Abstract
Temperature gradients in cities can cause inter-neighborhood differences in the timing of pollen release. However, most epidemiological studies examining allergenic pollen utilize daily measurements from a single pollen monitoring station with the implicit assumption that the measured time series of airborne pollen concentrations applies across the study areas, and that the temporal mismatch between concentrations at the counting station and elsewhere in the study area is negligible. This assumption is tested by quantifying temperature using satellite imagery, observing flowering times of oak (Quercus) and mulberry (Morus) trees at multiple sites, and collecting airborne pollen. Epidemiological studies of allergenic pollen are reviewed and temperatures within their study areas are quantified. In this one-year study, peak oak flowering time was well explained by average February nighttime temperature (R2 = 0.94), which varied by 6° C across Detroit. This relationship was used to predict flowering phenology across the study region. Peak flowering ranged from April 20 – May 13 and predicted a substantial portion of relative airborne oak pollen concentrations in Detroit (R2 = 0.46) and at the regional pollen monitoring station (R2 = 0.61). The regional pollen monitoring station was located in a cooler outlying area where peak flowering occurred around May 12 and peak pollen concentrations were measured on May 15. This provides evidence that the timing of pollen release varies substantially within a metropolitan area and challenges the assumption that pollen measurements at a single location are representative of an entire city. Across the epidemiological studies, 50% of study areas were not within 1° C (equal to a lag or lead of 4 days in flowering time) of temperatures at the pollen measurement location. Epidemiological studies using a single pollen station as a proxy for pollen concentrations are prone to significant measurement error if the study area is climatically variable.
Keywords: Aerobiology, Allergic rhinitis, Pollen concentrations, Pollen exposure, Quercus
1. INTRODUCTION
Pollen exposure is a major trigger of allergic rhinitis (AR), allergic conjunctivitis, and allergic asthma (Erbas et al., 2012; La Rosa et al., 2013; Linneberg et al., 2002; Salo et al., 2011). AR alone affects an estimated 10 – 23 % of people in North America and Europe (Bauchau and Durham, 2004; Mims, 2014), and rates are increasing globally (Björkstén et al., 2008). Pollen allergies reduce peoples’ quality of life, economic productivity, and place large burdens on healthcare systems (Meltzer, 2016; Meltzer et al., 2009; Nathan, 2007; Reed et al., 2004). Due to the importance of this public health issue, substantial efforts are made to quantify allergenic pollen exposures. A central component of this effort are networks of pollen counting stations, including 71 pollen counting stations currently comprising the National Allergy Bureau (NAB) network in the United States and ~400 active pollen counting stations in the European Aeroallergen Network. Pollen concentration measurements from these stations usually are assumed to be representative of the broader metropolitan areas in which they occur (e.g., Darrow et al. 2012, Qin et al. 2013, Osborne et al. 2017, Wang et al. 2017b) or the region (e.g., Gleason et al. 2014, Jariwala et al. 2014, Ito et al. 2015). Time series of daily pollen concentrations measured at pollen counting stations are the most commonly used proxy for pollen exposure in epidemiological studies.
Evidence suggests that most pollen only travels relatively short distances from source plants. Pollen measurements consistently show reduced pollen concentrations at farther distances from source plants (Adams-Groom et al., 2017; Bricchi et al., 2000; D. Frenz, 2000; Nowak et al., 2012; Raynor et al., 1970). Efforts to understand the spread of genetic material in agricultural systems have shown that pollen follows dispersal dynamics of other particles of similar size and density (Klein et al., 2003; J. Wang et al., 2017; Ye et al., 2016); these models predict low pollen concentrations at distances of tens to hundreds of meters due to settling and diffusion. Even though inter-regional transport of pollen is well documented and can be important in certain contexts (D’Amato et al., 2007; Zink et al., 2012), most evidence supports that pollen release from local plants is the best predictor of local airborne pollen concentrations. Thus, differences in pollen release over space and time are likely to contribute to heterogeneity in pollen levels across urban areas. This is further supported by studies showing substantial intra-urban variation in pollen concentrations driven by local plant community composition (Gonzalo-Garijo et al., 2006; Hjort et al., 2015; Katz and Carey, 2014; Weinberger et al., 2015; Werchan et al., 2017).
One potential source of variation in pollen concentrations is the timing of flowering (Devadas et al., 2018). Phenology (the timing of life history events) has been well studied (Polgar and Primack, 2011), and the flowering of temperate trees primarily depends on temperature although precipitation and photoperiod can play important roles (Gerst et al., 2017; Ibáñez et al., 2010; Wozniak and Steiner, 2017). Temperature is generally higher in urban areas than surrounding rural areas; the urban heat island effect is widespread and has been documented extensively (Imhoff et al., 2010; Rizwan et al., 2008). Because temperature is the main driver of temperate tree phenology, spring generally arrives earlier in cities than surrounding areas (Jochner and Menzel, 2015; Neil and Wu, 2006). Earlier flowering also has been observed for several tree species in urban areas compared to trees in nearby cooler rural areas (Lu et al., 2006; Massetti et al., 2015; Mimet et al., 2009; Roetzer et al., 2000). The amount of impervious surface area has been used as a proxy for temperature, and can predict spring phenology (Massetti et al., 2015). Thus, intra-urban temperature gradients can cause temporal mismatches in pollen release, potentially resulting in systematic heterogeneity in pollen concentrations within a city. If so, using pollen concentrations measured at a single station as a proxy for concentrations across a city could result in substantial measurement error of pollen exposure.
This study investigates the significance of intra-urban temperature gradients on differences in flowering time and airborne pollen concentrations and the potential impact on epidemiological analyses that include allergenic tree pollen. We hypothesize that: 1) trees in warmer neighborhoods within a city flower earlier; 2) airborne tree pollen concentrations are higher when more local trees are flowering; and 3) intra-urban temperature gradients within the study areas of epidemiological analyses are large enough to affect results. To test these hypotheses, we conduct a field study and characterize flowering times of two tree genera as a function of the intra-urban temperature gradient. Predicted flowering times are compared to pollen levels measured at 13 sites across the study area and at the regional NAB pollen monitoring station. We then assess the potential for temporal mismatches in airborne pollen concentrations between pollen monitoring stations and study areas in 11 epidemiological studies of allergenic pollen.
2. METHODS
2.1. STUDY AREA DESCRIPTION
In spring 2017, we conducted a field study in Detroit, Michigan, USA (42°19′N, 83°02′W), which covers 370 km2 with elevations from 175 to 205 m. Detroit has a humid continental climate: its annual average temperature is 10.6 °C (average monthly temperature of −3.2 °C in January and 23.6 °C in July), and its average precipitation is 940 mm/yr. Within the Detroit metropolitan area, air temperature during the summer is correlated with both impervious surface area and distance to water (Oswald et al., 2012).
2.2. PHENOLOGY FIELD CENSUS
We chose three regions in Detroit at varying distances from the Detroit River, and within each region selected three neighborhoods with high densities of oak trees using a street tree database (described below). In total, 115 oak (Quercus) trees were monitored at nine sites (SI 1), which had a variety of impervious surface area at different scales. Oaks were selected because they are common in urban areas, are frequently included in epidemiological analyses of allergenic pollen, and are estimated to cause over 21,000 emergency room visits in the United States each year (Anenberg et al., 2017). In addition, 22 white mulberry (Morus alba L.) trees were monitored; this allergenic species (Muñoz et al., 1995; Targow, 1971) is common in urban areas and flowers slightly after oaks. Within each of the nine neighborhoods, at least nine oak trees were monitored, selected by the following criteria: accessible for monitoring, not obviously unhealthy or damaged, and a diameter of >10 cm at 1.37 m height. Species composition of the selected trees included Quercus rubra L. (Northern red oak; n=67), Quercus palustris Munchh. (pin oak; n=28), Quercus alba L. (white oak; n=8), Quercus macrocarpa Michx. (burr oak; n=7), Quercus bicolor Willd. (swamp white oak; n=3), and Quercus velutina Lam. (black oak; n=2). Of these, 84 % were in the red oak group (Erythrobalanus) and the remainder were in the white oak group (Leucobalanus); this composition approximately matches oak street tree composition across Detroit (75% red oak group, SI 2). White mulberry trees were monitored in all nine neighborhoods, but the number of trees per neighborhood was lower (ranging from 1 – 8 trees).
Phenology measurements were made twice per week at each site. Foliar and floral phenological measurements of trees are often made by visually estimating the percentage of a plant at a particular phenological stage (e.g., Fotiou et al. 2011; Koenig et al. 2012; Lu et al. 2006; Vitasse et al. 2009). Here, we visually estimated the percentage of flowers that were immature (catkins not yet fully extended, anthers tightly closed), mature (catkins fully extended, some anthers open, anthers fully inflated), or senesced (anthers empty, open, and turning brown, catkins appears dried out) for each tree. To ensure consistency, observers would independently evaluate the phenological stage of a subset of trees, and arrive at a consensus estimate. Flower maturity varies substantially as a function of height within individual trees (Tal, 2011), so higher parts of the tree canopy were observed using binoculars. For 35 of the oak trees, we also selected 6 ground accessible twigs, and monitored the percentage of opened flowers within the catkins on those twigs. However, field observations showed that these low hanging flowers tended to flower later than the rest of the canopy (SI 3); due to that and the small sample size of trees we do not consider them further. Logistical constraints prevented us from repeatedly monitoring trees near the pollen counting station in Saint Claire Shores, but a phenology census of 23 oak trees was conducted on May 17. While one census does not allow us to be certain when peak flowering occurred, trees in Detroit had a similar percent flowering on May 7; if the duration of flowering is similar, peak flowering in Saint Claire Shores should be approximately May 13. Field measurements were recorded using Collector for ArcGIS (ESRI, Redlands, CA) on a tablet (Ipad Mini, Apple, Cupertino, USA), and locations were collected using a 1 m accurate GPS (GNSS Surveyor, Bad Elf, Tariffville, CT).
