Abstract
Of all brain regions, the 6-layered neocortex has undergone the most dramatic changes in size and complexity during mammalian brain evolution. These changes, occurring in the context of a conserved set of organizational features that emerge through stereotypical developmental processes, are considered responsible for the cognitive capacities and sensory specializations represented within the mammalian clade. The modern experimental era of developmental neurobiology, spanning 6 decades, has deciphered a number of mechanisms responsible for producing the diversity of cortical neuron types, their precise connectivity and the role of gene by environment interactions. Here, experiments providing insight into the development of cortical projection neuron differentiation and connectivity are reviewed. This current perspective integrates discussion of classic studies and new findings, based on recent technical advances, to highlight an improved understanding of the neuronal complexity and precise connectivity of cortical circuitry. These descriptive advances bring new opportunities for studies related to the developmental origins of cortical circuits that will, in turn, improve the prospects of identifying pathogenic targets of neurodevelopmental disorders.
1. INTRODUCTION
The mammalian neocortex is responsible for a wide array of nervous system functions spanning sensory, motor, cognitive and social-emotional domains. The complex cortical circuits that evolved to support these functions have been a central subject of neuroscience research for more than one hundred years and are currently being studied with an impressive degree of precision. Recent studies have begun to reveal surprising levels of neuronal cell-type diversity and specificity in the wiring of cortical circuits. These new findings raise intriguing questions about how such complexity and specificity emerge during the ontogeny of the neocortex. While addressing such issues promises to fulfill an intellectual curiosity, new studies using a combination of advanced technologies hold promise of identifying critical points of vulnerability in the construction of neocortical architecture that may be centrally involved in the pathogenesis of neurodevelopmental and psychiatric disorders.
The development of neocortical circuitry is rooted in evolutionarily conserved, stereotypical histogenic processes that include cell proliferation, neuron and glial production, neuronal migration, neuronal differentiation (molecular and structural), axon pathfinding and target innervation, synaptogenesis and maturation, synaptic pruning and cell death. In humans, cortical histogenesis is very protracted, beginning in the early first trimester and extending through puberty. In rodents, from which much of our mechanistic understanding arises, the process is much more rapid, as basic synaptic connectivity maps are established two to three weeks into postnatal development, less than one month after the first neurons of the cortex are produced during mid-gestation. This review focuses predominantly on aspects of cortical development that are under genetic control, but the development of the cortex is sensitive to environmental influences for periods that overlap with and extend beyond the initial aspects of circuit formation. Experiments demonstrating the effects of environmental perturbations on the developing cortex are lightly touched upon in this article, but we refer readers to excellent reviews on cortical plasticity (Larsen and Krubitzer, 2008; Espinosa and Stryker, 2012; Levelt and Hübener, 2012), as in depth discussion of this topic is beyond the scope of the present review. The goal here is to integrate current knowledge across interrelated epochs of development to provide a synthesis that highlights experimental opportunities related to genetic mechanisms of cortical circuit formation. Further, the article places an emphasis on the increasingly precise descriptions of cortical neuron diversity provided by application of advanced sequencing methods in the context of anatomically and electrophysiologically defined cell types (Cadwell et al., 2016; Fuzik et al., 2016; Klingler et al., 2018), and across the full range of connectivity discussed in the article (i.e. local and long-range cortical circuits). The review includes the current understanding of histogenic events in rodents and primates, though there is greater emphasis on the former. This is due to greatly improved cellular resolution combined with recent advances in genetic engineering to create opportunities for more detailed mechanistic studies of each of the four aspects of cortical development discussed in this article.
This article mainly discusses the development of excitatory cortical projection neurons that account for approximately 80% of all neurons in the cortex, and which interact in important ways with the less numerous inhibitory cortical interneurons that are not discussed in detail here. The paper delves into four key aspects of cortical ontogeny, the latter two being relatively immature in terms of a mechanistic understanding compared to knowledge regarding the earlier aspects of development. First, there is a brief review of the basic mechanisms by which distinct functional areas of the cortex are produced. Second, the mechanisms through which the diversity of cortical projection neurons is generated in defined cortical areas are discussed. Third, the developmental emergence of local synaptic connectivity is described and knowledge gaps noted. Lastly, current knowledge of long-distance intracortical connectivity is presented, and potential mechanisms that might guide its development are discussed.
2. PATTERNING THE CORTICAL AREA MAP
The cerebral cortex can be subdivided into many functionally distinct regions that are involved in processing specific forms of sensory information, generating motor output, integrating information across sensory modalities, or enabling higher-order cognitive and executive functions. This regionalization is reminiscent of the centuries old notion that specific functions can be localized to discrete regions of the cerebrum. In the mid 1800s, Paul Broca discovered a portion of the left frontal lobe critical to the production of language as indicated by its consistent atrophy in aphasic individuals (Broca, 1865). This provided some of the first scientific evidence for the localization of function within the brain. Half a century later, in 1909, Korbinian Brodmann published his influential comparative descriptions of cytoarchitectonic subdivisions of the cerebral cortices of humans, non-human primates, and other mammalian species (Brodmann, 1909). Recent technical advances have produced heightened efforts to refine the area maps; in both rodents and primates, a far more complex parcellation is emerging through both non-invasive and invasive connectomics studies in humans, monkeys and rodents (Gamanut et al., 2018; Somerville et al., 2018; Van Essen and Glasser, 2018), with several hundred areas in the primate identified. However, even in the context of this growing complexity, some organizational features, such as the presence of six layers and the relative size and density of the cells in each layer, are shared by most, but not all, subdivisions of the neocortex. Yet, the relative thickness and precise cellular composition of each layer, as well as the primary source of afferent and efferent axonal connectivity, varies across cortical areas. One commonly referenced area-specific feature is the lack of a clear histologically-identifiable layer 4 in prefrontal and motor cortices (but see (Yamawaki et al., 2014)). The developmental basis for the emergence of shared and unique features of the diverse cortical areas has been a subject of intense research over the past several decades.
The mechanisms responsible for the generation of the cortical area map were a major focus of mammalian developmental neurobiology in the final decades of the 20th century. Studies addressed two principle perspectives with contrasting mechanisms. One hypothesis held that cortical areas are predefined as a “protomap” within progenitors of the cortical primordium, which are then translated into the mature cortical area map through the prenatal migration of area-specified neurons that assemble into ontogenic columns derived from radial units (Rakic, 1988). The second hypothesis emphasized that equipotent cortical progenitors remain naive to areal positioning in the form of a “protocortex”, and that arrival of area-specific thalamic input, postnatally in the rodent and prenatally in primates, was responsible for driving the parcellation of functional cortical subdivisions (O'Leary, 1989). Much indirect, descriptive evidence bolstered the protomap hypothesis in the first decades of the debate. For example, several molecules were found to exhibit gradients of expression within the ventricular zone and cortical plate of the early cortical primordium prenatally, prior to the arrival of thalamic afferents (Suzuki et al., 1997; Rubenstein et al., 1999). Additionally, heterotopic cortical transplant experiments in rats demonstrated the persistence of a molecular signature of limbic cortical neurons when progenitors and neurons from limbic domains were transplanted into somatosensory cortex (Barbe and Levitt, 1991). Then, in the late 1990s and early 2000s, several studies provided more direct evidence in support of the protomap hypothesis. First, Rubenstein and colleagues demonstrated that Gbx2 mutant mice do not develop thalamocortical projections, yet develop normal patterns of cortical region-specific gene expression (Rubenstein et al., 1999). This provided the first definitive evidence that thalamic innervation was nonessential for generating the fundamental blueprint of the cortical area map, and suggested that the process of cortical area formation must depend on patterning mechanisms that operate within the telencephalon. This conclusion was supported by similar observations in the Mash1 mutant mouse reported later the same year (Nakagawa et al., 1999). Shortly thereafter, the experiments of Tomomi Shimogori and Elizabeth Grove discovered that a patterning center intrinsic to the telencephalon controlled the size and positioning of cortical areas. A secreted morphogen, fibroblast growth factor 8 (Fgf8), released from the commissural plate at the rostromedial end of the telencephalon, was shown to regulate cortical area size and position along the rostral-caudal axis (Figure 1) (Fukuchi-Shimogori and Grove, 2001). Expression of Fgf8 in this region begins at the earliest stages of mouse corticogenesis, around embryonic day 9 (Crossley and Martin, 1995). The necessity of this rostral signal for proper areal patterning of the cortex garnered its name, the rostral patterning center. Over-expression of Fgf8 from the rostral patterning center causes an enlargement of rostral (e.g. motor cortex) cortical areas and a posterior shift and shrinkage of caudal (e.g., somatosensory and visual cortex) territories (Fukuchi-Shimogori and Grove, 2001). Conversely, inhibiting Fgf8 signaling causes shrinking of rostral cortical domains and a rostral shift of caudal areas - all without changing the overall size of the cortex. Moreover, introducing an ectopic source of Fgf8 at the caudal pole of the cortex induces the formation of a second barrel field that is inverted relative to the primary map (Fukuchi-Shimogori and Grove, 2001; Assimacopoulos et al., 2012).
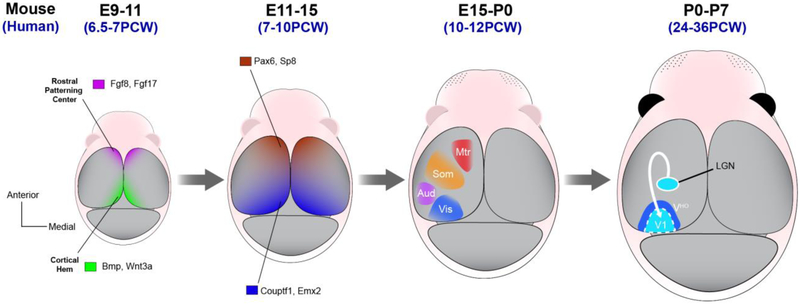
Figure 1. Patterning the cortical area map.
Illustrations of the dorsal surface of the developing mouse brain at different pre and postnatal stages. The first stage depicts the morphogen signals arising from the rostral patterning center (purple, Fgf8 and Fgf17) and cortical hem (green, Bmp and Wnt) beginning around embryonic day 9 in mice (corresponds approximately to human gestational week 6 or 7). These morphogens induce gradients of transcription factor (TF) expression, including Sp8, Pax6, Emx2 and Couptf1. The transcriptional programs regulated by these transcription factors establish cortical fields and the guidance cues that attract area-specific thalamic innervation. The innervation of cortex by thalamic axons, which happens postnatally in rodents (this occurs in the second and third trimester of human pregnancy), drives the sharpening of molecular and cytoarchitectural boundaries between primary sensory cortex (e.g. V1, dark blue) and adjacent higher order cortical areas (e.g. VHO, light blue).
Other morphogens have since been demonstrated to play complementary, yet distinct roles in patterning the cortex. For example, Fgf17 is expressed in an overlapping, but slightly larger area of the rostral patterning center (Cholfin and Rubenstein, 2007). Fgf17 mutant mice display a similar rostral shift in sensory cortical area positioning as that displayed by Fgf8 mutants, but specific frontal cortical regions appear differentially influenced by Fgf8 and Fgf17 (Cholfin and Rubenstein, 2008). Additionally, BMPs and Wnts are secreted from the cortical hem, another key patterning center that extends caudally along the midline of the cortical primordium (Figure 1) (Grove et al., 1998; He´ bert et al., 2002). In human, this same region, which also serves as the primary source of superficial Cajal-Retzius cells, is evident by 6.5-7 gestational weeks (Yoshida et al., 2006; Meyer, 2010; Van Essen and Glasser, 2018). Genetic ablation of the cortical hem causes shrinkage of the neocortex that is accompanied by rostral area expansion in a manner that resembles the phenotype induced by rostral Fgf8 overexpression (Caronia-Brown et al., 2014). This phenotype appears to depend on hem-derived Wnt3a, which antagonizes signaling downstream of Fgf8 (Caronia-Brown et al., 2014). Together, these studies demonstrate that the induction of cortical fields begins with the coordinated action of morphogens produced by patterning centers positioned at the edges of the dorsal telencephalon, during early prenatal development.
Under the influence of the morphogens secreted from the patterning centers at the rostral and caudal ends of the cortical primordium, several transcription factors develop graded expression patterns within the ventricular zone of the early cortical primordium, prior to the arrival of thalamic afferents. Like early patterning of the neural tube accomplished through a cascade of mutual repression, factors that are expressed in opposing gradients often serve as positive regulators of cell type-specific gene expression for the cells that have high expression, and at the same time, antagonize the influence of the counter molecular gradient. For example, Couptf1 and Emx2 are expressed in caudal-high to rostral-low gradients, whereas Pax6 and Sp8 are expressed in a reciprocal rostral-high to caudal-low counter-gradient. Mutation of Couptf1 causes dramatic expansion of rostral cortical territories and a corresponding shrinkage of caudal sensory-related areas (Zhou et al., 2000; Armentano et al., 2007), though the reduced sensory domains are positioned in the appropriate caudal locations relative to the enlarged areas within the cerebral hemisphere. Mutation of Emx2 or Pax6 causes reciprocal anterior-posterior shifts in the positioning of early markers of cortical areas (Bishop et al., 2000; Mallamaci et al., 2000), which result from mutual cross-repressive interactions between Emx2 and Pax6 (Hamasaki et al., 2004). Similarly, an interaction between Fgf8 and Emx2 is key to establishing the rostral-caudal axis of the cortical area map, as mutation of either gene results in the enlargement of the cortical territory expressing the other gene (Fukuchi-Shimogori and Grove, 2003; Garel, 2003; Cholfin and Rubenstein, 2008). Additionally, Emx2 appears to play an Fgf8-independent role in the direct specification of areal identity in cortical progenitors in a dose-dependent manner (Hamasaki et al., 2004). Importantly, recent studies of the transcription factor Pbx1 have shown that area identity is transcriptionally controled in dorsal pallial progenitors as well as their post-mitotic neuronal progeny (Golonzhka et al., 2015).
Despite the dramatic changes in area sizes and positions caused by the manipulation of some of these early patterning genes, the lamination and input-output connectivity of the resized and repositioned cortical areas appear to develop normally (Bishop et al., 2000; Shimogori and Grove, 2005; Armentano et al., 2007; Cholfin and Rubenstein, 2007). This suggests that the mechanisms responsible for patterning the cytoarchitectonic subdivisions of the neocortex also establish the guidance cues that attract proper area-specific thalamic input (Leingärtner et al., 2003; Shimogori and Grove, 2005). This conclusion is supported by early heterotopic transplant studies, which demonstrated that neurons committed to a limbic cortical fate attract thalamic inputs appropriate for the limbic cortex even when transplanted into somatosensory cortex (Barbe and Levitt, 1992). Thus, the aggregate of two decades of studies demonstrate that the initial establishment of a fundamental relationship between cortical area fate and area-specific thalamic innervation depends primarily on mechanisms intrinsic to the developing neocortex. Thus, there is conclusive support for the protomap hypothesis, yet it is unlikely to be so simple. There is a large body of literature demonstrating the capacity of afferent thalamic input to influence cortical cytoarchitecture phenotypes on many levels. One example of a relatively small-scale change induced by manipulations of peripheral sensory input comes from the classic studies of Van der Loos and Woolsey. Their studies demonstrated that cauterization of whisker follicles on the snout of neonatal mice dramatically alters the formation of the corresponding whisker barrels in the primary somatosensory cortex (Van der Loos and Woolsey, 1973) - a process that Crair and colleagues recently found to depend specifically on thalamocortical synaptic transmission (Li et al., 2013). Additionally, thalamocortical interactions have been shown to influence cortical organization more broadly. For example, prenatally, it has been shown that calcium waves propagate across thalamic nuclei of different sensory modalities, and that these waves influence the size of specific cortical fields (Moreno-Juan et al., 2017). In animals that undergo bilateral enucleation during development, primary visual cortical areas are reduced in size and develop responses to alternate sensory modalities, whereas adjacent cortical areas grow in surface area and may develop novel cytoarchitecture (Dehay et al., 1989; Rakic et al., 1991; Kahn and Krubitzer, 2002). Recent studies involving genetic ablation of specific thalamic nuclei in transgenic mice have further demonstrated that thalamic input is required to establish the genetic and functional distinctions between primary sensory cortex and adjacent higher order sensory cortex (Chou et al., 2013; Pouchelon et al., 2014). The cumulative evidence supports the conclusion that thalamocortical input provides an additional layer of mechanistic control over cortical area formation. Importantly, it seems that this extra level of control operates later, in postnatal development, after the prenatal, intrinsic prepatterning of the area blueprint, to define the final size of specific cortical territories and the sharpness of their boundaries. In addition to the robust effects that afferent thalamic innervation contribute to cortical organization, there is substantial evidence that spontaneous electrical activity within the cortex, which matures in the rodent from asynchronous, sparse patterns to synchronous, dense activity, contributes to the formation of cortical columns, neuronal survival and overall cortical organization (for reviews (Luhmann and Khazipov, 2018).
