Abstract
Non-invasive imaging, particularly echocardiography, plays a central role in the evaluation for heart failure with preserved ejection fraction (HFpEF). Echocardiography helps to rule in HFpEF among patients with unexplained dyspnea when the diagnosis is uncertain. In established HFpEF, echocardiography provides important insights into pathophysiology and phenotyping, such as isolated left ventricular diastolic dysfunction, left atrial dysfunction, abnormal right ventricular- pulmonary artery coupling, ischemia, or obesity phenotypes. Finally, imaging enables risk stratification for HFpEF. In this review, we will provide a critical appraisal of the role of echocardiography in the diagnosis and evaluation of HFpEF.
Keywords: diagnosis, diastolic function, echocardiography, filling pressure, heart failure, phenotyping, risk stratification
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is a common clinical syndrome that is increasing in prevalence coupled with the growing population burden of aging and comorbidities.1, 2 Over half of patients with unexplained exertional dyspnea referred for invasive evaluation are ultimately found to have HFpEF, and over 70% of patients with prevalent HF above the age of 65 years have normal EF.3, 4 Cardiovascular imaging plays a key role in the evaluation and management of HFpEF, particularly echocardiography.5
Echocardiography provides essential information on cardiac structure, function, and hemodynamics and is performed in essentially all patients where there is clinical suspicion for HFpEF.6 From a practical standpoint, the most important questions that can be addressed center on 1) diagnosis, determining whether a patient with unexplained dyspnea truly has HFpEF or an alternate cardiac or non-cardiac cause of dyspnea, and 2) management, where imaging can be used to evaluate hemodynamic status, determine underlying pathophysiologic phenotypes and 3) risk stratification for outcomes. In this review, we will provide a critical appraisal of the role of echocardiography crossing these 3 categories involved in the care of patients with or suspected of having HFpEF.
Case
A 72-year-old man was referred for evaluation of a two-year history of progressive exertional dyspnea with fatigue. He was obese (body mass index [BMI]: 36.2 kg/m2) and had chronic systemic hypertension treated with lisinopril and chlorthalidone. Jugular venous pressure was 8 cm, and there was no lower edema. N-terminal pro-B-type natriuretic peptide was 80pg/ml. Transthoracic echocardiography revealed normal left ventricular (LV) EF (62%), LV size (LV end-diastolic dimension 51 mm), left atrial (LA) volume (LA volume index [LAVI], 22 ml/m2), and right ventricular (RV) size, with normal systolic function. Transmitral inflow Doppler showed an E/A ratio of 1.0 with medial E/e’ of 12.9 and estimated right ventricular systolic pressure (RVSP) was 36 mmHg (peak tricuspid regurgitation [TR] velocity 2.8 m/sec). LV global longitudinal strain (GLS) was mildly reduced at −16.8%. A prominent epicardial fat pad was seen on echocardiography.
This common clinical presentation should raise clinical suspicion for HFpEF, and if present, it raises the question of what the underlying drivers of this patient’s HFpEF syndrome are. In the text below, we shall use this to frame what we seek from echocardiography in the evaluation of suspected HFpEF.
Diastolic Dysfunction and HFpEF
While the two terms are often used interchangeably, it is important to remember that diastolic dysfunction is not equivalent to HFpEF. HFpEF by definition requires the presence of elevated filling pressures either at rest or with exertion without which systemic perfusion cannot be maintained.7 Although diastolic dysfunction is a central feature in HFpEF, the pathophysiology is complex with variable contributions from diastolic dysfunction, impaired contractile reserve, impaired atrial function, relative pericardial restraint and abnormal ventricular vascular coupling which all contribute to the elevation in pulmonary venous and left sided filling pressures.8–10 Increases in LV filling pressures promote symptoms of dyspnea,11 impair exercise capacity,11, 12 and increase risk for HF hospitalization and mortality in HFpEF.13,14 Thus diastolic dysfunction is considered to be the cornerstone of HFpEF pathophysiology.8
Diastolic dysfunction is defined by prolongation of relaxation in early diastole, an increase in viscoelastic LV diastolic chamber stiffness, or some combination of the two.15 Declines in LV relaxation and compliance are part of normal aging, and accordingly not all patients with diastolic dysfunction have or will develop go on to develop clinical HFpEF.16–18 In one prospective cohort study, only 12% of subjects with severe diastolic dysfunction at initial evaluation developed clinical HFpEF over 6 years of follow up.19 Approximately one-third of patients with HFpEF enrolled in clinical trials lack echocardiographic evidence of diastolic dysfunction.20–22 Thus, while echocardiographic categorization of diastolic dysfunction is prognostic23 and useful to predict incident HFpEF,19 recent studies have suggested that they should not be used in isolation for diagnostic purposes3, 24.
Echocardiography to Identify Elevated Filling Pressure
The ultimate expression of abnormalities in diastolic function is an elevation in LV filling pressures. There are a number of echocardiographic indices that have been applied for the estimation of filling pressures, but the most studied (by far) is the ratio of early diastolic transmitral inflow velocity to mitral annular tissue velocity (E/e’).24–28 The diagnostic accuracy of the E/e’ ratio in HFpEF has recently been questioned, as a recent meta-analysis reported only a modest correlation between E/e’ and invasively-obtained resting filling pressures across studies (pooled r=0.56).5 Correlations between E/e’ and invasive filling pressure in subjects with preserved EF have been reported in 30 studies and vary widely in the strength of correlation (r=0.02 to 0.87) (Table 1). Despite its variable and often modest correlation with filling pressure, E/e’ has been reported to have prognostic value in patients with HFpEF.5, 21, 29
Table 1:
Correlations between E/e’ and invasive filling pressure in subjects with preserved EF
| Study | n | Subjects’ Characteristics | Timeframe | Echo | Invasive | r | p | Cutoff Cath | Cutoff Echo | Sens | Spec | Feasibility | Reproducibility* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nagueh 19971 | 60 | 45 ICU, 15 Cath lab | Simultaneous | E/e’ (sep) | PCWP | 0.87 | <0.001 | >15 mmHg | >10 | 97 | 78 | Intra/lnter 5±4/6±5% | |
| Ommen 20002 | 64 | 73% suspicious CAD | Simultaneous | E/e’ (avg) | MDP | 0.45 | 100% | ||||||
| E/e’ (sep) | MDP | 0.47 | >12 mmHg | >15 | 22% | 100% | 100% | ||||||