2.3. REMOTE SENSING DATA
Land surface temperature (LST) was quantified across Detroit using preprocessed 1 km2 resolution MODIS imagery (Wan, 2008; Wan et al., 2002) available through Google Earth Engine (Gorelick et al., 2016). LST is the temperature of observed surfaces including soil, vegetation, and buildings (Li et al., 2013). Many phenological studies have predicted plant phenology using LST (e.g., Chen et al. 2018; Hanes and Schwartz 2011; Zhang et al. 2004), often because LST is readily available from remote sensing imagery whereas air temperature is measured at sparsely-located weather stations. While air and land surface temperature are well correlated (Cai et al., 2017; Mildrexler et al., 2011), there is also a direct mechanistic link between LST and phenology: LST is directly related to energy exchanges between the atmosphere and soil (Quattrochi and Luvall, 1999; Simó et al., 2018) and soil temperature affects root phenology and plant reproductive phenology (Chen et al., 2016; Delpierre et al., 2016; Greer et al., 2006). LST measured by MODIS sensors on the Aqua and Terra satellites was extracted for each site (using coordinates of the site centroid) during each month from 2007 through 2017 and then reprojected to WGS84. The overpass time for Terra is approximately 10:30 and 22:30 whereas for Aqua the overpass times are 1:30 and 13:30; the imagery catalogues are MOD11A2 and MYD11A2 (version 5). The 10-year average for each month, a useful time frame for detecting spatial differences in temperature with MODIS data (Parmentier et al., 2014), was based on approximately 30 images, each of which is an average of clear sky imagery collected over an 8-day repeat cycle, with a one to two day revisit period. The 10-year average allows better comparisons between sites than MODIS images from a shorter period of time (e.g., the study year) would allow, as images often have missing data due to cloud cover and are sensitive to highly variable short-term conditions. As per Parmentier et al. (2014), these temperature averages may best be thought of as “monthly climatologies” and here are used to capture general patterns in differences in temperature over space. We also investigated whether changes in land use over the 10-year period could have affected our results by assessing differences between 10 and 5 year average temperatures. We focused on winter and spring given the importance of these seasons for oak spring phenology (Gerst et al., 2017).
2.4. POLLEN COLLECTION
In the field study, airborne pollen concentrations in Detroit were measured on 11 days between April 26 and May 23, 2017. Samplers were deployed to 13 sites where permission was obtained from land owners and placed at least 5 m from the nearest building (excluding small sheds). Sampling was conducted approximately three times per week from the late morning (11:00) to the early afternoon (2:00). Sampling was not conducted while raining; if rain occurred while sampling, samples were discarded. On each day, one particular section of the city (4 or 5 sites) was sampled; this sampling design decreased transportation time between samplers. Samplers were deployed sequentially at each site for two 90-minute periods and average concentrations across that period were used in the analysis; 48 samples were obtained.
Pollen was collected using custom-built rotorod-style samplers that have been used previously in similar studies (Huang et al., 2015; J. Wang et al., 2017; Ye et al., 2016). The samplers were attached to tripods at a height of 1.5 m above ground. The 2.9 × 20.0 mm sampling surface was a transparent acrylic rod covered with a thin layer of Trident Pure Silicone Grease (Trident Diving Equipment, Chatsworth, CA, USA). Rotation speed was measured using a digital tachometer (AGPTek, DT2234C) on all samplers before and after sampling. Rods were stored in custom air-tight boxes before and after deployment.
Quality assurance measures to assess the measurement reproducibility are detailed in SI 4. Briefly, field blanks controlled for field and lab contamination, collocated rotorods quantified the effects of minor differences in location, and precision was assessed with intra-sampler rod comparisons. Differences in collection efficiency between rotorods (which spin a sampling surface rapidly through the air) and Burkard samplers (which suck air through a small orifice) depend on wind conditions and particle size (Di-Giovanni, 1998; D. A. Frenz, 2000; Miki et al., 2017), but mean differences for the study conditions are expected to be < 20 % (D. A. Frenz, 2000).
For identification, sampling rods were placed in a customized holder, stained with Calberla solution, and viewed at 400 × using a Meiji ML 2000 microscope. Pollen of Quercus and Morus were identified using reference samples we collected and other pollen identification resources (Hepworth et al., 1983; Smith, 1984). Oak pollen was counted along three transects (0.46 × 20.00 mm; 48% of the collection surface) on each rod; all four rods from each sampler deployment were analyzed. Due to initial identification concerns, Morus pollen was counted separately in one short transect on each rod (0.46 × 2.90 mm; 16% of the collection surface); comparisons between rods indicated that this area was sufficient for this very abundant pollen (SI 4). In cases, there were substantial amounts of dust on the sampling surface; these samples were discarded as per standard rotorod sampling practices (Sterling and Lewis, 1998). When a rod was discarded, the other rods from that sampling location and date were used to determine airborne pollen concentrations; in only one case were all four rods discarded. All rods were glued to slides for archival purposes.
Average daily pollen concentration data were provided by the nearest NAB station, located in Saint Claire Shores (45.509, −82.905). Pollen is collected following the standard NAB protocol using a Burkard sampler located on the building roof, approximately 7 m above ground. Samples are collected approximately five days a week during the main pollen season, and pollen are identified to the genus level.
2.5. DATA AND STATISTICAL ANALYSIS
The airborne concentration of pollen grains, P (pollen m−3), was calculated as
where C = number of pollen grains on the slide; V = volume of air sampled for each revolution of the sampling arm (m3/revolution); R = rotational speed (RPM); M = sampling duration (min) (Frenz et al., 1996; Huang et al., 2015; J. Wang et al., 2017; Ye et al., 2016).
To identify causes of variation in the timing of flowering, the peak flowering day (i.e., the day with the largest observed portion of mature flowers) was calculated for each tree and we took the mean of this at each site. Differences in the percent of mature flowers at each site on each day were calculated using ANOVA and Tukey tests. To analyze peak flowering day as a function of temperature at the site level, we ran all possible single variable linear regressions (5 months of temperature climatologies for images collected at four times of day, for a total of 20 separate single variable models) and selected the model with the best fit using Akaike Information Criterion (AIC; Akaike 1974). The best single variable model was used to predict peak flowering time for the study area. All possible two and three variable regressions were also run using the OLSSR R package (Hebbali, 2018). The proportion of flowers that were active (i.e., fully mature but not senesced) across Detroit on each day was calculated as an empirical function of days before or after peak (reported in SI 5), based on an average across all sites. Individual tree flowering time was analyzed as a function of several variables including site temperature, impervious surface area surrounding each tree at several spatial scales (30, 50, 100, 200, 400, 800, and 1,600 m), and tree size. Oaks and mulberry were analyzed separately. Airborne oak and mulberry pollen concentrations in Detroit were analyzed as a function of estimated flowering intensity at that site and day using a linear model (a description of how flowering intensity was estimated is described above). Flowering intensity at the site was calculated as the average percent of estimated mature flowers within circles with the following radii: 0, 500, 1,000, 2,000, 3,000, 4,000, and 5,000 m; separate linear models were made for each spatial scale, and the model with the lowest AIC was selected. To account for potential bias caused by monitoring trees near roads (i.e., we did not include trees in backyards, which might systematically flower later), we also assessed models that included lags of 0, 1, 2, 3, and 4 days. To standardize for unknown differences in pollen production at each site (e.g., tree abundance) each site was scaled to the observed maximum value. The same approach was used to compare airborne pollen at the NAB station with estimated local flowering. The analyses of airborne pollen do not include the effects of weather, which is beyond the scope of this study.
Statistical analyses were conducted in R version 3.5.1 (R Core Team, 2013) and data visualizations were created using ggplot2 (Wickham, 2009). Mapping was conducted in R, ArcMap (ESRI, Redlands, CA), and Google Earth Engine (Gorelick et al., 2016).
2.7. ASSESSMENT OF POTENTIAL MEASUREMENT ERRORS ON THE LITERATURE
Temperature variation within the study area of an epidemiological investigation of allergenic pollen is rarely reported, but is needed to understand whether the study’s results could be affected by differences in the timing of pollen release. The potential for pollen exposure misclassification increases with large temperature differences within a study area or between a study area and the pollen measurement station, as was the case in Detroit. To assess potential impacts on the allergenic pollen epidemiological literature, we quantified the temperature variation in recent epidemiological studies that examined airborne pollen concentration time series. Studies were identified using a Google Scholar search on January 3, 2018 with keywords “allergenic pollen” & “pollen concentrations” & “health outcomes”. The resulting 214 articles were reviewed, and studies were retained if they analyzed a health outcome (e.g., hospital visits, medication usage, or medication prescription), included pollen as a main topic of analysis, specified location of the pollen monitoring station (required for our analysis), reported original data, were peer-reviewed, and were written in English. Studies were further restricted to those examining trees, the growth form included in our field study, and to studies using daily pollen measurements as larger time steps (e.g., weekly or monthly) might have significantly lower ability to resolve health responses attributable to pollen. The 11 selected studies are provided in Table 1. Although limited, this sample size should be sufficient to assess the relevance and applicability of our results to exposure misclassification. For each of the 11 studies, we delineated the study area (generally a municipal area) and determined coordinates of the study locations using ArcGIS 10.3 (ESRI, Redlands, CA, USA). For the four studies using hospital or clinic data that did not report the hospital catchment area, the study area was defined by a radius of 5 km surrounding the hospital (excluding major water bodies), which is similar to average distances traveled in urban areas (Buchmueller et al., 2006). Radii of 2 and 10 km were also evaluated. Temperatures were extracted for each study area and at the pollen station using methods detailed in the remote sensing section.
