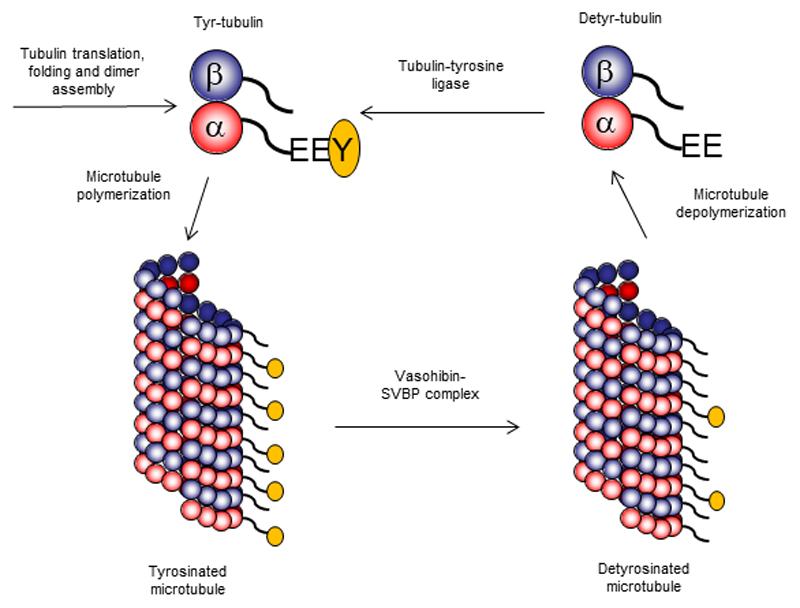
Microtubules are cytoskeletal filaments that drive chromosome segregation during cell division, control cell shape and motility, and serve as rails for motor-based intracellular transport. Microtubules are polymers built of highly conserved subunits, α- and β-tubulin, which contain a globular core and more variable C-terminal tails exposed at the microtubule surface. Although microtubules are structurally uniform, they display functional specialization due to the combination of different tubulin isoforms and multiple post-translational modifications - the so-called “tubulin code” (1). Many of these modifications occur within the C-terminal tubulin tails and affect microtubule interactions with motors proteins or regulatory factors. The first tubulin modifications to be discovered more than 40 years ago consist on the catalytic removal and reincorporation of the C-terminal tyrosine present in most α-tubulin isotypes (2–4) (Figure 1). Whereas re-tyrosination of soluble tubulin heterodimers is known to be mediated by tubulin-tyrosine ligase (5), α-tubulin detyrosination, which occurs preferentially on polymerized microtubules, is mediated by a carboxypeptidase activity that remained elusive until today. On pages XXX and XXX of this issue, two independent studies by Aillaud et al. (6) and Nieuwenhuis et al. (7) report the identification and characterization of Vasohibins as long-sought tubulin carboxypeptidases.
Figure.
Freshly translated α-tubulin contains a C-terminal tyrosine residue. After forming a dimer with β-tubulin, it assembles into microtubules. Vasohibin-SVBP complex removes C-terminal tyrosine residues from tubulin polymers and thus generates detyrosinated microtubules. When these microtubules disassemble, detyrosinated tubulin can be re-tyrosinated by tyrosine-tubulin ligase.
Vasohibins were originally identified as regulators of angiogenesis (8) and have a predicted protease fold with a non-canonical Cys-His-Ser/Thr catalytic triad (9). Mammalian genomes encode two Vasohibin paralogs, Vasohibin-1 and Vasohibin-2, but their proteolytic activity and molecular function had never been explored. Importantly, Vasohibins form a complex with the chaperone-like peptide Small Vasohibin-Binding Protein (SVBP) (10). This might explain why previous attempts to identify the tubulin carboxypeptidase have failed, since standard purification assays could result in dissociation of SVBP from Vasohibins, thereby compromising their stability and catalytic activity.