In summary, development of the mature cortical area map utilizes intrinsic and extrinsic biological mechanisms. The earliest phases of the process are initiated within the progenitors of the cortical primordium by morphogens that are secreted from patterning centers at the edges of the cortical sheet during mid-embryogenesis. These morphogens establish the anterior-posterior and mediolateral axes within the germinal zone of the prospective cortex by inducing reciprocal and orthogonal transcription factor gradients that serve as a coordinate system for progenitor cells. This early blueprint is then translated into distinct areal boundaries marked by differences in post-mitotic gene expression, which include guidance cues necessary for each cortical area to connect reciprocally with appropriate thalamic nuclei. Thalamic innervation refines areal boundaries by influencing the expression of some of the mature genetic, cytoarchitectural, and functional characteristics that distinguish cortical subdivisions. These anatomical and molecular events occur in the context of a dynamic landscape of electrical activity that progresses through phases of differing neuronal synchrony and dependence on electrical or chemical synaptic transmission.
3. CORTICAL NEURON SUBTYPE SPECIFICATION
The genetic and thalamic-input dependent processes that influence neocortical area organization are paralleled by a set of similarly complex mechanisms that contribute to the emergence of the remarkable diversity of cortical neuron types within each cortical area. These diverse neuron types can be subcategorized based on several interrelated features, including morphology, laminar position, input and output connectivity, membrane biophysical properties, and transcriptomes. Knowledge of the diversity of cortical neurons has improved dramatically in recent years, particularly due to technical advances that integrate neuroanatomical, electrophysiological, and molecular profiling methods. This section focuses primarily on mechanisms involved in the production and maturation of specific types of glutamatergic projection neurons, which outnumber inhibitory cortical interneurons by approximately five to one. Substantial progress also has been made in understanding the diversification and deployment of interneurons, which though smaller in number, are arguably even more diverse than excitatory neurons (Bandler et al., 2017; Wamsley and Fishell, 2017; Lim et al., 2018; Mayer et al., 2018).
In all mammals, excitatory pyramidal neurons are produced prenatally during a well-delineated period of neurogenesis, followed immediately by a stereotypical “inside-out” process of cell migration; the neurons that occupy the deep layers of cortex are born first, thus requiring that later-born neurons migrate radially through the deep layers before settling in more superficial positions (Angevine and Sidman, 1961; Rakic, 1974). Non-genetic factors, such as prenatal exposure to drugs or normal neurotransmitter signaling (e.g. GABA, 5-HT, glycine, glutamate, dopamine) prior to synaptogenesis emerged from developmental studies in the late 1990s and early 2000s as factors in early cortical histogenesis (LoTurco et al., 1995; Levitt, 1997; Behar et al., 1999; Owens and Kriegstein, 2002; Vitalis and Parnavelas, 2003; Wang et al., 2003; Rakic, 2006). Experiments in rodents and non-human primates indicate that neurotransmitters can increase or decrease neuron production and migration of excitatory neurons produced in the dorsal pallium and inhibitory neurons produced in the ganglionic eminences. Downstream changes in calcium and cyclic nucleotide signaling appear to be mechanisms through which the modulation of these events can occur (Bando et al., 2016). There is renewed interest in this area of investigation (Ascenzi and Bony, 2017), as most prior studies were performed before the availability of new tools that enable monitoring neurotransmitter effects on specific neuronal subtypes, including their molecular differentiation and connectivity.
Early in cortical neurogenesis, individual progenitor cells are multipotent and contribute neurons to multiple laminar and projection neuron subtypes through successive cell divisions (Luskin et al., 1988; Walsh and Cepko, 1988). As cortical development proceeds, cortical progenitor pools undergo pyramidal neuron lineage progression, giving rise primarily to neurons destined for superficial cortical layers late in development (McConnell, 1988; McConnell and Kaznowski, 1991; Frantz and McConnell, 1996). The seminal isochronic and heterochronic transplantation studies by McConnell and colleagues suggested a progressive reduction in responsiveness of progenitors to environmental cues as development proceeds (Desai and McConnell, 2000). However, a recent study revisited the question of whether or not all cortical progenitors undergo progressive lineage potential restriction. The study used a new tool, known as FlashTag, which enables the selective labeling and isolation of M-phase apical progenitors (Oberst et al., 2018). Surprisingly, this study found that heterochronic transplantation of late-stage (E15) apical progenitors into earlier-stage (E12) developing cortex (akin to the classic experiments by McConnell and colleagues) lead to reprogramming of the transplanted progenitors and the genesis of deep layer neurons appropriate to the transplanted host cortical progenitor pool. Thus, it seems that apical progenitors maintain responsivity to tissue environmental cues late into development, whereas intermediate progenitors (labeled by BrdU injections, given that they are in S-phase) lack this responsivity at late stages. Setting the lineage plasticity issue of different progenitor cell types aside, a preponderance of evidence currently supports the model that early neocortical progenitors are multipotent and that each generates a diverse population of neurons and glia, despite the heterogeneous expression of projection class-specific markers among some radial glial cells (Luskin et al., 1988; Guo et al., 2013; Gao et al., 2014; Eckler et al., 2015). It is noteworthy that there has been recent debate about the possible existence of fate-restricted cortical progenitors (Franco et al., 2012; Guo et al., 2013; Eckler et al., 2015; Gil-Sanz et al., 2015).
When and how are the many different subtypes of cortical projection neurons produced during the process of molecular and architectural differentiation? Early isochronic and heterochronic transplantation experiments demonstrated that a commitment to a deep or superficial layer neuron fate is made prior to the final cell division (McConnell and Kaznowski, 1991). However, several studies have identified genes first expressed post-mitotically that play critical roles in the specification of various cortical projection neuron subtypes (Arlotta et al., 2005; Alcamo et al., 2008; Britanova et al., 2008; Greig et al., 2013). Thus, the specification of neuron identities is a multistep process. This process requires coordination of intrinsic and extrinsic cues occurring at multiple stages as cortical progenitors progress toward specific cortical neuron fates.
Historically, laminar position of cortical neurons served as a central phenotypic read-out of cell fate. However, it is clear that the identity of a cortical projection neuron cannot be defined solely by its laminar position because multiple projection neuron subtypes can occupy the same cortical layer. Moreover, projection-based features of neuronal identity appear to be determined independently from laminar identity. For example, neurons that differentiate in ectopic laminar positions often develop projection phenotypes appropriate for their date of birth (Caviness, 1980; Jensen and Killackey, 1984; Lodato et al., 2011), rather than their new ectopic positions. Developmental studies have further demonstrated that distinct projection neuron subpopulations destined to occupy the same cortical layer can exhibit specific axonal projections at the earliest migratory stages, prior to neurons reaching their final laminar positions (Schwartz et al., 1991; Koester and O'Leary, 1993; Hatanaka et al., 2016). These observations place an emphasis on aspects of fate decisions that are made at the earliest stages of the differentiation process, perhaps prior to or shortly after the final cell division during initial neuronal migration. Experimental opportunities to investigate mechanisms operating during these early windows are afforded by the recent development of the FlashTag technique, which enables the capture and molecular profiling of isochronic populations of newborn cortical neurons (Telley et al., 2016; Govindan et al., 2018). It should be emphasized that early fate decisions are not final, but can be modified for an extended period of perinatal development. This has been demonstrated by studies involving reprogramming of cell identity through the ectopic overexpression of fate-specifying transcriptional regulators (described in detail below) (De la Rossa et al., 2013; Rouaux and Arlotta, 2013). Thus, while evidence suggests that some projection neurons begin to differentiate early along specific projection neuron lineages, the fate-selection process appears flexible, and likely requires the integration of multiple signals at different points during the development of projection neuron lineages.
At the lowest level of resolution, projection neurons (PNs) of the neocortex can be subdivided into three broad classes that are intermixed to varying degrees within individual cortical layers (Figure 2). The first class comprises the corticothalamic (CT) neurons, which are predominantly located within layer 6 and provide a feedback projection to the thalamus. Second, the pyramidal tract (PT) neurons are located exclusively within layer 5B and are named such because they extend axons toward the brainstem and spinal cord through the pyramidal tract. The third, and arguably the most heterogeneous class, are the intratelencephalic (IT) projection neurons that are present in layers 2-6, and extend axons toward targets in the contralateral and ipsilateral cortex, striatum, nucleus accumbens, and other dorsal pallium-derived structures, such as the septum and certain subnuclei of the amygdala. The IT-type neurons of layer 4 (e.g. spiny stellate, pyramidal, and star pyramidal neurons) are particularly unique within this class, as their synaptic outputs are confined to the local cortical area in which the neurons reside and they receive the predominant synaptic input from the thalamus. Importantly, IT-type neurons co-occupy the infragranular (below granular layer 4) layers of cortex with neighboring PT and CT neurons (Baker et al., 2018). Additionally, it is noteworthy that subsets of neurons within the primary classes can project to multiple targets within their respective projection domains. For example, some IT neurons send dual projections to two or more cortical areas and to the striatum (Mitchell and Macklis, 2005; Cederquist et al., 2013; MacDonald et al., 2018), and some PT-neurons send axon collaterals to higher-order nuclei in the thalamus as well as primary axons to the brainstem (Deschenes, 1994; Economo et al., 2018). Some of these dual-projecting subsets are marked by unique patterns of gene expression (Cederquist et al., 2013; MacDonald et al., 2018). Thus, while it is clear that the three first-order classes can be further subdivided, they are currently the most commonly referenced classes because of the unambiguous and categorical differentiation between them based on the anatomical features outlined above, as well as a number of class-specific electrophysiological, biochemical, and developmental properties (Harris and Shepherd, 2015). Importantly, each of these three classes is represented within each cortical area, but neuron number in each subclass, as well as specific cortical and subcortical areas targeted by each class, vary depending on the cytoarchitectonically-defined cortical area in which the neurons reside. For example, many PT type neurons in the motor cortex target the spinal cord, whereas those in the visual cortex instead target the superior colliculus (Harris and Shepherd, 2015). Additionally, it is commonly believed that prefrontal and motor cortices do not contain layer 4, but see (Yamawaki et al., 2014). There is a basic understanding of the developmental processes that underlie the differentiation of the three major classes of PNs. The mechanisms that influence the development of the array of PN subtypes that subdivide these major classes and that comprise later-maturing, area-specific circuits that underlie specific functions remain to be defined.
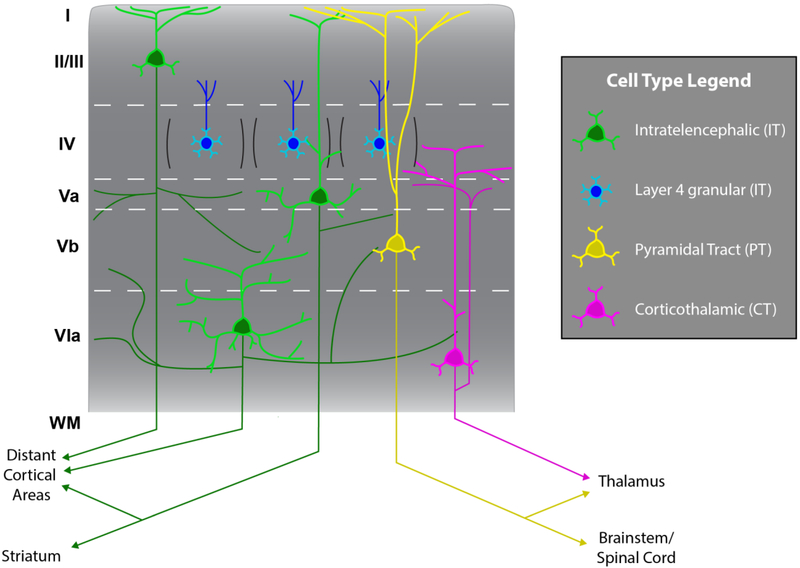
Figure 2. Cortical projection neuron diversity.
There are three primary classes of glutamatergic projection neurons in the cerebral cortex. Each class has a unique laminar distribution pattern. The corticothalamic neurons (CT, magenta) are located mostly within layer 6 and send axons to the thalamus and a narrow radial domain of the cortical column proximal to their cell bodies. The pyramidal tract neurons (PT, yellow) are positioned almost exclusively within layer 5B. These neurons project to the brainstem and spinal cord, and many issue colateral axons to other subcortical targets such as the thalamus. In contrast to the restricted laminar distribution of the first two classes, the intratelencephalic neurons (IT, green), which project axons only within the telecephalon, are distributed throughout all six layers. As noted in the text, these primary classes are divisible into secondary taxa, but consensus regarding more refined cell classes awaits further multi-dimensional, integrative analysis.
The molecular mechanisms governing the specification of the first-order classes of cortical projection neurons have started to be revealed in recent years. For instance, several transcription factors that function post-mitotically to regulate the specification of subtypes of projection neurons have been identified (Figure 3) (reviewed in detail here (Greig et al., 2013)). Some of the first major advances in this area came from a series of studies that identified projection class-specific genes through RNA microarray profiling of retrogradely-labeled and FACS-purified populations of discrete projection neuron subtypes (Arlotta et al., 2005; Molyneaux et al., 2005). Two transcription factors identified in these seminal studies, Ctip2 and Fezf2, are each required for the specification of PT-type corticospinal neurons (Arlotta et al., 2005; Chen et al., 2005a; Chen et al., 2005b; Molyneaux et al., 2005), with Ctip2 acting downstream of Fezf2 (Chen et al., 2008). Loss of Fezf2 causes many neurons originally destined for a PT-neuron fate to upregulate Satb2 and/or Tbr1, and to adopt the connectivity and electrophysiological characteristics of IT-type or CT-type neurons (Chen et al., 2008; McKenna et al., 2011; Srinivasana et al., 2012) (Figure 3). Similar cross-repressive functions, which resemble the cross-repressive regulation of cortical area formation, have been described for several other cell-class regulating transcription factors, such as Tbr1, Sox5, Ctip2 and Satb2, among others. Tbr1 and Sox5 enable layer 6 CT neuron development by binding to regulatory DNA elements near the Fezf2 locus and repressing its high-level expression to prevent CT and subplate neurons from sending inappropriate PT-like axonal projections toward the brainstem (Kwan et al., 2008; Lai et al., 2008; Han et al., 2011; McKenna et al., 2011). Likewise, direct repression of Ctip2 expression appears critical for Satb2 to properly specify IT-type callosal neurons that project to the contralateral cerebral hemisphere (Alcamo et al., 2008; Britanova et al., 2008; Srinivasana et al., 2012). Loss of Satb2 causes ectopic expression of Ctip2 in superficial cortical projection neurons, accompanied by the growth of their axons toward subcortical targets or through the anterior commissure rather than the corpus callosum. Thus, antagonistic interactions between the molecular determinants of alternate cortical projection neuron fates play an essential role in driving the diversification of and quantitative relations between the variety of PNs.
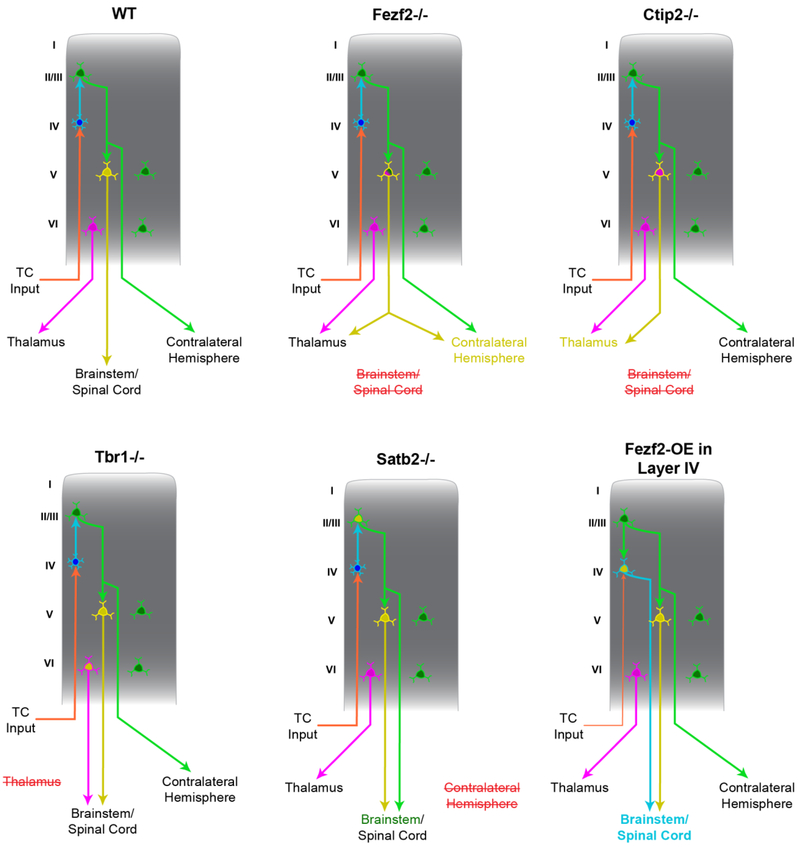
Figure 3. Transcriptional control of cortical projection neuron specification and wiring.