Table. 1:
Selected studies and temperature variability within them. The study area is the geographic area from where health outcomes were reported (* denotes studies that used health outcomes from particular hospital(s); calculation of the study area in these cases is described in the text). The calculation of the difference between the mean temperature of the study area and the pollen monitoring station used absolute values. Temperature is February night land surface temperature from Modis (Terra satellite).
| Study | Temperature of study area (mean and range, ° C) | Temperature of pollen monitoring station (° C) | Difference between study area and station (° C) | Area within 1° C of station (%) |
|---|---|---|---|---|
| Darrow et al. 2012 | 3.6 (1.5 – 7.2) | 4.5 | −0.9 | 57 |
| Gleason et al. 2014 | −3.3 (−9.3 – 4.6) | −3.4 | 0.2 | 29 |
| Guilbert et al. 2016 | −0.6 (−2.1 – 0.5) | −0.2 | −0.3 | 87 |
| Ito et al. 2015 | −1.9 (−3.8 – 2.9) | −4.5 | 2.7 | 0 |
| Jariwala et al. 2014* | −2.3 (−3.3 – −0.8) | −4.5 | 2.3 | 0 |
| Konishi et al. 2014* | 3.8 (2.4 – 5.5) | 1.6 | 2.2 | 1 |
| Osborne et al. 2017 | 0.7 (−1.9 – 3.1) | 1.3 | −0.6 | 71 |
| Qin et al. 2013 | −4.2 (−6.0 – 0.7) | −2.5 | −1.7 | 10 |
| Sakata et al. 2017* | 3.7 (2.5 – 6.0) | 3.3 | 0.4 | 84 |
| Sun et al. 2016 | 2.9 (1.2 – 5.9) | 3.0 | −0.1 | 90 |
| Wang et al. 2017* | −1.5 (−3.3 – −0.6) | −0.9 | −0.5 | 78 |
| mean | 0.4 | −0.2 | 1.1 | 46 |
3. RESULTS
3.1. FLOWERING PHENOLOGY VARIATION
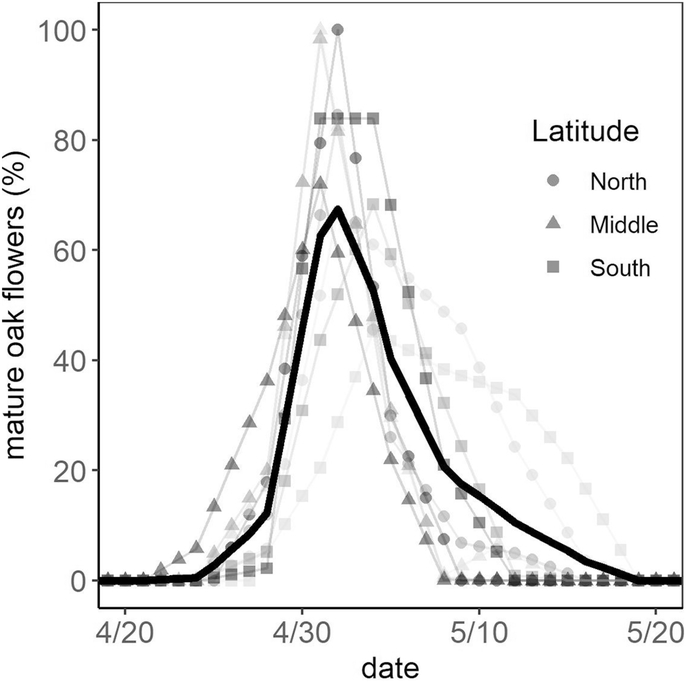
The timing of flowering among sites across Detroit differed substantially (Fig. 2) and significantly (SI 5). We also found substantial inter-individual variation in the timing of flowering, e.g., mature oak flowers were found between April 22 and May 16 (Fig. 2). The flowering period for individual oak trees varied from 4 – 10 days (SI 5); this is a coarse estimate because each individual was only observed twice a week.
Fig. 2.
Flowering period for monitored oak trees in each site (gray lines and symbols) and across all sites (thick black line).
3.2. FLOWERING PHENOLOGY, TEMPERATURE, AND ENVIRONMENT
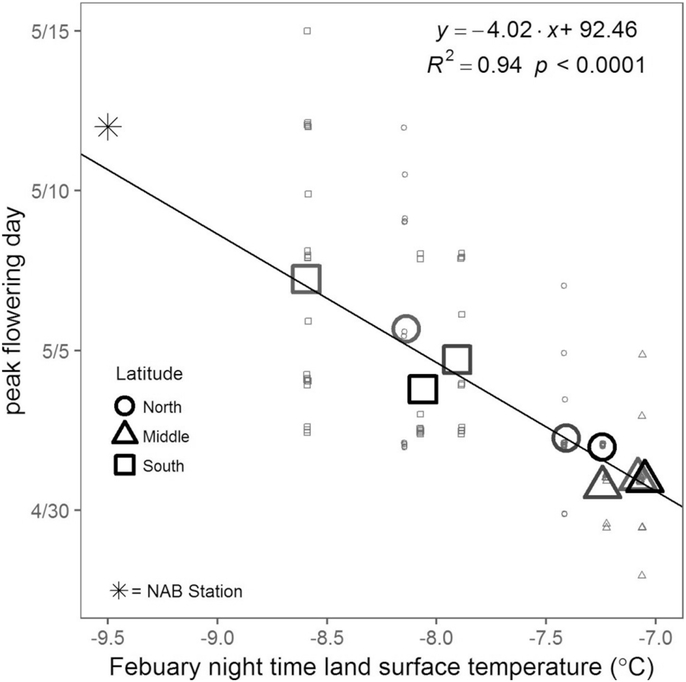
The day of peak flowering for oaks at a site was strongly correlated to temperature, and the temperature metric with the highest correlation was February nighttime land surface temperature from the Terra satellite (R2 = 0.94, p < 0.0001; Fig. 3). Temperatures from other months and from the Aqua satellite also had strong relationships (SI 6) and provided qualitatively similar predictions of flowering phenology (data not shown). Average temperature over five and ten years were quite similar, although the five-year average had substantially higher variability (SI 6). Only single variable models were considered given the small sample size (9 sites) and the incremental gain in model fit with additional covariates, e.g., the “best” two-variable model included Terra night time temperatures in February and March and increased R2 to 0.96. The day of peak flowering had little association with impervious surface area at all spatial scales or with tree diameter (R2 < 0.1; SI 7). Red oaks tended to flower before white oaks, but their responses to temperature were similar (SI 8).
Fig. 3.
Peak flowering for all monitored oaks (large symbols: site means, dots: individual trees) as a function of average February night-time temperature. Estimated day of peak flowering for oak trees near the pollen counting station is also displayed (asterisk), but not included in the linear regression.
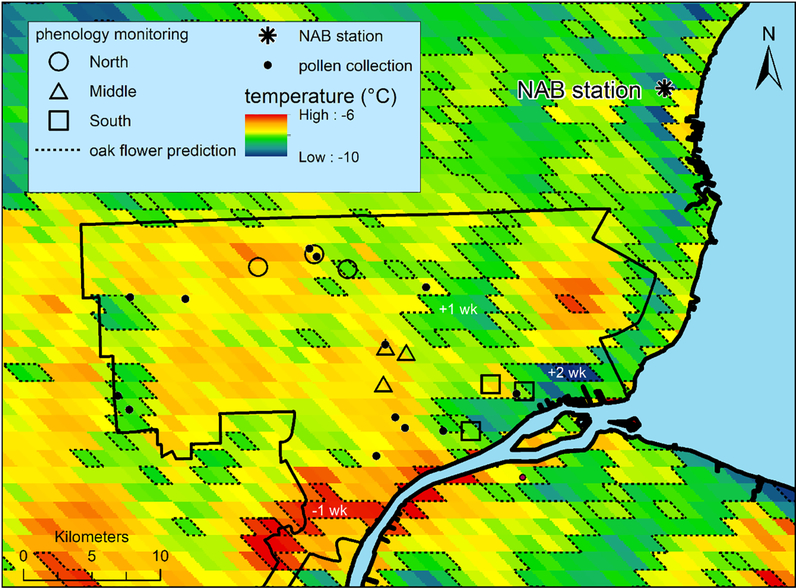
Using February night-time temperatures, we estimated peak flowering day for oak trees across the Detroit metropolitan region (Fig. 1, dashed isolines). February night-time temperature ranged from −10 – −4 °C (mean = −7.6 °C) throughout Detroit. Estimated peak flowering dates ranged from April 20 – May 13 (mean = May 3). February night temperatures in 17% of Detroit (by area) fell outside the range of temperatures at our measurement sites, thus requiring extrapolations from the data used. At the NAB station in Saint Claire Shores there was an excellent fit between predicted (May 11, based on temperature) and calculated day of peak flowering (May 12, based on field data from the census) as shown by the asterisk in Fig. 3 (note that this data point was not included in the linear regression).
