Abstract
Rickettsia parkeri, a causative agent of spotted fever rickettsiosis, is transmitted by Amblyomma maculatum (Gulf Coast tick), a tick that may also carry a nonpathogenic spotted fever group Rickettsia, “Candidatus Rickettsia andeanae”. Here, we evaluated R. parkeri and “Candidatus R. andeanae” in tissues from A. maculatum prior to, during, and after blood feeding on rabbits. Using colony-reared A. maculatum that were capillary-fed uninfected cells, R. parkeri, “Candidatus R. andeanae”, or both rickettsiae, we detected higher levels of Rickettsia spp. in the respective treatment groups. Rickettsial levels increased during blood feeding for both R. parkeri and “Candidatus R. andeanae”, with a greater increase in R. parkeri in co-infected ticks compared to singly-infected ticks. We detected transovarial transmission of “Candidatus R. andeanae” in egg and larval cohorts and confirmed vertical transmission of R. parkeri in one group of larvae. Rabbits from all Rickettsia-exposed groups seroconverted on immunofluorescent antibody testing using R. parkeri antigen. Visualization of “Candidatus R. andeanae” in tick salivary glands suggested potential transmission via tick feeding. Here, rickettsial levels in artificially infected ticks demonstrate changes during feeding and transovarial transmission that may be relevant for interpreting rickettsial levels detected in wild A. maculatum.
Keywords: Rickettsia parkeri, “Candidatus Rickettsia andeanae”, Gulf Coast tick, tick-feeding
Introduction
Rickettsia parkeri was not considered a human pathogen until 2002, when the first case of R. parkeri rickettsiosis was identified and later reported (Paddock et al., 2004). Since 2004, approximately 40 human cases of spotted fever rickettsiosis caused by R. parkeri have been reported in the southeastern United States (Cragun et al. 2010; Ekenna et al. 2014; Kaskas et al. 2014; Paddock et al. 2004, 2008; Myers et al. 2013; Straily et al. 2016; Paddock and Goddard 2015). The prevalence of R. parkeri in its primary vector, Amblyomma maculatum, typically varies from 10 – 56% (Ferrari et al. 2012; Mays et al. 2016; Nadolny et al. 2014; Paddock et al. 2010; Sumner et al. 2007; Varela-Stokes et al. 2011; Wright et al. 2011). This is significantly higher than the prevalence of Rickettsia rickettsii in Dermacentor ticks, where rates rarely exceed 1% (Philip et al. 1981). Amblyomma maculatum may also be infected with a presumably non-pathogenic spotted fever group Rickettsia (SFGR), “Candidatus Rickettsia andeanae” (Ferrari et al. 2012; Lee et al. 2017; Mays et al. 2016; Sumner et al. 2007). While low infection rates of “Candidatus R. andeanae” are typical within the southern range of A. maculatum, a high frequency of “Candidatus R. andeanae”-infected A. maculatum ticks was detected from populations in Kansas and Oklahoma, at 47% and 73%, respectively. Interestingly, R. parkeri was not detected from these two states in that survey (Paddock et al. 2015).
In general, single infections with R. parkeri and “Candidatus R. andeanae” in A. maculatum are more common than co-infections, though co-infections have been reported at variable rates (Ferrari et al. 2012; Flores-Mendoza et al. 2013; Lee et al. 2017, Varela-Stokes et al. 2011). In Mississippi A. maculatum populations, where high co-infection rates were previously reported, a subsequent survey by Lee et al. demonstrated low co-infection rates (Ferrari et al. 2012; Lee et al. 2017). This suggests that the dynamics among these two Rickettsia spp. at the population level may be more complex than either a synergism whereby presence of both species allows for increased occurrence of co-infections, or simple exclusion whereby presence of one prevents the occurrence of the other species. Within an individual tick, interactions among R. parkeri and “Candidatus R. andeanae” in unfed ticks and during vertebrate host feeding are unknown, and may affect population infection rates.
In this study, we evaluated rickettsial infection levels in feeding A. maculatum, using tick groups infected with either R. parkeri or “Candidatus R. andeanae”, or both Rickettsia species and an A. maculatum-Rickettsia-rabbit model to quantify rickettsiae in tick midgut and salivary gland tissues. We additionally assessed vertical transmission of rickettsiae in ticks and transmission to rabbits during tick feeding. Considering A. maculatum in populations from Kansas and Oklahoma were commonly infected with “Candidatus R. andeanae” whereas R. parkeri was absent, and “Candidatus R. andeanae”, but not R. parkeri, has been routinely detected in laboratory-reared colonies, we hypothesized that transovarial transmission would be more common for “Candidatus R. andeanae” than for R. parkeri.
Materials and methods
Source of Amblyomma maculatum
Laboratory-reared adult A. maculatum (Gulf Coast ticks) were purchased from Texas A & M University (TAMU), College Station, Texas and Oklahoma State University (OSU), Stillwater, Oklahoma. Both sources of A. maculatum were used because previous work in our laboratory demonstrated evidence of “Candidatus R. andeanae” in OSU colonies, and absence of rickettsiae in TAMU populations; however, that was not the case during this study as some level of rickettsial infection was detected in both sources immediately prior to beginning the study. To assess natural infection of rickettsiae in purchased ticks, portions of ticks from each source were individually tested by PCR for R. parkeri and “Candidatus R. andeanae” using previously published protocols targeting the outer membrane protein A gene (ompA) (Paddock et al. 2010, Wright et al. 2011). Amblyomma maculatum were maintained in a humidity chamber with saturated KNO3 (approximately 93% relative humidity), which was kept in the laboratory under an approximately 10: 14 (light: dark) cycle, and room temperature.
Preparation of cultivated rickettsial species
A strain of “Candidatus R. andeanae”, originally isolated in our laboratory from naturally infected A. maculatum, was propagated in co-culture with Vero cells or Ixodes scapularis embryonic cells (ISE6, provided by U.G. Munderloh, University of Minnesota, USA) as described (Ferrari et al. 2013). The Oktibbeha strain of R. parkeri transformed with plasmid pRAM18dRGA/Rif/GFPuv (R. parkeri GFPuv; provided by U.G. Munderloh, University of Minnesota, USA) (Burkhardt et al. 2011) was co-cultured in Vero cells. Infected and uninfected Vero cell cultures were maintained at 37°C with 5% CO2, in Eagle’s Minimal Essential Medium (MEM) with 10% fetal bovine serum. To prepare material to be used for capillary feeding ticks, cells from infected and uninfected flasks were harvested with a cell scraper and the cell suspension passed three times through a 21-gauge (G) needle and then an additional three times through a 30-G needle to release rickettsiae. The cell suspension was centrifuged at 50 x g for 5 minutes to pellet large clumps of cells and debris, and then rickettsial organisms were collected by centrifuging supernatant at 10,000 x g for 10 minutes and resuspending the pellet in cell culture media. An aliquot from this material was saved for DNA extraction (DNeasy Blood and Tissue Kit, Qiagen Inc. Valencia CA) and quantitative PCR (qPCR) using a TaqMan® protocol to specifically quantify R. parkeri and “Candidatus R. andeanae” prior to capillary feeding (described later in Methods under “DNA extraction and rickettsial DNA quantification by PCR”).
Vertebrate hosts for tick feeding
Thirty-six juvenile female New Zealand White rabbits were obtained from Charles River Laboratories, Massachusetts. Rabbits were 9–12 weeks old, in good body condition, and purchased in three separate groups of twelve for the three replicate trials. During the course of the study (approximately three months), average daily minimum and maximum temperatures in the animal room were 69.8°F (21.0°C) and 72.2°F (22.4°C), respectively. Average daily minimum and maximum humidity were 46% and 59%. Due to the air handling system causing a tendency for dry air, we supplemented room humidity using humidifiers. Rabbits were kept in individual standard wire bottom cages with environmental enrichment, and food and water ad libitum. These studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Mississippi State University, Protocol Number 13–002.