| Poerner 20033 | 85 | Subjects referred to CAG and E/A>0.9 | Mean 3 hours | E/e’ (sep) | EDP | 0.40 | <0.01 | ||||||
| E/e’ (lat) | EDP | 0.49 | <0.01 | ||||||||||
| E/e’ (avg) | EDP | 0.57 | <0.01 | ||||||||||
| Mansencal 20044 | 20 | CAD | <1 hour | E/e’ (lat) | Pre-A | 0.18 | >15 mmHg | >12 | 0% | 100% | 100% | ||
| Hadano 20055 | 65 | UA 6%, AS 5% | <3 hours | E/e’ (lat) | PCWP | 0.54 | <0.001 | >15 mmHg | >12 | 42% | 92% | ||
| Kidawa 20056 | 50 | Subjects referred to CAG | Simultaneous | E/e’ (lat) | EDP | 0.58 | <0.01 | >15 mmHg | >11 | 28% | 92% | ||
| E/e’ (sep) | EDP | 0.29 | NS | ||||||||||
| Kasner 20077 | 55 | 43 HFpEF and 12 controls | 3–5 hours | E/e’ (lat) | EDP | 0.71 | 0.001 | ||||||
| Min 20078 | 55 | Subjects referred to cath and E/e’ 8–15 | Simultaneous | E/e’ (sep) | EDP | 0.03 | 0.8 | ||||||
| Dokanish 20089 | 32 | Patients with dyspnea | Immediately after cath | E/e’ (avg) | Pre-A | 0.39 | <0.001 | >15 mmHg | >15 | 73% | 77% | ||
| Rudko 200810 | 39 | Elevated filling pressure or DD (77% CAD) | Simultaneous | E/e’ (sep) | EDP | 0.47 | <0.001 | ||||||
| Dokanish 201011 | 122 | Subjects referred to CAG | <20min | E/e’ (avg) | Pre-A | 0.63 | <0.001 | >15m mHg | >13 | 70% | 93% | ||
| Kasner 201012 | 33 | 21 HFpEF and 11 controls | Simultaneous | E/e’ (avg) | EDP | 0.57 | <0.001 | ||||||
| Maeder 201013 | 22 | 14 HFpEF and 8 controls | Simultaneous | E/e’ (sep) | PCWP | 0.19 | 0.39 | ||||||
| E/e’ (lat) | PCWP | 0.04 | 0.87 | ||||||||||
| E/e’ (avg) | PCWP | 0.12 | 0.59 | ||||||||||
| Hsiao 201114 | 100 | Stable CAD | Immediately after cath | E/e’ (sep) | Pre-A | 0.31 | 0.002 | ||||||
| E/e’ (lat) | Pre-A | 0.23 | 0.02 | ||||||||||
| Maeder 201115 | 36 | 15 HFpEF, 11 PAH, 10 healthy controls | Immediately after cath | E/e’ (sep) | PCWP | 0.23 | 0.2 | ||||||
| E/e’ (lat) | PCWP | −0.04 | 0.8 | ||||||||||
| E/e’ (avg) | PCWP | 0.13 | 0.5 | ||||||||||
| Bhella 201116 | 11 | 11 HFpEF | Simultaneous | E/e’ (avg) | PCWP | 0.64 | 0.04 | 59% | 92% | ||||
| Previtali 201217 | 57 | Subjects referred toCAG | <1 hour | E/e’ (lat) | EDP | 0.1 | 0.4 | Intra/lnter <10/20% | |||||
| E/e’ (avg) | EDP | NS | >15 mmHg | >12.1 (optim al) | 44% | 71% | Intra/lnter <10/20% | ||||||
| Manouras 201318 | 38 | Subjects with angina/dyspnea | Simultaneous | E/e’ (sep) | Pre-A | 0.02 | NS | ||||||
| E/e’ (lat) | Pre-A | 0.40 | <0.05 | ||||||||||
| E/e’ (avg) | Pre-A | 0.21 | <0.05 | >12 mmHg | >13 | 8% | 91% | ||||||
| Tatsumi 201419 | 22 | Subjects underwent 3D echo and cath | 0.1 ± 5.8 days | E/e’ (sep) | PCWP | 0.64 | 0.001 | ||||||
| Kasner 201520 | 23 | HFpEF | Simultaneous | E/e’ (avg) | EDP | 0.84 | <0.001 | ||||||
| Matsushita 201521 | 16 | Inpatient HFpEF | Same hospitalization | E/e’ (avg) | PCWP | 0.56 | 0.01 | >15 mmHg | >10 | 71% | 56% | ||
| Ma 201522 | 114 | 84 CAD and 30 controls | < 24 hours | E/e’ (sep) | EDP | 0.60 | <0.01 | ||||||
| Santos 201523 | 118 | Subjects with dyspnea | Immediately after cath | E/e’ (sep) | PCWP | 0.41 | <0.001 | >15 mmHg | ≥15 | 6% | 92% | 79% | |
| E/e’ (lat) | PCWP | 0.30 | <0.001 | >15 mmHg | ≥12 | 13% | 92% | 75% | |||||
| E/e’ (avg) | PCWP | 0.36 | <0.001 | >15 mmHg | ≥13 | 6% | 90% | 75% | |||||
| Cameli 201624 | 20 | 39% UA, 25% angina with positive stress test | 1 hour | E/e’ (avg) | EDP | 0.72 | <0.001 | 100% | |||||
| Rommel 201625 | 36 | 24 HFpEFand 12 controls | N/R | E/e’ (N/R) | EDP | 0.63 | <0.001 | ||||||
| Ma 201626 | 114 | 84 CAD and 30 controls | E/e’ (sep) | EDP | 0.60 | <0.01 | >15 mmHg | >10.9 | 91% | 68% | |||
| E/e’ (lat) | EDP | 0.29 | <0.01 | ||||||||||
| E/e’ (avg) | EDP | 0.41 | <0.01 | ||||||||||
| Hayashi 201627 | 47 | Cardiac diseases (CAD, OMI, HCM, HFpEF, etc) | <3 hours | E/e’ (avg) | MDP | 0.56 | <0.001 | ||||||
| Obokata 201628 | 74 | 50 HFpEF and 24 controls | Simultaneous | E/e’ (sep) | PCWP | 0.63 | <0.001 | 99% | |||||
| E/e’ (lat) | PCWP | 0.58 | <0.001 | 95% | |||||||||
| Lancellotti 201729 | 120 | Suspicious CAD | Simultaneous | E/e’ (avg) | EDP | 0.17 | 0.07 | ≥15 mmHg | ≥14 | 2.4% | 96% | ||
| E/e’ (sep) | EDP | 0.08 | 0.36 | ≥15 mmHg | ≥15 | 4.8% | 96% | ||||||
| Andersen 201730† | 450 | Subjects referred to right or left cath (EF<50% n=209) | Simultaneous or immediately after cath | E/e’ (avg) | PCWP | 0.65 | <0.001 |
Reproducibility represents percent variability, intra-class correlation coefficient, or mean difference. 3D, 3-dimentional; A, late diastolic mitral inflow velocity; AS, aortic stenosis; Avg, average; CAD, coronary artery disease; CAG, coronary angiography; cath, catheterization; DD, diastolic dysfunction; E, early diastolic mitral inflow velocity; e’, early diastolic mitral annular tissue velocity; Echo, echocardiography; EDP, left ventricular end-diastolic pressure; EF, ejection fraction; HCM, hypertrophic cardiomyopathy; HFpEF, heart failure with preserved ejection fraction; ICU, intensive care unit; lat, lateral; MDP, left ventricular mean diastolic pressure; N/R, not reported; NS, not significant; OMI, old myocardial infarction; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; Pre-A, left ventricular pressure during pre-atrial contraction; Sens, sensitivity; sep, septal; Spec, specificity; and UA, unstable angina.
This study pooled together both HFpEF and HFrEF patients.
Transmitral flow (TMF) is driven by the LA-LV pressure gradient during diastole and can be used for identification of elevated filling pressure in subjects with normal sinus rhythm. TMF is often graded as normal, impaired relaxation, pseudo-normal, and restrictive filling patterns. Since TMF is influenced by LA pressure, E/A ratio displays a U-shape relationship with LV filling pressure. The biphasic relationship of E/A ratio makes it difficult to differentiate normal and pseudo-normal patterns, and one must rely on other echocardiographic indices such as indexing E wave velocity to e’ septal tissue Doppler velocity.6
Other indices have also been related to LV filling pressures.6 Pulmonary vein (PV) Doppler flow reversals during atrial contraction provide a measure of end diastolic LV operative compliance and LVEDP. With increased impedance to end-diastolic atrial contraction, there is a prolongation of flow reversal into the PV relative to the duration of forward flow. Differences in these durations exceeding 20–30ms have been correlated with increased LVEDP,30–33 with a diagnostic sensitivity of 87% and specificity of 85%.34 Six studies have reported reasonable correlations between backward and forward PV flow duration and invasively-measured LV filling pressure in patients with preserved EF (r=0.39–0.70).27, 34–38 While these data appear favorable, diagnostic-quality recordings of the PV are often not technically feasible, and other PV parameters such as systolic and diastolic flow velocities are less robust.25, 27, 35 As such, PV Doppler indices have not gained substantial traction as indicators of filling pressure.
