Abstract
Objectives:
To improve the quality of invasive pulmonary aspergillosis (IPA) management for intensive care unit (ICU) patients using a practical diagnostic scoring model.
Methods:
This nested case-control study aimed to determine the incidence of IPA in 405 ICU patients, between July 2012 and June 2014, at 6 hospitals in Jakarta, Indonesia. Phenotypic identifications and galactomannan (GM) tests of sera and lung excreta were performed in mycology laboratory, Parasitology Department, Faculty of Medicine, Universitas Indonesia in Jakarta, Indonesia.
Results:
The incidence of IPA in the ICUs was 7.7% (31 of 405 patients). A scoring model used for IPA diagnosis showed 4 variables as the most potential risk factors: lung excreta GM index (score 2), solid organ malignancy (score 2), pulmonary tuberculosis (score 2), and systemic corticosteroids (score 1). Patients were included in a high-risk group if their score was >2, and in a low-risk group if their score was <2.
Conclusion:
This study provides a novel diagnosis scoring model to predict IPA in ICU patients. Using this model, a more rapid diagnosis and treatment of IPA may be possible. The application of the diagnosis scoring should be preceded by specified pre-requisites.
Invasive pulmonary aspergillosis (IPA) is a spectrum of invasive infection due to Aspergillus that may be life-threatening in immunodeficient patients.1,2 It is usually preceded by colonization of the airways with Aspergillus conidia.3 Prolonged neutropenia, hemato-oncological malignancy, bone marrow and solid organ transplant have been reported as IPA risk factors.4,5 The emergence of other risk factors in critically ill intensive care unit (ICU) patients such as systemic corticosteroid therapy, as well as chronic obstructive pulmonary disease (COPD), influenza, liver cirrhosis, and diabetes might contribute to the raised incidence of IPA.6,7
The incidence of IPA varies, depending on patient population and local epidemiology.8,9 In the ICUs of several European countries, the incidence rate has been reported to be between 0.3-6.9%.2,10 Diagnosis of IPA is considered difficult because of the atypical clinical symptoms, and the results of routine laboratory tests and radiological features of IPA are difficult to distinguish from other diseases.11,12 The standard diagnostic procedure is a cultured tissue biopsy. However, obtaining biopsies is almost impossible in critically ill patients and tissue biopsy culture is time-consuming.
Galactomannan (GM) antigen detection has shown promising results for the rapid diagnosis of IPA.13 Detection of GM in serum with an index of >0.5 is useful for early diagnosis of IPA in neutropenic patients, but it is not considered a suitable marker in non-neutropenic patients.14 Galactomannan antigen detection of lung excreta, for example, using broncho-alveolar lavage, bronchial washing, and endotracheal tube (ETT) aspirates, has a higher sensitivity than that of serum, both in neutropenic and non-neutropenic patients.14,15 Invasive pulmonary aspergillosis diagnosis consists of 3 categories, based on the guidelines of the European Organization for Research and Treatment of Cancer/Mycoses study group (EORTC/MSG), namely, proven, probable, and possible.16 These diagnostic criteria are less practical due to limited usefulness for research and due to certain risk factor conditions, and are difficult to apply in daily practice, including ICU patients.17,18 These limitations cause delays in treatment and worsen the prognosis.19
To address this situation, several methods of diagnosis have been proposed in the form of diagnostic algorithms and scoring models.17,18 These models are practical but have limitations because they require an Aspergillus culture of clinical materials, which is insensitive and time-consuming. There is no agreed model for diagnosis, and obstacles in establishing a diagnosis contribute to the high mortality rate in IPA.20 In ICU patients, mortality rates have been reported to be between 46-72%.2,21 Delaying antifungal treatment increases the risk of death in IPA patients, and results in high costs due to a longer hospital stay and an increased number of drugs and medical devices.21 This study aimed to improve the quality of IPA management in ICU patients, based on a scoring model of IPA diagnosis that could be used practically.
Methods
This study was part of a multicenter cohort study to determine the IPA incidence in ICU patients at 6 hospitals in Indonesia between July 2012 and June 2014. The study was approved by the Ethics Committee of the Faculty of Medicine in an appropriately accredited university in Jakarta, Indonesia, following the principles of the Helsinki Declaration.
