Abstract
A growing number of studies have examined associations of metal exposures with birth outcomes, however, results from these studies have been inconsistent, and hampered by methodological limitations. We measured direct fetal exposure to three metals (lead, manganese and zinc) during the second and third trimester and examined its association with birth weight and gestational age at delivery. Participants in the Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS), a population-based birth cohort established between September 2003 and December 2007, were invited to donate teeth to the study. Lead, manganese and zinc during the second and third trimesters were measured via high-resolution microspatial mapping of dentin growth rings, a validated biomarker for prenatal metal exposure. Gestational age at delivery and infant birth weight were obtained from the delivery medical record. A total of 145 children had tooth metal measurements and birth outcome data. Mean birth weight was 3431±472 g and mean gestational age at delivery was 39.0±1.3 weeks. Overall, there was a positive association between second (β=0.21, 95% CI: 0.05, 0.37, P=0.01) and third trimester (β=0.21, 95% CI: 0.05, 0.37, P=0.01) tooth manganese and birth weight Z-score; this remained statistically significant after covariate adjustment. There was also a negative association between second trimester tooth lead level and birth weight Z-score (β=−0.20, 95% CI: −0.38, −0.02, P=0.02), however, this was attenuated after adjusting for covariates. Mixture analysis revealed similar findings. There was evidence for a sex-specific effect of manganese with birth weight Z-score, with the association stronger in female compared to male infants. Overall, we found evidence suggesting that higher in utero manganese is associated with larger birth weight Z-scores and that these associations may vary by infant sex.
Keywords: Birth outcome, metals, birth weight, gestational age, teeth
1. Introduction
In contrast to other developed countries, rates of preterm birth and low birth weight are among the highest in the United States, at 9.6% and 8%, respectively, in 2015.1,2 As they undergo rapid and complex development, fetuses are at increased susceptibility to negative health effects of environmental exposures, including toxic metals.3-5 Prenatal exposure to toxic metals such as lead and atypical levels of essential metals such as manganese have been shown to be associated with adverse birth outcomes. In a large study in New York State, even low maternal blood lead levels were associated with decreased infant birth weight.6 Similarly, manganese is also associated with lower birth weight, however, this appears to be a non-linear relationship, with both low and high maternal manganese levels associated with lower birth weight.7,8 Less is known about the association of the essential element zinc with birth outcomes; in a systematic review, lower maternal blood zinc level was associated with lower birth weight, however, this was nearly entirely in populations where zinc deficiency is common, and the effects of excessive zinc levels could not be adequately studied.9
While studies have used surrogate measures of fetal exposures to metals (e.g. maternal blood sample or hair sample from pregnancy) or single measures of direct fetal exposure (cord blood), to our knowledge no study has examined if direct fetal exposure to metals in the second versus third trimester are associated with negative birth outcomes. We utilized elemental mapping of human primary teeth with laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) to examine an unbiased “map” of metal exposures (lead, manganese and zinc) during fetal development.10 This novel approach not only quantifies prenatal exposures, but also estimates the timing of the exposure (i.e. within trimester), as early as the 14th week of intrauterine development.10-13 The goal of this study was to examine if there are associations of direct fetal exposure to lead, manganese and zinc, by trimester (second or third), with birth outcomes, including birth weight and gestational age at delivery in the racially and socioeconomically diverse Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS).14-16
2. Methods
2.1. Study Population
WHEALS is a birth cohort study that recruited pregnant women with due dates from September 2003 through December 2007, who were seeing a Henry Ford Health System (HFHS) obstetrics practitioner at one of five clinics, were in their second trimester or later, were aged 21-49 years, and were living in a predefined geographic area in Wayne and Oakland counties that included the city of Detroit as well as the suburban areas immediately surrounding the city.14-16 WHEALS originally included 1,258 maternal-child pairs.
Details of the WHEALS Tooth Fairy Study have previously been reported.17 Briefly, advertisements for the WHEALS Tooth Fairy Study were placed in study newsletters, a save-the-tooth refrigerator magnet was sent in a holiday mailer to all participants, and families were asked if they wanted to donate a tooth for the study during planned recruitment phone calls for other WHEALS activities. A total of 373 teeth were received from 156 participants between December 2011 and January 2015. Teeth were selected for metal measurement if (1) the child had at least some outcome data available (birth outcomes and/or a 2-year clinic visit); and (2) the tooth was relatively intact. After excluding participants with missing data, the final sample sizes were 138 children with tooth metal measurements and birth weight data and 145 children with tooth metal measurements and gestational age at delivery data. All participants provided written, informed consent. Study protocols were approved by the HFHS Institutional Review Board.
2.2. Outcome Measurement
Prenatal and delivery records for WHEALS mothers were abstracted to obtain birth weight and gestational age at delivery. Sex- and gestational-age adjusted birth weight Z-scores were calculated using the US population as a reference.18 Low birth weight was defined as a birth weight <2,500 g and preterm delivery was defined as gestational age at delivery <37 weeks and are presented for descriptive purposes.
2.3. Covariate Measurement
Maternal race, household income, marital status, exposure to environmental tobacco smoke (ETS), smoking during pregnancy, year home was built, and exposure to indoor pets prenatally were self-reported. Year residence was built was dichotomized as 1980 or after or before 1980, to indicate risk of lead exposure due to lead-based paint.19 Address during pregnancy was recorded and used to define whether the fetus was mainly exposed to an urban residence (defined as within the confines of the city of Detroit) or a suburban residence. Prenatal and delivery records for WHEALS women were abstracted to obtain body mass index at the first prenatal care visit, prenatal hemoglobin levels, and delivery type. As done previously, prenatal maternal anemia was defined as the mother ever having a hemoglobin <10.5 g/dL during the WHEALS pregnancy.17
2.4. Analysis of metals in tooth samples
We directly measured metals in baby teeth using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) and assigned developmental times as detailed elsewhere.11,20 Teeth were sectioned and the neonatal line (a histological feature formed in enamel and dentine at the time of birth) and incremental markings were used to assign temporal information to sampling points. Second and third trimester metal levels were distinguished using previously validated methods that rely on incremental markings in teeth (akin to growth rings in trees).11,20 We used an ArF excimer laser ablation system (ESI, USA) attached to an Agilent Technologies 8800 triple-quad ICP-MS. Data were analysed as metal-to-calcium ratios (e.g. 208Pb:43Ca) to control for variations in mineral content within a tooth and between samples. Samples were analysed in two batches. Tooth attrition, which is the amount of tooth lost due to grinding or wear, was also measured; teeth with excessive attrition that would impede the chemical analysis are excluded. National Institute of Standards and Technology SRM 612 was used for calibration and quality control. The detection limit was 0.05 μg/g for lead, zinc and manganese. Across all time points and metals <5% are below the detection limit; values below the detection limit are excluded from statistical analysis.
