Abstract
Pharmacological strategies for pain management have primarily focused on dampening ascending neurotransmission and on opioid receptor-mediated therapies. Little is known about the contribution of endogenous descending modulatory systems to clinical pain outcomes and why some patients are mildly affected while others suffer debilitating pain-induced dysfunctions. Placebo effects that arise from patients’ positive expectancies and the underlying endogenous modulatory mechanisms may in part account for the variability in pain experience and severity, adherence to treatment, distinct coping strategies, and chronicity. Expectancy-induced analgesia and placebo effects in general have emerged as useful models to assess individual endogenous pain modulatory systems. Different systems and mechanisms trigger placebo effects that highly impact pain processing, clinical outcomes, and sense of well-being. This review illustrates critical elements of placebo mechanisms that inform the methodology of clinical trials, the discovery of new therapeutic targets, and the advancement of personalized pain management.
Keywords: nocebo, expectation, conditioning, modeling, dose-extending placebos, opioids, vasopressin
1. INTRODUCTION
The placebo effect is a powerful mechanism for modulating clinical outcomes. Linked to psychoneurobiological changes, placebo effects result from the expectancies of the patient, proxy, and provider (1, 2) and are distinct from regression to the mean, spontaneous remission, and fluctuations in symptoms. In randomized clinical trials, the inclusion of a no-intervention arm (3) and possibly a measurement of expectations (4) are critical design elements that can help separate placebo effects from these potential confounds (5).
This phenomenon has been particularly well investigated in the areas of experimental and clinical pain, but placebo effects can influence any treatment and any condition (6). Before the recent resurgence of interest in them, the increasing focus of modern medicine on specificity and mechanisms caused the placebo effect to be considered unscientific. Placebo treatments were believed to affect only subjective symptoms and not objective bodily processes, and thus they were commonly characterized, and often disregarded, as nonspecific effects. Yet as more rigorous and systematic research on the mechanisms of the placebo effect continued, scholars began to differentiate placebo effects from confounding variables as well as other individual and disease factors influencing symptom variability. New evidence continues to suggest that placebo effects influence physiological mechanisms and outcomes of pain. Placebo effects have been documented across different diseases, including symptoms ranging from itching to cancer-related fatigue, from Parkinson’s disease to attention deficit hyperactivity disorder (ADHD), from anxiety to social phobia, from depression to addiction, and from asthma to immune diseases (reviewed in 1, 7, 8).
2. HISTORICAL REMARKS
For hundreds of years, clinicians and other health care practitioners have known that different interventions with unclear mechanisms of action could still result in improvements of clinical symptoms. One of the earliest mentions of the administration of a placebo dates back to the 1770s when a physician described two instances of giving an inert medication to patients for end-of-life comfort (9). Furthermore, many placebo remedies appeared in the first London Pharmacopoeia issued by the Royal College of Physicians in 1618 (10). An estimated 5,000 ancient remedies with over 16,000 different prescriptions, from Gascoyne’s powder (i.e., coral, crabs’ eyes and claws, amber, bezoar, and pearls) to bezoar stones and animal gallstones (11), were initially used to please or placate and not for specific clinical effects. Placebos and their related therapeutic effects began to gain traction in the United States during the 1946 Cornell Conference on Therapy where, after reviewing European data regarding placebo action, it was declared for the first time that more research was needed given that “the placebo is a potent agent and in its actions can resemble almost any drug” (12, p. 1718). Although members of the community at this meeting commented that placebos had been commonly used, there were only a few documentations of this so-called pious fraud (13, 14). Beecher (15) pointed out that, despite the lack of scientific and clinical knowledge at that time, placebo effects may be present in experimental and clinical settings and that much of the action of a drug could be related to the individual’s process of suffering rather than the direct effects of the medication itself.
3. MECHANISMS OF PLACEBO EFFECTS
Pharmacological, neuroimaging, behavioral, and physiological approaches have been used to help understand the mechanisms of placebo effects in human research settings (Figure 1). Such studies have shown that placebo effects engage various neurobiological and physiological mechanisms, including the endogenous opioid, endocannabinoid, oxytocin, vasopressin, and dopamine systems. As such, its effects will depend on the target system and illness.
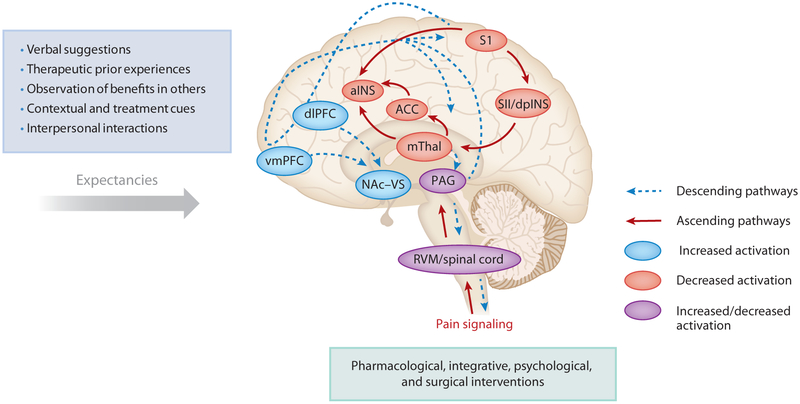
Figure 1.
Schematic representation of the psychophysiological mechanisms of placebo effects. Verbal suggestions (e.g., anticipation of a benefit), firsthand therapeutic experience of pain reduction (e.g., experiential learning and conditioning), observation of others (e.g., social learning), contextual and treatment cues (e.g., seeing a treatment), and interpersonal interactions (e.g., patient-clinician relationship) contribute to create expectancies that can trigger a set of psychoneurobiological changes. At the neural levels, placebo effects result in the release of neuropeptides (e.g., opioids) and the modulation of brain areas involved in the transmission of pain signaling and the formation of expectancies. Areas such as the spinal cord, RVM, PAG, mThal, ACC, SII-dpINS, and aINS (red) show reduced activation when placebo effects are observed. The generation of expectancies involves an increased activation of frontal areas including the vmPFC and dlPFC with a descending modulation of the NAc–VS, PAG, the spinal cord, and RVM (blue). PAG, the spinal cord, and RVM play a dual function with both increased and decreased activity (purple). The generated placebo effects influence responses to pharmacological, integrative, psychological, and surgical interventions (155). Abbreviations: aINS, anterior insula; ACC, anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; mThal, medial thalamus; NAc–VS, nucleus accumbens–ventral striatum; PAG, periaqueductal gray; RVM, rostroventral medulla; S1, primary somatosensory area; SII–dpINS, secondary somatosensory area and dorsal posterior insula; vmPFC, ventromedial prefrontal cortex. Figure adapted from Reference 55.
3.1. Human Pharmacological and Brain Imaging Approaches
In 1978, Levine and colleagues (16) demonstrated that placebo analgesic effects could be halted by the administration of the μ-opioid receptor antagonist naloxone. The last four decades of research have further linked placebo effects in the context of pain to the endogenous opioid system. Using the opioid antagonist naloxone (16–20) and in vivo receptor binding of μ-opioid receptors (21, 22), a series of neuropharmacological studies have corroborated and confirmed the notion that the opioid system is involved in the formation of placebo analgesia.
A placebo given after consecutive administrations of morphine occurring 1 day (20) or 1 week (23) apart induces a morphine-like effect on pain endurance that is antagonized by naloxone (10 mg/kg) (Figure 2). The importance of the opioid system for placebo effects is not surprising given that μ-opioid receptors are widely distributed, with the highest concentrations in the thalamus and periaqueductal gray (24), and are critical for the reduction of pain induced by therapeutic opioids. Endogenous opioids that are naturally made in the brain and exogenous opioid drugs that are synthetically manufactured activate opioid receptors in distinct locations (25). Uncovering the distinct features of the receptor binding mechanisms for endogenous opioids and exogenous opioids has important implications for drug development, the prevention of side effects, and better pain therapeutics.
Figure 2.
Opioids and placebo effects. A placebo (red) given after consecutive administrations of morphine occurring (a) 1 day or (b) 1 week apart induces a morphine-like effect on pain endurance, indicating that the placebo acts as a dose-extending agent despite the washout and the half-life of opioids. Data taken from References 20 and 23. Figure adapted from Reference 97.
Other systems, such as the cannabinoid and dopamine systems, have been explored using pharmacological antagonists to reverse the behavioral placebo effects. For example, pharmacological conditioning with the nonopioid nonsteroidal anti-inflammatory drug ketorolac induced a placebo effect when ketorolac was replaced by a placebo. The ketorolac-like effect was reversed by the cannabinoid type 1 receptor antagonist rimonabant, which suggests that the endogenous cannabinoid system can be trained to induce placebo effects.
In terms of the involvement of the dopamine system, there have been positive (26, 27) and negative results (28, 29) in pain research using brain imaging and pharmacological approaches, respectively. A PET imaging study with carbon-11-labeled raclopride as the radioligand (27) showed that the activation in the ventral basal ganglia, including the nucleus accumbens, is related to the perceived effectiveness of the treatment (which in reality was intravenous saline) and pain reduction, and this activation accounted for 25% of the variance in placebo analgesic effects (27).
