Abstract
Background:
Elevated systolic blood pressure (SBP) is potently associated with risk for recurrent strokes. In resource-limited settings, there is a dearth of data on the rates and determinants of uncontrolled SBP among stroke survivors at high risk of recurrent events.
Objectives:
To assess the rates and determinants of uncontrolled SBP over the first year post-stroke.
Methods:
This is a retrospective observational study involving stroke survivors who enrolled into an out-patient Neurology clinic in Kumasi, Ghana between January 2012 and June 2014. Baseline demographic features, clinical characteristics, antihypertensive medications prescribed at each clinic visit, treatment modifications and clinic blood pressure measurements were recorded. Predictors of uncontrolled SBP during follow-up were assessed using multivariable logistic regression model.
Results:
602 stroke survivors enrolled for follow-up within the study period of which 89.8% had hypertension. Up to 35% of subjects had SBP >140mmHg during follow-up clinic visits. Among those with uncontrolled SBP, 17% had antihypertensive treatment modifications during follow-up. Predictors of uncontrolled SBP were SBP at enrollment into clinic adjusted OR (95% CI) of 1.31 (1.17-1.47)/10mmHg increase and average number of anti-hypertensive medications prescribed adjusted OR (95% CI) of 1.30 (1.06-1.60) for increase in number of antihypertensive prescribed.
Conclusion:
A third of stroke survivors had SBP not on target during follow-up possibly due to a combination of therapeutic inertia, apparent treatment resistance and poor adherence to therapy. Longer-term prospective interventional studies on hypertension control among stroke survivors are warranted in sub-Saharan Africa.
Keywords: Systolic BP Control, Stroke survivors, Ghana, recurrence, therapeutic inertia, antihypertensive therapy, Resistant hypertension
INTRODUCTION
Hypertension is the premier modifiable risk factor for index and recurrent ischemic and hemorrhagic strokes (1–3). Among patients with recent non-cardio-embolic ischemic stroke, a clear association between systolic blood pressure (SBP) and risk for recurrent stroke has been established (4). Available evidence suggests successful reduction of systolic blood pressure results in significant reductions in risk for recurrent strokes (5,6) in developed countries. However, in sub-Saharan Africa where stroke incidence and prevalence are rapidly rising, achieving and sustaining blood pressure control is particularly challenging (7–10) due to a myriad of factors including misconceptions about hypertension, low literacy levels, medication access and affordability, non-adherence, inappropriate medication selection, clinical inertia and resistant hypertension (11–15).
There is limited published data on the rates and determinants of control of blood pressure among stroke survivors in sub-Saharan Africa (16,17) and yet still fewer trials designed (18) to evaluate locally tailored interventions to reduce this potent risk factor within these settings. It is well established that patients who experience a recent stroke are more motivated to adhere to their medications to achieve better risk factor control for secondary prevention. Given that having stroke increases risk of recurrent strokes (19, 20) and that antihypertensive medications are efficacious at reducing systolic blood pressure (5,6,21), we sought to evaluate the rates of control of SBP among stroke survivors enrolled into a neurology clinic in Kumasi, Ghana. Our objectives in this retrospective study were to assess the rates of uncontrolled SBP over the first year after enrolling at the Neurology clinic with a diagnosis of stroke, to document the anti-hypertensive medications prescribed for stroke patients and treatment intensifications undertaken for patients not on target for SBP during clinic appointments in a resource-limited setting.
METHODS
This retrospective study was approved by the Committee on Human Research Publication and Ethics (CHRPE) of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, and the Komfo Anokye Teaching Hospital, Kumasi, Ghana. The study was conducted at the Neurology Clinic of the Komfo Anokye Teaching Hospital in Kumasi, Ghana. Kumasi is the second largest city in Ghana with an estimated population of 4 million inhabitants. The Neurology clinic was established in 2011 by FSS and runs once a week receiving referrals for adults >16 years with neurologic disorders from 6 out of the 10 administrative regions of Ghana and serves an estimated population of 10 million as previously described (22).