Study participants who met the inclusion criteria comprised 405 ICU patients ≥15 years old suspected as having IPA. The inclusion criteria comprised patients with one of the following conditions: patients with fever unresponsive to adequate antibiotics, neutropenic hemato-oncology patients with signs of infection, cancer chemotherapy recipients within the last 3 months prior to ICU admission, patients having received corticosteroid therapy for at least 7 days prior to ICU admission or having received cumulative doses over 250 mg of methylprednisolone in the last 3 months, patients undergoing a prolonged ICU stay (>21 days), patients suffering from liver cirrhosis, patients with COPD, HIV-infected patients with impaired/lung disease, or patients receiving other immunosuppressive treatments (for example, tacrolimus, cyclosporine, or methotrexate). Patients who did not meet these criteria but who showed clinical symptoms of invasive mycoses (pleuritic pain, pleural rub), or who had one of the symptoms of lower respiratory tract infection (such as increased sputum secretion, shortness of breath, or hemoptysis), or patients with new infiltrates evident on chest plain radiographs were also included in the study. Exclusion criteria included patients/families who declined to participate and those having received systemic antifungal therapy within the previous month. A scoring model for IPA diagnosis was determined using a nested case-control design. The case group comprised patients who met the diagnostic criteria of probable/putative IPA, whereas the control group was determined through randomization of patients who did not suffer from IPA. Phenotypic identifications and GM antigen detections of sera and lung excreta were undertaken in a medical department mycology laboratory in Indonesia. Molecular typing and identification were performed in a microbiology laboratory in the Netherlands.
A scoring model for IPA diagnosis was obtained using multivariate logistic regression analysis, Statistical Package for Social Sciences Version 19.0 (IBM Corp., Armonk, NY, USA). Variables with a p-value of <0.2 were included in the multivariate analysis, while variables with a p-value of >0.2 could still be included in the analysis if they were considered to affect the analysis or epidemiological data. Odds ratios (OR) and 95% confidence intervals (CI) were used to estimate the relative risk (RR). The scoring model was created by entering a value proportional to the OR and using adjusted logistic regression models.
Results
Of the eligible patients, 405 ICU patients completed the study and of these, 31 (7.7%) were diagnosed with IPA. Therefore, the case group comprised 31 patients, and the control group comprised 124 patients. The incidence rate of IPA in this study was slightly higher than in European countries as it included patients with established and non-established risk factors. The incidence rates for IPA in developing countries tend to be higher due to the massive and less selective use of antibiotics and corticosteroids, the higher rate of tuberculosis (TB) cases as the underlying disease in IPA, and the lack of data from autopsies.
There was no statistically significant difference between the case and control groups in terms of gender (p=0.871) and age (p=0.772 and p=0.812). Most of the patients in both groups were aged >60 years. Older patients are more at risk because of immunosenescence, dysregulation of immune function that renders elderly patients more susceptible to infections, autoimmune diseases, and malignancies.
Furthermore, a bivariate analysis was conducted and variables with a p<0.2 were included in the multivariate analysis. Certain variables that were considered to affect the prediction of IPA diagnosis were also included in the analysis, despite having a p>0.2, according to previous studies.18 Sixteen variables were included to build a scoring model to predict IPA (Table 1).
Table 1.
Variables included in the model analysis to predict IPA.
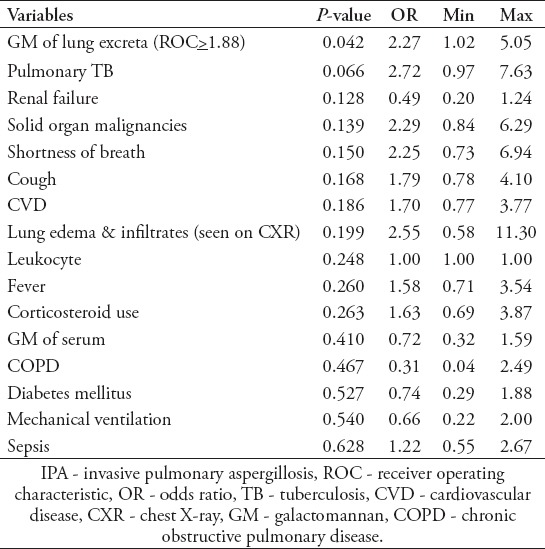
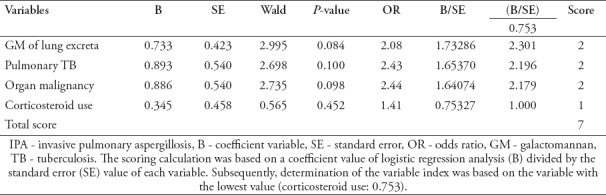
Multivariate analysis was conducted, and the backward method was applied to obtain the variables considered as high-risk factors for IPA, namely, the GM value of lung excreta, solid organ malignancies, pulmonary TB, and the use of systemic corticosteroids. The scoring model for IPA diagnosis was then conducted to facilitate a practical application. The scores were as follows: lung excreta GM: 2.301=2, pulmonary TB: 2.196=2, solid organ malignancy: 2.179-2, and systemic corticosteroid use score: 1. If the study participant had more than one variable determinant, then the score was calculated with a maximum total of 7 (Table 2).