Pharmacological approaches with dopamine antagonists and agonists did not show an effect on placebo analgesia in healthy participants, and the dopamine antagonist haloperidol did not reverse placebo analgesia (29) in neuropathic chronic pain patients (28). Although these results indicate that dopamine antagonists and agonists may not be essential for eliciting placebo-induced pain reduction, they do modulate expectancies and the desire of pain relief (28) and the efficacy of recalled placebo effects (30). Conversely, dopamine-like effects have been observed in studies with Parkinson’s patients using either in vivo single neural activity recording and pharmacological conditioning (e.g., apomorphine) (31–33) or in PET studies (34, 35), suggesting that placebo effects are in part mediated by the dopamine system in Parkinson’s disease.
3.2. Using Agonist Agents to Enhance Placebo Effects
Recently it has been shown that it is possible to increase placebo effects pharmacologically, with potential benefits for improving pain management and coping. Both oxytocin and vasopressin agonists have been given intranasally to enhance expectancy-induced analgesia (36, 37). The central nervous system distribution of these two peptide hormones suggests that they are pivotal for regulating social behaviors across different species (38, 39) and with sex-specific effects (39–44). In male animals, vasopressin promotes aggression, probably acting at the level of the septum, anterior hypothalamus, and central gray. In female animals, it promotes affiliative behaviors via actions in the septum and ventral pallidum (40). Human studies suggest that vasopressin plays a role in conciliatory behaviors (45) as well as in interpersonal communication (46, 47), favoring tend-and-befriend behavioral patterns of women toward other women and fight-or-flight in men toward other men (47). We explored the effects of arginine vasopressin, a nonselective agonist of the arginine vasopressin 1a (Avp1a) and 1b (Avp1b) receptors, against no treatment, oxytocin, and saline in a randomized, placebo-controlled, double-blind, parallel design trial. We implemented a model of expectancy-induced hypoanalgesia in which verbal suggestions of pain reduction along with the administration of a sham intervention were applied. We used a relatively low dose of oxytocin (24 IU) compared to the dosage (40 IU) used in a study by Kessner et al. (37), who reported an increase of placebo effects in men. When given intranasally, synthetic vasopressin reaches the central nervous system through the nasal mucous membranes and achieves a steady state within 30–50 min, and the peptide concentration in the cerebrospinal fluid collected in humans, as measured by radioimmunoassay, remains stable for about 80 min (48).
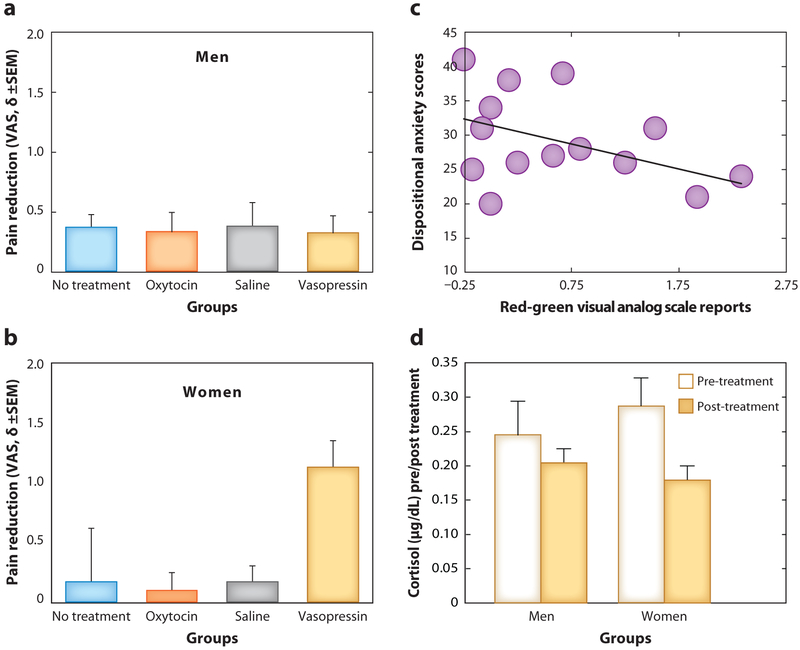
Arginine vasopressin, given intranasally, increases verbally induced placebo effects with a significant sex-by-treatment interaction. Vasopressin enhanced the effect of the placebo analgesic treatment in women but not in men, relative to the no treatment, oxytocin, and saline groups. Moreover, larger placebo effects were seen in those women with lower acute cortisol levels and lower dispositional anxiety (Figure 3).
Figure 3.
Arginine vasopressin and placebo analgesic effects. (a,b) Vasopressin induces a significant increase in placebo effects as compared to oxytocin, saline, and no treatment. The effect size is significantly larger (Cohen’s δ = 0.603) in women as compared with a smaller and nonsignificant effect of vasopressin on placebo effects in men. In women, (c) dispositional anxiety and (d) the significant acute salivary cortisol changes correlate negatively with the magnitude of vasopressin-induced enhancement of placebo effects. Red-green refers to the difference in pain reports when two distinct visual cues are presented as part of the placebo manipulation. The VAS runs from 0 = no pain to 10 = maximum tolerable pain. Abbreviation: VAS, Visual Analog Scale. Data from Reference 38.
This finding suggests the potential of using vasopressin agonists as pharmacological therapeutic targets. This possible link between psychophysiological changes and behaviors could be a way to better understand drug (and placebo) responsiveness (49). These results are also consistent with animal and human studies (50–52). Receptor autography studies in the socially monogamous coppery titi monkey demonstrated that Avp1a receptors are crucially involved in social behaviors and expressed at the level of the cortex (cingulate, insular, and occipital), central amygdala, nucleus accumbens, caudate, putamen, endopiriform nucleus, and hippocampus (53). The activation of brain reward and salience circuits has also been shown in men and women (47). Vasopressin most likely shaped the meaning of the instructions, resulting in an enhancement of expectancy-induced analgesic responses, emphasizing the role of response to meaning in forming placebo effects (54). Future brain imaging studies could identify the brain circuitries involved, thus opening up the possibility of using these hormones clinically.
3.3. Triggering Placebo Effects Behaviorally
Behavioral and neurobiological placebo effects are triggered by verbal suggestions, classical and nonclassical conditioning, and social interactions, including observation and complex interpersonal interactions (55–57). Verbal suggestions can induce the anticipation of positive outcomes and placebo effects.
During a postsurgical window, overt administration of morphine, combined with telling a patient that the treatment they are going to receive is a potent pain treatment, induces substantially larger benefits than covert administration of morphine, that is, by leaving the patient unaware of its administration while delivering the medication through a computerized infusion pump (6). The benefits of morphine and many other pain treatments are linked to both the medication and expectancy responses.
Various degrees of exposure to analgesic benefits through prior pharmacological and nonpharmacological interventions create subsequent neurobiological placebo effects. In particular, the length of training (58), prior experience with either an effective or an ineffective treatment (59), the schedule (1 day apart versus 1 week apart) (Figure 2) (23), and the conditioning paradigm (continuous versus partial reinforcement) (60) can all influence the occurrence and magnitude of placebo effects. Partial reinforcement refers to the situation in which the unconditioned stimulus is associated with the neutral stimulus in either a random fashion or according to a schedule, but not for every association response as is the case with the full continuous reinforcement paradigm. Prior positive therapeutic experiences augment placebo-induced analgesic effects, and negative previous experiences diminish them. A group of healthy study participants received a nonpharmacological treatment that was made effective (e.g., the pain intensity was surreptitiously reduced to simulate analgesia), and a second group received a treatment that was made ineffective (e.g., the pain intensity was kept at the same painful level); when tested for placebo effects, those subjects who were preexposed to a positive outcome showed a pain reduction of 49.3%, while those who had a negative therapeutic experience only experienced a 9.7% reduction in pain (59). When the subjects were retested a week afterwards with the other treatment (i.e., those assigned to the positive outcome received the negative treatment and vice versa), placebo effects following the effective procedure were significantly higher than those observed after the ineffective treatment (29% versus 18% pain reduction), suggesting sequential effects. These findings may help clarify variability in clinical outcomes and placebo effects when patients are switched from one treatment to another.
Positive and negative treatment histories affect not only behavioral placebo effects but also induced brain changes (61). A history of negative therapeutic experiences reduces placebo analgesia and is paralleled by higher activation of the bilateral posterior insulae that regulate afferent nociceptive (pain arising from the stimulation of the nerve cells) processes and lower activation of the right dorsolateral prefrontal cortex, which plays a role in the formation and maintenance of placebo effects (62, 63).
The duration and extent of previous pain relief experiences impact the magnitude of placebo analgesia, as indicated in a study that varied the conditioning (acquisition phase) from 10 to 40 exposures to analgesic treatment. The observed size and extinction of placebo and nocebo [referring to the impact of negative expectancies on outcomes (64)] effects were linked to the length of exposures to prior effective and ineffective (65) interventions (58). Observing a benefit in another person also plays a role in the formation of placebo effects (66–68). First postulated by Bootzin & Caspi in 2002 (69), observational learning generates expectancies that lead to placebo analgesic effects of similar sizes. This was subsequently demonstrated by Colloca & Benedetti (67) in observational learning and conditioning arms of a randomized parallel-arm trial; participants’ pain was reduced by 39.18% and 43.35%, respectively, in the two arms.
