Abstract
Background
Pulmonary complications are often seen during the postoperative period following lung resection for patients with lung cancer. Some situations such as intubation, a long stay in the intensive care unit, the high cost of antibiotics and mortality may be avoided with the prevention of postoperative pulmonary complications. Non‐invasive positive pressure ventilation (NIPPV) is widely used in hospitals, and is thought to reduce the number of pulmonary complications and mortality after this type of surgery. Therefore, a systematic review is needed to critically assess the benefits and harms of NIPPV for patients undergoing lung resection. This is an update of a Cochrane review first published in 2015.
Objectives
To assess the effectiveness and safety of NIPPV for preventing complications in patients following pulmonary resection for lung cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS and PEDro until 21 December 2018, to identify potentially eligible trials. We did not use any date or language restrictions in the electronic searches. We searched the reference lists of relevant papers and contacted experts in the field for information about additional published and unpublished studies. We also searched the Register of Controlled Trials (www.controlled‐trials.com) and ClinicalTrials.gov (clinicaltrials.gov) to identify ongoing studies.
Selection criteria
We considered randomised or quasi‐randomised clinical trials that compared NIPPV in the immediate postoperative period after pulmonary resection with no intervention or conventional respiratory therapy.
Data collection and analysis
Two authors collected data and assessed trial risk of bias. Where possible, we pooled data from the individual studies using a fixed‐effect model (quantitative synthesis), but where this was not possible we tabulated or presented the data in the main text (qualitative synthesis). Where substantial heterogeneity existed, we applied a random‐effects model.
Main results
Of the 190 references retrieved from the searches, 7 randomised clinical trials (RCTs) (1 identified with the new search) and 1 quasi‐randomised trial fulfilled the eligibility criteria for this review, including a total of 486 patients. Five studies described quantitative measures of pulmonary complications, with pooled data showing no difference between NIPPV compared with no intervention (RR 1.03; 95% CI 0.72 to 1.47). Three studies reported intubation rates and there was no significant difference between the intervention and control groups (RR 0.55; 95% CI 0.25 to 1.20). Five studies reported measures of mortality on completion of the intervention period. There was no statistical difference between the groups for this outcome (RR 0.60; 95% CI 0.24 to 1.53). Similar results were observed in the subgroup analysis considering ventilatory mode (bi‐level versus continuous positive airway pressure (CPAP). No study evaluated the postoperative use of antibiotics. Two studies reported the length of intensive care unit stay and there was no significant difference between the intervention and control groups (MD ‐0.75; 95% CI ‐3.93 to 2.43). Four studies reported the length of hospital stay and there was no significant difference between the intervention and control groups (MD ‐0.12; 95% CI ‐6.15 to 5.90). None of the studies described any complications related to NIPPV. Of the seven included studies, four studies were considered as 'low risk of bias' in all domains, two studies were considered 'high risk of bias' for the allocation concealment domain, and one of these was also considered 'high risk of bias' for random sequence generation. One other study was considered ‘high risk of bias’ for including participants with more severe disease. The new study identified could not be included in the meta‐analysis as its intervention differed from the other studies (use of pre and postoperative NIPPV in the same population).
Authors' conclusions
This review demonstrated that there was no additional benefit of using NIPPV in the postoperative period after pulmonary resection for all outcomes analysed (pulmonary complications, rate of intubation, mortality, postoperative consumption of antibiotics, length of intensive care unit stay, length of hospital stay and adverse effects related to NIPPV). However, the quality of evidence is 'very low', 'low' and 'moderate' since there were few studies, with small sample size and low frequency of outcomes. New well‐designed and well‐conducted randomised trials are needed to answer the questions of this review with greater certainty.
Plain language summary
Non‐invasive positive pressure ventilation for prevention of complications after pulmonary resection in lung cancer patients
Review question: Is the use of non‐invasive positive pressure ventilation (NIPPV) safe and effective to prevent complications in the postoperative period in patients who underwent pulmonary resection for lung cancer?
Background: Death after major lung surgery for lung cancer resection is usually caused by complications, particularly lung complications, in the first week or so. Some experts recommend the use of NIPPV, a technique that provides pressurised gas to the airway, inflating the lungs through a mask without using any tubes put into the nostrils or main airway.
Search date: The search was last updated on December 21, 2018.
Study characteristics: We found seven randomised clinical trials and one quasi‐randomised trial suitable for this review, involving a total of 486 patients.
Key results: When we put all the results together we could not show that using NIPPV was any better than not using it at preventing complications such as death, breathing problems, the need for extra breathing tubes or the length of stay in intensive care unit or in hospital. However, we thought the studies had problems with their methods and that the quality of evidence was either 'very low' or 'moderate'.
Summary of findings
Summary of findings for the main comparison. NIPPV vs no NIPPV for prevention of complications after pulmonary resection in lung cancer patients.
| NIPPV versus no NIPPV for prevention of complications after pulmonary resection in lung cancer patients | ||||||
| Patient or population: lung cancer patients Settings: postoperative pulmonary resection Intervention: NIPPV versus no NIPPV | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | NIPPV versus no NIPPV | |||||
| Pulmonary complications | 254 per 1000 | 277 per 1000 (183 to 421) | RR 1.03 (0.72 to 1.47) | 238 (4 studies) | ⊕⊕⊝⊝ low1,2 | |
| Rate of intubation | Study population | RR 0.55 (0.25 to 1.2) | 69 (2 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 371 per 1000 | 204 per 1000 (93 to 446) | |||||
| Moderate | ||||||
| 296 per 1000 | 163 per 1000 (74 to 355) | |||||
| Mortality | 115 per 1000 | 54 per 1000 (20 to 152) | RR 0.60 (0.24 to 1.53) | 151 (4 studies) | ⊕⊕⊕⊝ moderate2 | |
| Length of intensive care unit stay | The mean length of intensive care unit stay in the intervention groups was 0.75 lower (3.93 lower to 2.43 higher) | 69 (2 studies) | ⊕⊕⊝⊝ low3,4 | |||
| Length of hospital stay | The mean length of hospital stay in the intervention groups was 1.01 higher (9.81 lower to 11.84 higher) | 101 (3 studies) | ⊕⊝⊝⊝ very low3,5,6,7 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: One of the studies is a quasi‐RCT 2 Imprecision: Low number of events 3 Risk of bias: One study included participants with more severe disease (acute hypoxaemic respiratory insufficiency) 4 Imprecision: The pooled effect confidence interval includes both reduction and increase of length of ICU stay 5 Risk of bias: The frequency of pulmonary complication in one study was higher in the intervention group 6 Imprecision: The pooled effect confidence interval includes both reduction and increase of length of hospital stay
7 Inconsistency: Important heterogeneity (I2 = 83%)
Background
Description of the condition
Lung cancer is the most common malignant tumour and accounted for 1.37 million deaths worldwide in 2008 according to the World Health Organization (WHO). It is one of the malignant tumours that causes the most deaths internationally, being the second most common cancer in men, after prostate cancer, and in women, after breast cancer. In over 85% of cases, smoking is related to the diagnosis and is the main risk factor for disease development (Ferlay 2010; Jemal 2011; NICE 2011).
There are several different types of lung cancer, but it is usually classified into two major groups: non‐small‐cell lung carcinoma (NSCLC) and small‐cell lung carcinoma (SCLC). The most common treatment with curative intent for NSCLC is surgery. It is indicated for patients with stages I and II disease and in some cases for those with stage III disease who are fit enough for surgery. An overall risk score, such as Thoracoscore, is used in order to estimate the risk of operative death. The treatment of choice is lobectomy (either open or thoracoscopic) (Rivera 2007; NICE 2011) or pneumonectomy depending on the extent of the tumour.