An alternative method of assessing the impact of elevated left sided filling pressures chronically is to determine their downstream effects on the LA. Atrial operating compliance and atrial volume are linked to LV diastolic function through atrioventricular coupling; whereby chronic impedance to LA emptying secondary to LV diastolic dysfunction causes LA remodeling and dysfunction.39–42 LA volume is believed to reflect the chronic effects of LV filling pressure elevation over time, rather than instantaneous pressures.
Because this is a chronic marker, correlations between LA volume index and ambient LV filling pressures are lower than what has been reported for other indices such as E/e’ and PV Doppler (r=0.10 to 0.49).28, 43–46 In contrast to E/e’, LA volume index is not strongly associated with outcome in HFpEF.21, 47–49 This does not mean that cumulative effects of filling pressure does not contribute to outcome in HFpEF, but rather emphasizes the need for an alternative parameter to evaluate LA burden such as LA reservoir strain, which we will be discuss below.
Earlier studies suggested that patients with HFpEF display concentric hypertrophy, which leads to increased passive chamber stiffness and thus elevated filling pressure.50 Indeed, LV mass index has been reported to be modestly correlated with invasively-measured LV filling pressure (r=0.41–0.48, p<0.001).43, 44 Current ESC guidelines include increased LV mass index as one of the criteria for the diagnosis of HFpEF.51 However, community-based studies, as well as trial ancillary studies, have shown that many patients with HFpEF have either concentric remodeling in the absence of hypertrophy, or even normal LV geometry.22, 52, 53
Consistent with this observation, it was recently demonstrated that LV hypertrophy was highly specific (88%) but poorly sensitive (26%) for the diagnosis of HFpEF and therefore its absence cannot be used to rule out the diagnosis.3 When evaluating LV morphology, care should be taken to exclude other differential diagnoses that mimic HFpEF (Table 2). Whenever significant LV hypertrophy is identified, the diagnosis of amyloidosis must be considered, particularly in the presence of a pericardial effusion or apical sparing pattern of LV strain.54 In a series of consecutive patients with LVH≥12 mm, amyloidosis represented 13% of hospitalized “HFpEF”.55 This distinction from HFpEF is particularly important now that new treatments are becoming available for cardiac amyloid.56
Table 2.
Differential Diagnoses of HFpEF and Their Echocardiographic Clues
| Differential Diagnosis | Echocardiographic Clues |
|---|---|
| Hypertrophic | Asymmetric hypertrophy, ↑↑LV wall thickness, |
| cardiomyopathy | LVOT obstruction, SAM |
| Restrictive | Small LV cavity, ↑LV wall thickness, Sparkling |
| cardiomyopathy | myocardium, Apical sparing, Severely reduced tissue Doppler, PE |
| Pulmonary arterial | ↑RVSP with no sign of elevated LV filling |
| hypertension | pressure, Isolated right heart dilation, PA dilation, RVOT Doppler midsystolic notch |
| Constrictive pericarditis | Pericardial thickening, Septal bounce, annulus paradoxus and annulus reversus, ↑Respiratory variation in mitral/tricuspid flow, Absence of IVC collapse |
| Valvular heart disease | Morphological valvular abnormalities, Color Doppler |
| Coronary artery disease | Regional wall motion abnormality and thinning |
| Chronic thromboembolic | ↑RVSP with no sign of elevated LV filling |
| pulmonary hypertension | pressure, Isolated right heart dilation, PA dilation, RVOT Doppler midsystolic notch |
| High output heart failure | ↑Doppler-derived cardiac output |
IVC, inferior vena cava; LV, left ventricular; LVOT, left ventricular outflow obstruction; PA, pulmonary artery; RVSP, estimated right ventricular systolic pressure; PE, pericardial effusion; RVOT, right ventricular outflow; SAM, systolic anterior motion of the mitral valve; and other abbreviations as in Table 1.
Strain and strain rate imaging have also been evaluated to estimate LV filling pressure. The ratio of mitral E velocity to longitudinal diastolic strain rate during early diastole (E/SRE) correlated moderately with invasively-obtained filling pressure, with high sensitivity and specificity (E/SRE >11.5, 91% and 78%, respectively).57–59 One study reported that E/SRE predicted cardiovascular outcomes better than E/e’.60 Smaller studies have demonstrated correlations between LV GLS and filling pressures.57, 61, 62 Left atrial longitudinal strain during ventricular systole represents atrial reservoir function and is reduced in HFpEF.63 One study has demonstrated a high correlation between LA reservoir strain and invasive filling pressure (r=−0.79) in patients with preserved EF,45 but its discriminatory ability to diagnose HFpEF from non-cardiac dyspnea remains unexplored. On the other hand, decreased GLS (>−16%) has been reported to be associated with adverse outcomes in HFpEF.29
Optimal Use of Echocardiography in Diagnosis of HFpEF
The diagnosis of HFpEF is obvious in the patient with overt congestion at rest, where jugular vein distention, peripheral edema and pulmonary congestion are present, and echocardiography is not necessary to establish the clinical diagnosis. In contrast, evaluation of the euvolemic patient with exertional dyspnea presents a greater diagnostic challenge.3, 24, 64 Correlative analyses are important to demonstrate strength of association between two variables, and as described above, numerous echocardiographic indices are correlated with filling pressures. However, from a diagnostic perspective, it is more important to consider the ability of a test to discriminate cases from controls rather than simple correlative analyses.
In this regard, an elevated E/e’ ratio has been reported to have excellent specificity for identifying high LV filling pressure (77–100%), suggesting that it may be useful to ‘rule in’ the diagnosis of HFpEF when elevated.3, 24, 37, 38, 44, 46, 58, 65–68 However, the E/e’ ratio displays poor sensitivity (range 0–73%), meaning it is not an effective test to exclude HFpEF.24, 37, 38, 44, 46, 65–67 Because impaired relaxation is expected to accompany high filling pressures, it has been proposed that elevation in E/e’ be coupled with an impairment in the e’ velocity.51 This more stringent requirement may improve specificity, but will only further compromise sensitivity.24
Expert consensus guidelines have recommended use of an elevated LA volume index at a cutpoint of >34 ml/m2 as another indicator of diastolic dysfunction.69–71 When prospectively evaluated, an enlarged LA volume index (>34ml/m2) is indeed specific (83%) for HFpEF, but like E/e’, it is poorly sensitive (49%).3, 46 One potential concern is the appropriate method of allometrically scaling LA volume to body size in obese patients, who represent the majority of the HFpEF population.72 With obesity, a linear adjustment of LA volume index to body surface area may result in underestimation of LA remodeling, because the quotient will be lower as body mass increases. Another complicating issue in the evaluation of LA volume is the presence of atrial fibrillation.73 Despite this, recent data have shown that the presence of atrial fibrillation in the patient with dyspnea is highly predictive of the presence of underlying HFpEF, making this less of an issue, at least as it pertains to diagnosis.3, 74
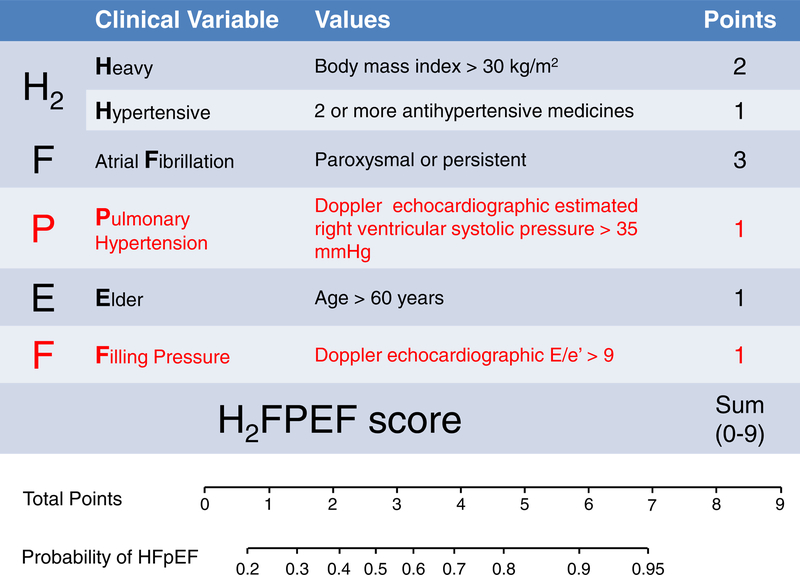
The current guidelines have recommended a combination of different indices of diastolic function to diagnose HFpEF. While these approaches have been found to display high specificity, sensitivity is poor.3, 24 We recently developed a simple score to predict the presence of HFpEF among more than 500 patients with unexplained dyspnea.3 While many echocardiographic variables were predictive of HFpEF diagnosis in isolation (Table 3), we found that the combination of elevated E/e’ (>9) and RVSP (>35 mmHg) were additive to clinical characteristics, including older age, larger body mass index, number of antihypertensive drugs, and history of atrial fibrillation in multivariable analyses (H2FPEF score, Figure 1).3 This scheme was then validated in an independent test cohort where it retained excellent discriminatory capacity (AUC 0.886; p<0.0001). Thus, while numerous echocardiographic indicators are related to the presence or absence of HFpEF (Table 2), it appears that the combination of E/e’ and RVSP is optimal to inform the noninvasive diagnosis.