Tick feeding trials
For each of the three replicate trials, twelve rabbits were divided into four experimental groups, with three rabbits per group. All rabbits were infested with adult A. maculatum. Groups were defined according to what was used for capillary-feeding A. maculatum: (1) “Candidatus R. andeanae” culture, (2) R. parkeri culture, (3) mixture of “Candidatus R. andeanae” and R. parkeri, and (4) uninfected Vero cell culture (control). For groups capillary-fed either Rickettsia sp., we used a live cultured rickettsial suspension containing a known concentration of R. parkeri GFPuv (2×106 copy number/mL) or “Candidatus R. andeanae” (2×104 copy number/mL) in 10-μL glass capillary tubes positioned on adult male and female A. maculatum as described in Macaluso et al. (2001). Feeding lasted approximately 2 hours in a 32°C incubator. For ticks that were capillary-fed both rickettsiae, we mixed equal volumes of R. parkeri and “Candidatus R. andeanae”, with each Rickettsia species itself at half the final concentration of that used in single species exposures. Because “Candidatus R. andeanae” levels were generally lower than R. parkeri levels for capillary feeding, we additionally selected OSU A. maculatum for “Candidatus R. andeanae” singly- and co-infected groups, which we determined to be naturally infected with “Candidatus R. andeanae” immediately prior to the study. We did not adjust R. parkeri and “Candidatus R. andeanae” concentrations to be equal for capillary feeding in part because higher levels of R. parkeri compared to “Candidatus R. andeanae” have been detected in wild, questing A. maculatum (Lee et al. 2017) and volumes of “Candidatus R. andeanae” cultures were limited. The control group of rabbits was infested with A. maculatum (TAMU source) capillary-fed with uninfected Vero cells, which were processed in the same manner as infected cells, tested for rickettsiae by qPCR, and resuspended in the cell culture media. Approximately 800 A. maculatum were capillary-fed for the three experimental groups in each trial; this took into account potential mortality and provided sufficient numbers of ticks to place 36 on each of 12 rabbits and set aside 30 ticks for day 0 sampling. After capillary feeding, A. maculatum were kept in a humidity chamber for one week to allow for rickettsial replication and dissemination in tissues.
On day 0 post tick-placement (DPT 0), one week after capillary feeding, 30 A. maculatum (15 male: 15 female) were removed from each of the four experimental tick groups and dissected to collect tissues for qPCR and microscopy, as described below. Another 36 A. maculatum (18 male: 18 female) from each group were placed on each of three individual rabbits, with 12 total rabbits used in each trial, and three trials performed sequentially. Rabbits were anesthetized during tick placement (DPT 0) and removal (DPT 6 and 12) using a combination of ketamine (15 mg/kg), dexmedetomidine (0.125 mg/kg), butorphanol (0.2 mg/kg), and glycopyrrolate (0.01 mg/kg). When procedures were completed, we used atipamazole (intramuscular injection) for reversal of dexmedetomidine, and monitored recovery of rabbits. We placed ticks in a chamber measuring ~60 cm2, constructed using a design based on Embers et al. (2013) and secured to the shaved dorsum of each rabbit using skin bond (Osto-Bond, by Montreal Ostomy) adhesive. Rabbits were fitted with Elizabethan collars during tick feeding to discourage chewing of the tick chamber.
On DPT 6, nine male and nine female A. maculatum were removed from each rabbit and dissected for collection of tissues. On DPT 12, the remaining A. maculatum were removed from each chamber and a subset processed as above. The number of live ticks removed on DPT 12 varied and increased over the three trials. For each rabbit, up to three replete female A. maculatum collected on DPT 12 were placed in wells of sterile 6-well culture plates for oviposition and kept in a humidity chamber under the same conditions as for other ticks in the study. After oviposition, egg masses were divided, with a portion of the eggs (approximately 300–500) processed for DNA extraction and rickettsial qPCR. The remainder of the egg mass was kept in the humidity chamber to hatch for subsequent qPCR testing of larvae. A subset of the larvae (approximately 100–300) was processed in the same way as for the egg mass and tested by rickettsial qPCR.
Blood was collected in heparin tubes from each rabbit on DPT 0, 6, 12, and twice a week thereafter until DPT 33. Additionally, rabbit skin biopsies were taken from the tick feeding sites for cell culture and qPCR assay on DPT 12. A sample of plasma was archived (−20°C) for serological assays (indirect immunofluorescent antibody test; IFA) and whole blood was archived (−20°C) for DNA extraction and subsequent qPCR. On DPT 33, rabbits were anesthetized with the injectable anesthetics as described above and then euthanized by barbiturate overdose using Beuthanasia®, as per our IACUC protocol. We performed necropsies on the same day of euthanasia and collected organs, including skin, popliteal lymph node, axillary lymph node, mesenteric lymph node, spleen, lung, heart, liver, and kidney. Samples from these organs were saved at −20°C for future PCR testing or placed in 10% formalin at room temperature for histological examination. For evaluation of rickettsiae in tick tissues, we collected salivary glands, midgut and ovaries (females) from A. maculatum on DPT 0, 6, and 12 and pooled tissues from three separate ticks by tissue type, then gently mixed tissues with PBS, and redistributed them into three separate tubes for (1) qPCR assay; (2) microscopy by combined fluorescence in situ hybridization and immunohistochemistry (FISH/IHC); (3) and transmission electron microscopy (TEM), Thus, each technique included representative tissue from all three ticks. Tick tissues for DNA extraction and subsequent qPCR were placed in tubes containing PBS and stored at −20°C until DNA extraction. Samples for FISH/IHC were placed in tubes containing 10% formalin; tissues for TEM were placed in Karnovsky’s fixative and were stored at 4°C until processing, described below. Additionally, all legs were removed from individual ticks and pooled as above. These were used for DNA extraction and qPCR to evaluate rickettsial infection in the hemolymph.
DNA extraction and rickettsial DNA quantification by PCR
Genomic DNA was extracted from tissues (tick salivary glands, midgut, female ovaries, legs, and rabbit tissues) using a DNeasy Blood and Tissue Kit (Qiagen Inc., Valencia CA) and stored at −20°C until qPCR testing. Negative (water) control samples were included with each set of tissues extracted. Additionally, we included both negative control extracts and non-template water controls in qPCR assays to test for presence of environmental contamination with rickettsial DNA or qPCR products.
We quantified the ratio of Rickettsia DNA relative to tick DNA in A. maculatum tissues using a TaqMan® multiplex qPCR assay as previously described (Lee et al. 2017). To test for rickettsial DNA in rabbit skin biopsies collected on DPT 12, approximately 5–15 mg of tissue was used for DNA extraction. For tissues harvested at necropsy, all tissues used for extraction were between 25–30 mg, with the exception of the spleen, which was 8–10 mg. Samples tested by qPCR included the skin biopsy sample collected on DPT 12, and axillary lymph node, skin and spleen collected at necropsy (DPT 33) as well as DNA extracts from whole blood samples taken on DPT 0, 6 and 12. The rickettsial qPCR assay using rabbit extracts was similar to that for tick extracts, with the replacement of a rabbit gene target (12S rRNA), and corresponding primers, probe and plasmid controls to determine relative quantities (Table 1). All samples were analyzed in duplicate. Non-template (water) controls were included in each run for quality control. We accepted qPCR data only when assay efficiencies were between 90% and 110% for all three targets and R-squared values for the standard curve were above or equal to 0.985; these data were then used for evaluating rickettsial levels in ticks and rabbits. We calculated ratios and used these values for statistical analyses.
Table 1.