A small number of children (N=17) had two teeth analyzed for quality control procedures; in these cases, metals levels were averaged over teeth within each child. We have previously shown moderate to high agreement in second and third trimester lead (intraclass correlation coefficient [ICC]=0.55 for second trimester and ICC=0.74 for third trimester) and manganese (ICC=0.84 for second trimester and IC=0.59 for third trimester, after removing one tooth pair with a manganese outlier) in children with two tooth measures in the study.17 There was also good to excellent agreement in zinc measures from children contributing two teeth to the analysis (second trimester ICC=0.82; third trimester ICC=0.72).
2.5. Statistical Analysis
Our primary outcomes of interest were the continuous variables birth weight Z-score and gestational age at delivery; normality assumptions were met. Each metal was centred and standardized within batch before statistical analysis. Differences in metal levels between the second and third trimester was examined with a paired t-test.
Linear regression modelling was used with birth weight Z-score and gestational age as separate outcomes and individual centred and standardized metal measures in the second and third trimesters as the exposures. Models were fit separately for the second and third trimester. All analyses were adjusted for batch, tooth attrition, and tooth type. Additional potential clinical and demographic confounders of birth weight Z-score and gestational age were incrementally added to the model if they were potentially associated with birth outcomes and/or metal levels, including race, maternal age, maternal anemia, urban residence, year the house was built and ETS.21,22 Because many of these variables could also be considered to be on the causal pathway (i.e. urban residence, maternal anemia, maternal age, year the house was built, ETS) for the main analysis we fit models without (Model 2) and with (Model 3) these variables. In order to compare whether the effect of a metal in the second and third trimester was the same, a contrast statement of effect estimates from the second trimester and third trimester was used. Interaction terms and stratified models were also fit to explore potential differences by race and sex.
We used weighted quantile sum (WQS) regression to study the mixture effects of metals on birth outcomes. WQS was developed23 to assess mixture effects in a single coherent model, thereby minimizing Type 1 errors, while also quantifying the contribution of individual “bad actors” among a multitude of correlated exposures. This is done by the calculation of a weighted index, WQS=Σ Wjqij, which integrates an empirically-estimated weight (w) for each exposure variable (j) with the ranked concentration of that exposure per subject (qij). The WQS index calculated for each subject thus captures the extent of their exposure to a chemical mixture, and the significance of this mixture with respect to health outcomes can be tested in the standard linear model Yi = α0 + β1WQS + α1Xi + εi, with a powerful single degree of freedom test of the significance of β1 measuring the “mixture effect” related to the health outcome Yi. The model intercept, α0, and parameters for covariates, α1Xi , and model error, εi, are interpreted as in standard linear models. In models where the mixture effect is significant, the identification of “bad actors” can be assessed by a comparison of the weights, w, estimated for each chemical, with heavily-weighted components contributing most strongly to the mixtures effect on the modeled health outcome. WQS regression is particularly robust against multicollinearity because of the constraints imposed in parameter estimation, in that all weights are constrained to an estimate between 0 and 1, and to sum to 1, thereby capturing a normalized effect estimate. The standardization of exposures to rankings (generally quartiles or deciles) further reduces the impact of extreme values and improves interpretability. WQS was fit constraining all parameters in the positive direction (i.e. to estimate effect on increased birth weight Z-score) or in the negative direction (i.e. to estimate the effect on decreased birth weight Z-score).
For all analyses, a P-value <0.05 was used to define statistical significance. Interaction P-value <0.1 was used to define statistical significance for interactions.24
3. Results
Among the 145 children included in analyses, mean birth weight was 3431±472 g and mean birth weight Z-score was 0.06±0.96, with 3 (2.1%) children born low birth weight. Mean gestational age at delivery was 39.0±1.3 weeks and 6 (4.1%) children were born preterm. Participant characteristics are provided in Table 1. Race was statistically significantly associated with birth weight Z-score, with African-American mothers having lower birth weight infants than white mothers (P=0.034). No other covariates were statistically significantly associated with birth weight Z-score or gestational age at birth.
Table 1.
