Abstract
Background:
Many patients with Stage I-II pancreatic adenocarcinoma (PDAC) do not undergo resection. We hypothesized that: (1) clinical staging underestimates nodal involvement, causing Stage IIB to have a higher percent of resected patients, and (2) this stage-shift causes discrepancies in observed survival.
Methods:
The Surveillance, Epidemiology, and End-results (SEER) Research Database was used to evaluate cause-specific survival (CSS) in patients with PDAC from 2004–2012. Survival was compared using log-rank test. Single-center data on 105 patients that underwent resection of PDAC without neoadjuvant treatment was used to compare clinical and pathologic nodal staging.
Results:
In SEER data, medium-term survival in Stage IIB was superior to IB and IIA, with median CSS of 14, 9, and 11 months respectively (P<0.001). Seventy-two percent of Stage IIB patients underwent resection vs. 28% in IB and 36% in IIA (P<0.001). In our institutional data, 12.4% of patients had clinical evidence of nodal involvement vs. 69.5% by pathologic staging (P<0.001). Among clinical stage IA-IIA patients, 71.6% had nodal involvement by pathologic staging.
Conclusion:
Both SEER and institutional data support substantial underestimation of nodal involvement by clinical staging. This finding has implications in decisions regarding neoadjuvant therapy and analysis of outcomes in the absence of pathologic staging.
INTRODUCTION
A fundamental expectation in the care of cancer patients is that staging systems should stratify patients by extent of disease and predict outcome. For example, the preface to the American Joint Commission on Cancer (AJCC) Staging Manual states that “staging provides those with cancer and their physicians the critical benchmark for defining prognosis and the likelihood of overcoming the cancer and for determining the best treatment approach for their cases”.1 In pancreatic ductal adenocarcinoma (PDAC) clinical staging is used to make the decision about neoadjuvant therapy versus upfront resection. However, PDAC is unique among solid tumors in that approximately half of Stage I-II PDAC patients do not undergo resection.2–3 Because of this; comparisons of survival in early-stage patients involve both resected and non-resected patients.
Previous evaluation of the National Cancer Database (NCDB) from 1992–1998 found that the staging system was largely accurate with respect to survival, as evidenced by a concordance index (c-index) of 0.613.4 The study noted one discrepancy; unresected patients with Stage IIB disease had superior survival compared to Stage IIA patients in the first 2 years after diagnosis. The authors hypothesized that this was because of inconsistent clinical staging in unresected patients, who do not undergo pathologic staging.4
Our primary objective was to evaluate the predictive ability of the 7th edition AJCC staging system in a contemporary cohort of patients from the Surveillance, Epidemiology and End-Results (SEER) Research Database, particularly in Stages I-II. Once discrepancies in survival between Stages IIA-IIB were confirmed in the newer data, we sought to substantiate the hypothesis of Bilimoria et al: that unresected patients are understaged because the difference between Stages IIA and IIB (nodal involvement) is difficult to discern based on imaging.4 Since SEER documents clinical stage when resection was not performed and pathologic stage when it was, we explored the accuracy of clinical nodal (N) staging by comparing clinical and pathologic staging in a cohort of patients from our institution that underwent “surgery first” treatment of clinical Stage I-II PDAC. We also used SEER data to test two possible alternative explanations: that the inclusion of patients with T1N1M0 disease into Stage IIB may positively skew the survival of this stage, and that there may be a tendency to treat Stage IIB patients more aggressively than those with stages IB-IIA.
MATERIALS AND METHODS
SEER Research Database Cohort
Patients age 18 and older diagnosed between January 1, 2004 and December 31, 2012 with PDAC (ICD-O, 3rd Edition histology codes 8140–2, 8144, 8490, 8500–1, 8503–4, and 8507) were extracted from the SEER Research Database. The SEER Program contains approximately 26% of the population in the United States.5 Patients with previous cancers and those with unknown stage were not included in the data extraction. Patients with unknown resection status (N=221), Stage II NOS (not otherwise specified) disease (N=370) and those diagnosed at autopsy (N=241) were excluded, leaving 45,147 patients available for analysis. An additional 504 patients had missing data regarding receipt of radiation, leaving 44,643 patients available for analysis regarding receipt of radiation. Patient demographic and clinical data are reported as counts and percentages for categorical data and median and interquartile range (IQR) for continuous variables. Fisher’s exact test was used to examine the percent of patients in Stage IIB that had undergone resection compared separately to those in Stages IA-IIA.