Stroke survivors are referred to the neurology clinic upon discharge from the ward as in-patients or from surrounding hospitals and clinics for follow-up care mainly for secondary prevention and rehabilitation. At enrollment into the clinic, patient charts from in-patient are used at the neurology clinic for follow-up. Data collected for the present analysis include age, gender, marital status, occupation, religion, type of stroke, blood pressure measurements on admission and discharge as in-patients and vascular risk factors as well as anti-hypertensive medications prescribed on discharge. At each clinic visit, patients’ systolic and diastolic blood pressures are measured three times by a trained nurse using a mercury sphygmomanometer and the last two recordings averaged by a trained clinic nurse and documented in the patient’s chart. Changes made to anti-hypertensive medications are recorded in the patients’ chart and were captured in the present study. Typically stroke patients are scheduled for follow-up visits on months 1, 3, 6 and 12 with non-scheduled visits where necessary. Stroke types were determined for those with cranial CT scans performed within 10 days post-stroke and at the neurology clinic, ischemic strokes were sub-typed according to the Oxford Community Stroke Project (OCSP) (23) while the SMASH-U was used to sub-type hemorrhagic stroke etiologically into structural, medication-associated, amyloid angiopathy, systemic diseases, hypertensive or undetermined (24). Patients were seldom able to afford the cost of carotid Doppler and echocardiography for etiological sub-types of ischemic strokes. Adherence to anti-hypertensive medications was assessed by asking patient if they had missed doses of their blood pressure medications within the last week but this data was inconsistently documented in patient charts.
Definition of terms:
Systolic Blood Pressure was deemed to be on target of clinic SBP was <140mmHg according to JNC-8 guidelines (25).
The present analysis involves 608 stroke survivors who enrolled into the Neurology clinic between January 2012 to June 2014 and data was closed for analysis in June 2015.
Statistical Analysis:
Means and medians were compared using the Student’s t-test or Mann-Whitney’s U-test for paired comparisons and Analysis of variance or Kruskal Wallis tests for more than 2 group comparisons. Proportions were compared using the Chi-squared test. A multivariable logistic regression model was employed to assess the determinants of clinic SBP ≥140mmHg during follow-up. In this model, variables such as age, gender, stroke type (ischemic, hemorrhagic, not-typed), Systolic blood pressure at enrollment, average number of antihypertensive medications prescribed over the course of follow-up were selected as independent variables based on their known associations with hypertensive control. In bivariable analysis, factors associated with the dependent variable at a p-value level of 0.10 were included in the multivariable model. In all analysis, two-tailed p-values <0.05 were considered statistically significant with no adjustments for multiple comparisons. Statistical analysis was performed using SPSS version 19.
RESULTS
Demographic Characteristics:
Six hundred and eight (608) patients with stroke enrolled into the Neurology clinic between January 2012 and June 2014 but 28 subjects were excluded from further analysis because they did not have documented baseline blood pressure in medical charts. Three hundred and six (306) representing 50.3% were females. The mean ± SD age of study subjects was 59.9 ± 13.9 years, higher for females compared with males- 61.2 ± 15.3 years versus 58.6 ± 12.2 years, p=0.02. Two hundred and eighty patients (66.2%) were married, 77 (18.2%) were widows/widowers, 35 (8.3%) were divorced and 31 (7.3%) were single out of 423 patients with data available on marital status. 88.6%, 8.7% and 2.7% (n=528 with data available) were Christians, Muslims and Pagans respectively.
451 patients were referred to the neurology clinic after being discharged from the medical wards with stroke and 155 were referred from the general clinic at KATH or from a peripheral hospital/clinic for follow-up.
Risk factor profiles:
Vascular risk factors patients were aware they had were hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease and family history of stroke at frequencies of 72.8%, 20.1%, 5.6%, 1.3% and 2.6% respectively. Upon evaluating patients in the clinic, we identified 89.8% had hypertension, 23.5% had dyslipidemia, 20.2% had diabetes mellitus, 14.5% used alcohol in excess, 3.6% smoked cigarette, 1.8% were obese and <1% had atrial fibrillation, biventricular failure, sickle cell anemia and transient ischemic attacks.
Stroke Types:
381 (62.7%) had cranial CT scans done at stroke onset of which 247 were ischemic, 114 were hemorrhagic and 20 had normal scans possibly due to lacunar infarcts. Among subjects with ischemic strokes, 91 had LACI, 62 had PACI, 23 had POCI and 9 had TACI sub-types according to the OCSP classification. Etiologic subtypes of hemorrhagic strokes included 67% with hypertensive, 9% with aneurysmal, 6% with amyloid angiopathy, 1 with vasculitis and 25% undetermined but likely to be of hypertensive cause using the SMASH-U classification. Out of 86 subjects with hemorrhagic strokes, 58 were non-lobar in location comprising of 46 in the basal ganglia, 8 thalamic, 3 pontine, and 1 ventricular while 28 were lobar, 27 cortical and 1 cerebellar.