Distribution of maternal and delivery characteristics and their univariable association with birth weight Z-score and gestational age at delivery
| Characteristic | N (%) or mean±std |
Birth weight Z-score | Gestational age at delivery (in weeks) |
|---|---|---|---|
| mean±std or r | mean±std or r | ||
| Age (years) | 31.0±5.1 | r=0.22 | r=−0.12 |
| Race | |||
| White | 54 (36.5%) | 0.32±1.06 | 38.9±1.6 |
| African-American | 65 (43.9%) | −0.07±0.89* | 39.0±1.3 |
| Other | 29 (19.6%) | −0.08±0.85 | 39.3±1.0 |
| Education | |||
| College degree or higher | 67 (45.3%) | 0.15±0.9 | 39.2±1.3 |
| Less than college degree | 81 (54.7%) | −0.02±1.0 | 38.9±1.4 |
| Household income | |||
| <$80K | 87 (58.8%) | −0.10±0.88 | 39.0±1.3 |
| >$80K | 42 (28.4%) | 0.26±1.09 | 39.1±1.3 |
| Refused to answer | 19 (12.8%) | 0.35±0.88 | 39.1±1.5 |
| Marital Status | |||
| Married | 106 (71.6%) | 0.06±0.97 | 39.1±1.4 |
| Not Married | 42 (28.4%) | 0.07±0.94 | 38.9±1.2 |
| Residence | |||
| Urban | 63 (42.6%) | −0.12±0.87 | 38.9±1.3 |
| Suburban | 85 (57.4%) | 0.20±1.00 | 39.1±1.3 |
| Smoked during pregnancy | |||
| Yes | 9 (6.1%) | −0.21±0.87 | 39.0±1.4 |
| No | 139 (93.9%) | 0.08±0.96 | 39.0±1.3 |
| Prenatal ETS exposure | |||
| Yes | 32 (21.6%) | −0.23±0.84 | 39.0±1.2 |
| No | 116 (78.4%) | 0.14±0.97 | 39.0±1.4 |
| Prenatal indoor pets | |||
| Yes | 64 (43.2%) | 0.17±0.91 | 39.1±1.3 |
| No | 84 (56.8%) | −0.02±0.99 | 39.0±1.3 |
| Ever anemic prenatally | |||
| Yes | 21 (14.2%) | 0.45±0.79 | 39.5±1.1 |
| No | 127 (85.1%) | −0.002±0.97 | 39.0±1.4 |
| First BMI measured in pregnancy (kg/m2) | 29.6±8.1 | r=−0.05 | r=−0.13 |
| Parity | |||
| Nulliparous | 57 (38.5%) | −0.13±0.93 | 39.1±1.5 |
| Multiparous | 91 (61.5%) | 0.17±0.96 | 39.0±1.2 |
| Infant sex | |||
| Male | 72 (48.7%) | 0.15±0.98 | 38.9±1.5 |
| Female | 76 (51.4%) | −0.02±0.93 | 39.2±1.1 |
| Year house built | |||
| 1980 or after | 25 (16.9%) | 0.14±1.11 | 39.2±1.6 |
| Before 1980 | 116 (78.4%) | 0.04±0.94 | 39.0±1.3 |
| Missing | 7 (4.7%) | 0.12±0.78 | 39.1±0.7 |
P-value comparing African-American to white (P=0.034)
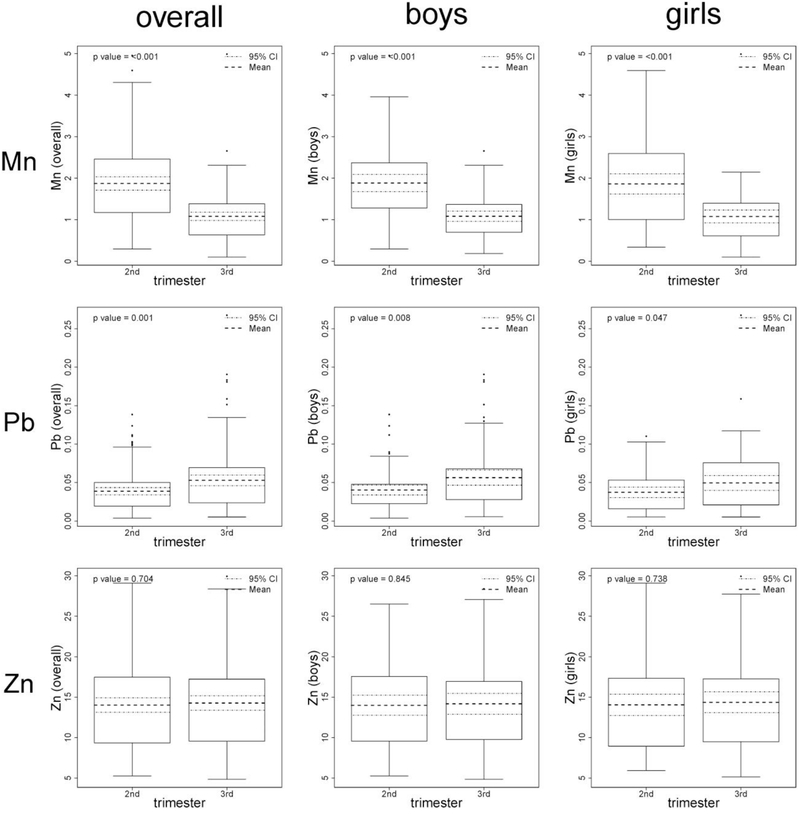
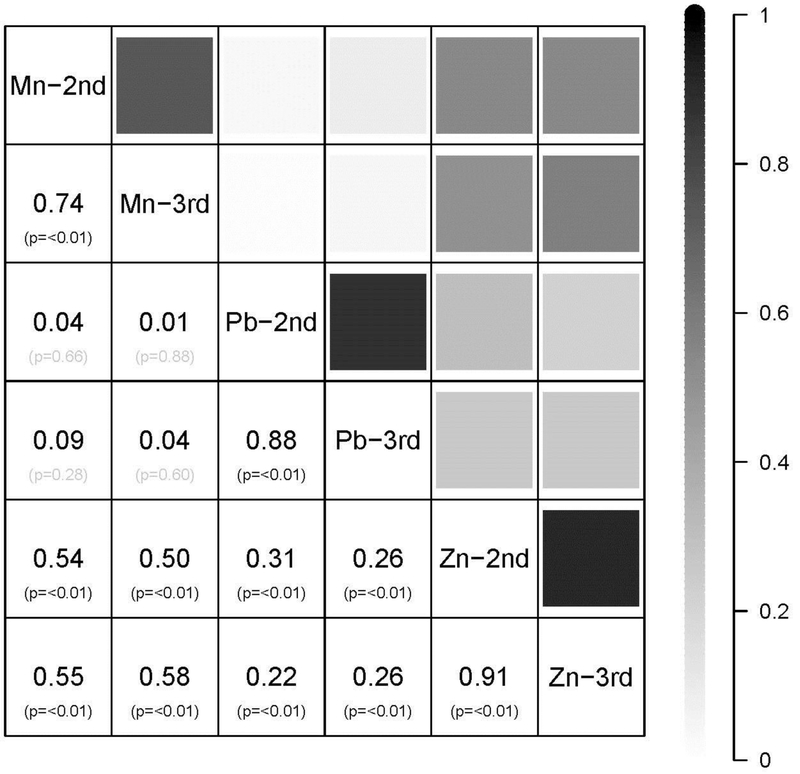
The distribution of manganese, lead and zinc in teeth, by trimester, is presented in Figure 1 in metal-to-calcium ratio of ion counts. Tooth zinc levels in the second (mean±standard deviation (SD)=14.02±5.47) and third trimester (mean±SD=14.27±5.63) were similar (P=0.70). Tooth manganese levels decreased between the second (mean±SD=1.87±0.97) and third trimester (mean±SD=1.08±0.61; P<0.001). In contrast, tooth lead levels increased between the second (mean±SD=0.04±0.03) and third trimester (mean±SD=0.05±0.04; P=0.001). Results were similar by sex (Figure 1). The correlation across tooth metals was also examined (Figure 2); as expected, for each metal, there was high correlation across trimester.