Cause-specific survival (CSS) was calculated using the SEER cause-specific death classification data. Kaplan-Meier product limit methods were used to estimate cause-specific survival and to calculate median cause-specific survival and 5-year cause specific survival. Log-rank test was used for comparisons of survival. Stage-specific survival was examined for patients that underwent surgical resection as well as the overall cohort of both resected and unresected patients. Survival was also compared between the different subgroups that comprise Stage IIB (T1N1M0, T2N1M0, and T3N1M0), Stage IB (T2N0M0) and Stage IIA (T3N0M0) to determine whether inclusion of T1N1M0 patients may be partially responsible for improved survival observed in Stage IIB as compared to IB and IIA.
Concordance (c) index was calculated to evaluate the discriminatory power of the staging system using a Cox proportional hazards model with stage as the only variable. C-index was calculated for the SEER cohort and recalculated with T1N1M0 patients reclassified as Stage IIA. To evaluate the alternative hypothesis that cancers of increasing stage may be more likely to be treated with radiation therapy, logistic regression analysis was performed using stage as an ordered variable to test for trend in both resected and unresected Stage IA-III patients.
University of Utah Data
We examined patients at the University of Utah Health Sciences Center who underwent resection of PDAC from January 2001 to December 2014. Patients receiving neoadjuvant treatment (N=58) were excluded since they have a lower rate of lymph node involvement than patients resected upfront, making a comparison between clinical and pathologic N-stages invalid.6 Patients without a preoperative diagnosis of PDAC were also excluded (N=39). After exclusion criteria were applied, a cohort of 105 patients with both clinical and pathologic stage was available for analysis. Wilcox rank sum test was used with clinical and pathologic stages scored as ordered categorical variables to determine if pathologic stage was consistently higher than clinical stage. Fisher’s exact test was used to compare the number of patients that had nodal involvement by clinical and pathologic stage.
All statistical analysis was performed using R 3.2.1 statistical programming software (The R Foundation for Statistical Computing, Vienna, Austria). A p value of <0.05 was considered to be statistically significant for all analyses and all p-values were two-sided. The University of Utah Health Sciences Institutional Review Board approved this research.
RESULTS
Lack of discrimination of survival in Stages IB-IIA
A total of 45,147 patients diagnosed between 2004 and 2012 with a first cancer diagnosis of PDAC were identified from the SEER database and 20.7% of patients underwent resection (Table 1). Patents were followed from diagnosis until time of death or to a median of 9 months (IQR 3, 23) after diagnosis for the 13.8% for patients that were still alive at the time of last follow up. The expected inverse relationship between increasing stage and survival was observed when resected patients were evaluated (Figure 1A). However, when all patients (resected and unresected) were analyzed, the inverse relationship between stage and survival was lost (Figure 1B). In fact, median cause-specific survival for Stage IIB was longer than IB and IIA, and survival for IIA was longer than IB (Table 2). The survival of Stage IIB patients was better than IB and IIA patients until 37 months after diagnosis. The c-index of the overall cohort was 0.656 (95% confidence interval (CI) 0.653–0.660), which is similar to the value of 0.631 (95% CI 0.614–0.647) reported in the 2007 validation.4
Table 1.
