Abstract
Traditionally the skin was believed to be devoid of B cells, and studies of the skin immune system have largely focused on other types of leukocytes. Exciting recent data show that B cells localize to healthy skin of humans and other mammalian species with likely homeostatic functions in host defense, regulation of microbial communities and wound healing. Distinct skin-associated B cell subsets drive or suppress cutaneous inflammatory responses with important clinical implications. Localized functions of skin-associated B cell subsets during inflammation include antibody production, interactions with skin T cells, tertiary lymphoid tissue formation, production of pro-inflammatory cytokines, but also immunosuppression by providing IL-10. In this review, we delve into the intriguing new roles of skin-associated B cells in homeostasis and inflammation.
Introduction
The skin is an essential barrier organ exposed to a variety of daily insults that range from simple physical injuries like cuts and UV damage to infections with sophisticated skin pathogens such as herpes simplex virus or Staphylococcus aureus. In addition to these external threats, the skin is the frequent target of allergy, autoimmunity and cancer. The skin is colonized with microbes and consists of protective layers that encompass physical and cellular components as well as antimicrobial proteins and microbial metabolites (reviewed in (1)). The outermost layer of the skin is the keratinized epidermis, and the dermis and subcutaneous adipose tissue lay below it, and leukocytes populate all of these anatomical skin sites (reviewed in (2)). To enter the skin from blood, leukocytes migrate through postcapillary venules in the dermis from where they can access microenvironments within the dermis or traverse further into the epidermis (reviewed in detail in (3)). To egress from skin, leukocytes have to reach dermal afferent lymph vessels, which will transport them via larger collecting lymph vessels to the draining lymph node (reviewed in (4)).
In the past decades, knowledge about the skin immune system has been rapidly expanding by defining the protective and pathological roles of different skin-associated leukocyte subsets (e.g. T cells) as well as other skin cells such as keratinocytes (reviewed in (5)). In contrast, the existence of skin-localized B cell functions remained largely unknown, despite a recognized systemic importance of B cells for skin immunity and inflammation. Specifically, B cells that differentiate into antibody-secreting cells give rise to systemic antibody titers that enforce defense against cutaneous infections but also support cutaneous allergies and autoimmune diseases. Today, there is growing evidence for a localized function of B cells within skin in driving disease through multiple mechanisms. Subsets of skin-associated B cells have also emerged as critical negative regulators of skin inflammation. In addition, there is evidence that skin-associated B cells are involved in skin homeostasis and repair by regulating wound healing and the cutaneous microbiome. Here we review skin-associated B cells as novel players in cutaneous homeostasis and inflammation as illustrated in Figures 1 and 2, respectively. A role for B cells in skin cancers has been reviewed elsewhere (6).
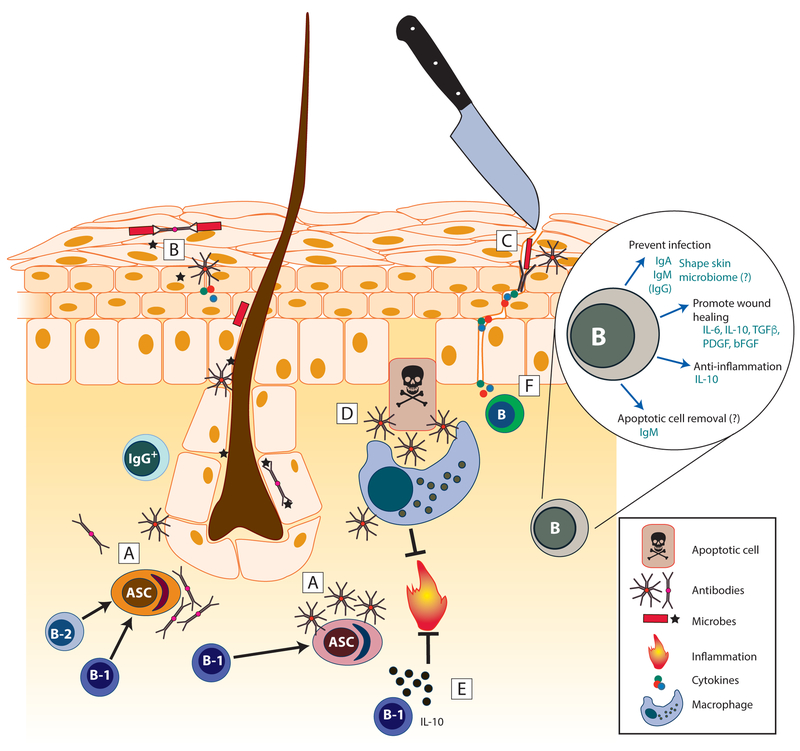
Figure 1. Skin-associated B cells maintain normal healthy skin.
B-1 and B-2 lineage cells as well as antibody-secreting cells localize to healthy skin. [A -C] Secretory IgM and IgA are produced in healthy skin [A] and bind skin commensals [B] as well as invading microbes after barrier breach [C]. [D] Secreted natural IgM binds to and enhances uptake of apoptotic cells (efferocytosis) by macrophages, a process that induces anti-inflammatory programming in macrophages. [E] Skin-associated B cells, primarily B-1-like B cells, produce IL-10 to limit inflammation. [F] Additional cytokines and growth factors are produced by B cells and support wound healing. Inset, Summary of mechanisms by which B cells influence skin homeostasis. ASC, antibody-secreting cell; PDGF, platelet-derived growth factor; bFGF, basic fibroblast growth factor.
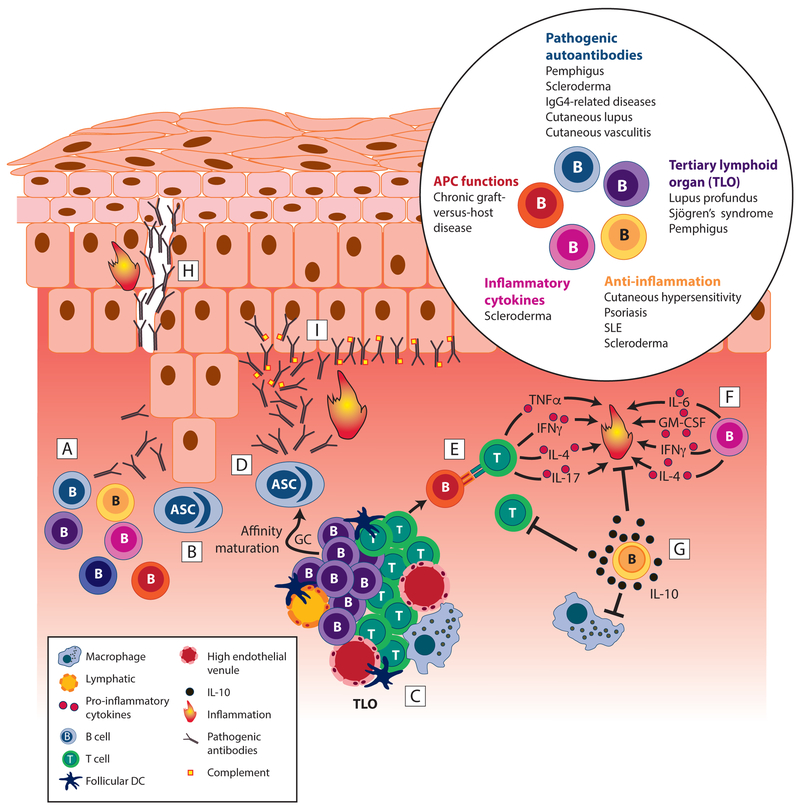
Figure 2. Skin-associated B cells are critical to driving and suppressing inflammation.