Several transcription factors that contribute to the differentiation of the three first-order classes of cortical projection neurons (CT, PT, and IT neurons) have been identified. Connections made by these cortical neurons are depicted in the context of the wild-type (WT) mouse cortex, along with the associated connectivity changes caused by the mutation (or ectopic overexpression) of these developmentally important transcription factors (please see text for references; knockout mutations are denoted by the gene symbol followed by −/−, e.g. Fezf2−/−). Fezf2 is a PT neuron selector gene that regulates the expression of many functionally important genes. When Fezf2 is deleted, the cortex no longer sends projections to the spinal cord, but, instead, the PT neurons upregulate genes that promote CT and IT neuron phenotypes. Accordingly, these mutant PT neurons send ectopic projections to the thalamus or across the corpus callosum. Ctip2 also contributes to the development of PT-type neurons, as projections from the cortex to the spinal cord are disrupted in Ctip2−/− mice. Tbr1 promotes the development of CT neurons, as evidenced by the fate-conversion of CT neurons into PT neurons in Tbr1−/− mice. In Tbr1−/− mutant mice, CT neurons upregulate Fezf2 and project toward the brainstem and spinal cord. Satb2 is a critical regulator of IT neurons, as Satb2−/− mice do not send axons through the corpus callosum to the contralateral hemisphere. Instead, upper layer neurons upregulate Ctip2 and project subcortically. When Fezf2 is ectopically expressed in layer IV IT neurons, these neurons are reprogrammed into PT neurons; they adopt several molecular and connectivity phenotypes that are characteristic of PT neurons, but normally excluded from layer IV IT neurons. Red, strikethrough font indicates the loss of projections from the cortex to the indicated structure (e.g. Fezf2−/− mice lose projects from cortex to spinal cord).
Recent studies have begun to illuminate the interaction between transcriptional control of cortical area formation and cell-type specification to evaluate how these developmental processes relate to one another. A clear example comes from two studies investigating the transcription factor, Ctip1 (Greig et al., 2016; Woodworth et al., 2016). During postnatal development, Ctip1 expression becomes enriched in sensory cortical domains, such as somatosensory and visual cortex, compared to motor related areas. Removal of Ctip1 function leads to the “motorization” of sensory cortical areas as measured by connectivity and molecular markers (Greig et al., 2016). Importantly, this arealization phenotype is accompanied by an expansion of layer 5 (which is normally thicker in motor cortex than in somatosensory cortex) and overproduction of PT type neurons at the expense of layer 6 CT neurons (Woodworth et al., 2016). The balance between the normal production of PT and CT neurons appears to depend on mutual transcriptional repression between Ctip2 expressed in PT neurons, and Ctip1 expressed in CT and IT neurons. Thus, Ctip1 regulates both arealization and cell-type specification. More mechanistic examples of the link between cortical area formation and cell type production are likely to emerge and thus further emphasize the need to recognize cortical area formation and the underlying processes of cell-type production and maturation as interdependent, rather than entirely separate developmental phenomena.
Beyond the cross-repressive transcriptional logic that is central to the balanced production of neurons belonging to the three first-order classes (i.e. IT, PT, and CT neurons), downstream effector molecules that instruct the development of certain class-specific phenotypes have been identified. Fez2, which specifies PT-type corticospinal motor neurons, serves as a clear example linking a master transcriptional regulator to several phenotypes of a specific class of projection neurons. Fezf2 was found to bind directly to and activate the transcription of a large group of PT-specific genes, while simultaneously repressing the expression of many IT-type genes (Lodato et al., 2014). As a demonstration of its key role in the development of defining anatomical features of corticospinal motor neurons, Fezf2 was shown to directly promote the expression of EphB1, which is critical for the proper extension of axons toward the spinal cord. Additionally, Fezf2 was found to promote a glutamatergic identity and inhibit GABAergic identity through direct activation or repression of Vglut1 and Gad1, respectively (Rouaux and Arlotta, 2010; Lodato et al., 2014). Lastly, Fezf2 has been shown to regulate the local input connectivity of cortical neurons (De la Rossa et al., 2013; Ye et al., 2015) (discussed in the following section on development of local cortical microcircuitry). A second example of the development of projection class-specific features is the expression of axon guidance receptors that operate downstream of the repressive interaction between Satb2 and Ctip2. The proper expression of the Netrin1 receptors DCC and Unc5C was found to depend on regulation by Satb2 and Ctip2, respectively. Aberrant expression patterns of these receptors were shown to contribute to the atypical axonal pathfinding of IT-type callosal neurons observed in Satb2 mutant mice (Srivatsa et al., 2014). It is important to emphasize that while new details that link the activity of fate-determining transcription factors to downstream molecular signaling processes are an intense area of current research, there is presently little known mechanistically about how class-defining characteristics (other than axon targeting), including morphology, membrane properties, and afferent connectivity, develop within each cortical projection neuron class.
These examples highlight some of the major progress that has been made toward the identification of the transcriptional programs responsible for the diversification of IT, PT, and CT neurons. Yet, recent molecular analyses of cortical projection neurons highlight what may be vast diversity among these first-order neurons, with an absence of a basic understanding of how subclasses emerge developmentally (Molyneaux et al., 2009; Zeisel et al., 2015). To emphasize this point, a recent single-cell RNA-sequencing study identified 19 distinct transcriptomic signatures for glutamatergic cortical neurons in the visual cortex of adult mice (Tasic et al., 2016), and data generated from more recent single cell sequencing studies suggest that the transcriptomic signatures of glutamatergic neurons varies substantially across cortical areas (Tasic et al., 2017). An open question relating to these data is whether each of these transcriptomic signatures represents a unique cell type, or reflects heterogeneous transcriptional states within an individual projection neuron class that could be driven by a range of neural activity, among other factors (Chen and Arlotta, 2016; Chevee et al., 2018); a definitive answer will likely require more cohesive definitions of cell types, through linking transcriptomic profiles with additional phenotypes, including morphology, electrophysiolgical properties, and function (Zeng and Sanes, 2017). Indeed, this type of poly-phenotypic approach has been critical to achieving accuracy and completeness in the identification of retinal neuron types (Seung and Sumbul, 2014).
Assuming that specific subtypes of IT, PT or CT neurons will be identified (such as the two distinct types of PT neurons recently identified in motor cortex (Economo et al., 2018), efforts to determine how these new subtypes emerge during development could be facilitated by investigating the transcriptomes of cortical projection neurons at single-cell resolution during the period of neuronal differentiation. Progressive refinement of cortical neuron identity appears to continue at least through the first week of postnatal development for some projection neuron classes (Azim et al., 2009). Single cell transcriptomic data that could address heterogeneity within the three first-order classes of projection neurons during this postnatal window of development are currently limited, which may complicate the identification of key phenotypically-related molecules because of the dynamic nature of gene expression over this period (Arlotta et al., 2005; Molyneaux et al., 2005; Judson et al., 2009). Filling this void should be a top priority, as identifying genes expressed in specific cell-types during the rodent postnatal period and primate mid-late prenatal period will inform hypotheses about how cortical circuits form (discussed in following section).
To emphasize the point of continued developmental refinement of projection neuron phenotypes postnatally, a recent set of studies focusing on the connectivity and molecular phenotypes of neurons that express the MET receptor tyrosine kinase during postnatal development revealed that, in the somatosensory cortex, only subsets of IT and PT neurons express Met (Kast et al., 2017). Such heterogeneity has implications for cortical neuron subtype refinement, as exemplified by the role that MET signaling plays in the differentiation of specific subtypes of nociceptive sensory neurons in the dorsal root ganglion (DRG) (Gascon et al., 2010). This study showed that MET receptor signaling drives the downregulation of Runx1 expression that is required for the relatively late differentiation of peptidergic nociceptive neurons from the nonpeptidergic lineage within the DRG. During normal development of the DRG, TrkA and Runx1 are initially co-expressed in a population of immature cells, but these markers segregate as development proceeds and become mutually exclusive phenotypes of peptidergic and non-peptidergic neurons, respectively. Loss of MET signaling in this context causes incomplete segregation of TrkA and Runx1 expression and a concomitant reduction in the number of CGRP+ peptidergic neurons, all without influencing the total number of sensory neurons. Thus, MET signaling has the capacity to influence important cell fate decisions in the peripheral nervous system. It is possible that heterogeneous MET signaling operates in a similar manner to influence the differentiation of subsets of IT or PT neurons in the cerebral cortex. Such a role could be assessed by determining whether the typical diversity of IT and PT neuron subtypes is present in Met mutant cortices, by methods such as single-cell RNA-sequencing or more targeted evaluation of specific molecular markers. It is important to note that there also may be more complex non-cell autonomous influences of MET receptor signaling on cortical cell-type differentiation, similar to what has been shown for medium spiny neurons in the striatum and in pools of branchial motor neurons (Helmbacher et al., 2003; Judson et al., 2010). In the spinal cord, MET signaling is critical for the proper expansion of specific motor neuron subpopulations that don’t normally express the gene but which may be influenced through an intermediary effector. With single cell RNA sequencing providing a transcriptomic inventory, it should be possible to determine how MET signaling, and presumably signaling through other receptors, influence cellular differentiation in subsets of neurons that express the receptor as well as those that do not.
In summary, cortical projection neurons appear to be far more diverse than expected. Substantial progress has been made in our understanding of the mechanisms by which some core phenotypes of primary projection neuron classes emerge during development. Ongoing efforts to generate an accurate and complete catalog of mature cortical projection neurons will inform questions regarding the mechanisms through which these neurons further diversify. Additionally, descriptions of the transcriptional profiles of single neurons during the period that projection neurons undergo lineage bifurcations will illuminate the dynamic developmental infrastructure of the cortex as initial microcircuit assembly occurs, which ultimately underlie cortical computations. These later aspects of cortical circuit development are the focus of the following sections. These maturation processes begin to elaborate late prenatally (in human) and continue postnatally (in both rodent and human), over a very extended period of time in primates (Kostovic et al., 2014). The relationship between microcircuit development and cell-type specification should be recognized as interdependent, given that the processes share some temporal overlap, and there are now mechanistic examples linking cell-fate specification and re-programming to specific microcircuit patterns of connectivity.
4. DEVELOPMENT OF LOCAL EXCITATORY CORTICAL MICROCIRCUITRY
The many molecules that regulate the early diversification of cortical neuron subtypes function within the nucleus of the developing cell to drive transcriptional programs that lead to the differentiation of specific neuronal classes (Greig et al., 2013). However, the mechanisms responsible for the highly-stereotyped local and long-range connectivity that forms between these diverse neuron classes must involve cell-cell interactions occurring at the surface of the developing neurons (de Wit and Ghosh, 2016). A modest number of molecules, such as certain receptor tyrosine kinases, are known to play important roles in some aspects of class-specific axon guidance (Torii and Levitt, 2005; Torii et al., 2013a; Fothergill et al., 2014; Lodato et al., 2014; Srivatsa et al., 2014). However, there are likely additional molecules that regulate the development of other key aspects of synaptic specificity, which remain to be discovered. Recent progress has been made in defining details of mature local synaptic connectivity in specific cortical areas at higher resolution (Harris and Shepherd, 2015). This has created opportunities to investigate developmental mechanisms in a more systematic fashion (Huang, 2014). Novel and unexpected details regarding intracortical synaptic organization and cell-type characteristics have emerged with new levels of resolution. The newest findings in this research area continue to highlight a theme of this review – the diversity of cortical neurons extends far beyond our current classification scheme. Given that there is still much to be learned about more discrete cortical neuron subtypes, this section focuses on what is known about the development of canonical cortical circuits. We emphasize that these circuit motifs are described in terms of cell classes that will likely be parsed further, leading to more precise understanding of cortical development and function.
The canonical diagram of the cortical microcircuit is characterized by sensory-specific thalamocortical neurons terminating densely in layer 4 of their appropriate partner primary sensory cortical region. The axons of core-type thalamic neurons make monosynaptic connections with excitatory (and inhibitory) neurons within layer 4 of primary sensory cortices (Figure 4). These layer 4 neurons then provide parallel input to layer 2/3 pyramidal neurons. Layer 2/3 then provides dense output to layer 5 via collateralization of axons targeting the contralateral hemisphere. The layer 5 PT neurons are the final node in the cerebral cortical microcircuit as these neurons serve as the principle output conduit for information transmitted to subcortical targets. Yet this microcircuit wiring diagram has become significantly more complicated over the years. For example, layer 4 is no longer considered the sole target of core-type thalamocortical input. Layer 5B and 6A also receive dense projections from core-type thalamus and are activated in vivo on a similar timescale and with a similar response magnitude as layer 4 neurons (Constantinople, 2013). Moreover, second-order thalamic nuclei project to primary sensory cortex, but mostly target layer 5a and layer 1 (Petreanu et al., 2009; Wimmer et al., 2010). Thus, there are multiple input and output channels to a single cortical column. In this section, descriptions focus on summarizing some of the recent progress toward mapping the developmental emergence of the cortical wiring diagram. A recent observation is that substantial remodeling of connectivity patterns between specific cell populations, such as subplate neurons (see below), of the immature cortex is a core feature of microcircuit development. Thus, perturbation of normally transient connections can have long-lasting effects on features of cortical microcircuitry, including thalamocortical connectivity (Marques-Smith et al., 2016; Tuncdemir et al., 2016). In this section, the few molecular mechanisms contributing to synaptic maturation discovered to date are discussed, accompanied by the presentation of some current opportunities for exploring neuron class-specific mechanisms that may contribute to the maturation of stereotyped cortical circuitry.
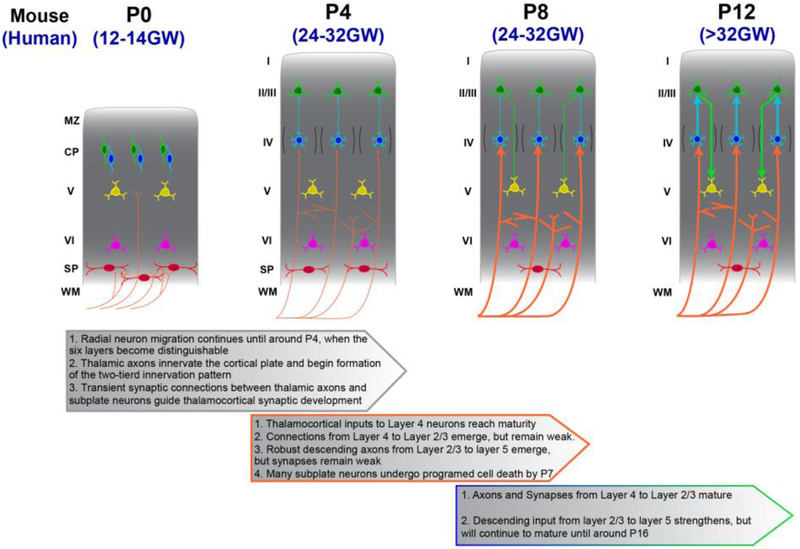
Figure 4. The temporal development of local coritcal microcircuity.
In mice, thalamocortical (TC) axons (orange) begin to innervate the developing cortex around birth (P0) before superficial layer neurons (blue and green) have finished migrating (this begins around the 12th week of gestation in humans). Radial migration concludes around P4, in mice (in humans the six layers of cortex are fully distinguishable by the 28th week of gestation), a timepoint at which immature synapses between TC axons and layer IV neurons are present. These TC to layer IV synapses mature through AMPA-receptor insertion between P4 and P8 (denoted by thickening of orange lines, and appearance of arrowheads at P8; increases in TC innervation of the human cortical plate continues between the 24th and 30th week of gestation). Meanwhile immature connections between layer IV and layer II/III, and between layer II/III and layer V begin to develop. These later developing synapsese mature between P8 and P16 (denoted by thickening of green and blue lines, and appearance of arrowheads; in humans, these later processes occur from approximately the 32nd week of gestation through several months of postnatal development). MZ, marginal zone; CP, cortical plate; WM, white matter.
The concept of the cortical column has existed for approximately 80 years since the histological studies of Lorente de No in the 1930s (Lorente de No, 1933, 1938). Vernon Mountcastle followed roughly 20 years later with the physiological description of the shared response properties of vertically aligned neurons observed in extracellular recordings of the cat somatosensory cortex (Mountcastle, 1957). The associated “canonical microcircuit” of cortical columns is typically defined as comprising an intermingled set of mini-columns, of unspecified number, which are thought to be elementary units of cortical development, organization and information processing (Mountcastle, 1997; Mountcastle, 2003). It is important to note that while the defining anatomical representation of the ‘minicolumn’ may be recognizable across species (Geschwind and Rakic, 2013; Harris and Shepherd, 2015), there remains disagreement regarding i) a precise definition, ii) its presence across all of the cerebral cortex (da Costa and Martin, 2010; Rockland, 2010; Defelipe et al., 2012), and iii) evidence for it being the smallest functional unit of the canonical column (Horton and Adams, 2005). Irrespective of the reasonable debates related to a single defining concept, columnar organization of the neocortex exists, and neuroscientists have sought a detailed account of the synaptic organization of neurons that make up the cortical minicolumn (Douglas et al., 1989; Harris and Mrsic-Flogel, 2013; Harris and Shepherd, 2015). Efforts to define this local circuitry have employed diverse methods, including three-dimensional anatomical reconstruction of individual neurons and their relation to thalamic afferents, in vivo and ex vivo electrophysiological recordings, and most recently, channel-rhodopsin assisted circuit mapping (CRACM), among others (Petreanu et al., 2007; Petreanu et al., 2009; Ko et al., 2011; Oberlaender et al., 2012). These efforts provide a detailed map of the remarkably consistent and highly precise wiring of cortical neuron subtypes that are vertically aligned across the six layers of the neocortex. The translation of how this microcircuitry is established during development may be relevant for understanding the hypothesized disease vulnerabilities of minicolumn organization and glutamatergic neuron subtypes based on their molecular and microcircuit identities (Parikshak et al., 2013; Willsey et al., 2013; Hutsler and Casanova, 2016).