Demographic, tumor and treatment characteristics of 45,147 patients with pancreatic adenocarcinoma, SEER Research Database 2004–2012.
| All Patients (N=45,147) |
Resected Patients (N=9,343) |
Unresected Patients (N=35,804) |
|
|---|---|---|---|
| Variable | No. (%) | No. (%) | No. (%) |
| Sex | |||
| Male | 23,226 (51.4%) | 4,706 (50.4%) | 18,520 (51.7%) |
| Female | 21,921 (48.6%) | 4,637 (49.6%) | 17,284 (48.3%) |
| Age, median (IQR) | 67 (59, 76) | 66 (58, 74) | 68 (59, 77) |
| Race | |||
| White | 35,925 (79.6%) | 7,665 (82.0%) | 28,260 (78.9%) |
| Black | 5,649 (12.5%) | 969 (10.4%) | 4,680 (13.1%) |
| Asian/Pacific Islander | 3,182 (7.0%) | 643 (6.9%) | 2,539 (7.1%) |
| American Indian/Alaska Native | 268 (0.6%) | 43 (0.5%) | 225 (0.6%) |
| Unknown | 123 (0.3%) | 23 (0.2%) | 100 (0.3%) |
| Ethnicity | |||
| Hispanic | 4,523 (10.0%) | 825 (8.8%) | 3,698 (10.3%) |
| Non-Hispanic | 40,624 (90.0%) | 8,518 (91.2%) | 32,106 (89.7%) |
| Tumor Location | |||
| Head | 23,483 (52.0%) | 6,987 (74.8%) | 16,496 (46.1%) |
| Body | 5,547 (12.2%) | 562 (6.0%) | 4,985 (13.9%) |
| Tail | 5,804 (12.9%) | 844 (9.0) | 4,960 (13.9%) |
| Overlapping/unspecified/other | 10,313 (22.8%) | 950 (10.2%) | 9,363 (26.2%) |
| Tumor Grade | (N=17,397) | (N=8,518) | (N=8,879) |
| Low | 9,712 (55.8%) | 5,283 (62.0%) | 4,429 (49.9%) |
| High | 7,685 (44.2%) | 3,235 (38.0%) | 4,450 (50.1%) |
| Stage at Presentation | |||
| IA | 646 (1.4%) | 373 (4.0%) | 273 (0.8%) |
| IB | 2,109 (4.7%) | 595 (6.4%) | 1,514 (4.2%) |
| IIA | 5,345 (11.8%) | 1,931 (20.7%) | 3,414 (9.5%) |
| IIB | 7,335 (16.2%) | 5,283 (56.5%) | 2,052 (5.7%) |
| III | 4,693 (10.4%) | 456 (4.9%) | 4,237 (11.8%) |
| IV | 25,019 (55.4%) | 705 (7.5%) | 24,314 (67.9%) |
| Received external beam radiation | (N=44,643) | (N=9,153) | (N=35,490) |
| Yes | 8,773 (19.7%) | 3,510 (38.3%) | 5,263 (14.8%) |
| No | 35,870 (80.3%) | 5,643 (61.7%) | 30,227 (85.2%) |
Figure 1.
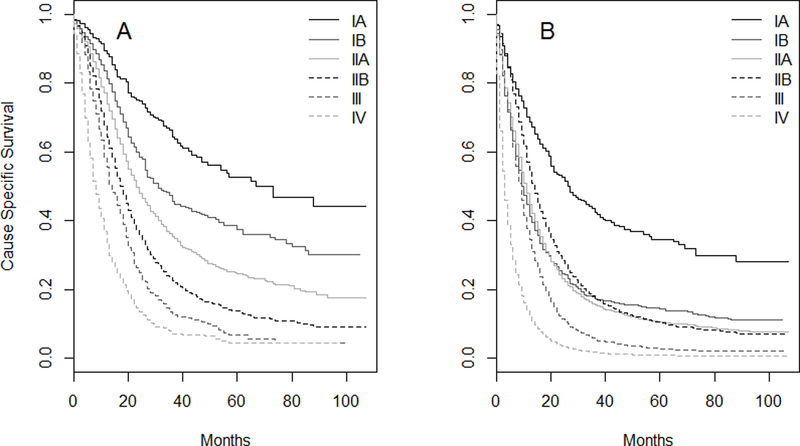
Cause-specific survival for resected patients (N=9,343) (A) and all patients (N=45,147) (B) from the SEER Research Database.