Blood Pressure Control Rates at Enrollment into the Neurology Clinic:
Of the 580 patients with documented blood pressure measurements at baseline visit, 402 (69.3%) had SBP <140mmHg. As shown in Table 1, stroke survivors with systolic blood pressure ≥140mmHg were significantly younger, more likely to be employed, with a propensity for hemorrhagic stroke type compared with those with SBP below 140mmHg. The median (IQR) number of anti-hypertensive medications taken by subjects with SBP ≥140mmHg of 3 (2-3) was significantly more than 2 (1-3) taken by those with SBP <140mmHg, p<0.0001. The classes of anti-hypertensive medications used by stroke survivors at enrolment in decreasing frequency were Calcium channel blockers (n=454), ACE-Inhibitors (n=308), Angiotensin Receptor Blockers (n=249), diuretics (n=149), Methyldopa (n=116), Beta-blockers (n=39) and Hydralazine (n=34).
Table 1.
Demographic and Clinical Characteristics of 580 Stroke Survivors at enrollment visit into the Neurology Clinic according to Systolic Blood Pressure.
| Variable | SBP ≤ 140mmHg n = 402 | SBP > 140mmHg n = 178 | P-value |
|---|---|---|---|
| Age, median (IQR) | 61 (50 – 72) | 56 (50 - 68) | 0.04 |
| Males, n (%) | 203 (50.5) | 85 (47.8) | 0.6 |
| Occupation, n (%) | 0.005 | ||
| Employed | 205 (51.0) | 104 (58.4) | |
| Unemployed/Retiree | 138 (34.3) | 38 (21.3) | |
| Missing data | 59 (14.7) | 36 (20.2) | |
| Religion, n (%) | 0.11 | ||
| Christian | 321 (79.9) | 128 (71.9) | |
| Moslem | 29 (7.2) | 14 (7.9) | |
| Pagan | 9 (2.2) | 4 (2.2) | |
| Others | 43 (10.7) | 32 (18.0) | |
| Stroke Type, n (%) | 0.04 | ||
| Ischemic | 172 (42.8) | 60 (33.7) | |
| Hemorrhagic | 65 (16.2) | 42 (23.6) | |
| Unclassified | 165 (41.0) | 76 (42.7) | |
| CV Risk Factors, n (%) | |||
| Hypertension | 379 (94.2) | 176 (98.9) | 0.01 |
| Dyslipidemia* | 108 (44.4) | 51 (47.2) | 0.63 |
| Diabetes Mellitus | 82 (20.4) | 38 (21.3) | 0.79 |
| Alcohol | 57 (14.2) | 30 (16.9) | 0.63 |
| Smoking | 16 (4.1) | 5 (2.9) | 0.63 |
| Cardiac disease | 6 (1.5) | 1 (0.6) | 0.68 |
| Classes of Anti-hypertensive Medications, n (%) | |||
| ACE-Inhibitors | 194 (48.3) | 105 (60.0) | 0.02 |
| Angiotensin Receptor Blockers | 156 (38.8) | 83 (46.6) | 0.08 |
| Beta-Blockers | 25 (6.2) | 13 (7.3) | 0.49 |
| Calcium channel Blockers | 281 (69.9) | 156 (87.6) | <0.0001 |
| Diuretics | 86 (21.4) | 58 (32.6) | 0.004 |
| Methyldopa | 61 (15.1) | 48 (27.0) | 0.0008 |
| Hydralazine | 14 (3.5) | 20 (11.2) | 0.0002 |
| Number of Anti-hypertensive subject is taking, n (%) | |||
| 0 | 37 (9.2) | 3 (1.7) | <0.0001 |
| 1 | 73 (18.2) | 8 (4.5) | |
| 2 | 172 (42.8) | 73 (41.0) | |
| 3 | 86 (21.4) | 57 (32.0) | |
| 4 | 29 (7.2) | 28 (15.7) | |
| 5 or more | 5 (1.2) | 9 (5.1) | |
| Statin therapy, n (%) | 199 (49.5) | 90 (50.6) | 0.81 |
| Antiplatelet therapy, n (%) | 174 (43.3) | 70 (39.3) | 0.37 |
Blood Pressure Control Rates during follow-up:
The SBP and DBP at enrollment and during follow-up remained stable with no differences between baseline mean ± SD of SBP and DBP of 137.7 ± 21.8mmHg and 88.1 ± 12.8mmHg versus 140.6 ± 21.0mmHg and 89.2 ± 11.4mmHg at month 12 of follow-up. The proportion of subjects with SBP <140mmHg at enrollment (n=580), months 1 (n=256), 3 (n=219), 6 (n=214) and 12 (n=315) were 69.3%, 64.5%, 63.0%, 65.0% and 67.0% respectively as shown in figure 1 but lower than 77.7% before discharge from the ward. Although the proportion of subjects with SBP ≥ 160mmHg progressively increased during follow-up from 8.8% to 12.4%, statistical significance was not achieved chi-square test for trend, p=0.30.
FIGURE 1.