Primers and probes used for rabbit host in TaqMan® Multiplex qPCR assay for quantification of rickettsial levels.
| Primer/Probe Name | Sequence (5’→ 3’) | Final Concentration (nM) |
|---|---|---|
| Rab12S-F | CTCTAAGAGCCAAAGGAGGATTT | 300 |
| Rab12S-R | T GTCACTTGAGGAGGGTGA | 300 |
| Rab 12S Cy5 | ATTGAACAAGGCCATGAAGCACGC | 200 |
Fluorescence in situ hybridization and immunohistochemistry (FISH/IHC) of tick tissues
Tick tissues were fixed in 10% neutral buffered formalin for 20–24 hours, then routinely processed, embedded in paraffin, and 5 μm sections placed onto charged slides. To distinguish R. parkeri GFPuv and “Candidatus R. andeanae” under microscopy, we optimized a protocol combining fluorescent in situ hybridization using a RNA probe (riboprobe) targeting “Candidatus R. andeanae” and fluorescent immunohistochemistry using an antibody against GFPuv. Immunohistochemistry was used to detect R. parkeri GFPuv due to loss of GFPuv expression during tissue processing. To generate the riboprobe, we used primers designed in the laboratory for amplifying the “Candidatus R. andeanae” region of 23S-5S intergenic spacer region including T7 promoter site, and based on sequences generated using previously published primers (Stothard et al. 1994). Our in situ 23S primers for amplifying the 300 bp riboprobe were as follows, Rick 23S_F 5’-CCATTAGAGCCGTGGAAGAC-3’ and Rick23S_R_T7 5’-TAATACGACTCACTATAGGGCCACCAAGCTAGCAATACAA-3’. The resultant amplicon shared 97% identity with R. parkeri Portsmouth (CP003341). To prepare the riboprobe, the “Candidatus R. andeanae” 23S amplicon was labeled with digoxigenin-UTP by in vitro transcription with T7 RNA polymerase (Roche Diagnostics Corporation, Indianapolis, IN). Sections were first deparaffinized and then steamed at 100°C for 20 minutes in 10 mM sodium citrate buffer (pH 6) to begin antigen retrieval. Sections were agitated in 0.3% Triton™ X-100 (Sigma, St. Louis, MO) at room temperature (RT) for 10 minutes and digested with 5 μg/mL of proteinase K at 37°C for 20 minutes. Post-fixation in 4% formaldehyde at 4°C for 10 minutes was followed by acetylation (0.25% acetic anhydride) using 0.1M triethanolamine at RT for 10 minutes, and subsequent denaturation in 70% formamide at 70°C for 10 minutes. The sections were hybridized with a digoxigenin-labeled 23S ribosomal RNA probe specific for “Candidatus R. andeanae” at 43°C overnight. The next day, the sections were blocked with bovine serum albumin (BSA) diluted to 5% (Blocker™ BSA (10%) in PBS, Thermo Scientific, Rockford, IL) at RT for 20 minutes. Sections were then incubated with sheep anti-digoxigenin primary antibody (1: 800, Roche Diagnostics, Indianapolis, IN) and mouse anti-GFPuv primary antibody (1: 500, R&D systems, Minneapolis, MN) in PBS with 5% BSA at RT for 60 minutes. For the secondary antibody, slides were incubated with donkey anti-sheep IgG antibody, Cy 3 conjugate (1: 500; EMD Millipore Corporation, Temecula, CA) in PBS with 5% BSA at RT for 60 minutes and then subsequently incubated with goat anti-mouse IgG antibody, Cy2 conjugate (1: 250; Abcam Inc., Cambridge, MA) in PBS with 5% BSA at RT for 60 minutes. The sections were covered with mounting media (KPL Inc., Gaithersburg, MD) and observed using an Olympus BX60 fluorescence microscope (Olympus Corporation of the Americas, Center Valley, PA) and then a ZEISS LSM 510 confocal laser scanning microscope for selected slides (Zeiss Light Microscopy, Gottingen, Germany). Uninfected Vero cells and Vero cells infected with R. parkeri and “Candidatus R. andeanae” were used as negative and positive controls, respectively. Cell cultures of rickettsiae were first pelleted and resuspended in molten agarose, then plugs placed in separate histological cassettes, paraffin embedded, and processed alongside tick tissue slides.
Transmission electron microscopy (TEM) of tick tissues
Tick tissue samples (salivary gland, midgut, and female ovaries) from each group of A. maculatum were fixed in Karnovsky’s fixative (5% glutaraldehyde and 4% paraformaldehyde) containing 1% DMSO in 0.1 M cacodylate buffer at pH 7.2. Tick tissues were rinsed 4 times with 0.1 M cacodylate buffer at pH 7.2 for 15 minutes on ice and then were post-fixed in 2% osmium tetroxide in 0.1 M cacodylate buffer at pH 7.2 for 2 hours. After rinsing with distilled water, tick tissues were partially dehydrated in a graded ethanol series and then stained at the same time (en bloc) with 2% uranyl acetate in 70% ethanol overnight. The next morning, tissues were further dehydrated with ethanol, embedded in Spurr’s resin (Electron Microscopy Science, Hatfield, PA), and the resin blocks polymerized overnight at 68–70°C. Sections (60–80 nm) were cut from the block using a Reichert-Jung Ultracut E Ultramicrotome (Vienna, Austria) and placed on formvar coated copper grids. Tissue sections were stained with alcoholic uranyl acetate followed by lead citrate. Sections were observed on a JEOL JEM-1230 Transmission Electron Microscope (JEOL USA, Peabody, MA) at 80 kV. Cultured R. parkeri and “Candidatus R. andeanae” were suspended in 1% of agarose as positive controls and processed as tissue samples. Tick tissues confirmed negative for rickettsiae by qPCR were used for negative tissue controls.
Isolation of rickettsiae from skin by co-culture
For rickettsial isolation from rabbit skin, a full thickness 6 mm diameter skin (biopsy punch, Miltex Inc., York, PA, USA) was collected within the tick chamber of each rabbit on DPT 12, briefly cleaned with 70% ethanol, rinsed with autoclaved distilled water and then dried briefly. The sample was divided into portions for DNA extraction, histology, and cell culture; cell culture portions were frozen at −80°C until processing. For processing, a small amount of skin (~50 mg) was triturated into small pieces in fresh MEM (pH 7.3; Sigma-Aldrich, St. Louis, MO), supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA), 100 U/mL of penicillin, 100 μg/mL of streptomycin (Pen Strep, Gibco by Life Technologies, St. Louise, MO), and 2.5 μg/mL of amphotericin (Sigma-Aldrich, St. Louis, MO). Suspensions were inoculated into a 8090% confluent flask of Vero cells and maintained for 2 months, with media replaced twice weekly. Small areas of cultured Vero cells were harvested using a cell scraper (BD Biosciences, Bedford, MA) for DNA extraction and rickettsial PCR using species-specific primers targeting the rickettsial outer membrane protein A (rompA) gene for R. parkeri and “Candidatus R. andeanae” (Paddock et al. 2010; Varela-Stokes et al. 2011). Amplicons were visualized in 2% agarose gels stained with SYBR® Safe DNA gel stain (Invitrogen, Carlsbad, CA, 92008), and examined under ultraviolet light. We used microscopy of stained cytospins to monitor cultures periodically for evidence of rickettsiae. Cytospins were stained with acridine orange (BD, Maryland, US) or Diff-Quick (Dade Behring, Delaware, US) and examined under fluorescence and light microscopy, respectively.