Table 2.
AJCC 7th edition staging System for pancreatic adenocarcinoma and observed cause-specific survival in patients with pancreatic adenocarcinoma, SEER* Research Database 2004–2012.
| Stage | AJCC 7th Edition TNM Staging System |
Number of Patients |
Median Survival (months) |
5 Year Survival (%) |
|---|---|---|---|---|
| IA | T1 N0 M0 | 646 | 26 | 34.6% |
| IB | T2 N0 M0 | 2,109 | 9 | 14.2% |
| IIA | T3 N0 M0 | 5,345 | 11 | 10.4% |
| IIB | T1-T3 N1 M0 | 7,335 | 14 | 10.4% |
| T1N1M0 | 236 | 19 | 19.0% | |
| T2N1M0 | 951 | 13 | 11.6% | |
| T3N1M0 | 6,143 | 14 | 10.4% | |
| III | T4 N0-N1 M0 | 4,693 | 9 | 2.7% |
| IV | T1-T4 N0-N1 M1 | 25,019 | 3 | 0.8% |
SEER, Surveillance, Epidemiology, and End Results Program
TMN, Tumor Node Metastasis. T1: a tumor ≤ 2 cm is confined to the pancreas, T2: a tumor confined to the pancreas and > 2 cm, T3: a tumor that has grown outside of the pancreas but not into vessels, T4: a tumor with vascular involvement.
Stage-shift results in fewer resected patients in Stages IB-IIA
In the SEER data, there were almost as many patients with Stage IIB disease as with IA-IIA added together; 7,335 versus 8,100. The number of resected patients in Stage IIB was 5,283 compared to 2,899 in IA-IIA. The rate of resection was 72% in Stage IIB, which was higher than any other stage (P<0.001 for IIB compared separately to IB, IIA, and IIA, Figure 2). This corresponded to a rate of resection in Stage IIB that was 1.25 times higher than in IA, over 2.5 times higher than in IB, and 2 times higher than in IIA.
Figure 2.
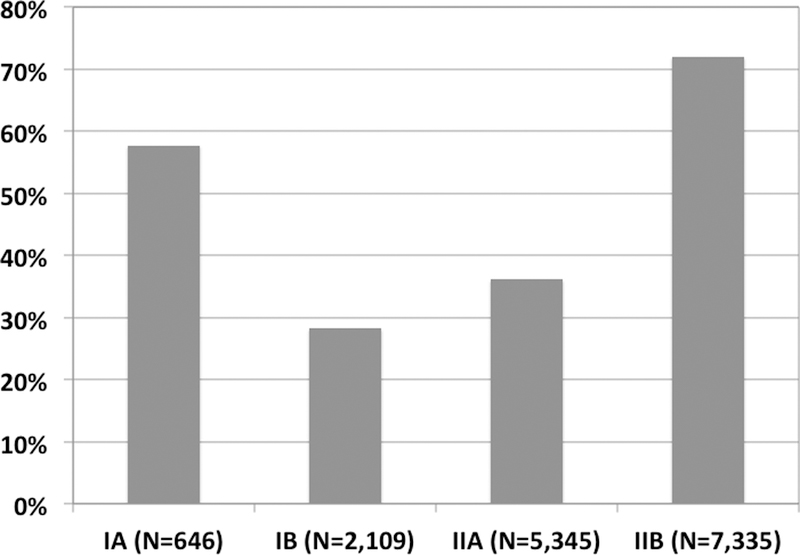
Percentage of patients with Stage I-II PDAC with resection performed, SEER Research Database. Stages IA, IB, and IIA are compared separately to Stage IIB (P<0.001 for all comparisons, Fisher’s test).