Figure 1.
Distribution of manganese (Mn), lead (Pb), and zinc (Zn) by trimester of measurement, overall and by sex. Tooth metal data are presented in metal-to-calcium ratio of ion counts and are not centered/standardized for graphical purposes. P-value is for difference by trimester. CI, confidence interval.
Figure 2.
Correlations between centered and standardized tooth metals across trimester of measure. Shading indicates strength of correlation. Data are correlation coefficient (P-value). Mn, manganese; Pb, lead; Zn, zinc; 2nd, second trimester; 3rd, third trimester.
3.1. Association of tooth metals with birth weight Z-score and gestational age at delivery
Multivariable regression modeling revealed associations between tooth metal measures and birth weight Z-score (Table 2). Specifically, after adjusting for batch, tooth attrition and tooth type, manganese in the second (β=0.21, 95% CI: 0.05, 0.37, P=0.01) and third trimester (β=0.21, 95% CI: 0.05, 0.37, P=0.01) were positively associated with birth weight Z-score and lead in the second trimester was negatively associated with birth weight Z-score (β=−0.20, 95% CI: −0.38, −0.02, P=0.02). Results were similar for manganese after covariate adjustment (Table 2 Models 2 and 3). However, after full covariate adjustment, results were attenuated and no longer statistically significant for lead. There was no evidence for a trimester-specific effect of each metal with birth weight Z-score (Table 2; all P-values for effect by time > 0.26).
Table 2.
Association of centered and standardized tooth metals measured in second or third trimester with birth weight Z-score and gestational age at delivery. Bolded values highlight all P-values <0.05.
| Metal | Second trimester | Third trimester | Effect by time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | β* | 95% CI | P | |
| Birth Weight Z-score | |||||||||
| Model 1 | |||||||||
| Mn | 0.21 | (0.05, 0.37) | 0.01 | 0.21 | (0.05, 0.37) | 0.01 | −0.02 | (−0.49, 0.45) | 0.92 |
| Pb | −0.20 | (−0.38, −0.02) | 0.02 | −0.13 | (−0.29, 0.03) | 0.12 | −0.34 | (−0.93, 0.25) | 0.26 |
| Zn | 0.12 | (−0.06, 0.30) | 0.16 | 0.12 | (−0.04, 0.28) | 0.16 | 0.14 | (−0.72, 1.00) | 0.76 |
| Model 2 | |||||||||
| Mn | 0.19 | (0.03, 0.35) | 0.02 | 0.16 | (0.00, 0.32) | 0.05 | 0.10 | (−0.35, 0.55) | 0.68 |
| Pb | −0.12 | (−0.32, 0.08) | 0.23 | −0.04 | (−0.22, 0.14) | 0.69 | −0.34 | (−0.93, 0.25) | 0.26 |
| Zn | 0.09 | (−0.07, 0.25) | 0.27 | 0.09 | (−0.07, 0.25) | 0.31 | 0.23 | (−0.61, 1.07) | 0.59 |
| Model 3 | |||||||||
| Mn | 0.17 | (0.01, 0.33) | 0.03 | 0.16 | (0.00, 0.32) | 0.05 | 0.03 | (−0.42, 0.48) | 0.89 |
| Pb | −0.15 | (−0.35, 0.05) | 0.12 | −0.06 | (−0.24, 0.12) | 0.53 | −0.31 | (−0.90, 0.28) | 0.30 |
| Zn | 0.07 | (−0.09, 0.23) | 0.39 | 0.07 | (−0.09, 0.23) | 0.42 | 0.16 | (−0.66, 0.98) | 0.70 |
| Gestational Age at Delivery | |||||||||
| Model 1 | |||||||||
| Mn | 0.03 | (−0.19, 0.25) | 0.80 | 0.01 | (−0.21, 0.23) | 0.90 | 0.05 | (−0.60, 0.70) | 0.87 |
| Pb | 0.01 | (−0.23, 0.25) | 0.96 | 0.08 | (−0.14, 0.30) | 0.48 | −0.27 | (−1.07, 0.53) | 0.51 |
| Zn | −0.05 | (−0.29, 0.19) | 0.66 | −0.04 | (−0.28, 0.20) | 0.72 | −0.22 | (−1.40, 0.96) | 0.71 |
| Model 2 | |||||||||
| Mn | 0.03 | (−0.19, 0.25) | 0.79 | 0.03 | (−0.21, 0.27) | 0.80 | 0.04 | (−0.61, 0.69) | 0.90 |
| Pb | 0.07 | (−0.20, 0.34) | 0.62 | 0.10 | (−0.14, 0.34) | 0.42 | −0.15 | (−0.97, 0.67) | 0.73 |
| Zn | −0.03 | (−0.27, 0.21) | 0.79 | −0.02 | (−0.26, 0.22) | 0.85 | −0.14 | (−1.32, 1.04) | 0.82 |
| Model 3 | |||||||||
| Mn | 0.02 | (−0.22, 0.26) | 0.86 | 0.00 | (−0.24, 0.24) | 1.00 | 0.11 | (−0.56, 0.78) | 0.76 |
| Pb | 0.08 | (−0.19, 0.35) | 0.55 | 0.14 | (−0.11, 0.39) | 0.26 | −0.22 | (−1.08, 0.64) | 0.61 |
| Zn | −0.03 | (−0.27, 0.21) | 0.83 | −0.01 | (−0.25, 0.23) | 0.92 | −0.11 | (−1.31, 1.09) | 0.86 |
β for the effect by time is the difference in effect estimates from the 2nd and 3rd trimesters
Mn, manganese; Pb, lead; Zn, Zinc; β, Beta; CI, Confidence Interval
Model 1: Adjusted for batch, tooth attrition, and tooth type
Model 2: Adjusted for batch, tooth attrition, tooth type, and race
Model 3: Adjusted for batch, tooth attrition, tooth type, race, urban, ETS, anemic, maternal age, and year house built
When we examined the effects of the three metals jointly as a mixture we saw significant associations with birth weight Z-score (Table 3). In our WQS regression analyses, when we constrained the association of metals with birth weight Z-score to be positive (i.e. to see which metals increased birth weight), we observed that second trimester metal mixture was significantly associated with birth weight Z-score (P=0.047). This association was almost entirely attributed to manganese (75% of observed association) and zinc (23%). The third trimester metal mixture was also driven largely by manganese and zinc but was not statistically significant (P=0.085). When we constrained the association of metals with birth weight Z-score to be negative (i.e. to see which metals decreased birth weight Z-score), much of the association, albeit not statistically significant, was driven by lead. Results were similar after full covariate adjustment (Table 3, Model 2).