Skin-associated B cells play several important roles in skin inflammation. [A] B cell subsets with various functions accumulate in inflamed skin. [B] Antibody-secreting cells accumulate in inflamed skin and secrete antibodies with reactivity to cutaneous antigens, including autoantigens and skin-associated allergens. [C] Aggregates of collaborating B and T cells form, and B cells initiate further development of tertiary lymphoid organs that support activation and differentiation of pathogenic B and T cells. [D] High affinity autoreactive plasma cells and IgGs emerge from GC reaction in TLO, exacerbating inflammation. [E] B cells act as APCs inducing T cell activation and pro-inflammatory cytokine production. [F] B cells produce pro-inflammatory cytokines, like IL-6, and potentially GM-CSF, IFN-γ, or IL-4, all of which promote local inflammation and, in case of IL-6, fibrosis via stimulation of fibroblasts. [G] B cells secrete IL-10 that suppresses activation of other leukocytes, including T cells and macrophages, thereby counteracting inflammation. [H] Pathogenic autoantibody deposition can destroy cell-cell junctions creating acantholysis or gaps between cells as in pemphigus. [I] Autoantibodies can accumulate at the dermoepidermal junction as in cutaneous lupus erythematosus, leading to complement activation and other inflammatory downstream effector functions. Inset, Summary of disease settings in which skin-associated B cells modulate skin inflammation. ASC, antibody secreting cell; SLE, systemic lupus erythematosus. GC, germinal center; TLO, tertiary lymphoid organ.
Both recirculating (mobile) and resident T cell subsets exist in skin, and T cells resident for prolonged periods of time are termed “tissue-resident memory T cells” and have distinct surface markers (i.e. CD103 and CD69 on CD8+ T cells) as well as differentiation programming (reviewed in (7)). While it is clear that subsets of B cells egress from skin by entering afferent lymph vessels after some time of residency (8), it is currently unknown for how long B cells dwell in skin. In this review, we use the term “skin-resident” for B cells that have left the vascular space and reside in the skin and “skin-associated” for B cells that are either skin-recirculating (present in skin-draining lymph) or skin-resident.
Innate-like and conventional B cells localize to healthy skin
While initial histological studies were unable to detect B cells in healthy human skin (9, 10), cannulation of afferent lymph vessels draining healthy skin in sheep (8, 11) and humans (12, 13) demonstrated the existence of skin-recirculating B cells. More recent studies that employ flow cytometry identified and characterized B cells residing in healthy skin of mice and humans (14, 15). Skin-associated B cells are evolutionarily conserved as the skin of lower vertebrates, such as teleost fish, contains B cells and Ig secreting cells (16). In mammalian skin, B cells localize to the dermis where they are sparsely scattered as individual cells during homeostasis, and often as cell clusters or more organized lymphoid structures during inflammation (14, 17–20)
Skin-associated B cells are a heterogenous population with both conventional (B-2) and innate-like (B-1-like) B cells (8, 14). Relative to their blood-borne counterparts, IgG+ skin-infiltrating human B-2 B cells have a lower representation of the IgG1 subclass and show a preferred usage of certain Vh genes, suggesting the existence of skin-specific B cell subsets (15). While the majority of skin B cells resemble conventional B-2 B cells, innate-like (B-1-like) B cells are enriched in skin compared to blood in humans and mice (14). Also, a population of ovine skin-recirculating B cells in afferent lymph expresses high levels of IgM and myeloid markers such as CD11b indicative of B-1-like B cells (8). Innate-like B cells often recognize conserved pathogen structures and produce natural antibodies without previous antigenic exposure as well as mount rapid T cell-independent antibody responses early after infection (reviewed in (21, 22)). Their often polyreactive antibodies (mostly IgM and IgA) are therefore an effective first line of defense against infection (21, 22). In mice, B-1 B cells have a high propensity to migrate into skin (14) and other barrier sites such as the respiratory tract or the small intestinal mucosa, where they limit early infections by secreting IgA and IgM and innate-stimulatory cytokines such as GM-CSF (23–25). Thus, innate-like B cell localization to mammalian barrier organs represents a form of barrier enforcement and first line of defense against invading pathogens following barrier breach.
Antibody production in healthy skin potentially modulates skin barrier function and microbiomes
While most antibodies act systemically by blood distribution throughout the body and diffusion into tissues, tissue titers can be enhanced by localized antibody production. For example, tissue-resident plasma cells secrete multimeric IgA and IgM, which are transcytosed through epithelial barriers from abluminal to luminal side via polymeric IgR (reviewed in (26)). As a result, they reach glandular excretions and coat mucosal surfaces as secretory Igs (26). The localized role of IgA, and to some degree that of IgM, is well appreciated as a first line of defense against toxins and pathogen invasion of mucosal barriers (26–28). In contrast, studies analyzing antibody production in mammalian skin are limited. To this end, antibody secretion in healthy skin has been demonstrated for humans and sheep (8, 29). In human skin, IgA is secreted in eccrine sweat glands and appears in sweat and sebum, and antibodies of IgG and IgM isotype reach the skin surface by undefined mechanisms (29, 30). Our own studies found that the isotype predominantly produced by skin-resident plasma cells of naïve mice is IgM (GFD and SEM; unpublished observations), which may reflect the relative lack of eccrine sweat glands in mouse skin. More extensive studies are necessary to determine how each Ig isotype reaches distinct skin compartments in different mammalian species.
During inflammation, the presence of antibody-secreting plasma cells in lesional human skin has been well documented for decades. Moreover, plasma cells, identified histologically, facilitate dermatopathological diagnosis of several inflammatory entities e.g. necrobiosis lipoidica and acne keloidalis nuchae (31). The isotype (i.e. subclasses of IgG, IgA, IgM and/or IgE) produced by plasma cells in lesional skin varies depending on the skin site and type of inflammation (for examples see References (32–34)). Ig-secreting cell accumulation in inflamed skin leads to higher Ig titers in lymph draining the skin site, reflecting an increase in tissue titers (8). While localized antibody production is likely an effective, or sometimes desperate, attempt to control skin pathogens, it is destructive in the case of pathogenic autoantibody production to cutaneous autoantigens (e.g. in pemphigus (18); further discussed below).
Diverse microbes colonize throughout the skin, including epidermal surfaces and appendages such as hair follicles (35), and colonization reportedly reaches as deep as the dermis and dermal (subcutaneous) adipose tissue (36). The microbial communities of the skin are critically involved in orchestrating local cutaneous physiological and pathophysiologic processes, including wound healing, inflammation, immune responses to skin pathogens (37–39), and even susceptibility to skin cancer (40). On the other hand, skin colonization with pathogenic bacteria such as methicillin-resistant Staphylococcus aureus can cause serious disease upon skin barrier breach (41). In the intestine, antibody, in particular thymus-independent secretory IgM and IgA, bind to commensal bacteria and facilitate symbiotic host-microbe interactions (42–44), and the question arises whether there is a similar role for antibody in shaping skin microbiomes. A study in RAG-1-deficient mice, which lack all B and T cells as well as antibodies, was unable to detect alterations in the ear skin surface microbiome using 16S rRNA sequencing (45). However, electron microscopy studies using immuno-gold labeling of human skin samples showed that skin microbes are coated with IgA, IgG and IgM (46), supporting a role for cutaneous antibodies in regulating microbial colonization of skin microenvironments. In addition, IgM in human sweat from healthy skin can bind S. aureus (46). Thus, it is possible that, akin to their role in the intestine, secretory Igs influence microbial communities in the skin. Future studies are warranted that test the role of antibodies for their ability to modulate microbial colonization of distinct skin compartments, including that of deeper skin layers.