Factors such as the length of surgery or anaesthesia and mechanical ventilation may contribute to postoperative morbidity and mortality. The type and extent of surgery can reduce these complications. For example, video‐assisted thoracic surgery is less invasive than open thoracotomy and may result in fewer complications. One retrospective descriptive study conducted at the Siriraj University Hospital, Thailand, between 2006 and 2010 studied 512 patients. The objective was to determine the morbidity and mortality related to pulmonary resection surgery. About 70% of all patients with lung cancer underwent pulmonary resection and 39.5% experienced morbidity or mortality (Kutlu 2000; Igai 2009; Suksompong 2012).
Mortality and morbidity after lung resection are usually caused by pulmonary complications during the postoperative period, such as acute lung injury, acute respiratory distress syndrome and pneumonia. To minimise these postoperative respiratory complications, some authors recommend the use of non‐invasive positive pressure ventilation (NIPPV) (Brooks‐Brunn 1995; Kutlu 2000; Lorut 2005; Perrin 2007).
Description of the intervention
NIPPV can be defined as a technique that provides pressurised gas to the airway, promoting increased transpulmonary pressure, inflating the lungs through a mask or interface, and which does not use an invasive route (e.g. endotracheal tube, oronasal tube or tracheostomy). Physiologically, this technique promotes an increase in functional residual capacity and recruitment of collapsed airways, providing better oxygenation, reducing carbon dioxide (CO2) retention and decreasing the work of breathing (Brochard 2002; Schettino 2007).
There are two types of NIPPV: continuous positive airway pressure (CPAP), where only one pressure level is employed at end‐expiration, and bi‐level positive airway pressure, which employs two pressure levels (inspiration/end‐expiration). The main difference is that the bi‐level type can increase the tidal volume and may help during the inspiratory phase (Schettino 2007; Vital 2008).
Some studies have shown benefits from the use of NIPPV in the treatment of acute respiratory failure, the most common postoperative complication following abdominal or thoracic surgery (Martin 2000).
Published clinical trials have suggested that the use of NIPPV can reduce the number of pulmonary complications and mortality. This makes it possible to reduce the length of hospital stay and hospital costs (Nakagawa 2001; Arozullah 2003; Lorut 2005).
How the intervention might work
The use of NIPPV (CPAP or bi‐level) promotes increased lung volumes because of recruitment of collapsed airways, leading to better oxygenation of tissues and increased lung capacity. Using NIPPV may reduce hypercapnia, hypoxaemia, atelectasis, pleural fistula, respiratory muscle dysfunction, lung infections, and bronchial congestion, helping to prevent consequent acute respiratory failure (Auriant 2001; Brochard 2002).
The intervention aims to minimise the risk of complications, reduce the length of intensive care and hospital stay, the need for reintubation and postoperative mortality.
Why it is important to do this review
NIPPV is widely used after lung cancer resection. A systematic review is needed to establish its effectiveness and safety.
Objectives
To assess the effectiveness and safety of NIPPV for preventing complications in patients who have undergone pulmonary resection for lung cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs with no restriction regarding intervention and follow‐up length. We did not include cross‐over trials. We also excluded trials that only compared ventilatory modes of non‐invasive ventilation.
Types of participants
Patients aged above 18 years of both genders, who underwent any type of lung resection (pneumectomy, lobectomy, segmentectomy) for lung cancer (small‐cell lung carcinoma (SCLC) or non‐small‐cell lung carcinoma (NSCLC)).
Types of interventions
We included studies in which the intervention was non‐invasive positive pressure ventilation (NIPPV) started in the immediate postoperative period (defined as the first 24 hours after surgery) applied through a nasal or face mask. Two modalities of NIPPV were used: either continuous positive airway pressure (CPAP) or bi‐level positive airway pressure mode.
We included studies which used as a control one or a combination of the following interventions: oxygen therapy in order to obtain pulse oximetry oxygen saturation (SpO2) ≥ 92% or arterial partial pressure of oxygen (PaO2) ≥ 65 mmHg; chest physiotherapy techniques for removing secretions; breathing exercises; incentive spirometry; no intervention.
Types of outcome measures
Primary outcomes
Pulmonary complication rate (i.e. pulmonary infections, bronchial congestion, atelectasis, acute lung injury, pleural fistula).
Rate of intubation.
Mortality.
Secondary outcomes
Postoperative use of antibiotics.
Length of intensive care unit stay.
Length of hospital stay.
Adverse effects related to NIPPV (i.e. skin damage, pulmonary aspiration, gastric distension, vomiting, asphyxia, pneumothorax, conjunctivitis, sinusitis, mask discomfort, claustrophobia).
Search methods for identification of studies
Electronic searches
We searched the following databases to identify potentially eligible trials:
The Cochrane Central Register of Controlled Trials (CENTRAL, issue 12, 2018) (Appendix 1)
MEDLINE (accessed via PubMed) (1946 to 21 December 2018) (Appendix 2)
Embase (Ovid Embase Classic + Embase) (1947 to 21 December 2018) (Appendix 3)
LILACS (accessed via BIREME) (1980 to 21 December 2018) (Appendix 4)
PEDro (1999 to 21 December 2018) (Appendix 5)
The Cochrane Lung Cancer Group Information Specialists developed the search strategies for the three main databases: CENTRAL, MEDLINE and Embase. The search string for MEDLINE was developed according to the Cochrane Highly Sensitive Search Strategy, sensitivity maximising version (2008 version) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.b of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted it for use in LILACS and PEDro.
The search was not limited by language or publication status
Searching other resources
We searched the reference lists of primary studies and review articles.
We checked conference proceedings and abstracts from 28 February 2013 to 21 January 2019.
We contacted authors and experts in the field to identify published and unpublished trials.
To identify ongoing studies we searched www.controlled‐trials.com and clinicaltrials.gov.
We included RCTs published only as abstracts and contacted the authors for further information.
Data collection and analysis
Selection of studies
Two review authors (MFST; APVC) independently assessed the titles and abstracts of studies retrieved from the search in order to ascertain whether or not they represented potentially relevant trials. Based on this first assessment, we obtained the full text of all potentially relevant articles. A third review author (RR or GP) resolved disagreements.
Data extraction and management
We extracted data using data extraction forms, based on the standardised form described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) adapted to suit the review. The form was developed and applied by two review authors (MFST, APVC) working independently. The following data were included in the form:
Characteristics of the study: first author, year of publication and design;
Participants: inclusion criteria (age and gender, type of lung cancer, type of lung resection), number enrolled in each comparison group;
Interventions and controls: NIPPV (CPAP or bi‐level), type of physiotherapy (chest physiotherapy techniques for removing secretions, breathing exercises, incentive spirometry), frequency and duration of therapy, oxygen therapy and co‐interventions;
Outcomes: types of outcome measures, timing of outcomes measures, adverse effects.
Data related to 'Risk of bias' assessment (i.e. randomisation method, allocation concealment, blinding, withdrawals, method used for analysing data ‐ i.e. intention‐to‐treat)
Two review authors (MFST, APVC) extracted full data independently and a third review author (RR or GP) resolved disagreements.
Assessment of risk of bias in included studies
Two review authors (MFST; APVC) assessed the risk of bias of each study independently using the tool and guidance described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For each 'Risk of bias' domain detailed below, the authors assessed the study as having 'low risk of bias', 'high risk of bias' or 'unclear risk of bias'.
1. Random sequence generation
The appropriate use of randomisation methods for generating allocation such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots or minimisation.
2. Allocation concealment
The appropriate use of methods for ensuring the allocation concealment without previous knowledge of intervention assignments.
3. Blinding of participants and personnel
We cannot assess blinding of participants, since it is not possible to blind the patients with this type of intervention, nor is it possible to prevent the personnel from becoming aware of the interventions during the studies.
4. Blinding of outcome assessment
Were the evaluators aware of the results of the outcomes of the assigned interventions? This item was evaluated at the outcome level.
5. Incomplete outcome data
Did the study describe the completeness of outcome data for each main outcome, including attrition and exclusions from the study?
6. Selective reporting
How was the possibility of selective outcome reporting examined by the review authors, and what was found?
7. Other sources of bias
Have important questions about bias not addressed in other areas of the tool been indicated? For example if the study was funded by any company that could influence the results, or description of any adverse effects.