Table 3.
Weighted quantile sum (WQS) index for metals mixtures (using decile score) during second and third trimesters, related to birth weight Z-score. Bolded values highlight all P-values <0.05.
| Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive Direction* | Negative Direction* | Positive Direction* | Negative Direction* | ||||||
| Second trimester |
Third trimester |
Second trimester |
Third trimester |
Second trimester |
Third trimester |
Second trimester |
Third trimester |
||
| Beta | 0.075 | 0.067 | −0.03 | −0.003 | 0.116 | 0.134 | −0.09 | −0.025 | |
| SE | 0.038 | 0.039 | 0.04 | 0.04 | 0.069 | 0.073 | 0.06 | 0.067 | |
| P-value | 0.047 | 0.085 | 0.348 | 0.946 | 0.094 | 0.065 | 0.148 | 0.711 | |
| Metal | Weights | Weights | Weights | Weights | Weights | Weights | Weights | Weights | |
| Mn | 0.75 | 0.83 | 0.01 | 0.05 | 0.93 | 0.84 | 0.00 | 0.02 | |
| Pb | 0.02 | 0.07 | 0.98 | 0.85 | 0.00 | 0.03 | 0.98 | 0.93 | |
| Zn | 0.23 | 0.10 | 0.01 | 0.10 | 0.07 | 0.13 | 0.01 | 0.05 | |
Positive direction indicates the association of metals with birth weight Z-score constrained to be positive; Negative direction indicates the association of metals with birth weight Z-score constrained to be negative
SE, standard error; Mn, manganese; Pb, lead; Zn, Zinc
Model 1: Adjusted for batch, tooth attrition, tooth type, and race
Model 2: Adjusted for batch, tooth attrition, tooth type, race, urban, ETS, anemic, maternal age, and year house built
None of the metals showed a significant association with gestational age at delivery (Table 2). Similarly, we did not observe any appreciable mixture effects with gestational age (data not shown).
3.2. Sex and race specific effects of tooth metals on birth outcomes
Mean birth weight was slightly higher in male (3514.4±509.9 g) compared to female infants (3354.6±424.3 g) (P= 0.045). As expected, there was no difference by sex in birth weight Z-score (0.15±0.98 in males and −0.02±0.93 in females; P=0.29). There was also no difference in gestational age at delivery by sex (38.9±1.5 in males and 39.2±1.1 weeks in females; P=0.16). There were no sex differences in any metal level in either trimester (all P>0.13).
There was evidence for sex-specific associations of tooth metals with birth weight Z-score (Supplemental Table). There was a statistically significant sex-by-trimester tooth lead interaction with birth weight Z-score (P=0.07 in the model adjusted for batch, attrition and tooth type; P=0.09 in the fully adjusted model). After adjusting for batch, tooth attrition, and tooth type, tooth lead level in the second trimester was negatively associated with birth weight Z-score among male (β=−0.39, 95 % CI: −0.64, −0.14, P=0.01) but not female (β=−0.10, 95 % CI: −0.34, 0.14, P=0.42) infants. In the fully adjusted model, however, the male-specific effect of lead on birth weight was attenuated and no longer statistically significant (β=−0.20, 95 % CI: −0.47, 0.07, P=0.15). While there was no evidence for a manganese-sex interaction with birth weight Z-score (all P>0.45), in stratified models there were potential differences by sex in the manganese-birth weight Z-score association. Among females, tooth manganese levels in both the second (β=0.28, 95 % CI: 0.08, 0.48, P=0.01) and third trimester (β=0.24, 95 % CI: 0.04, 0.44, P=0.02) were positively associated with birth weight Z-score; there was no association with second (β=0.11, 95 % CI: −0.16, 0.38, P=0.44) or third trimester (β=0.16, 95 % CI: −0.15, 0.47, P=0.33) manganese in males. In females, after full covariate adjustment, the association of manganese in the second trimester (β=0.23, 95 % CI: 0.01, 0.45, P=0.04) remained statistically significant with birth weight Z-score while the association of third trimester manganese (β=0.19, 95 % CI: −0.03, 0.41, P=0.07) with birth weight Z-score was slightly attenuated and no longer statistically significant. There was no evidence for a sex-specific effect of zinc on birth weight Z-score.
There was no evidence for a race-specific effect of tooth metal levels with either birth weight Z-score or gestational age at delivery (all interaction P>0.11).
4. Discussion
In the current study, we utilized the novel elemental bio-imaging of human primary teeth to examine direct fetal exposure to zinc, lead and manganese with birth outcomes, including birth weight and gestational age at delivery. We found evidence consistent with previous studies showing positive associations between prenatal tooth manganese with birth weight Z-score and inverse associations of tooth lead with birth weight Z-score, however, after covariate adjustment, the associations with lead were attenuated. Associations between tooth metals and birth outcomes appeared to be sex-specific.
Potential mechanisms linking prenatal lead exposure with impaired fetal growth and preterm delivery include competition of lead with calcium for deposition into bone and impact on collagen synthesis which may impact chrorioamniotic membrane structure.6 While a number of studies have shown that lead increases the risk of poorer birth outcomes, not all studies have consistently demonstrated such associations.6,25-29 In the current study, before covariate adjustment, we did find an association between tooth lead level in the second trimester, however, after covariate adjustment, this association was attenuated. These findings are similar to those found in Project Viva, which showed that mid-pregnancy maternal red blood cell lead level was not associated with infant birth weight or gestational age at delivery after adjusting for confounding factors.25 In the CANDLE study which had maternal blood lead level measures in the second and third trimester as well as cord blood lead levels in 98 women, after adjusting for gravidity, marital status and gestational age at blood draw, second trimester maternal blood lead level was associated with a marginally statistically significant lower birth weight and statistically significant greater odds of preterm delivery.27 Similarly, in the Project Viva study, maternal red blood cell lead level was associated with increased risk of preterm delivery.25 With only 6 children in our sample born preterm, we were unable to examine if tooth metals were associated with preterm delivery.
