Abstract
Purpose
GOLD guidelines classify COPD patients into A–D groups based on health status as assessed by COPD Assessment Test (CAT) or mMRC tools and exacerbations and recommend single or dual long-acting bronchodilators as maintenance therapy, with additional inhaled corticosteroids (ICS) if the disease remains uncontrolled. We aimed to classify primary care COPD patients into A–D groups, assess usual treatment and adherence to guidelines, potential mismatches between CAT-and mMRC-based classification and described symptoms within groups.
Patients and methods
A total of 257 primary care COPD patients were enrolled between 2015 and 2016 in Greece. Physicians used structured interviews to collect cross-sectional data including demographics, symptoms, CAT, mMRC scores, and medications. Patients were classified into A–D groups based on CAT and mMRC, and prevalence of symptoms and medication was estimated within A–D groups. Interviews with physicians were also performed to explore additional issues about treatment and adherence to guidelines.
Results
Mean (SD) age was 65 (12.3) years with 79% males. The majority of patients reported uncontrolled symptoms (91% and 61% with ≥10 CAT or ≥2 mMRC scores, respectively). Thirty-seven percentage had $2 exacerbations in the past year. Group B was the largest followed by Groups D, A, and C. Patients were classified as more severe by CAT than by mMRC. In all groups, the majority were treated with combined long-acting beta agonist/ICS (> 50%). When patients were asked to report their main symptoms, dyspnea and cough were the most important symptoms mentioned, and there was a great variation between the A–D groups. However, Groups A–C reported mainly morning symptoms, whereas Group D suffered symptoms all day. Physicians reported a significant number of barriers to implementing guidelines, eg, frequent lack of guideline updates, access to diagnostic procedures, and prescription-reimbursement issues.
Conclusion
Our study confirms poor adherence to guidelines regarding treatment with an overuse of ICS and important barriers to implementation. A mismatch in classification occurs depending on the tool used, which can mislead clinicians in their choice of treatment.
Keywords: adherence, COPD, GOLD guidelines, usual care, classification, CAT, mMRC, symptoms
Introduction
COPD is the most common lung disease.1 The importance of symptoms has been outlined in the GOLD 2018,1 where the definition has been updated and enriched as follows: “characterized by persistent respiratory symptoms and airflow limitation”. Moreover, patient classification in A–D groups as defined by the GOLD 2018 guidelines1 is now based only on health status and exacerbations, while spirometry has been left out. Given the fact that spirometry is considered difficult in primary care – in terms of time, performance, interpretation, and reimbursement2 – we expect the primary care physicians’ everyday clinical practice to be facilitated by the new classification.1 GOLD suggests the use of either COPD Assessment Test (CAT) or modified Medical Research Council Dyspnea Scale (mMRC),3 although at the same time clearly states that CAT is preferable. However, studies show that there is a great discrepancy on whether symptoms should be assessed with mMRC or a specific COPD health status tool such as CAT.4,5 Patients do not only suffer from dyspnea but they also suffer from other symptoms such as cough, chest tightness, and wheezing, all included in the eight CAT item questions, highlighting a need for a clear statement from guidelines on its use instead of the mMRC.
Until 2016, GOLD suggested inhaled corticosteroids (ICS) use in Groups C and D in addition to long-acting bronchodilators.6 Evidence from a big randomized control trial has changed the scene and therefore GOLD now suggests dual bronchodilation (such as long-acting beta agonist [LABA] and long-acting muscarinic antagonist [LAMA]) as the cornerstone of treatment in Groups B through D,7 with ICS now recommended as an add-on therapy when maximum control with dual bronchodilation is not achieved. Specifically, GOLD 2018 guidelines recommend long-acting bronchodilators as maintenance therapy while ICS are proposed only for use in patients with severe/very severe disease and/or frequent exacerbations. Evidence suggests that ICS should be prescribed with caution as they are associated with significant adverse events; however, international studies show that ICS are still considered a cornerstone of treatment independently of the GOLD classification/recommendations not only by primary care physicians but also by pulmonologists.8–10
Apart from the GOLD recommendations for COPD management, the majority of European countries have their own national guidelines for primary care. The International Primary Care for Respiratory Group (IPCRG) has recently tried to depict the different guidelines in an attempt to provide with evidence to the majority of its members and share expertise and experiences.11 Although the value of guidelines has been outlined for several years, internationally and nationally, it is not known whether those are really followed by the average general practitioner (GP). It seems that primary care physicians often do not adhere to the guidelines, resulting in a significant gap in applying evidence to practice. Indeed, research shows that adherence to the guidelines in the COPD management is poor.12,13 Several studies have shown inappropriate treatment in great discordance with the suggested guidelines with inappropriate prescription or overprescription of ICS up to 50%.14–17
The primary aim of the current study was to classify the Greek COPD patients who participated into a cross-sectional primary care study into GOLD 2018 A–D groups and assess how those patients were treated and whether they were treated according to the guidelines. Our secondary aims were to assess mismatch of classification depending on whether CAT or mMRC was used, to identify the most prevalent symptoms of patients within A–D groups and in which part of the day those symptoms are more prominent, as well as to explore reasons for poor adherence.