Fig. 1.
Pollen collection sites (black dots), phenology monitoring sites (hollow symbols), and the NAB station (*) are shown. The city of Detroit’s boundary is shown in black. Colors show average February night time land surface temperatures (°C) in Detroit. Dashed lines show 1 week differences in flowzering time, based on model predictions.
For mulberry, the 22 monitored trees reached peak flowering between May 4 and May 20, with an average of May 14 (SI 9). May night-time temperature was the temperature metric that best predicted individual peak mulberry flowering time (R2 = 0.22, p < 0.05), which we used to estimate flowering time across the city (SI 9). Predicted peak flowering in Detroit ranged from days May 9 – May 25 (mean = May 15), but note that the relationship this is based on had low predictive power. Figures comparing NAB pollen concentrations and observed flowering phenology in Detroit are reported in SI 10.
3.3. AIRBORNE POLLEN CONCENTRATIONS
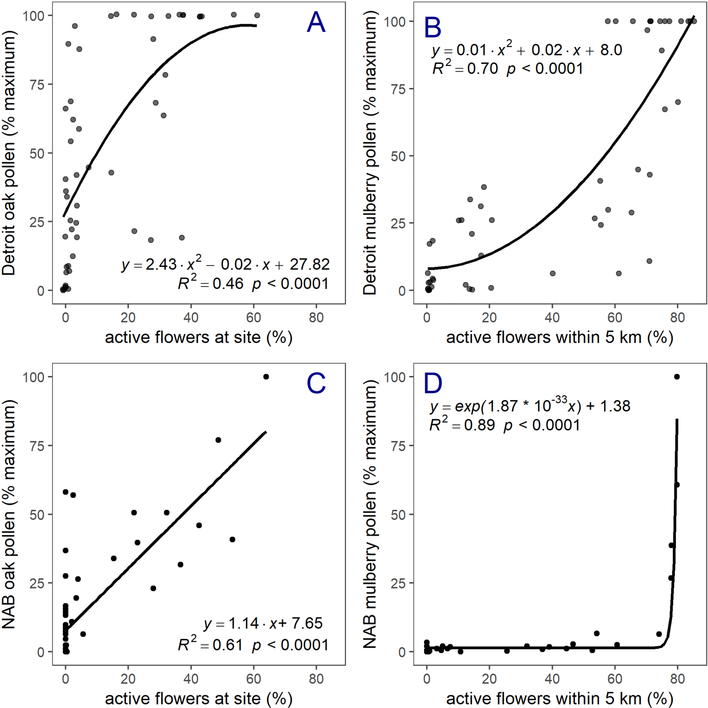
Airborne pollen concentrations in Detroit were significantly correlated with estimated flowering at the collection site for both oak (R2 = 0.46, p < 0.001) and mulberry (R2 = 0.68, p < 0.0001; Fig. 4, panels A and B; comparisons for each pollen monitoring site are reported in SI 11). Correlations were strongest for oak when flowering was estimated at the exact site of pollen collection, but for mulberry the strongest correlation was for flowering within 5 km of the site (SI 12). Including a lag of 3 days in estimated flowering intensity improved the model fit for oak (R2 = 0.46, p < 0.0001 vs. R2 = 0.25, p < 0.001) but lags did not improve model fit for mulberry (SI 13). Absolute concentrations of oak pollen in Detroit averaged 207 grains/m3 (range: 0.5 – 1,220 grains/m3) and peaked between April 26 and May 11 (SI 14). Mulberry pollen in Detroit averaged 1,475 grains/m3 (range: 0 – 10,352 grains/m3) and was abundant between May 2 and May 18 (SI 14). Pollen concentrations at the NAB station were significantly correlated with active flowering (Fig. 4, panels C and D) for oak (R2 = 0.61, p < 0.0001) and mulberry (R2 = 0.89, p < 0.0001). As for Detroit, active flowering had the most explanatory power at the same spatial scales (oak: 0 m, mulberry: 5 km) and with the same lags (oak: 3 days, mulberry: 0 days). The highest pollen concentrations of oak were measured at the NAB station on May 15 and May 13 (compared to a predicted peak day of flowering of May 11 without the lag or May 14 with the lag, and the estimate of May 12 based on the local field census). For mulberry, the predicted day of peak flowering at the NAB station was May 25 but peak pollen concentrations were measured at the NAB station on May 17 (not including the 5 km buffer). While these analyses account for some spatial differences in pollen levels (e.g., by scaling to the site maximum), they do not address effects of wind speed, wind direction, temperature, precipitation, and possibly other variables that varied during the sampling period (SI 15). While many variables can affect the relationship between airborne pollen and flowering time, Fig. 4 suggests that maximum airborne pollen concentrations are most likely when flowers are mature. The figure illustrates this relationship using several different curves.
Fig. 4.
Airborne oak pollen in Detroit and at the NAB station as a function of active flowers. Each point represents pollen collected at a particular site and day. A three-day lag in flowering is included for oaks and a 5 km buffer for flowering is included for mulberry.
3.4. EVALUATION OF POLLEN MEASUREMENTS IN THE LITERATURE
Table 1 provides differences in temperature between study locations and pollen stations in the 10 selected epidemiological studies of tree pollen. The range of temperatures within study areas ranged from 2.7 – 13.8° C (average = 5.0° C). The proportion of study area within ±1° C of the pollen counting station (an equivalent of ± 4 days for oak flowering in our study) ranged from 0 – 90 % (average = 55 %). Changing the size of the study area radius (for the four studies that did not provide this) to 2 and 10 km changed the across-study average proportion of study area within 1° C to 55 % (2 km) and 50 % (10 km); more details on temperatures at those two distances are included in SI 16. Within studies, the difference in temperature between the station and the mean of the study area ranged from −0.9 to 2.3° C (mean of absolute values = 1.0° C). Cooler temperatures at the study area compared to at the pollen counting station would lead to later than expected flowering, whereas warmer temperatures at the study area compared to the pollen station would lead to earlier than expected flowering. Lags or average pollen over multiple days was used in all but two of the studies (Jariwala et al., 2014; X. Wang et al., 2017). The lags and day averages extended as long as 10 days before the health outcome was measured (Guilbert et al., 2016); no studies used leads.
4. DISCUSSION
4.1. TEMPERATURE AND PHENOLOGY
Oak floral phenology is well predicted by temperature (Fu et al., 2012; Massetti et al., 2015; Mimet et al., 2009). Early spring temperatures are especially important determinants of oak tree flowering phenology in Eastern North America (Gerst et al., 2017), and others have reported strong associations between February and March temperatures and tree flowering time (Chmielewski and Rotzer, 2001; Fitter et al., 1995). Plant phenology responds differently to temperature at different times of day (Kalcsits et al., 2009; Lu et al., 2006; Yin et al., 1996), which may explain the relative predictive power of the temperature metrics (e.g., temperature measured at 1:30 vs. 22:30), but the sensitivity of oak phenology to these differences is unknown. The mean phenological responses of oak trees to temperature are consistent between our study (4-day shift in flowering per degree Celsius; hereafter referred to as days °C−1) and other studies: 4.2 days °C−1 (Menzel, 2003, based on time series from many locations), 4.1 – 4.4 days °C−1 (Polgar et al., 2014, based on time series from one location) 6.5 days °C−1 (Vitasse et al., 2009, based on time series from many locations). This result is similar to the average phenological response to temperature for many common woody plants in eastern North American, 5 days °C−1 (Polgar et al. 2014). Temperature variation attributed to the Detroit River may affect the timing of phenology and explain why impervious surface area was not a strong predictor of phenology, contrary to Massetti et al., (2015) where a strong relationship between impervious surface area and flowering time was found. In our studyhe correlation between February night temperature and impervious surface area was weak. While we studied a single year, the underlying urban heat island effect that causes these patterns is relatively stable across years (Liu et al., 2007; Wilby et al., 2011). Similarly, while the date of budburst can vary substantially among years, the order in which particular trees develop within a year is very consistent (Cole and Sheldon, 2017; Wesołowski and Rowiński, 2006), so these intra-urban patterns are expected to be consistent across years. Individual tree phenology within sites was unpredictable, potentially due to unmeasured variables such as tree genetics and microclimates (Fotiou et al., 2011). High inter-tree variability and the low number of monitored mulberry trees likely explains why temperature was not a better predictor of mulberry phenology.