To provide evidence for a phenomenon of upstaging between clinical and pathologic staging, we evaluated 105 patients at our institution that underwent resection of PDAC without neoadjuvant therapy (Table 3). Only 13 patients (12.4%) had nodal involvement documented by clinical staging versus 73 (69.5%) by pathologic staging (P<0.001). Only 38 patients (36.2%) had N-status correctly classified by clinical staging. In cases of incorrect classification of N-status, 60 of 67 patients (89.6%) were upstaged from N0 to N1 and only 7 (10.4%) were downstaged from N1 to N0. Of 81 clinical stage IA-IIA patients, 58 (71.6%) had nodal involvement by pathologic stage. Overall, AJCC 7th edition pathologic stage was significantly higher than clinical stage (Table 4, P<0.001, Wilcox rank sum test). Importantly, 87 patients (83%) underwent preoperative endoscopic ultrasound (EUS). The remainder had only cross-sectional imaging.
Table 3.
Demographic, oncologic and treatment data for 105 patients at University of Utah that underwent resection of pancreatic adenocarcinoma without neoadjuvant therapy
| Variable | |
|---|---|
| Age, median (IQR) | 69 (60, 74) |
| Male sex, No. (%) | 57 (54.3%) |
| Caucasian race, No. (%) | 88 (83.8%) |
| Operation type, No. (%) | |
| Whipple | 90 (85.7%) |
| Distal pancreatectomy | 14 (13.3%) |
| Total pancreatectomy | 1 (0.95%) |
| Tumor size, cm, mean ± SD (N=99) | 3.6 ± 2.0 |
| Grade, No. (%) | |
| Well-moderately differentiated | 54 (51.4%) |
| Poorly differentiated | 51 (48.6%) |
| Adjuvant chemotherapy, No. (%) | 56 (53.3%) |
| Adjuvant radiation, No. (%) | 37 (35.2%) |
Table 4.
Clinical vs. Pathologic Staging for 105 patients at University of Utah that underwent upfront resection of pancreatic adenocarcinoma without neoadjuvant therapy.
| Clinical Stage | Pathologic Stage | P | |
|---|---|---|---|
| Stage IA | 12 (11.4%) | 5 (4.8%) | <0.001 |
| Stage IB | 37 (35.2%) | 4 (3.8%) | |
| Stage IIA | 32 (30.5%) | 19 (18.1%) | |
| Stage IIB | 17 (16.2%) | 67 (63.8%) | |
| Stage III | 7 (6.7%) | 8 (7.6%) | |
| Stage IV | 0 | 2 (1.9%) | |
T1N1M0 patients do not account for superior survival of Stage IIB
Using the SEER data, we compared the survival of patients in the 3 subgroups that comprise Stage IIB (T1N1M0, T2N1M0, and T3N1M0) to each other and to IB and IIA. In resected patients, T1N1M0 had similar survival to Stage IIA (Figure 3A). When all patients (resected and unresected) were considered, those with T1N1M0 disease had better survival than Stage IB, IIA and the other subgroups (T2-T3N1M0) in IIB (Figure 3B). However, the number of T1N1M0 patients was small (N=237, or 3.2% of all Stage IIB patients). Reclassifying T1N1M0 cases as Stage IIA did not cause a significant change in the c-index due to the small number of T1N1M0 patients.
Figure 3.
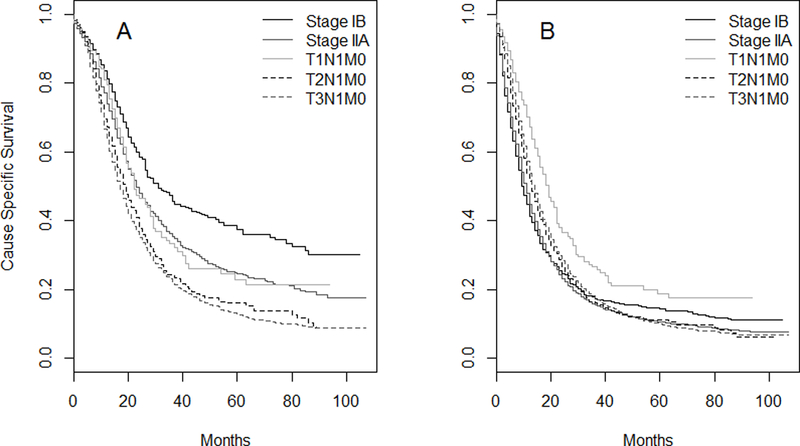
Cause-specific survival for resected patients (A) and all (B) patients with Stages IB-IIB PDAC, SEER Research Database. Stage IIB is subdivided into T1N1M0, T2N1M0 and T3N1M0.