Measures of treatment effect
For continuous outcomes, we presented mean difference (MD) for measures in the same scale, and standardised mean difference (SMD) for measures that used different scales. For dichotomous outcomes, we used risk ratio (RR). For each outcome, we calculated summary estimates of treatment effect (with 95% confidence interval (CI) for each comparison.
Unit of analysis issues
We considered the individual patient as the unit of analysis.
Dealing with missing data
Where data were missing or unsuitable for analysis, we contacted the authors to request further information. Where data were missing, we presented the results in the context of the findings.
Assessment of heterogeneity
We conducted a visual inspection of forest plots to assess heterogeneity between studies. Additionally we investigated heterogeneity in the included studies using the I2 statistic. We considered as substantial heterogeneity I2 values above 50% (Higgins 2011). We investigated the following types of statistical heterogeneity:
Clinical heterogeneity: including differences in the study location and setting, full characteristics of participants (e.g. age, gender, smoking history, histological type of tumour, type of surgery), co‐morbidity (e.g. cardiovascular disease, chronic obstructive pulmonary disease, diabetes, chronic renal disease) and treatments that participants received following trial entry (i.e. neoadjuvant or adjuvant chemotherapy, radiotherapy, or a combination). We considered the definition of outcomes (i.e. mild, moderate, severe; short, middle and long term ‐ both classifications as defined by included studies), and how they were measured and recorded. Depending upon the extent of the clinical diversity, we analysed studies separately or presented the results using a narrative approach.
Methodological heterogeneity: including assessment of the randomisation process, study quality and analytical method.
Assessment of reporting biases
As it was not possible to pool more than ten studies, funnel plots were not used to investigate possible publication biases.
Assessing the quality of evidence and presenting 'Summary of Findings' tables
We assessed the quality of the evidence for the primary outcomes according to the GRADE criteria (section 12.2, Higgins 2011). We prepared a 'Summary of findings' table considering the following outcomes: pulmonary complication rate (i.e. pulmonary infections, bronchial congestion, atelectasis, acute lung injury, pleural fistula); rate of intubation; mortality; length of intensive care unit stay and length of hospital stay.
Data synthesis
To synthesise data we used Review Manager software (RevMan 2014) producing forest plots using a fixed‐effect model. When we found substantial heterogeneity , a random‐effects model was applied. For dichotomous outcomes, the Mantel‐Haenszel method was used, while continuous outcomes were combined using the inverse variance method. If data aggregation was not possible, the results of individual studies were presented in tables or graphs and discussed.
Subgroup analysis and investigation of heterogeneity
The following subgroups were considered if possible:
smoking history;
type of resection (segmentectomy, lobectomy, pneumectomy);
duration of the NIPPV;
intermittent or continuous NIPPV approach;
ventilatory mode (CPAP or bi‐level).
Sensitivity analysis
We conducted sensitivity analyses excluding trials at high risk of bias (Higgins 2011). We considered and discussed the results of these analyses compared to the overall findings.
Results
Description of studies
Refer to Characteristics of included studies and Characteristics of excluded studies for complete details of studies which were classified as included or excluded.
Results of the search
We retrieved 190 references using the strategy search described above (MEDLINE 38 references, Embase 92 references, CENTRAL 58 references, LILACS 2 references, and PEDro 0 references). We identified three duplicate studies, resulting in a total of 187 references. After screening titles and abstracts, we identified 12 potentially eligible studies (Ingwersen 1993; Aguilo 1997; Auriant 2001; Perrin 2007; Liao 2010; Ludwig 2011; Barbagallo 2012; Danner 2012; Nery 2012; Garutti 2014; Roceto 2014; Hernández 2018). See Figure 1.
1.
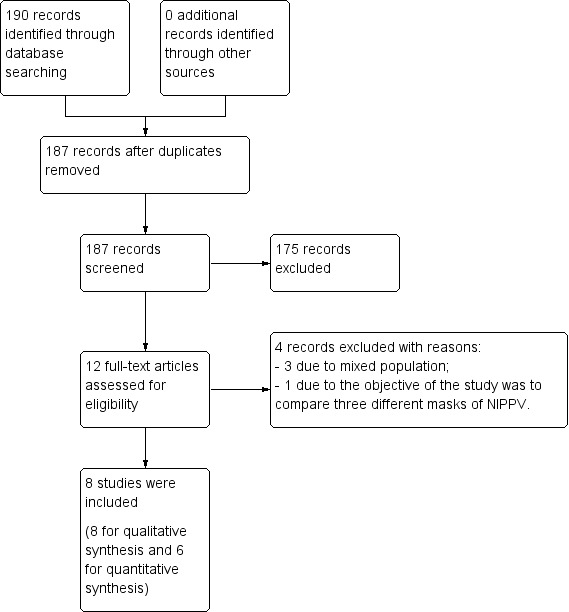
Study flow diagram.
Included studies
See: Characteristics of included studies
Study
The review included eight studies reporting seven randomised controlled trials (Auriant 2001; Perrin 2007; Barbagallo 2012; Danner 2012; Garutti 2014; Roceto 2014; Hernández 2018) and one quasi‐randomised controlled trial (Ludwig 2011), including a total of 486 participants.
Population
Patients who underwent lung resection (pneumectomy, bi‐ or lobectomy, segmentectomy) for lung cancer.
Setting
Seven studies were performed in Europe: two in France (Auriant 2001; Perrin 2007), two in Germany (Ludwig 2011; Danner 2012), one in Italy (Barbagallo 2012) and two in Spain (Garutti 2014; Hernández 2018). One study was performed in Latin America, Brazil (Roceto 2014).
Intervention
There were several variations in the interventions in terms of duration and frequency of use and in terms of ventilatory mode. Two studies used the NIPPV for two days and the time of use per day ranged from 2 to 14 hours (Auriant 2001; Danner 2012). Two studies applied NIPPV for two hours during three consecutive days (Perrin 2007; Roceto 2014) and Barbagallo 2012 applied the intervention for two hours twice a day, but did not report the number of days on which the intervention was performed. Garutti 2014 applied NIPPV for seven consecutive hours in the first postoperative day. One study used the intervention for a longer period than in the other studies, applying NIPPV three times a day during the entire hospital stay (Ludwig 2011). One study applied NIPPV one week before surgery and could therefore not be included in our meta‐analysis. NIPPV was applied for 30 minutes every two hours until 24:00h after surgery and then one more time for another 30 minutes during the night (Hernández 2018).
Concerning the ventilatory mode, four studies used bi‐level mode (Auriant 2001; Perrin 2007; Danner 2012; Hernández 2018) and four studies used CPAP mode (Ludwig 2011; Barbagallo 2012; Garutti 2014; Roceto 2014).
Control groups received a combination of the following: oxygen support, antibiotic prophylaxis, bronchodilators, pain control, thrombosis prophylaxis with heparin, early mobilisation, breathing exercises and chest physiotherapy.
Excluded studies
See: Characteristics of excluded studies.
Four studies were excluded for the following reasons:
mixed population with few participants who underwent lung resection for NSCLC (Aguilo 1997; Liao 2010; Nery 2012);
the objective of the study was to compare three different masks for NIPPV (Ingwersen 1993).
Risk of bias in included studies
Four of the eight included studies had a low risk of bias in all seven domains of the Cochrane 'Risk of bias' tool (Barbagallo 2012; Danner 2012; Garutti 2014; Perrin 2007). Three domains were rated as low risk of bias for all studies (blinding of outcome assessment, incomplete outcome data, selective reporting). See Figure 2.
2.
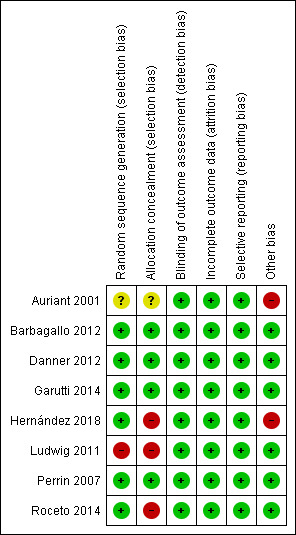
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For the generation of the randomisation sequence, six studies had a low risk of bias (Barbagallo 2012; Danner 2012; Garutti 2014; Perrin 2007; Roceto 2014; Hernández 2018). One study did not describe the method of randomisation (Auriant 2001) and one study used an inappropriate method of randomisation based on the birth date of the participants and hence was considered to be a quasi‐randomised controlled trial (Ludwig 2011).