Skin-resident B cells drive skin inflammation –B cell accumulation
In a number of autoimmune diseases with cutaneous manifestations, the role of B cells and antibodies has generally been considered systemic, i.e. pathogenic autoantibodies produced in lymphoid tissue enter blood and diffuse into skin where they cause disease. Recently, B cells are increasingly recognized as an important part of the infiltrating immune compartment in autoimmune and inflammatory skin diseases. B cell numbers are elevated in lesional skin relative to control skin specimens in a number of inflammatory diseases, including, but not limited to, psoriasis (47), pemphigus (18), lupus profundus (19, 20), systemic sclerosis (scleroderma) (48), discoid lupus erythematosus (49, 50), Sjögren’s syndrome (51), IgG4-related skin diseases (33, 34), atopic dermatitis (52), and allergic contact dermatitis (53).While an increase in skin-infiltrating B cells suggests a role for B cells in cutaneous inflammation, the therapeutic success of systemic B cell depletion with rituximab confirms a pathogenic role for B cells in a number of these diseases (54–56). In pemphigus, atopic dermatitis, and scleroderma, disease severity and progression are positively correlated with the number of skin-infiltrating B cells (18, 48, 52). Specifically, in scleroderma, a chronic connective tissue disorder that often progresses to extensive cutaneous and vascular fibrosis, only those patients with skin-infiltrating B cells advanced in disease severity over a period of 12 months (48). Furthermore, a pathogenic role for B cells in the initiation phase of skin fibrosis was confirmed using B cell depletion therapy in scleroderma patients and mouse models of the disease (57–59). Collectively, the recent reports of skin-infiltrating B lineage cells in skin pathologies raise the question whether B cells directly contribute to cutaneous inflammation through local effector functions (as discussed in the sections below). Of note, skin-infiltrating B cells are not always pathogenic, e.g. psoriasis does not usually improve with B cell depletion (60), and rituximab treatment can induce psoriasis in some individuals (61), suggesting a protective role of B cells (discussed below). Czarnowicki et al. investigated B cell subsets in two skin diseases in which B cells appear to have opposing roles: atopic dermatitis and psoriasis (52), which are driven by Th2 and Th17/Th1 cell responses, respectively (62). The authors suggested that a higher B cell activation status reflected accumulative antigen exposure and ability to contribute to T cell activation and other pathogenic mechanisms, such as antibody production and IgE switching (52). Thus, it is likely that BCR specificity for skin-associated antigens (autoantigens, allergens, or microbiota) in combination with B cell-effector functions (discussed below) dictates the distinct roles of skin-associated B cells in different types of cutaneous inflammation.
Skin-resident B cells drive skin inflammation –Localized antibody production
A prominent example in which skin-infiltrating B cells may drive disease is pemphigus vulgaris, a severe autoimmune blistering disease that affects mucosal membranes and skin. Pemphigus is characterized by circulating anti-desmoglein 1/3 (Dsg1/3) autoantibodies, which recognize desmosomal adhesion proteins on epidermal keratinocytes. In humans, placental transfer of anti-Dsg autoantibody from diseased mothers induces pemphigus symptoms in their newborns (reviewed in (63)). Likewise, passive transfer of the autoantibodies into newborn mice is sufficient to induce pemphigus pathology, confirming that pathogenic autoantibody is the main etiology of the disease (63). The vast majority of pemphigus patients accumulate anti-Dsg1/3 autoantibodies in skin; however, circulating Dsg1/3 autoantibodies can be found in patients without skin lesions and in some healthy controls, indicating that in addition to systemic autoantibodies, other factors determine disease (reviewed in (64)). The pathogenic potential of anti-Dsg1/3 antibodies after transfer into mice correlates with serum titers (65). An increase in cutaneous autoantibody titers could be due to enhanced localized production and/or diffusion from systemic antibodies, ultimately boosting localized binding to cutaneous autoantigens. In support of this notion, Yuan et al. recently showed that autoreactive Dsg1- and Dsg3-specific antibody-secreting cells and B cells localize to lesional relative to healthy skin, suggesting a pathogenic role in situ by these autoreactive cells (18). Akin to pemphigus, the majority of scleroderma patients present with both systemic and local autoantibodies as well as skin lesion-infiltrating B cells and plasma cells (48). Finally, IgG4-related skin diseases, which are fibroinflammatory disorders affecting multiple organs including skin, are induced by accumulation of terminally differentiated IgG4+ plasma cells and antibody deposition in affected skin (33, 34). Taken together, localized antibody production is a feature of several skin diseases including those in which antibody binding to cutaneous autoantigens is a known driver of disease.
While in some instances, such as pemphigus vulgaris, dissociation of tissue due to autoantibody binding alone causes pathology (63), many times complement fixation and/or Fc-mediated Ab effector functions are necessary for tissue destruction. For example, in cutaneous manifestations of the autoimmune disease lupus erythematosus, the lupus band test on skin biopsies reveals the deposition of autoantibody-antigen complexes as well as complement proteins along the dermoepidermal junction (66). In cutaneous vasculitis, immunoglobulins and complement proteins are deposited around dermal vessel walls (67). Complement activation in the skin has the potential to exacerbate inflammatory damage by direct cell lysis and/or via recruitment and activation of innate immune cells, such as macrophages and neutrophils. Thus, autoantibody production in skin is a powerful means for localized destruction and inflammation through complement and/or other downstream mechanisms.
Skin-resident B cells drive skin inflammation –Cutaneous tertiary lymphoid organs (TLOs) and B cell aggregates
Formation of ectopic lymphoid tissue or TLOs in the skin may provide a local microenvironment for skin-associated B cells to promote skin inflammation. TLOs are considered outposts of secondary lymphoid organs as they possess similar characteristics, such as organized B cell and T cell areas with antigen presenting dendritic cells on a network of fibroblasts as well as high endothelial venules, lymph vessels, and germinal center (GC)-like structures (68). TLOs are found in or near sites of chronic inflammation e.g. during autoimmunity, infection or transplant rejection. In extracutaneous lesions of autoimmunity, such as in systemic lupus erythematosus (SLE) and rheumatoid arthritis, TLOs are considered pathogenic as they perpetuate local inflammation by enhancing autoantibody production and autoreactive T cell activation (reviewed in (69, 70)). Even though TLO-like structures that support interactions between skin B and T cells exist in certain inflammatory skin diseases, little is known about the role of TLOs in the pathogenesis of cutaneous pathologies. In lupus erythematosus panniculitis (lupus profundus), a form of cutaneous lupus that is typified by inflammation of the subcutaneous adipose tissue, aggregates of B cells and plasma cells, or lymphoid follicles containing distinct germinal centers are found in the majority of cutaneous lesions (19, 20). Kogame et al. showed that additional components of a TLO are present in these lupus profundus lesions, including CXCL13+ cells, peripheral node addressin+ (PNAd+) high endothelial venules (HEVs), and podoplanin+ lymphatic vessels (20). These findings confirm that TLO structures develop in lupus profundus. In pemphigus skin lesions, B cells and IL-21+ CD4+ T cells interact closely in aggregate structures that resemble developing TLOs (18). Aggregates of B cells and T cells have also been noted in skin manifestations of Sjögren’s syndrome (51), an autoimmune disorder of exocrine glands. Skin-localized GC-like structures likely enhance pathogenic Ig affinity maturation, class switching to different IgG subclasses, as well as antibody production, thereby augmenting humoral responses to skin-associated antigens. Localized activation of effector T cells and Ab production in lymphoid aggregates and TLOs could help explain why in one individual with systemic autoantibodies that target skin, some skin areas are affected while others are spared from pathology. Notably, B cells are drivers of lymphoid neogenesis including TLOs by provision of lymphotoxin (70). Therefore, when skin-resident B cells initiate formation of a local TLO this may be the tipping point that accelerates disease in many cases of chronic skin inflammation.