These studies have relevance in terms of considering an individual’s treatment history and using learning and modeling manipulations to maximize or minimize treatment responses depending on the context (clinical practice versus treatment development), and they have facilitated a better understanding of the overall placebo phenomenon.
4. AN INTEGRATIVE MODEL OF PLACEBO EFFECTS
Despite the numerous attempts to explain placebo effects with theories and frameworks that have included the expectancy theory (70), classical conditioning (71), other learning principles (60, 72), contextual effects (73), and the meaning response (54), none of them alone seems to account for the variety of placebo effects observed in clinical trials and practice.
Colloca & Miller (56) recently proposed an integrative model that focuses on instructional, experiential, and social learning mechanisms that can trigger conscious and unconscious expectancies that generate placebo effects. Although instructional (e.g., a suggestion that a certain painkiller reduces pain), experiential (e.g., the exposure to an effective pain treatment), and social (e.g., the observation of others perceiving pain relief) learning vary in terms of their nature, these processes convey information that is dynamically integrated with contextual cues, prior beliefs, and therapeutic histories to create placebo effects (Figure 1). This integrative view of empirical findings related to placebo effects also facilitates the conceptualization of a model of placebo effects, rather than a reliance on discrete mechanisms (e.g., conditioning versus expectations) that are rarely separable in clinical settings. An integrative model also facilitates the study of placebo effects through influential error prediction models including Bayesian (74), Rescorla-Wagner (75), and others (76). It is likely that cues from the clinical encounter are integrated with personal experience to make inferences and predict the likelihood of future outcomes.
Two aspects are worth mentioning when considering this integrative model. First, the isolation of verbal, conditioned, and social cues is not possible in the usual patient-clinician interaction. The clinician shows attention (e.g., the ritual elements of providing a treatment) and uses verbal and nonverbal communication strategies, including reassurance, suggestions for positive therapeutic expectations, empathic listening, and encouragement. The patient-clinician relationship, as Colloca & Miller (56, p. 1864) suggested, is “a process of interaction in which patients and doctors continually, mutually and reciprocally influence each other.” For example, the clinician’s expectation about the effectiveness of a treatment may in turn influence the patient’s expectation of benefit (56). A clinician who understands their patient modifies their behavior as a healer based on the patient’s behavior as a convalescent.
Second, expectations are typically seen as a reportable anticipation of a future event (e.g., an expectation that a patient’s pain will be 6 out of 10). Expectancies, on the contrary, do not necessarily entail consciousness and are instead anticipatory and predictive states that may or may not be consciously accessible depending on the body system and the phylogenetic level (56, 77). For example, placebo-induced pain reduction is an experience consciously accessible, whereas placebo (conditioned) hormonal responses are unaffected by expectations (78). There is evidence that nonhuman animals learn to predict and expect outcomes, showing placebo effects both in general with the expectancy of reward, observed in honeybees (79), and in the context of placebo manipulations (80, 81).
5. ANIMAL RESEARCH
The investigation of the placebo effect has been flourishing in human laboratory settings more than in animal research. This is not surprising given that placebo effects in animals can only be studied through physiological or pharmacological paradigms, whereas in humans verbally induced responses can also be investigated (reviewed in 82). The animal studies that have been conducted appear to corroborate the results from human studies.
Following one of the first seminal works using scopolamine and conditioning (81), it was subsequently demonstrated in rats that when environmental (83) and gustatory (84) cues were paired with morphine (the unconditioned stimulus), replacing the morphine with a placebo could induce morphine-like placebo analgesic effects (85). Although in a few instances a single administration of an opioid (e.g., fentanyl) given in a novel context has been reported to create a placebo effect (86), the general understanding is that the repetitive administration of the unconditioned stimulus is critical for creating associations and learned placebo responses. In another study, animals were tested for tolerance to noxious heat on a hot plate and given repetitive injections of morphine over 4 days in a chamber with a grid floor and a blue light. On day 5, replacing the morphine with saline induced a placebo analgesic effect as expressed by an increased latency of the nociceptive responses. This effect was abolished by the administration of the opioid receptor antagonist naloxone (87). Conversely, when the placebo effect was evoked by an exposure to aspirin (400 mg/kg), this effect was not blocked by naloxone, which mirrors results in humans (20).
The placebo effect appears to be transferable from one system to another (e.g., from pain to depression) as suggested by the observation that conditioning of the opioid system induces an antidepressant effect as well. The antidepressant effect was comparable to that elicited by the serotonin-norepinephrine reuptake inhibitor clomipramine (88).
At the brain level, placebo analgesia induced by classical conditioning is blocked by naloxone delivered systemically at a relatively high dosage (5 mg/kg), and this blockage is paralleled by a modulation of the rostral anterior cingulate cortex (89). By contrast, injections of the dopamine receptor antagonist haloperidol but not of naloxone immediately before testing prevented the expression of the learned responses when a conditioned preference model of placebo analgesia was used, suggesting that endogenous dopamine and opioids are involved depending on the context (e.g., motivation versus expectancy of analgesia) (90).
Apart from pain research, pharmacological conditioning has also been used with immunomodulating substances (91–93). Early discoveries by Ader & Cohen (94) demonstrated an increase in antibody titers when rats were conditioned to the immunosuppressive properties of cyclophosphamide. Subsequently, many studies in animals have revealed that a variety of immunological parameters can be manipulated by conditioning protocols in which a flavor or odor is typically employed as a conditioned stimulus along with a pharmacological agent used as the unconditioned stimulus. For example, immunosuppressive responses were observed when rats that were conditioned with a formulation of saccharin that contained cyclosporine A were reexposed to the saccharin alone, resulting in changes in the level of the production of T helper type 1 cytokine and the calcineurin activity in CD4+ Tlymphocytes (95). Other parameters that can be conditioned are antibody responses, mitogen-induced lymphocyte proliferation, leukocyte counts, circulation of lymphocyte subpopulations, the activity of natural killer cells, and acute phase reactions (reviewed in 96). This phenomenon, the behavioral conditioning of immune (and other) functions, has the potential for use in human pain, immune, and other system-related applications via reinforced partial learning paradigms and dose-extending placebos.
6. TRANSLATION-RELATED ASPECTS
6.1. Dose-Extending Placebos
The term dose-extending placebos refers to a placebo given after the repetitive administration of an effective medication. This tool harnesses the body’s innate capacity to create learned conditioned responses and trigger the activation of the opioid and nonopioid endogenous pain modulatory systems.
The novel idea of interspersing placebos with a proven medication rather than using the medication only (or placebos alone; see below) to treat a disease is in line with the pharmacological and nonpharmacological research on placebo effects and learning. The full and partial reinforcement approaches have been tested in both laboratory and clinical settings (reviewed in 97), and the results suggest new therapeutic strategies that may result in the reduction of the total intake of pain medications, the side effects associated with these medications, and, finally, costs associated with the treatments.
Recently, some clinical trials have been conducted with dose-extending placebos that resulted in clinically relevant reductions of opioids, corticosteroids, zolpidem, and amphetamines given to patients with pain, psoriasis, insomnia, and ADHD, respectively. In dose-extending placebo studies, participants receive a repetitive administration of the medication (e.g., opioids) that will be conditioned creating a pharmacological memory and a drug-like induced body response (97) (Figure 2).
For example, psoriasis patients were treated with corticosteroids interspersed with placebos. Those who received placebos had a reduction of one-quarter or one-half in the total amount of corticosteroids they were currently prescribed, and the remainder was filled in by identical placebos (dose-extending arm). The outcomes in the dose-extending arm were compared with the control arm in which the same drug reduction occurred but without giving any placebos and with the arm receiving a full dose of corticosteroids. The relapse of psoriasis symptoms was 26.7% in those who received the half dose of corticosteroids along with the dose-extending placebos, 61.5% in those in the control arm (reduction without placebos), and 22.2% in the arm treated with the full dose of corticosteroids (98).
Chronic insomnia improved when patients were given a combination of active medication (10 mg zolpidem pill) and placebo pills for 12 weeks compared to groups randomized to either 10 mg, 5 mg, or intermittent 10 mg nightly control doses (99). Children with ADHD who were randomly assigned to a group receiving placebos paired with a 50% reduction in the dose of amphetamine showed similar benefits to those children who received the full dose (100). Antihistamine-like effects can be elicited by conditioning the action of antihistamine drugs in patients with allergic rhinitis (101). Recently, 30 kidney transplant patients treated with the immunosuppressive drug cyclosporine A or tacrolimus underwent a conditioning procedure that induced a significant conditioned inhibition of T cell proliferative capacity (102). Therefore, active treatments can potentially be interspersed with placebo to optimize therapeutic regimens, especially in conditions in which patients depend on the treatment for the rest of their lives with severe adverse effects.