Table 3: Operating Characteristics of Echocardiographic Parameters for the Diagnosis of HFpEF.
Data from Reddy YNV, Carter RE, Obokata M, et al. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–870.
| AUC | P | Sensitivity | Specificity | |
|---|---|---|---|---|
| Ejection fraction <55% | 0.52 | 0.09 | 8% | 96% |
| LV hypertrophy | 0.57 | 0.0006 | 26% | 88% |
| LA volume Index >34 ml/m2 | 0.66 | <0.0001 | 49% | 83% |
| E/e’ ratio (septal) >9 | 0.69 | <0.0001 | 78% | 59% |
| E/e’ ratio (septal) >13 | 0.66 | <0.0001 | 46% | 86% |
| Septal e’ velocity <7 cm/s | 0.62 | <0.0001 | 48% | 76% |
| Right atrial pressure >10 mmHq | 0.56 | <0.0001 | 16% | 97% |
| RV systolic pressure >35mmHq | 0.66 | <0.0001 | 46% | 86% |
| RV fractional area change <48% | 0.64 | <0.0001 | 39% | 88% |
| Tricuspid annular plane systolic excursion <16 mm | 0.54 | 0.0008 | 9% | 99% |
| Visual RV dysfunction | 0.58 | <0.0001 | 22% | 94% |
| Visual RV dilation | 0.60 | <0.0001 | 32% | 88% |
Figure 1. The H2FPEF score to Aide in Diagnosis HFpEF.
In this score, the echocardiographic parameters that were independently predictive for heart failure with preserved ejection fraction (HFpEF) (E/e’ >9 and right ventricular systolic pressure >35mmHg) are incorporated in tandem with clinical characteristics to determine the probability that HFpEF is present in patients presenting with unexplained dyspnea. (Adapted from Reddy YNV, Carter RE, Obokata M, et al. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–870; with permission.)
According to the approach,3 the findings in this case on echocardiography (elevated E/e’ and RVSP) along with older age, obesity, and use of 2 antihypertensive drugs indicate HFpEF is the likely cause of exertional dyspnea with 92% probability.
In contrast, patients with very low probability can be excluded and work-up for other causes will be required. Dynamic stress testing to evaluate abnormal elevation in filling pressure will be required to establish the cause of exertional dyspnea, as will be discussed in the later section (Fig. 2).24 In this case, exercise catheterization study demonstrated a normal PCWP at rest (11 mmHg) but markedly increased filling pressures during exertion (30 mmHg) which confirmed the diagnosis of HFpEF.
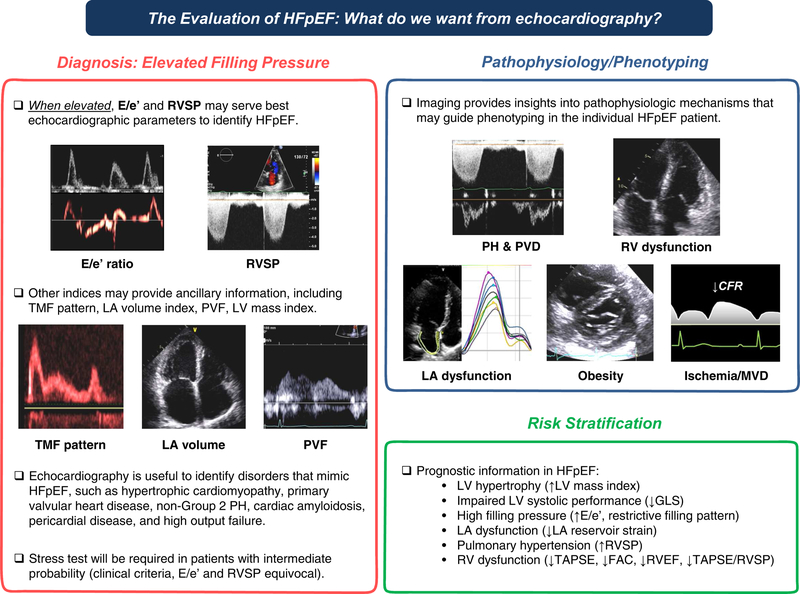
Figure 2. Summary of the Role of Non-Invasive Imaging in the Evaluation of Heart Failure with Preserved Ejection Fraction.
E/e’, the ratio of early diastolic mitral inflow to mitral annular tissue velocities; FAC, right ventricular fractional area change; GLS, left ventricular global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; MVD, microvascular dysfunction; PH, pulmonary hypertension; PVD, pulmonary vascular disease; PVF, pulmonary venous flow; and RV, right ventricular; RVEF, right ventricular ejection fraction; RVSP, estimated right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion; and TMF, transmitral flow.
Diastolic Stress Echocardiography for the Diagnosis of HFpEF
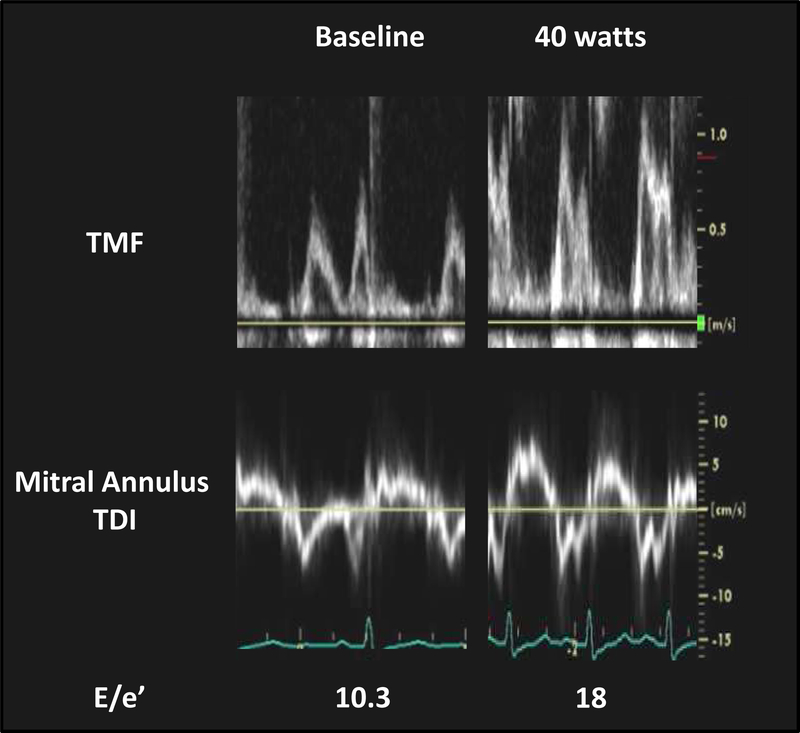
Part of the difficulty in diagnosing HFpEF is related to the fact that filling pressures are often normal at rest, but become elevated only during the stress of exercise.3, 24, 64 Because of this fact, invasive cardiopulmonary exercise testing has emerged as the gold standard to definitively identify or exclude HFpEF as the cause of dyspnea.3, 24, 64, 75, 76 Recent studies have evaluated whether similar data can be obtained non-invasively using diastolic stress echocardiography (Fig. 3).24
Figure 3. Typical Case of Diastolic Stress Echocardiography.