Skin-resident B cells drive skin inflammation –Local APC function and cytokine secretion
B cells are increasingly recognized as key effector cells in inflammation and infection by secretion of inflammatory cytokines such as IL-6, GM-CSF, IFN-γ and IL-4 (71), and B cell cytokine secretion in skin is a new area of investigation. Recently, Matsushita and colleagues showed that uninflamed skin of mice harbors IL-6+ B cells, which drastically increase in inflamed skin in the bleomycin-induced scleroderma model (59). Importantly, the authors found that skin pathology, including, skin fibrosis and thickening were significantly reduced in mice whose B cells lack the ability to express IL-6, demonstrating a pathogenic role for IL-6+ B cells in fibrosing skin inflammation (59). Furthermore, blockade of the cytokine BAFF (a B cell targeting approach used to treat SLE (72)) inhibited expansion of IL-6+ but not that of anti-inflammatory IL-10+ B cells, leading to reduced pathology in bleomycin-induced skin fibrosis (59). These findings stress the importance to further define skin-associated B cells and their functions as this may reveal novel tools to selectively target distinct B cell subsets.
Antigen-presentation to effector T cells is another antibody-independent effector function of B cells that is increasingly recognized to drive inflammation. Specifically, effector T cells that recognize antigen on B cell MHCII release potent inflammatory cytokines and subsequently expand. For example, mice with B cells that cannot present antigen to T cells are protected from neuroinflammation in a mouse model of multiple sclerosis due to reduced pathogenic T cell responses (73). Studies by our lab showed that skin-resident and skin-recirculating B cells express high levels of MHCII and costimulatory molecules (CD80/86 and CD1), and are therefore well equipped for T cell activation (8). While studies of B cells as APCs in skin inflammation are limited, Young et al. showed a pathogenic role for B cells in a mouse model of chronic graft-versus-host disease (74), a serious complication of allogeneic hematopoietic stem cell transplantation. Specifically, donor B cells upregulated MHCII and costimulatory molecules and augmented donor CD4+ T cell expansion, alloreactivity, and survival, which in turn promoted chronic graft-versus-host disease in skin and lung (74). While the authors concluded that B cell-T cell crosstalk occurred in secondary lymphoid tissues (74), it is possible that such interactions were also present directly in skin and/or skin-associated TLOs. Effector T cell activation by skin-associated B cells and downstream inflammation caused by T cell cytokines, such as IFN-γ, IL-17, and IL-4 has the potential to exacerbate many inflammatory skin disorders. Therefore, additional studies into the APC function of skin-associated B cells are highly warranted.
Cutaneous regulatory B cells
B cells with the capacity to suppress immune responses, termed regulatory B cells (Bregs), have recently received much attention as they limit inflammatory and autoimmune processes in many organ systems and diseases (reviewed in (75, 76)). Notably, B cells with the ability to suppress skin inflammation were already described more than 40 years ago (77, 78). The suppressive mechanisms employed by Bregs are varied and include expansion and maintenance of regulatory T cells (Tregs), adenosine generation, and secretion of the suppressive cytokines IL-35 and IL-10 (reviewed in (79, 80)). In skin, B cells that produce IL-10 are critical to limiting inflammation, and the significance of other Breg functions has not been explored to date.
In mouse models, IL-10+ Bregs (aka “B10 cells”) limit inflammation in cutaneous hypersensitivity (CHS) (81, 82), scleroderma (59), and psoriasis-like inflammation (83, 84). Earlier studies suggested that splenic Bregs suppress T cell priming and/or polarization in the initiation phase and that innate-like Bregs (e.g. peritoneal B-1 B cells) are important in the remission phase of inflammation with all Bregs acting within lymphoid tissues (81, 82). Our lab recently showed that IL-10+ B cells reside in normal mouse and human skin, and analysis of IL-10 reporter mice revealed that B cells actively transcribe IL-10 in the skin even in the absence of inflammation (14). Moreover, IL-10+ B-1 B cells, which are potent suppressors of inflammation in both cutaneous and intestinal inflammation (82, 85), are recruited into inflamed skin (14). Matsushita et al. also showed that IL-10+ Bregs reside in skin and are critical in limiting pathology in a scleroderma model (59). Therefore, IL-10+ Bregs are well positioned to suppress inflammation in the skin itself in addition to acting in lymphoid tissue. Breg presence and active IL-10 transcription in uninflamed skin (14) suggest that skin Bregs fulfill a steady state function by suppressing aberrant responses to physical insults and/or skin commensal microbiota.
A potential pathway to induce skin Bregs is through cutaneous UV exposure. Specifically, moderate UV light exposure of skin leads to enrichment of skin lymph node-resident B cells with the capacity to inhibit DC activation of T cells in CHS models in an IL-10-dependent manner (86, 87) as well as IL-10-negative Bregs that suppress experimental autoimmune encephalitis (88). It will be interesting to further investigate this pathway as well as potential differences between individual mammalian species with inherent differences in sun (UV) light exposure (e.g. caused by hair density and nocturnal vs. diurnal chronotypes).
In humans, B cell depleting therapy (i.e. rituximab, anti-CD20) is able to induce or exacerbate the inflammatory skin disease psoriasis (reviewed in (61)). This apparent paradoxical effect of an immunosuppressive therapy supports a protective role for B cells in this largely Th1 and Th17 cell-mediated disease. Interestingly, psoriasis is associated with low levels of dermal IL-10 and is clinically responsive to locally administered IL-10 (89, 90), possibly reflecting a niche for regulation by IL-10+ cell types such as IL-10+ Bregs. Extending the mouse studies that demonstrate a critical role for IL-10+ B cells in suppressing psoriasiform inflammation (83, 84), individuals with psoriasis have reduced numbers of IL-10+ B cells in their blood (91, 92). Other cutaneous inflammatory diseases, such as the skin blistering disease pemphigus and scleroderma can also be associated with reduced numbers of circulating IL-10+ Bregs during active disease (93–96) and an increase in disease remission (97). Notably, IL-10+ B cells localize to the inflamed skin in SLE (98). It is therefore possible that skin-associated Bregs limit skin inflammation even in B cell- and autoantibody-mediated inflammation.