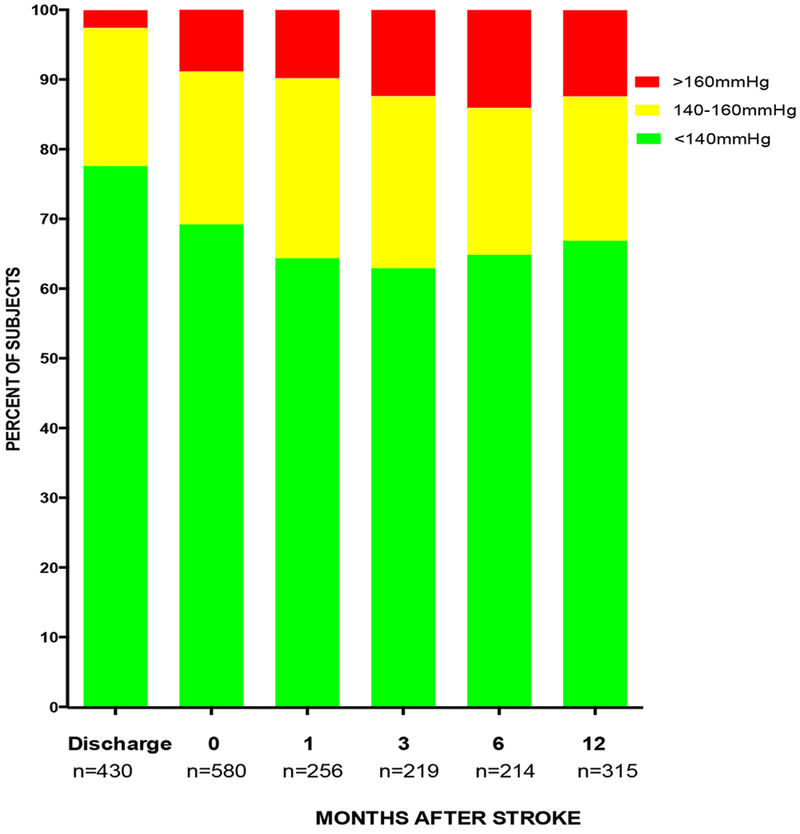
Systolic Blood Pressures at discharge, entry and follow-up at Neurology Clinic for Stroke Survivors. Number of subjects who attended clinic at time points are indicated. Some patients missed clinic appointments, were not scheduled for clinic visits at some time points, or were lost to follow-up.
Comparison of Blood Pressure Control Rates among Ischemic versus Hemorrhagic stroke survivors:
Ischemic stroke survivors had higher proportions of the subjects with SBP <140mmHg compared with hemorrhagic stroke survivors during follow-up. Cumulatively, there were 190 SBP measurements among Hemorrhagic stroke survivors and 388 among ischemic stroke patients during follow-up after the enrollment visit. The proportions with SBP <140mmHg, 140-160mmHg and >160mmHg were 70.4%, 18.6% and 11.0% respectively among ischemic stroke survivors compared with 57.9%, 26.8% and 15.3% respectively among hemorrhagic stroke survivors, p=0.01. Furthermore, significantly more antihypertensive medications were prescribed for subjects with hemorrhagic strokes compared with ischemic stroke survivors the median (IQR) being 3 (2-4) vs 2 (2-3), p<0.0001 at month 1, 2.5 (2-3) vs 2.0 (2-3), p=0.05 at month 3, 3 (2-4) vs 2 (2-3), p=0.006 at month 6 and 3 (2-4) vs 2 (2-3), p<0.0001 at month 12.
Antihypertensive medication prescriptions during follow-up:
The mean ± SD SBP increased progressively with number of anti-hypertensive medications prescribed being 133.0 ± 19.0mmHg, 138.4 ± 19.0mmHg, 143.9 ± 22.0mmHg, 148.3 ± 23.4mmHg and 156.3 ± 16.1mmHg, p<0.0001 for patients prescribed 1, 2, 3, 4, and 5 anti-hypertensive medications respectively during follow-up. CCB, ACE-I and ARBs were most frequently used during follow-up with a median (IQR) of 2 (2-3) antihypertensive prescribed per patient overall as shown in table 2. However among patients whose SBP were ≥140mmHg at each clinic visit the median (IQR) number of antihypertensive medications prescribed was 3 (2-3) compared with 2 (2-3) among those with SBP <140mmHg, p<0.0001.