Increasing stage is associated with use of radiation
There was a significant association between increasing stage and receipt of radiation during the treatment course in both resected and unresected Stage IA-III patients in the SEER cohort (Figure 4, P<0.001).
Figure 4.
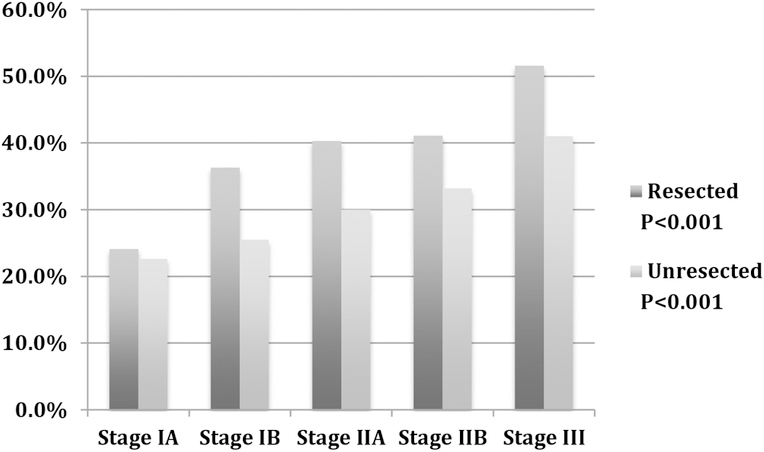
Percent of patients within each stage that received external beam radiation during treatment course, SEER Research Database.
DISCUSSION
The major finding in this study is that inaccuracy of clinical N-staging causes Stage IIB to be comprised of a disproportionately high number of resected patients compared to IA-IIA (Figure 2). The number of resected patients in IIB was 1.8 times higher than the number in IA-IIA added together. This explains why Stage IIB patients have better early to medium-term survival. PDAC is unique among other common solid organ tumors in that only about half of patients with early stage, resectable tumors undergo resection.2–3 Thus, when comparing the survival of the overall population of PDAC patients, the percent of resected patients in each stage makes a substantial impact on observed stage-specific survival.
We found that the rate of nodal involvement by pathologic staging was over 5 times higher than that measured by clinical staging. Of patients that were clinically staged as IA-IIA, 71.6% had nodal positivity by pathologic staging. Our findings are consistent with other studies showing that pathologic nodal involvement (i.e. Stage IIB) is the rule rather than the exception in resectable PDAC. A recent study used a uniform lymph node harvesting protocol in 227 consecutive pancreaticoduodenectomies found an actual lymph node positivity rate of 77%.7 They also found that 91% of patients had involvement of the peri-pancreatic soft tissue, classifying the patients as pT3. An additional 4.5% qualified as pT3 due to extension to adjacent sites. Thus, less than 5% of cases actually qualified as T1-T2 tumors.8 These findings show that a node-negative PDAC confined to the pancreas (T1-T2) is truly a rare occurrence, and support the concept of PDAC as an advanced disease in most cases.