Transmitral inflow velocities (TMF) and mitral annular tissue Doppler velocities at rest and during 40 watts supine ergometer exercise in an invasively-proven HFpEF patient (pulmonary capillary wedge pressure during exercise 27 mmHg). At baseline, transthoracic echocardiography demonstrates normal EF (70%), left atrial volume index (30 ml/m2), normal E/e’ (average 10.3), and an estimated right ventricular systolic pressure of 28 mmHg. With exercise up to 40 watts, mitral E increases dramatically without significant change in e’, resulting in an increase E/e’ ratio. Tricuspid regurgitant velocity increases from 2.5 to 3.5 m/sec during exercise. TDI, tissue Doppler imaging and other abbreviations as in Figure 1.
A recent study using simultaneous catheterization-echocardiographic evaluation at rest and during exercise in patients being evaluated for exertional dyspnea (EF≥50%) demonstrated that addition of E/e’ during exercise improved sensitivity for diagnosis of HFpEF compared to resting assessment alone, but at the cost of a decreased specificity.24 However, only 74 patients were enrolled in this single-center study, and other groups have not observed as favorable results in HFpEF with exercise echocardiography.67, 77–79 Some studies have raised questions with the ability of E/e’ to track changes in filling pressure during exercise, particularly since E/e’ increases far less than directly measured filling pressures.24, 67, 79 Given the discrepant results in the totality of studies published to date and lack of reproducibility, additional validation, preferably using multicenter designs, are required to clarify the role for noninvasive diastolic stress echocardiography in the evaluation of HFpEF.80
Abnormal LV systolic and diastolic responses to exercise assessed by LV longitudinal strain or strain rate and E/e’ have been reported to improve risk prediction over clinical and resting measurements in HFpEF, though this usage also requires additional confirmation in larger, multicenter studies.81, 82
Echocardiography to Identify HFpEF Phenotypes
It has recently been recognized that HFpEF is a heterogeneous syndrome, and treatments applying the “one size fits all” approach have uniformly failed to date when tested in clinical trials.83 Accordingly, there is an unmet need to categorize different phenotypes within the broader spectrum of HFpEF into pathophysiologically homogenous groups, and cardiac imaging may be a very useful tool to enable this characterization. Candidate phenotypes that might be used for deeper characterization by echocardiography in HFpEF are described below.
Left Atrial Dysfunction Phenotype
Left atrial remodeling and dysfunction secondary to increased LV filling pressure are associated with worse symptoms of dyspnea, more pulmonary vascular disease, greater RV dysfunction, depressed exercise capacity, and adverse outcomes in HFpEF.39, 42, 84, 85 Thus, LA hypertension/dysfunction can be a potential sub-phenotype of HFpEF. Multiple recent studies have shown the utility of LA reservoir strain assessed by speckle-tracking echocardiography to identify LA dysfunction, help diagnosis, and predict outcomes in HFpEF42, 63, 85, 86
Pulmonary Hypertension and Pulmonary Vascular Disease Phenotype
PH is common in patients with HFpEF, and is associated with worse exercise capacity and clinical outcomes.48, 87, 88 While PH is predominantly related to left atrial hypertension in the majority of HFpEF patients, a number of patients develop pulmonary vascular disease, manifest by elevation in pulmonary vascular resistance and reduction in pulmonary arterial compliance.89 HFpEF patients with pulmonary vascular disease is associated with reduced exercise capacity, impaired RV systolic reserve, and worse outcomes, suggesting a different phenotype in the HFpEF spectrum.90 The presence of pulmonary vascular disease can be suspected from mid systolic notching in the RV outflow Doppler profile, along with a short acceleration time caused by increased pulmonary arterial impedance with enhanced early wave reflection.91, 92 There is increasing recognition of the importance of RV and pulmonary vascular coupling (RV-PA coupling) and a recent study has reported that RV-PA coupling assessed by tricuspid annular plane systolic excursion (TAPSE) to RVSP (<0.36 mm/mmHg) predicts the pulmonary vascular disease in HFpEF.93
Right Ventricular Dysfunction Phenotype
The presence of PH causes RV systolic dysfunction in HFpEF, but recent data have shown that RV-PA coupling is even more important.87, 88 TAPSE, RV fractional area change, free wall strain, tricuspid annular s’ velocity, and RV index of myocardial performance can be measured as indices of RV systolic function.94, 95 RV-PA coupling can then be assessed by the ratio of RV function to RVSP,94, 95 and lower TAPSE/RVSP ratio (<0.36 mm/mmHg) is associated with adverse outcomes in HFpEF.93, 94, 96
RV dysfunction is associated with RV remodeling. Echocardiography allows for assessments of RV dilation (RV basal, mid, and longitudinal dimensions and areas), RV hypertrophy, as well as right atrial (RA) dilation. Increased RV diameter, area, and RV wall thickness have been shown to predict adverse outcome in HFpEF.47, 87 RV and RA dilatation lead to tricuspid annular dilation and resultant tricuspid insufficiency, which may further promote systemic venous congestion and impair left heart filling, particularly during exercise.97 Thus, the severity of tricuspid insufficiency should be assessed in all patients with HFpEF.
Obesity Phenotype
Obesity is now recognized as an important phenotype of HFpEF.72 As compared to patients with non-obese HFpEF, patients with the obese phenotype display a number of key differences, including greater relationships between body weight and cardiac filling pressures, greater plasma volume expansion, more ventricular remodeling, more adverse hemodynamics, altered right ventricular-pulmonary artery coupling, worse exercise capacity, and enhanced pericardial restraint.72 Assessments of septal configuration in the short axis can provide non-invasive estimates of the degree of relative pericardial restraint which contributes to the PCWP elevation in HFpEF obese phenotype as well as patients with pulmonary vascular phenotype and those with severe tricuspid insufficiency (Fig. 4).72, 97, 98
Figure 4. Typical Case of Obese HFpEF.
An echocardiographic parasternal short-axis view at end-diastole demonstrates the Dshaped septum in a patient with obese HFpEF (body mass index [BMI] 44 kg/m2).Cardiac catheterization reveals severely elevated right atrial pressure (17 mmHg) relative to pulmonary capillary wedge pressure (21 mmHg). Abbreviations as in Figure 1.
Visceral adiposity and ectopic fat deposit can contribute to the obesity phenotype by altering hemodynamics, inducing systemic and local inflammation, and causing mechanistic compression exaggerating pericardial restraint. Abdominal obesity is associated with epicardial fat and has recently been found to be associated with increased mortality in HFpEF.99 Measurements of epicardial thickness are feasible by echocardiography (Fig. 5), but are more accurately performed using other modalities such as CT and MRI.