Patients and methods
This study consists of the Greek national branch of the Uncovering and Noting Long-term Outcomes in COPD and asthma to enhance Knowledge (UNLOCK), an international collaboration of primary care researchers to coordinate and share data sets of relevant diagnostic and follow-up variables for COPD and asthma management in primary care. It was set up by IPCRG and the first author Ioanna Tsiligianni was responsible for developing the Greek database. The protocol summary was published in the study by Chavannes et al.18
A convenient sampling method was used to select COPD patients living in rural and semiurban areas served by primary care facilities across Greece. The participation rate varied from 78% to 91%. A sample of 257 COPD patients, first diagnosed by spirometry performed by chest physicians, was enrolled between 2015 and 2016 from 53 primary care facilities in Greece. GPs practising at primary health care units in the selected areas performed structured interviews with the aim to collect cross-sectional information including demographic characteristics, medical history, lifestyle, CAT19 and mMRC3,20 scores, annual number of exacerbations and hospitalizations due to respiratory illness, and medication used for COPD management. The degree of dyspnea in the mMRC scale was rated and assigned to five grades: “Grade 0: breathless with strenuous exercise; Grade 1: short of breath when hurrying on level ground or walking up a slight hill; Grade 2: walked slower than people of the same age on level ground, and experienced breathlessness or the need to stop to breathe when walking on level ground at their own pace; Grade 3: stop to breathe after walking about 100 yards, or after a few minutes on level ground; Grade 4: too breathless to leave the house, or breathless when dressing or undressing”. An exacerbation was defined based on GOLD as “an event in the natural course of the disease characterized by a change in the patient’s baseline dyspnea, cough, and/or sputum that is beyond normal day-to-day variations, is acute in onset, and may warrant a change in regular medication to a patient with underlying COPD”, and patients were specifically asked whether they underwent such a change in the past 12 months. COPD patients were classified according to the GOLD 2018 guidelines with ABCD grading system.1 The A–D grading system considers COPD health status, assessed by CAT or mMRC, along with exacerbation frequency and need for hospitalization (A is better, D is worse). We classify COPD patients into A–D groups based on both CAT and mMRC tools and cross-tabulated the two methods to assess agreement. Based on electronic or paper-based prescription records, we collected data on brand/commercial name of inhaler(s) used. Consequently, we documented whether the patient received short-acting beta agonist (SABA) and/or LABA and/or short-acting muscarinic antagonist (SAMA) and/or LAMA and/or ICS. Type of inhaled medication was then cross-tabulated with A–D groups.
Additionally, we addressed three questions to the patients as follows: which are the symptoms that bother them, which is the most important for them, and in what part of the day those occurred. Finally, this study also had a qualitative part with semistructured interviews of GPs, where open-ended questions were used to explore GPs’ views on adherence to guidelines.
Statistics
Descriptive statistics for baseline data were presented as percentage or mean and SD. If the distribution of continuous data was not normal, then median (minimum–maximum) was used instead. We tested for correlation between CAT and mMRC scores with Pearson rho coefficient. A P-value of <0.05 was considered to indicate statistically significant associations. Data management and statistical analyses were performed using IBM-SPSS Statistics software (version 23).