This line of research may pave the way to new therapeutic strategies for the treatment of acute chronic pain and addiction (103) by reducing the intake of opioids. In particular, harnessing placebo effects (e.g., via dose-extending placebos) can be a novel strategy to help face the opioid epidemic crisis in the United States (and elsewhere), resulting in 16 deaths per day in the United States (104). The majority are noncancer chronic pain patients who misuse and abuse painkiller prescriptions that were dispensed to them to manage acute perioperative pain. One out of 550 chronic opioid users dies within approximately 2.5 years of their first opioid prescription that was given to treat acute pain (105). A well-done study documented that 6% of 36,000 opioid-naive patients undergoing elective surgery presented with persistent opioid use (defined as prescription fulfillment between 90 and 180 days after the surgery) regardless of the type of surgery (minor versus major) (106). The prospect of optimizing acute pain management while opioids are reduced (or avoided) is worthy of future investigation.
6.2. Open-Label Placebos
Recent clinical research suggests that certain types of patients who receive placebos and are told that placebos are substances without active components but are also told that the treatment will still be effective show significant clinical improvements. These few recent studies challenge the assumption that deception is necessary to trigger placebo effects (103). Park & Covi (107) first administered open-label placebos to 15 neurotic outpatients and reported improvement with the placebo treatment and, in some cases, desire to continue the placebos. Open-label placebos have also been tested in small clinical trials in patients with irritable bowel syndrome (108), chronic low-back pain (109), major depression (110), ADHD (100), rhinitis (111), and cancer-related fatigue (112). Patients enrolled in these published studies have been informed that the placebo effect is indeed a powerful phenomenon that depends on the patient simply taking the pills diligently as instructed, whether or not the patient believes that they will work. This explanation seems sufficient to maintain a reduction in symptoms, as suggested by a recent study that found that an open-label placebo given in an experimental setting with a rationale for its benefit was as effective as a placebo deceptively described as an active treatment (113).
Alternatively, the act of taking a pill or receiving a local treatment might serve as a conditioned cue in line with learned placebo effects. For instance, analgesia was still experienced by healthy participants in one study even after it was revealed that the cream they received during the conditioning paradigm was only Vaseline (114). The effects were not correlated with participants’ expectations of pain relief, indicating that other processes may mediate placebo effects. Such a nondeceptive approach may facilitate the adoption of placebos in clinical contexts given that some ethical dilemmas such as the preservation of the patient’s autonomy and the threatening of deception in patent-clinician relationships are circumvented. Evidence-based research is needed to guide therapeutic decisions to provide the best available treatment.
Currently published open-label placebo studies are small, ranging from 15 to 83 patients, and although a no-intervention arm is included, they often lack the inclusion of the best treatment arm as a comparator (115). This omission makes it difficult to assess the clinical effectiveness of open-label placebos. The promise of a safe and ethically sound method of administering placebos warrants further rigorous research into the value of open-label placebos.
7. PLACEBO EFFECTS AND THEIR IMPLICATIONS FOR CLINICAL TRIALS
As explained in Section 1, to disentangle placebo effects from natural history, biases, Hawthorne effects, regression to the mean, and other nonspecific effects, clinical trial designs that include a no-intervention arm are necessary to distinguish changes seen in an untreated group from those seen in a placebo arm (Figure 4).
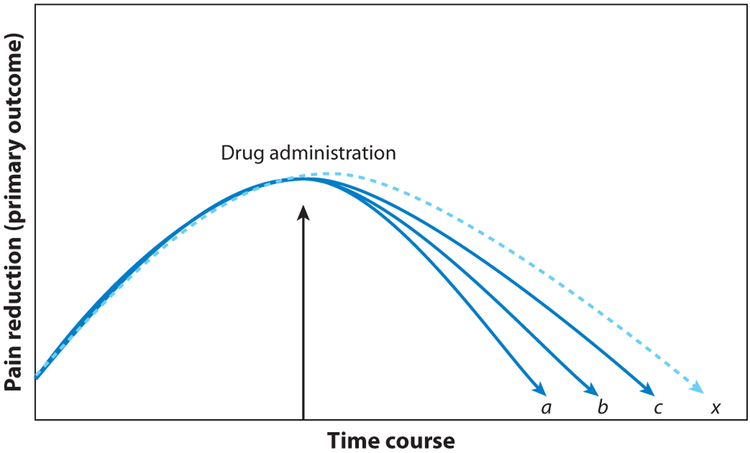
Figure 4.
Reduction of pain (primary outcome) in a hypothetical clinical trial that compares two treatments, a and b, and a placebo, c. The treatments a and b are two classes of analgesics. The no-intervention arm, x, is needed to detect a genuine placebo effect, i.e., the difference between the placebo arm, c, and the no-treatment arm, x.
There are situations in which no-intervention arms are difficult to implement. For example, some clinical trial designs such as the Sequential Parallel Comparison (116) and enrichment designs (117) may help harmonize clinical and ethical requirements with the principles of placebo research.
Another possibility, perhaps more feasible, is to introduce an assessment of participants’ [and, for children, proxy’s (118)] expectations in the context of clinical trials and practice. Expectations can influence the course of different medical conditions (8, 119) from long-term mortality (120) to outcomes for surgical interventions (121). It is plausible, therefore, to consider expectations as a potential predictor of outcomes. Expectations can be measured with a simple to-be question: “What do you expect your level of pain intensity to be? Please rate from 0 = no pain to 100 = maximum imaginable pain” (122). Such a question can be integrated with measurements of credibility, such as “How effective do you think this treatment will be for you?” (123), and post-treatment allocation questions, including “Which arm of the trial do you think you have been assigned to? Treatment A, B … Placebo?” Given that patients often show treatment benefits that are not reflected in objective measurements (e.g., 124), asking patients about the perceived effectiveness of the treatment they received can be a precious covariate used in data analyses (30, 125). A recent clinical trial showed that albuterol but not the two placebo interventions improved forced expiratory volume in 1 s in asthmatic patients, but placebos and not albuterol provided incremental benefit with respect to the self-reported outcomes (124), suggesting that the combined therapies (i.e., albuterol and placebos) may achieve the best overall benefit.
The gold standard of efficacy in randomized clinical trials assumes that drug and placebo responses are additive and, as such, the removal of placebo responders from the trial is not the correct way to detect an intervention effect. However, nonadditive effects have also been shown using a 2 × 2 balanced placebo design in which participants receive instructions about a drug (i.e., they are told either about a drug or about a placebo) and then are administered the actual drug (i.e., they are given either a drug or a placebo). The effect of lidocaine/prilocaine on subjective pain ratings and neural responses at the level of the anterior insula, the rostral anterior cingulate cortex, and the ventral striatum showed an interactive effect (126). Clinical findings have also suggested that placebo and drug effects may not be merely additive (6). The additive versus interactive effect may depend on the mechanisms of action of the given treatment. Future mechanistic (e.g., PET with radiotracers) and clinical research is needed to address the issue of additivity.
8. PLACEBO EFFECTS IN CLINICAL PRACTICE
Different individuals experience and cope with chronic pain differently; some are mildly affected, while others suffer debilitating dysfunction. Individuals also vary substantially in their responses to therapeutic interventions; for some, pharmacological treatments are highly efficacious, and in others only modest reductions in pain occur. Similarly, some respond to placebos over time (127), whereas others do not respond to pain-related placebo manipulations in clinical as well as in experimental settings. This large variability in placebo phenotypes can be attributed in part to expectancy-induced analgesia, which refers to differences due to patients’ and providers’ beliefs and desires (128). These effects are important in optimizing not only clinical trial designs but also plans for the therapeutic management of pain since those who are placebo responders may benefit to a larger extent from nonpharmacological and psychological interventions.
Many painkillers are discarded after phase II/III clinical trials because they do not outperform placebos (129). In 2011, Clinicaltrials.gov (https://clinicaltrials.gov/) listed over 4,000 pain trials, yet in the last few years, the only new approvals were for just five existing drugs, e.g., duloxetine, oxycodone, and fentanyl, in new formulations or dosage forms (130). In clinical trials for cancer and neuropathic pain, the failure rate for pharmacological treatment has been over 90% in the past 10 years (130, 131). In laboratory settings, placebo effects have also been explored with chronic pain populations affected by different pain disorders, including chronic idiopathic pain (pain arising spontaneously or from an unknown cause), neuropathic pain (132–134), low-back pain (109, 135), knee osteoarthritis (136, 137), irritable bowel syndrome (122, 138, 139), fibromyalgia (140), and migraine (141). Experimental research suggests that the modality (thermal versus chemical) and duration (phasic versus tonic) of painful stimulations can affect placebo effects (142). Moreover, research has indicated that features such as price (a higher price leads to larger placebo effects) (141, 143), labeling (generic versus brand) (144), and route of administration (sham acupuncture versus oral placebo) (145) influence the occurrence and magnitude of placebo effects and perceived side effects.
Expectancy and placebo effects are largely (and often exclusively) responsible for the therapeutic effects of nonpharmacological treatments such as homeopathy (146) and other integrative treatments (147). This is not surprising given that expectancy and placebo effects also optimize the response to different opioid and nonopioid treatments (e.g., buprenorphine, tramadol, ketorolac, and metamizol) (55) and, remarkably, account for up to 50% of the effectiveness of pain treatments (6). These effects depend on an interaction between modulatory central nervous systems and peripheral pain mechanisms. A few studies suggest that the placebo effect may increase the half-life of substances such as caffeine (148) and interact with medications (149), therefore biasing the results of clinical trials and challenging the concept that placebo and drug effects are additive. Yet it remains to be fully elucidated how being aware of receiving a pain treatment might alter the drug’s pharmacokinetics.