Manganese is an essential nutrient and as such, is necessary for proper growth and development, however, manganese at higher levels can also be toxic.30,31 Similar to lead, there is a growing body of literature showing the importance of manganese in healthy birth outcomes. Data from 172 mother-infant-pairs born in Shanghai showed both lower and higher maternal manganese levels in blood measured near delivery were associated with lower birth weight.32 Similar findings were obtained in a case-control study conducted in Hubei Province, China, which showed both lower and higher maternal urinary manganese measured at delivery was associated with low birth weight.30 In contrast, in a study in Taiwan, first and second, but not third trimester maternal erythrocyte manganese levels were inversely associated with infant birth weight.33 We found that tooth manganese levels in the second and third trimester were positively associated with birth weight, even after adjustment for covariates. Future studies that establish ideal manganese levels during pregnancy may be needed.
Like manganese, zinc is an essential nutrient that is needed for cell growth, differentiation and survival.34,35 A systematic review of the role of maternal zinc status and pregnancy complications revealed that there is sufficient evidence to suggest there is an association between maternal circulating zinc levels and low birth weight in populations where zinc deficiency is prevalent, but insufficient studies examining the association of maternal zinc with preterm birth.9 In the China-Anhui birth cohort study, lower maternal serum zinc level in the first but not second trimester was associated with greater risk for preterm birth.34 In a recent study conducted in Spain, cord blood zinc levels were higher in infants with appropriate-for-age growth compared to those with intrauterine growth restriction or small-for-gestational-age growth.36 In the National Birth Defects Prevention Study, lower maternal self-reported zinc intake was associated with greater odds of preterm delivery (before 32 weeks gestation).37 Overall, we found no association of tooth zinc level with birth outcomes in the WHEALS cohort.
We explored potential race and sex specific effects of metals in teeth with birth outcomes. In a study of infants born in Shanghai there was a statistically significant inverse association of cord blood lead level with birth weight among male but not female infants.38 Similar results were found in the Project Viva cohort, showing higher maternal blood lead level was associated with lower birth weight in male but not female infants; however, this was attenuated after covariate adjustment.25 In the Project Viva cohort, maternal blood lead level was also statistically significantly associated with preterm birth and suggestively associated with decreased gestational age at delivery in male but not female infants.25 In contrast, there was no sex-specific relationship of cord blood lead and birth weight in the Tohoku Study of Child Development.39 Before covariate adjustment, we found that second trimester tooth lead level was inversely associated with birth weight Z-score in male but not female infants, however, this was attenuated after adjusting for covariates. Wang et al (2017) postulate that male fetuses have a higher growth rate and nutritional demands, and thus may be at higher risk of poorer growth with lead exposure if lead negatively impacts placental function.38 Previous studies have shown that higher maternal blood lead is inversely associated with placental thickness.40 In contrast, we found that higher manganese level was associated with greater birth weight Z-score in female but not male infants. In a study in Hubei Province, China, maternal pre-delivery urine manganese levels demonstrated a curvilinear association with low birth weight in female infants but a linear association in male infants.30 Additional work to confirm our findings is needed.
Data from NHANES suggests that only 59.4% of pregnant women (1988-1994) in the US had adequate zinc intake.41 In the US, NHANES data also suggest that reproductive age women have higher blood manganese levels than similarly aged men.42 Among US women 20 to 44 years, pregnant women also have higher blood manganese levels than their non-pregnant counterparts.42 There is no evidence to suggest that women/fetuses from WHEALS would have zinc or manganese intake that differs from the rest of the United States. Prenatal tooth manganese level in WHEALS is higher than that from a cohort in Mexico, however, which may reflect underlying dietary differences in mothers from the US compared to Mexico.43 Finally, we have previously shown, based on data from routine blood lead screening, that children from the WHEALS cohort have rates of elevated blood lead levels that are similar to that of the US,44,45 suggesting that our population is neither disproportionately burdened by, nor protected from, lead exposure.
The current study has a number of strengths and limitations. We have previously shown that children from WHEALS who donated a tooth to the study were of slightly older gestational age at delivery (39.1±1.3 weeks compared to 38.7±1.8 weeks) and higher birth weight Z-score (0.06±0.95 compared to −0.15±0.99 weeks) than WHEALS children who did not donate a tooth to the study.17 As reported elsewhere, the propensity for participation in research studies by healthier volunteers (with respect to both outcomes of interest and potential exposures) may bias the results of a study,46 potentially toward the null. There are known sex differences in the vulnerability to prenatal exposures;47 we found evidence for sex-specific effects of tooth manganese on birth weight Z-score in stratified models but not when tested with interaction terms, which may suggest that sex acts as an effect modifier rather than an interaction variable.48 However, we also may be underpowered to detect some associations, particularly those that are sex or race specific, which may be why we have several associations that are marginally statistically significant. Further, in our fully-adjusted models, the associations of some metals were attenuated and no longer statistically significant; it is possible that we adjusted for covariates that are on the causal pathway from exposure to outcome, and thus we have over-adjusted our models and biased the results to the null.49 Utilizing the novel tooth-matrix methodology overcomes several limitations of previous studies, including precluding the need for multiple measures of maternal blood or urine over time and the direct measurement of fetal exposure rather than relying on maternal exposure as a surrogate. Tooth biomarkers have been used in earlier studies and have shown differences in exposure to lead and other metals.50-59 However, levels of metals reported by our methods are not directly comparable to those studies using other analytical methods. It is also known that the distribution of elements in dental hard tissue is heterogeneous.60 Our racially diverse cohort is a strength, as well as the distribution of residence across both urban and suburban areas.
4.1. Conclusions
Overall, we found evidence suggesting that higher in utero manganese is associated with larger birth weight Z-scores and that these associations may vary by infant sex. Future studies are needed to better understand the mechanisms by which manganese could impact birth weight differentially by sex.
Supplementary Material
Highlights.
145 children had 2nd and 3rd trimester tooth metal measurements and birth outcome data
Higher manganese and lower lead in teeth associated with greater birth weight
Sex-specific association of tooth manganese with birth weight
Acknowledgments
Funding Sources
This study was supported by the National Institutes of Health [R21 ES022321, R01 AI050681, R01 HL113010, P01 AI089473, P30 ES020957, K99 HD087523, P30 ES017885, T42 OH008455 and DP2 ES025453] and the Fund for Henry Ford Hospital. Study sponsors had no role in the study design, data collection or interpretation, or in the writing of this manuscript.