The two groups seeking for the elusive tubulin carboxypeptidase converged on Vasohibins using completely different unbiased approaches. Aillaud et al. employed chemical proteomics: they developed a potent irreversible inhibitor of tubulin carboxypeptidase, and combined it with mass spectrometry-based analysis of fractionated mouse brain lysates to identify Vasohibin-1 as the strongest candidate for the tubulin carboxipeptidase (6). In a complementary approach, Nieuwenhuis et al. employed a genetic screen for regulators of tubulin detyrosination using gene-trapping mutagenesis in a haploid human cell line. Reassuringly, they have not only identified tubulin-tyrosine ligase as the strongest negative regulator of tubulin detyrosination, but also isolated SVBP as the most significant positive regulator of this process (7).
Both groups went on to characterize the complexes of Vasohibins with SVBP at the biochemical and functional level. They found that SVBP binds to Vasohibins and enhances their tubulin carboxypeptidase activity both in cells and in assays with purified proteins (6, 7). As expected, the cysteine residues in the predicted catalytic sites of the two Vasohibins were indeed important for the activity (6, 7). Consistent with previous studies of microtubule detyrosination, Vasohibin-SVBP complexes displayed higher carboxypeptidase activity towards polymerized microtubules compared to unpolymerized tubulin dimers. Loss of function studies in cultured neurons, cell lines and mouse embryos revealed a redundant role of the two Vasohibins in tubulin detyrosination. Importantly, some detyrosinated tubulin was still present when both Vasohibins were depleted. This could not be attributed to partial depletion of Vasohibins or the expression of the α-tubulin isoform TUBA4A, which lacks the C-terminal tyrosine (7). Thus, cells contain an additional tubulin detyrosinating activity that remains to be identified.
Tubulin (de)tyrosination is intimately linked to microtubule dynamics: freshly polymerized microtubules are typically tyrosinated, whereas more stable, long-lived microtubules can be preferentially detyrosinated (11). Furthermore, the affinity of some regulators of microtubule stability is modulated by detyrosination (1). The tyrosination state of microtubules can also affect the binding of motor proteins: some kinesins bind better to detyrosinated microtubules compared with tyrosinated ones, while cytoplasmic dynein with its cofactors displays an opposite preference (1). As a result, intracellular cargos linked to particular motors can either preferentially use or avoid detyrosinated microtubules, resulting in differential cargo transport along particular microtubule tracks. Through such mechanisms, tubulin detyrosination plays a role in vital cellular functions, including cell division (12), cardiomyocyte contraction (13) and neuronal physiology (14).
In neuronal cells, the depletion of both Vasohibins or SVBP, which reduced, but did not abolish the levels of detyrosinated tubulin, delayed neuronal differentiation, induced morphological abnormalities and affected neuronal migration in mouse embryos (6). These results are consistent with the previous work on the neuronal function of the enzyme performing the reverse reaction, tubulin-tyrosine ligase (14). Curiously, contrary to other experimental perturbations that attenuate tubulin detyrosination during mitosis (12), Vasohibin deficiency did not seem to compromise chromosome congression, despite a clear reduction in microtubule detyrosination (7). This discrepancy might be explained by functional redundancy with yet unidentified tubulin carboxypeptidases that could ensure sufficient tubulin detyrosination or by redundancy in the process of chromosome congression per se. Future works will be necessary to further address this issue, and the availability of powerful new tools, such as more potent and specific tubulin carboxypeptidase inhibitors and knock out cell lines developed in the present studies, will certainly play an important role.
The discovery of Vasohibins as major tubulin detyrosinating enzymes will help to understand how cells deploy and use the tubulin code to control cytoskeletal organization and transport. This discovery also raises several exciting questions. Previous work on Vasohibins indicated that they might be secreted through an unconventional mechanism (8, 10) and that they can differentially affect angiogenesis and might also have a role in cancer (8). It would be interesting to know whether secreted Vasohibins maintain their tubulin detyrosinating activity and whether they can participate in intercellular communication mechanisms affecting cytoskeletal organization and trafficking. Another important question is whether tubulin is the only substrate of these enzymes. The work of Aillaud et al. showed that Vasohibin-2, but not Vasohibin-1, could remove the tyrosine from the C-terminus of the microtubule-regulating protein EB1, although the functional implication of such modification is unclear. Future structural studies of Vasohibins will be important to address how these enzymes interact with their substrates such as microtubules. Finally, the work on knockout mice lacking Vasohibin-1 or Vasohibin-2 revealed relatively mild phenotypes (15), and more detailed analysis of these mice, as well as the generation of animals completely lacking the Vasohibin/SVBP function would be needed to address the role of these proteins and tubulin detyrosination in the context of the whole organism.