Collectively, there is accumulating evidence that IL-10+ B cells suppress skin inflammation not only in mouse models but also in humans. Additional studies are needed to dissect the regulatory B cell subsets at cutaneous and extracutaneous sites and IL-10-dependent and -independent mechanisms by which they suppress different types and phases (i.e. acute vs. chronic; initiation vs. remission) of skin inflammation. In particular in B cell-mediated autoimmune diseases it would be advantageous to be able to deplete disease-driving B cells selectively, while sparing Bregs. Conversely, tools to selectively target Bregs that impede effective cutaneous immune responses, based on differential surface marker expression for example, could potentially be desirable when treating cutaneous tumors or chronic infections. To complicate matters, Bregs that make IL-10 do not appear to be a separate B cell lineage and are rather an activation or differentiation state of several traditional B cells subsets (99). Similarly, in T cells IL-10 is produced not only by bona fide FoxP3+ Tregs but also by effector T cells and represents an essential mechanism for limiting immunopathology at effector sites (100, 101). For example, during influenza virus infection IFN-γ+ CD4 and CD8 effector T cells acquire the ability to secrete IL-10 critical for host survival by limiting tissue destruction and pulmonary inflammation (102). Therefore, further studies are needed to decode the signals that induce IL-10 or other anti-inflammatory properties in distinct skin-associated B cell subsets, allowing for selective therapeutic manipulation.
Skin B cells potentially support wound healing
As a barrier organ with various physical insults that happen on a daily basis, there is a vast need for repair of small and larger skin wounds. B cells localize to cutaneous wounds (103, 104) and are potentially involved in wound healing (105). Specifically, mice that overexpress BCR co-receptor CD19 (CD19-transgenic mice) exhibit accelerated, while CD19-deficent mice have delayed, wound healing (105). The authors of the study suggested a TLR4-initiated increase in cytokines, such as IL-6, IL-10, TGF-β, platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF) as the mechanism of B cell-enhanced wound healing (105). However, CD19-deficient and transgenic mice have greatly altered B cell subsets with a relative lack or increase of innate-like B cells (i.e. MZ and B-1 B cells), respectively (106–108). As B-1-like B cells, which are CD19hi, localize to skin (14), it is tempting to speculate that these cells promote wound healing. In further support of a role of B cells in wound healing is an observation that exogenous application of splenic B cells to wounds accelerates the healing process in WT and diabetic mice (109). Future studies are needed to dissect the mechanism by which different subsets of skin-associated B cells may contribute to the wound healing process and how this may be targeted for therapeutic purposes.
Conclusions
With the relatively recent discovery that B cells are part of the skin immune system we are just beginning to understand the capacity of skin-associated B cells in modulating cutaneous immune responses. The newly uncovered pathogenic and protective functions of B cells in skin and that of B cells at other extralymphoid sites e.g. the joints and synovia, the central nervous system, and intestinal mucosa offer a glimpse of the power of skin-associated B cells in disease settings. Thus, further illuminating the functions of skin B cell subsets provides the opportunity to develop novel approaches to modulate cutaneous immune responses in homeostasis, inflammation, infection, and cancer. We anticipate that exciting new developments in cutaneous B cell biology are on the horizon.
Acknowledgements
The authors thank Neda Nikbakht, Gregg Silverman, and members of the Debes Lab for critical comments on the manuscript and stimulating discussions. We also thank John Stanley for advice and discussion of pemphigus pathogenesis. The authors are indebted to Paul Schiffmacher and Tim Flanagan for figure illustrations.
This work was supported in parts by NIH grants R01AR067751 and R01AI127389 to GFD and a Percival E. and Ethel Brown Foerderer Foundation Fellowship from Thomas Jefferson University to SEM.
Abbreviations used:
- Dsg1/3
desmoglein 1/3
- TLO
tertiary lymphoid organ
- GC
germinal center
- SLE
systemic lupus erythematosus
- Tregs
regulatory T cells
- CHS
cutaneous hypersensitivity
References
- 1.Chen YE, Fischbach MA, and Belkaid Y. 2018. Skin microbiota-host interactions. Nature 553: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lian CG a. M. G. F. 2015. Histology of the Skin In Lever’s Histopathology of the Skin, 11 ed Elder DE, ed. Wolters Kluwer, Philadelphia: 8–75. [Google Scholar]
- 3.Schön MP, Zollner TM, and Boehncke WH. 2003. The molecular basis of lymphocyte recruitment to the skin: clues for pathogenesis and selective therapies of inflammatory disorders. J. Invest. Dermatol 121: 951–962. [DOI] [PubMed] [Google Scholar]
- 4.Lund AW, Medler TR, Leachman SA, and Coussens LM. 2016. Lymphatic Vessels, Inflammation, and Immunity in Skin Cancer. Cancer Discov. 6: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasparakis M, Haase I, and Nestle FO. 2014. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol 14: 289–301. [DOI] [PubMed] [Google Scholar]
- 6.Egbuniwe IU, Karagiannis SN, Nestle FO, and Lacy KE. 2015. Revisiting the role of B cells in skin immune surveillance. Trends Immunol. 36: 102–111. [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt T, Palendira U, Tscharke DC, and Bedoui S. 2018. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev 283: 54–76. [DOI] [PubMed] [Google Scholar]
- 8.Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, Alt C, Young AJ, Hay JB, and Debes GF. 2012. The skin, a novel niche for recirculating B cells. J. Immunol 188: 6027–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bos JD, and Teunissen MB. 2008. Innate and Adaptive Immunity In Clinical and Basic Immunodermatolgy Gaspari AA, and Tyring SK, eds. Springer, London: 17–30. [Google Scholar]
- 10.Bos JD, Zonneveld I, Das PK, Krieg SR, van der Loos CM, and Kapsenberg ML. 1987. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. J. Invest. Dermatol 88: 569–573. [DOI] [PubMed] [Google Scholar]
- 11.Mackay CR, Kimpton WG, Brandon MR, and Cahill RN. 1988. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J. Exp. Med 167: 1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olszewski WL, Grzelak I, Ziolkowska A, and Engeset A. 1995. Immune cell traffic from blood through the normal human skin to lymphatics. Clin. Dermatol 13: 473–483. [DOI] [PubMed] [Google Scholar]
- 13.Yawalkar N, Hunger RE, Pichler WJ, Braathen LR, and Brand CU. 2000. Human afferent lymph from normal skin contains an increased number of mainly memory / effector CD4(+) T cells expressing activation, adhesion and co-stimulatory molecules. Eur. J. Immunol 30: 491–497. [DOI] [PubMed] [Google Scholar]