Footnotes
Declarations of Interest: none.
Human Subjects Protections
This study was reviewed and approved by the Henry Ford Health System Institutional Review Board (Federal Wide Assurance Number 00005846; IRB# 00000253 and IRB# 00008660); IRB approval #1881. Parents/guardians provided written, informed consent.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. The Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital Stat Rep. 2017;66(1):1. [PubMed] [Google Scholar]
- 3.Perera FP, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect. 1999;107 Suppl 3:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perera FP, Illman SM, Kinney PL, Whyatt RM, Kelvin EA, Shepard P, Evans D, Fullilove M, Ford J, Miller RL, Meyer IH, Rauh VA. The challenge of preventing environmentally related disease in young children: community-based research in New York City. Environ Health Perspect. 2002;110(2):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grason HA, Misra DP. Reducing exposure to environmental toxicants before birth: moving from risk perception to risk reduction. Public Health Rep. 2009;124(5):629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM. Maternal low-level lead exposure and fetal growth. Environ Health Perspect. 2010;118(10):1471–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, Wright RO. Maternal blood manganese levels and infant birth weight. Epidemiology. 2009;20(3):367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eum JH, Cheong HK, Ha EH, Ha M, Kim Y, Hong YC, Park H, Chang N. Maternal blood manganese level and birth weight: a MOCEH birth cohort study. Environ Health. 2014;13(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RL, Grieger JA, Bianco-Miotto T, Roberts CT. Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy Complications: A Systematic Review. Nutrients. 2016;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hare D, Austin C, Doble P, Arora M. Elemental bio-imaging of trace elements in teeth using laser ablation-inductively coupled plasma-mass spectrometry. J Dent. 2011;39(5):397–403. [DOI] [PubMed] [Google Scholar]
- 11.Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, Smith DR. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ Sci Technol. 2012;46(9):5118–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora M, Kennedy BJ, Elhlou S, Pearson NJ, Walker DM, Bayl P, Chan SW. Spatial distribution of lead in human primary teeth as a biomarker of pre- and neonatal lead exposure. Sci Total Environ. 2006;371(1-3):55–62. [DOI] [PubMed] [Google Scholar]
- 13.Sabel N, Johansson C, Kuhnisch J, Robertson A, Steiniger F, Noren JG, Klingberg G, Nietzsche S. Neonatal lines in the enamel of primary teeth--a morphological and scanning electron microscopic investigation. Arch Oral Biol. 2008;53(10):954–963. [DOI] [PubMed] [Google Scholar]
- 14.Cassidy-Bushrow A, Wegienka G, Barone C, Valentini R, Yee J, Havstad S, Johnson C. Race-specific relationship of birth weight and renal function among healthy young children. Pediatr Nephrol. 2012;27(8):1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havstad S, Wegienka G, Zoratti EM, Lynch SV, Boushey HA, Nicholas C, Ownby DR, Johnson CC. Effect of prenatal indoor pet exposure on the trajectory of total IgE levels in early childhood. J Allergy Clin Immunol. 2011;128(4):880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wegienka G, Havstad S, Joseph CL, Zoratti E, Ownby D, Woodcroft K, Johnson CC. Racial Disparities in Allergic Outcomes in African Americans Emerge as Early as Age 2 Years. Clinical & Experimental Allergy. 2011;42(6):909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassidy-Bushrow AE, Sitarik AR, Havstad S, Park SK, Bielak LF, Austin C, Johnson CC, Arora M. Burden of higher lead exposure in African-Americans starts in utero and persists into childhood. Environ Int. 2017;108:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon SL, Gaitens JM, Jacobs DE, Strauss W, Nagaraja J, Pivetz T, Wilson JW, Ashley PJ. Exposure of U.S. children to residential dust lead, 1999-2004: II. The contribution of lead-contaminated dust to children's blood lead levels. Environ Health Perspect. 2009;117(3):468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora M, Austin C, Sarrafpour B, Hernandez-Avila M, Hu H, Wright RO, Tellez-Rojo MM. Determining prenatal, early childhood and cumulative long-term lead exposure using micro-spatial deciduous dentine levels. PLoS One. 2014;9(5):e97805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institute of Medicine. 1985. Preventing Low Birthweight: Summary. Washington, DC: The National Academies Press; 10.17226/512. [DOI] [PubMed] [Google Scholar]
- 22.Shiue I, Bramley G. Environmental chemicals mediated the effect of old housing on adult health problems: US NHANES, 2009-2010. Environ Sci Pollut Res Int. 2015;22(2):1299–1308. [DOI] [PubMed] [Google Scholar]
- 23.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. Journal of Agricultural, Biological, and Environmental Statistics. 2015;20(1):100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvin S Statistical Analysis of Epidemiologic Data, 2nd edn. New York: Oxford University Press; 1996. [Google Scholar]
- 25.Perkins M, Wright RO, Amarasiriwardena CJ, Jayawardene I, Rifas-Shiman SL, Oken E. Very low maternal lead level in pregnancy and birth outcomes in an eastern Massachusetts population. Ann Epidemiol. 2014;24(12):915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor CM, Golding J, Emond AM. Adverse effects of maternal lead levels on birth outcomes in the ALSPAC study: a prospective birth cohort study. BJOG. 2015;122(3):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabito FA, Kocak M, Werthmann DW, Tylavsky FA, Palmer CD, Parsons PJ. Changes in low levels of lead over the course of pregnancy and the association with birth outcomes. Reprod Toxicol. 2014;50:138–144. [DOI] [PubMed] [Google Scholar]
- 28.Xie X, Ding G, Cui C, Chen L, Gao Y, Zhou Y, Shi R, Tian Y. The effects of low-level prenatal lead exposure on birth outcomes. Environ Pollut. 2013;175:30–34. [DOI] [PubMed] [Google Scholar]