Serological testing using indirect fluorescent antibody assay (IFA)
Plasma was separated from an aliquot of heparinized blood collected on the nine sample days, and then stored at −80°C until serologic testing by indirect fluorescence antibody assay (IFA). For antigen slides, 12-well-slides were prepared using R. parkeri (Portsmouth) grown in Vero cells, and stored in −80°C until use. Rabbit plasma samples were screened at 1:32 and 1:64 dilutions as previously described (Moraru et al. 2013). For detection, we used FITC (fluorescein isothiocyanate)-conjugated goat anti-rabbit IgG (KPL Inc., Gaithersburg, MD). Slides were counterstained with Eriochrome™ Black T (Fisher Scientific, Waltham, MA, USA), mounted using Vectashield (Vector Laboratories, Inc., Burlingame, CA, USA), and then observed with a fluorescence microscope. For quality control, positive and negative sera from known exposed and unexposed cattle were applied at 1:32 or 1:64 dilutions to separate control wells on each slide; the secondary antibody was FITC-conjugated goat anti-bovine IgG (KPL Inc., Gaithersburg, MD). Samples were considered seropositive if they demonstrated reactivity at the 1:64 dilution as well as 1:32 dilution; these samples were then serially diluted two-fold to determine end-point titers.
Statistical Analyses
Because of the large number of samples negative for rickettsiae by qPCR, and therefore having ratios of rickettsial to tick DNA (“rickettsial levels”) equal to “0”, data were not normally distributed. Thus, to determine the effect of experimental group, sample day, tissues, and sex on rickettsial levels, we dichotomized the rickettsial levels so that samples were considered either positive or negative for rickettsia, and then used mixed model logistic regression using PROC GLIMMIX in SAS for Windows 9.4 (SAS Institute, Inc., Cary, NC, USA). Separate models were fit for “Candidatus R. andeanae” DNA ratio and R. parkeri DNA ratio. The explanatory variables were selected for a multivariable model by manual forward selection starting with experimental groups. The interaction terms of the main effects were tested and removed, if not significant. The random effects included in the models were day (rabbit trial), rabbit (trial), and trial. When analyzing serological responses, the data were not normally distributed due to the large numbers of seronegative samples. Thus, the effects of experimental group and sample day on IFA titers, dichotomized to positive and negative, also were assessed by mixed model logistic regression. Rabbit (trial) and trial were included as random effects. The residual option was used in a random statement with a first order autoregressive covariance structure to account for the repeated measures of rabbits. We reported results as odds ratios. An alpha level of 0.05 was used to determine statistical significance.
Results
While initially screening A. maculatum from the TAMU source, which had been previously negative for both rickettsial species, we detected rickettsiae at infection rates of approximately 5–10%. Consequently, subsets of A. maculatum from OSU and TAMU that were infected with “Candidatus R. andeanae” were preferentially selected for the “Candidatus R. andeanae”-infected group or the “Candidatus R. andeanae and R. parkeri co-infected group. The control group received A. maculatum from the TAMU source. Unfortunately, despite our selection of a previously negative source of ticks, we did not have a group that was Rickettsia-free in this study.
Rickettsial levels in tick tissues
In salivary gland and midgut tissue extracts from A. maculatum capillary-fed R. parkeri alone (R. parkeri singly-infected group), the average ratio (AR) increased minimally from DPT 0 to DPT 12 (Table 2). The AR for R. parkeri in tissue extracts from A. maculatum capillary-fed both rickettsial species also increased by DPT 12. While the AR of R parkeri demonstrated an increasing trend from DPT 0 to DPT 12, statistical analysis using logistic regression showed that when comparing presence or absence of R. parkeri between DPT 0, 6, and 12, differences were not significant. Overall, the odds of detecting R. parkeri in the midgut tissue was 2.7 times greater than in the salivary gland (P<0.0001, CL=1.9 and 3.9), irrespective of groups, likely reflecting acquisition of R. parkeri from capillary feeding. Similarly, we observed an increase in the AR for “Candidatus R. andeanae” in salivary glands and midguts during the feeding period for A. maculatum capillary-fed “Candidatus R. andeanae” alone or in combination with R. parkeri (Table 3). Using logistic regression based on positive and negative tissue frequency, there was no significant association in detection frequency (presence or absence of rickettsiae) among the salivary gland, midgut and leg extracts. Overall, there was a significant association for detection frequency among experimental groups (p<0.0001) and days tested (p=0.0014).
Table 2.
Ratio of R. parkeri copy number to tick MIF copy number in salivary gland, midgut and leg (hemolymph).
| DPTc 0 | DPT 6 | DPT 12 | ||
|---|---|---|---|---|
| Control | Salivary gland | 0.003±0.002a (30)b |
0.078±0.047 (50) |
0.400±0.400 (41) |
| (media only) | Midgut | 0.014 ± 0.004 (30) |
0.027±0.014 (50) |
0.054±0.053 (41) |
| Leg (hemolymph) | 0.003±0.003 (30) |
0 (50) |
0.001±0.000 (41) |
|
| “Candidatus R. andeanae” | Salivary gland | 0 (30) |
0 (53) |
0 (47) |
| (singly-infected) | Midgut | 0.001±0.001 (30) |
0.012±0.008 (53) |
0.006±0.004 (47) |
| Leg (hemolymph) | 0 (30) |
0 (53) |
0.001±0.001 (47) |
|
| R. parkeri | Salivary gland | 0.003±0.002 (30) |
0.146±0.067 (46) |
0.351±0.218 (39) |
| (singly-infected) | Midgut | 0.005±0.002 (30) |
0.140±0.066 (46) |
1.076±0.630 (39) |
| Leg (hemolymph) | 0.002±0.001 (30) |
0.001±0.001 (46) |
0.092±0.080 (39) |
|
| “Candidatus R. andeanae” | Salivary gland | 0 (30) |
1.084±0.509 (50) |
83.320±43.761 (43) |
| and R. parkeri | Midgut | 0.125±0.053 (30) |
3.699±1.820 (50) |
35.621±13.490 (43) |
| (co-infection) | Leg (hemolymph) | 0.001±0.001 (30) |
1.543±1.083 (50) |
69.582±42.487 (43) |
Average ± Standard Error
Numbers of specimen
DPT: Day post tick-placement on rabbits
Table 3.
Ratio of “Candidatus R. andeanae” copy number to tick MIF copy number in salivary gland, midgut and leg (hemolymph).