4.2. AIRBORNE POLLEN
Airborne pollen concentrations in Detroit were predicted by estimated flowering time for oak trees (R2 = 0.46) and mulberry (R2 = 0.70). Thus, intra-urban differences in flowering phenology result in systematic differences in airborne pollen concentrations, which can be observed with even intermittent pollen sampling. The true relationships between predicted flowering time and airborne pollen concentrations are expected to be stronger than reported here, given that we do not account for the substantial inter-daily variation in airborne pollen caused by meteorology (Hamid et al., 2014; Martin et al., 2010; Menut et al., 2014; Negrini et al., 2011). Daily weather conditions also would act to synchronize anther anthesis and pollen release, and therefore airborne pollen concentrations at the NAB station and at sampling locations in Detroit, but the importance of this process appears low compared to temporal differences in floral maturation. Although a direct comparison of pollen measurements in Detroit and the NAB station is limited due to differences in sampling time (3 hours vs 24 hours), sampler height (1.5 m vs. 7 m), and method (Rotorod vs Burkard), it is noteworthy how poorly these time series align; in linear regressions NAB pollen measurements explained very little variation in pollen measurements in Detroit for oak (R2 = 0.07) and mulberry (R2 = 0.04). The underlying relationships between floral phenology and airborne pollen concentrations may also be somewhat masked by limitations of the pollen sampling methodology (e.g., scaling pollen concentrations within each site, sampling for only three hours a day, and using predictions of flowering rather than observed flowering). Introducing a lag in flowering time of 3 days substantially improved the ability of the phenological model to predict airborne oak pollen concentrations in both Detroit (R2 = 0.46 vs. R2 = 0.25) and at the NAB station (R2 = 0.61 vs. R2 = 0.34). This suggests that the monitored oak trees in Detroit flowered systematically earlier than unmonitored trees; this fits with other observations of earlier development for trees near more pavement (Chen et al., 2016). Ideally, future work will include congeneric tree abundance as a covariate; anecdotally, sites with the highest oak pollen concentrations were surrounded by oak trees. Future studies would benefit from 24 hour sampling periods as pollen concentrations can vary substantially on hourly time scales (Norris-Hill and Emberlin, 1991; Wang and Yang, 2009). Even with these pollen sampling limitations, there were strong associations between estimated flowering and airborne pollen at the NAB station for oak and mulberry (respectively, R2 = 0.61 and R2 = 0.89), which corroborates the relationships found among temperature, flowering, and airborne pollen concentrations.
Airborne pollen concentrations are determined by pollen inputs from both local and external plants; the larger the ratio of local to external pollen, the more intra-urban differences in flowering phenology will matter. Pollen input from external regions depends on the quantity of pollen produced and its transport to the study area. Oaks are very abundant in forests across much of the United States and are expected to be some of the most prolific producers of pollen on a national scale (Wozniak and Steiner, 2017), potentially leading to relatively high external inputs of oak pollen. In cities, absolute oak abundance is low to moderate (Pennington et al., 2010; White et al., 2014; Woodall et al., 2010), potentially resulting in a relatively lower ratio of local to external pollen compared to other genera. Our results show evidence of some long-distance transport of pollen (i.e., the moderate concentrations of oak pollen recorded at the NAB station from April 10 to 17 were well before local flowering began). In contrast, pollen from genera that tend towards urban areas, e.g., mulberry (Pennington et al., 2010; White et al., 2014; Woodall et al., 2010), may be driven more by local release of pollen. This could explain why mulberry pollen in Detroit was better predicted by estimated local flowering than oak pollen was. Overall though, peak pollen concentrations in Detroit for both oaks and mulberries are best explained in the context of local pollen release.
Relatively large external pollen inputs could overwrite local differences in pollen release; evidence for this could potentially be detected by mismatches between floral phenology and airborne pollen. In general though, local phenology and airborne pollen tend to be strongly correlated (Hidalgo et al., 2003; Jato et al., 2002; Latorre, 1999; Latorre and Bianchi, 1998; Veriankaite et al., 2010). Observed mismatches between floral phenology and pollen are likely caused by long-distance pollen dispersal overwhelming the signal of local pollen release (Latorre, 1999; Latorre and Bianchi, 1998), but alternate processes such as insufficient phenological monitoring of local populations (i.e., unobserved flowering) or unmeasured intra-genus variation in flowering phenology (Fairley and Batchelder, 1986; Latorre and Bianchi, 1998) could lead to the same pattern. Observed floral phenology tends to match local pollen concentrations, implying that local pollen production is generally more important than external inputs for tree pollen at breathing height. The relative importance of local pollen is also expected to be affected by the sampling height (Fernández-Rodríguez et al., 2014; Soldevilla et al., 1995; Spieksma et al., 2000), but monitoring station altitudes (5 – 15 m according to NAB guidelines or 15 – 20 m according to European Aeroallergen Network guidelines) often are not substantially higher than tree canopies (e.g., oak trees in our study system frequently reach 25 m). Thus, measurements of tree pollen as used in epidemiological studies are expected to be strongly influenced by local trees and may not be representative of a broader area.
4.3. IMPLICATIONS FOR EPIDEMIOLOGICAL STUDIES OF ALLERGENIC POLLEN
Time series of flowering phenology and relative pollen concentrations varied substantially across Detroit. This highlights the problem with the standard practice of analyzing health outcomes throughout a heterogeneous metropolitan area or region based on measurements of pollen concentrations from a single location. In this case, a typical allergenic pollen epidemiological analysis would have falsely assumed that mulberry pollen concentrations in Detroit were negligible until May 16 (the beginning of peak pollen concentrations at the NAB station; SI 10). In fact, pollen measurements in Detroit show very high mulberry pollen concentrations in Detroit as early as May 2, and many sites had declining pollen concentrations by the time of peak pollen concentrations at the NAB station. This measurement error could have incorrectly led to the conclusion that mulberry pollen is a less important allergen than it is. Given that Juglans and grasses flower soon after oaks and mulberries, it would be easy to confound their effects with oaks and mulberry.
The large temperature differences between pollen collection locations and substantial proportions of study areas found in six of the eleven reviewed studies (Darrow et al., 2012; Gleason et al., 2014; Ito et al., 2015; Jariwala et al., 2014; Konishi et al., 2014; Qin et al., 2013) may have resulted in exposure measurement errors similar to estimates described for Detroit. Most studies reviewed here (7 out of 11) included time lags in the analyses in order to account for delays between exposures and health outcomes. This practice could also help correct for directional bias between pollen counting stations and the study area. However, choosing universal time lags (usually of up to a few days) will not be able to account for large differences within a study area. This is problematic in large study regions and in study areas that have strong temperature gradients (e.g., coastal cities). Although none of the studies we reviewed here did so, our findings suggest that lead effects should be investigated in models to account for pollen stations that are phenologically behind study areas, as is the case in Detroit, New York City (Ito et al., 2015; Jariwala et al., 2014), and Tokyo (Konishi et al., 2014). Large temperature differences between the station and the study area could result in differences in pollen composition, potentially confounding associations between particular tree genera and health outcomes (e.g., Ito et al. 2015). In general, the temporal mismatches we document here are likely to be especially important for trees, which tend to have much shorter flowering periods than weeds and grasses (Hepworth et al., 1983).
Supplementary Material
HIGHLIGHTS.
Intra-urban temperature gradients cause large differences in tree flowering time
Flowering time predicts airborne pollen concentrations
Epidemiological investigations of allergenic pollen may experience substantial measurement error due to differences in temperature, flowering time, and airborne pollen within cities
ACKNOWLEDGEMENTS
This work could not have been completed without the considerable efforts of a research assistant, John Kost, the volunteers who allowed us to collect pollen on their property, and Dr. Junming Wang for the loan of sampling equipment.