Table 2:
Frequencies of Classes & Number of Anti-hypertensive Medications prescribed to Stroke Survivors during 1st year follow-up in Neurology Clinic
| Classes of Anti-hypertensive Medications Prescribed | |||||||
|---|---|---|---|---|---|---|---|
| Month of visit | Calcium Channel Blockers, n (%) | Angiotensin Receptor Blockers, n (%) | Angiotensin Converting Enzyme Inhibitors, n (%) | Diuretics, n (%) | Methyldopa n (%) | Beta blockers n (%) | Hydralazine n(%) |
| 1 (n=267) | 196 (73.4) | 127 (47.6) | 125 (46.8) | 68 (25.5) | 53 (19.9) | 20 (7.5) | 15 (5.6) |
| 3 (n=225) | 167 (74.2) | 97 (43.1) | 111 (49.3) | 57 (25.3) | 37 (16.4) | 13 (5.8) | 9 (4.0) |
| 6 (n=217) | 169 (77.9) | 96 (44.2) | 104 (47.9) | 84 (38.7) | 48 (22.1) | 15 (6.9) | 7 (3.2) |
| 12 (n=325) | 254 (78.2) | 157 (48.3) | 152 (46.8) | 101 (31.1) | 70 (21.5) | 27 (8.3) | 12 (3.7) |
| Number of Anti-hypertensive Medications Prescribed per patient | |||||||
| Month of visit | 0, n (%) | 1, n (%) | 2, n (%) | 3, n (%) | 4, n (%) | 5, n (%) | Total |
| 1 | 20 (7.5) | 39 (14.6) | 103 (38.6) | 68 (25.5) | 31 (11.6) | 6 (2.2) | 267 |
| 3 | 15 (6.7) | 33 (14.7) | 94 (41.8) | 64 (28.4) | 17 (7.6) | 2 (0.9) | 225 |
| 6 | 14 (6.4) | 24 (11.1) | 76 (35.0) | 72 (33.2) | 25 (11.5) | 6 (2.8) | 217 |
| 12 | 12 (3.7) | 39 (12.0) | 134 (41.2) | 99 (30.5) | 36 (11.1) | 5 (1.5) | 325 |
Treatment modifications when SBP were not on target during follow-up :
Out of 1,034 clinic visits after the enrollment visit, there were 351 (33.9%) episodes where SBP was ≥140mmHg. Generally, intensification of blood pressure medications was undertaken in only 63 (17.9%) out 351 opportunities for modification. Treatment intensification involved addition of new classes of anti-hypertensive medications (n=42), substitution of anti-hypertensive classes (n=19) and increase in dosage of existing anti-hypertensive medications depicted in Table 3. Of the 1,034 visits, 135 had single, 407 had dual, 303 had triple, 108 had quadruple, 19 had quintuple anti-hypertensive medications prescribed respectively with no anti-hypertensive medication prescribed for 60 patient visits.
Table 3:
Anti-hypertensive Treatment Modifications/Intensifications Among Stroke Survivors during follow-up
| Month of visit | # of patients on visit | # (%) of subjects with Anti-HTN medication modifications | # (%) of patients with SBP>140mmHg | # (%) of subjects with Anti-HTN medication Intensification | Types of Anti-hypertensive treatment intensification for subjects with SBP >140mmHg |
|---|---|---|---|---|---|
| 1 | 267 | 56 (21.0) | 91 (34.1) | 25 (27.4) | 1. Addition of new class of anti-hypertensive, n=17 2. Increase in dosage of anti-hypertensive, n=1 3. Substitution of anti-hypertensive classes, n=7 |
| 3 | 225 | 30 (13.3) | 81 (36.0) | 14 (17.3) | 1. Addition of new class of anti-hypertensive, n=11 2. Increase in dosage of anti-hypertensive, n=1 3. Substitution of anti-hypertensive classes, n=2 |
| 6 | 217 | 30 (13.8) | 75 (34.6) | 13 (17.3) | 1. Addition of new class of anti-hypertensive, n=7 2. Increase in dosage of anti-hypertensive, n=0 3. Substitution of anti-hypertensive classes, n=6 |
| 12 | 325 | 33 (10.1) | 104 (32.0) | 11 (10.6) | 1. Addition of new class of anti-hypertensive, n=7 2. Increase in dosage of anti-hypertensive, n=0 3. Substitution of anti-hypertensive classes, n=4 |
| TOTAL | 1,034 | 149 (14.4) | 351 (33.9) | 63 (17.9) | 1. Addition of new class of anti-hypertensive, n=42 2. Increase in dosage of anti-hypertensive, n=2 3. Substitution of anti-hypertensive classes, n=19 |
Determinants of SBP ≥140mmHg during follow-up:
The independent predictors of ≥ 1 SBP of ≥140mmHg post-enrollment on multivariable logistic regression analysis were systolic blood pressure at enrollment into clinic and the average number of anti-hypertensive medications prescribed over the course of follow-up with adjusted OR (95% CI) of 1.31 (1.17-1.47) and 1.30 (1.06-1.60) respectively as shown in Table 4.