| DPTc 0 | DPT 6 | DPT 12 | ||
|---|---|---|---|---|
| Control | Salivary gland | 0.007±0.003a (30)b |
0.153±0.072 (50) |
0.099±0.076 (41) |
| (media only) | Midgut | 0.012±0.009 (30) |
0.068±0.039 (50) |
0.155±0.125 (41) |
| Leg (hemolymph) | 0.024±0.011 (30) |
0.004±0.002 (50) |
0.004±0.003 (41) |
|
| “Candidatus R. andeanae” | Salivary gland | 0.903±0.193 (30) |
20.544±7.051 (53) |
23.636±7.393 (47) |
| (singly-infected) | Midgut | 2.403±0.605 (30) |
17.310±6.193 (53) |
22.851±9.147 (47) |
| Leg (hemolymph) | 7.728±1.491 (30) |
3.338±0.428 (53) |
7.203±1.532 (47) |
|
| R. parkeri | Salivary gland | 0.001±0.000 (30) |
0.927±0.743 (46) |
0.102±0.071 (39) |
| (singly-infected) | Midgut | 0.061±0.028 (30) |
0.010±0.009 (46) |
1.639±1.103 (39) |
| Leg (hemolymph) | 0.078±0.042 (30) |
0.010±0.005 (46) |
0.041±0.040 (39) |
|
| “Candidatus R. andeanae” | Salivary gland | 0.582±0.180 (30) |
4.643±1.473 (50) |
24.102±11.928 (43) |
| and R. parkeri | Midgut | 2.863±0.966 (30) |
11.180±6.753 (50) |
10.385±3.894 (43) |
| (co-infection) | Leg (hemolymph) | 14.247±4.213 (30) |
4.899±1.459 (50) |
4.709±0.999 (43) |
Average ± Standard Error
Number of samples
DPT: Day post tick-placement on rabbits
The AR for R. parkeri in extracts of female A. maculatum ovaries remained low during the three sample days for the group fed R. parkeri alone; transovarial transmission was not detected (Table 4A). In comparison, the AR for R. parkeri increased during feeding in the co-infected group. Surprisingly, one larval pool from an egg clutch produced by a single replete female in the “Candidatus R. andeanae”-fed group and one from a female in the co-infected group were positive for R. parkeri by qPCR, confirmed by PCR testing using the rompA gene target. A second larval pool from the same replete females in the “Candidatus R. andeanae”-fed and co-infected groups was determined to be negative and the other was positive, respectively, for R. parkeri. Both larval pools from both treatment groups were also positive for “Candidatus R. andeanae” by PCR, demonstrating presence of both rickettsiae in larvae from, at least, one female in the co-infected group. The odds of detecting R. parkeri in ovary extracts from the co-infected group was 2.9 times greater than for detecting it in the R. parkeri capillary-fed group; this was not statistically significant (P=0.1501, CL 0.680 and 12.094). The AR for “Candidatus R. andeanae” in ovary extracts from both A. maculatum capillary-fed “Candidatus R. andeanae” only and in the co-infected group increased similarly from DPT 0 to 12. Transovarial transmission was detected in approximately 69% (18/26) and 59% (13/22) of egg pools from the “Candidatus R. andeanae”-fed and co-infected group, respectively (Table 4B). In the uninfected control and R. parkeri capillary-fed groups, we detected a relatively low AR for “Candidatus R. andeanae.” Additionally, transovarial transmission of “Candidatus R. andeanae” was consistently detected at low levels in egg and larval pools from these two groups, demonstrating presence of natural infection in the tick population used in these studies.
Table 4.
Ratio of R. parkeri (A) and “Candidatus R. andeanae” (B) copy number to tick MIF copy number in ovary extracts.
| A. Rickettsia parkeri | |||||
| DPTd 0 | DPT 6 | DPT 12 | Eggs | Larvae | |
| Control | 0 (15)b |
0.001±0.001a (25) |
0 (17) |
0/26c | 0/20 |
| “Candidatus R. andeanae” | 0 (15) |
0 (27) |
0 (23) |
0/26 | 1(0)/22+ |
| R. parkeri | 0.009±0.009 (15) |
0.114±0.079 (20) |
0.173±0.164 (14) |
0/16 | 0/10 |
| Co-infected group | 0.002±0.001 (15) |
0.217±0.130 (24) |
5.950±2.996 (21) |
0/22 | 1(1)/15+ |
| B. “Candidatus Rickettsia andeanae” | |||||
| DPT 0 | DPT 6 | DPTd 12 | Eggs | Larvae | |
| Control | 0.012±0.012a (15)b |
0.080±0.064 (25) |
0.048±0.048 (17) |
5/26c | 2/20 |
| “Candidatus R. andeanae” | 1.563±0.504 (15) |
1.886±0.694 (27) |
3.996±1.158 (23) |
18/26 | 14/22 |
| R. parkeri | 0.032±0.016 (15) |
0.186±0.115 (20) |
0.531±0.445 (14) |
2/16 | 2/10 |
| Co-infected group | 0.333±0.139 (15) |
2.711 ±2.031 (24) |
2.536±0.913 (21) |
13/22 | 9/15 |
Average ± Standard error
Numbers of specimen
Numbers of positive masses/Numbers of egg or larvae masses tested
DPT: Day post tick-placement on the rabbits
Subsets were positive by qPCR and then retested to confirm (results in parentheses).
Microscopic evidence of rickettsiae using FISH/IHC and TEM
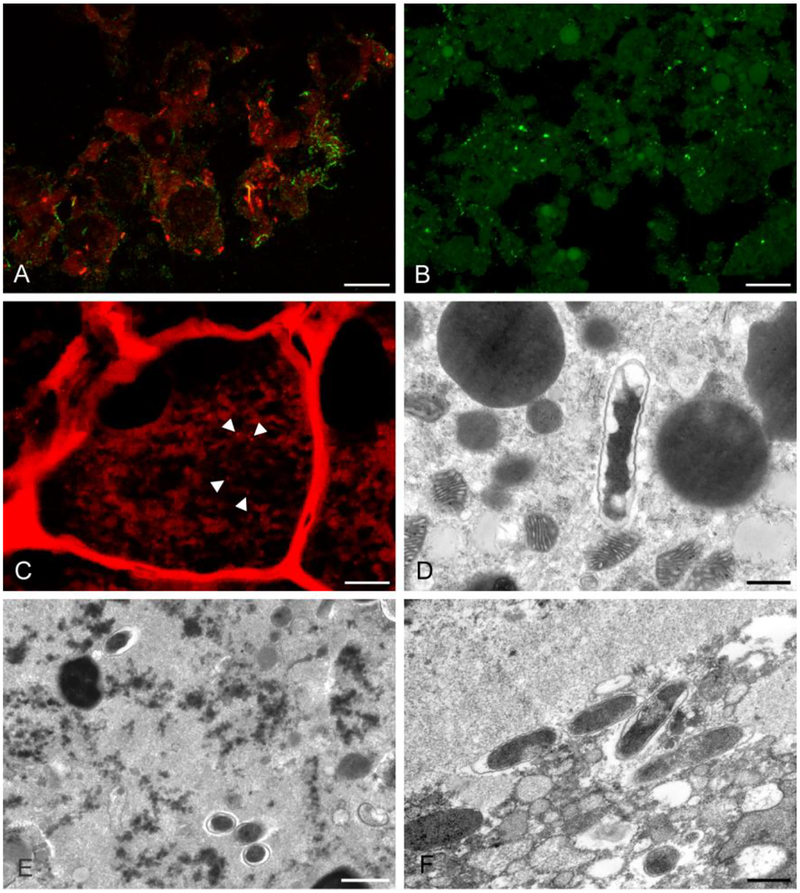
In order to confirm our ability to differentiate rickettsiae using the combined FISH/IHC technique, and to confirm the presence of rickettsiae in those tissues where high rickettsial ratios were detected, we selected A. maculatum tissues based on qPCR results. Specifically, the AR range for rickettsial levels in selected tissues was between 10 and 300. Using FISH/IHC, we were able to detect fluorescent signal consistent with R. parkeri and “Candidatus R. andeanae” (Fig. 1A) in slides containing sections from Vero cell cultures infected with either rickettsial species; we did not see any representative signal in uninfected Vero cells (control). We detected rod-shaped organisms, approximately 1×0.5 μm, consistent with R. parkeri in salivary gland tissue in the co-infected group on DPT 12 (Fig. 1B). In addition, signal consistent with “Candidatus R. andeanae” was detected in developing eggs in the ovarian tissue from the co-infected group on DPT 12 (Fig. 1C). Similarly, R. parkeri signal was detected in the tissues of salivary and midgut in the R. parkeri and co-infected groups. Similar rod-shaped organisms analogous to “Candidatus R. andeanae” were confirmed in tissues including salivary gland, midgut and ovary from the “Candidatus R. andeanae” and coinfected groups. However, the two different organisms, R. parkeri and “Candidatus R andeanae”, were not observed simultaneously in one tick tissue in the co-infected experimental group.
Fig. 1.
Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) [A-C] and transmission electron microscopy (TEM) [D-F] revealed rickettsial organisms in the experimentally infected tick tissue. (A) R. parkeri (green) and “Candidatus R. andeanae” (orange) were detected in Vero cells (FISH and IHC, scale bar = 10 μm). (B) R. parkeri (green) were detected in salivary gland in the co-infected group on DPT 12 (IHC, scale bar = 10 μm). (C) “Candidatus R. andeanae” (orange) were detected in developing eggs in an ovary from the co-infected group on DPT 12 (FISH, scale bar = 10 μm). (D) A single rickettsial organism in a developing egg in the ovary of a “Candidatus R. andeanae” singly-infected tick from DPT 12 (TEM, scale bar = 600 nm). (E) Rickettsial organisms in midgut tissue from a tick in the co-infected group sampled on DPT 12 (TEM, scale bar = 1 μm) (F) Multiple rickettsial organisms in salivary gland tissue from a tick in the co-infected group sampled on DPT 12 (TEM, scale bar = 600 nm).
Tissue samples including salivary gland, midgut and ovary were also selected for transmission electron microscopy (TEM) in the same manner as for FISH/IHC visualization. TEM could reveal approximately 1×0.5 μm rod-shaped electron-dense bacteria with halo zone and trilaminar cell wall consistent with Rickettsia spp. (Silverman et al. 1978; Hayes et al. 1979). Tissues including salivary gland, midgut, and ovary from both singly-infected and co-infected groups contained evidence of rickettsial organisms by TEM. A single rickettsial organism was observed in a developing egg in ovarian tissue from the “Candidatus R. andeanae”-infected group on DPT 12 (Fig. 1D). We also observed clusters of rickettsial organisms in midgut tissues (Fig. 1E) and in salivary glands from the co-infected group on DPT 12 (Fig. 1F).
Rickettsial transmission to rabbits
All rabbits from the three trials were seronegative on DPT 0. Some rabbits from treatment groups seroconverted (titer 64) on DPT 6, and most were seropositive, by DPT 12, with end-point titers that decreased by the end of the study. The geometric mean titers of rabbits in the three trials for the control group, “Candidatus R. andeanae” group, R. parkeri group, and co-infected group on DPT 12 were 117, 59, 128 and 30, respectively, demonstrating exposure to Rickettsia-infected A. maculatum in the control as well as treatment groups. The odds of being positive among experimental groups were not statistically significant by logistic regression (P=0.8648).
Discussion
Using laboratory-reared A. maculatum that were capillary-fed uninfected cell culture, cultured R. parkeri, cultured “Candidatus R. andeanae”, or a mixture of both Rickettsia spp., we identified some trends in rickettsial levels during rabbit feeding and transmission of rickettsiae. Both R. parkeri and “Candidatus R. andeanae” DNA levels (based on ratios of rickettsial to tick DNA; AR) demonstrated an increasing trend from DPT 0, when ticks were subsampled a week after capillary feeding, to DPT 6 and 12, during which time they fed on rabbit hosts. While R. parkeri levels in ticks that were capillary-fed R. parkeri alone were generally low, levels of R. parkeri in co-infected ticks exposed to both rickettsial species were higher in all three tissues tested. We observed mostly consistent trends in the variation of rickettsial levels both in ticks from rabbits within an experimental group from a single trial and among the three trials that we conducted.
The TAMU laboratory-reared A. maculatum colony that was previously determined to be Rickettsia-negative by PCR and thus selected for these studies, was found to have low levels of rickettsiae and low infection rates. The ticks selected for capillary feeding in the co-infected group were from OSU, where colonies were known to be naturally infected with “Candidatus R. andeanae” at high infection rates. After capillary-feeding and prior to host feeding, we observed a mortality rate of approximately 30% in A. maculatum (TAMU source) that were capillary-fed R. parkeri, in comparison to approximately 10% in the control group. As we did not calculate mortality rates for all groups, it is unclear whether the introduction of cultured R. parkeri was directly detrimental to tick fitness. While there may be speculation that R. parkeri has some degree of lethal effect in A. maculatum, we used a modified strain (R. parkeri Oktibbeha GFPuv) and detected low levels of R. parkeri by qPCR in this treatment group. We did not test dead ticks to determine infection status. The two sources of laboratory-reared A. maculatum populations may also naturally differ in fitness.
While we detected a high Rickettsia level for R. parkeri on DPT 12 in tissues from the co-infected group, levels of “Candidatus R. andeanae” in that group were lower and similar to levels in the group capillary-fed only “Candidatus R. andeanae”. Notably, ticks in both the “Candidatus R. andeanae” and co-infected groups were preferentially selected from OSU in order to ensure presence of “Candidatus R. andeanae”. Thus, the “Candidatus R. andeanae” levels we detected may be more representative of natural infection than the levels of R. parkeri detected in ticks that were capillary-fed higher amounts. However, this would not account for the higher levels of R. parkeri in the co-infected group compared to the singly-infected group, and may suggest an interaction to be explored further. Still, the odds of detecting R. parkeri were not statistically different between the R. parkeri singly-infected group and the co-infected group. Interestingly, the odds of detecting “Candidatus R. andeanae” in the “Candidatus R. andeanae” singly-infected group was nearly two times greater than in the co-infected group. Nonetheless, “Candidatus R. andeanae” was detectable in both groups.
We observed transovarial transmission of “Candidatus R. andeanae” in groups capillary-fed this organism alone or mixed with R. parkeri. In contrast, rare transovarial transmission was detected with R. parkeri. Natural infection with “Candidatus R. andeanae” potentially enabled this observation. These findings may have also been affected by the modified strain of R. parkeri we used or by the reliance on capillary feeding R. parkeri one week prior, which may not have been sufficient to infect ovaries. However, we are not aware of published studies that demonstrate a difference in transformed and wild type R. parkeri (Oktibbeha). Unfortunately, the female carcasses were not saved for DNA extraction and testing. There is a possibility that the introduction of R. parkeri in the co-infected group was in the presence of “Candidatus R. andeanae”, rather than naïve female ticks exposed simultaneously to both rickettsial species. Considering transovarial transmission of R. parkeri has been reported in A. americanum and A. triste (Nieri-Bastos et al. 2013; Goddard 2003), and was expected to occur here, the initial presence of “Candidatus R. andeanae” in some ticks, late introduction of R. parkeri via capillary feeding adults, and the Amblyomma species may all have contributed to the different findings in this study. The late introduction of R. parkeri in the presence of “Candidatus R. andeanae” may be comparable to a co-feeding event. Presence of Rickettsia amblyommii in 11 Amblyomma americanum, compared to 11 uninfected A. americanum, was associated with a lower likelihood of R. parkeri acquisition during co-feeding with R. parkeri-infected A. maculatum (Wright et al. 2015a) and some evidence of exclusion occurred for “Candidatus R. andeanae”-infected nymphs co-fed with R. parkeri-infected adult A. maculatum (Lee et al., 2018). Levin et al., also noted slightly lower frequency, though not statistically significant, of transovarial transmission of R. rickettsii for A. americanum in ticks already infected with R. amblyommatis (2018). Further, the use of R. parkeri (Oktibbeha), genetically modified to express GFPuv, while important as a tool for visualizing organism microscopically, may have affected acquisition by and transovarial transmission in the tick, as well as transmissibility of R. parkeri to the host. Other Rickettsia spp. that lack plasmids have been similarly transformed with no apparent effect on viability, though it is unclear whether the same holds for R. parkeri (Hauptmann et al. 2017).