Table 2.
Model conversion to a scoring model for the diagnosis of IPA.
The next step was to determine the discrimination value to predict IPA based on receiver operating characteristic (ROC) curve analysis. Further calculations were performed to determine the diagnostic value of various intersection points. Our analysis determined a diagnostic cut-off of 2 for IPA. This cut-off point showed high sensitivity (77.4%) and an acceptable specificity (48.4%). High sensitivity is useful to predict an early diagnosis of IPA and provides the basis for early antifungal administration. However, a score of 3 produced higher specificity (82.3%), but the sensitivity was lower (48.4%) and could cause diagnostic delay (Figure 1).
Figure 1.
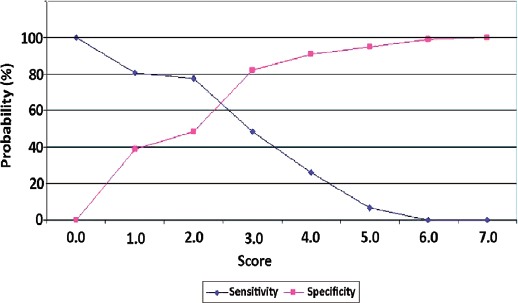
Sensitivity and specificity curves. Sensitivity and specificity curves were used to determine the optimal cut-off value. There were 2 alternative points of intersection: a score of 2 (sensitivity 77.4%, specificity 48.4%) and a score of 3 (48.4% sensitivity, 82.3% specificity).
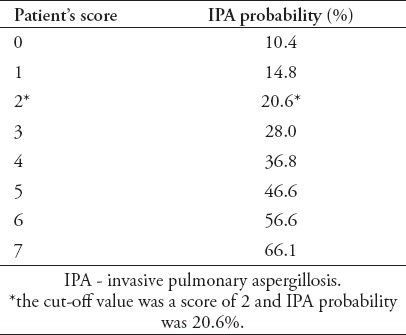
The prediction of a patient’s probability to suffer from IPA was determined based on regression analysis of the various scores (Table 3). If a patient had a score of 2, the IPA probability was 20.6%. In that case, the antifungal administration should be considered since IPA mortality is remarkably high (Appendix 1).
Table 3.
Association of a possible patient score and IPA probability.
Appendix 1.
Clinical application of the scoring model for diagnosis of invasive pulmonary aspergillosis (IPA). In this scenario, the patient had 2 risk factors: a galactomannan (GM) index of endotracheal tube (ETT) aspirates >1.88, and pulmonary tuberculosis (TB) was positive. Therefore, the IPA score was 4 and the IPA probability was 36.8% (high-risk group). Antifungal administration is recommended for the patient.

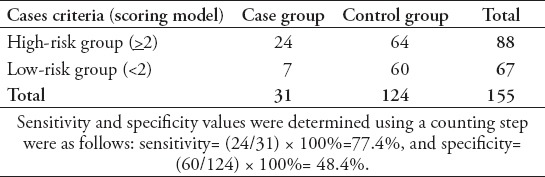
The suitability of the scoring model with a diagnosis in the case group (patients with IPA) and the control group (patients who did not suffer from IPA) is shown in Table 4. The case group comprised 31 patients. There were 24 patients with a total score of >2 included in the high-risk group and we recommended these patients to start appropriate antifungal therapy, 7 patients with a total score of <2 were classified into the low-risk group and antifungal therapy was not required. This was in accordance with the internal validation test results based on the area under the curve (AUC) value (AUC: 0.685, 95% CI: 0.571-0.789).
Table 4.
The suitability of a diagnosis using the scoring model.