Four studies described methods used to conceal allocation (Barbagallo 2012; Danner 2012; Garutti 2014; Perrin 2007) and were considered to have a low risk of bias for this domain. The method was not described in one study and was considered to have an unclear risk of bias (Auriant 2001). Three studies (Ludwig 2011; Roceto 2014; Hernández 2018) were considered as being at high risk of bias for allocation concealment.
Blinding
Blinding of participants and personnel was not possible in this type of intervention. Therefore, this domain was not considered.
The main outcomes were considered unlikely to be influenced by a lack of blinding. For this reasons, all studies were assessed as presenting low risk of bias regarding assessor blinding.
Incomplete outcome data
Four studies had no losses or missing data and were considered as having a low risk of bias for this domain (Auriant 2001; Ludwig 2011; Danner 2012; Hernández 2018). The other four had few losses and the reasons were described (Perrin 2007; Barbagallo 2012; Garutti 2014; Roceto 2014) and were also considered as presenting a low risk of bias.
Selective reporting
Although we identified no protocols for the included studies, all studies evaluated the proposed and/or relevant outcomes and were considered as presenting a low risk of bias for selective reporting.
Other potential sources of bias
Two studies (Auriant 2001; Hernández 2018) were considered as having a high risk of bias. Auriant 2001 included participants with more severe disease compared to patients in other studies. This study included only patients with a diagnosis of acute hypoxaemic respiratory insufficiency in the postoperative period that resulted in low blood oxygen levels and respiratory muscle failure. Thus, these patients may have a reduced chance of successful NIPPV compared to patients without this diagnosis. Hernández 2018 included participants with better respiratory function than the majority of the patients included in the other studies. It resulted in a lower incidence of clinically significant postoperative complications compared to other analyses.
Effects of interventions
See: Table 1
Primary outcomes
I. Pulmonary complication rate
Six studies described quantitative measures of pulmonary complications but we combined five studies in a meta‐analysis by bi‐level and CPAP ventilation mode (Perrin 2007; Ludwig 2011; Barbagallo 2012; Danner 2012; Garutti 2014) (Figure 3). We observed no difference between the NIPPV and the non‐NIPPV group (RR 1.03; 95% CI 0.72 to 1.47) (Analysis 1.1).
3.
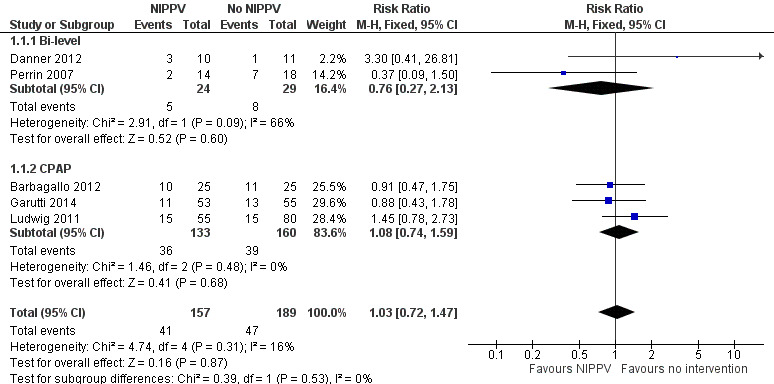
Forest plot of comparison: 1 NIPPV vs no NIPPV, outcome: 1.1 Pulmonary complications rate.
1.1. Analysis.
Comparison 1 NIPPV versus no NIPPV, Outcome 1 Pulmonary complications.
We found no difference in the subgroup analysis considering ventilatory mode (bi‐level or CPAP) and no difference between interventions after removing the quasi‐randomised trial (Ludwig 2011).
We did not include Hernández 2018 in the meta‐analysis because of the difference of intervention (preoperative use of NIPPV). The most frequent pulmonary complication was atelectasis, present in 24% of patients (6 in each group). There was no statistical difference between the groups (p > 0.05).
II. Rate of intubation
Three studies reported the rate of intubation (Auriant 2001; Barbagallo 2012; Danner 2012) and there was no significant difference between the NIPPV group and the non‐NIPPV group (RR 0.55; 95% CI 0.25 to 1.20) (Analysis 1.2).
1.2. Analysis.
Comparison 1 NIPPV versus no NIPPV, Outcome 2 Rate of intubation.
The Auriant 2001 study reported that the use of NIPPV was able to reduce the need for endotracheal intubation with a consequent decrease in length of intensive care unit and hospital stay. The patients in the study were more severely ill than those included in the other studies because of the study's inclusion criteria: only patients with a diagnosis of acute hypoxaemic respiratory insufficiency in the postoperative period.
In Barbagallo 2012 study no patients needed to be intubated in either group, thus we did not include these data in the meta‐analysis.
In Danner 2012 study two patients were intubated in NIPPV group and one in the control group (Figure 4).
4.
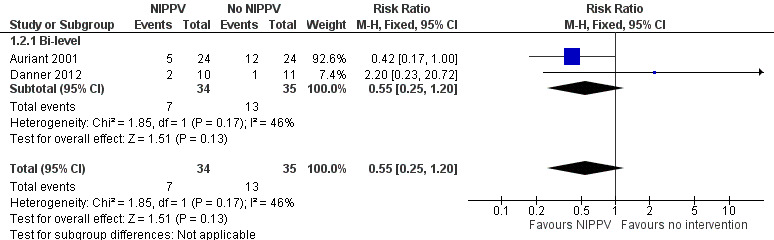
Forest plot of comparison: 1 NIPPV vs no NIPPV, outcome: 1.2 Rate of intubation.
III. Mortality
We performed a meta‐analysis of this outcome with five studies (Auriant 2001; Perrin 2007; Barbagallo 2012; Danner 2012; Garutti 2014). At the end of the intervention period, there was no difference in mortality between the NIPPV group and the non‐NIPPV group (RR 0.60; 95% CI 0.24 to 1.53) (Analysis 1.3), even considering ventilatory mode (bi‐level or CPAP). Two studies did not report mortality (Perrin 2007; Barbagallo 2012). Auriant 2001 showed lower mortality in the NIPPV group but without a statistical difference (Figure 5).
1.3. Analysis.
Comparison 1 NIPPV versus no NIPPV, Outcome 3 Mortality.
5.
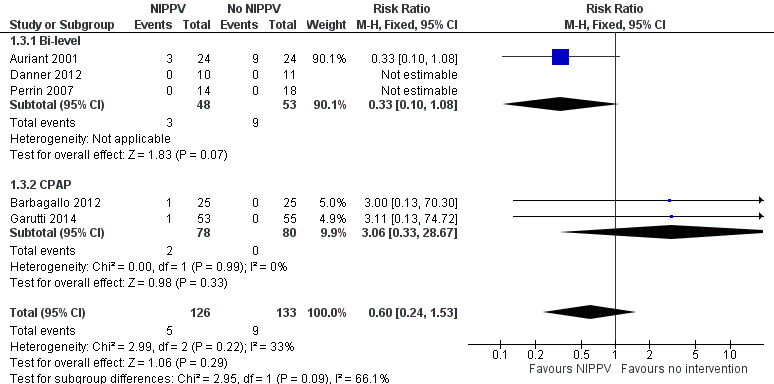
Forest plot of comparison: 1 NIPPV vs no NIPPV, outcome: 1.3 Mortality.
Secondary outcomes
IV. Postoperative use of antibiotics
No study reported this outcome.