Table 4:
Multiple Logistic Regression Analysis for determinants of Uncontrolled SBP among Stroke Survivors during follow-up at Neurology Clinic.
| Variable | Unadjusted Odds ratio 95% CI | p-value | Adjusted Odds ratio 95% CI | p-value |
|---|---|---|---|---|
| Age | ||||
| For each 10 years older | 1.00 (0.88 - 1.15) | 0.96 | - | - |
| Gender | ||||
| Female | 1.00 | 0.95 | - | - |
| Male | 1.01 (0.70 – 1.46) | |||
| Stroke Type | ||||
| Ischemic stroke | 1.00 | 1.00 | ||
| Hemorrhagic stroke | 1.19 (0.71-2.02) | 0.50 | 0.88 (0.49-1.59) | 0.88 |
| Not typed | 1.21 (0.99-1.49) | 0.07 | 1.16 (0.74-1.82) | 0.52 |
| Systolic BP at enrollment | ||||
| For every 10mmHg increase | 1.37 (1.23-1.53) | <0.0000 | 1.31 (1.17-1.47) | <0.0000 |
| Average number of classes of anti-hypertensive prescribed | ||||
| For each increase in class | 1.60 (1.33-1.93) | <0.0000 | 1.30 (1.06-1.60) | 0.01 |
Clinical Events during follow-up:
There was one recorded recurrent stroke and three patients were re-admitted with hypertensive emergencies.
DISCUSSION
We found that 90% of stroke survivors enrolling into a Neurology clinic in this resource-limited setting had systemic arterial hypertension as a modifiable vascular risk factor. Amidst an attrition rate of 25%, we found on average that systolic blood pressure was on target among 65-70% of patients at clinic visits during follow-up. Also, SBP control rates among ischemic stroke survivors were higher and required fewer antihypertensive medications to achieve SBP targets compared with hemorrhagic stroke survivors. Notably, <20% of patients who were not on target at clinic visits had treatment intensification performed indicative of therapeutic inertia on the part of clinicians.
Prior to enrollment into the Neurology clinic, nearly 90% of stroke survivors were on at one or more combinations of antihypertensive medications initiated while on hospital admission with stroke, 50% were on statins and 45% were on anti-platelet therapy. Consequently, 70% of stroke survivors in this series had SBP on target at baseline visit. This is in conformity with the 2014 American Heart Association/American Stroke Association Guidelines for the prevention of stroke in patients with stroke and transient ischemic attacks where the need to initiate or resume hypertension treatment after the acute phases of stroke in neurologically stable patients with blood pressures of >140/90mmHg has been emphasized (26). Recently, the in-patient management of acute strokes at this study site occurs at a Stroke unit where dedicated nursing care and treatment guidelines are followed (27, 28). The prompt, incisive and aggressive initiation of secondary prevention strategies within this cohort particularly in our settings is salutary and addresses deficiencies identified commonly after cerebrovascular events (29–31).
On subsequent clinic visits, 30-35% of patients had ≥1 elevated outpatient SBP measurement during the first year post-stroke. This is comparable with 38% observed among 3153 predominantly male ischemic stroke survivors in a US cohort (32). A significant potential contributor to this observation is therapeutic inertia wherein only 17% of subjects presenting to clinic with uncontrolled SBP had documented evidence of medication alterations. Among patients who were uncontrolled, 47% were either on single or dual combination of antihypertensive medications with some on sub-optimal doses. This clinical inertia and under-treatment of high risk patients is quite common in clinical practice and are often driven by physician beliefs such as awaiting for full drug effect, patients almost near target, poor compliance, side effects and good self-measurements or white-coat hypertension (33) and on occasions lack of awareness of treatment guidelines.
There is a proven association between cardiovascular events and consistency of blood pressure control. (4) The high rates of initiation and persistence of secondary preventive therapeutic measures initiated after stroke in particular reference to antihypertensive therapy is comparable to reports from European and North American cohorts (34–37) where ischemic strokes are preponderant but better than those among Chinese cohort (38) where ischemic and hemorrhagic stroke distribution and stroke outcomes are similar to our cohort. Indeed we noted that hemorrhagic stroke survivors were more likely to present to clinics with SBP not on target and required more antihypertensive medications than ischemic stroke survivors. This information would be useful for practitioners managing hemorrhagic stroke survivors and the underlying mechanisms for these difference remains to be elucidated.