Based on the impact of expectancies and placebo effects in acute postoperative pain, chronic pain, and experimental pain, future research should identify biomarkers (genetic, psychosocial, and neural) of variability in clinical outcomes in individuals suffering from pain. It would be helpful to understand how the physiopathology of pain (neuropathic versus non-neuropathic pain), the source of pain (surgical versus inflammatory pain), associated comorbidities (psychiatric versus systemic diseases), and psychological factors (resilience versus neuroticism) can affect placebo effects.
9. PLACEBO AND FRAMING EFFECTS
Clinical and laboratory research studies have demonstrated that expectancies, when optimized or silenced via the overt-covert (also known as open-hidden) paradigm, significantly and in a bidirectional manner influence the response to various treatments such as morphine, anxiolytic diazepam, deep brain stimulation (reviewed in 6), intravenous remifentanil (150), topical lidocaine (133), and acupuncture (151).
In patient populations, nonadherence to treatments and the need for higher doses or alternative treatment prescriptions are often related to the lack of placebo effects (152). Leveraging placebo effects and minimizing so-called nocebo effects are essential in daily clinical practice (64, 152). Framing patient-clinician communication in a way that empowers realistic, yet still cooperative, expectations can help promote mindsets that are positive despite the nature of pain and associated diseases. Although placebo effects depend at least partially on psychological factors, the recipient’s disposition, and prior therapeutic experiences, there are many examples of patient-clinician communications that reduce pain burden and opioid use in the postoperative setting. A significant 50% reduction in postoperative pain and narcotic use was shown in patients who received elective intra-abdominal surgeries and were informed before the surgery about postoperative pain. Half of the patients were informed about postoperative pain, its duration, and severity by the physician and educated about coping strategies (i.e., breathing). This reduced the amount of postoperative pain and the need for narcotics and was labeled as active placebo action (153). Perhaps it is time to reintroduce such active placebo actions as part of acute postoperative pain management and in the education of future clinicians.
Verbal instructions given with drug administration, prior therapeutic experiences, interactions with health practitioners, and interactions with other patients all contribute to the complexity of individual placebo effects. Yet knowledge on mechanisms of placebo effects is seen as a field apart from drug development. Despite the observation that thousands of clinical trials fail because interventions do not outperform placebo responses, research on placebo effects and placebo responses has been kept as a separate component of research. This review attempts to merge mechanisms and translational components of placebo research. Given that placebo effects are ubiquitous in daily clinical practice and part of all therapies for pain, understanding how to harness these placebo responses using framing effects, social context, and open dose-extending placebos interspersed with pharmacological regimes should be considered for optimal pain management approaches.
SUMMARY POINTS.
The placebo effect is an important factor that modulates clinical outcomes. It is linked to psychoneurobiological changes occurring as the result of the patient’s, proxy’s, and provider’s expectations.
For several years, placebo effects have been dismissed and considered a nuisance in trials despite the fact that clinicians and other health care practitioners have known that these effects can and do result in improvements of clinical symptoms.
A series of neuropharmacological studies have corroborated and confirmed the notion that the opioid system is involved in the formation of placebo-induced reductions in pain.
Behavioral and neurobiological placebo effects are triggered by verbal suggestions, classical and nonclassical conditioning, and social interactions (including observation and complex interpersonal interactions).
Vasopressin (and oxytocin), given intranasally, boosts placebo effects that most likely depend on social aspects and show a dimorphic effect.
Positive therapeutic encounters, empathic listening, and encouragements shape the effectiveness of treatment and the expectancies of patients, clinicians, and proxies.
Dose-extending placebos that harness the body’s capacity to create learned conditioned responses and pharmacological memories can in turn trigger the activation of opioid and nonopioid endogenous pain modulatory systems.
Verbal instructions and the way in which information is framed impact both the duration and severity of postoperative pain, indicating that physicians and patients should be educated about coping strategies.
FUTURE ISSUES.
Existing evidence shows that genetic factors may contribute to placebo effects and responses: Twin studies are a useful approach to determine the heritability of placebo-related phenotypes and the contribution of genetic and environmental factors.
The relationship between placebo effects and pain processing must be determined: Systematic, participant-level meta-analyses of published brain imaging studies (154) will help corroborate current knowledge on neural responses to placebos.
Further animal research with pharmacological conditioning and distinct types of pain models will help elucidate the molecular mechanisms of pharmacological memories that in turn elicit behavioral and bodily responses.
Large studies that investigate sex, age, and race as biological variables are needed to better account for interindividual variability in placebo responsiveness.
New research in conditions other than pain is needed to fully understand how expectancy and placebo effects impact symptoms and diseases.
The use of opioids for the management of acute and chronic pain raises serious concerns: Large studies for tapering opioid medication with dose-extending placebos would help explore alternative treatment options.
Future translational efforts should focus on patient-centered research that would help create new policies favoring the clinical applications of placebos, ranging from doseextending placebos to educational programs for new generations of clinicians.
Placebo effect: powerful determinant of health outcomes across many different diseases and encounters; the placebo effect is due to the expectancy of positive treatment outcomes
Expectation: constructs that refer to anticipation of outcome that are verbalized and measurable via validated scales
Regression to the mean: phenomenon by which a variable tends to move closer to the center of its distribution from initial to later measurements
Spontaneous remission: a catch-all term to describe the phenomenon when the symptoms of a condition improve naturally without any interventions
Vasopressin: nonselective small polypeptide that binds arginine vasopressin Avp1a and Avp1b receptors in the central nervous system
Expectancy: psychophysical predictor that can be present in humans and animals without full awareness (implicit expectancies)
Expectancy-induced analgesia: reduction in pain experience in an individual that results from the inhibition of nociceptive stimulations via the activation of descending neural pathways
Nocebo effects: negative expectancies that contribute to the occurrence of side effects and influence both clinical outcomes and patients’ adherence to medication
Classical conditioning: a neutral stimulus (syringe) that induces a conditioned response (hypoalgesia) after pairing it with an unconditioned stimulus (morphine)
Social learning: ability to acquire information from social observations and interpersonal contexts and thus generate treatment expectations that in turn alter clinical response
Placebo response: outcome changes that are due to natural history, biases, Hawthorne effects, regression to the mean, and other nonspecific effects
Dose-extending placebos: placebos or subtherapeutic doses of treatments (e.g., opioids) that are interspersed with the target treatments in accordance with reinforcement learning principles
Endogenous pain modulatory systems: the inner systems within the body (e.g., the opioid system) that can modulate individuals’ experiences of pain
Pharmacological memory: bodily response(s) occurring after repetitive administrations of a medication that elicits conditioned drug-like effects
Open-label placebos: saline, talc pills, or other dummy interventions that are given to patients and presented overtly as placebos
No-intervention arm: also called natural history, this arm includes patients assigned to a waitlist who are observed for the course of the symptoms in the absence of any treatments
Hawthorne effect: a patient’s response related to the mere fact of being enrolled in a clinical trial or study
Overt-covert (open-hidden) paradigm: medication administered by informing patients that a treatment has been given as compared to a covert computer-controlled treatment administration
Mindset: a patient’s set of attitudes and beliefs about a symptom or intervention, which can in turn evoke a biological and behavioral response in their body
Framing effects: outcome perceptions related to the description of logically equivalent information about the risk/benefit ratio of an intervention
ACKNOWLEDGMENTS
This research was supported by the University of Maryland, Baltimore, and the National Institute of Dental and Craniofacial Research (NIDCR R01DE025946). The author would like to thank Dr. Pedro E. Martinez and Kathleen Hand for their helpful comments.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Colloca L, Benedetti F. 2005. Placebos and painkillers: Is mind as real as matter? Nat. Rev. Neurosci. 6:545–52 [DOI] [PubMed] [Google Scholar]
- 2.Colloca L, ed. 2018. Neurobiology of the Placebo Effect, Volume I-II Cambridge, MA: Elsevier/Academic Press [Google Scholar]; This two-volume book emphasizes recent findings and new directions in placebo research.
