells through down-regulating the JAK/STAT sipgnaling pathway. Am J Transl Res. 2017;9(2):803–811. [PMC free article] [PubMed] [Google Scholar]
- 35.Menheniott TR, Peterson AJ, O’Connor L, et al. A novel gastrokine, Gkn3, marks gastric atrophy and shows evidence of adaptive gene loss in humans. Gastroenterology. 2010;138(5):1823–1835. doi: 10.1053/j.gastro.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 36.Geahlen JH, Lapid C, Thorell K, et al. Evolution of the human gastrokine locus and confounding factors regarding the pseudogenicity of GKN3. Physiol Genomics. 2013;45(15):667–683. doi: 10.1152/physiolgenomics.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menheniott TR, Kurklu B, Giraud AS. Gastrokines: stomach-specific proteins with putative homeostatic and tumor suppressor roles. Am J Physiol Gastrointest Liver Physiol. 2013;304(2):G109–G121. doi: 10.1152/ajpgi.00374.2012. [DOI] [PubMed] [Google Scholar]
- 38.Rippa E, La Monica G, Allocca R, Romano MF, de Palma M, Arcari P. Overexpression of gastrokine 1 in gastric cancer cells induces Fas-mediated apoptosis. J Cell Physiol. 2011;226(10):2571–2578. doi: 10.1002/jcp.22601. [DOI] [PubMed] [Google Scholar]
- 39.Yoon JH, Choi YJ, Choi WS, Nam SW, Lee JY, Park WS. Functional analysis of the NH2-terminal hydrophobic region and BRICHOS domain of GKN1. Biochem Biophys Res Commun. 2013;440(4):689–695. doi: 10.1016/j.bbrc.2013.09.123. [DOI] [PubMed] [Google Scholar]
- 40.Yan GR, Xu SH, Tan ZL, Yin XF, He QY. Proteomics characterization of gastrokine 1-induced growth inhibition of gastric cancer cells. Proteomics. 2011;11(18):3657–3664. doi: 10.1002/pmic.201100215. [DOI] [PubMed] [Google Scholar]
- 41.Yoshihara T, Kadota Y, Yoshimura Y, et al. Proteomic alteration in gastic adenocarcinomas from Japanese patients. Mol Cancer. 2006;5(1):75. doi: 10.1186/1476-4598-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo XY, Dong L, Qin B, Jiang J, Shi AM. Decreased expression of gastrokine 1 in gastric mucosa of gastric cancer patients. World J Gastroenterol. 2014;20(44):16702–16706. doi: 10.3748/wjg.v20.i44.16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao W, Chen J, Peng TL, Yin XF, Chen LZ, Chen MH. Helicobacter pylori infection and administration of non-steroidal anti-inflammatory drugs down-regulate the expression of gastrokine-1 in gastric mucosa. Turk J Gastroenterol. 2012;23(3):212–219. doi: 10.4318/tjg.2012.0345. [DOI] [PubMed] [Google Scholar]
- 44.Nardone G, Martin G, Rocco A, et al. Molecular expression of gastrokine 1 in normal mucosa and in Helicobacter pylori-related preneoplastic and neoplastic gastric lesions. Cancer Biol Ther. 2008;7(12):1890–1895. doi: 10.4161/cbt.7.12.6936. [DOI] [PubMed] [Google Scholar]
- 45.Walsh-Reitz MM, Huang EF, Musch MW, et al. AMP-18 protects barrier function of colonic epithelial cells: role of tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G163–G171. doi: 10.1152/ajpgi.00013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rippa E, Altieri F, di Stadio CS, et al. Ectopic expression of gastrokine 1 in gastric cancer cells up-regulates tight and adherens junction proteins network. Pathol Res Pract. 2015;211(8):577–583. doi: 10.1016/j.prp.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Toback FG, Walsh-Reitz MM, Musch MW, et al. Peptide fragments of AMP-18, a novel secreted gastric antrum mucosal protein, are mitogenic and motogenic. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G344–G353. doi: 10.1152/ajpgi.00455.2002. [DOI] [PubMed] [Google Scholar]
- 48.Yoon JH, Kang YH, Choi YJ, et al. Gastrokine 1 functions as a tumor suppressor by inhibition of epithelial-mesenchymal transition in gastric cancers. J Cancer Res Clin Oncol. 2011;137(11):1697–1704. doi: 10.1007/s00432-011-1051-8. [DOI] [PubMed] [Google Scholar]
- 49.Xing R, Cui JT, Xia N, Lu YY, Yy L. GKN1 inhibits cell invasion in gastric cancer by inactivating the NF-kappaB pathway. Discov Med. 2015;19(103):65–71. [PubMed] [Google Scholar]
- 50.Yoon JH, Choi WS, Kim O, et al. Gastrokine 1 inhibits gastric cancer cell migration and invasion by downregulating RhoA expression. Gastric Cancer. 2017;20(2):274–285. doi: 10.1007/s10120-016-0617-1. [DOI] [PubMed] [Google Scholar]