Results
Table 1 presents the main population characteristics. There were three variables that had >5% of missing data including number of exacerbations and hospitalizations and occupational status; however, we used the Little’ s Missing Completely at Random test and showed that the missed data were missing at random (chi-square value =22.6, P-value=0.09), which should not cause any systematic bias. Mean (SD) population age was 65 (12.3) years with 79.4% males. The great majority were married/with partner (84.2%) and retired (55.4%). 55.6% were current smokers. CAT was positively correlated with mMRC score (rho=0.55; P-value>0.001). With the use of CAT and mMRC tools, uncontrolled/poor health status was reported in 91.1% and 60.6%, respectively. 37.2% of the patients had at least two exacerbations in the last 12 months. The majority of patients reported dyspnea as their main symptom. Based on the GOLD 2018 guidelines, Group B was the largest followed by Group D as given by CAT (Group B, 56.4%; Group D, 36.8%) or mMRC (Group B, 34.3%; Group D, 28.5%)-based tool. The majority of patients in Groups A and C as given by mMRC were reclassified into Groups B (45 out of 55 [82%]) and D (17 out of 20 [85%]), respectively, when the classification included CAT as index of health status, whereas patients in Groups B and D remained in the same group with either tool (Figure 1).
Table 1.
Population characteristics
| N=257 | |
|---|---|
|
| |
| Age in years; mean (SD) | 65 (12.3) |
|
| |
| Gender, n (%) | |
| Male | 204 (79.4) |
| Female | 53 (20.6) |
|
| |
| Marital status, n (%) | |
| Married/with partner | 208 (84.2) |
| Widower | 24 (9.7) |
| Divorced or never married | 15 (6.1) |
|
| |
| Occupational status, n (%) | |
| Employed | 74 (33.3) |
| Unemployed/housewife | 25 (11.3) |
| Retired | 123 (55.4) |
|
| |
| Smoking status, n (%) | |
| Current | 143 (55.6) |
| Ex | 83 (32.3) |
| Never | 31 (12.1) |
|
| |
| Pack-years; median (min–max) | 40 (10–200) |
|
| |
| CAT, mean (SD) | 17.3 (6.5) |
|
| |
| mMRC, mean (SD) | 1.9 (1.2) |
|
| |
| Main symptom, n (%) | |
| Cough | 96 (37.9) |
| Dyspnea | 135 (53.4) |
| Others (wheezing, chest tightness, and phlegm) | 22 (8.7) |
|
| |
| CAT, n (%) | |
| <10 | 22 (8.9) |
| ≥10 | 224 (91.1) |
|
| |
| mMRC, n (%) | |
| 0–1 | 100 (39.4) |
| ≥2 | 154 (60.6) |
|
| |
| Number of exacerbations in the last 12 months, median (min–max) | 1 (0–4) |
|
| |
| Number of exacerbations in the last 12 months, n (%) | |
| <2 | 130 (62.8) |
| ≥2 | 77 (37.2) |
|
| |
| Number of hospitalization in the last 12 months, median (min–max) | 0 (0–3) |
|
| |
| GOLD 2018–CAT/mMRC based, n (%) | |
| A | 11 (5.4)/57 (27.5) |
| B | 115 (56.4)/71 (34.3) |
| C | 3 (1.5)/20 (9.7) |
| D | 75 (36.8)/59 (28.5) |
Abbreviations: CAT, COPD Assessment Test; max, maximum; min, minimum; mMRC, Modified Medical Research Council Dyspnea Scale.
Figure 1.
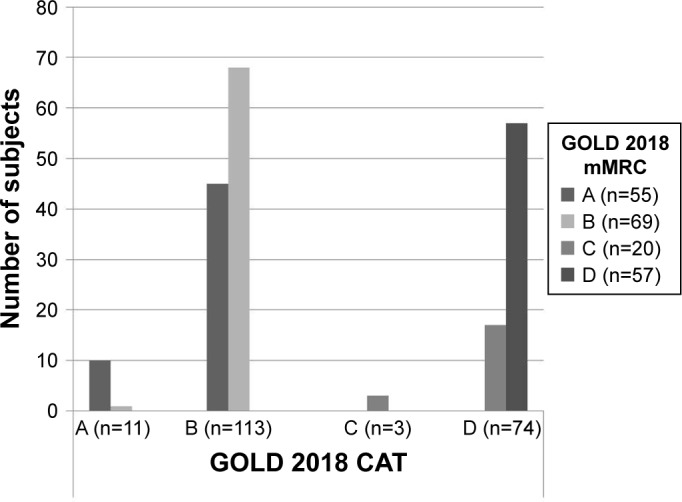
Agreement between CAT-and mMRC-based GOLD 2018 A–D classification.
Abbreviations: CAT, COPD Assessment Test; mMRC, Modified Medical Research Council Dyspnea Scale.