- 3.Ernst E, Resch KL. 1995. Concept of true and perceived placebo effects. BMJ 311:551–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vase L, Amanzio M, Price DD. 2015. Nocebo versus placebo: the challenges of trial design in analgesia research. Clin. Pharmacol. Ther. 97:143–50 [DOI] [PubMed] [Google Scholar]
- 5.Colloca L 2017. Treatment of pediatric migraine. N. Engl. J. Med. 376:1387–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colloca L, Lopiano L, Lanotte M, Benedetti F. 2004. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol. 3:679–84 [DOI] [PubMed] [Google Scholar]
- 7.Colloca L, Lopiano L, Benedetti F, Lanotte M. 2005. The placebo response in conditions other than pain. Semin. Pain Med. 3:43–47 [Google Scholar]
- 8.Benedetti F 2008. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu. Rev. Pharmacol. Toxicol. 48:33–60 [DOI] [PubMed] [Google Scholar]
- 9.Raicek JE, Stone BH, Kaptchuk TJ. 2012. Placebos in 19th century medicine: a quantitative analysis of the BMJ. BMJ 345:e8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berman A. 1972. The Pharmacopoeia Reformata of London (1744) and its anonymous author. Ohio State Med. J. 68:774–75 [PubMed] [Google Scholar]
- 11.Shapiro AK. 1959. The placebo effect in the history of medical treatment: implications for psychiatry. Am. J. Psychiatry 116:298–304 [DOI] [PubMed] [Google Scholar]
- 12.Wolff HG, Dubois EF, Cattell M. 1946. Conferences on therapy: the use of placebos in therapy. New York State J. Med. 46:1718–27 [PubMed] [Google Scholar]
- 13.Jefferson T 1905. The Works of Thomas Jefferson, Vol. 10: Correspondence and Papers 1803–1807, ed. Ford PL. New York/London: G.P. Putnam’s Sons [Google Scholar]
- 14.Miller FG. 2013. The concept and significance of the placebo effect In The Placebo: A Reader, ed. Miller FG, pp. 1–9. Baltimore, MD: Johns Hopkins Univ. Press [Google Scholar]
- 15.Beecher HK. 1955. The powerful placebo. J. Am. Med. Assoc. 159:1602–6 [DOI] [PubMed] [Google Scholar]
- 16.Levine JD, Gordon NC, Fields HL. 1978. The mechanism of placebo analgesia. Lancet 2:654–57. [DOI] [PubMed] [Google Scholar]; Pioneering study that stimulated research in the area of placebo and its underlying endogenous mechanisms.
- 17.Eippert F, Finsterbusch J, Bingel U, Buchel C. 2009. Direct evidence for spinal cord involvement in placebo analgesia. Science 326:404. [DOI] [PubMed] [Google Scholar]
- 18.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, et al. 2009. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63:533–43 [DOI] [PubMed] [Google Scholar]; Naloxone and functional magnetic resonance imaging illustrate the brain areas involved in placebo-induced analgesia.
- 19.Benedetti F 1996. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain 64:535–43 [DOI] [PubMed] [Google Scholar]
- 20.Amanzio M, Benedetti F. 1999. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J. Neurosci. 19:484–94 [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration of the differential role of the opioid system for verbally induced and conditioned placebo effects
- 21.Wager TD, Scott DJ, Zubieta JK. 2007. Placebo effects on human μ-opioid activity during pain. PNAS 104:11056–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, et al. 2005. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J. Neurosci. 25:7754–62 [DOI] [PMC free article] [PubMed] [Google Scholar]; This was the first in vivo brain imaging demonstration of the involvement of endogenous opioids.
- 23.Benedetti F, Pollo A, Colloca L. 2007. Opioid-mediated placebo responses boost pain endurance and physical performance: Is it doping in sport competitions? J. Neurosci. 27:11934–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oroszi G, Goldman D. 2004. Alcoholism: genes and mechanisms. Pharmacogenomics 5:1037–48 [DOI] [PubMed] [Google Scholar]
- 25.Stoeber M, Jullie D, Lobingier BT, Laeremans T, Steyaert J, et al. 2018. A genetically encoded biosensor reveals location bias of opioid drug action. Neuron 98(5):963–76.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]; Opioid drugs produce a pattern of activation that differs from the modulation driven by opioids that are naturally released.
- 26.Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. 2009. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J. Neurosci. 29:4882–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. 2008. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry 65:220–31 [DOI] [PubMed] [Google Scholar]
- 28.Skyt I, Moslemi K, Baastrup C, Grosen K, Benedetti F, et al. 2017. Dopaminergic tone does not influence pain levels during placebo interventions in patients with chronic neuropathic pain. Pain 159(2):261–72 [DOI] [PubMed] [Google Scholar]
- 29.Wrobel N, Wiech K, Forkmann K, Ritter C, Bingel U. 2014. Haloperidol blocks dorsal striatum activity but not analgesia in a placebo paradigm. Cortex 57:60–73 [DOI] [PubMed] [Google Scholar]
- 30.Jarcho JM, Feier NA, Labus JS, Naliboff B, Smith SR, et al. 2016. Placebo analgesia: self-report measures and preliminary evidence of cortical dopamine release associated with placebo response. Neuroimage Clin. 10:107–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, et al. 2004. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat. Neurosci. 7:587–88 [DOI] [PubMed] [Google Scholar]
- 32.Benedetti F, Lanotte M, Colloca L, Ducati A, Zibetti M, Lopiano L. 2009. Electrophysiological properties of thalamic, subthalamic and nigral neurons during the anti-parkinsonian placebo response. J. Physiol. 587:3869–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercado R, Constantoyannis C, Mandat T, Kumar A, Schulzer M, et al. 2006. Expectation and the placebo effect in Parkinson’s disease patients with subthalamic nucleus deep brain stimulation. Mov. Disord. 21:1457–61 [DOI] [PubMed] [Google Scholar]
- 34.Lidstone SC, Schulzer M, Dinelle K, Mak E, Sossi V, et al. 2010. Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch. Gen. Psychiatry 67:857–65 [DOI] [PubMed] [Google Scholar]
- 35.de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. 2001. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science 293:1164–66 [DOI] [PubMed] [Google Scholar]
- 36.Colloca L, Pine DS, Ernst M, Miller FG, Grillon C. 2016. Vasopressin boosts placebo analgesic effects in women: a randomized trial. Biol. Psychiatry 79:794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provided the first evidence that the nanopeptide vasopressin increases placebo effects in women.
- 37.Kessner S, Sprenger C, Wrobel N, Wiech K, Bingel U. 2013. Effect of oxytocin on placebo analgesia: a randomized study. JAMA 310:1733–35 [DOI] [PubMed] [Google Scholar]; This study provided the first evidence that the nanopeptide oxytocin increases placebo effects in men.
- 38.Kogan A, Saslow LR, Impett EA, Oveis C, Keltner D, Rodrigues Saturn S. 2011. Thin-slicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition. PNAS 108:19189–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donaldson ZR, Young LJ. 2008. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322:900–4 [DOI] [PubMed] [Google Scholar]
- 40.Bielsky IF, Hu SB, Young LJ. 2005. Sexual dimorphism in the vasopressin system: lack of an altered behavioral phenotype in female V1a receptor knockout mice. Behav. Brain Res. 164:132–36 [DOI] [PubMed] [Google Scholar]
- 41.Ebstein RP, Israel S, Lerer E, Uzefovsky F, Shalev I, et al. 2009. Arginine vasopressin and oxytocin modulate human social behavior. Ann. N. Y. Acad. Sci. 1167:87–102 [DOI] [PubMed] [Google Scholar]
- 42.Heinrichs M, Domes G. 2008. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog. Brain Res. 170:337–50 [DOI] [PubMed] [Google Scholar]
- 43.Heinrichs M, von Dawans B, Domes G. 2009. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 30:548–57 [DOI] [PubMed] [Google Scholar]
- 44.Young LJ, Wang Z. 2004. The neurobiology of pair bonding. Nat. Neurosci. 7:1048–54 [DOI] [PubMed] [Google Scholar]
- 45.Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, et al. 2015. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 9:754–64 [DOI] [PubMed] [Google Scholar]
- 46.Thompson R, Gupta S, Miller K, Mills S, Orr S. 2004. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology 29:35–48 [DOI] [PubMed] [Google Scholar]
- 47.Thompson RR, George K, Walton JC, Orr SP, Benson J. 2006. Sex-specific influences of vasopressin on human social communication. PNAS 103:7889–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. 2002. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 5:514–16 [DOI] [PubMed] [Google Scholar]
- 49.Meissner K, Bingel U, Colloca L, Wager TD, Watson A, Flaten MA. 2011. The placebo effect: advances from different methodological approaches. J. Neurosci. 31:16117–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodson JL, Thompson RR. 2010. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin. Neurobiol. 20:784–94 [DOI] [PubMed] [Google Scholar]
- 51.Ferris CF, Melloni RH Jr., Koppel G, Perry KW, Fuller RW, Delville Y. 1997. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J. Neurosci. 17:4331–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gobrogge KL, Liu Y, Young LJ, Wang Z. 2009. Anterior hypothalamic vasopressin regulates pairbonding and drug-induced aggression in a monogamous rodent. PNAS 106:19144–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, et al. 2014. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus). Neuroscience 273:12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moerman DE, Jonas WB. 2002. Deconstructing the placebo effect and finding the meaning response. Ann. Intern. Med. 136:471–76 [DOI] [PubMed] [Google Scholar]
- 55.Colloca L, Klinger R, Flor H, Bingel U. 2013. Placebo analgesia: psychological and neurobiological mechanisms. Pain 154:511–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colloca L, Miller FG. 2011. How placebo responses are formed: a learning perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366:1859–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colloca L, Flaten MA, Meissner K, eds. 2013. Placebo and Pain: From Bench to Bedside. Oxford, UK: Elsevier [Google Scholar]
- 58.Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. 2010. How the number of learning trials affects placebo and nocebo responses. Pain 151:430–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colloca L, Benedetti F. 2006. How prior experience shapes placebo analgesia. Pain 124:126–33 [DOI] [PubMed] [Google Scholar]
- 60.Au Yeung ST, Colagiuri B, Lovibond PF, Colloca L. 2014. Partial reinforcement, extinction, and placebo analgesia. Pain 155:1110–17 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that partial reinforcement prevents extinction of placebo analgesic effects over time.