Funding: This work was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award F32 ES026477 from the National Institute of Environmental Health Sciences. It was also supported by the Michigan Institute for Clinical Health Research through the Postdoctoral Translational Scholars Program (Grant Number UL1 TR002240).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
WORKS CITED
- Adams-Groom B, Skjoth CA, Baker M, Welch TE, 2017. Modelled and observed surface soil pollen deposition distance curves for isolated trees of Carpinus betulus, Cedrus atlantica, Juglans nigra and Platanus acerifolia. Aerobiologia (Bologna). 33, 1–10. 10.1007/s10453-017-9479-128255194 [DOI] [Google Scholar]
- Akaike H, 1974. A new look at the statistical model identification. IEEE Trans. Automat. Contr 19, 716–723. [Google Scholar]
- Anenberg S, Weinberger KR, Roman H, Neumann J, Crimmins A, Fann N, Martinich J, Kinney P, 2017. Impacts of oak pollen on allergic asthma in the United States and potential influence of future climate change. GeoHealth 1, 80–92. 10.1002/2017GH000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchau V, Durham SR, 2004. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur. Respir. J 24, 758–764. 10.1183/09031936.04.00013904 [DOI] [PubMed] [Google Scholar]
- Björkstén B, Clayton T, Ellwood P, Stewart A, Strachan D, Ait-Khaled N, Anderson HR, Asher MI, Beasley R, Bjorkstén B, Brunekreef B, Cookson W, Crane J, Ellwood P, Foliaki S, Keil U, Lai CKW, Mallol J, Robertson C, Mitchell EA, Montefort S, Odhiambo J, Pearce N, Shah J, Stewart AW, Strachan DP, Von Mutius E, Weiland SK, Williams H, 2008. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr. Allergy Immunol. 19, 110–124. 10.1111/j.1399-3038.2007.00601.x [DOI] [PubMed] [Google Scholar]
- Bricchi E, Frenguelli G, Mincigrucci G, 2000. Experimental results about Platanus pollen deposition. Aerobiologia (Bologna). 16, 347–352. [Google Scholar]
- Buchmueller TC, Jacobson M, Wold C, 2006. How far to the hospital?. The effect of hospital closures on access to care. J. Health Econ 25, 740–761. 10.1016/j.jhealeco.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Cai Y, Chen G, Wang Y, Yang L, 2017. Impacts of land cover and seasonal variation on maximum air temperature estimation using MODIS imagery. Remote Sens. 9 10.3390/rs9030233 [DOI] [Google Scholar]
- Chen Y, Wang X, Jiang B, Li L, 2018. The leaf phenophase of deciduous species altered by land pavements. Int. J. Biobeteorology 62, 949–959. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang X, Jiang B, Yang N, Li L, 2016. Pavement induced soil warming accelerates leaf budburst of ash trees. Urban For. Urban Green 16, 36–42. 10.1016/j.ufug.2016.01.014 [DOI] [Google Scholar]
- Chmielewski FM, Rotzer T, 2001. Response of tree phenology to climate change across Europe. Agric. For. Meteorol 108, 101–112. 10.1016/S0168-1923(01)00233-7 [DOI] [Google Scholar]
- Cole EF, Sheldon BC, 2017. The shifting phenological landscape: Within- and between-species variation in leaf emergence in a mixed-deciduous woodland. Ecol. Evol. 1–13. 10.1002/ece3.2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, Liccardi G, Popov T, van Cauwenberge P, 2007. Allergenic pollen and pollen allergy in Europe. Allergy 62, 976–90. 10.1111/j.1398-9995.2007.01393.x [DOI] [PubMed] [Google Scholar]
- Darrow L. a, Hess J,Rogers C. a, Tolbert PE, Klein M,Sarnat SE., 2012. Ambient pollen concentrations and emergency department visits for asthma and wheeze. J. Allergy Clin. Immunol 130, 630–638.e4. 10.1016/j.jaci.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre N, Vitasse Y, Chuine I, Guillemot J, Bazot S, Rutishauser T, Rathgeber CBK, 2016. Temperate and boreal forest tree phenology: from organ-scale processes to terrestrial ecosystem models. Ann. For. Sci. 73, 5–25. 10.1007/s13595-015-0477-6 [DOI] [Google Scholar]
- Devadas R, Huete AR, Vicendese D, Erbas B, Beggs PJ, Medek D, Haberle SG, Newnham RM, Johnston FH, Jaggard AK, Campbell B, Burton PK, Katelaris CH, Newbigin E, Thibaudon M, Davies JM, 2018. Dynamic ecological observations from satellites inform aerobiology of allergenic grass pollen. Sci. Total Environ 633, 441–451. 10.1016/j.scitotenv.2018.03.191 [DOI] [PubMed] [Google Scholar]
- Di-Giovanni F, 1998. A review of the sampling efficiency of rotating-arm impactors used in aerobiological studies. Grana 37, 164–171. 10.1080/00173139809362661 [DOI] [Google Scholar]
- Erbas B, Akram M, Dharmage SC, Tham R, Dennekamp M, Newbigin E, Taylor P, Tang MLK, Abramson MJ, 2012. The role of seasonal grass pollen on childhood asthma emergency department presentations. Clin. Exp. Allergy 42, 799–805. 10.1111/j.1365-2222.2012.03995.x [DOI] [PubMed] [Google Scholar]
- Fairley D, Batchelder GL, 1986. A study of oak-pollen production and phenology in northern California: Prediction of annual variation in pollen counts based on geographic and meterologic factors. J. Allergy Clin. Immunol 78, 300–307. 10.1016/S0091-6749(86)80080-X [DOI] [PubMed] [Google Scholar]
- Fernández-Rodríguez S, Tormo-Molina R, Maya-Manzano JM, Silva-Palacios I, Gonzalo-Garijo Á, 2014. A comparative study on the effects of altitude on daily and hourly airborne pollen counts. Aerobiologia (Bologna). 30, 257–268. 10.1007/s10453-014-9325-7 [DOI] [Google Scholar]
- Fitter A, Fitter R, Harris I, Williamson M, 1995. Relationships between first flowering date and temperature in the flora of a locality in central England. Funct. Ecol. 9, 55–60. [Google Scholar]
- Fotiou C, Damialis A, Krigas N, Halley JM, Vokou D, 2011. Parietaria judaica flowering phenology, pollen production, viability and atmospheric circulation, and expansive ability in the urban environment: Impacts of environmental factors. Int. J. Biometeorol 55, 35–50. 10.1007/s00484-010-0307-3 [DOI] [PubMed] [Google Scholar]
- Frenz D, 2000. Interpreting atmospheric pollen counts for use in clinical allergy: spatial variability. Ann. Allergy, Asthma Immunol 84, 481–9. 10.1016/S1081-1206(10)62506-9 [DOI] [PubMed] [Google Scholar]
- Frenz DA, 2000. The effect of windspeed on pollen and spore counts collected with the Rotorod Sampler and Burkard spore trap. Ann. Allergy, Asthma Immunol 85, 392–394. 10.1016/S1081-1206(10)62553-7 [DOI] [PubMed] [Google Scholar]
- Frenz DA, Scamehorn RT, Hokanson JM, Murray LW, 1996. A brief method for analyzing rotorod samples for pollen content. Aerobiologia (Bologna). 12, 51–54. [Google Scholar]
- Fu YH, Campioli M, Deckmyn G, Janssens IA, 2012. The impact of winter and spring temperatures on temperate tree budburst dates: Results from an experimental climate manipulation. PLoS One 7 10.1371/journal.pone.0047324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst KL, Rossington NL, Mazer SJ, 2017. Phenological responsiveness to climate differs among four species of Quercus in North America. J. Ecol 1610–1622. 10.1111/1365-2745.12774 [DOI] [Google Scholar]
- Gleason JA, Bielory L, Fagliano JA, 2014. Associations between ozone, PM2.5, and four pollen types on emergency department pediatric asthma events during the warm season in New Jersey: A case-crossover study. Environ. Res 132, 421–429. 10.1016/j.envres.2014.03.035 [DOI] [PubMed] [Google Scholar]
- Gonzalo-Garijo MA, Tormo-Molina R, Muñoz-Rodríguez AF, Silva-Palacios I, 2006. Differences in the spatial distribution of airborne pollen concentrations at different urban locations within a city. J. Investig. Allergol. Clin. Immunol 16, 37–43. [PubMed] [Google Scholar]
- Gorelick N, Hancher M, Dixon M, Ilyushchenko S, Thau D, Moore R, 2016. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ 202, 18–27. 10.1016/j.rse.2017.06.031 [DOI] [Google Scholar]
- Greer DH, Wünsche JN, Norling CL, Wiggins HN, 2006. Root-zone temperatures affect phenology of bud break, flower cluster development, shoot extension growth and gas exchange of “Braeburn” (Malus domestica) apple trees. Tree Physiol. 26, 105–111. 10.1093/treephys/26.1.105 [DOI] [PubMed] [Google Scholar]
- Guilbert A, Simons K, Hoebeke L, Packeu A, Hendrickx M, De Cremer K, Buyl R, Coomans D, Van Nieuwenhuyse A, 2016. Short-term effect of pollen and spore exposure on allergy morbidity in the BrusselsCapital Region. Ecohealth 13, 303–315. 10.1007/s10393-016-1124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid N, Ali SM, Talib F, Sadiq I, Ghufran MA, 2014. Spatial and temporal variations of pollen concentrations in Islamabad (Pakistan): effect of meteorological parameters and impact on human health. Grana 3134, 1–15. 10.1080/00173134.2014.967289 [DOI] [Google Scholar]
- Hanes JM, Schwartz MD, 2011. Modeling land surface phenology in a mixed temperate forest using MODIS measurements of leaf area index and land surface temperature. Theor. Appl. Climatol 105, 37–50. 10.1007/s00704-010-0374-8 [DOI] [Google Scholar]
- Hebbali A, 2018. OLSSR: Tools for building OLS Regression models.