The precise synaptic connections of the cortex are established during the first weeks of postnatal development (in rodents), as reflected at a coarse level by the dramatic increase in cortical synapse density during the second postnatal week of life (Micheva and Beaulieu, 1996). Details regarding the timing and progression of the development of specific connections have started to be characterized. Beginning with thalamocortical innervation, these details are described here in the serial order of the sequential pathways (‘hodology’) underlying common descriptions of the canonical microcircuit (Figure 4) (Harris and Shepherd, 2015).
The ontogeny and specialized functions of the transient subplate neurons, which are among the first neurons to be generated in the developing cortex, have been realized through four decades of studies in many mammalian species (Kostovic and Rakic, 1990; Kanold and Luhmann, 2009; Kostovic and Judas, 2010; Hoerder-Suabedissen and Molnar, 2015; Duque et al., 2016). Subplate neurons reside at the interface between the deepest layers of the cortical plate and the subcortical white matter during development, but undergo programmed cell death in early postnatal development to a variable extent across species (Hoerder-Suabedissen and Molnar, 2015). In rodents, thalamic axons reach the subplate in the final days of prenatal development and invade the cortical plate approximately at the time of birth (Agmon et al., 1993; Lopez-Bendito and Molnar, 2003) (In humans, this occurs much earlier, approximately at the end of the first trimester (Krsnik et al., 2017)). The earliest synapses formed in the developing cortex are transient synapses between thalamocortical axons and subplate neurons, which appear critical to subsequent formation of connectivity between the thalamus and cortex, as ablation of subplate neurons early in development impairs thalamocortical innervation patterns and the maturation of thalamus to layer 4 synapses (Ghosh et al., 1990; Ghosh and Schatz, 1994; Kanold et al., 2006a). The importance of the transient thalamus to subplate synapses is partly emphasized by the fact that, in ferrets, subplate neurons are the first cortical neurons to respond to peripheral sensory input (Wess et al., 2017). Additionally, glutamatergic synaptic connections between subplate neurons and cortical plate neurons undergo dynamic remodeling during early postnatal development in rodents (in humans, during the third trimester) that contributes to the later maturation of functional connections between thalamus and layer 4 (Friauf and Shatz, 1991; Hanganu et al., 2002; Kanold et al., 2006b; Tolner et al., 2012; Viswanathan et al., 2012; Nagode et al., 2017). In neonates, subplate cells are coupled by gap junctions with other neurons in the same cortical columns and are required for the acetylcholinegenerated oscillations in the beta frequency range that precede NMDA receptor-driven columnar activity (Dupont et al., 2006; Hanganu et al., 2009). In rodents, many subplate neurons undergo a process of programmed cell death during early postnatal development leaving a very sparse population of cells at the interface between layer 6 and the subcortical white matter (Price et al., 1997; Hoerder-Suabedissen and Molnar, 2013; Marx et al., 2017), sometimes referred to as layer 6B. In primates, a larger population survives as superficial and deep interstitial neurons that are situated in subcortical white matter (Kostovic and Rakic, 1980; Judas et al., 2010; Judas et al., 2013; Mortazavi et al., 2017). As some rodent subplate/layer 6B neurons project axons tangentially over long intracortical distances (Mitchell and Macklis, 2005; Kast et al., 2017), the process of subplate programmed cell death may contribute to remodeling of long-distance intra-areal connectivity that is discussed in the following section on the development of long-range intracortical connectivity.
The thalamocortical axons continue their growth past the subplate and into the cortical plate forming a two-tiered innervation pattern, with arborizations at the interface between layers 5 and 6 (lower tier), and a prominent arborization within layer 4 (upper tier) (Agmon et al., 1993). The thalamocortical axons branch extensively in the lower tier between postnatal (P) 1 and P5, while the arborization in the upper tier starts to form around P3 and continues to expand through axon branching until at least P12 (Agmon et al., 1993). Early layer 4 thalamocortical connections are characterized as “silent synapses”, containing NMDA receptors, but not AMPA receptors (Isaac et al., 1997). These thalamocortical synapses in layer 4 mature through a long term potentiation (LTP)-like process involving postsynaptic AMPA receptor insertion and alteration of NMDA receptor kinetics until P8, when their strength plateaus and becomes insensitive to experimentally-induced LTP (Crair and Malenka, 1995; Isaac et al., 1997). In mice, it has been shown that the connection between thalamus and layer 4 neurons strengthens during the second postnatal week relative to thalamic inputs to layer 6, as individual layer 4 neurons begin to receive convergent input from multiple thalamocortical axons (Crocker-Buque et al., 2015). Additionally, recent studies in the mouse suggest that the typical maturation of thalamic input to layer 4 neurons requires remodeling of transient patterns of connectivity involving infragranular somatostatin-positive (SST) interneurons (Marques-Smith et al., 2016; Tuncdemir et al., 2016). Strong, but transient, thalamic input to these SST interneurons in the first postnatal week (Tuncdemir et al., 2016) and the formation and subsequent disassembly of reciprocal connections between layer 4 neurons and the infragranular SST interneurons (Marques-Smith et al., 2016) appear critical to the typical maturation of thalamic input to parvalbumin-positive interneurons and glutamatergic layer 4 neurons. Thus, integration of thalamic inputs by cortical microcircuits is a complex process that undergoes substantial synaptic remodeling. In mice, this remodeling takes place primarily in the first 10 days of postnatal development. Importantly, it appears that specific cell types, such as subplate neurons and infragranular SST+ neurons, provide transient synaptic scaffolds that guide the later development of stable thalamocortical circuits. Thus, improper specification of these important neuron types would likely impact the formation of later developing thalamocortical circuits in a lasting manner, which further highlights the complex, interdependent link between cell-type specification and local circuit wiring.
The development of the unidirectional layer 4 to layer 2/3 pathway is best understood in rodents, and occurs in an overlapping, but slightly later time window compared to the thalamocortical connection. Specifically, laser-scanning photostimulation (LSPS) experiments in the rat barrel cortex demonstrated that the strength of layer 4 to layer 2/3 connectivity increases dramatically between P8 and P16 (Bureau et al., 2004). This increase in synaptic input is paralleled by a major increase in arborization of layer 4 axons in layer 2/3 over the same period (Bender et al., 2003; Bureau et al., 2004). The strength of layer 4 input to layer 2/3 neurons varies as a function of layer 2/3 neuron depth, with neurons in lower layer 2/3 receiving relatively stronger input from layer 4 than neurons in the upper portion of layer 2/3 (Bureau et al., 2004; Staiger et al., 2015). As with the thalamocortical synapses described in layer 4, synapses onto layer 2/3 neurons gradually transition from a silent state to their mature form through postsynaptic AMPA receptor insertion and NMDA receptor subunit switching, with silent synapses largely absent by P12 in rats and mice (Mierau et al., 2004; Busetto et al., 2008). Interestingly, layer 2/3 synapses exhibit a unique developmental trajectory in maturation compared to deeper layer neurons (Rumpel et al., 2004). Specifically, layer 2/3 neurons in rat visual cortex have been shown to form functionally active synapses early postnatally, and only form silent synapses later, which eventually transition to maturity through AMPA receptor insertion. The functional implications of this pattern are unknown.
The development of connectivity between layer 2/3 and layer 5 occurs concurrent with the formation of the layer 4 to layer 2/3 pathway. The patterns of maturation after initial synaptogenesis are complex, with differences defined by sub-laminar location of layer 5 target neurons. Initially, weak, yet functional, AMPA receptor-containing synapses connect layer 2/3 and layer 5 neurons as early as P5 in mice (Anastasiades and Butt, 2012). These observations are consistent with the robust collateralization of descending layer 2/3 axons within layer 5, which begins around P3 and is nearly complete by P7 (Srivatsa et al., 2015). However, the local input connectivity of layer 5 neurons appears diffuse, with equivalent input coming from layer 5 and layer 2/3 during the first postnatal week. Moreover, many of the synapses between layer 2/3 and layer 5 neurons are functionally silent, consisting mostly of NMDA receptors at P5 (Anastasiades and Butt, 2012). Input from layer 2/3 strengthens during the second postnatal week to become the predominant intracortical excitatory drive to layer 5 neurons by P13, thus resembling the mature circuit. The strength of layer 2/3 input also has been reported to depend on the identity of the postsynaptic layer 5 neuron in terms of both sublaminar position and projection identity (Anderson et al., 2010). Specifically, corticostriatal neurons positioned in the lower half of layer 5A, and corticospinal neurons positioned in upper layer 5B, receive strong inputs from layer 2/3. In contrast, retrogradely-labeled corticostriatal and corticospinal neurons located in other subcompartments of layer 5 received almost no layer 2/3 input. Thus, the local wiring of cortical projection neuron subtypes is highly complex, with pairing of pre- and post-synaptic partners relating to multiple features of cell identity, including, at least, laminar position and efferent projections.
There is compelling evidence that the development of differences in the local connectivity of cortical neuron subtypes is under molecular control. This evidence comes from studies involving the reprogramming of cortical neuron identity by ectopic expression of the corticospinal selector gene, Fezf2 (De la Rossa et al., 2013; Rouaux and Arlotta, 2013; Ye et al., 2015). High-level Fezf2 expression is normally restricted to PT-type neurons of layer 5B, and regulates the proper development of their efferent axonal development (Molyneaux et al., 2005). These layer 5 neurons receive strong local synaptic input from layer 2/3, whereas layer 4 neurons do not (Petreanu et al., 2007). Remarkably, overexpression of Fezf2 in postnatal layer 4 IT neurons, well after their generation prenatally, results in the acquisition of many phenotypes of PT-type neurons (Figure 3, see preceding text on cell-type specification). This includes the receipt of synaptic inputs from layer 2/3 (De la Rossa et al., 2013). The recruitment of layer 2/3 synapses by Fezf2-expressing neurons (either in layer 5B, or ectopically in layer 4) is likely mediated by cell-cell interactions occurring between layer 2/3 axons and the dendrites of Fezf2-positive neurons. Thus, one potentially fruitful avenue for identifying the mechanism responsible for the formation of this circuit motif may involve identifying cell surface proteins that are normally enriched in layer 5 neurons, are induced by Fezf2-overexpression in layer 4, and that are capable of recruiting layer 2/3 synaptic input when ectopically expressed in layer 4.
The molecular mechanisms downstream of Fezf2 responsible for local circuit wiring remain unclear, but one study identified a pair of proteins critical to the formation of the layer 2/3 to layer 5 connections. Specifically, the complementary expression of the secreted protein, Sonic hedgehog (Shh), and its membrane-bound receptor, Boc, are important for establishing this circuit (Harwell et al., 2012). Shh is expressed selectively by corticofugal neurons in layer 5B, whereas Boc is expressed in neurons of layers 2/3, 4, and 5a. Deletion of either molecule causes a selective and marked reduction in the strength of connectivity between layer 2/3 and layer 5, whereas intralaminar connectivity between layer 2/3 neurons is unaffected. The impaired connectivity appears to involve a reduction in the number of presynaptic specializations formed by descending layer 2/3 axons, as well as reduced dendritic arborization and spine formation by layer 5 neurons. It unknown whether the Shh and Boc interaction in this circuit operates directly downstream of Fezf2, or if separate Fezf2-dependent mechanisms operate in parallel to control formation of this local circuit motif. As proposed for the identification of molecules downstream of Fezf2 that mediate circuit wiring, a clear next step is to determine whether ectopic expression of Shh in layer 4 neurons can induce the formation of atypical layer 2/3 to layer 4 synaptic connectivity.
The genetic mechanisms regulating other components of the canonical microcircuit are less well understood. However, some genes that regulate the formation of synapses onto specific classes of cortical neurons have been identified, with the clearest examples involving cortical inhibitory interneurons. One well-characterized case involves Neuregulin-1 (Nrg-1) signaling through its tyrosine kinase receptor, Erbb4. Erbb4 is selectively expressed by parvalbumin (PV)-positive interneurons in the neocortex and hippocampus (Fazzari et al., 2010). Mutation of Erbb4 leads to reduced excitatory input onto PV-positive interneurons through a cell-intrinsic mechanism. Additionally, Erbb4 mutation causes reduced axo-axonic GABAergic synapses formed by PV+ chandelier cells at the axon initial segment of pyramidal neurons (Fazzari et al., 2010; Del Pino et al., 2013). The phenotypes involving PV+ interneurons of Erbb4 mutant mice occur in the absence of altered excitatory connectivity between neighboring pyramidal neurons. Therefore, this ligand-receptor pair influences specific sets of synapses formed by a discrete class of cortical neurons, while sparing connectivity between other classes of neurons.
Selective genetic control over the development of specific cortical synapses also occurs via the interaction of the cytokine Cxcl12 with its receptors Cxcr4 and Cxcr7. Cxcl12 expression is highly enriched in layer 5 neurons during postnatal development, and becomes almost completely restricted to layer 5 of medial prefrontal cortex after P14 (Wu et al., 2016). The cytokine receptors Cxcr4 and Cxcr7 are expressed by PV interneurons. Conditional deletion of Cxcl12 from layer 5 neurons leads to a selective reduction of perisomatic inhibitory synapses onto layer 5 neurons. This selective deficit was apparent as a reduction in PV+ and Gad65+ terminals around the somata of layer 5 neurons, a decrease in the probability of connectivity (measured in paired recordings) between pairs of PV+ interneurons and layer 5 pyramidal cells, and reduced inhibitory postsynaptic potentials recorded in layer 5 neurons (Wu et al., 2016). It remains to be determined whether this mechanism regulates the formation of the same layer 5 inhibitory microcircuit motif in other cortical areas, but this is plausible given that Cxcl12 is broadly expressed in layer 5 neurons in other regions of the cortex during postnatal development.
While there is an assumption that different PN subclasses exist in all cortical areas, discoveries relating to the mechanisms that drive the formation of the highly-stereotyped circuitry within each cortical area will be facilitated by achieving consensus regarding the classification of discrete projection neuron classes based on multiple phenotypic criteria (Tasic et al., 2016; Kast et al., 2017; Zeng and Sanes, 2017). Despite the current lack of a mechanistic understanding, developmental studies suggest that molecules implicated in late aspects of neuronal maturation (e.g. cell-type differentiation, dendritic elaboration, and synaptogenesis) are, in some cases, expressed heterogeneously within current subclasses of projection neurons during the period of circuit formation, as recently demonstrated (Kast et al., 2017). Recent studies that defined the developmental expression patterns of the MET receptor tyrosine kinase reveal the potential complexity of heterogeneous expression of a maturation-related gene in cortical projection neurons. MET is expressed by limited subsets of IT and PT projection neurons during postnatal development (Kast et al., 2017). There is mounting evidence that cortical neurons in Met mutant mice display atypical dendritic and synaptic phenotypes (Eagleson et al., 2017). Biochemical and immunohistological analyses indicate that expression of MET protein in the neocortex is highly dynamic, beginning late prenatally, rising dramatically to its peak between P7-10, and then decline over the next week (Judson et al., 2009; Eagleson et al., 2016; Peng et al., 2016). Ultrastructural studies further indicate that MET protein is enriched in developing cortical neuropil, particularly axons and immature pre- and postsynaptic elements (Eagleson et al., 2013). Because MET appears to function locally within nascent synapses of specific subtypes of IT and PT cortical neurons, it is positioned to directly regulate the stabilization and strengthening of their synaptic contacts (Qiu et al., 2014; Eagleson et al., 2016; Peng et al., 2016; Xie et al., 2016). It is also possible that signaling through the MET receptor influences the differentiation of specific IT and PT neuron subtypes, in a similar way to Fezf2 mutants, which could be accompanied by shifts in cell-type specific dendritic and synaptic phenotypes. Consistent with such a cell-type differentiation function, MET signaling is critical to relatively late stages of cell fate determination in neurons of the dorsal root ganglion (Gascon et al., 2010). Efficient means of teasing apart such mechanisms in the cortex would be made clearer by working toward an accurate and complete categorization of subtypes of IT, PT and CT neurons both in maturity and during development, and thereafter defining expression of genes of interest within more refined categories. This categorization would provide new opportunities to determine whether the appropriate projection neuron subtypes develop in mutant mice (such as Met and other mutants), and provide opportunities to apply advanced targeting strategies for these same neuron types to be investigated electrophysiologically across genotypes. This is particularly important to interpreting the function of genes such as Met, for which non-cell-autonomous effects are apparent in mutant mice, which may obscure primary cellular gene functions (Helmbacher et al., 2003; Judson et al., 2010).