V. Length of intensive care unit stay
Two studies reported measures of this outcome and the ventilatory mode of both of them was bi‐level mode (Auriant 2001; Danner 2012). We found no significant difference between the NIPPV group and the non‐NIPPV group (MD ‐0.75; 95% CI ‐3.93 to 2.43) (Analysis 1.4).
1.4. Analysis.
Comparison 1 NIPPV versus no NIPPV, Outcome 4 Length of intensive care unit stay.
VI. Length of hospital stay
Five studies reported measures of the length of hospital stay (Auriant 2001; Perrin 2007; Danner 2012; Garutti 2014; Hernández 2018). The study conducted by Danner 2012 suggested results in favour of the control group. We found no statistically significant difference between the NIPPV group and the non‐NIPPV group (MD ‐0.12; 95% CI ‐6.15 to 5.90) (Figure 6) (Analysis 1.5). We found no difference in the subgroup analysis by ventilatory mode (bi‐level or CPAP).
6.
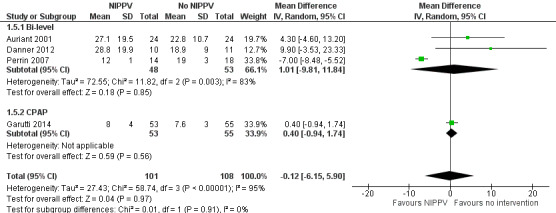
Forest plot of comparison: 1 NIPPV vs no NIPPV, outcome: 1.5 Length of hospital stay.
1.5. Analysis.
Comparison 1 NIPPV versus no NIPPV, Outcome 5 Length of hospital stay.
Hernández 2018 was not included in the meta‐analysis because the intervention was different; they used pre and postoperative NIPPV in the same group of participants. They found that the postoperative hospital stay was similar in both groups (NIPPV group: 6.60 ± 4 days versus non‐NIPPV group: 6.84 ± 3.94 days (p = 0.63)).
VII. Adverse effects related to NIPPV
None of the studies described any adverse effects related to NIPPV (Auriant 2001; Perrin 2007; Ludwig 2011; Barbagallo 2012; Danner 2012; Garutti 2014; Roceto 2014; Hernández 2018).
Discussion
Summary of main results
This systematic review aimed to assess the effectiveness and safety of non‐invasive positive pressure ventilation (NIPPV) to prevent postoperative complications in patients undergoing pulmonary resection for lung cancer.
Data from eight RCTs with a total of 486 patients were included. We excluded studies that included patients undergoing pulmonary resection for reasons other than lung cancer (such as chronic obstructive pulmonary disease (COPD), bronchiectasis, benign lung tumour or residual tuberculosis lesions).
In this review we present meta‐analyses of the following five outcomes: pulmonary complications, rate of intubation, mortality, length of intensive care unit stay and length of hospital stay.
Subgroup analysis was possible for ventilatory mode (bi‐level mode or continuous positive airway pressure (CPAP) in meta‐analyses of the following four outcomes: pulmonary complications, rate of intubation, mortality and length of hospital stay. The results of these analyses did not show statistical differences in the use of NIPPV with different ventilatory modes. However, for the outcome of intubation rate, we did not include Barbagallo 2012 in the meta‐analysis since for this study the rate was zero in both groups.
One study was not included in our meta‐analyses because the intervention consisted of both pre and postoperative NIPPV to the same group of participants while the other included studies were all reporting on postoperative NIPPV (Hernández 2018). The study did not find statistical differences between the groups for pulmonary complications, length of hospital stay, spirometric values, arterial blood gases and radiographic findings.
The quality of evidence was considered 'very low' to 'moderate' since there were few studies, all with small sample sizes and there was a low frequency of all outcomes. The meta‐analyses suggest no benefit of NIPPV compared with control.
Overall completeness and applicability of evidence
The patients analysed in this review underwent pulmonary resection for lung cancer and received protocols of NIPPV compared to no NIPPV.
There was a large variation in the timing and duration of NIPPV across the studies, resulting in different protocols (Danner 2012 from 2 to 14 hours during 2 days; Ludwig 2011 for 3 times a day during the entire stay; Perrin 2007 and Roceto 2014 for 2 hours during 3 days; Barbagallo 2012 for 2 hours twice a day; Garutti 2014 for 7 consecutive hours in the first postoperative day). It is possible that these different protocols affected the results.
We found no data for the following subgroup analyses: smoking, type of resection (segmentectomy, lobectomy, pneumectomy), duration of the NIPPV, intermittent or continuous NIPPV approach.
Adverse effects of NIPPV were analysed but none of the patients experienced any of these events, such as skin damage, pulmonary aspiration, gastric distension, vomiting, asphyxia, pneumothorax, conjunctivitis, sinusitis, mask discomfort and claustrophobia.
We believe that the number of studies, sample size for each study and the variation in timing and duration of the intervention may have influenced the results.
Quality of the evidence
The quality of evidence provided by the included studies has been rated as low overall, based on the GRADE approach. For one of the outcomes, the studies provided 'very low' quality of evidence, two 'low' quality of evidence and two 'moderate' quality of evidence, as follows:
Pulmonary complication rate: 4 studies; 238 participants; RR 1.03 CI 95% 0.72 to 1.47; evidence of low quality; quality downgraded because one of the studies is a quasi‐RCT and there was a low number of events.
Rate of intubation: 2 studies; 69 participants; RR 0.55 CI 95% 0.25 to 1.2; evidence of moderate quality; quality downgraded because of the low number of events.
Mortality: 4 studies; 151 participants; RR 0.60 CI 95% 0.24 to 1.53; evidence of moderate quality; quality downgraded because of the low number of events.
Length of intensive care unit (ICU) stay: 2 studies; 69 participants; MD ‐0.75; 95% CI ‐3.93 to 2.43; evidence of low quality; quality downgraded because one study included participants with more severe disease (acute hypoxaemic respiratory insufficiency) and the pooled effect confidence interval included both a reduction and an increase in the length of ICU stay.
Length of hospital stay: 3 studies; 101 participants; MD ‐0.12; 95% CI ‐6.15 to 5.90; evidence of very low quality; quality downgraded because one study included participants with more severe disease (acute hypoxaemic respiratory insufficiency); the frequency of pulmonary complications in one study was higher in the intervention group; the pooled effect confidence interval includes both a reduction and an increase of length of hospital stay; and inconsistency (important heterogeneity among studies).
Potential biases in the review process
We excluded three studies because they included mixed populations with few participants who underwent lung resection for NSCLC. The authors did not respond to our requests for individual data.
As this review includes seven trials for meta‐analysis, we could not assess the publication bias using a funnel plot.
Agreements and disagreements with other studies or reviews
We did not find any non‐Cochrane systematic review assessing the same clinical question.
Authors' conclusions
Implications for practice.
This systematic review shows that the use of non‐invasive positive pressure ventilation (NIPPV) to prevent pulmonary complications in the postoperative period for patients who underwent lung resection has no statistically significant benefit compared with control, although no additional adverse effects were observed. However the likelihood of a type two error exists because of the small number of included patients, and so the findings must be confirmed by trials with higher statistical power.
Implications for research.
This systematic review demonstrates the need for trials of better methodological quality, including an appropriate sample size to detect any difference between the interventions regarding the main outcomes and to increase the frequency of events observed. Standardisation of NIPPV procedures (frequency, duration and timing) is also required along with further randomised trials to allow pooling of the data through meta‐analysis. Additionally the effects of NIPPV applied for a longer period should also be evaluated.