- 14.Geherin SA, Gomez D, Glabman RA, Ruthel G, Hamann A, and Debes GF. 2016. IL-10+ Innate-like B Cells Are Part of the Skin Immune System and Require alpha4beta1 Integrin To Migrate between the Peritoneum and Inflamed Skin. J. Immunol 196: 2514–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saul L, Ilieva KM, Bax HJ, Karagiannis P, Correa I, Rodriguez-Hernandez I, Josephs DH, Tosi I, Egbuniwe IU, Lombardi S, Crescioli S, Hobbs C, Villanova F, Cheung A, Geh JL, Healy C, Harries M, Sanz-Moreno V, Fear DJ, Spicer JF, Lacy KE, Nestle FO, and Karagiannis SN. 2016. IgG subclass switching and clonal expansion in cutaneous melanoma and normal skin. Sci. Rep 6: 29736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salinas I, Zhang YA, and Sunyer JO. 2011. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol 35: 1346–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafyatis R, Kissin E, York M, Farina G, Viger K, Fritzler MJ, Merkel PA, and Simms RW. 2009. B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 60: 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan H, Zhou S, Liu Z, Cong W, Fei X, Zeng W, Zhu H, Xu R, Wang Y, Zheng J, and Pan M. 2017. Pivotal Role of Lesional and Perilesional T/B Lymphocytes in Pemphigus Pathogenesis. J. Invest. Dermatol 137: 2362–2370. [DOI] [PubMed] [Google Scholar]
- 19.Massone C, Kodama K, Salmhofer W, Abe R, Shimizu H, Parodi A, Kerl H, and Cerroni L. 2005. Lupus erythematosus panniculitis (lupus profundus): clinical, histopathological, and molecular analysis of nine cases. J. Cutan. Pathol 32: 396–404. [DOI] [PubMed] [Google Scholar]
- 20.Kogame T, Yamashita R, Hirata M, Kataoka TR, Kamido H, Ueshima C, Matsui M, Nomura T, and Kabashima K. 2018. Analysis of possible structures of inducible skin-associated lymphoid tissue in lupus erythematosus profundus. J. Dermatol [DOI] [PubMed] [Google Scholar]
- 21.Baumgarth N 2011. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol 11: 34–46. [DOI] [PubMed] [Google Scholar]
- 22.Kearney JF 2005. Innate-like B cells. Springer Semin. Immunopathol 26: 377–383. [DOI] [PubMed] [Google Scholar]
- 23.Choi YS, and Baumgarth N. 2008. Dual role for B-1a cells in immunity to influenza virus infection. J. Exp. Med 205: 3053–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki K, Maruya M, Kawamoto S, and Fagarasan S. 2010. Roles of B-1 and B-2 cells in innate and acquired IgA-mediated immunity. Immunol. Rev 237: 180–190. [DOI] [PubMed] [Google Scholar]
- 25.Weber GF, Chousterman BG, Hilgendorf I, Robbins CS, Theurl I, Gerhardt LM, Iwamoto Y, Quach TD, Ali M, Chen JW, Rothstein TL, Nahrendorf M, Weissleder R, and Swirski FK. 2014. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J. Exp. Med 211: 1243–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandtzaeg P 2013. Secretory IgA: Designed for Anti-Microbial Defense. Front. Immunol 4: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantis NJ, Rol N, and Corthesy B. 2011. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrenstein MR, and Notley CA. 2010. The importance of natural IgM: scavenger, protector and regulator. Nat. Rev. Immunol 10: 778–786. [DOI] [PubMed] [Google Scholar]
- 29.Okada T, Konishi H, Ito M, Nagura H, and Asai J. 1988. Identification of secretory immunoglobulin A in human sweat and sweat glands. J. Invest. Dermatol 90: 648–651. [DOI] [PubMed] [Google Scholar]
- 30.Metze D, Jurecka W, Gebhart W, Schmidt J, Mainitz M, and Niebauer G. 1989. Immunohistochemical demonstration of immunoglobulin A in human sebaceous and sweat glands. J. Invest. Dermatol 92: 13–17. [DOI] [PubMed] [Google Scholar]
- 31.Elston DM F. T; K. CJ Peckham S; High WA; DiCaudo DJ; Bhuta S 2014. Dermatopathology. Elservier Limited. [Google Scholar]
- 32.Lai AFRF, Cormane RH, and van Furth R. 1974. An immunohistopathological study on the synthesis of immunoglobulins and complement in normal and pathological skin and the adjacent mucous membranes. Br. J. Dermatol 90: 123–136. [DOI] [PubMed] [Google Scholar]
- 33.Tokura Y, Yagi H, Yanaguchi H, Majima Y, Kasuya A, Ito T, Maekawa M, and Hashizume H. 2014. IgG4-related skin disease. Br. J. Dermatol 171: 959–967. [DOI] [PubMed] [Google Scholar]
- 34.Hsiao PF, and Wu YH. 2016. Characterization of Cutaneous Plasmacytosis at Different Disease Stages. Dermatology 232: 738–747. [DOI] [PubMed] [Google Scholar]
- 35.SanMiguel A, and Grice EA. 2015. Interactions between host factors and the skin microbiome. Cell. Mol. Life Sci 72: 1499–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakatsuji T, Chiang HI, Jiang SB, Nagarajan H, Zengler K, and Gallo RL. 2013. The microbiome extends to subepidermal compartments of normal skin. Nat Commun 4: 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grice EA, Snitkin ES, Yockey LJ, Bermudez DM, Liechty KW, and Segre JA. 2010. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc. Natl. Acad. Sci. U. S. A 107: 14799–14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, and Belkaid Y. 2012. Compartmentalized control of skin immunity by resident commensals. Science 337: 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weyrich LS, Dixit S, Farrer AG, Cooper AJ, and Cooper AJ. 2015. The skin microbiome: Associations between altered microbial communities and disease. Australas. J. Dermatol 56: 268–274. [DOI] [PubMed] [Google Scholar]
- 40.Nakatsuji T, Chen TH, Butcher AM, Trzoss LL, Nam SJ, Shirakawa KT, Zhou W, Oh J, Otto M, Fenical W, and Gallo RL. 2018. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci Adv 4: eaao4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang ES, Tan J, Eells S, Rieg G, Tagudar G, and Miller LG. 2010. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin. Microbiol. Infect 16: 425–431. [DOI] [PubMed] [Google Scholar]
- 42.Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, Ishizuka IE, Dent AL, Wilson PC, Jabri B, Antonopoulos DA, and Bendelac A. 2015. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity 43: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato LM, Kawamoto S, Maruya M, and Fagarasan S. 2014. The role of the adaptive immune system in regulation of gut microbiota. Immunol. Rev 260: 67–75. [DOI] [PubMed] [Google Scholar]
- 44.Magri G, Comerma L, Pybus M, Sintes J, Llige D, Segura-Garzon D, Bascones S, Yeste A, Grasset EK, Gutzeit C, Uzzan M, Ramanujam M, van Zelm MC, Albero-Gonzalez R, Vazquez I, Iglesias M, Serrano S, Marquez L, Mercade E, Mehandru S, and Cerutti A. 2017. Human Secretory IgM Emerges from Plasma Cells Clonally Related to Gut Memory B Cells and Targets Highly Diverse Commensals. Immunity 47: 118–134 e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scholz F, Badgley BD, Sadowsky MJ, and Kaplan DH. 2014. Immune mediated shaping of microflora community composition depends on barrier site. PLoS One 9: e84019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metze D, Kersten A, Jurecka W, and Gebhart W. 1991. Immunoglobulins coat microorganisms of skin surface: a comparative immunohistochemical and ultrastructural study of cutaneous and oral microbial symbionts. J. Invest. Dermatol 96: 439–445. [DOI] [PubMed] [Google Scholar]
- 47.Mahmoud F, Abul H, al Saleh Q, Hassab-el Naby H, Kajeji M, Haines D, Burleson J, and Morgan G. 1999. Elevated B-lymphocyte levels in lesional tissue of non-arthritic psoriasis. J. Dermatol 26: 428–433. [DOI] [PubMed] [Google Scholar]