As a result of such studies, changes have been made in the recently released 8th Edition AJCC staging system.9 The current T-stage system is abandoned in favor of a size-based system in T1-T3 and nodal substaging is introduced. In the 8th edition N1 tumors are those with 1–3 positive nodes, and those with ≥ 4 nodes are classified as N2. N1 tumors define Stage IIB and N2 tumors are classified as part of Stage III. This reclassification of patients with ≥ 4 positive nodes recognizes the importance of nodal burden of disease on survival, and acknowledges that patients with high nodal disease burden behave like locally advanced tumors rather than localized tumors. A multi-institution validation study of the 8th edition system has evaluated the changes made by the 8th edition system, and found that the median survival of N2 patients was 16.3 months, which is comparable to the reported survival 13–17 months of Stage III tumors.10 This validation study also found that the 8th edition results in a more even distribution of patients among Stages I-IIB disease. By 8th edition staging 8% had Stage IA, 22% IB, 4% IIA, 40%, IIB, and 26% III versus 5% IA, 7% IB, 22% IIA, and 67% IIB by 7th edition stage.10 However, it is important to note that the phenomenon of nodal upstaging that we describe will occur to the same extent with the 8th edition system as it did with the 7th edition. The more equal stage distribution in the 8th edition system is partially because some of the Stage IIB patients are shifted in to Stage III. The 70% of patients our data show will be upstaged from N-negative to N-positive will now simply be more evenly distributed between Stages IIB and III.
Our findings emphasize that clinicians are essentially blind as to whether a patient has nodal involvement at the time of the decision for neoadjuvant therapy versus upfront resection. Currently, many institutions utilize neoadjuvant therapy in cases of borderline resectable disease. A smaller number utilize a neoadjuvant approach in all patients with resectable disease.11 Better strategies are needed to identify which patients will benefit from neoadjuvant therapy. As an example, a recent study using the NCDB found that carbohydrate antigen (CA) 19–9 elevation at time of diagnosis independently predicts decreased survival in these patients.12 However, patients with CA 19–9 elevation that received neoadjuvant therapy had comparable survival to those with normal CA 19–9. Further such efforts to identify patients with more aggressive biology in the face of radiographically resectable tumors are needed to better select patients for neoadjuvant therapy. Creation of a preoperative nomogram to predict pathologic lymph node positivity could be a useful strategy.
We explored two alternative explanations as to why the staging system does not discriminate survival in Stages IB-IIB. Since Stage IIB is composed of T1-T3 tumors, we hypothesized that T1N1M0 patients might experience better survival than the rest of IIB patients. In resected patients, T1N1M0 patients experienced survival comparable to Stage IIA. In the overall group (resected and unresected) T1N1M0 patients had superior survival to Stage IB, IIA and the other groups (T2-T3) within IIB. However, when T1N1M0 patients were reclassified as Stage IIA, this had no significant impact on the concordance index of the overall staging system due to the small number of such patients. Thus, while it may be appropriate for T1N1M0 patients to be classified as Stage IIA for the purpose of improved prognostication for individual patients, the small contribution of better survival in T1N1M0 cases cannot be solely responsible for the observed discrepancies in survival in Stages IB-IIB. Our other alternative hypothesis was that patients with Stage IIB disease might be more likely to be treated with chemoradiation, prolonging survival compared to patients of lower stages. Increasing stage was associated with increasing rates of receipt of radiation in Stages IA-III in the SEER data (Figure 4). SEER does not contain information on chemotherapy. However, a study using NCDB data showed that, after pancreatectomy, 85% of patients that received radiation also received chemotherapy, whereas only 10% of patients who did not undergo adjuvant radiation received chemotherapy13. There is likely also a similar trend present for increasing chemotherapy usage rate with increasing stage. It is difficult to quantify exactly how much impact this has on the survival of each stage, but it seems likely that this effect is small relative to the impact of the high utilization of resection in Stage IIB.
This study is limited by its retrospective nature. The SEER database documents clinical stage when surgery is not performed, and pathologic stage when it is. Thus, a direct comparison between clinical and pathologic stage using SEER data was not possible. Although the SEER database maintains a 98% accuracy rate, incorrect coding or erroneous data are also possible.5 We could not quantify the extent to which the trend of treating more patients with radiation and chemotherapy (which is not recorded in SEER) improved survival in higher stage patients. The number of patients from our institution was relatively small, and larger studies should confirm the exact relationship between clinical and pathologic staging.