Discussion
The scoring model for IPA diagnosis in this study differs from that of previous studies as it does not include positive Aspergillus culture as a predictor variable.16,18 This is an advantage since cultures are time-consuming with low sensitivity. However, Aspergillus cultures should be pursued in laboratories with complete facilities to exclude the cross-reactivity of GM detection, increase the accuracy of IPA diagnosis and detect azole resistance.22,23
One limitation in this study was that serial GM testing could not be performed due to financial considerations. It is necessary to perform serial GM testing for re-assessment of risk factors because the disease course could be progressive.24 In such circumstances, an unstable clinical situation might occur which could change the IPA score from low-risk (<2) to high-risk (>2). Patients initially considered in the low-risk IPA group without specific clinical symptoms and radiological abnormalities were classified as having ‘silent IPA’ (an early disease course). With clinical deterioration, re-scoring could be performed to include repetition of the GM test and re-evaluation of other risk factors (systemic corticosteroid therapy, solid organ malignancy or pulmonary tuberculosis). If re-scoring showed that patients needed to be re-classified into a high-risk group, then patient management could be changed and antifungal therapy administered.
Galactomannan antigen detection can be performed for early detection of IPA as the results are much more quickly obtained than tissue culturing. Invasive pulmonary aspergillosis can be detected in blood from 5-8 days prior to the onset of clinical symptoms, and from 7-12 days prior to the appearance of computed tomography (CT) scan abnormalities. Galactomannan antigen detection in bronchial secretions is recommended as the preferred screening test for IPA in high-risk patients as it has an excellent negative predictive value. Sensitivity and specificity of GM detection vary due to many factors, such as age, underlying diseases, certain antimicrobial use and duration of antifungal therapy.14,25 The serum GM index cut-off point used in this study was 0.51. The p-value of serum GM in the case group compared to the control was not significant (p=0.41) and the OR of 0.72 indicated that GM serum in this study was not an effective predictor of IPA. Lung excreta GM had a cut-off point of 1.88 and compared to the control group was significant (p=0.041), and the OR of 2.27 indicated that the value of lung excreta GM was a much better predictor of IPA compared to serum GM.
The existence of GM in lung excreta is related to Aspergillus invasion into the airways, while the presence of GM in serum is related to further penetration of Aspergillus hyphae through the endothelial cell wall.26 In non-neutropenic patients, Aspergillus does not always invade blood vessels, so the GM does not readily enter the bloodstream and neutrophils could have eliminated the GM. In non-neutropenic patients, serum GM was detected in lower concentration levels and was not reliable to predict IPA.27 However, in neutropenic patients, Aspergillus infection might cause angioinvasion, so that GM could be easily released into the bloodstream.27,28 In a neutropenic condition, GM is not properly eliminated from the bloodstream, so the serum GM is detected in higher concentrations and potentially predicts IPA in neutropenic patients; whereas, in non-neutropenic patients, serum GM is detected in lower concentrations or remains negative and is therefore, unlikely to predict IPA.27 The GM concentration might reflect fungal burden; therefore, the examination of serial GM could be used to predict the prognosis or monitoring of the treatment response. However, GM detection should be combined with other tests such as culture, a CT scan, or polymerase chain reaction (PCR) testing for an optimal benefit.29 The accuracy of IPA diagnosis could prevent excessive use of antifungal drugs, health care costs could be decreased, and the emergence of resistant strains could be prevented.
The high-resolution CT (HRCT) scan of the chest actually provides a more specific radiology result and diagnostic value. However, due to limited facilities and financial issues in carrying out the HRCT, this study only use the chest X-ray instead of the CT scan. Some other study groups are also aware with the limited access to HRCT, especially in low-middle income country (LMIC), so that the use of chest X-ray is still acceptable for the diagnosis.17,30
This scoring model might be used to screen for IPA but should be accompanied with more definite reference tests undertaken in appropriate health facilities, including the standard culture-based method to isolate Aspergillus as well as non-culture-based PCR methods.31,32 To avoid excessive diagnosis that may lead to over-treatment due to the application of this scoring model, it is necessary to consider the pre-requisite conditions of inclusion criteria that must be met by each patient. The use of this scoring model in different hospitals or other patient groups, also needs the external validation to be performed.
With a more precise diagnosis, selective antifungal drugs could be given to prevent excessive health costs. If the initial scoring showed patients to be included in a high-risk category, systemic antifungal drugs should be given but when culture results show no growth of Aspergillus and clinical conditions improve then administration of antifungal drugs should be reconsidered through taking into account all clinical aspects. Clinical monitoring should be continued until the patient’s condition is stable and improving. External validation of the scoring model of IPA diagnosis is necessary to determine its validity before it could be applied regularly. Studies regarding the susceptibility profile of Aspergillus derived from the environment also need to be performed to anticipate the emergence of resistant species that could complicate IPA management.