- 51.Àngels Díaz-Ramos, Roig-Borrellas A, García-Melero A, López-Alemany R. α -Enolase, a multifunctional protein: its role on pathophysiological situations. J Biomed Biotechnol. 2012;2012(7):1–12. doi: 10.1155/2012/156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai ST, Chien IH, Shen WH, et al. ENO1, a potential prognostic head and neck cancer marker, promotes transformation partly via chemokine CCL20 induction. Eur J Cancer. 2010;46(9):1712–1723. doi: 10.1016/j.ejca.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Franco AT, Israel DA, Washington MK, et al. Activation of -catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA. 2005;102(30):10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu F, Tempera I, Lee HT, Dewispelaere K, Lieberman PM. EBNA1 binding and epigenetic regulation of gastrokine tumor suppressor genes in gastric carcinoma cells. Virol J. 2014;11(1):12. doi: 10.1186/1743-422X-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altieri F, di Stadio CS, Federico A, et al. Epigenetic alterations of gastrokine 1 gene expression in gastric cancer. Oncotarget. 2017;8(10):16899–16911. doi: 10.18632/oncotarget.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nardone G, Rippa E, Martin G, et al. Gastrokine 1 expression in patients with and without Helicobacter pylori infection. Dig Liver Dis. 2007;39(2):122–129. doi: 10.1016/j.dld.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Xiao JW, Chen JH, Ren MY, Tian XB, Wang CS. Relationship between expression of gastrokine 1 and clinicopathological characteristics in gastric cancer patients. Asian Pac J Cancer Prev. 2012;13(11):5897–5901. doi: 10.7314/apjcp.2012.13.11.5897. [DOI] [PubMed] [Google Scholar]
- 58.Yoon JH, Seo HS, Choi SS, et al. Gastrokine 1 inhibits the carcinogenic potentials of Helicobacter pylori CagA. Carcinogenesis. 2014;35(11):2619–2629. doi: 10.1093/carcin/bgu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kotzev AI, Draganov PV. Carbohydrate antigen 19-9, carcinoembryonic antigen, and carbohydrate antigen 72-4 in gastric cancer: is the old band still playing? Gastrointest Tumors. 2018;5(1-2):1–13. doi: 10.1159/000488240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu MX, Cui HJ, Yao TL, Gui YF. Clinical value of combined tests for tumor markers for gastric cancer. J Biol Regul Homeost Agents. 2018;32(2):263–268. [PubMed] [Google Scholar]
- 61.Ning S, Wei W, Li J, et al. Clinical significance and diagnostic capacity of serum TK1, CEA, CA 19-9 and Ca 72-4 levels in gastric and colorectal cancer patients. J Cancer. 2018;9(3):494–501. doi: 10.7150/jca.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng F, Tian Y, Xu G, et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17(1):737. doi: 10.1186/s12885-017-3738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen C, Chen Q, Zhao Q, Liu M, Guo J. Value of combined detection of serum CEA, CA72-4, CA19-9, CA15-3 and CA12-5 in the diagnosis of gastric cancer. Ann Clin Lab Sci. 2017;47(3):260–263. [PubMed] [Google Scholar]
- 64.Virgilio E, Giarnieri E, Montagnini M, et al. Analyzing gastric lavage of gastric cancer patients: a prospective observational study on cytopathology and determination of intragastric CEA, CA 19.9, Ca 72.4, and CA 50. Acta Cytol. 2016;60(2):161–166. doi: 10.1159/000445765. [DOI] [PubMed] [Google Scholar]
- 65.Liang Y, Wang W, Fang C, et al. Clinical significance and diagnostic value of serum CEA, CA19-9 and CA72-4 in patients with gastric cancer. Oncotarget. 2016;7(31):49565–49573. doi: 10.18632/oncotarget.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rehena Z, Ghosh CK, Afroz F, et al. Comparison of serum CA72-4 and CEA levels in patient with endoscopically suspected gastric carcinoma. Mymensingh Med J. 2015;24(3):542–549. [PubMed] [Google Scholar]
- 67.Yu J, Zhang S, Zhao B. Differences and correlation of serum CEA, CA19-9 and CA72-4 in gastric cancer. Mol Clin Oncol. 2016;4(3):441–449. doi: 10.3892/mco.2015.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin LK, Sun XQ, Mou DZ. Value of combined detection of serum CEA, CA72-4, CA19-9 and TSGF in the diagnosis of gastric cancer. Asian Pac J Cancer Prev. 2015;16(9):3867–3870. doi: 10.7314/apjcp.2015.16.9.3867. [DOI] [PubMed] [Google Scholar]
- 69.Yang AP, Liu J, Lei HY, et al. CA72-4 combined with CEA, CA125 and CAl9-9 improves the sensitivity for the early diagnosis of gastric cancer. Clin Chim Acta. 2014;437:183–186. doi: 10.1016/j.cca.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 70.Villano V, di Stadio CS, Federico A, et al. Gastrokine 1 mRNA in human sera is not informative biomarker for gastric cancer. J Negat Results Biomed. 2016;15(1):14. doi: 10.1186/s12952-016-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoon JH, Ham IH, Kim O, et al. Gastrokine 1 protein is a potential theragnostic target for gastric cancer. Gastric Cancer. 2018;21(6):956–967. doi: 10.1007/s10120-018-0828-8. [DOI] [PubMed] [Google Scholar]
- 72.Lalkhen AG, McCluskey A. Clinical tests: sensitivity and specificity. BJA Educ. 2008;8(4):143–146. [Google Scholar]