- 61.Kessner S, Wiech K, Forkmann K, Ploner M, Bingel U. 2013. The effect of treatment history on therapeutic outcome: an experimental approach. JAMA Intern. Med. 173:1468–69 [DOI] [PubMed] [Google Scholar]; Positive and negative prior therapeutic experiences shape not only behavioral but also brain responses.
- 62.Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA. 2010. Neural bases of conditioned placebo analgesia. Pain 151:816–24 [DOI] [PubMed] [Google Scholar]
- 63.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, et al. 2004. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science 303:1162–67 [DOI] [PubMed] [Google Scholar]
- 64.Colloca L 2017. Nocebo effects can make you feel pain. Science 358:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colloca L, Sigaudo M, Benedetti F. 2008. The role of learning in nocebo and placebo effects. Pain 136:211–18 [DOI] [PubMed] [Google Scholar]
- 66.Schenk LA, Krimmel SR, Colloca L. 2017. Observe to get pain relief: current evidence and potential mechanisms of socially learned pain modulation. Pain 158:2077–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colloca L, Benedetti F. 2009. Placebo analgesia induced by social observational learning. Pain 144:28–34 [DOI] [PubMed] [Google Scholar]; First demonstration that observing a therapeutic outcome in others elicits placebo effects.
- 68.Hunter T, Siess F, Colloca L. 2014. Socially induced placebo analgesia: a comparison of a pre-recorded versus live face-to-face observation. Eur. J. Pain 18:914–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bootzin RR, Caspi O. 2002. Explanatory mechanisms for placebo effects: cognition, personality and social learning In The Science of the Placebo: Toward an Interdisciplinary Research Agenda, ed. Guess HA, Kleinman A, Kusek JW, Engel LW, pp. 108–32. London: BMJ Books [Google Scholar]
- 70.Kirsch I 1985. Response expectancy as a determinant of experience and behavior. Am. Psychol. 40:1189–202 [Google Scholar]
- 71.Wickramasekera I. 1980. A conditioned response model of the placebo effect predictions from the model. Biofeedback Self Regul. 5:5–18 [DOI] [PubMed] [Google Scholar]
- 72.Colloca L 2014. Placebo, nocebo, and learning mechanisms. Handb. Exp. Pharmacol. 225:17–35 [DOI] [PubMed] [Google Scholar]
- 73.Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. 2001. Influence of context effects on health outcomes: a systematic review. Lancet 357:757–62 [DOI] [PubMed] [Google Scholar]
- 74.Kruschke JK. 2006. Locally Bayesian learning with applications to retrospective revaluation and highlighting. Psychol. Rev. 113:677–99 [DOI] [PubMed] [Google Scholar]
- 75.Schenk LA, Sprenger C, Onat S, Colloca L, Buchel C. 2017. Suppression of striatal prediction errors by the prefrontal cortex in placebo hypoalgesia. J. Neurosci. 37:9715–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiech K 2016. Deconstructing the sensation of pain: the influence of cognitive processes on pain perception. Science 354:584–87 [DOI] [PubMed] [Google Scholar]
- 77.Colloca L, Miller FG. 2011. Role of expectations in health. Curr. Opin. Psychiatry 24:149–55 [DOI] [PubMed] [Google Scholar]
- 78.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. 2003. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J. Neurosci. 23:4315–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gil M 2010. Reward expectations in honeybees. Commun. Integr. Biol. 3:95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ader R, Cohen N. 1982. Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus. Science 215:1534–36 [DOI] [PubMed] [Google Scholar]
- 81.Herrnstein RJ. 1962. Placebo effect in the rat. Science 138:677–78 [DOI] [PubMed] [Google Scholar]
- 82.Keller AA, Akintola T, Colloca L. 2018. Placebo analgesia in rodents: current and future research. See Reference 2, pp. 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krank MD, Hinson RE, Siegel S. 1981. Conditional hyperalgesia is elicited by environmental signals of morphine. Behav. Neural Biol. 32:148–57 [DOI] [PubMed] [Google Scholar]
- 84.Valone JM, Randall CK, Kraemer PJ, Bardo MT. 1998. Olfactory cues and morphine-induced conditioned analgesia in rats. Pharmacol. Biochem. Behav. 60:115–18 [DOI] [PubMed] [Google Scholar]
- 85.Bardo MT, Valone JM. 1994. Morphine-conditioned analgesia using a taste cue: dissociation of taste aversion and analgesia. Psychopharmacology 114:269–74 [DOI] [PubMed] [Google Scholar]
- 86.Bryant CD, Roberts KW, Culbertson CS, Le A, Evans CJ, Fanselow MS. 2009. Pavlovian conditioning of multiple opioid-like responses in mice. Drug Alcohol Depend. 103:74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo JY, Wang JY, Luo F. 2010. Dissection of placebo analgesia in mice: the conditions for activation of opioid and non-opioid systems. J. Psychopharmacol. 24:1561–67 [DOI] [PubMed] [Google Scholar]
- 88.Guo JY, Yuan XY, Sui F, Zhang WC, Wang JY, et al. 2011. Placebo analgesia affects the behavioral despair tests and hormonal secretions in mice. Psychopharmacology 217:83–90 [DOI] [PubMed] [Google Scholar]
- 89.Zhang RR, Zhang WC, Wang JY, Guo JY. 2013. The opioid placebo analgesia is mediated exclusively through μ-opioid receptor in rat. Int. J. Neuropsychopharmacol. 16:849–56 [DOI] [PubMed] [Google Scholar]
- 90.Lee IS, Lee B, Park HJ, Olausson H, Enck P, Chae Y. 2015. A new animal model of placebo analgesia: involvement of the dopaminergic system in reward learning. Sci. Rep. 5:17140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schedlowski M, Pacheco-Lopez G. 2010. The learned immune response: Pavlov and beyond. Brain Behav. Immun. 24:176–85 [DOI] [PubMed] [Google Scholar]
- 92.Bermudez-Rattoni F 2004. Molecular mechanisms of taste-recognition memory. Nat. Rev. Neurosci. 5:209–17 [DOI] [PubMed] [Google Scholar]
- 93.Ader R 2003. Conditioned immunomodulation: research needs and directions. Brain Behav. Immun. 17(Suppl. 1):S51–57 [DOI] [PubMed] [Google Scholar]
- 94.Ader R, Cohen N. 1975. Behaviorally conditioned immunosuppression. Psychosom. Med. 37:333–40 [DOI] [PubMed] [Google Scholar]
- 95.Pacheco-Lopez G, Riether C, Doenlen R, Engler H, Niemi MB, et al. 2009. Calcineurin inhibition in splenocytes induced by pavlovian conditioning. FASEB J. 23:1161–67 [DOI] [PubMed] [Google Scholar]
- 96.Hadamitzky M, Sondermann W, Benson S, Schedlowski M. 2018. Placebo effects in the immune system. Int. Rev. Neurobiol. 138:39–59 [DOI] [PubMed] [Google Scholar]
- 97.Colloca L, Enck P, DeGrazia D. 2016. Relieving pain using dose-extending placebos: a scoping review. Pain 157:1590–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ader R, Mercurio MG, Walton J, James D, Davis M, et al. 2010. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom. Med. 72:192–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perlis M, Grandner M, Zee J, Bremer E, Whinnery J, et al. 2015. Durability of treatment response to zolpidem with three different maintenance regimens: a preliminary study. Sleep Med. 16:1160–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sandler AD, Bodfish JW. 2008. Open-label use of placebos in the treatment of ADHD: a pilot study. Child Care Health Dev. 34:104–10 [DOI] [PubMed] [Google Scholar]
- 101.Goebel MU, Meykadeh N, Kou W, Schedlowski M, Hengge UR. 2008. Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychother. Psychosom. 77:227–34 [DOI] [PubMed] [Google Scholar]
- 102.Kirchhof J, Petrakova L, Brinkhoff A, Benson S, Schmidt J, et al. 2018. Learned immunosuppressive placebo responses in renal transplant patients. PNAS 115:4223–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Belcher AM, Ferre S, Martinez PE, Colloca L. 2018. Role of placebo effects in pain and neuropsychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 87(Pt. B):298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, et al. 2015. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 162:276–86 [DOI] [PubMed] [Google Scholar]
- 105.Kaplovitch E, Gomes T, Camacho X, Dhalla IA, Mamdani MM, Juurlink DN. 2015. Sex differences in dose escalation and overdose death during chronic opioid therapy: a population-based cohort study. PLOS ONE 10:e0134550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, et al. 2017. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park LC, Covi L. 1965. Nonblind placebo trial: an exploration of neurotic patient’s responses to placebo when its inert content is disclosed. Arch. Gen. Psychiatry 12:36–45 [PubMed] [Google Scholar]
- 108.Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, et al. 2010. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLOS ONE 5:e15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, Kirsch I. 2016. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain 157:2766–72 [DOI] [PMC free article] [PubMed] [Google Scholar]; Chronic low-back pain patients showed long-lasting pain reduction when pills were labeled as placebos.