In conclusion, this scoring model for diagnosing IPA in the ICU was based on 4 variables with the highest potential risk factors, namely, the value of lung excreta GM (score 2), solid organ malignancy (score 2), pulmonary tuberculosis (score 2), and the use of systemic corticosteroids (score 1). Patients were regarded at high risk of developing IPA if they scored >2, whereas a score <2 revealed a low risk of developing IPA. The application of this scoring model should be preceded with certain pre-requisites. External validation is necessary prior to this model being applied regularly.
Acknowledgment
The authors gratefully acknowledge Ganda Sibabiat, Radja Nasution for patient recruitment in Jakarta, Indonesia. Mrs. Ridhawati Sjam, Mrs. Mulyati, R. Adawiyah and Findra Setianingrum for laboratory work in Jakarta, Indonesia. Mrs. Ilse C. Breuker for laboratory work in CWZ laboratory in Nijmegen, The Netherlands.
Footnotes
References
- 1.Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. doi: 10.1086/513943. quiz 804-805. [DOI] [PubMed] [Google Scholar]
- 2.Taccone FS, Van den Abeele AM, Bulpa P, Misset B, Meersseman W, Cardoso T, et al. Epidemiology of invasive aspergillosis in critically ill patients:clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7. doi: 10.1186/s13054-014-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270–277. doi: 10.1136/thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]
- 5.Vallabhaneni S, Benedict K, Derado G, Mody RK. Trends in hospitalizations related to invasive Aspergillosis and Mucormycosis in the United States, 2000-2013. Open Forum Infect Dis. 2017;4:ofw268. doi: 10.1093/ofid/ofw268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Yang Q, Huang J, Li L. Clinical findings in 19 cases of invasive pulmonary aspergillosis with liver cirrhosis. Multidiscip Respir Med. 2014;9:1–5. doi: 10.1186/2049-6958-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van de Veerdonk FL, Kolwijck E, Lestrade PP, Hodiamont CJ, Rijnders BJ, van Paassen J, et al. Influenza-Associated Aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201612-2540LE. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Schwarz C, Brandt C, Whitaker P, Sutharsan S, Skopnik H, Gartner S, et al. Invasive pulmonary fungal infections in cystic fibrosis. Mycopathologia. 2018;183:33–43. doi: 10.1007/s11046-017-0199-4. [DOI] [PubMed] [Google Scholar]
- 9.Rotjanapan P, Chen YC, Chakrabarti A, Li RY, Rudramurthy SM, Yu J, et al. Erratum:Epidemiology and clinical characteristics of invasive mould infections:A multicenter, retrospective analysis in five Asian countries. Med Mycol. 2018;56:387. doi: 10.1093/mmy/myx055. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos G, Frantzeskaki F, Poulakou G, Armaganidis A. Invasive aspergillosis in the intensive care unit. Ann N Y Acad Sci. 2012;1272:31–39. doi: 10.1111/j.1749-6632.2012.06805.x. [DOI] [PubMed] [Google Scholar]
- 11.Bassetti M, Bouza E. Invasive mould infections in the ICU setting:complexities and solutions. J Antimicrob Chemother. 2017;72:i39–i47. doi: 10.1093/jac/dkx032. [DOI] [PubMed] [Google Scholar]
- 12.Bassetti M, Garnacho-Montero J, Calandra T, Kullberg B, Dimopoulos G, Azoulay E, et al. Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intensive Care Med. 2017;43:1225–1238. doi: 10.1007/s00134-017-4731-2. [DOI] [PubMed] [Google Scholar]
- 13.Leeflang MM, Debets-Ossenkopp YJ, Wang J, Visser CE, Scholten RJ, Hooft L, et al. Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev. 2015:CD007394. doi: 10.1002/14651858.CD007394.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou W, Li H, Zhang Y, Huang M, He Q, Li P, et al. Diagnostic value of galactomannan antigen test in serum and bronchoalveolar lavage fluid samples from patients with nonneutropenic invasive pulmonary aspergillosis. J Clin Microbiol. 