This study has limitations worth noting. The study was conducted in a single center among stroke survivors in a neurology clinic and thus may not be generalizable to stroke population outside these settings. The lack of complete information on adherence <25% precluded its inclusion in models for evaluating determinants of poor SBP control. There was significant loss-to-follow up and defaulter rates which could have been due to functional limitations imposed by stroke symptomatology, long distances travelled for clinic visits, lack of finances, utilization of alternative therapies by stroke survivors. Finally, clinical outcomes such as rates of new CVDs, recurrent strokes, hospitalizations and deaths were not reported in the present study to assess the impact of SBP control on disease outcomes. Indeed, the rates of hospitalizations and recurrent strokes we have reported may be an underestimation of the actual rates of these events due to the lack of electronic databases to ascertain these events in defaulting subjects.
In spite of these limitations, this study raises several important questions which are pertinent for risk factor management among stroke survivors in resource-limited settings where professional medical doctors are in short supply. In this milieu, efficacious BP control programs that are feasible, acceptable, timely and sustainable are urgently needed amidst a rising epidemic of stroke and other CVDs. In this regard mobile health (m-Health) technology offers a promising potential approach to meet this need (39 – 41). Task shifting strategies such as employing nurses who will visit stroke survivors at home or remotely via telecommunication needs to be tested for efficacy and acceptability in achieving sustained BP control among stroke survivors in our environs.
In conclusion we have shown that two-thirds of stroke survivors attending a neurology clinic in a resource limited setting have their SBP on target during office visits with a third of those not on target due to therapeutic inertia, apparent treatment resistant hypertension and possibly poor adherence to therapy. Prospective studies among stroke survivors to assess the impact of therapeutic inertia, treatment resistant hypertension and medication adherence on vascular outcomes in sub-Saharan Africa are warranted.
Acknowledgments
Source of funding: National Institute of Health- National Institute of Neurological Disorders & Stroke; R21 NS094033.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None to declare
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics- 2014 update: A report from the American Heart Association. Circulation 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, et al. Prospective Studies Collaboration: age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002; 360 (9349):1903.doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Biffi A, Anderson CD, Battey TW, et al. Association between blood pressure and Risk of Recurrent intracerebral hemorrhage. JAMA. 2015; 314(9):904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Towfighi A, Markovic D, Ovbiagele B. Consistency of blood pressure control after ischemic stroke: prevalence and prognosis. Stroke. 2014. May; 45(5):1313–7. [DOI] [PubMed] [Google Scholar]
- 5.PROGRESS Collaborative Group Randomized trial of a perindopril-based pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischemic attack. Lancet 2001;358 (9287):1003 Doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Algra A, Amarenco P. Medical treatment in acute and long-term secondary prevention after transient ischemic attack and ischemic stroke. Lancet. 2011;377:1681–92. [DOI] [PubMed] [Google Scholar]
- 7.Owolabi MO. Taming the burgeoning stroke epidemic in Africa: Stroke quadrangle to the rescue. West Indian Med J. 2011; 60:412–421. [PubMed] [Google Scholar]
- 8.BeLue R, Okoror TA, Iwelunmor J, et al. An overview of cardiovascular risk factor in sub-Saharan African countries: a socio-cultural perspective. Global Health. 2009;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemogoum D, Degaute JP, Bovet P. Stroke prevention, treatment, and rehabilitation in sub-Saharan Africa. Am J Prev Med. 2005;29:95–101. [DOI] [PubMed] [Google Scholar]
- 10.Oke DA, Bandele EO. Misconceptions of hypertension. J Natl Med Assoc. 2004; 96:1221–1224. [PMC free article] [PubMed] [Google Scholar]
- 11.Dusing R Overcoming barriers to effective blood pressure control in patients with hypertension. Current Medical Research and Opinion. 2006; 22:1545–1553. [DOI] [PubMed] [Google Scholar]
- 12.Borzecki AM, Oliveria SA, Berlowitz DR. Barriers to hypertension control. American Heart Journal 2005;149: 785–794. [DOI] [PubMed] [Google Scholar]
- 13.Okonofua EC, Simpson KN, Jesri A, et al. Therapeutic inertia is an impediment to achieving the healthy people 2010 blood pressure control goals. Hypertension. 2006; 47: 345–351. [DOI] [PubMed] [Google Scholar]
- 14.Andrade SE, Gurwitz JH, Field TS, et al. Hypertension management: The care gap between clinical guidelines and clinical practice. The American Journal of Managed Care. 2004;10:481–486. [PubMed] [Google Scholar]