The neuronal heterogeneity described by these studies underscores the importance of future efforts to define the developmental diversity of projection neuron phenotypes and the mechanisms responsible for influencing expression of uniquely combined structural, electrophysiological and molecular features. These data will be particularly important to understand the substantial heterogeneity reported in the mature cortex (Harris and Shepherd, 2015; Zeisel et al., 2015; Tasic et al., 2018), which appears to subdivide the three distinct classes of projection neurons that have been the primary focus of developmental studies (Greig et al., 2013). As more detailed descriptions of cortical neuron types begin to provide clearer means of targeting specific cortical neuron types in a consistent manner, it will be important to re-evaluate current concepts about the organization of cortical microcircuits as exemplified by new insights regarding infragranular SST+ neurons (Naka et al., 2018). Moreover, this information will provide new opportunities to explore how the fine-grained details of local and long-range circuits emerge developmentally and become disrupted in disease states.
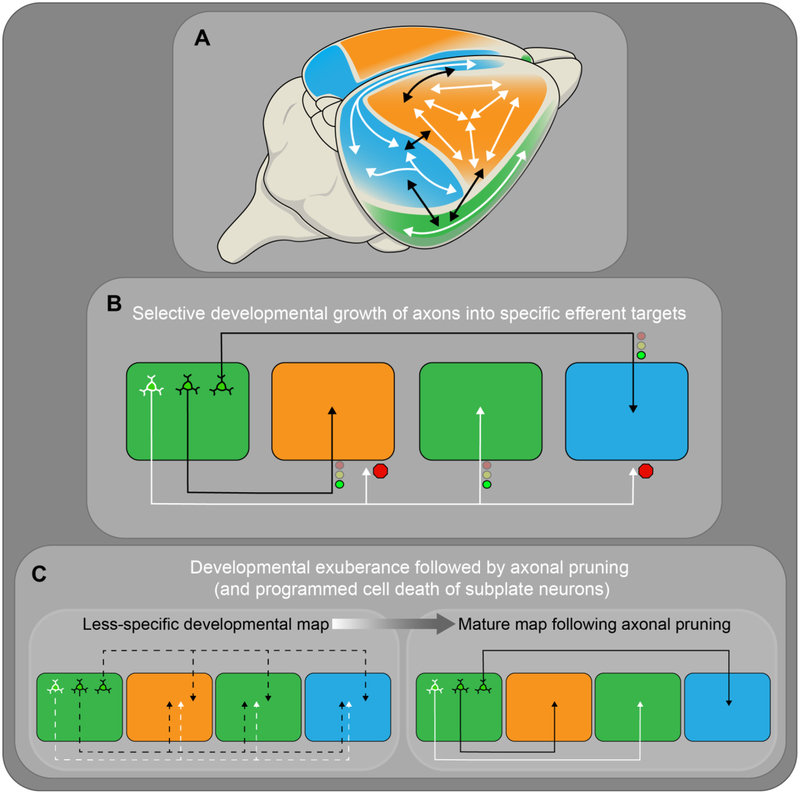
5. DEVELOPMENT OF LONG-RANGE CORTICAL CONNECTIVITY
So far, the development of the neocortex has been described at several levels of resolution, ranging from macro-level cortical area production to cell fate specification and wiring of cortical neuron subtypes. An equally impressive and functionally important level of cortical organization is the highly-stereotyped pattern of connectivity that forms between tangentially distributed cortical areas. Comprehensive characterization of such corticocortical connectivity has been generated in rats and mice (Oh et al., 2014; Zingg et al., 2014; Bota et al., 2015; Swanson et al., 2017; Gamanut et al., 2018), contributing to a well-defined macro-level “cortical connectome”. Despite the substantial improvements in the resolution and completeness of intracortical connectivity maps, knowledge regarding the mechanisms by which these maps emerge during development remains very limited. Much of the literature describing the developmental progression of intracortical connectivity focuses on a small number of cortical areas, such as the visual cortex, and primarily involves the interhemispheric connections formed through the corpus callosum. But, in fact, the number of distinct association connections formed between ipsilateral cortical areas (on the order of two thousand distinct connections) outnumber those formed between areas in contralateral hemispheres approximately four to one (Swanson et al., 2017). Nonetheless, studies of callosal development and of corticofugal projections have begun to reveal mechanisms that regulate development of cortical projection topography, and thus may provide insight into how cortical networks emerge. This section reflects on these topics, with a focus on rodent data, and highlights some investigative opportunities that could be addressed by integrating concepts from the developmental literature with recent data describing mature cortical connectivity. It should be noted that in humans, the expansion of association areas and the concomitant connectivity, for which emergence is evident by the end of the second trimester and continues postnatally (Kostovic et al., 2014), also reflects the importance of investing more effort to gain a more detailed understanding of the mechanisms that build cortical networks (Raznahan et al., 2012; Geschwind and Rakic, 2013).
In rodents, the connectivity that forms between tangentially separated cortical areas develops over the first two to three weeks of postnatal life (Ivy and Killackey, 1982; Ozaki and Wahlsten, 1998; Mitchell and Macklis, 2005; Berezovskii et al., 2011). Within specific strains of rats and mice, patterns of connectivity are highly consistent across individuals. To what degree such stereotyped connectivity is genetically constrained or sensitive to experience is unclear. At present, there are examples that provide evidence to support contributions of both intrinsic genetic programming and extrinsic influences (Innocenti and Frost, 1979; Huffman et al., 2004; Karlen et al., 2006; Larsen et al., 2009), but mechanistic details remain sparse. One potential approach to address the genetic mechanisms is to interrogate the cortical area and cell-type specific expression of genes with demonstrated importance in axon guidance, dendritic development, and synapse formation (Sanes and Yamagata, 2009; de Wit and Ghosh, 2016). For example, the classic cadherins and non-clustered protocadherins are known to exhibit region- and cell-type specific expression in the developing and mature cortex (Suzuki et al., 1997; Krishna et al., 2011). The important role that these proteins can play in the development of stereotyped connectivity is exemplified by recent studies of retinal circuit wiring (Duan et al., 2014). Thus, investigating these, and other cell adhesion molecules in developing cortical circuitry ought to be a priority.
To provide a framework for understanding how this connectivity might develop and to contextualize the limited, but relevant mechanistic examples, it is useful to outline some basic phenomena that have been observed during cerebral cortical development. First, it should be acknowledged that there are several strategies through which stereotyped patterns of intracortical connectivity could form. One possibility is that, during development, exuberant axonal projections from each cortical area develop in a diffuse and non-specific manner to many cortical areas, but only a subset of the initially promiscuous projections stabilizes to form the persistent connections observed in the mature cortical connectome. Alternatively, there could be pre-determined and highly directed growth of axons from cortical regions to only those specific areas that will maintain their inputs in the mature map. These scenarios represent the extremes of possible developmental mechanisms, and the current lack of comprehensive developmental analysis during the period of formation of these connections limits research-supported conclusions. However, evidence from a select number of cortical areas exists to support the involvement of some aspects of both developmental exuberance and selective growth, suggesting that intracortical connectivity develops through a combination of strategies.
The earliest examples of large scale exuberance in the developing cortex came from studies that identified an abundance of callosal projection neurons located in cortical regions during development that contain little to no callosal projections in the adult (Innocenti, 1981; Ivy and Killackey, 1981; O'Leary et al., 1981). These early studies, and those that followed, demonstrated that the “dropping out” of callosal neurons from these cortical areas during development is due to the selective elimination of callosal axons, rather than the death of the neurons that had formed the transient callosal projections (O'Leary et al., 1981). Interestingly, some of these transient callosal neurons maintain an ipsilateral projection to the frontal cortex (Ivy and Killackey, 1982), suggesting that the pruning process involves the selective deletion of a subset of axon collaterals. Whereas substantial programmed cell death of postmitotic projection neurons has not been reported in the developing cortical plate, programmed cell death of subplate neurons is prominent, particularly in primates, and could contribute to developmental refinement of cortical connectivity [Figure 5C; (Kostovic and Rakic, 1990; Hoerder-Suabedissen and Molnar, 2015)]. The developmental principle of selective pruning of collateral axons has been shown in many brain circuits, including ipsilateral corticocortical as well as corticofugal projections (Stanfield et al., 1982; Price and Blakemore, 1985; Callaway and Katz, 1990; O'Leary, 1992). Thus, there appears to be some degree of “non-specific”, exuberant projections that forms during the elaboration of cortical connections, which is later refined to produce the mature pattern of connectivity (Figure 5C) (Price and Blakemore, 1985; Innocenti and Price, 2005).
Figure 5. Development of intracortical association networks of the rodent neocortex.
Recent graph-theoretical meta-analyses of cortical association connections in rats (Swanson et al., 2017) and comprehensive tracing of homologous connecitons in mice (Zingg et al., 2014) suggest a core-shell arrangement of cortical connections that consists of at least 3 networks distinguished by increased within network interconnectivity (white arrows). The core (orange) is surrounded by the shell, which is comprised of medial (blue) and lateral portions (green). Importantly, connections between the three distinct networks are common, but tend to be less pronounced, with the exception of those relayed through hub regions such as the entorhinal, temporal association, and posterior parietal cortices (between network connections denoted by black arrows). B) Restricted and selective axonal growth of developing intracortical neurons into the cortical targets that receive input from those neurons in adulthood. The green circles (green light beneath dimmed yellow and red circles) represent the presence of growth permissive or growth promoting signals for appropriate axons, whereas the red octagons (stop signs) indicate the absence of growth promoting signals for some axons or potential presence of axonal growth inhibiting signals. C) Widespread developmental axon outgrowth of intracortical projection neurons, followed by refinement of connectivity maps through selective axonal pruning.
Despite these clear examples of developmentally exuberant projection patterns, there is evidence to suggest that restricted and selective axonal growth also plays a role in the development of intracortical circuitry (Figure 5B). For example, early anterograde labeling studies demonstrated that callosal axons densely innervate only the areas of the developing cortex that will retain callosal innervation in the adult (Innocenti, 1981; Ivy and Killackey, 1981). These studies demonstrated that transient axonal projections branch extensively within the subplate and white matter in a less specific manner, but axonal projections that will persist innervate appropriate layers of the cortex proper in areas that will receive persistent afferent projections in the adult (Innocenti and Price, 2005). This phenomenon of selective axonal growth into specific gray matter territories of the contralateral cortex also has been observed in more recent studies employing in utero electroporation of fluorescent protein reporters (Fenlon et al., 2017). Additionally, one study in the developing primate found that association projections between ipsilateral area V2 and V4 form through a process of directed axonal growth and target selection (Barone et al., 1996). In this study, projections from V2 to V4 were shown to arise from clusters of neurons located in acetylcholinesterase (AChE) dense bands within area V2, and that these clusters could be observed from very early stages of development. There were only very modest V2 to V4 transient projections arising from the AChE-negative “interbands”, and these interband neurons were primarily observed in early development in injection cases that involved tracer penetration of the white matter. Together, these observations were interpreted as an accumulation of developmentally transient axons in the white matter beneath V4, followed by target selection and selective growth of axons derived from neurons clustered in the AChE-dense bands and retraction of axons derived from the AChE-negative interband neurons. The factors that drive the competence of specific axons to grow into select gray matter regions, such as axons from the neurons of the AChE-dense bands into V4, and other axons less able to do so, are unknown. Yet these data suggest that molecular interactions between the axons and the recipient cortical area may be involved.
Although there are currently few examples of molecular mechanisms guiding the development of specific patterns of intracortical connectivity, studies of thalamocortical and corticothalamic projections have identified specific membrane-bound receptors and their ligands that are required to establish proper patterns of afferent and efferent cortical connectivity with subcortical structures (Vanderhaeghen et al., 2000; Dufour et al., 2003; Lopez-Bendito and Molnar, 2003; Torii and Levitt, 2005). Some of the most striking examples come from early work on Eph receptor tyrosine kinases and their ephrin ligands in instructing reciprocal wiring between the cortex and thalamus. Ephs and ephrins are expressed in complementary gradients within the cortex and thalamus (Vanderhaeghen et al., 2000; Sestan et al., 2001). These gradients are critical for the proper topography of connections from thalamic nuclei to their target cortical areas (Dufour et al., 2003; Cang et al., 2005), as well as the reciprocal topography of the feedback projections from cortex to thalamus (Torii and Levitt, 2005; Torii et al., 2013a). In most cases, perturbations of the normal EphA or ephrin-A expression gradients cause expansion or compression of the topographic representation of axonal inputs within otherwise appropriate cortical or thalamic areas (Cang et al., 2005; Torii and Levitt, 2005; Torii et al., 2013a). However, ectopic wiring of thalamocortical projections to distinct cortical areas (rather than topographic intra-areal remapping) also has been observed in some Eph and ephrin mutant mice (Uziel et al., 2002; Dufour et al., 2003). Together, these studies emphasize at least two distinct roles for Eph/ephrin signaling in the development of extrinsic cortical connections. One mechanism involves directed growth of axon pathways at intermediate stages, and a second mechanism involves instructing the topographic innervation by incoming axons during the final stage of target selection. Whether Ephs and ephrins play a similar role in instructing intracortical connectivity (within the developing cortex proper) is unclear. It is highly plausible, however, given the differential expression of these molecules across the cortical mantle and the role of these molecules in instructing many CNS circuit maps beyond those described here (Flanagan, 2006; Torii et al., 2013b).
Together, the examples described above illustrate several processes that should be considered in formulating models of how intracortical connectivity develops (Figure 5). First, a certain degree of exuberance in the connectivity of cortical neurons seems to occur, with transient projections reaching, at least, the white matter and subplate of non-target cortical areas (Figure 5C). These transient projections are eventually eliminated, contributing to the well-known refinement of intracortical connectivity postnatally. Second, an interaction between growing axons and potential cortical targets seems to mediate a target selection process in which axons penetrate primarily those cortical areas in which innervation will persist in the adult (Figure 5B). Third, evidence suggests that a final phase of selective axonal elaboration and stabilization within specific layers of cortical target areas is dissociable from each of the first two phases described (Fenlon et al., 2017). These final stages of corticocortical axon elaboration and stabilization within proper targets uniquely depend on spontaneous, and sensory-evoked, electrical activity of developing cortical projection neurons (Mizuno et al., 2007; Wang et al., 2007; Suarez et al., 2014; Fenlon et al., 2017). Changes in electrical activity underlie plasticity of cortical wiring in the context of altered sensory experience (Suarez et al., 2014) and are under transcriptional control during normal cortical development (Rodriguez-Tornos et al., 2016). Experiments that highlight the experience-dependent malleability of cortical wiring demonstrate the capacity of experience (with major contributions from altered patterns of neural activity) to epigenetically modulate circuit phenotypes that are encoded in the genome. Such epigentic flexibility has been proposed to provide adaptability of developing cortical circuits that may serve to maximize the computational capacities that are specifically relevant to the environment in which an organism develops (Krubitzer and Prescott, 2018). However, even in the context of a clear capacity for experience-dependent change, significant constriants on cortical phenotypes are imposed by genetically encoded developmental processes (Krubitzer and Prescott, 2018). Although the mechanisms that control each of the foundational phases of long-distance cortical wiring have yet to be fully elucidated, the concepts outlined here provide a framework for designing experiments to probe such mechanisms.
Each mature cortical area has efferent projections that reach a larger number of multiple cortical target areas than previously appreciated (Figure 5A) (Zingg et al., 2014; Swanson et al., 2017). Few studies, however, have addressed the degree to which divergent output projections arise from distinct neurons projecting to distinct subsets of downstream cortical areas, or divergent collateral axon projections arising from a common population of cortical neurons residing in a specific cortical area. There are examples demonstrating the former scenario of parallel cortical output channels arising from distinct neuron populations (Berezovskii et al., 2011; Yamashita et al., 2013; Fenlon et al., 2017). However, there are also examples of projection neurons forming collaterals to multiple cortical areas (Schwartz and Goldman-Rakic, 1982; Bai et al., 2004; Mitchell and Macklis, 2005; Cederquist et al., 2013). Traditionally, comprehensive evaluation of the degree to which individual cortical neurons send outputs to single versus multiple cortical target areas was labor intensive and technically very challenging. However, the recent development of a method known as multiplexed analysis of projections by sequencing (MAPseq) has enabled high-throughput analysis of single-cell projection phenotypes (Kebschull et al., 2016). Application of MAPseq to primary visual cortex revealed that most layer 2/3 cortical projection neurons target multiple cortical areas, and that there is non-random organization of the outputs to these divergent cortical targets (Han et al., 2018). The data from this study support the conclusion that there is substantial diversity of IT-type cortical projection neurons that selectively innervate subsets of interconnected cortical areas. From this example, it seems that most cortical projection neurons target multiple areas, but do so with some degree of selectivity. Thus, a critical next step will involve defining the molecules that specific subtypes of IT-type projection neurons express during the period when selective innervation patterns are established, as well as the intrinsic and experience-dependent mechanisms that drive the complexity of IT axon targeting. Toward this end, a recent study combined single cell RNA-sequencing and MAPseq analysis of developing intracortical projection neurons to integrate cellular resolution gene expression to emerging connectivity motifs (Klingler et al., 2018). This type of approach promises to inform hypothesis-driven experiments regarding the genetic mechanisms that regulate the development of intracortical networks.