- Hepworth W., Vinay P, Zenger V, 1983. Airborne and allergenic pollen of North America, Airborne and Allergenic Pollen of North America. Johns Hopkins University Press, Baltimore. [Google Scholar]
- Hidalgo PJ, Galan C, Dominguez E, 2003. Male phenology of three species of Cupressus: correlation with airborne pollen. Trees-Structure Funct. 17, 336–344. 10.1007/s00468-002-0243-x [DOI] [Google Scholar]
- Hjort J, Hugg TT, Antikainen H, Rusanen J, Sofiev M, Jaakkola MS, Jaakkola JJK, 2015. Fine-scale exposure to allergenic pollen in the urban environment: Evaluation of land use regression approach. Environ. Health Perspect 124, 619–626. 10.1289/ehp.1509761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ye R, Qi M, Li X, Miller DR, Stewart CN, Dubois DW, Wang J, 2015. Wind-mediated horseweed (Conyza canadensis) gene flow: Pollen emission, dispersion, and deposition. Ecol. Evol 5, 2646–2658. 10.1002/ece3.1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez I, Primack RB, Miller-Rushing AJ, Ellwood E, Higuchi H, Lee SD, Kobori H, Silander JA, 2010. Forecasting phenology under global warming. Philos. Trans. R. Soc. B Biol. Sci 365, 3247–3260. 10.1098/rstb.2010.0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff ML, Zhang P, Wolfe RE, Bounoua L, 2010. Remote sensing of the urban heat island effect across biomes in the continental USA. Remote Sens. Environ. 114, 504–513. 10.1016/j.rse.2009.10.008 [DOI] [Google Scholar]
- Ito K, Weinberger KR, Robinson GS, Sheffield PE, Lall R, Mathes R, Ross Z, Kinney PL, Matte TD, 2015. The associations between daily spring pollen counts, over-the-counter allergy medication sales, and asthma syndrome emergency department visits in New York City, 2002–2012. Environ. Heal 14, 71 10.1186/s12940-015-0057-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwala S, Toh J, Shum M, de Vos G, Zou K, Sindher S, Patel P, Geevarghese A, Tavdy A, Rosenstreich D, 2014. The association between asthma-related emergency department visits and pollen and mold spore concentrations in the Bronx, 2001–2008. J. Asthma 51, 79–83. 10.3109/02770903.2013.853779 [DOI] [PubMed] [Google Scholar]
- Jato V, Méndez J, Rodríguez-Rajo J, Seijo C, 2002. The relationship between the flowering phenophase and airborne pollen of Betula in galicia (N.W. Spain). Aerobiologia (Bologna). 18, 55–64. 10.1023/A:1014987325946 [DOI] [Google Scholar]
- Jochner S, Menzel A, 2015. Urban phenological studies - Past, present, future. Environ. Pollut 203, 250–261. 10.1016/j.envpol.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Kalcsits LA, Silim S, Tanino K, 2009. Warm temperature accelerates short photoperiod-induced growth cessation and dormancy induction in hybrid poplar (Populus × spp.). Trees 23, 971–979. 10.1007/s00468-009-0339-7 [DOI] [Google Scholar]
- Katz DSW, Carey TS, 2014. Heterogeneity in ragweed pollen exposure is determined by plant composition at small spatial scales. Sci. Total Environ. 485, 435–440. 10.1016/j.scitotenv.2014.03.099 [DOI] [PubMed] [Google Scholar]
- Klein E, Lavigne C, Foueillassar X, Gouyon P, Laredo C, 2003. Corn pollen dispersal: quasi-mechanistic models and field experiments. Ecol. Monogr 73, 131–150. [Google Scholar]
- Koenig WD, Funk KA, Kraft TS, Carmen WJ, Barringer BC, Knops JMH, 2012. Stabilizing selection for within-season flowering phenology confirms pollen limitation in a wind-pollinated tree. J. Ecol 100, 758–763. 10.1111/j.1365-2745.2011.01941.x [DOI] [Google Scholar]
- Konishi S, Ng CFS, Stickley A, Nishihata S, Shinsugi C, Ueda K, Takami A, Watanabe C, 2014. Particulate matter modifies the association between airborne pollen and daily medical consultations for pollinosis in Tokyo. Sci. Total Environ 499, 125–132. 10.1016/j.scitotenv.2014.08.045 [DOI] [PubMed] [Google Scholar]
- La Rosa M, Lionetti E, Reibaldi M, Russo A, Longo A, Leonardi S, Tomarchio S, Avitabile T, Reibaldi A, 2013. Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr 39, 18 10.1186/1824-7288-39-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre F, 1999. Differences between airborne pollen and flowering phenology of urban trees with reference to production, dispersal and interannual climate variability. Aerobiologia (Bologna). 15, 131–141. 10.1023/A:1007523316024 [DOI] [Google Scholar]
- Latorre F, Bianchi MM, 1998. Relationships between flowering development of Ulmus pumila and Fraxinus excelsior and their airborne pollen. Grana 37, 37–41. 10.1080/00173139809362672 [DOI] [Google Scholar]
- Li Z-L, Tang B-H, Wu H, Ren H, Yan G, Wan Z, Trigo IF, Sobrino JA, 2013. Satellite-derived land surface temperature: Current status and perspectives. Remote Sens. Environ 131, 14–37. 10.1016/j.rse.2012.12.008 [DOI] [Google Scholar]
- Linneberg A, Henrik Nielsen N, Frølund L, Madsen F, Dirksen A, Jørgensen T, 2002. The link between allergic rhinitis and allergic asthma: A prospective population-based study. The Copenhagen Allergy Study. Allergy Eur. J. Allergy Clin. Immunol 57, 1048–1052. 10.1034/j.1398-9995.2002.23664.x [DOI] [PubMed] [Google Scholar]
- Liu W, Ji C, Zhong J, Jiang X, Zheng Z, 2007. Temporal characteristics of the Beijing urban heat island. Theor. Appl. Climatol 87, 213–221. 10.1007/s00704-005-0192-6 [DOI] [Google Scholar]
- Lu P, Yu Q, Liu J, Lee X, 2006. Advance of tree-flowering dates in response to urban climate change. Agric. For. Meteorol 138, 120–131. 10.1016/j.agrformet.2006.04.002 [DOI] [Google Scholar]
- Martin MD, Chamecki M, Brush GS, 2010. Anthesis synchronization and floral morphology determine diurnal patterns of ragweed pollen dispersal. Agric. For. Meteorol 150, 1307–1317. 10.1016/j.agrformet.2010.06.001 [DOI] [Google Scholar]
- Massetti L, Petralli M, Orlandini S, 2015. The effect of urban morphology on Tilia×europaea flowering. Urban For. Urban Green 14, 187–193. 10.1016/j.ufug.2014.10.005 [DOI] [Google Scholar]
- Meltzer EO, 2016. Allergic rhinitis: Burden of illness, quality of life, comorbidities, and control. Immunol. Allergy Clin. North Am 36, 235–248. 10.1016/j.iac.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, Simmons AL, Wingertzahn MA, Boyle JM, 2009. Burden of allergic rhinitis: Results from the Pediatric Allergies in America survey. J. Allergy Clin. Immunol 124, 43–70. 10.1016/j.jaci.2009.05.013 [DOI] [PubMed] [Google Scholar]
- Menut L, Vautard R, Colette a, Khvorostyanov D, Potier a, Hamaoui-Laguel L, Viovy N, Thibaudon M, 2014. A new model of ragweed pollen release based on the analysis of meteorological conditions. Atmos. Chem. Phys. Discuss 14, 10891–10927. 10.5194/acpd-14-10891-2014 [DOI] [Google Scholar]
- Menzel A, 2003. Plant phenologƖcal anomalƖes in Germany and theƖr relatƖon to aƖr temperature and NAO. Clim. Change 57, 243–263. [Google Scholar]
- Miki K, Kawashima S, Fujita T, Nakamura K, Clot B, 2017. Effect of micro-scale wind on the measurement of airborne pollen concentrations using volumetric methods on a building rooftop. Atmos. Environ 158, 1–10. 10.1016/j.atmosenv.2017.03.015 [DOI] [Google Scholar]
- Mildrexler DJ, Zhao M, Running SW, 2011. A global comparison between station air temperatures and MODIS land surface temperatures reveals the cooling role of forests. J. Geophys. Res. Biogeosciences 116, 1–15. 10.1029/2010JG001486 [DOI] [Google Scholar]
- Mimet a, Pellissier V, Quénol H, Aguejdad R, Dubreuil V, Rozé F, 2009. Urbanisation induces early flowering: evidence from Platanus acerifolia and Prunus cerasus. Int. J. Biometeorol 53, 287–98. 10.1007/s00484-009-0214-7 [DOI] [PubMed] [Google Scholar]
- Mims JW, 2014. Epidemiology of allergic rhinitis. Int. Forum Allergy Rhinol 4, 18–20. 10.1002/alr.21385 [DOI] [PubMed] [Google Scholar]
- Muñoz FJ, Delgado J, Palma JL, Giménez MJ, Monteserín FJ, Conde J, 1995. Airborne contact urticaria due to mulberry (Morus alba) pollen. Contact Dermatitis 32, 61–61. 10.1111/j.1600-0536.1995.tb00855.x [DOI] [PubMed] [Google Scholar]
- Nathan R, 2007. The burden of allergic rhinitis. Allergy Asthma Proc. 28, 3–9. 10.2500/aap.2007.28.2934 [DOI] [PubMed] [Google Scholar]
- Negrini AC, Negrini S, Giunta V, Quaglini S, Ciprandi G, 2011. Thirty-year survey on airborne pollen concentrations in Genoa, Italy: Relationship with sensitizations, meteorological data, and air pollution. Am. J. Rhinol. Allergy 25, 232–241. 10.2500/ajra.2011.25.3729 [DOI] [PubMed] [Google Scholar]
- Neil K, Wu J, 2006. Effects of urbanization on plant flowering phenology : A review. Urban Ecosyst. 9, 243–257. 10.1007/s11252-006-9354-2 [DOI] [Google Scholar]
- Norris-Hill J, Emberlin J, 1991. Diurnal variation of pollen concentration in the air of north-central London. Grana 3134, 37–41. 10.1080/00173139109427803 [DOI] [Google Scholar]
- Nowak M, Szymanśka A, Grewling Ł, 2012. Allergic risk zones of plane tree pollen (Platanus sp.) in Poznan. Postep. Dermatologii i Alergol 29, 156–160. [Google Scholar]
- Osborne NJ, Alcock I, Wheeler BW, Hajat S, Sarran C, Clewlow Y, McInnes RN, Hemming D, White M, Vardoulakis S, Fleming LE, 2017. Pollen exposure and hospitalization due to asthma exacerbations: daily time series in a European city. Int. J. Biometeorol 61, 1837–1848. 10.1007/s00484-017-1369-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald EM, Rood RB, Zhang K, Gronlund CJ, O’Neill MS, White-Newsome JL, Brines SJ, Brown DG, 2012. An investigation into the spatial variability of near-surface air temperatures in the Detroit, Michigan, metropolitan region. J. Appl. Meteorol. Climatol 51, 1290–1304. 10.1175/JAMC-D-11-0127.1 [DOI] [Google Scholar]
- Parmentier B, McGill B, Wilson AM, Regetz J, Jetz W, Guralnick RP, Tuanmu MN, Robinson N, Schildhauer M, 2014. An assessment of methods and remote-sensing derived covariates for regional predictions of 1 km daily maximum air temperature. Remote Sens. 6, 8639–8670. 10.3390/rs6098639 [DOI] [Google Scholar]
- Pennington DN, Hansel JR, Gorchov DL, 2010. Urbanization and riparian forest woody communities: Diversity, composition, and structure within a metropolitan landscape. Biol. Conserv 143, 182–194. 10.1016/j.biocon.2009.10.002 [DOI] [Google Scholar]
- Polgar C, Gallinat A, Primack RB, 2014. Drivers of leaf-out phenology and their implications for species invasions: Insights from Thoreau’s Concord. New Phytol. 202, 106–115. 10.1111/nph.12647 [DOI] [PubMed] [Google Scholar]
- Polgar CA, Primack RB, 2011. Leaf-out phenology of temperate woody plants: From trees to ecosystems. New Phytol. 191, 926–941. 10.1111/j.1469-8137.2011.03803.x [DOI] [PubMed] [Google Scholar]
- Qin P, Waltoft BL, Mortensen PB, Postolache TT, 2013. Suicide risk in relation to air pollen counts: a study based on data from Danish registers. BMJ Open 3, e002462 10.1136/bmjopen-2012-002462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrochi D, Luvall J, 1999. Thermal infrared remote sensing for analysis of landscape ecological processes: Methods and applications. Landsc. Ecol 14, 577–598. 10.1023/A [DOI] [Google Scholar]
- R Core Team, 2013. R: A Language and Environment for Statistical Computing.