- 48.Bosello S, Angelucci C, Lama G, Alivernini S, Proietti G, Tolusso B, Sica G, Gremese E, and Ferraccioli G. 2018. Characterization of inflammatory cell infiltrate of scleroderma skin: B cells and skin score progression. Arthritis Res. Ther 20: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Brien JC, Hosler GA, and Chong BF. 2017. Changes in T cell and B cell composition in discoid lupus erythematosus skin at different stages. J. Dermatol. Sci 85: 247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussein MR, Aboulhagag NM, Atta HS, and Atta SM. 2008. Evaluation of the profile of the immune cell infiltrate in lichen planus, discoid lupus erythematosus, and chronic dermatitis. Pathology 40: 682–693. [DOI] [PubMed] [Google Scholar]
- 51.Roguedas AM, Pers JO, Lemasson G, Devauchelle V, Tobon GJ, Saraux A, Misery L, and Youinou P. 2010. Memory B-cell aggregates in skin biopsy are diagnostic for primary Sjogren’s syndrome. J. Autoimmun 35: 241–247. [DOI] [PubMed] [Google Scholar]
- 52.Czarnowicki T, Gonzalez J, Bonifacio KM, Shemer A, Xiangyu P, Kunjravia N, Malajian D, Fuentes-Duculan J, Esaki H, Noda S, Estrada Y, Xu H, Zheng X, Krueger JG, and Guttman-Yassky E. 2016. Diverse activation and differentiation of multiple B-cell subsets in patients with atopic dermatitis but not in patients with psoriasis. J. Allergy Clin. Immunol 137: 118–129 e115. [DOI] [PubMed] [Google Scholar]
- 53.van Beek N, Schulze FS, Zillikens D, and Schmidt E. 2016. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev. Clin. Immunol 12: 267–277. [DOI] [PubMed] [Google Scholar]
- 54.Nagel A, Hertl M, and Eming R. 2009. B-cell-directed therapy for inflammatory skin diseases. J. Invest. Dermatol 129: 289–301. [DOI] [PubMed] [Google Scholar]
- 55.Thiebaut M, Launay D, Riviere S, Mahevas T, Bellakhal S, Hachulla E, Fain O, and Mekinian A. 2018. Efficacy and safety of rituximab in systemic sclerosis: French retrospective study and literature review. Autoimmun Rev 17: 582–587. [DOI] [PubMed] [Google Scholar]
- 56.Joly P, Maho-Vaillant M, Prost-Squarcioni C, Hebert V, Houivet E, Calbo S, Caillot F, Golinski ML, Labeille B, Picard-Dahan C, Paul C, Richard MA, Bouaziz JD, Duvert-Lehembre S, Bernard P, Caux F, Alexandre M, Ingen-Housz-Oro S, Vabres P, Delaporte E, Quereux G, Dupuy A, Debarbieux S, Avenel-Audran M, D’Incan M, Bedane C, Beneton N, Jullien D, Dupin N, Misery L, Machet L, Beylot-Barry M, Dereure O, Sassolas B, Vermeulin T, Benichou J, Musette P, and d. French study group on autoimmune bullous skin. 2017. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet 389: 2031–2040. [DOI] [PubMed] [Google Scholar]
- 57.Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz JD, Uchida J, Fujimoto M, Matsushita T, Matsushita Y, Horikawa M, Komura K, Takehara K, Sato S, and Tedder TF. 2006. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am. J. Pathol 169: 954–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y, Distler O, and E. R. s. group. 2015. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann. Rheum. Dis 74: 1188–1194. [DOI] [PubMed] [Google Scholar]
- 59.Matsushita T, Kobayashi T, Mizumaki K, Kano M, Sawada T, Tennichi M, Okamura A, Hamaguchi Y, Iwakura Y, Hasegawa M, Fujimoto M, and Takehara K. 2018. BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci Adv 4: eaas9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jimenez-Boj E, Stamm TA, Sadlonova M, Rovensky J, Raffayova H, Leeb B, Machold KP, Graninger WB, and Smolen JS. 2012. Rituximab in psoriatic arthritis: an exploratory evaluation. Ann. Rheum. Dis 71: 1868–1871. [DOI] [PubMed] [Google Scholar]
- 61.Kersh AE, and Feldman RJ. 2018. Autoimmune Sequelae Following Rituximab Therapy: A Review of the Literature and Potential Immunologic Mechanisms. J. Clin. Rheumatol [DOI] [PubMed] [Google Scholar]
- 62.Guttman-Yassky E, and Krueger JG. 2017. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr. Opin. Immunol 48: 68–73. [DOI] [PubMed] [Google Scholar]
- 63.Hammers CM, and Stanley JR. 2016. Mechanisms of Disease: Pemphigus and Bullous Pemphigoid. Annu. Rev. Pathol 11: 175–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed AR, Carrozzo M, Caux F, Cirillo N, Dmochowski M, Alonso AE, Gniadecki R, Hertl M, Lopez-Zabalza MJ, Lotti R, Pincelli C, Pittelkow M, Schmidt E, Sinha AA, Sprecher E, and Grando SA. 2016. Monopathogenic vs multipathogenic explanations of pemphigus pathophysiology. Exp. Dermatol 25: 839–846. [DOI] [PubMed] [Google Scholar]
- 65.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, and Diaz LA. 1982. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N. Engl. J. Med 306: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 66.Wallim LR, Nisihara R, Skare T, Mocelin V, and Messias-Reason IJ. 2014. Mannose binding lectin deposition in skin of lupus erythematosus patients: a case series. Hum. Immunol 75: 629–632. [DOI] [PubMed] [Google Scholar]
- 67.Grunwald MH, Avinoach I, Amichai B, and Halevy S. 1997. Leukocytoclastic vasculitis--correlation between different histologic stages and direct immunofluorescence results. Int. J. Dermatol 36: 349–352. [DOI] [PubMed] [Google Scholar]
- 68.Ruddle NH 2016. High Endothelial Venules and Lymphatic Vessels in Tertiary Lymphoid Organs: Characteristics, Functions, and Regulation. Front. Immunol 7: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones GW, Hill DG, and Jones SA. 2016. Understanding Immune Cells in Tertiary Lymphoid Organ Development: It Is All Starting to Come Together. Front. Immunol 7: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alsughayyir J, Pettigrew GJ, and Motallebzadeh R. 2017. Spoiling for a Fight: B Lymphocytes As Initiator and Effector Populations within Tertiary Lymphoid Organs in Autoimmunity and Transplantation. Front. Immunol 8: 1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen P, and Fillatreau S. 2015. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol 15: 441–451. [DOI] [PubMed] [Google Scholar]
- 72.Hahn BH 2013. Belimumab for systemic lupus erythematosus. N. Engl. J. Med 368: 1528–1535. [DOI] [PubMed] [Google Scholar]
- 73.Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod’homme T, Varrin-Doyer M, Shetty A, Linington C, Slavin AJ, Hidalgo J, Jenne DE, Wekerle H, Sobel RA, Bernard CC, Shlomchik MJ, and Zamvil SS. 2013. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J. Exp. Med 210: 2921–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young JS, Wu T, Chen Y, Zhao D, Liu H, Yi T, Johnston H, Racine J, Li X, Wang A, Todorov I, and Zeng D. 2012. Donor B cells in transplants augment clonal expansion and survival of pathogenic CD4+ T cells that mediate autoimmune-like chronic graft-versus-host disease. J. Immunol 189: 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Candando KM, Lykken JM, and Tedder TF. 2014. B10 cell regulation of health and disease. Immunol. Rev 259: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mauri C, and Menon M. 2017. Human regulatory B cells in health and disease: therapeutic potential. J. Clin. Invest 127: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katz SI, Parker D, and Turk JL. 1974. B-cell suppression of delayed hypersensitivity reactions. Nature 251: 550–551. [DOI] [PubMed] [Google Scholar]