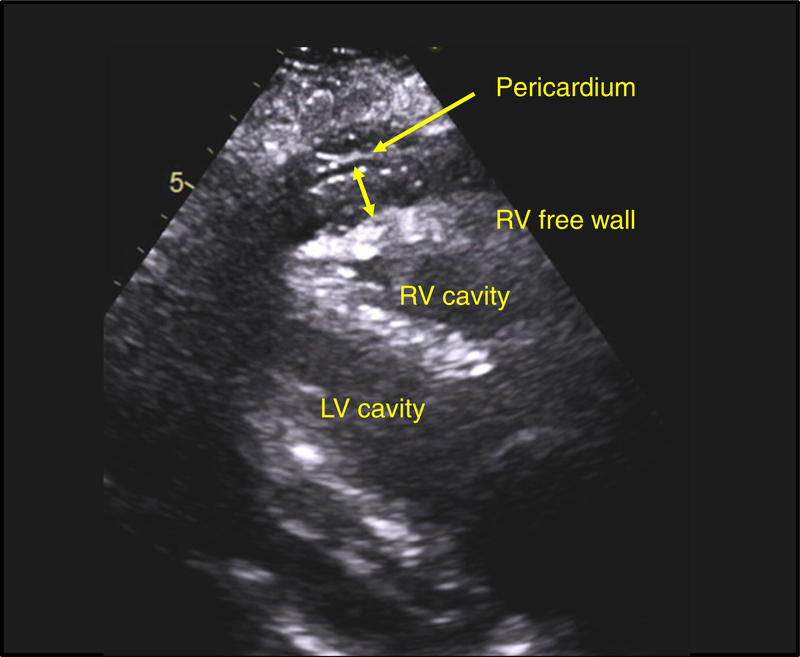
Figure 5. Example of Prominent Epicardial Fat in Obese HFpEF.
Parasternal long-axis view at end-systole in an obese HFpEF patient (BMI 38 kg/m2). Note the increased epicardial fat thickness (14 mm) identified between the right ventricular (RV) free wall and the pericardium. LV, left ventricular; and other abbreviations as in Figures 1 and 4.
Ischemia/Microvascular Dysfunction Phenotype
The presence of epicardial coronary artery disease identifies a distinct HFpEF phenotype in view of its high prevalence, worse prognosis, and importantly a possibility of improving outcomes through revascularization.100 Stress imaging, including echocardiography has been shown to be less accurate in patients with HFpEF, with high rates of false positive and false negative tests.100 This may reflect the fact that subendocardial ischemia may also develop in the absence of epicardial coronary stenosis in HFpEF, caused by the combination of coronary microvascular dysfunction and hemodynamic derangements that compromise subendocardial perfusion.101
Patients with HFpEF developing greater myocardial injury during exercise in tandem with myocardial supply-demand mismatch, and those with greater burden of ischemia and injury display the most profound limitations in LV systolic and diastolic reserve, higher filling pressures during exercise, and more impaired exercise capacity.101 A recent study has shown that adenosine stress echocardiography can be used to assess coronary flow reserve in these patients, and this may be an important non-invasive phenotyping tool, particularly if new treatments are developed targeting microvascular function.102 Other groups have used nuclear and MRI-based imaging to evaluate for coronary microvascular dysfunction in HFpEF,103 and there is hope that novel therapies targeted to microvascular dysfunction may be properly targeted to the right patients using the different imaging modalities.
Conclusions and Future Directions
Echocardiography is clearly essential in the evaluation for HFpEF and provides valuable information to estimate LV filling pressure and understand pathophysiology and improve both evaluation for both diagnosis and prognosis (Summary Figure). Together with clinical characteristics, echocardiography can help determine the likelihood that HFpEF is present, and allow for more informed decision making regarding the need for more advanced testing. However, echocardiography alone is often insufficient to make or refute the diagnosis of HFpEF, and in many cases, invasive hemodynamic exercise testing is required. Categorizing HFpEF patients based upon underlying pathophysiological phenotypes represents a key next step providing individualized medicine in this field, and echocardiography plays a crucial role in this regard, though the optimal ways to categorize patients remain unknown. Finally, echocardiographic parameters provide prognostic information reflecting specific pathophysiologic abnormalities in HFpEF. Further study is required to standardize diagnostic criteria for HFpEF, determine roles for different modalities in its evaluation, establish the potential value for diastolic stress echocardiography, and identify the optimal roles of noninvasive imaging along with other clinical markers for HFpEF phenotyping.
KEY POINTS:
Heart failure with preserved ejection fraction (HFpEF) is a common clinical syndrome that is increasing in prevalence coupled with the growing population burden of aging and comorbidities.
Cardio-vascular imaging plays a key role in the evaluation and management of HFpEF, particularly echocardiography.
Echocardiography provides essential information on cardiac structure, function, and hemodynamics and is performed in essentially all patients where there is clinical suspicion for HFpEF.
From a practical standpoint, the most important questions that can be addressed center on 1) diagnosis, determining whether a patient with unexplained dyspnea truly has HFpEF or an alternate cardiac or non-cardiac cause of dyspnea, and 2) management, where imaging can be used to evaluate hemodynamic status, determine underlying pathophysiologic phenotypes and 3) risk stratification for outcomes.
Acknowledgments
Funding
Dr. Borlaug is supported by the National Institutes of Health (R01 HL128526, R01 HL 126638, U01 HL125205 and U10 HL110262). Dr. Obokata is supported by a research fellowship from the Uehara Memorial Foundation, Japan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
The Authors have nothing to disclose.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259 [DOI] [PubMed] [Google Scholar]
- 2.Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, Agarwal SK, Konety SH, Rodriguez CJ, Rosamond WD. Trends in hospitalizations and survival of acute decompensated heart failure in four us communities (2005–2014): Aric study community surveillance. Circulation. 2018;138:12–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kucharska-Newton A, Sueta CA, Mosley TH, Wright JD, Coresh J, Heiss G, Folsom AR, Solomon SD. Heart failure stages among older adults in the community: The atherosclerosis risk in communities study. Circulation. 2017;135:224–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 esc heart failure guidelines and in the 2016 ase/eacvi recommendations: A systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2018 [DOI] [PubMed] [Google Scholar]
- 6.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29:277–314 [DOI] [PubMed] [Google Scholar]
- 7.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515 [DOI] [PubMed] [Google Scholar]
- 9.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obokata M, Olson TP, Reddy YN, Melenovsky V, Kane GC, Borlaug BA. Hemodynamics, dyspnea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39:2810–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944 [DOI] [PubMed] [Google Scholar]
- 14.Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B, Neumann FJ. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–3112 [DOI] [PubMed] [Google Scholar]
- 15.Borlaug BA, Kass DA. Invasive hemodynamic assessment in heart failure. Cardiol Clin. 2011;29:269–280 [DOI] [PubMed] [Google Scholar]
- 16.Nayor M, Cooper LL, Enserro DM, Xanthakis V, Larson MG, Benjamin EJ, Aragam J, Mitchell GF, Vasan RS. Left ventricular diastolic dysfunction in the community: Impact of diagnostic criteria on the burden, correlates, and prognosis. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah AM, Claggett B, Kitzman D, Biering-Sorensen T, Jensen JS, Cheng S, Matsushita K, Konety S, Folsom AR, Mosley TH, Wright JD, Heiss G, Solomon SD. Contemporary assessment of left ventricular diastolic function in older adults: The atherosclerosis risk in communities study. Circulation. 2017;135:426–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy YNV, Borlaug BA. What do you want from your echocardiogram? J Am Heart Assoc. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr., Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, Ostergren J, McKelvie RS. Diastolic dysfunction in heart failure with preserved systolic function: Need for objective evidence:Results from the charm echocardiographic substudy-charmes. J Am Coll Cardiol. 2007;49:687–694 [DOI] [PubMed] [Google Scholar]
- 21.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: Findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist (topcat) trial. Circ Heart Fail. 2014;7:740–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501 [DOI] [PubMed] [Google Scholar]
- 23.Aljaroudi W, Alraies MC, Halley C, Rodriguez L, Grimm RA, Thomas JD, Jaber WA. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125:782–788 [DOI] [PubMed] [Google Scholar]
- 24.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: A simultaneous invasive-echocardiographic study. Circulation. 2017;135:825–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533 [DOI] [PubMed] [Google Scholar]
- 26.Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue doppler imaging. Circulation. 1998;98:1644–1650 [DOI] [PubMed] [Google Scholar]