Finally, recent analysis of intracortical connectivity in the rat identified a network topology consisting of three modules in each cerebral hemisphere that are organized in a core-shell arrangement (Bota et al., 2015; Swanson et al., 2017). Each module is composed of cortical areas that are more densely interconnected with one another than they are with cortical areas in the other modules, but interconnections between modules also are an important feature that tends to route through highly-connected “hub” regions (Figure 5). Remarkably, although the modules were identified in a manner naive to topographical relationships between cortical areas, each module was found to consist of cortical areas that were topographically continuous across the cortical mantle. The first module forms the core of the core-shell arrangement and includes many cortical areas that are dedicated to processing sensory information and producing motor output, such as somatosensory, visual, auditory, and motor cortices as well as the posterior parietal and temporal association cortices. The second module forms the lateral aspect of the shell and comprises cortical areas that are centrally involved in interoceptive processing and short-term memory, among other functions. Some of the cortical areas in module 2 include gustatory, visceral, agranular insula and medial prefrontal cortex, as well as most of the hippocampal formation. The third module forms the medial bank of the shell component of the core-shell arrangement, and comprises a network that overlaps in many ways with the default mode network (Raichle et al., 2001). The cortical areas in module 3 include orbital and premotor areas, retrosplenial and anterior cingulate cortex, and the pre-, post-, and parasubiculum (Swanson et al., 2017). It is noteworthy that a similar network topology has been described in the mouse neocortex (Zingg et al., 2014), with some differences that might be attributable to the stricter focus on isocortical areas, in contrast to the inclusion of allocortical regions in the analysis of the rat connectome.
The complex, network level organization described above has yet to be explored in a developmental context, but this must form rapidly in the rodent, given our understanding of the timing of the onset and completion of intracortical connectivity formation. There has been some speculation about distinct classes of intracortical neurons potentially contributing to a modular developmental process that could underlie the assembly of intracortical connectivity motifs, with an emphasis on sensory cortical hierarchies (Harris and Shepherd, 2015; Han et al., 2018). However, more detailed consideration of the macro-level cortical connectivity described in recent network analysis may reveal additional entry points for identifying how cortical networks are produced during development. For example, do neurons that project to cortical areas outside of their home module also send collateral axons to other cortical areas within their own module? Or, are there distinct populations of neurons that route information across module “boundaries” and others that project strictly to other areas within the same module? Are there distinct sets of neurons that project to specific subsets of areas within the same network module? This last question has been partially addressed in some cortical areas. For example, in primary visual cortex it appears that most IT type neurons project to multiple higher visual areas (Han et al., 2018). In primary somatosensory cortex, distinct projection neuron subtypes have been identified that project to secondary somatosensory cortex or primary motor cortex (Yamashita et al., 2013). Similarly, in higher visual areas, distinct projection neurons send feedforward or feedback projections, but not both (Berezovskii et al., 2011). This level of detail is important because the role of genetics in establishing connectivity likely operates at the level of individual cortical neurons, in which molecules can dictate the responsivity of genetically-defined axons to cues in potential target areas. Thus, a focused analysis of intracortical connectivity at the single neuron level, bearing in mind the organization of the macro-level network modules, may provide novel insights regarding the construction of cortical networks. Additionally, mapping gene expression onto the network module framework during the period of circuit formation could provide additional insight. In this regard, it seems important to determine whether there are molecular signatures unique to each module. Such signatures could function to restrict the growth of most axons to territories within the home module, or to promote the cross-module growth or stabilization of axons that project in such a manner. Once the degree of selectivity and divergence of efferent projections has been established, correlating gene expression with unique neuronal projection patterns through retrograde tracing and molecular profiling would further inform hypotheses regarding the mechanisms underlying intracortical development.
6. CONCLUDING REMARKS
From the initial neuron birthdating reports in the early 1960s, approximately sixty years of modern studies on cerebral cortical development have led to a remarkable accumulation of descriptions, in many mammalian species, that have focused on neuron production and migration. These descriptions naturally led to mechanistic studies identifying the genes that underlie the regulation of neuronal specification regarding their laminar fate and general projection classes. Factors have been discovered that regulate cell migration behavior during the very important period of neuronal deployment to their final position in the developing cerebral cortex. The general rules that govern neuronal targeting, through the establishment of major axon pathways and innervation patterns has been established. Yet, as this review has highlighted, the diversity of mature projection neuron types appears to far exceed the original taxonomy, based on two types of evidence – large-scale connectomics analyses and transcriptomics with single cell resolution. Selective disruption of distinct cortical circuits due to genetic or environmental insults may contribute to the unique pathophysiological processes that distinguish various neurodevelopmental disorders, but hope of identifying selective phenotypes in various disease states will depend on more detailed descriptions of cortical neurons and the circuits in which they are embedded. New methods, such as MAPseq, are being used to provide high-throughput targeted analysis of connectivity of single projection neurons. These data will help determine the connectivity rules. Efforts in mapping gene expression onto a cortical network module framework will yield important information that will help determine relationships between a cell’s connectome and transcriptome. Yet, these efforts must be applied ultimately during the period of circuit formation to understand heterogeneity, the degree of selectivity and divergence of efferent and afferent cortical projections and addressing hypotheses regarding the mechanisms underlying development and maturation of circuits that ultimately underlie complex cognitive, social and emotional functions. The developmental details are particularly important given the growing application of model systems such as brain organoids generated from iPS cells produced from cells harvested from typical, neurologic and psychiatric patients (Quadrato and Arlotta, 2017; Marsoner et al., 2018; Pasca, 2018). Both the promise and limitations of the models will emerge from understanding the details of cortical networks and the neurons from which they arise.
Acknowledgements:
We are grateful to our colleagues Drs. Kathie Eagleson, Simona Lodato, Ron McKay, and Alexandra Lanjewar for their suggestions and critical reading of the manuscript. This work was supported by the Simms/Mann Chair in Developmental Neurogenetics, WM Keck Chair in Neurogenetics and NIH grant R01 MH067842 (PL) and Children’s Hospital Los Angeles Research Career Development Fellowship (RJK).
ABBREVIATIONS
- PN
Projection Neuron
- CT
Corticothalamic Neuron
- PT
Pyramidal Tract Neuron
- IT
Intratelencephalic Neuron
- DRG
Dorsal Root Ganglion
- LTP
Long Term Potentiation
- LSPS
Laser Scanning Photostimulation
- SST
Somatostatin
- PV
Parvalbumin
- AChE
Acetylcholinesterase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmon A, Yang LT, O’Dowd DK, Jones EG (1993) Organized Growth of Thalamocortical Axons from the Deep Tier of Terminations into Layer IV of Developing Mouse Barrel Cortex. The Journal of Neuroscience 13:5365–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farin I, Grosschedl R, McConnell SK (2008) Satb2 Regulates Callosal Projection Neuron Identity in the Developing Cerebral Cortex. Neuron 57:364–377. [DOI] [PubMed] [Google Scholar]
- Anastasiades PG, Butt SJB (2012) A Role for Silent Synapses in the Development of the Pathway from Layer 2/3 to 5 Pyramidal Cells in the Neocortex. The Journal of Neuroscience 32:13085–13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CT, Sheets PL, Kiritani T, Shepherd GM (2010) Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat Neurosci 13:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine JJ, Sidman R (1961) Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse.. Nature:766–768. [DOI] [PubMed] [Google Scholar]
- Arlotta PM BJ;, Chen JI J; Kominami R;, Macklis J (2005) Neuronal Subtype-Specific Genes that Control Corticospinal Motor Neuron Development In Vivo. Neuron 45:207–211. [DOI] [PubMed] [Google Scholar]
- Armentano M, Chou SJ, Tomassy GS, Leingartner A, O'Leary DD, Studer M (2007) COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci 10:1277–1286. [DOI] [PubMed] [Google Scholar]
- Ascenzi M, Bony G (2017) The building of the neocortex with non-hyperpolarizing neurotransmitters. Dev Neurobiol 77:1023–1037. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos S, Kao T, Issa NP, Grove EA (2012) Fibroblast growth factor 8 organizes the neocortical area map and regulates sensory map topography. J Neurosci 32:7191–7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Shnider SJ, Cederquist GY, Sohur US, Macklis JD (2009) Lmo4 and Clim1 progressively delineate cortical projection neuron subtypes during development. Cereb Cortex 19 Suppl 1: i62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai WZ, Ishida M, Arimatsu Y (2004) Chemically defined feedback connections from infragranular layers of sensory association cortices in the rat. Neuroscience 1:257–267. [DOI] [PubMed] [Google Scholar]
- Baker A, Kalmbach B, Morishima M, Kim J, Juavinett A, Li N, Dembrow N (2018) Specialized Subpopulations of Deep-Layer Pyramidal Neurons in the Neocortex: Bridging Cellular Properties to Functional Consequences. Journal of Neuroscience 38:5441–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler RC, Mayer C, Fishell G (2017) Cortical interneuron specification: the juncture of genes, time and geometry. Curr Opin Neurobiol 42:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando Y, Irie K, Shimomura T, Umeshima H, Kushida Y, Kengaku M, Fujiyoshi Y, Hirano T, Tagawa Y (2016) Control of Spontaneous Ca2+ Transients Is Critical for Neuronal Maturation in the Developing Neocortex. Cereb Cortex 26:106–117. [DOI] [PubMed] [Google Scholar]
- Barbe MF, Levitt P (1991) The Early Commitment of Fetal Neurons to the Limbic Cortex. The Journal of Neuroscience 11:519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MF, Levitt P (1992) Attraction of specific thalamic input by cerebral grafts depends on the molecular identity of the implant. PNAS 89:3706–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P, Dehay C, Berland M, Kennedy H (1996) Role of Directed Growth and Target Selection in the Formation of Cortical Pathways: Prenatal Development of the Projection of Area V2 to Area V4 in the Monkey. The Journal of Coparative Neurology 1:1–20. [DOI] [PubMed] [Google Scholar]
- Behar T, Scott C, Greene C, Wen X, Smith S, Maric D, Liu Q, Colton C, Barker J (1999) Glutamate Acting at NMDA Receptors Stimulates Embryonic Cortical Neuronal Migration. Journal of Neuroscience 19:4449–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KJ, Rangel J, Feldman DE (2003) Development of Columnar Topography in the Excitatory Layer 4 to Layer 2/3 Projection in Rat Barrel Cortex. The Journal of Neuroscience 23:8759–8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezovskii VK, Nassi JJ, Born RR (2011) Segregation of Feedforward and Feedback Projections in Mouse Visual Cortex. The Journal of Coparative Neurology 18:3672–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O'Leary DDM (2000) Regulation of Area Identity in the Mammalian Neocortex by Emx2 and Pax6. Science 288:344–349. [DOI] [PubMed] [Google Scholar]
- Bota M, Sporns O, Swanson LW (2015) Architecture of the cerebral cortical association connectome underlying cognition. Proc Natl Acad Sci U S A 112:E2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, Sestan N, Molnar Z, Tarabykin V (2008) Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron 57:378–392. [DOI] [PubMed] [Google Scholar]
- Broca P (1865) Sur le siège de lafaculté du langage articulé.. Bull Soc Anthropol Paris 337–393. [Google Scholar]
- Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde (Comparative localization in the Cerebral Cortex).. Leipzig: Johann Ambrosius Barth. [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K (2004) Precise development of functional and anatomical columns in the neocortex. Neuron 42:789–801. [DOI] [PubMed] [Google Scholar]
- Busetto G, Higley MJ, Sabatini BL (2008) Developmental presence and disappearance of postsynaptically silent synapses on dendritic spines of rat layer 2/3 pyramidal neurons. J Physiol 586:1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, Reimer J, Shen S, Bethge M, Tolias KF, Sandberg R, Tolias AS (2016) Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol 34:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC (1990) Emergence and refinement of clustered horizontal connections in cat striate cortexway and Katz. The Journal of Neuroscience 4:1134–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Kaneko M, Yamada J, Woods G, Stryker MP, Feldheim DA (2005) Ephrin-as guide the formation of functional maps in the visual cortex. Neuron 48:577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caronia-Brown G, Yoshida M, Gulden F, Assimacopoulos S, Grove EA (2014) The cortical hem regulates the size and patterning of neocortex. Development 141:2855–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness V (1980) The developmental consequences of abnormal cell position in the reeler mouse. Trends in Neurosciences 3:31–33. [Google Scholar]
- Cederquist GY, Azim E, Shnider SJ, Padmanabhan H, Macklis JD (2013) Lmo4 establishes rostral motor cortex projection neuron subtype diversity. J Neurosci 33:6321–6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK (2005a) Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. PNAS 102:17184–17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK (2008) The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. PNAS 105:11382–11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Arlotta P (2016) Seq-ing the cortex one neuron at a time. Nat Neurosci 19:179–181. [DOI] [PubMed] [Google Scholar]
- Chen J-G, Rašin M-R, Kwan KY, Šestan N (2005b) Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. PNAS 102:17792–17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevee M, Robertson JJ, Cannon GH, Brown SP, Goff LA (2018) Variation in Activity State, Axonal Projection, and Position Define the Transcriptional Identity of Individual Neocortical Projection Neurons. Cell Rep 22:441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL (2007) Patterning of frontal cortex subdivisions by Fgf17. PNAS 104:7652–7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL (2008) Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17, and Emx2. J Comp Neurol 509:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S-J, Babot Z, Leingärtner A, Studer M, Nakagawa Y, O’Leary DDM (2013) Geniculocortical Input Drives Genetic Distinctions Between Primary and Higher-Order Visual Areas. Science 340:1239–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CMB, Randy M. (2013) Deep Cortical Layers Are Activated Directly by Thalamus. Science 340:1591–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Malenka RC (1995) A critical period for long-term potentiation of thalamocortical synapses. Nature 375:325–328. [DOI] [PubMed] [Google Scholar]
- Crocker-Buque A, Brown SM, Kind PC, Isaac JT, Daw MI (2015) Experience-Dependent, Layer-Specific Development of Divergent Thalamocortical Connectivity. Cereb Cortex 25:2255–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley P, Martin G (1995) The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121:439–451. [DOI] [PubMed] [Google Scholar]
- da Costa NM, Martin KA (2010) Whose Cortical Column Would that Be? Front Neuroanat 4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Rossa A, Bellone C, Golding B, Vitali I, Moss J, Toni N, Luscher C, Jabaudon D (2013) In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nat Neurosci 16:193–200. [DOI] [PubMed] [Google Scholar]
- de Wit J, Ghosh A (2016) Specification of synaptic connectivity by cell surface interactions. Nat Rev Neurosci 17:22–35. [DOI] [PubMed] [Google Scholar]
- Defelipe J, Markram H, Rockland K (2012) The neocortical column. Front Neuroanat 6:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay C, Horsburgh G, Berland M, Killackey H, Kennedy H (1989) Maturation and connectivity of the visual cortex in monkey is altered by prenatal removal of retinal input. Nature 337:265–267. [DOI] [PubMed] [Google Scholar]
- Del Pino I, Garcia-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martinez de Lagran M, Ciceri G, Gabaldon MV, Moratal D, Dierssen M, Canals S, Marin O, Rico B (2013) Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron 79:1152–1168. [DOI] [PubMed] [Google Scholar]
- Desai AR, McConnell SK (2000) Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development:2863–2872. [DOI] [PubMed] [Google Scholar]
- Deschenes MB J; Pinault D (1994) Corticothalamic projections from layer V cells in rat are collaterals of long-range corticofugal axons Brain Research:215–219. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KAC, Whitteridge D (1989) A Canonical Microcircuit for Neocortex. Neural Computation:480–488. [Google Scholar]
- Duan X, Krishnaswamy A, De la Huerta I, Sanes JR (2014) Type II cadherins guide assembly of a direction-selective retinal circuit. Cell 158:793–807. [DOI] [PubMed] [Google Scholar]
- Dufour A, Seibt J, Passante L, Depaepe V, Ciossek T, Frisén J, Kullander K, Flanagan JG, Polleux F, Vanderhaeghen P (2003) Area Specificity and Topography of Thalamocortical Projections Are Controlled by ephrin/Eph Genes. Neuron 39:453–465. [DOI] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ (2006) Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature 439:79–83. [DOI] [PubMed] [Google Scholar]