2017;55:2153–2161. doi: 10.1128/JCM.00345-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder M, Simon M, Katchanov J, Wijaya C, Rohde H, Christner M, et al. Does galactomannan testing increase diagnostic accuracy for IPA in the ICU?A prospective observational study. Crit Care. 2016;20:139. doi: 10.1186/s13054-016-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 18.Huang L, He H, Jin J, Zhan Q. Is Bulpa criteria suitable for the diagnosis of probable invasive pulmonary Aspergillosis in critically ill patients with chronic obstructive pulmonary disease?A comparative study with EORTC/ MSG and ICU criteria. BMC Infect Dis. 2017;17:209. doi: 10.1186/s12879-017-2307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hope WW, Petraitis V, Petraitiene R, Aghamolla T, Bacher J, Walsh TJ. The initial 96 hours of invasive pulmonary aspergillosis:histopathology, comparative kinetics of galactomannan and (1->3) β-d-glucan and consequences of delayed antifungal therapy. Antimicrob Agents Chemother. 2010;54:4879–4886. doi: 10.1128/AAC.00673-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakrabarti A, Chatterjee SS, Das A, Shivaprakash MR. Invasive aspergillosis in developing countries. Med Mycol. 2011;49:S35–S47. doi: 10.3109/13693786.2010.505206. [DOI] [PubMed] [Google Scholar]
- 21.Baddley JW, Stephens JM, Ji X, Gao X, Schlamm HT, Tarallo M. Aspergillosis in Intensive Care Unit (ICU) patients:epidemiology and economic outcomes. BMC Infect Dis. 2013;13:29. doi: 10.1186/1471-2334-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tortorano AM, Esposto MC, Prigitano A, Grancini A, Ossi C, Cavanna C, et al. Cross-reactivity of Fusarium spp. in the Aspergillus Galactomannan enzyme-linked immunosorbent assay. J Clin Microbiol. 2012;50:1051–1053. doi: 10.1128/JCM.05946-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. Azole Resistance in Aspergillus fumigatus:Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin Infect Dis. 2016;62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuturlar Y, Ozkalemkas F, Ener B, Serin SO, Kazak E, Ozcelik T, et al. Serum galactomannan levels in the diagnosis of invasive aspergillosis. Korean J Intern Med. 2015;30:899–905. doi: 10.3904/kjim.2015.30.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Racil Z, Kocmanova I, Toskova M, Buresova L, Weinbergerova B, Lengerova M, et al. Galactomannan detection in bronchoalveolar lavage fluid for the diagnosis of invasive aspergillosis in patients with hematological diseases-the role of factors affecting assay performance. Int J Infect Dis. 2011;15:e874–e881. doi: 10.1016/j.ijid.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Hope WW, Kruhlak MJ, Lyman CA, Petraitiene R, Petraitis V, Francesconi A, et al. Pathogenesis of Aspergillus fumigatus and the kinetics of galactomannan in an in vitro model of early invasive pulmonary aspergillosis:implications for antifungal therapy. J Infect Dis. 2007;195:455–466. doi: 10.1086/510535. [DOI] [PubMed] [Google Scholar]
- 27.Cordonnier C, Botterel F, Ben Amor R, Pautas C, Maury S, Kuentz M, et al. Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis. Clin Microbiol Infect. 2009;15:81–86. doi: 10.1111/j.1469-0691.2008.02122.x. [DOI] [PubMed] [Google Scholar]
- 28.Maertens J, Verhaegen J, Lagrou K, Van Eldere J, Boogaerts M. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients:a prospective validation. Blood. 2001;97:1604–1610. doi: 10.1182/blood.v97.6.1604. [DOI] [PubMed] [Google Scholar]
- 29.Heng SC, Chen SC, Morrissey CO, Thursky K, Manser RL, De Silva HD, et al. Clinical utility of Aspergillus galactomannan and PCR in bronchoalveolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis in patients with haematological malignancies. Diagn Microbiol Infect Dis. 2014;79:322–327. doi: 10.1016/j.diagmicrobio.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Denning DW, Page ID, Chakaya J, Jabeen K, Jude CM, Cornet M, et al. Case Definition of Chronic Pulmonary Aspergillosis in Resource-Constrained Settings. Emerg Infect Dis. 2018:24. doi: 10.3201/eid2408.171312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morton CO, White PL, Barnes RA, Klingspor L, Cuenca-Estrella M, Lagrou K, et al. Determining the analytical specificity of PCR-based assays for the diagnosis of IA:What is Aspergillus? Med Mycol. 2017;55:402–413. doi: 10.1093/mmy/myw093. [DOI] [PubMed] [Google Scholar]
- 32.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases:executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24:e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]