- 27.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of doppler echocardiography and tissue doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous doppler-catheterization study. Circulation. 2000;102:1788–1794 [DOI] [PubMed] [Google Scholar]
- 28.Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha JW, Xu J, Klein AL, Nagueh SF. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol. 2017;69:1937–1948 [DOI] [PubMed] [Google Scholar]
- 29.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto K, Nishimura RA, Burnett JC Jr., Redfield MM. Assessment of left ventricular end-diastolic pressure by doppler echocardiography: Contribution of duration of pulmonary venous versus mitral flow velocity curves at atrial contraction. J Am Soc Echocardiogr. 1997;10:52–59 [DOI] [PubMed] [Google Scholar]
- 31.Buffle E, Kramarz J, Elazar E, Aviram G, Ingbir M, Nesher N, Biner S, Keren G, Topilsky Y. Added value of pulmonary venous flow doppler assessment in patients with preserved ejection fraction and its contribution to the diastolic grading paradigm. Eur Heart J Cardiovasc Imaging. 2015;16:1191–1197 [DOI] [PubMed] [Google Scholar]
- 32.Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22:1972–1982 [DOI] [PubMed] [Google Scholar]
- 33.Kuecherer HF, Muhiudeen IA, Kusumoto FM, Lee E, Moulinier LE, Cahalan MK, Schiller NB. Estimation of mean left atrial pressure from transesophageal pulsed doppler echocardiography of pulmonary venous flow. Circulation. 1990;82:1127–1139 [DOI] [PubMed] [Google Scholar]
- 34.Poerner TC, Goebel B, Unglaub P, Suselbeck T, Kaden JJ, Borggrefe M, Haase KK. Non-invasive evaluation of left ventricular filling pressures in patients with abnormal relaxation. Clin Sci. 2004;106:485–494 [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Nishimura RA, Chaliki HP, Appleton CP, Holmes DR Jr., Redfield MM. Determination of left ventricular filling pressure by doppler echocardiography in patients with coronary artery disease: Critical role of left ventricular systolic function. Journal of the American College of Cardiology. 1997;30:1819–1826 [DOI] [PubMed] [Google Scholar]
- 36.Poerner TC, Goebel B, Unglaub P, Sueselbeck T, Strotmann JM, Pfleger S, Borggrefe M, Haase KK. Detection of a pseudonormal mitral inflow pattern: An echocardiographic and tissue doppler study. Echocardiography. 2003;20:345–356 [DOI] [PubMed] [Google Scholar]
- 37.Hadano Y, Murata K, Liu J, Oyama R, Harada N, Okuda S, Hamada Y, Tanaka N, Matsuzaki M. Can transthoracic doppler echocardiography predict the discrepancy between left ventricular end-diastolic pressure and mean pulmonary capillary wedge pressure in patients with heart failure? Circ J. 2005;69:432–438 [DOI] [PubMed] [Google Scholar]
- 38.Kidawa M, Coignard L, Drobinski G, Krzeminska-Pakula M, Thomas D, Komajda M, Isnard R. Comparative value of tissue doppler imaging and m-mode color doppler mitral flow propagation velocity for the evaluation of left ventricular filling pressure. Chest. 2005;128:2544–2550 [DOI] [PubMed] [Google Scholar]
- 39.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303 [DOI] [PubMed] [Google Scholar]
- 40.Zakeri R, Moulay G, Chai Q, Ogut O, Hussain S, Takahama H, Lu T, Wang XL, Linke WA, Lee HC, Redfield MM. Left atrial remodeling and atrioventricular coupling in a canine model of early heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Roeder M, Rommel KP, Kowallick JT, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuss G, Lucke C, Gutberlet M, Schuler G, Schuster A, Lurz P. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2017;10 [DOI] [PubMed] [Google Scholar]
- 42.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen-Torvik LJ, Maganti K, Shah SJ. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: Importance of left atrial strain. Circ Cardiovasc Imag. 2016;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschope C. Utility of doppler echocardiography and tissue doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: A comparative doppler-conductance catheterization study. Circulation. 2007;116:637–647 [DOI] [PubMed] [Google Scholar]
- 44.Dokainish H, Nguyen JS, Sengupta R, Pillai M, Alam M, Bobek J, Lakkis N. Do additional echocardiographic variables increase the accuracy of e/e’ for predicting left ventricular filling pressure in normal ejection fraction? An echocardiographic and invasive hemodynamic study. J Am Soc Echocardiogr. 2010;23:156–161 [DOI] [PubMed] [Google Scholar]
- 45.Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, D’Ascenzi F, Focardi M, Favilli R, Pierli C, Fineschi M, Mondillo S. Correlation of left atrial strain and doppler measurements with invasive measurement of left ventricular enddiastolic pressure in patients stratified for different values of ejection fraction. Echocardiography. 2016;33:398–405 [DOI] [PubMed] [Google Scholar]
- 46.Lancellotti P, Galderisi M, Edvardsen T, Donal E, Goliasch G, Cardim N, Magne J, Laginha S, Hagendorff A, Haland TF, Aaberge L, Martinez C, Rapacciuolo A, Santoro C, Ilardi F, Postolache A, Dulgheru R, Mateescu AD, Beladan CC, Deleanu D, Marchetta S, Auffret V, Schwammenthal E, Habib G, Popescu BA. Echo-doppler estimation of left ventricular filling pressure: Results of the multicentre eacvi euro-filling study. Eur Heart J Cardiovasc Imaging. 2017;18:961–968 [DOI] [PubMed] [Google Scholar]
- 47.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donal E, Lund LH, Oger E, Bosseau C, Reynaud A, Hage C, Drouet E, Daubert JC, Linde C. Importance of combined left atrial size and estimated pulmonary pressure for clinical outcome in patients presenting with heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. 2017;18:629–635 [DOI] [PubMed] [Google Scholar]
- 50.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959 [DOI] [PubMed] [Google Scholar]
- 51.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 esc guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (esc) developed with the special contribution of the heart failure association (hfa) of the esc. Eur Heart J. 2016;37:2129–2200 [DOI] [PubMed] [Google Scholar]
- 52.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from olmsted county, minnesota. Circulation. 2007;115:1982–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phelan D, Collier P, Thavendiranathan P, Popovic ZB, Hanna M, Plana JC, Marwick TH, Thomas JD. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–1448 [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L, Garcia-Pavia P. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594 [DOI] [PubMed] [Google Scholar]
- 56.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016 [DOI] [PubMed] [Google Scholar]
- 57.Kasner M, Gaub R, Sinning D, Westermann D, Steendijk P, Hoffmann W, Schultheiss HP, Tschope C. Global strain rate imaging for the estimation of diastolic function in hfnef compared with pressure-volume loop analysis. Eur J EChocardiogr. 2010;11:743–751 [DOI] [PubMed] [Google Scholar]
- 58.Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Usefulness of new diastolic strain and strain rate indexes for the estimation of left ventricular filling pressure. Am J Cardiol. 2008;101:1504–1509 [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Khoury DS, Thohan V, Torre-Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115:1376–1383 [DOI] [PubMed] [Google Scholar]
- 60.Lassen MCH, Biering-Sorensen SR, Olsen FJ, Skaarup KG, Tolstrup K, Qasim AN, Mogelvang R, Jensen JS, Biering-Sorensen T. Ratio of transmitral early filling velocity to early diastolic strain rate predicts long-term risk of cardiovascular morbidity and mortality in the general population. Eur Heart J. 2018 [DOI] [PubMed] [Google Scholar]
- 61.Hayashi T, Yamada S, Iwano H, Nakabachi M, Sakakibara M, Okada K, Murai D, Nishino H, Kusunose K, Watanabe K, Ishizu T, Wakami K, Yamada H, Dohi K, Seo Y, Ohte N, Mikami T, Tsutsui H. Left ventricular global strain for estimating relaxation and filling pressure- a multicenter study. Circ J. 2016;80:1163–1170 [DOI] [PubMed] [Google Scholar]
- 62.Ma H, Wu WC, Xie RA, Gao LJ, Wang H. Correlation of global strain rate and left ventricular filling pressure in patients with coronary artery disease: A 2-d speckle-tracking study. Ultrasound Med Biol. 2016;42:413–420 [DOI] [PubMed] [Google Scholar]