- Duque A, Krsnik Z, Kostovic I, Rakic P (2016) Secondary expansion of the transient subplate zone in the developing cerebrum of human and nonhuman primates. Proc Natl Acad Sci U S A 113:9892–9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Xie Z, Levitt P (2017) The Pleiotropic MET Receptor Network: Circuit Development and the Neural-Medical Interface of Autism. Biol Psychiatry 81:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Milner TA, Xie Z, Levitt P (2013) Synaptic and extrasynaptic location of the receptor tyrosine kinase met during postnatal development in the mouse neocortex and hippocampus. J Comp Neurol 521:3241–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Lane CJ, McFadyen-Ketchum L, Solak S, Wu HH, Levitt P (2016) Distinct intracellular signaling mediates C-MET regulation of dendritic growth and synaptogenesis. Dev Neurobiol 76:1160–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckler MJ, Nguyen TD, McKenna WL, Fastow BL, Guo C, Rubenstein JLR, Chen B (2015) Cux2-positive radial glial cells generate diverse subtypes of neocortical projection neurons and macroglia. Neuron 86:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economo MN, Viswanathan S, Tasic B, Bas E, Winnubst J, Menon V, Graybuck LT, Nguyen TN, Smith KA, Yao Z, Wang L, Gerfen CR, Chandrashekar J, Zeng H, Looger LL, Svoboda K (2018) Distinct descending motor cortex pathways and their roles in movement. Nature 563:79–84. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP (2012) Development and plasticity of the primary visual cortex. Neuron 75:230–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B (2010) Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 464:1376–1380. [DOI] [PubMed] [Google Scholar]
- Fenlon LR, Suarez R, Richards LJ (2017) The anatomy, organisation and development of contralateral callosal projections of the mouse somatosensory cortex. Brain and Neuroscience Advances:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JG (2006) Neural map specification by gradients. Curr Opin Neurobiol 16:59–66. [DOI] [PubMed] [Google Scholar]
- Fothergill T, Donahoo AL, Douglass A, Zalucki O, Yuan J, Shu T, Goodhill GJ, Richards LJ (2014) Netrin-DCC signaling regulates corpus callosum formation through attraction of pioneering axons and by modulating Slit2-mediated repulsion. Cereb Cortex 24:1138–1151. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, Muller U (2012) Fate-restricted neural progenitors in the mammalian cerebral cortex. Science 337:746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz GD, McConnell S (1996) Restriction of Late Cerebral Cortical Progenitors to an Upper-Layer Fate. Neuron 17:55–61. [DOI] [PubMed] [Google Scholar]
- Friauf E, Shatz C (1991) Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. Journal of Neurophysiology 66:2059–2071. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA (2001) Neocortex Patterning by the Secreted Signaling Molecule FGF8. Science 294:1071–1074. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA (2003) Emx2 patterns the neocortex by regulating FGF signaling. Nature Neuroscience 6. [DOI] [PubMed] [Google Scholar]
- Fuzik J, Zeisel A, Mate Z, Calvigioni D, Yanagawa Y, Szabo G, Linnarsson S, Harkany T (2016) Integration of electrophysiological recordings with single-cell RNA-seq data identifies neuronal subtypes. Nat Biotechnol 34:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamanut R, Kennedy H, Toroczkai Z, Ercsey-Ravasz M, Van Essen DC, Knoblauch K, Burkhalter A (2018) The Mouse Cortical Connectome, Characterized by an Ultra-Dense Cortical Graph, Maintains Specificity by Distinct Connectivity Profiles. Neuron 97:698–715 e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Postiglione MP, Krieger TG, Hernandez L, Wang C, Han Z, Streicher C, Papusheva E, Insolera R, Chugh K, Kodish O, Huang K, Simons BD, Luo L, Hippenmeyer S, Shi SH (2014) Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 159:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S (2003) Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development 130:1903–1914. [DOI] [PubMed] [Google Scholar]
- Gascon E, Gaillard S, Malapert P, Liu Y, Rodat-Despoix L, Samokhvalov IM, Delmas P, Helmbacher F, Maina F, Moqrich A (2010) Hepatocyte growth factor-Met signaling is required for Runx1 extinction and peptidergic differentiation in primary nociceptive neurons. J Neurosci 30:12414–12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind D, Rakic P (2013) Cortical Evolution: Judge the Brain by its Cover. Neuron 80:633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Schatz CJ (1994) Segregation of geniculocortical afferents during the crticial period: a role for subplate neurons. The Journal of Neuroscience 6:3862–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ (1990) Requirement for subplate neurons in the formation of thalamocortical connections. Nature 347:179–181. [DOI] [PubMed] [Google Scholar]
- Gil-Sanz C, Espinosa A, Fregoso SP, Bluske KK, Cunningham CL, Martinez-Garay I, Zeng H, Franco SJ, Muller U (2015) Lineage Tracing Using Cux2-Cre and Cux2-CreERT2 Mice. Neuron 86:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golonzhka O, Nord A, Tang Paul LF, Lindtner S, Ypsilanti Athena R, Ferretti E, Visel A, Selleri L, Rubenstein John LR (2015) Pbx Regulates Patterning of the Cerebral Cortex in Progenitors and Postmitotic Neurons. Neuron 88:1192–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan S, Oberst P, Jabaudon D (2018) In vivo pulse labeling of isochronic cohorts of cells in the central nervous system using FlashTag. Nat Protoc 13:2297–2311. [DOI] [PubMed] [Google Scholar]
- Greig LC, Woodworth MB, Greppi C, Macklis JD (2016) Ctip1 Controls Acquisition of Sensory Area Identity and Establishment of Sensory Input Fields in the Developing Neocortex. Neuron 90:261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD (2013) Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci 14:755–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L-w, Ragsdale CW (1998) The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3 deficient mice. Development 125. [DOI] [PubMed] [Google Scholar]
- Guo C, Eckler MJ, McKenna WL, McKinsey GL, Rubenstein JL, Chen B (2013) Fezf2 expression identifies a multipotent progenitor for neocortical projection neurons, astrocytes, and oligodendrocytes. Neuron 80:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T, Leingartner A, Ringstedt T, O'Leary DD (2004) EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron 43:359–372. [DOI] [PubMed] [Google Scholar]
- Han W, Kwan KY, Shim SS, Lam MMS, Shin Y, Xu X, Zhu Y, Li M, Šestan N (2011) TBR1 directly represses Fezf2 to control the laminar origin and development of the corticospinal tract. PNAS 108:3041–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kebschull JM, Campbell RAA, Cowan D, Imhof F, Zador AM, Mrsic-Flogel TD (2018) The logic of single-cell projections from visual cortex. Nature 556:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ (2002) Functional Synaptic Projections onto Subplate Neurons in Neonatal Rat Somatosensory Cortex. Journal of Neuroscience 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Okabe A, Lessmann V, Luhmann HJ (2009) Cellular mechanisms of subplate-driven and cholinergic input-dependent network activity in the neonatal rat somatosensory cortex. Cereb Cortex 19:89–105. [DOI] [PubMed] [Google Scholar]
- Harris KD, Mrsic-Flogel TD (2013) Cortical connectivity and sensory coding. Nature 503:51–58. [DOI] [PubMed] [Google Scholar]
- Harris KD, Shepherd GM (2015) The neocortical circuit: themes and variations. Nat Neurosci 18:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwell CC, Parker PR, Gee SM, Okada A, McConnell SK, Kreitzer AC, Kriegstein AR (2012) Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron 73:1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y, Namikawa T, Yamauchi K, Kawaguchi Y (2016) Cortical Divergent Projections in Mice Originate from Two Sequentially Generated, Distinct Populations of Excitatory Cortical Neurons with Different Initial Axonal Outgrowth Characteristics. Cereb Cortex 26:2257–2270. [DOI] [PubMed] [Google Scholar]
- He´ bert JM, Mishina Y, McConnell SK (2002) BMP Signaling Is Required Locally to Pattern the Dorsal Telencephalic Midline. Neuron 35:1029–1041. [DOI] [PubMed] [Google Scholar]
- Helmbacher F, Dessaud E, Arber S, deLapeyrière O, Henderson CE, Klein R, Maina F (2003) Met Signaling Is Required for Recruitment of Motor Neurons to PEA3-Positive Motor Pools. Neuron 39:767–777. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Molnar Z (2013) Molecular diversity of early-born subplate neurons. Cereb Cortex 23:1473–1483. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Molnar Z (2015) Development, evolution and pathology of neocortical subplate neurons. Nat Rev Neurosci 16:133–146. [DOI] [PubMed] [Google Scholar]
- Horton JC, Adams DL (2005) The cortical column: a structure without a function. Philos Trans R Soc Lond B Biol Sci 360:837–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ (2014) Toward a genetic dissection of cortical circuits in the mouse. Neuron 83:1284–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman KJ, Garel S, Rubenstein JL (2004) Fgf8 Regulates the Development of Intra-Neocortical Projections. The Journal of Neuroscience 41:8917–8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler JJ, Casanova MF (2016) Review: Cortical construction in autism spectrum disorder: columns, connectivity and the subplate. Neuropathol Appl Neurobiol 42:115–134. [DOI] [PubMed] [Google Scholar]
- Innocenti GM (1981) Growth and Reshaping of Axons in the Establishment of Visual Callosal Connections. Science 212:824–827. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Frost DO (1979) Effects of visual experience on the maturation of the efferent system to the corpus callosum. Nature 280:231–234. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ (2005) Exuberance in the development of cortical networks. Nat Rev Neurosci 6:955–965. [DOI] [PubMed] [Google Scholar]
- Isaac JTR, Crair MC, Nicoll RA, Malenka RC (1997) Silent Synapses during Development of Thalamocortical Inputs. Neuron 18:269–280. [DOI] [PubMed] [Google Scholar]
- Ivy GO, Killackey HP (1981) The Ontogeny of the Distribution of Callosal Projection Neurons in the Rat Parietal Cortex. The Journal of Coparative Neurology 1981:367–389. [DOI] [PubMed] [Google Scholar]
- Ivy GO, Killackey H (1982) Ontogenetic changes in the projections of neocortical neurons The Journal of Neuroscience 2:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KF, Killackey HP (1984) Subcortical projections from ectopic neocortical neurons. PNAS 81:964–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judas M, Pletikos M, Jovanov-Milosevic N (2010) Populations of subplate and interstitial neurons in fetal and adult human telencephalon. Journal of Anatomy 217:381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judas M, Sedmak G, Kostovic I (2013) The significance of the subplate for evolution and developmental plasticity of the human brain. Fronteirs in Human Neuroscience 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Eagleson KL, Wang L, Levitt P (2010) Evidence of cell-nonautonomous changes in dendrite and dendritic spine morphology in the met-signaling-deficient mouse forebrain. J Comp Neurol 518:4463–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Bergman MY, Campbell DB, Eagleson KL, Levitt P (2009) Dynamic gene and protein expression patterns of the autism-associated met receptor tyrosine kinase in the developing mouse forebrain. J Comp Neurol 513:511–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn DM, Krubitzer L (2002) Massive cross-modal cortical plasticity and the emergence of a new cortical area in developmentally blind mammals. pNaS 99:11429–11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ (2009) The Subplate and Early Cortical Circuits. Annual Review of Neuroscience 33:23–48. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Schatz CJ (2006a) Role of Subplate Neurons in Functional Maturation of Visual Cortical Columns. Science 301:521–525. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ (2006b) Role of Subplate Neurons in Functional Maturation of Visual Cortical Columns. Science 301:521–525. [DOI] [PubMed] [Google Scholar]
- Karlen SJ, Kahn DM, Krubitzer L (2006) Early blindness results in abnormal corticocortical and thalamocortical connections. Neuroscience 142:843–858. [DOI] [PubMed] [Google Scholar]
- Kast RJ, Wu HH, Levitt P (2017) Developmental Connectivity and Molecular Phenotypes of Unique Cortical Projection Neurons that Express a Synapse-Associated Receptor Tyrosine Kinase. Cereb Cortex:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebschull JM, Garcia da Silva P, Reid AP, Peikon ID, Albeanu DF, Zador AM (2016) High-Throughput Mapping of Single-Neuron Projections by Sequencing of Barcoded RnA. Neuron 91:975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler E, Prados J, Kebschull JM, Dayer A, Zador AM, Jabaudon D (2018) Single-cell molecular connectomics of intracortically-projecting neurons. BioRxIV. [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD (2011) Functional specificity of local synaptic connections in neocortical networks. Nature 473:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester SE, O'Leary DDM (1993) Connectional Distinction Between Callosal and Subcortical Projection Neurons is Determined Prior to Axon Extension. Developmental Biology 160:1–14. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P (1980) Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. 1980 9:219–242. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P (1990) Developmental History of the Transient Subplate Zone in the Visual and Somatosensory Cortex of the Macaque Monkey and Human Brain. The Journal of Coparative Neurology 297:441–470. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M (2010) The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr 99:1119–1127. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Jovanov-Milosevic N, Rados M, Sedmak G, Benjak V, Kostovic-Srzentic M, Vasung L, Culjat M, Rados M, Huppi P, Judas M (2014) Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Struct Funct 219:231–253. [DOI] [PubMed] [Google Scholar]
- Krishna KK, Hertel N, Redies C (2011) Cadherin expression in the somatosensory cortex: evidence for a combinatorial molecular code at the single-cell level. Neuroscience 175:37–48. [DOI] [PubMed] [Google Scholar]
- Krsnik Z, Majic V, Vasung L, Huang H, Kostovic I (2017) Growth of Thalamocortical Fibers to the Somatosensory Cortex in the Human Fetal Brain. Front Neurosci 11:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Prescott TJ (2018) The Combinatorial Creature: Cortical Phenotypes within and across Lifetimes. Trends Neurosci 41:744–762. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Lam MMS, Krsnik Z, Kawasawa* YI, Lefebvre V, Sestan N (2008) SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. PNAS 105:16021–16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD (2008) SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron 57:232–247. [DOI] [PubMed] [Google Scholar]
- Larsen DD, Krubitzer L (2008) Genetic and epigenetic contributions to the cortical phenotype in mammals. Brain Res Bull 75:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen DD, Luu JD, Burns ME, Krubitzer L (2009) What are the Effects of Severe Visual Impairment on the Cortical Organization and Connectivity of Primary Visual Cortex? Front Neuroanat 3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leingärtner A, Richards LJ, Dyck RH, Akazawa C, O'Leary DD (2003) Cloning and Cortical Expression of Rat Emx2 and Adenovirus-mediated Overexpression to Assess its Regulation of Area-specific Targeting of Thalamocortical Axons. Cereb Cortex 13:648–660. [DOI] [PubMed] [Google Scholar]
- Levelt C, Hübener M (2012) Critical-Period Plasticity in the Visual Cortex. Annual Review of Neuroscience 35:309–330 [DOI] [PubMed] [Google Scholar]
- Levitt P (1997) New evidence for neurotransmitter influences on brain development. Trends in Neurosciences 20:269–293. [DOI] [PubMed] [Google Scholar]
- Li H, Fertuzinhos S, Mohns E, Hnasko TS, Verhage M, Edwards R, Sestan N, Crair MC (2013) Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron 79:970–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Mi D, Llorca A, Marin O (2018) Development and Functional Diversification of Cortical Interneurons. Neuron 100:294–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Rouaux C, Quast KB, Jantrachotechatchawan C, Studer M, Hensch TK, Arlotta P (2011) Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron 69:763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Molyneaux BJ, Zuccaro E, Goff LA, Chen HH, Yuan W, Meleski A, Takahashi E, Mahony S, Rinn JL, Gifford DK, Arlotta P (2014) Gene co-regulation by Fezf2 selects neurotransmitter identity and connectivity of corticospinal neurons. Nat Neurosci 17:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Molnar Z (2003) Thalamocortical development: how are we going to get there? Nat Rev Neurosci 4:276–289. [DOI] [PubMed] [Google Scholar]
- Lorente de No R (1933) Studies on the structure of the cerebral cortex I. The area entorhinalis. J Psychol Neurol 45:381–438. [Google Scholar]
- Lorente de No R (1938) Architectonics and structure of the cerebral cortex.: Oxford University Press. [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJS, Davis MBE, Kriegstein AR (1995) GABA and Glutamate Depolarize Cortical Progenitor Cells and Inhibit DNA Synthesis. Neuron 15:1287–1298. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Khazipov R (2018) Neuronal activity patterns in the developing barrel cortex. Neuroscience 368:256–267. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Pearlman AL, Sanes JR (1988) Cell lineage in the Cerebral Cortex of Mouse Studied In Vivo and In Vitro with a Recombinant Retrovirus. Neuron 1:635–647. [DOI] [PubMed] [Google Scholar]
- MacDonald JL, Fame RM, Gillis-Buck EM, Macklis JD (2018) Caveolin1 Identifies a Specific Subpopulation of Cerebral Cortex Callosal Projection Neurons (CPN) Including Dual Projecting Cortical Callosal/Frontal Projection Neurons (CPN/FPN). eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallamaci A, Muzio L, Chan C-H, Parnavelas J, Boncinelli E (2000) Area identity shifts in the early cerebral cortex of Emx2−/− mutant mice. Nature Neuroscience 3:679–686. [DOI] [PubMed] [Google Scholar]
- Marques-Smith A, Lyngholm D, Kaufmann AK, Stacey JA, Hoerder-Suabedissen A, Becker EB, Wilson MC, Molnar Z, Butt SJ (2016) A Transient Translaminar GABAergic Interneuron Circuit Connects Thalamocortical Recipient Layers in Neonatal Somatosensory Cortex. Neuron 89:536–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsoner F, Koch P, Ladewig J (2018) Cortical organoids: why all this hype? Curr Opin Genet Dev 52:22–28. [DOI] [PubMed] [Google Scholar]