- 29.Kaji M, Nishi Y. Lead and Growth. Clinical Pediatric Endocrinology. 2006;15(4):123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia W, Zhou Y, Zheng T, Zhang B, Bassig BA, Li Y, Wise JP Sr., Zhou A, Wan Y, Wang Y, Xiong C, Zhao J, Li Z, Yao Y, Hu J, Pan X, Xu S. Maternal urinary manganese and risk of low birth weight: a case-control study. BMC Public Health. 2016;16:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR in Biomedicine. 2004;17(8):544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Ding G, Gao Y, Wang P, Shi R, Huang H, Tian Y. Manganese concentrations in maternal-infant blood and birth weight. Environ Sci Pollut Res Int. 2014;21(9):6170–6175. [DOI] [PubMed] [Google Scholar]
- 33.Tsai MS, Liao KW, Chang CH, Chien LC, Mao IF, Tsai YA, Chen ML. The critical fetal stage for maternal manganese exposure Environ Res. 2015;137:215–221. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Hu YF, Hao JH, Chen YH, Wang Y, Zhu P, Zhang C, Xu YY, Tao FB, Xu DX. Maternal Serum Zinc Concentration during Pregnancy Is Inversely Associated with Risk of Preterm Birth in a Chinese Population. J Nutr. 2016;146(3):509–515. [DOI] [PubMed] [Google Scholar]
- 35.Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv Immunol. 2008;97:149–176. [DOI] [PubMed] [Google Scholar]
- 36.Sabra S, Malmqvist E, Saborit A, Gratacos E, Gomez Roig MD. Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction. PLoS One. 2017;12(10):e0185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmichael SL, Yang W, Shaw GM. Maternal dietary nutrient intake and risk of preterm delivery. Am J Perinatol. 2013;30(7):579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Gao ZY, Yan J, Ying XL, Tong SL, Yan CH. Sex differences in the effects of prenatal lead exposure on birth outcomes. Environ Pollut. 2017;225:193–200. [DOI] [PubMed] [Google Scholar]
- 39.Tatsuta N, Kurokawa N, Nakai K, Suzuki K, Iwai-Shimada M, Murata K, Satoh H. Effects of intrauterine exposures to polychlorinated biphenyls, methylmercury, and lead on birth weight in Japanese male and female newborns. Environ Health Prev Med. 2017;22(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Saleh I, Shinwari N, Mashhour A, Rabah A. Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. Int J Hyg Environ Health. 2014;217(2-3):205–218. [DOI] [PubMed] [Google Scholar]
- 41.Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. Zinc Intake of the U.S. Population: Findings from the Third National Health and Nutrition Examination Survey, 1988-1994. The Journal of Nutrition. 2000;130(5):1367S–1373S. [DOI] [PubMed] [Google Scholar]
- 42.Oulhote Y, Mergler D, Bouchard MF. Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011-2012. Environmental Health. 2014;13(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunier RB, Arora M, Jerrett M, Bradman A, Harley KG, Mora AM, Kogut K, Hubbard A, Austin C, Holland N, Eskenazi B. Manganese in Teeth and Neurodevelopment in Young Mexican-American Children. Environmental research. 2015;142:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler W, Brown M. Blood lead levels in children aged 1-5 years - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2013;62(13):245–248. [PMC free article] [PubMed] [Google Scholar]
- 45.Cassidy-Bushrow AE, Havstad S, Basu N, Ownby DR, Park SK, Ownby DR, Johnson CC, Wegienka G. Detectable Blood Lead Level and Body Size in Early Childhood. Biol Trace Elem Res. 2016;171(1):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Froom P, Melamed S, Kristal-Boneh E, Benbassat J, Ribak J. Healthy Volunteer Effect in Industrial Workers. Journal of Clinical Epidemiology. 1999;52(8):731–735. [DOI] [PubMed] [Google Scholar]
- 47.DiPietro JA, Voegtline KM. The gestational foundation of sex differences in development and vulnerability. Neuroscience. 2017;342:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.VanderWeele TJ. On the distinction between interaction and effect modification. Epidemiology. 2009;20(6):863–871. [DOI] [PubMed] [Google Scholar]
- 49.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gulson BL. Tooth analyses of sources and intensity of lead exposure in children. Environ Health Perspect. 1996;104(3):306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Waternaux C. Low-level lead exposure and children's cognitive function in the preschool years. Pediatrics. 1991;87(2):219–227. [PubMed] [Google Scholar]
- 52.Kim R, Hu H, Rotnitzky A, Bellinger D, Needleman H. Longitudinal relationship between dentin lead levels in childhood and bone lead levels in young adulthood. Arch Environ Health. 1996;51(5):375–382. [DOI] [PubMed] [Google Scholar]
- 53.Leviton A, Bellinger D, Allred EN, Rabinowitz M, Needleman H, Schoenbaum S. Pre- and postnatal low-level lead exposure and children's dysfunction in school. Environ Res. 1993;60(1):30–43. [DOI] [PubMed] [Google Scholar]
- 54.Needleman HL. The current status of childhood low-level lead toxicity. Neurotoxicology. 1993;14(2-3):161–166. [PubMed] [Google Scholar]
- 55.Needleman HL, Allred E, Bellinger D, Leviton A, Rabinowitz M, Iverson K. Antecedents and correlates of hypoplastic enamel defects of primary incisors. Pediatr Dent. 1992;14(3):158–166. [PubMed] [Google Scholar]
- 56.Needleman HL, Davidson I, Sewell EM, Shapiro IM. Subclinical lead exposure in philadelphia schoolchildren. Identification by dentine lead analysis. N Engl J Med. 1974;290(5):245–248. [DOI] [PubMed] [Google Scholar]
- 57.Needleman HL, Gatsonis CA. Low-level lead exposure and the IQ of children. A meta-analysis of modern studies. JAMA. 1990;263(5):673–678. [PubMed] [Google Scholar]
- 58.Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN. The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. N Engl J Med. 1990;322(2):83–88. [DOI] [PubMed] [Google Scholar]
- 59.Needleman HL, Shapiro IM. Dentine lead levels in asymptomatic Philadelphia school children: subclinical exposure in high and low risk groups. Environ Health Perspect. 1974;7:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Souza-Guerra C, Barroso RC, de Almeida AP, Peixoto IT, Moreira S, de Sousa FB, Gerlach RF. Anatomical variations in primary teeth microelements with known differences in lead content by micro-Synchrotron Radiation X-Ray Fluorescence (mu-SRXRF) - A preliminary study. J Trace Elem Med Biol. 2014;28(2):186–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.