- 15.Thorogood M, Connor MD, Hundt GL, et al. Understanding and managing hypertension in an African sub-district: a multidisciplinary approach. Scand J Public Health Suppl. 2007; 69:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abubakar SA, Obiako OR, Isa MS, et al. Blood pressure control in long-term stroke survivors evaluated one year post stroke. Niger Postgrad Med J. 2015; 22(1):56–60. [PubMed] [Google Scholar]
- 17.Thorogood M, Connor MD, Lewando-Hundt G, et al. SASPI Project Team. Secondary prevention of stroke-results from the South Africa Stroke Prevention Initiative (SASPI) study. Bull World Health Organ. 2004; 82(7): 503–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Owolabi MO, Akinyemi RO, Gebregziabher M, et al. Randomized controlled trial of a multipronged intervention to improve blood pressure control among stroke survivors in Nigeria. Int J Stroke. 2014; 9(8):1109–16. [DOI] [PubMed] [Google Scholar]
- 19.Hong KS, Yegiaian S, Lee M, et al. Declining stroke and vascular event recurrence rates in secondary prevention trial over the past 50 years and consequences of trial design. Circulation 2011; 123: 2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003; 34: 2741–2748. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Wolf PA, McGee DL, et al. Systolic blood pressure, arterial rigidity, and risk of stroke. The Framingham study. JAMA 1981; 245; 1225–1229. [PubMed] [Google Scholar]
- 22.Sarfo FS, Akassi J, Badu E, et al. Profile of neurological disorders in an adult neurology clinic in Kumasi, Ghana. eNeurologicalSci. 2016; 3:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991; 337:1521–26. [DOI] [PubMed] [Google Scholar]
- 24.Meretoja A, Strbian D, Putaala J, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke 2012; 43(10): 2592–7. [DOI] [PubMed] [Google Scholar]
- 25.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014; 311(5): 507–20. [DOI] [PubMed] [Google Scholar]
- 26.Kernan WN, Ovbiagele B, Black HR, et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke. 2014; 45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 27.Sarfo FS, Awuah DO, Nkyi C, et al. Recent patterns and predictors of neurological mortality among hospitalized patients in Central Ghana. J Neurol Sci. 2016; 363:217–24. [DOI] [PubMed] [Google Scholar]
- 28.Sarfo FS, Akassi J, Awuah D, et al. Trends in stroke admission and mortality rates from 1983 to 2013 in Central Ghana. J Neurol Sci. 2015; 357 (1-2):240–5. [DOI] [PubMed] [Google Scholar]
- 29.Rudd AG, Lowe D, Hoffman A, et al. Secondary prevention for stroke in the United Kingdom: results from the National Sentinel Audit of Stroke. Age Ageing. 2004;33:280–286. [DOI] [PubMed] [Google Scholar]
- 30.Xu G, Liu X, Wu W, et al. Recurrence after ischemic stroke in Chinese patients: impact of uncontrolled modifiable risk factors. Cerebrovasc Dis. 2007; 23:117–120. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan RC, Tirschwell DL, Longstreth WT Jr, et al. Vascular events, mortality, and preventive therapy following ischemic stroke in the elderly. Neurology 2005; 65:835–842. [DOI] [PubMed] [Google Scholar]
- 32.Roumie CL, Zillich AJ, Bravata DM, et al. Hypertension treatment intensification among stroke survivors with uncontrolled blood pressure. Stroke 2015; 46(2): 465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrari P; National Coordinators for the Reasons for not Intensifying Antihypertensive Treatment (RIAT) trial12. Reasons for therapeutic inertia when managing hypertension in clinical practice in non-Western countries. J Hum Hypertens. 2009; 23(3): 151–9. [DOI] [PubMed] [Google Scholar]
- 34.Sappok T, Faulstich A, Stuckert E, et al. Compliance with secondary prevention of ischemic stroke: a prospective evaluation. Stroke. 2001;32(8):1884–1889. [DOI] [PubMed] [Google Scholar]
- 35.Lummis HL, Sketris IS, Gubitz GJ, et al. Medication persistence rates and factors associated with persistence in patients following stroke: a cohort study. BMC Neurol. 2008; 8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bushnell CD, Olson DM, Zhao X, et al. Secondary preventive medication persistence and adherence 1 year after stroke. Neurology. 2011;77(12):1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glader EL, Sjolander M, Eriksson M, et al. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke. 2010;41(2):397–401. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Ju Y, Wang C, et al. Patterns and predictors of antihypertensive medication used 1 year after ischemic stroke or TIA in urban China. Patient Prefer Adherence. 2013; 7: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bastawrous A, Armstrong MJ. Mobile health use in low- and high-income countries: an overview of the peer-reviewed literature. Journal of the Royal Society of Medicine 2013; 106:130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leon N, Schneider H, Daviaud E. Applying a framework for assessing the health system challenges to scaling up mHealth in South Africa. BMC Medical Informatics and decision making. 2012;12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vodopivec-Jamsek V, de Jongh T, Gurol-Urganci I, et al. Mobile phone messaging for preventive health care. The Cochrane database of systematic reviews 2012; 12: CD007457. [DOI] [PMC free article] [PubMed] [Google Scholar]