- Marx M, Qi G, Hanganu-Opatz IL, Kilb W, Luhmann HJ, Feldmeyer D (2017) Neocortical Layer 6B as a Remnant of the Subplate - A Morphological Comparison. Cereb Cortex 27:1011–1026. [DOI] [PubMed] [Google Scholar]
- Mayer C, Hafemeister C, Bandler RC, Machold R, Batista Brito R, Jaglin X, Allaway K, Butler A, Fishell G, Satija R (2018) Developmental diversification of cortical inhibitory interneurons. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S (1988) Fates of Visual Cortical Neurons in the Ferret After lsochronic and Heterochronic Transplantation. The Journal of Neuroscience 8:945–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S, Kaznowski CE (1991) Cell Cycle Dependence of Laminar Determination in Developing Neocortex. Science 254:282–285. [DOI] [PubMed] [Google Scholar]
- McKenna WL, Betancourt J, Larkin KA, Abrams B, Guo C, Rubenstein JL, Chen B (2011) Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci 31:549–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G (2010) Building a human cortex: the evolutionary differentiation of Cajal-Retzius cells and the cortical hem. J Anat 217:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C (1996) Quantitative Aspects of Synaptogenesis in the Rat Barrel Field Cortex With Special Reference to GABA Circuitry. The Journal of Coparative Neurology 373:340–354. [DOI] [PubMed] [Google Scholar]
- Mierau SB, Meredith RM, Upton AL, Paulsen O (2004) Dissociation of experience-dependent and -independent changes in excitatory synaptic transmission during development of barrel cortex. PNAS 101:15518–15523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BD, Macklis JD (2005) Large-scale maintenance of dual projections by callosal and frontal cortical projection neurons in adult mice. J Comp Neurol 482:17–32. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Hirano T, Tagawa Y (2007) Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. J Neurosci 27:6760–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD (2005) Fezl is required for the birth and specification of corticospinal motor neurons. Neuron 47:817–831. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Fame RM, MacDonald JL, MacQuarrie KL, Macklis JD (2009) Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci 29:12343–12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Juan V, Filipchuk A, Antón-Bolaños N, Mezzera C, Gezelius H, Andrés B, Rodríguez-Malmierca L, Susín R, Schaad O, Iwasato T, Schüle R, Rutlin M, Nelson S, Ducret S, Valdeolmillos M, Rijli FM, López-Bendito G (2017) Prenatal thalamic waves regulate cortical area size prior to sensory processing. Nature Communications 8:14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi F, Romano S, Rosene D, Rockland K (2017) A Survey of White Matter Neurons at the Gyral Crowns and Sulcal Depths in the Rhesus Monkey. Fronteirs in Neuroanatomy 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle V (1957) Modality and Topographic Properties of Single Neurons of Cat's Somatosensory Cortex. Journal of Neurophysiology:408–434. [DOI] [PubMed] [Google Scholar]
- Mountcastle V (2003) Introduction. Computation in Coritcal Columns. Cereb Cortex 13:2–4. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB (1997) The columnar organization of the neocortex. Brain 120:701–722. [DOI] [PubMed] [Google Scholar]
- Nagode DA, Meng X, Winkowski DE, Smith E, Khan-Tareen H, Kareddy V, Kao JPY, Kanold PO (2017) Abnormal Development of the Earliest Cortical Circuits in a Mouse Model of Autism Spectrum Disorder. Cell Rep 18:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka A, Veit J, Shababo B, Chance R, Risso D, Stafford D, Snyder B, Egladyous A, Chu D, Sridharan S, Paninski L, Ngai J, Adesnik FI (2018) Complementary networks of cortical somatostatin interneurons enforce layer specific control. BioRxIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Johnson JE, O’Leary DDM (1999) Graded and Areal Expression Patterns of Regulatory Genes and Cadherins in Embryonic Neocortex Independent of Thalamocortical Input. Journal of Neuroscience 19:10877–10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DD (1989) Do Cortical Areas Emerge from a Protocortex? Trends in Neurosciences 12:400–406. [DOI] [PubMed] [Google Scholar]
- O'Leary DD (1992) Development of connectional diversity and the mammalian brain by the pruning of projections. Current Opinion in Neurobiology 2:70–77. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Stanfield BB, Cowan WM (1981) Evidence that the early postnatal restriction of the cells of origin of the callosal projection is due to the elimination of axonal collaterals rather than the death of neurons. Developmental Brain Research 1:607–617. [DOI] [PubMed] [Google Scholar]
- Oberlaender M, de Kock CP, Bruno RM, Ramirez A, Meyer HS, Dercksen VJ, Helmstaedter M, Sakmann B (2012) Cell type-specific three-dimensional structure of thalamocortical circuits in a column of rat vibrissal cortex. Cereb Cortex 22:2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst P, Fièvre S, Baumann N, Concetti C, Jabaudon D (2018) Apical progenitors remain multipotent throughout cortical neurogenesis. BioRxIV. [Google Scholar]
- Oh SW et al. (2014) A mesoscale connectome of the mouse brain. Nature 508:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR (2002) Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 3:715–727. [DOI] [PubMed] [Google Scholar]
- Ozaki HS, Wahlsten D (1998) Timing and Origin of the First Cortical Axons to Project Through the Corpus Callosum and the Subsequent Emergence of Callosal Projection Cells in Mouse. The Journal of Coparative Neurology:197–206. [DOI] [PubMed] [Google Scholar]
- Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V, Horvath S, Geschwind DH (2013) Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155:1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca S (2018) The rise of three-dimensional human brain cultures. Nature 553:437–445. [DOI] [PubMed] [Google Scholar]
- Peng Y, Lu Z, Li G, Piechowicz M, Anderson M, Uddin Y, Wu J, Qiu S (2016) The autism-associated MET receptor tyrosine kinase engages early neuronal growth mechanism and controls glutamatergic circuits development in the forebrain. Mol Psychiatry 21:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K (2007) Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10:663–668. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K (2009) The subcellular organization of neocortical excitatory connections. Nature 457:1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouchelon G, Gambino F, Bellone C, Telley L, Vitali I, Luscher C, Holtmaat A, Jabaudon D (2014) Modality-specific thalamocortical inputs instruct the identity of postsynaptic L4 neurons. Nature 511:471–474. [DOI] [PubMed] [Google Scholar]
- Price D, Aslam S, Tasker L, Gillies K (1997) Fates of the Earliest Generated Cells in the Developing Murine Neocortex. J Comp Neurol 377:414–422. [PubMed] [Google Scholar]
- Price DJ, Blakemore C (1985) Regressive events in the postnatal development of association projections in the visual cortex. Nature 316:721–724. [DOI] [PubMed] [Google Scholar]
- Qiu S, Lu Z, Levitt P (2014) MET receptor tyrosine kinase controls dendritic complexity, spine morphogenesis, and glutamatergic synapse maturation in the hippocampus. J Neurosci 34:16166–16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrato G, Arlotta P (2017) Present and future of modeling human brain development in 3D organoids. Curr Opin Cell Biol 49:47–52. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P (1974) Neurons in Rhesus Monkey Visual Cortex: Systematic Relation between Time of Origin and Eventual Disposition. Science 183:425–427. [DOI] [PubMed] [Google Scholar]
- Rakic P (1988) Specification of Cerebral Cortical Areas. Science 241:170–176. [DOI] [PubMed] [Google Scholar]
- Rakic P (2006) A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex 16 Suppl 1:i3–17. [DOI] [PubMed] [Google Scholar]
- Rakic P, Suner I, Williams RW (1991) A novel cytoarchitectonic area induced experimentally within the primate visual cortex. PNAS 88:2083–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Greenstein D, Lee N, Clasen L, Giedd J (2012) Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc Natl Acad Sci U S A 109:11366–11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS (2010) Five points on columns. Front Neuroanat 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Tornos F, Briz C, Weiss L, Sebastian-Serrano A, Ares S, Navarrete M, Frangeul L, Galazo M, Jabaudon D, Esteban J, Nieto M (2016) Cux1 Enables Interhemispheric Connections of Layer II/III Neurons by Regulating Kv1 Dependent Firing. Neuron 89:494–506. [DOI] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P (2010) Fezf2 directs the differentiation of corticofugal neurons from striatal progenitors in vivo. Nat Neurosci 13:1345–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P (2013) Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat Cell Biol 15:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Anderson S, Shi L, Miyashita-Lin EM, Bulfone A, Hevner R (1999) Genetic Control of Cortical Regionalization and Connectivity. Cereb Cortex 9:524–532. [DOI] [PubMed] [Google Scholar]
- Rumpel S, Kattenstroth G, Gottmann K (2004) Silent Synapses in the Immature Visual Cortex: Layer-Specific Developmental Regulation. Journal of Neurophysiology 91:1097–1101. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M (2009) Many paths to synaptic specificity. Annu Rev Cell Dev Biol 25:161–195. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Goldman-Rakic P (1982) Single cortical neurones have axon collaterals to ipsilateral and contralateral cortex in fetal and adult primates. Nature 299:154–155. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Rakic P, Goldman-Rakic PS (1991) Early phenotype expression of cortical neurons: Evidence that a subclass of migrating neurons have callosal axons. Proc Natl Acad Sci U S A 88:1354–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestan N, Rakic P, Donoghue MP (2001) Independent parcellation of the embryonic visual cortex and thalamus revealed by combinatorial Eph/ephrin gene expression. Current Biology 11:39–43. [DOI] [PubMed] [Google Scholar]
- Seung HS, Sumbul U (2014) Neuronal Cell Types and Connectivity: Lessons from the Retina. Neuron 83:1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogori T, Grove EA (2005) Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J Neurosci 25:6550–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Bookheimer SY, Buckner RL, Burgess GC, Curtiss SW, Dapretto M, Elam JS, Gaffrey MS, Harms MP, Hodge C, Kandala S, Kastman eK, Nichols TE, Schlaggar BL, Smith SM, Thomas KM, Yacoub E, Van Essen DC, Barch DM (2018) The Lifespan Human Connectome Project in Development: A large-scale study of brain connectivity development in 5-21 year olds. Neuroimage 183:456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasana K, Leonea DP, Batesona RK, Dobrevab G, Kohwic Y, Kohwi-Shigematsuc TG, Rudolf, McConnell SK (2012) A network of genetic repression and derepression specifies projection fates in the developing neocortex. PNAS 109:19071–19078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsa S, Parthasarathy S, Molnar Z, Tarabykin V (2015) Sip1 downstream Effector ninein controls neocortical axonal growth, ipsilateral branching, and microtubule growth and stability. Neuron 85:998–1012. [DOI] [PubMed] [Google Scholar]
- Srivatsa S, Parthasarathy S, Britanova O, Bormuth I, Donahoo AL, Ackerman SL, Richards LJ, Tarabykin V (2014) Unc5C and DCC act downstream of Ctip2 and Satb2 and contribute to corpus callosum formation. Nat Commun 5:3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger JF, Bojak I, Miceli S, Schubert D (2015) A gradual depth-dependent change in connectivity features of supragranular pyramidal cells in rat barrel cortex. Brain Struct Funct 220:1317–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield BB, O'Leary DD, Fricks C (1982) Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature 298:371–373. [DOI] [PubMed] [Google Scholar]
- Suarez R, Fenlon LR, Marek R, Avitan L, Sah P, Goodhill GJ, Richards LJ (2014) Balanced interhemispheric cortical activity is required for correct targeting of the corpus callosum. Neuron 82:1289–1298. [DOI] [PubMed] [Google Scholar]
- Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M (1997) Neuronal Circuits Are Subdivided by Differential Expression of Type-II Classic Cadherins in Postnatal Mouse Brains. Molecular and Cellular Neursocience 9:433–447. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hahn JD, Sporns O (2017) Organizing principles for the cerebral cortex network of commissural and association connections. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B et al. (2016) Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 19:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B et al. (2017) Shared and distinct transcriptomic cell types across neocortical areas. BioRxIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B et al. (2018) Shared and distinct transcriptomic cell types across neocortical areas. Nature 563:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telley L, Govindan S, Prados J, Stevant I, Nef S, Dermitzakis E, Dayer A, Jabaudon D (2016) Sequential transcriptional waves direct the differentiation of newborn neurons in the mouse neocortex. Science 351:1443–1446. [DOI] [PubMed] [Google Scholar]
- Tolner EA, Sheikh A, Yukin AY, Kaila K, Kanold PO (2012) Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J Neurosci 32:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Levitt P (2005) Dissociation of corticothalamic and thalamocortical axon targeting by an EphA7-mediated mechanism. Neuron 48:563–575. [DOI] [PubMed] [Google Scholar]
- Torii M, Rakic P, Levitt P (2013a) Role of EphA/ephrin--a signaling in the development of topographic maps in mouse corticothalamic projections. J Comp Neurol 521:626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Hackett TA, Rakic P, Levitt P, Polley DB (2013b) EphA signaling impacts development of topographic connectivity in auditory corticofugal systems. Cereb Cortex 23:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncdemir SN, Wamsley B, Stam FJ, Osakada F, Goulding M, Callaway EM, Rudy B, Fishell G (2016) Early Somatostatin Interneuron Connectivity Mediates the Maturation of Deep Layer Cortical Circuits. Neuron 89:521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel D, Muhlfriedel S, Zarbalis K, Wurst W, Levitt P, Bolz J (2002) Miswiring of Limbic Thalamocortical Projections in the Absence of Ephrin-A5. The Journal of Neuroscience 21:9352–9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA (1973) Somatosensory Cortex: Structural Alterations following Early Injury to Sense Organs. Science 179:395–398. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF (2018) Parcellating Cerebral Cortex: How Invasive Animal Studies Inform Noninvasive Mapmaking in Humans. Neuron 99:640–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen P, Lu Q, Prakash N, Frisén J, W CA, Frostig RD, Flanagan JG (2000) A mapping label required for normal scale of body representation in the cortex. Nature Neuroscience 3. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, Bandyopadhyay S, Kao JP, Kanold PO (2012) Changing microcircuits in the subplate of the developing cortex. J Neurosci 32:1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Parnavelas J (2003) The Role of Serotonin in Early Cortical Development. Developpmental Neuroscience 25:245–256. [DOI] [PubMed] [Google Scholar]
- Walsh C, Cepko CL (1988) Clonanlly Related Cortical Cells Show Several Migration Patterns. Science 241:1342–1245. [DOI] [PubMed] [Google Scholar]
- Wamsley B, Fishell G (2017) Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat Rev Neurosci 18:299–309. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang L, Zhou Y, Zhou J, Yang X, Duan S-QX, and Yu-Qiang Ding (2007) Activity-Dependent Development of Callosal Projections in the Somatosensory Cortex. The Journal of Neuroscience 27:11334–11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A (2003) GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol 550:785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess JM, Isaiah A, Watkins PV, Kanold PO (2017) Subplate neurons are the first cortical neurons to respond to sensory stimuli. Proc Natl Acad Sci U S A 114:12602–12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsey AJ et al. (2013) Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Bruno RM, de Kock CP, Kuner T, Sakmann B (2010) Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex. Cereb Cortex 20:2265–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth MB, Greig LC, Liu KX, Ippolito GC, Tucker HO, Macklis JD (2016) Ctip1 Regulates the Balance between Specification of Distinct Projection Neuron Subtypes in Deep Cortical Layers. Cell Rep 15:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PR, Cho KK, Vogt D, Sohal VS, Rubenstein JL (2016) The Cytokine CXCL12 Promotes Basket Interneuron Inhibitory Synapses in the Medial Prefrontal Cortex. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Eagleson KL, Wu HH, Levitt P (2016) Hepatocyte Growth Factor Modulates MET Receptor Tyrosine Kinase and beta-Catenin Functional Interactions to Enhance Synapse Formation. eNeuro 3:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Pala A, Pedrido L, Kremer Y, Welker E, Petersen CC (2013) Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron 80:1477–1490. [DOI] [PubMed] [Google Scholar]
- Yamawaki N, Borges K, Suter BA, Harris KD, Shepherd GM (2014) A genuine layer 4 in motor cortex with prototypical synaptic circuit connectivity. Elife 3:e05422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Mostajo-Radji MA, Brown JR, Rouaux C, Tomassy GS, Hensch TK, Arlotta P (2015) Instructing Perisomatic Inhibition by Direct Lineage Reprogramming of Neocortical Projection Neurons. Neuron 88:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA (2006) Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development 133:537–545. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Muñoz-Manchado A, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, Linnarsson S (2015) Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347:1138–1142. [DOI] [PubMed] [Google Scholar]
- Zeng H, Sanes JR (2017) Neuronal cell-type classification: challenges, opportunities and the path forward. Nat Rev Neurosci 18:530–546. [DOI] [PubMed] [Google Scholar]
- Zhou C, Tsai SY, Tsai M-J (2000) COUP-TFI: an intrinsic factor for early regionalization of the neocortex. Genes and Development:2054–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Hintiryan H, Gou L, Song MY, Bay M, Bienkowski MS, Foster NN, Yamashita S, Bowman I, Toga AW, Dong HW (2014) Neural networks of the mouse neocortex. Cell 156:1096–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]