What's new
| Date | Event | Description |
|---|---|---|
| 5 February 2019 | New search has been performed | New search ran 21 December 2018. |
| 5 February 2019 | New citation required but conclusions have not changed | One new study that could not be included in the meta‐analysis identified (Hernández 2018). Conclusions not changed. |
Acknowledgements
We appreciate the help that the Cochrane Lung Cancer Group gave us during the implementation of the protocol, in particular Sera Tort, Ivan Solà, Ramon Rami Porta, Mia Schmidt‐Hansen, Tajender Vasu, Marta Roqué and Desiree West (consumer). For the development of the review, we appreciate the help received from Corynne Marchal, Virginie Westeel, Fergus Macbeth, Jean‐Paul Sculier, Frederic Fiteni, Ramon Rami Porta, Paul Van Schil, Marta Roqué, Sophie Paget‐Bailly and Ivan Solà. We also thank the Brazilian Cochrane Center team for their methodological support.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (The Cochrane Library)
| Search | |
| #1 | MeSH descriptor: [Positive‐Pressure Respiration] explode all trees |
| #2 | positive pressure ventilation:ti,ab |
| #3 | pressure support ventilation:ti,ab |
| #4 | noninvasive ventilatory support:ti,ab |
| #5 | non invasive ventilatory support:ti,ab |
| #6 | NIVS:ti,ab |
| #7 | NPPV:ti,ab |
| #8 | NIPSV:ti,ab |
| #9 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 |
| #10 | MeSH descriptor: [Pneumonectomy] explode all trees |
| #11 | lung resection*:ti,ab |
| #12 | lobectom*:ti,ab |
| #13 | pneumonectom*:ti,ab |
| #14 | #10 or #11 or #12 or #13 |
| #15 | #9 and #14 |
Appendix 2. MEDLINE search strategy
| Search | |
| #1 | Positive‐Pressure Respiration[MeSH] |
| #2 | positive pressure ventilation[tiab] |
| #3 | pressure support ventilation[tiab] |
| #4 | noninvasive ventilatory support[tiab] |
| #5 | non invasive ventilatory support[tiab] |
| #6 | NIVS[tiab] |
| #7 | NPPV[tiab] |
| #8 | NIPSV[tiab] |
| #9 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 |
| #10 | Pneumonectomy[MeSH] |
| #11 | lung resection*[tiab] |
| #12 | lobectom*[tiab] |
| #13 | pneumonectom*[tiab] |
| #14 | #10 OR #11 OR #12 OR #13 |
| #15 | #9 AND #14 |
| #16 | (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT (humans[mh] AND animals[mh])) |
| #17 | #15 AND #16 |
Appendix 3. EMBASE (Ovid Embase Classic+Embase)
| Search | |
| 1 | exp positive end expiratory pressure/ |
| 2 | positive pressure ventilation.ti,ab. |
| 3 | pressure support ventilation.ti,ab. |
| 4 | noninvasive ventilatory support.ti,ab. |
| 5 | non invasive ventilatory support.ti,ab. |
| 6 | NIVS.ti,ab. |
| 7 | NPPV.ti,ab. |
| 8 | NIPSV.ti,ab. |
| 9 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 |
| 10 | exp lung resection/ |
| 11 | lung resection*.ti,ab. |
| 12 | lobectom*.ti,ab. |
| 13 | pneumonectom*.ti,ab. |
| 14 | 10 or 11 or 12 or 13 |
| 15 | 9 and 14 |
| 16 | random:.tw. or clinical trial:.mp. or exp health care quality/ |
| 17 | 15 and 16 |
Appendix 4. LILACS (accessed via BIREME)
| Search | |
| #1 | MH:E02.041.625.790$ OR MH:E02.880.820.790$ OR MH:"Positive‐Pressure Respiration" OR TW:"Positive End‐Expiratory Pressure" OR TW:"Respiración por Presión Positiva Continua" OR TW:"Presión Positiva Espiratoria Final" OR TW:"Respiração por Pressão Positiva Contínua" OR TW:"Pressão Positiva Expiratória Final" OR TW:"Pressão Expiratória Final Positiva" OR TW:"positive pressure ventilation" OR TW:"pressure support ventilation" OR TW:"noninvasive ventilatory support" OR TW:"non invasive ventilatory support" OR TW:NIVS OR TW:NPPV OR TW:NIPSV MH:E04.928.600.600$ OR MH:Pneumonectomy OR TW:"Lung Volume Reduction" OR TW:"Lobectomía Pulmonar" OR TW:"Reducción de Volumen Pulmonar" OR TW:"Lobectomia Pulmonar" OR TW:"Reduçao do Volume do Pulmão" OR TW:"Redução do Volume Pulmonar" (MH:E02.041.625.790$ OR MH:E02.880.820.790$ OR MH:"Positive‐Pressure Respiration" OR TW:"Positive End‐Expiratory Pressure" OR TW:"Respiración por Presión Positiva Continua" OR TW:"Presión Positiva Espiratoria Final" OR TW:"Respiração por Pressão Positiva Contínua" OR TW:"Pressão Positiva Expiratória Final" OR TW:"Pressão Expiratória Final Positiva" OR TW:"positive pressure ventilation" OR TW:"pressure support ventilation" OR TW:"noninvasive ventilatory support" OR TW:"non invasive ventilatory support" OR TW:NIVS OR TW:NPPV OR TW:NIPSV) AND (MH:E04.928.600.600$ OR MH:Pneumonectomy OR TW:"Lung Volume Reduction" OR TW:"Lobectomía Pulmonar" OR TW:"Reducción de Volumen Pulmonar" OR TW:"Lobectomia Pulmonar" OR TW:"Reduçao do Volume do Pulmão" OR TW:"Redução do Volume Pulmonar") |
Appendix 5. PEDro
| Simple Search: Non invasive ventilation lung resection |
| Advanced search Abstract & Title: Non invasive ventilation Therapy: [no appropriate value in this field] Problem: [no appropriate value in this field] Body part: [no appropriate value in this field] Subdiscipline: oncology Topic: [no appropriate value in this field] Method: clinical trial Author/Association: ‐‐‐‐‐‐‐‐‐‐ Title only: ‐‐‐‐‐‐‐‐‐‐ Source: ‐‐‐‐‐‐‐‐‐‐ Published since: ‐‐‐‐‐‐‐‐‐ New records added since: ‐‐‐‐‐‐‐‐‐ Score of at least: ‐‐‐‐‐‐‐‐‐‐ When searching: • Match all search terms (AND) Match any search terms (ON) |
Data and analyses
Comparison 1. NIPPV versus no NIPPV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulmonary complications | 5 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.72, 1.47] |
| 1.1 Bi‐level | 2 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.13] |
| 1.2 CPAP | 3 | 293 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.74, 1.59] |
| 2 Rate of intubation | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.20] |
| 2.1 Bi‐level | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.20] |
| 3 Mortality | 5 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.24, 1.53] |
| 3.1 Bi‐level | 3 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.08] |
| 3.2 CPAP | 2 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.33, 28.67] |
| 4 Length of intensive care unit stay | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐3.93, 2.43] |
| 4.1 Bi‐level | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐3.93, 2.43] |
| 5 Length of hospital stay | 4 | 209 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐6.15, 5.90] |
| 5.1 Bi‐level | 3 | 101 | Mean Difference (IV, Random, 95% CI) | 1.01 [‐9.81, 11.84] |
| 5.2 CPAP | 1 | 108 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.94, 1.74] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Auriant 2001.
| Methods | RCT May 1999 ‐ July 2000 |
|
| Participants | 48 patients Patients who were admitted for acute hypoxaemic respiratory insufficiency following pulmonary resection for lung cancer |
|
| Interventions | Intervention: NIPPV (bi‐level mode) + oxygen supplementation to achieve an SpO2 above 90% + bronchodilators + patient‐controlled analgesia (PCA) + chest physiotherapy Control: Oxygen supplementation to achieve an SpO2 above 90% + bronchodilators + PCA + chest physiotherapy |
|
| Outcomes | Pulmonary complication rate Rate of intubation Mortality Length of intensive care unit (ICU) stay Length of hospital stay PaO2/FiO2 ratio |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 48 patients were enrolled and 48 finished the study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | The study included participants with more severe disease (acute hypoxaemic respiratory insufficiency) |
Barbagallo 2012.