- 110.Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, Fava M. 2012. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother. Psychosom. 81:312–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schaefer M, Harke R, Denke C. 2016. Open-label placebos improve symptoms in allergic rhinitis: a randomized controlled trial. Psychother. Psychosom. 85:373–74 [DOI] [PubMed] [Google Scholar]
- 112.Hoenemeyer TW, Kaptchuk TJ, Mehta TS, Fontaine KR. 2018. Open-label placebo treatment for cancer-related fatigue: a randomized-controlled clinical trial. Sci. Rep. 8:2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Locher C, Nascimento AF, Kirsch I, Kossowsky J, Meyer A, Gaab J. 2017. Is the rationale more important than deception? A randomized controlled trial of open-label placebo analgesia. Pain 158:2320–28 [DOI] [PubMed] [Google Scholar]
- 114.Schafer SM, Colloca L, Wager TD. 2015. Conditioned placebo analgesia persists when subjects know they are receiving a placebo. J. Pain 16:412–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Colloca L, Howick J. 2018. Placebos without deception: outcomes, mechanisms, and ethics. Int. Rev. Neurobiol. 138:219–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fava M, Evins AE, Dorer DJ, Schoenfeld DA. 2003. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother. Psychosom. 72:115–27 [DOI] [PubMed] [Google Scholar]
- 117.Staud R, Price DD. 2008. Role of placebo factors in clinical trials with special focus on enrichment designs. Pain 139:479–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simmons K, Ortiz R, Kossowsky J, Krummenacher P, Grillon C, et al. 2014. Pain and placebo in pediatrics: a comprehensive review of laboratory and clinical findings. Pain 155:2229–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gramling R, Epstein R. 2011. Optimism amid serious disease: clinical panacea or ethical conundrum? Comment on “Recovery expectations and long-term prognosis of patients with coronary heart disease.” Arch. Intern. Med. 171:935–36 [DOI] [PubMed] [Google Scholar]
- 120.Barefoot JC, Brummett BH, Williams RB, Siegler IC, Helms MJ, et al. 2011. Recovery expectations and long-term prognosis of patients with coronary heart disease. Arch. Intern. Med. 171:929–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Auer CJ, Glombiewski JA, Doering BK, Winkler A, Laferton JA, et al. 2016. Patients’ expectations predict surgery outcomes: a meta-analysis. Int. J. Behav. Med. 23:49–62 [DOI] [PubMed] [Google Scholar]
- 122.Vase L, Robinson ME, Verne GN, Price DD. 2003. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain 105:17–25 [DOI] [PubMed] [Google Scholar]
- 123.Devilly GJ, Borkovec TD. 2000. Psychometric properties of the credibility/expectancy questionnaire. J. Behav. Ther. Exp. Psychiatry 31:73–86 [DOI] [PubMed] [Google Scholar]
- 124.Wechsler ME, Kelley JM, Boyd IO, Dutile S, Marigowda G, et al. 2011. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N. Engl. J. Med. 365:119–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pecina M, Stohler CS, Zubieta JK. 2013. Role of μ-opioid system in the formation of memory of placebo responses. Mol. Psychiatry 18:135–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schenk LA, Sprenger C, Geuter S, Buchel C. 2014. Expectation requires treatment to boost pain relief: an fMRI study. Pain 155:150–57 [DOI] [PubMed] [Google Scholar]
- 127.Morton DL, Watson A, El-Deredy W, Jones AK. 2009. Reproducibility of placebo analgesia: effect of dispositional optimism. Pain 146:194–98 [DOI] [PubMed] [Google Scholar]
- 128.Vase L, Vollert J, Finnerup NB, Miao X, Atkinson G, et al. 2015. Predictors of the placebo analgesia response in randomized controlled trials of chronic pain: a meta-analysis of the individual data from nine industrially sponsored trials. Pain 156:1795–802 [DOI] [PubMed] [Google Scholar]
- 129.Dolgin E 2010. Fluctuating baseline pain implicated in failure of clinical trials. Nat. Med. 16:1053. [DOI] [PubMed] [Google Scholar]
- 130.Bartfai T, Lees GV. 2011. Pharma TARP: a troubled asset relief program for novel, abandoned projects in the pharmaceutical industry. ScientificWorldJournal 11:454–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Katz J, Finnerup NB, Dworkin RH. 2008. Clinical trial outcome in neuropathic pain: relationship to study characteristics. Neurology 70:263–72 [DOI] [PubMed] [Google Scholar]
- 132.Vase L, Petersen GL, Lund K. 2014. Placebo effects in idiopathic and neuropathic pain conditions. Handb. Exp. Pharmacol. 225:121–36 [DOI] [PubMed] [Google Scholar]
- 133.Petersen GL, Finnerup NB, Grosen K, Pilegaard HK, Tracey I, et al. 2014. Expectations and positive emotional feelings accompany reductions in ongoing and evoked neuropathic pain following placebo interventions. Pain 155:2687–98 [DOI] [PubMed] [Google Scholar]
- 134.Petersen GL, Finnerup NB, Norskov KN, Grosen K, Pilegaard HK, et al. 2012. Placebo manipulations reduce hyperalgesia in neuropathic pain. Pain 153:1292–300 [DOI] [PubMed] [Google Scholar]
- 135.Hashmi JA, Baria AT, Baliki MN, Huang L, Schnitzer TJ, Apkarian AV. 2012. Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain 153:2393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tetreault P, Mansour A, Vachon-Presseau E, Schnitzer TJ, Apkarian AV, Baliki MN. 2016. Brain connectivity predicts placebo response across chronic pain clinical trials. PLOS Biol. 14:e1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kong J, Wang Z, Leiser J, Minicucci D, Edwards R, et al. 2018. Enhancing treatment of osteoarthritis knee pain by boosting expectancy: a functional neuroimaging study. Neuroimage Clin. 18:325–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, et al. 2008. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 336:999–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vase L, Robinson ME, Verne GN, Price DD. 2005. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain 115:338–47 [DOI] [PubMed] [Google Scholar]
- 140.Kosek E, Rosen A, Carville S, Choy E, Gracely RH, et al. 2017. Lower placebo responses after long-term exposure to fibromyalgia pain. J. Pain 18(7):835–43 [DOI] [PubMed] [Google Scholar]
- 141.Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, et al. 2014. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci. Transl. Med. 6:218ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vase L, Petersen GL, Riley JL 3rd, Price DD. 2009. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain 145:36–44 [DOI] [PubMed] [Google Scholar]
- 143.Waber RL, Shiv B, Carmon Z, Ariely D. 2008. Commercial features of placebo and therapeutic efficacy. JAMA 299:1016–17 [DOI] [PubMed] [Google Scholar]
- 144.Faasse K, Martin LR, Grey A, Gamble G, Petrie KJ. 2016. Impact of brand or generic labeling on medication effectiveness and side effects. Health Psychol. 35:187–90 [DOI] [PubMed] [Google Scholar]
- 145.Meissner K, Fassler M, Rucker G, Kleijnen J, Hrobjartsson A, et al. 2013. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern. Med. 173:1941–51 [DOI] [PubMed] [Google Scholar]
- 146.Mathie RT, Ramparsad N, Legg LA, Clausen J, Moss S, et al. 2017. Randomised, double-blind, placebo-controlled trials of non-individualised homeopathic treatment: systematic review and meta-analysis. Syst. Rev. 6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vickers AJ, Cronin AM, Maschino AC, Lewith G, MacPherson H, et al. 2012. Acupuncture for chronic pain: individual patient data meta-analysis. Arch. Intern. Med. 172:1444–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hammami MM, Al-Gaai EA, Alvi S, Hammami MB. 2010. Interaction between drug and placebo effects: a cross-over balanced placebo design trial. Trials 11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hammami MM, Hammami S, Al-Swayeh R, Al-Gaai E, Farah FA, De Padua SJ. 2016. Drug*placebo interaction effect may bias clinical trials interpretation: hybrid balanced placebo and randomized placebo-controlled design. BMC Med. Res. Methodol. 16:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, et al. 2011. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci. Transl. Med. 3:70ra14. [DOI] [PubMed] [Google Scholar]
- 151.Linde K, Witt CM, Streng A, Weidenhammer W, Wagenpfeil S, et al. 2007. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain 128:264–71 [DOI] [PubMed] [Google Scholar]
- 152.Colloca L, Miller FG. 2011. The nocebo effect and its relevance for clinical practice. Psychosom. Med. 73:598–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Egbert LD, Battit GE, Welch CE, Bartlett MK. 1964. Reduction of postoperative pain by encouragement and instruction of patients. a study of doctor-patient rapport. N. Engl. J. Med. 270:825–27 [DOI] [PubMed] [Google Scholar]
- 154.Zunhammer M, Bingel U, Wager TD. 2018. Placebo effects on the neurologic pain signature: a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurol. 75(11):1321–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wager TD, Atlas LY. 2015. The neuroscience of placebo effects: connecting context, learning and health. Nat. Rev. Neurosci. 16(7):403–18 [DOI] [PMC free article] [PubMed] [Google Scholar]