Footnotes
Auxiliary Image
Microtubule diversity in human cells. Stable detyrosinated microtubules are highlighted in green. Image credits: Luísa Ferreira, Chromosome Instability and Dynamics Lab, Institute for Molecular Cell Biology (IBMC), Porto, Portugal.
References
- 1.Gadadhar S, Bodakuntla S, Natarajan K, Janke C. J Cell Sci. 2017;130:1347. doi: 10.1242/jcs.199471. [DOI] [PubMed] [Google Scholar]
- 2.Arce CA, Rodriguez JA, Barra HS, Caputo R. Eur J Biochem. 1975;59:145. doi: 10.1111/j.1432-1033.1975.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 3.Raybin D, Flavin M. Biochem Biophys Res Commun. 1975;65:1088. doi: 10.1016/s0006-291x(75)80497-9. [DOI] [PubMed] [Google Scholar]
- 4.Hallak ME, Rodriguez JA, Barra HS, Caputto R. FEBS Lett. 1977;73:147. doi: 10.1016/0014-5793(77)80968-x. [DOI] [PubMed] [Google Scholar]
- 5.Ersfeld K, Wehland J, Plessmann U, Dodemont H, Gerke V, Weber K. J Cell Biol. 1993;120:725. doi: 10.1083/jcb.120.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aillaud C, Bosc C, Peris L, Bosson A, Heemeryck P, Van Dijk J, Le Friec J, Boulan B, Vossier F, Sanman LE, Syed S, et al. Science. 2017 doi: 10.1126/science.aao4165. [DOI] [PubMed] [Google Scholar]
- 7.Nieuwenhuis J, Adamopoulos A, Bleijerveld OB, Mazouzi A, Stickel E, Celie P, Altelaar M, Knipscheer P, Perrakis A, Blomen VA, Brummelkamp TR. Science. 2017 doi: 10.1126/science.aao5676. [DOI] [PubMed] [Google Scholar]
- 8.Sato Y. J Biochem. 2013;153:5. doi: 10.1093/jb/mvs128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Pulido L, Ponting CP. Bioinformatics. 2016;32:1441. doi: 10.1093/bioinformatics/btv761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki Y, Kobayashi M, Miyashita H, Ohta H, Sonoda H, Sato Y. J Cell Sci. 2010;123:3094. doi: 10.1242/jcs.067538. [DOI] [PubMed] [Google Scholar]
- 11.Gundersen GG, Bulinski JC. Eur J Cell Biol. 1986;42:288. [PubMed] [Google Scholar]
- 12.Barisic M, Silva e Sousa R, Tripathy SK, Magiera MM, Zaytsev AV, Pereira AL, Janke C, Grishchuk EL, Maiato H. Science. 2015;348:799. doi: 10.1126/science.aaa5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robison P, Caporizzo MA, Ahmadzadeh H, Bogush AI, Chen CY, Margulies KB, Shenoy VB, Prosser BL. Science. 2016;352:aaf0659. doi: 10.1126/science.aaf0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erck C, Peris L, Andrieux A, Meissirel C, Gruber AD, Vernet M, Schweitzer A, Saoudi Y, Pointu H, Bosc C, Salin PA, et al. Proc Natl Acad Sci U S A. 2005;102:7853. doi: 10.1073/pnas.0409626102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura H, Miyashita H, Suzuki Y, Kobayashi M, Watanabe K, Sonoda H, Ohta H, Fujiwara T, Shimosegawa T, Sato Y. Blood. 2009;113:4810. doi: 10.1182/blood-2008-07-170316. [DOI] [PubMed] [Google Scholar]