- Raynor G, Ogden E, Hayes J, 1970. Dispersion and deposition of ragweed pollen from experimental sources. J. Appl. Meteorol 9, 885–895. [Google Scholar]
- Reed S, Lee T, McCrory D, 2004. The economic burden of allergic rhinitis: a critical evaluation of the literature. Pharmacoeconomics 22, 345–61. [DOI] [PubMed] [Google Scholar]
- Rizwan AM, Dennis LYC, Liu C, 2008. A review on the generation, determination and mitigation of urban heat island. J. Environ. Sci. 20, 120–128. 10.1016/S1001-0742(08)60019-4 [DOI] [PubMed] [Google Scholar]
- Roetzer T, Wittenzeller M, Haeckel H, Nekovar J, 2000. Phenology in central Europe - differences and trends of spring phenophases in urban and rural areas. Int. J. Biometeorol 44, 60–66. 10.1007/s004840000062 [DOI] [PubMed] [Google Scholar]
- Salo P, Calatroni A, Gergen P, Hoppin J, Sever M, Jaramillo R, Arbes S, Zeldin D, 2011. Allergy-related outcomes in relation to serum IgE: Results from the National Health and Nutrition Examination Survey 2005–2006. J. Allergy Clin. Immunol. 127, 1226–1235. 10.1016/j.jaci.2010.12.1106.Allergy-related [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simó G, Martínez-Villagrasa D, Jiménez MA, Caselles V, Cuxart J, 2018. Impact of the surface–atmosphere variables on the relation between air and land surface temperatures. Pure Appl. Geophys. 10.1007/s00024-018-1930-x [DOI] [Google Scholar]
- Smith G, 1984. Sampling and identifying allergenic pollens and moulds. Blewstone Press, San Antonio, Texas. Soldevilla, C.G., Alcfizar-Teno, P., Dominguez-Vilches, E., 1995. Airborne pollen grain concentrations at two different heights. Aerobiol. Aerobiol. Internalional J. Aerobiol 11, 105–109. 10.1007/BF02738275 [DOI] [Google Scholar]
- Spieksma FTM, Van Noort P, Nikkels H, 2000. Influence of nearby stands of Artemisia on street-level versus roof-top-level ratio’s of airborne pollen quantities. Aerobiologia (Bologna). 16, 21–24. 10.1023/A:1007618017071 [DOI] [Google Scholar]
- Sterling DA, Lewis RD, 1998. Pollen and fungal spores indoor and outdoor of mobile homes. Ann. Allergy, Asthma Immunol 80, 279–285. 10.1016/S1081-1206(10)62971-7 [DOI] [PubMed] [Google Scholar]
- Sun X, Waller A, Yeatts KB, Thie L, 2016. Pollen concentration and asthma exacerbations in Wake County, North Carolina, 2006–2012. Sci. Total Environ 544, 185–191. 10.1016/j.scitotenv.2015.11.100 [DOI] [PubMed] [Google Scholar]
- Tal O, 2011. Flowering phenological pattern in crowns of four temperate deciduous tree species and its reproductive implications. Plant Biol. 13, 62–70. 10.1111/j.1438-8677.2010.00386.x [DOI] [PubMed] [Google Scholar]
- Targow A, 1971. The mulberry tree: A neglected factor in respiratory allergy in Southern California. Ann. Allergy 29, 318–322. [PubMed] [Google Scholar]
- Veriankaite L, Sauliene I, Bukantis A, 2010. Analysis of changes in flowering phases and airborne pollen dispersion of the genus Betula (birch). J. Environ. Eng. Landsc. Manag 18, 137–144. 10.3846/jeelm.2010.16 [DOI] [Google Scholar]
- Vitasse Y, Porté AJ, Kremer A, Michalet R, Delzon S, 2009. Responses of canopy duration to temperature changes in four temperate tree species: Relative contributions of spring and autumn leaf phenology. Oecologia 161, 187–198. 10.1007/s00442-009-1363-4 [DOI] [PubMed] [Google Scholar]
- Wan Z, 2008. New refinements and validation of the collection-6 MODIS land-surface temperature/emissivity product. Remote Sens. Environ 112, 59–74. 10.1016/j.rse.2013.08.027 [DOI] [Google Scholar]
- Wan Z, Zhang Y, Zhang Q, Li Z liang, 2002. Validation of the land-surface temperature products retrieved from terra moderate resolution imaging spectroradiometer data. Remote Sens. Environ 83, 163–180. 10.1016/S0034-4257(02)00093-7 [DOI] [Google Scholar]
- Wang J, Qi M, Huang H, Ye R, Li X, Neal Stewart C, 2017. Atmospheric pollen dispersion from herbicide-resistant horseweed (Conyza canadensis L.). Aerobiologia (Bologna). 33, 393–406. 10.1007/s10453-017-9477-3 [DOI] [Google Scholar]
- Wang J, Yang X, 2009. Improved method for nondestructive measurement of dynamic pollen source strength from transgenic crops using sonic anemometer. Int. J. Agric. Biol. Eng 2, 33–39. 10.3965/j.issn.1934-6344.2009.01.033-039 [DOI] [Google Scholar]
- Wang X, Tian Z, Ning H, Wang X, 2017. The ambient pollen distribution in Beijing urban area and its relationship with consumption of outpatient anti-allergic prescriptions. Eur. Rev. Med. Pharmacol. Sci 21, 108–115. [PubMed] [Google Scholar]
- Weinberger KR, Kinney PL, Lovasi GS, 2015. A review of spatial variation of allergenic tree pollen within cities. Arboric. Urban For 41, 57–68. [Google Scholar]
- Werchan B, Werchan M, Mücke H-G, Gauger U, Simoleit A, Zuberbier T, Bergmann K-C, 2017. Spatial distribution of allergenic pollen through a large metropolitan area. Environ. Monit. Assess 189, 169 10.1007/s10661-017-5876-8 [DOI] [PubMed] [Google Scholar]
- Wesołowski T, Rowiński P, 2006. Timing of bud burst and tree-leaf development in a multispecies temperate forest. For. Ecol. Manage 237, 387–393. 10.1016/j.foreco.2006.09.061 [DOI] [Google Scholar]
- White RJ, Carreiro MM, Zipperer WC, 2014. Woody plant communities along urban, suburban, and rural streams in Louisville, Kentucky, USA. Urban Ecosyst 17, 1061–1094. 10.1007/s11252-014-0376-x [DOI] [Google Scholar]
- Wickham H, 2009. ggplot2: elegant graphics for data analysis. Springer, New York, NY. [Google Scholar]
- Wilby RL, Jones PD, Lister DH, 2011. Decadal variations in the nocturnal heat island of London. Weather 66, 59–64. 10.1002/wea.679 [DOI] [Google Scholar]
- Woodall CW, Nowak DJ, Liknes GC, Westfall J. a., 2010. Assessing the potential for urban trees to facilitate forest tree migration in the eastern United States. For. Ecol. Manage 259, 1447–1454. 10.1016/j.foreco.2010.01.018 [DOI] [Google Scholar]
- Wozniak MC, Steiner A, 2017. A prognostic pollen emissions model for climate models (PECM1.0). Geosci. Model Dev 1–36. [Google Scholar]
- Ye R, Huang H, Alexander J, Liu W, Millwood RJ, Wang J, Stewart CN, 2016. Field studies on dynamic pollen production, deposition, and dispersion of glyphosate-resistant horseweed (Conyza canadensis). Weed Sci. 64, 101–111. 10.1614/WS-D-15-00073.1 [DOI] [Google Scholar]
- Yin X, Kropff MJ, Goudriaan J, 1996. Differential effects of day and night temperature on development to flowering in rice. Ann. Bot 77, 203–213. 10.1006/anbo.1996.0024 [DOI] [Google Scholar]
- Zhang X, Friedl MA, Schaaf CB, Strahler AH, 2004. Climate controls on vegetation phenological patterns in northern mid- and high latitudes inferred from MODIS data. Glob. Chang. Biol 10, 1133–1145. 10.1111/j.1365-2486.2004.00784.x [DOI] [Google Scholar]
- Zink K, Vogel H, Vogel B, Magyar D, Kottmeier C, 2012. Modeling the dispersion of Ambrosia artemisiifolia L. pollen with the model system COSMO-ART. Int. J. Biometeorol 56, 669–80. 10.1007/s00484-011-0468-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.