| Methods | RCT March 2007 ‐ December 2008 |
|
| Participants | 50 patients Patients who underwent elective lung lobectomy for non‐small‐cell lung cancer |
|
| Interventions | Intervention: 2 CPAP cycles of 2 hours for 1 week + oxygen support + antibiotic prophylaxis + aerosol therapy + thrombosis prophylaxis with heparin + rapid mobilisation and chest physiotherapy once daily Control: Oxygen support + antibiotic prophylaxis + aerosol therapy + thrombosis prophylaxis with heparin + rapid mobilisation and chest physiotherapy once daily |
|
| Outcomes | Pulmonary complication rate Rate of intubation Mortality Length of ICU stay Length of hospital stay PaO2/FiO2 ratio |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes with the order of distribution determined by computer generated randomised numbers |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was one person lost from each group |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Danner 2012.
| Methods | RCT April 2005 ‐ June 2009 |
|
| Participants | 21 patients Patients with severely impaired pulmonary function and indication for major pulmonary resection for lung cancer |
|
| Interventions | Intervention: NIPPV (bi‐level mode) autonomously for 10 hours in the course of 2 consecutive days and for another 6 hours on the third day + pain control + chest physiotherapy + early mobilisation + breathing exercises Control: Pain control + chest physiotherapy + early mobilisation + breathing exercises |
|
| Outcomes | Pulmonary complication rate Rate of intubation Mortality Length of ICU stay Length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by having the patients of the study group draw from prepared sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21 patients were enrolled and 21 finished the study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Garutti 2014.
| Methods | RCT April 2011 ‐ May 2012 |
|
| Participants | 110 patients Patients undergoing pulmonary resection by lateral thoracotomy |
|
| Interventions | Intervention: NIPPV (CPAP mode) via facial mask in the Post Anesthesia Care Unit after extubation maintaining a pressure of 5 to 7 cmH2O continuously for the following 6 hours Control: Oxygen support (using Venturi oxygen mask) |
|
| Outcomes | Pulmonary complication rate Mortality Non‐pulmonary complication rate Length of hospital stay PaO2/FiO2 ratio Cardiac complications |
|
| Notes | Fundación para la Investigación biomédica del Hospital Gragorio Marañon de Madrid received 6000 euros from Vygon® to cover the insurance costs of the study patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes with the order of distribution determined by computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses: 2. Reasons for exclusion after randomisation were described |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Hernández 2018.
| Methods | RCT January 2012 ‐ June 2013 |
|
| Participants | 50 patients Patients who had undergone elective lung resection (segmentectomy, lobectomy or pneumonectomy) by posterolateral thoracotomy for lung cancer, lung metastasis and bronchiectasis surgery |
|
| Interventions | Intervention: Preoperative period: respiratory rehabilitation 2 weeks before surgery + NIPPV one week before surgery Postoperative period: NIPPV (bi‐level mode) with IPAP 10‐12 cmH2 and an EPAP 4‐5 cmH2 for 30 min every 2 h until 24:00. Later, during the night, it was administered only once from 4:00 to 4:30 am. After this last session, treatment with BiPAP was terminated + chest physiotherapy + 500 mg of nebulized ipratropium bromide diluted in 3 ml of saline every 8 h for the first 3 days Control: Preoperative period: respiratory rehabilitation 2 weeks before surgery Postoperative period: venturi mask with FiO2 40% + 500 mg of nebulized ipratropium bromide diluted in 3 ml of saline every 8 h for the first 3 days |
|
| Outcomes | Pulmonary complication Length of hospital stay Spirometric values Arterial blood gases Radiographic findings |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation |
| Allocation concealment (selection bias) | High risk | Both groups were separate according to the use or not of prophylactic BiPAP |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50 patients were enrolled and 50 finished the study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | The study included participants with better respiratory function than the majority of the patients included in other studies. It results in low incidence of clinically significant postoperative complications compared to other analyses |
Ludwig 2011.
| Methods | Quasi‐RCT June 2007 ‐ March 2009 |
|
| Participants | 135 patients Patients who underwent anatomic resection with curative intent for bronchial carcinoma |
|
| Interventions | Intervention: NIPPV (CPAP mode) 3 times a day + diaphragmatic breathing + postural correction + stretching + shoulder girdle motion Control: Diaphragmatic breathing + postural correction + stretching + shoulder girdle motion |
|
| Outcomes | Pulmonary complications Length of hospital stay Oxygen consumption 6‐minute walk test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The sequence of allocation was performed preoperatively according to year of birth |
| Allocation concealment (selection bias) | High risk | No description of allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 135 patients were enrolled and 135 finished the study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Perrin 2007.
| Methods | RCT January 2001 ‐ January 2004 |
|
| Participants | 32 patients Patients with preoperative FEV1< 70% of the predicted value and scheduled for elective lobectomy related to lung cancer |
|
| Interventions | Intervention: NIPPV (bi‐level mode) 1 hour per day + bronchodilators + oral ambroxol + chest physiotherapy Control: Bronchodilatators + oral ambroxol + chest physiotherapy |
|
| Outcomes | Pulmonary complication rate (atelectasis) Mortality Length of hospital stay Adverse effects related to NIPPV Arterial blood gases Pulmonary function (FVC and FEV1) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes with the order of distribution determined by computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses: 7. Reasons for exclusion after randomisation were described |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Roceto 2014.
| Methods | RCT October 2007 ‐ November 2009 |
|
| Participants | 60 patients Patients who underwent pulmonary resection for lung cancer |
|
| Interventions | Intervention: NIPPV (CPAP) for three days starting in the immediate postoperative period + chest physiotherapy Control: Chest physiotherapy |
|
| Outcomes | Oxygen index Air leaks Dyspnea scale Pain scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Opaque and sealed envelopes |
| Allocation concealment (selection bias) | High risk | Both the investigators and the patient knew to which group the patient was allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses: 20. Reasons for exclusion after randomisation were described |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aguilo 1997 | Mixed population with few participants who underwent lung resection for NSCLC |
| Ingwersen 1993 | The interventions compared did not fulfil the criteria for this review. The study compared 3 different types of NIPPV with no control group. |
| Liao 2010 | Mixed population with few participants who underwent lung resection for NSCLC |
| Nery 2012 | Mixed population with few participants who underwent lung resection for NSCLC |
Differences between protocol and review
After publication of the protocol (Torres 2013), we decided to include quasi‐randomised controlled trials in the review.
We added a 'Summary of findings' table to show the findings of the included studies in the review in a summarised format. We used the five GRADE criteria (risk of bias, inconsistency, imprecision, indirectness and publication bias) to assess the quality of the body of evidence.
Number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH) were not used for meta‐analysis, contrary to what was planned in the protocol (Torres 2013).
Contributions of authors
Conceiving the review: MFST; GP; APVC; RR.
Designing the review: MFST; RR.
Co‐ordinating the review: RR.
Data collection for the review: MFST; APVC.
Designing search strategies: MFST.
Undertaking searches: MFST; APVC.
Screening search results: MFST; APVC.
Organising retrieval of papers: MFST.
Screening retrieved papers against eligibility criteria: MFST.
Appraising quality of papers: MFST; GP.
Extracting data from papers: MFST; GP.
Writing to authors of papers for additional information: MFST.
Providing additional data about papers: MFST.
Obtaining and screening data on unpublished studies: MFST.
Data management for the review: MFST; GP.
Entering data into RevMan: MFST; GP.
Analysis of data: MFST; GP.
Interpretation of data: MFST; GP; RR.
Providing a methodological perspective: MFST; GP; RR.
Providing a clinical perspective: MFST; GP; RR.
Providing a policy perspective: MFST.
Providing a consumer perspective: MFST; GP; RR.
Writing the review (or protocol): MFST; GP; RR.
Providing general advice on the review: MFST; RR.
Securing funding for the review: not applied.
Performing previous work that was the foundation of the current review: MFST.
Update: MFST; RR.
Sources of support
Internal sources
-
Brazilian Cochrane Center, Brazil.