In summary, we examined recent SEER data and found that median survival is unexpectedly longer for Stage IIB patients than IIA, and longer for IIA than IB. This is primarily because over 70% of Stage IIB patients undergo resection, which is more than in lower stages. Clinical staging of PDAC significantly underestimates nodal involvement, and approximately 70% of patients that were clinically node negative had nodal involvement after pathologic evaluation. The recently released AJCC 8th Edition Staging System will classify some of these upstaged patients (those with ≥ 4 positive nodes) as Stage III. These findings add to the modern understanding of PDAC as a systemic disease, and point to the need for better strategies to identify which patients will benefit from neoadjuvant therapy. If techniques were available to preoperatively identify patients with significant nodal involvement then the utility of using clinical nodal positivity as an indication for neoadjuvant therapy could be explored.
Acknowledgements
This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067–02 (formerly 8UL1TR000105 and UL1RR025764).
This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067–02 (formerly 8UL1TR000105 and UL1RR025764).
Footnotes
Presented as an oral presentation at the 11th Annual Academic Surgical Congress: February 3, 2016. Jacksonville, FL.
Conflicts of interest: The authors have no financial or other potential conflicts of interest to disclose.
References
- 1.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A AJCC Cancer Staging Manual. New York (NY): Springer-Verlag; 2007. [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strohl MP, Raigani S, Ammori JB, Hardacre JM, Kim JA. Surgery of localized pancreatic cancer: the trend is not improving. Pancreas. 2016;45:687–693. [DOI] [PubMed] [Google Scholar]
- 4.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP et al. Validation of the 6th Edition AJCC Pancreatic Cancer Staging System: Report From the National Cancer Database. Cancer. 2007;110:738–44. [DOI] [PubMed] [Google Scholar]
- 5.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2014 Sub (1973–2012) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.
- 6.Estrella JS, Rashid A, Fleming JB, Katz MH, Lee JE, Wolf RA et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:268–77. [DOI] [PubMed] [Google Scholar]
- 7.Basturk O, Saka B, Balci S, Postlewait LM, Knight J, Goodman M et al. Substaging of Lymph Node Status in Resected Pancreatic Ductal Adenocarcinoma Has Strong Prognostic Correlations: Proposal for a Revised N Classification for TNM Staging. Ann Surg Oncol. 2015;Suppl3:S1187–1195. [DOI] [PubMed] [Google Scholar]
- 8.Saka B, Balci S, Basturk O, Bagci P, Postlewait LM, Maithel S et al. Pancreatic Ductal Adenocarcinoma is Spread to the Peripancreatic Soft Tissue in the Majority of Resected Cases, Rendering the AJCC T-Stage Protocol (7th Edition) Inapplicable and Insignificant: A Size-Based Staging System (pT1: ≤2, pT2: >2-≤4, pT3: >4 cm) is More Valid and Clinically Relevant. Ann Surg Oncol. 2016. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakar S, Pawlik TM, Allen PJ, et al. Exocrine Pancreas. Pancreatic adenocarcinoma In: Amin MB, editor. AJCC Cancer Staging Manual.. 8th ed, New York: Springer-Verlag; 2016. [Google Scholar]
- 10.Allen PJ, Kuk D, Fernandez-del Castillo C, Basturk O, Wolfgang CL, Cameron JL, Lillemoe KD et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients with Pancreatic Adenocarcinoma. Ann Surg. 2016; published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fathi A, Christians KK, George B, Ritch PS, Erickson BA, Tolat P et al. Neoadjuvant therapy for localize pancreatic cancer: guiding principles. J Gastrointest Oncol. 2015;6:418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berquist JR, Puig C, Shubert C, Groeschl RT, Habermann EB, Kendrick ML, et al. Carbohydrate antigen 19–9 elevation in anatomically resectable, early-stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: a national cancer database study. J Am Coll Surg. 2016;223:52–65.. [DOI] [PubMed] [Google Scholar]
- 13.Bilimoria KY, Stewart AK, Tomlinson JS, Gay EG, Ko CY, Talamonti et al. Impact of adjuvant radiation on survival: a note of caution when using cancer registry data to evaluate adjuvant treatments. Ann Surg Oncol. 2007;14:3321–7. [DOI] [PubMed] [Google Scholar]