Organisms consistent with rickettsiae in treatment groups for “Candidatus R. andeanae” and R. parkeri were visualized by the FISH/IHC, providing additional evidence of rickettsial presence in tick tissues that demonstrated high rickettsial copy numbers. Numerous organisms corresponding to R. parkeri were detected in the salivary gland and midgut, however they were not observed within developing eggs in the ovaries. This was consistent with rare transovarial transmission of R. parkeri that we observed. We rarely detected “Candidatus R. andeanae” in the salivary gland and midgut by microscopy, but “Candidatus R. andeanae” was frequently observed within the developing eggs in ovaries. The ultrastructure of SFGR is similar among species and makes differentiation by TEM not possible. TEM was included here to confirm presence of organisms with characteristics expected for SFGR, including electron dense rod to elliptical shaped 1 μm long bacteria that had an electron-lucent halo zone (“slime layer”) and adjacent trilaminar cell wall (Silverman et al. 1978; Hayes et al. 1979). Clusters of Rickettsia-like organisms were visualized in the developing eggs, midguts, and salivary glands; SFGR characteristics of these organisms were sufficient to distinguish these from other bacteria possibly present in the ticks (Niebylski et al. 1997). While other prokaryotes may have been detected in A. maculatum using TEM, we limited our search to organisms likely to be SFGR.
We were unable to cultivate rickettsiae from the samples of skin biopsied on DPT 12, with negative culture results based on lack of detectable rickettsial DNA by PCR and lack of detectable organism in culture cytospins. DNA extracts from blood samples on DPT 0 and 6 and rabbit tissues, including axillary lymph node, skin and spleen, on DPT 33 were also negative by qPCR for rickettsial DNA. Despite the absence of detectable rickettsiae in sampled tissues, exposure to rickettsiae was documented in all treatment groups by serology; specifically, antibodies reactive to the R. parkeri antigen. Once rabbits seroconverted, most were seropositive throughout the study. Based on these findings, it was suspected that rickettsial transmission could occur whether ticks were capillary-fed cultured organisms, naturally infected, or both. Detection of “Candidatus R. andeanae” in the salivary glands of the “Candidatus R. andeanae” singly-infected group and seroconversion (to R. parkeri antigen) in that group suggests the potential for transmission via tick feeding. As no rickettsiae were detected in rabbit tissues tested approximately one month after tick infestation or from skin biopsies taken 12 days after infestation, we could not determine whether transmitted rickettsiae were viable, or whether infection, in addition to exposure, occurred for either “Candidatus R. andeanae” or R. parkeri. Histopathological examination of rabbit tissue was unremarkable. Rickettsial DNA from rabbit tissues was not detected in any experimental group.
In this study, specific tissue tropism was not observed for “Candidatus R. andeanae”, whether ticks were singly-infected or co-infected with R. parkeri, and the presence of natural infection in some ticks used likely confounded this. However, the odds of detecting R. parkeri in the midgut tissue, irrespective of experimental group, was nearly 2.7 times greater than in salivary gland. This was likely due to levels of R. parkeri present initially during capillary feeding. Exposure of adult ticks via capillary feeding may also not produce the same tissue tropism as natural acquisition. In this study, we found that both R. parkeri and “Candidatus R. andeanae” were detectable in salivary glands one week after capillary-feeding (DPT 0). However, the lower AR of R. parkeri in comparison to “Candidatus R. andeanae” in salivary glands suggests that naturally infected ticks begin with a higher level of rickettsiae. High levels of R. parkeri were detected in the co-infected group, though evidence of vertical transmission of R. parkeri was rare. Our sample size was small here, but results differ substantially from the transovarial transmission rates of R. parkeri in A. maculatum observed by Wright et al., where the efficiency ranged from approximately 67% to 100% over three generations of ticks that were also fed on New Zealand white rabbits (2015b). “Candidatus R. andeanae”, a SFGR that is presumed non-pathogenic, was commonly detected in tick tissues and vertically transmitted. Transovarial interference is well-documented for R. rickettsii and other Rickettsia spp., with the presence of one Rickettsia sp. blocking vertical transmission of another (for example, Burgdorfer 1988, Macaluso et al., 2002). Evidence of potential transovarial interference is also suggested by disparate infection rates of different Rickettsia spp. in tick populations (Burgdorfer et al., 1981, Paddock et al., 2015). Whether interference occurred here is unclear, as the number of replete females was low and not all offspring were tested, however “Candidatus R. andeanae” was also detected in co-infected ticks. While there were limitations to this study, establishing transmission dynamics for the two A. maculatum- associated SFGR at early time points in feeding may better define effects between rickettsiae that share a tick species, when simultaneously present in the tick.
Acknowledgments
Funding for this work was provided from the National Institutes of Health (NIH) 1R15A 1099928–01A1, which supported design of the study and collection, analysis, and interpretation of data and in writing the manuscript. The A.V.S. laboratory was also supported in part by NIH COBRE P20GM103646 during this study. We thank all staff of the LARAC, especially Ms. Jamie Walker for critical care, all animal assistance, and support for this project. We appreciate laboratory assistance from Katie Graham and Jacob Hughes.
References
- Banajee KH, Embers ME, Langohr IM, Doyle LA, Hasenkampf NR, Macaluso KR (2015) Amblyomma maculatum feeding augments Rickettsia parkeri infection in a rhesus macaque model: A pilot study. PLoS One 10:e0135175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PJ, Jiang J, Schoeler GB, Moron C, Anaya E, Cespedes M, Cruz C, Felices V, Guevara C, Mendoza L, Vilaseca P, Sumner JW, Richards AL, Olson JG (2004) Characterization of spotted fever group rickettsiae in flea and tick specimens from northern Peru. J Clin Microbiol 42:4961–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Hayes SF, Mavros AJ (1981) Nonpathogenic rickettsiae in Dermacentor andersoni : a limiting factor for the distribution of Rickettsia rickettsii, pp. 585–594. In Burgdorfer W Anacker RL [eds.], Rickettsiae and rickettsial diseases. Academic Press, Inc., New York. [Google Scholar]
- Burgdorfer W (1988) Ecological and epidemiological considerations of Rocky Mountain spotted fever and scrub typhus, pp. 33–50. In Walker DH [ed.], Biology of rickettsial diseases, vol. I CRC, Inc., Boca Raton, FL. [Google Scholar]
- Burkhardt NY, Baldridge GD, Williamson PC, Billingsley PM, Heu CC, Felsheim RF, Kurtti TJ, Munderloh UG (2011) Development of shuttle vectors for transformation of diverse Rickettsia species. PLoS One 6:e29511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragun WC, Bartlett BL, Ellis MW, Hoover ZZ, Tyring SK, Mendoza N, Vento TJ, Nicholson WL, Eremeeva ME, Olano JP, Rapini RP, Paddock CD (2010) The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol 146:641–648. [DOI] [PubMed] [Google Scholar]
- Ekenna O, Paddock CD, Goddard J (2014) Gulf coast tick rash illness in Mississippi caused by Rickettsia parkeri. J Miss State Med Assoc 55:216–219. [PubMed] [Google Scholar]
- Embers ME, Grasperge BJ, Jacobs MB, Phillipp MT (2013) Feeding of ticks on animals for transmission and xenodiagnosis in Lyme disease research. J Vis Exp 78:e50617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari FAG, Goddard J, Paddock CD, Varela-Stokes AS (2012) Rickettsia parkeri and “Candidatus Rickettsia andeanae” in Gulf Coast ticks, Mississippi, USA. Emerg Infect Dis 18:1705–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari FAG, Goddard J, Moraru GM, Smith WE, Varela-Stokes AS (2013) Isolation of “Candidatus Rickettsia andeanae” (Rickettsiales: Rickettsiaceae) in embryonic cells of naturally infected Amblyomma maculatum (Ixodida: Ixodidae). J Med Entomol 50:1118–1125. [DOI] [PubMed] [Google Scholar]