Cochrane methodology support and statistical training
External sources
No sources of support supplied
Declarations of interest
Maria FS Torres: none known
Gustavo JM Porfírio: none known
Alan PV Carvalho: none known
Rachel Riera: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Auriant 2001 {published data only}
- Auriant I, Jallot A, Herve P, Cerrina J, Ladurie FLR, Fournier JL, et al. A comparison of non‐invasive positive pressure ventilation (NPPV) and conventional therapy in patients with acute hypoxemic respiratory failure (AHRF) following lung resection. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A:248. [Google Scholar]
- Auriant I, Jallot A, Hervé P, Cerrina J, Roy Ladurie F, Fournier JL, et al. Non‐invasive ventilation reduces mortality in acute respiratory failure following lung resection. American Journal of Respiratory and Critical Care Medicine 2001;164(7):1231‐5. [DOI] [PubMed] [Google Scholar]
Barbagallo 2012 {published data only}
- Barbagallo M, Ortu A, Spadini E, Salvadori A, Ampollini L, Internullo E, et al. Prophylactic use of helmet CPAP after pulmonary lobectomy: a prospective randomized controlled study. Respiratory Care 2012;57(9):1418‐24. [DOI] [PubMed] [Google Scholar]
Danner 2012 {published data only}
- Danner BC, Koerber W, Emmert A, Olgemoeller U, Doerge H, Quintel M, et al. Non‐invasive pressure support ventilation in major lung resection for high risk patients: does it matter?. Open Journal of Thoracic Surgery 2012;2:63‐71. [Google Scholar]
Garutti 2014 {published data only}
- Garutti I, Puente‐Maestu L, Laso J, Sevilla R, Ferrando A, Frias I, et al. Comparison of gas exchange after lung resection with a Boussignac CPAP or Venturi mask. British Journal of Anaesthesia 2014;112(5):929‐35. [DOI] [PubMed] [Google Scholar]
Hernández 2018 {published data only}
- Hernández EG, Pérez AR, Gilard JF, Álamo MNM, Socorro ME, Suárez PR, et al. Prophylactic use of non‐invasive mechanical ventilation in lung resection. European Review for Medical and Pharmacological Sciences 2018;22:190‐8. [DOI] [PubMed] [Google Scholar]
Ludwig 2011 {published data only}
- Ludwig C, Angenendt S, Martins R, Mayer V, Stoelben E. Intermittent positive pressure breathing after lung surgery. Asian Cardiovascular & Thoracic Annals 2011;19(1):10‐13. [DOI] [PubMed] [Google Scholar]
Perrin 2007 {published data only}
- Perrin C, Jullien V, Vénissac N, Berthier F, Padovani B, Guillot F, et al. Prophylactic use of non‐invasive ventilation in patients undergoing lung resectional surgery. Respiratory Medicine 2007;101:1572‐8. [DOI] [PubMed] [Google Scholar]
Roceto 2014 {published data only}
- Roceto LS, Galhardo FDM, Saad IAB, Toro IFC. Continuous positive airway pressure (CPAP) after lung resection: a randomized clinical trial [Pressão positiva contínua nas vias aéreas (CPAP) após ressecção pulmonar: ensaio clínico randomizado]. Sao Paulo Medical Journal 2014;132(1):41‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aguilo 1997 {published data only}
- Aguilo R, Togores B, Pons S, Rubi M, Barbe F, Agusti AGN. Non‐invasive ventilatory support after lung resectional surgery. Chest 1997;112(1):117‐21. [DOI] [PubMed] [Google Scholar]
Ingwersen 1993 {published data only}
- Ingwersen UM, Larsen KR, Bertelsen MT, Nielsen KK, Laub M, Sandermann J, et al. Three different mask physiotherapy regimens for prevention of post‐operative pulmonary complications after heart and pulmonary surgery. Intensive Care Medicine 1993;19:294‐8. [DOI] [PubMed] [Google Scholar]
Liao 2010 {published data only}
- Liao G, Chen R, He J. Prophylactic use of non‐invasive positive pressure ventilation in post‐thoracic surgery patients: a prospective randomized control study. Journal of Thoracic Diseases 2010;2:205‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nery 2012 {published data only}
- Nery F, Lopes AJ, Domingos DN, Cunha RF, Peixoto MG, Higa C, et al. CPAP increases 6‐minutes walk distance after lung resection surgery. Respiratory Care 2012;57(3):363‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Arozullah 2003
- Arozullah AM, Conde MV, Lawrence VA. Preoperative evaluation for postoperative pulmonary complications. Medical Clinics of North America 2003;87(1):153‐73. [DOI] [PubMed] [Google Scholar]
Brochard 2002
- Brochard L, Mancebo J, Elliott MW. Non‐invasive ventilation for acute respiratory failure. European Respiratory Journal 2002;19(4):712‐21. [DOI] [PubMed] [Google Scholar]
Brooks‐Brunn 1995
- Brooks‐Brunn JA. Postoperative atelectasis and pneumonia. Heart & Lung 1995;24:94‐115. [PubMed] [Google Scholar]
Ferlay 2010
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127:2893‐917. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Igai 2009
- Igai H, Takahashi M, Ohata K, Yamashina A, Matsuoka T, Kameiama K, et al. Surgical treatment for non small cell lung cancer in octogenarians ‐ the usefulness of video‐assisted thoracic surgery. Interactive Cardiovascular and Thoracic Surgery 2009;9:274‐7. [DOI] [PubMed] [Google Scholar]
Jemal 2011
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
Kutlu 2000
- Kutlu CA, Williams EA, Evans TW, Pastorino U, Goldstraw P. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Annals of Thoracic Surgery 2000;69:376‐80. [DOI] [PubMed] [Google Scholar]
Lorut 2005
- Lorut C, Rabbat A, Chatelier G, Lefevre A, Roche N, Regnard JF, et al. The place of routine immediate non‐invasive ventilation following pulmonary resection in preventing pulmonary complications in patients with COPD (POPVNI Trial) [Intérêt de la ventilation non invasive (VNI) systématique in post‐opératoire immédiat d'une résection pulmonaire pour prévenir les complications pulmonaires chez les patients BPCO (essai POPVNI)]. Revue des Maladies Respiratoires 2005;22(1 Pt 1):127‐34. [DOI] [PubMed] [Google Scholar]
Martin 2000
- Martin TJ, Hovis JD, Costantino JP, Bierman MI, Donahoe MP, Rogers RM, et al. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. American Journal of Respiratory & Critical Care Medicine 2000;161(3 Pt 1):807‐13. [DOI] [PubMed] [Google Scholar]
Nakagawa 2001
- Nakagawa M, Tanaka H, Tsukuma H, Kishi Y. Relationship between the duration of the preoperative smoke‐free period and the incidence of postoperative pulmonary complications after pulmonary surgery. Chest 2001;120(3):705‐10. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence (NICE). Lung cancer. The diagnosis and treatment of lung cancer, 2011. http://guidance.nice.org.uk/CG121. (accessed 26 November 2012).
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivera 2007
- Rivera MP, Mehta AC. Initial diagnosis of lung cancer: ACCP evidence‐based clinical practice guidelines. Chest 2007;132:131S‐48S. [DOI] [PubMed] [Google Scholar]
Schettino 2007
- Schettino GP, Reis MA, Galas F, Park M, Franca S, Okamoto V. Non‐invasive positive pressure ventilation [Ventilação mecânica não invasiva com pressão positiva]. Jornal Brasileiro de Pneumologia 2007;33 Suppl 2:92‐105. [DOI] [PubMed] [Google Scholar]
Suksompong 2012
- Suksompong S, Thamtanavit S, Bormann B, Thongcharoen P. Thoracic surgery mortality and morbidity in a university hospital. Asian Cardiovascular & Thoracic Annals 2012;20(2):182‐7. [DOI] [PubMed] [Google Scholar]
Vital 2008
- Vital FM, Sarconato H, Ladeira MT, Sen A, Hawkes CA, Soares B, et al. Non‐invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD005351.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Torres 2013
- Torres MFS, Carvalho APV, Riera R. Non‐invasive positive pressure ventilation for prevention of complications after pulmonary resection in lung cancer patients. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010355] [DOI] [PubMed] [Google Scholar]