- 63.Obokata M, Negishi K, Kurosawa K, Arima H, Tateno R, Ui G, Tange S, Arai M, Kurabayashi M. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6:749–758 [DOI] [PubMed] [Google Scholar]
- 64.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mansencal N, Bouvier E, Joseph T, Farcot JC, Pilliere R, Redheuil A, Lacombe P, Jondeau G, Dubourg O. Value of tissue doppler imaging to predict left ventricular filling pressure in patients with coronary artery disease. Echocardiography. 2004;21:133–138 [DOI] [PubMed] [Google Scholar]
- 66.Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, Kocka V. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: A pressure-volume loop analysis. Journal of the American College of Cardiology. 2010;55:1701–1710 [DOI] [PubMed] [Google Scholar]
- 67.Bhella PS, Pacini EL, Prasad A, Hastings JL, Adams-Huett B, Thomas JD, Grayburn PA, Levine BD. Echocardiographic indices do not reliably track changes in left-sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2011;4:482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manouras A, Nyktari E, Sahlen A, Winter R, Vardas P, Brodin LA. The value of e/em ratio in the estimation of left ventricular filling pressures: Impact of acute load reduction: A comparative simultaneous echocardiographic and catheterization study. Int J Cardiol. 2013;166:589–595 [DOI] [PubMed] [Google Scholar]
- 69.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270 [DOI] [PubMed] [Google Scholar]
- 70.Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez De Diego JJ, Hagendorff A, Henri C, Hristova K, Lopez T, Magne J, De La Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Donal E, Lang RM, Badano LP, Lancellotti P. Echocardiographic reference ranges for normal cardiac chamber size: Results from the norre study. Eur Heart J Cardiovasc Imaging. 2014;15:680–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nistri S, Galderisi M, Ballo P, Olivotto I, D’Andrea A, Pagliani L, Santoro A, Papesso B, Innelli P, Cecchi F, Mondillo S. Determinants of echocardiographic left atrial volume: Implications for normalcy. Eur J Echocardiogr. 2011;12:826–833 [DOI] [PubMed] [Google Scholar]
- 72.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lam CS, Rienstra M, Tay WT, Liu LC, Hummel YM, van der Meer P, de Boer RA, Van Gelder IC, van Veldhuisen DJ, Voors AA, Hoendermis ES. Atrial fibrillation in heart failure with preserved ejection fraction: Association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail. 2017;5:92–98 [DOI] [PubMed] [Google Scholar]
- 74.Reddy YNV, Obokata M, Gersh BJ, Borlaug BA. High prevalence of occult heart failure with preserved ejection fraction among patients with atrial fibrillation and dyspnea. Circulation. 2018;137:534–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maron BA, Cockrill BA, Waxman AB, Systrom DM. The invasive cardiopulmonary exercise test. Circulation. 2013;127:1157–1164 [DOI] [PubMed] [Google Scholar]
- 76.Givertz MM, Fang JC, Sorajja P, Dimas V, Forfia PR, Kapur NK, Kern MJ, Naidu SS, Borlaug BA. Executive summary of the scai/hfsa clinical expert consensus document on the use of invasive hemodynamics for the diagnosis and management of cardiovascular disease. J Card Fail. 2017;23:487–491 [DOI] [PubMed] [Google Scholar]
- 77.Sharifov OF, Gupta H. What is the evidence that the tissue doppler index e/e’ reflects left ventricular filling pressure changes after exercise or pharmacological intervention for evaluating diastolic function? A systematic review. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santos M, Rivero J, McCullough SD, West E, Opotowsky AR, Waxman AB, Systrom DM, Shah AM. E/e’ ratio in patients with unexplained dyspnea: Lack of accuracy in estimating left ventricular filling pressure. Circ Heart Fail. 2015;8:749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863 [DOI] [PubMed] [Google Scholar]
- 80.Obokata M, Borlaug BA. The strengths and limitations of e/e’ in heart failure with preserved ejection fraction. Eur J Heart Fail. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Fang F, Wai-Kwok Yip G, Sanderson JE, Feng W, Xie JM, Luo XX, Lee AP, Lam YY. Left ventricular long-axis performance during exercise is an important prognosticator in patients with heart failure and preserved ejection fraction. Int J Cardiol. 2015;178:131–135 [DOI] [PubMed] [Google Scholar]
- 82.Kosmala W, Przewlocka-Kosmala M, Rojek A, Mysiak A, Dabrowski A, Marwick TH. Association of abnormal left ventricular functional reserve with outcome in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2017 [DOI] [PubMed] [Google Scholar]
- 83.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: A multiorgan roadmap. Circulation. 2016;134:73–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban baltimore community: The role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207 [DOI] [PubMed] [Google Scholar]
- 85.Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, Voors AA, Lefkowitz M, Bransford T, Shi V, Packer M, McMurray JJ, Shah AM, Solomon SD. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, Shah AM. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9:e002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mohammed SF, Hussain I, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: A community-based study. Circulation. 2014;130:2310–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorter TM, Obokata M, Reddy YN, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Borlaug BA, Obokata M. Is it time to recognize a new phenotype? Heart failure with preserved ejection fraction with pulmonary vascular disease. Eur Heart J. 2017;38:2874–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arkles JS, Opotowsky AR, Ojeda J, Rogers F, Liu T, Prassana V, Marzec L, Palevsky HI, Ferrari VA, Forfia PR. Shape of the right ventricular doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:268–276 [DOI] [PubMed] [Google Scholar]
- 92.Takahama H, McCully RB, Frantz RP, Kane GC. Unraveling the rv ejection doppler envelope: Insight into pulmonary artery hemodynamics and disease severity. JACC Cardiovasc Imaging. 2017;10:1268–1277 [DOI] [PubMed] [Google Scholar]
- 93.Gorter TM, van Veldhuisen DJ, Voors AA, Hummel YM, Lam CSP, Berger RMF, van Melle JP, Hoendermis ES. Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs. Post-capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2018;19:425–432. [DOI] [PubMed] [Google Scholar]
- 94.Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:H1373–1381 [DOI] [PubMed] [Google Scholar]
- 95.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the american society of echocardiography endorsed by the european association of echocardiography, a registered branch of the european society of cardiology, and the canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786–688 [DOI] [PubMed] [Google Scholar]
- 96.Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJ, Shah SJ. Rv contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: Stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10:1211–1221 [DOI] [PubMed] [Google Scholar]
- 97.Andersen MJ, Nishimura RA, Borlaug BA. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail. 2014;7:911–917 [DOI] [PubMed] [Google Scholar]
- 98.Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39:2825–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with hfpef. J Am Coll Cardiol. 2017;70:2739–2749 [DOI] [PubMed] [Google Scholar]
- 100.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827 [DOI] [PubMed] [Google Scholar]
- 101.Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P, Borlaug BA. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2018;72:29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Fermer ML, Broberg MA, Gan LM, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: Promis-hfpef. Eur Heart J. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mohammed SF, Majure DT, Redfield MM. Zooming in on the microvasculature in heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9 [DOI] [PMC free article] [PubMed] [Google Scholar]