- 78.Neta R, and Salvin SB. 1974. Specific suppression of delayed hypersensitivity: the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J. Immunol 113: 1716–1725. [PubMed] [Google Scholar]
- 79.Mauri C, and Menon M. 2015. The expanding family of regulatory B cells. Int. Immunol 27: 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray A, and Dittel BN. 2017. Mechanisms of Regulatory B cell Function in Autoimmune and Inflammatory Diseases beyond IL-10. J Clin Med 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, and Tedder TF. 2008. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28: 639–650. [DOI] [PubMed] [Google Scholar]
- 82.Nakashima H, Hamaguchi Y, Watanabe R, Ishiura N, Kuwano Y, Okochi H, Takahashi Y, Tamaki K, Sato S, Tedder TF, and Fujimoto M. 2010. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J. Immunol 184: 4637–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yanaba K, Kamata M, Ishiura N, Shibata S, Asano Y, Tada Y, Sugaya M, Kadono T, Tedder TF, and Sato S. 2013. Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation. J. Leukoc. Biol 94: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alrefai H, Muhammad K, Rudolf R, Pham DA, Klein-Hessling S, Patra AK, Avots A, Bukur V, Sahin U, Tenzer S, Goebeler M, Kerstan A, and Serfling E. 2016. NFATc1 supports imiquimod-induced skin inflammation by suppressing IL-10 synthesis in B cells. Nat Commun 7: 11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maseda D, Candando KM, Smith SH, Kalampokis I, Weaver CT, Plevy SE, Poe JC, and Tedder TF. 2013. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-gamma+CD4+ T cell numbers during colitis development in mice. J. Immunol 191: 2780–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Byrne SN, and Halliday GM. 2005. B cells activated in lymph nodes in response to ultraviolet irradiation or by interleukin-10 inhibit dendritic cell induction of immunity. J. Invest. Dermatol 124: 570–578. [DOI] [PubMed] [Google Scholar]
- 87.Matsumura Y, Byrne SN, Nghiem DX, Miyahara Y, and Ullrich SE. 2006. A role for inflammatory mediators in the induction of immunoregulatory B cells. J. Immunol 177: 4810–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kok LF, Marsh-Wakefield F, Marshall JE, Gillis C, Halliday GM, and Byrne SN. 2016. B cells are required for sunlight protection of mice from a CNS-targeted autoimmune attack. J. Autoimmun 73: 10–23. [DOI] [PubMed] [Google Scholar]
- 89.Nickoloff BJ, Fivenson DP, Kunkel SL, Strieter RM, and Turka LA. 1994. Keratinocyte interleukin-10 expression is upregulated in tape-stripped skin, poison ivy dermatitis, and Sezary syndrome, but not in psoriatic plaques. Clin. Immunol. Immunopathol 73: 63–68. [DOI] [PubMed] [Google Scholar]
- 90.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, Volk HD, and Docke WD. 1998. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J. Clin. Invest 101: 783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hayashi M, Yanaba K, Umezawa Y, Yoshihara Y, Kikuchi S, Ishiuji Y, Saeki H, and Nakagawa H. 2016. IL-10-producing regulatory B cells are decreased in patients with psoriasis. J. Dermatol. Sci 81: 93–100. [DOI] [PubMed] [Google Scholar]
- 92.Mavropoulos A, Varna A, Zafiriou E, Liaskos C, Alexiou I, Roussaki-Schulze A, Vlychou M, Katsiari C, Bogdanos DP, and Sakkas LI. 2017. IL-10 producing Bregs are impaired in psoriatic arthritis and psoriasis and inversely correlate with IL-17- and IFNgamma-producing T cells. Clin. Immunol 184: 33–41. [DOI] [PubMed] [Google Scholar]
- 93.Kabuto M, Fujimoto N, and Tanaka T. 2016. Increase of interleukin-10-producing B cells associated with long-term remission after i.v. immunoglobulin treatment for pemphigus. J. Dermatol 43: 815–818. [DOI] [PubMed] [Google Scholar]
- 94.Kabuto M, Fujimoto N, Takahashi T, and Tanaka T. 2017. Decreased level of interleukin-10-producing B cells in patients with pemphigus but not in patients with pemphigoid. Br. J. Dermatol 176: 1204–1212. [DOI] [PubMed] [Google Scholar]
- 95.Matsushita T, Hamaguchi Y, Hasegawa M, Takehara K, and Fujimoto M. 2016. Decreased levels of regulatory B cells in patients with systemic sclerosis: association with autoantibody production and disease activity. Rheumatology (Oxford) 55: 263–267. [DOI] [PubMed] [Google Scholar]
- 96.Mavropoulos A, Simopoulou T, Varna A, Liaskos C, Katsiari CG, Bogdanos DP, and Sakkas LI. 2016. Breg Cells Are Numerically Decreased and Functionally Impaired in Patients With Systemic Sclerosis. Arthritis Rheumatol 68: 494–504. [DOI] [PubMed] [Google Scholar]
- 97.Colliou N, Picard D, Caillot F, Calbo S, Le Corre S, Lim A, Lemercier B, Le Mauff B, Maho-Vaillant M, Jacquot S, Bedane C, Bernard P, Caux F, Prost C, Delaporte E, Doutre MS, Dreno B, Franck N, Ingen-Housz-Oro S, Chosidow O, Pauwels C, Picard C, Roujeau JC, Sigal M, Tancrede-Bohin E, Templier I, Eming R, Hertl M, D’Incan M, Joly P, and Musette P. 2013. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci. Transl. Med 5: 175ra130. [DOI] [PubMed] [Google Scholar]
- 98.Yang X, Yang J, Chu Y, Xue Y, Xuan D, Zheng S, and Zou H. 2014. T follicular helper cells and regulatory B cells dynamics in systemic lupus erythematosus. PLoS One 9: e88441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lykken JM, Candando KM, and Tedder TF. 2015. Regulatory B10 cell development and function. Int. Immunol 27: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trinchieri G 2007. Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med 204: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maynard CL, and Weaver CT. 2008. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol. Rev 226: 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun J, Madan R, Karp CL, and Braciale TJ. 2009. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med 15: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cowin AJ, Brosnan MP, Holmes TM, and Ferguson MW. 1998. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev. Dyn 212: 385–393. [DOI] [PubMed] [Google Scholar]
- 104.Richards AM, Floyd DC, Terenghi G, and McGrouther DA. 1999. Cellular changes in denervated tissue during wound healing in a rat model. Br. J. Dermatol 140: 1093–1099. [DOI] [PubMed] [Google Scholar]
- 105.Iwata Y, Yoshizaki A, Komura K, Shimizu K, Ogawa F, Hara T, Muroi E, Bae S, Takenaka M, Yukami T, Hasegawa M, Fujimoto M, Tomita Y, Tedder TF, and Sato S. 2009. CD19, a response regulator of B lymphocytes, regulates wound healing through hyaluronan-induced TLR4 signaling. Am. J. Pathol 175: 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rickert RC, Rajewsky K, and Roes J. 1995. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 376: 352–355. [DOI] [PubMed] [Google Scholar]
- 107.Martin F, and Kearney JF. 2000. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 12: 39–49. [DOI] [PubMed] [Google Scholar]
- 108.Sato S, Ono N, Steeber DA, Pisetsky DS, and Tedder TF. 1996. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J. Immunol 157: 4371–4378. [PubMed] [Google Scholar]
- 109.Sirbulescu RF, Boehm CK, Soon E, Wilks MQ, Ilies I, Yuan H, Maxner B, Chronos N, Kaittanis C, Normandin MD, El Fakhri G, Orgill DP, Sluder AE, and Poznansky MC. 2017. Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen. 25: 774–791. [DOI] [PMC free article] [PubMed] [Google Scholar]