- Flores-Mendoza C, Florin D, Felices V, Pozo EJ, Graf PC, Burrus RG, Richards AL (2013) Detection of Rickettsia parkeri from within Piura, Peru, and the first reported presence of “Candidatus Rickettsia andeanae” in the tick Rhipicephalus sanguineus. Vector Borne Zoonotic Dis 13:505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J (2003) Experimental infection of lone star ticks, Amblyomma americanum (L.), with Rickettsia parkeri and exposure of guinea pigs to the agent. J Med Entomol 40:686–689. [DOI] [PubMed] [Google Scholar]
- Grasperge BJ, Reif KE, Morgan TD, Sunyakumthorn P, Bynog J, Paddock CD, Macaluso KR (2012) Susceptibility of inbred mice to Rickettsia parkeri. Infect. Immun 80, 1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann M, Burkhardt N, Munderloh U, Kuehl S, Richardt U, Krasemann S, Hartmann K, Krech T, Fleischer B, Keller C, Osterloh A (2017). GFPuv-expressing recombinant Rickettsia typhi: a useful tool for the study of pathogenesis and CD8+ T cell immunology in R. typhi infection. Infect Immun 85:e00156–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SF, Burgdorfer W (1979) Ultrastructure of Rickettsia rhipicephali, a new member of the spotted fever group rickettsiae in tissues of the host vector Rhipicephalus sanguineus. J Bacteriol 137:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskas NM, Ledet JJ, Wong A, Muzny CA, Elopre L, Hughey L (2014) Rickettsia parkeri: eschar diagnosis. J Am Acad Dermatol 3:e87–89. [DOI] [PubMed] [Google Scholar]
- Kocan KM, Yoshioka J, Sonenshine DE, de la Fuente J, Ceraul SM, Blouin EF, Almazan C (2005) Capillary tube feeding system for studying tick-pathogen interactions of Dermacentor variabilis (Acari: Ixodidae) and Anaplasma marginale (Rickettsiales: Anaplasmataceae). J Med Entomol 42:864–874. [DOI] [PubMed] [Google Scholar]
- Lee JK, Moraru GM, Stokes J, Wills R, Mitchell E, Unz E, Moore-Henderson B, Harper AB, Varela-Stokes AS (2017) Rickettsia parkeri and “Candidatus Rickettsia andeanae” in questing Amblyomma maculatum (Gulf Coast tick) from Mississippi. J Med Entomol 54:476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Stokes JV, Moraru GM, Harper AB, Smith CL, Wills RW, Varela-Stokes AS (2018) Transmission of Amblyomma maculatum-associated Rickettsia spp. during cofeeding on cattle. Vector Borne Zoonotic Dis Oct 2018. Ahead of print 10.1089/vbz.2017.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, Schumacher LBM, Snellgrove A (2018) Effects of Rickettsia amblyommatis infection on the vector competence of Amblyomma americanum ticks for Rickettsia rickettsii. Vector Borne Zoonotic Dis Nov 2018. Ahead of print 10.1089/vbz.2018.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF (2001) Infection and transovarial transmission of Rickettsiae in Dermacentor variabilis ticks acquired by artificial feeding. Vector Borne Zoonotic Dis 1:45–53. [DOI] [PubMed] [Google Scholar]
- Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF (2002) Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second rickettsia. J Med Entomol 39:809–813. [DOI] [PubMed] [Google Scholar]
- Mays SE, Houston AE, Trout RTT (2016) Specifying pathogen associations of Amblyomma maculatum (Acari: Ixodidae) in Western Tennessee. J Med Entomol 53:435–440. [DOI] [PubMed] [Google Scholar]
- Moraru GM, Goddard J, Paddock CD, Varela-Stokes A, (2013) Experimental infection of cotton rats and bobwhite quail with Rickettsia parkeri. ParasitVectors 6:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers T, Lalani T, Dent M, Jiang J, Daly PL, Maguire JD, Richards AL (2013) Detecting Rickettsia parkeri infection from eschar swab specimens. Emerg Infect Dis 19:778–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadolny RM, Wright CL, Sonenshine DE, Hynes WL, Gaff HD (2014) Ticks and spotted fever group rickettsiae of southeastern Virginia. Ticks Tick Borne Dis 5:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebylski ML, Schrumpf ME, Burgdorfer W, Fischer ER, Gage KL, Schwan TG (1997) Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int J Syst Bacteriol 47:446–452. [DOI] [PubMed] [Google Scholar]
- Nieri-Bastos FA, Szabo MP, Pacheco RC, Soares JF, Soares HS, Moraes-Filho J, Dias RA, Labruna MB (2013) Comparative evaluation of infected and noninfected Amblyomma triste ticks with Rickettsia parkeri, the agent of an emerging rickettsiosis in the New World. BioMed Res Int 2013:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieri-Bastos FA, Lopes MG, Cancado PH, Rossa GA, Faccini JL, Gennari SM, Labruna MB (2014) “Candidatus Rickettsia andeanae”, a spotted fever group agent infecting Amblyomma parvum ticks in two Brazilian biomes. Mem Inst Oswaldo Cruz 109:259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Sumner JW, Corner JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SL, Tamminga CL, Ohl CA (2004) Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis 38:805–811. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME (2008) Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 47:1188–1196. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Fournier PE, Sumner JW, Goddard J, Elshenawy Y, Metcalfe MJ, Loftis AD, Varela-Stokes A (2010) Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl Environ Microbiol 76:2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Denison AM, Dryden MW, Noden BH, Lash RR, Abdelghani SS, Evans AE, Kelly AR, Hecht JA, Karpathy SE, Ganta RR, Little SE (2015) High prevalence of “Candidatus Rickettsia andeanae” and apparent exclusion of Rickettsia parkeri in adult Amblyomma maculatum (Acari: Ixodidae) from Kansas and Oklahoma. Ticks Tick Borne Dis 6:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Goddard J (2015) The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae). J Med Entomol 52:230–252. [DOI] [PubMed] [Google Scholar]
- Philip RN, Casper EA (1981) Serotypes of spotted fever group rickettsiae isolated from Dermacentor andersoni (Stiles) ticks in western Montana. Am J Trop Med Hyg 30:230–238. [DOI] [PubMed] [Google Scholar]
- Silverman DJ, Wisseman CL Jr., Waddell AD, Jones M (1978) External layers of Rickettsia prowazekii and Rickettsia rickettsii: occurrence of a slime layer. Infect Immun 22:233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard DR, Clark JB, Fuerst PA (1994) Ancestral divergence of Rickettsia bellii from the spotted fever and typhus groups of Rickettsia and antiquity of the genus Rickettsia. Int J Syst Bacteriol 44:798–804. [DOI] [PubMed] [Google Scholar]
- Straily A, Feldpausch A, Ulbrich C, Schell K, Casillas S, Zaki S, Denison AM, Condit ME, Gabel J, Paddock CD (2016) Rickettsia parkeri Rickettsiosis -Georgia, 2012–2014. Morb Mortal Wkly Rep 65:718–719. [DOI] [PubMed] [Google Scholar]
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Paddock CD (2007) Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis 13:751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Stokes AS, Paddock CD, Engber B, Toliver M (2011) Rickettsia parkeri in Amblyomma maculatum ticks, North Carolina, USA, 2009–2010. Emerg Infect Dis 17:2350–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Nadolny RM, Jiang J, Richards AL, Sonenshine DE, Gaff HD, Hynes WL (2011) Rickettsia parkeri in Gulf Coast Ticks, Southeastern Virginia, USA. Emerg Infect Dis 17:896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Sonenshine DE, Gaff HD, Hynes WL (2015a) Rickettsia parkeri transmission to Amblyomma americanum by cofeeding with Amblyomma maculatum (Acari: Ixodidae) and potential for spillover. J Med Entomol 52:1090–1095. [DOI] [PubMed] [Google Scholar]
- Wright CL, Gaff HD, Soneshine DE, Hynes WL (2015b) Experimental vertical transmission of Rickettsia parkeri in the Gulf Coast tick, Amblyomma maculatum. Ticks Tick Borne Dis 6:568–573. [DOI] [PubMed] [Google Scholar]