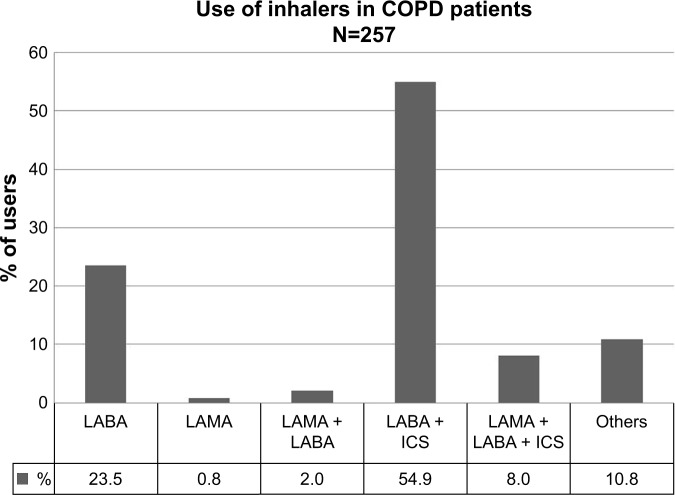
Table 2 presents the inhaled medication combinations used by COPD patients. The majority of patients were treated with LABA combined with ICS (54.9%) or LABA alone (23.5%) (Figure 2). However, there was a large variation in the combination of inhaled medications.
Table 2.
Inhaled medication used by COPD patients (N=257)
| n (%) | |
|---|---|
| ICS | 6 (2.4) |
| LABA | 60 (23.5) |
| LAMA | 2 (0.8) |
| LABA + ICS | 141 (55.3) |
| LAMA + ICS | 2 (0.8) |
| LAMA + LABA | 4 (1.6) |
| LAMA + LABA + ICS | 14 (5.5) |
| SABA | 2 (0.8) |
| SABA + ICS | 3 (1.2) |
| SABA + LAMA + LABA | 1 (0.4) |
| SABA + LAMA + LABA + ICS | 6 (2.4) |
| SAMA + SABA | 2 (0.8) |
| SAMA + SABA + ICS | 8 (3.1) |
| All | 2 (0.8) |
| None | 2 (0.8) |
Abbreviations: ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting beta agonist; SAMA, short-acting muscarinic antagonist.
Figure 2.
Inhaled medication combinations used by COPD patients.
Notes: CAT (missing n=53); mMRC (missing n=50).
Abbreviations: CAT, COPD Assessment Test; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; mMRC, Modified Medical Research Council Dyspnea Scale; SABA, short-acting beta agonist; SAMA, short-acting muscarinic antagonist.
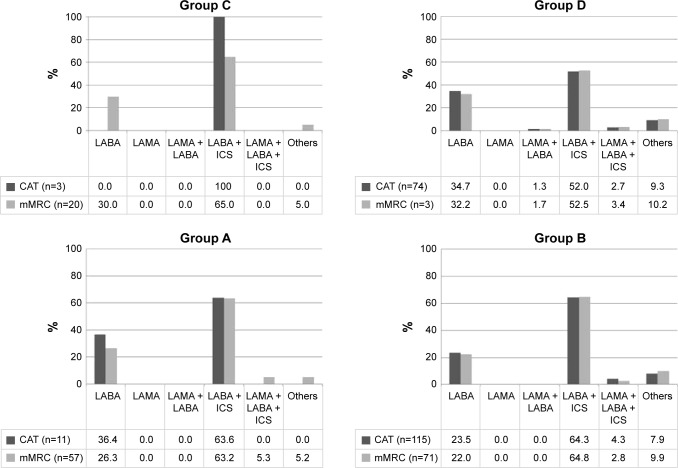
Figure 3 presents inhaled medication used within the A–D groups. Inhaled medication used was similar for all groups with the majority of patients being treated with LABA combined with ICS (range 52%–65%) or LABA alone (range 22%–36%). Use of short-acting bronchodilator was limited in Group A and only a small number of subjects (<5.5%) used LABA–LAMA–ICS triple therapy, even in Group D (~3%). Trends in prescribed inhaled medication did not differ much between CAT and mMRC based A-D GOLD 2018 categorization.
Figure 3.
Inhaled medication used in the GOLD 2018 A–D groups.
Notes: CAT (missing n=53); mMRC (missing n=50).
Abbreviations: CAT, COPD Assessment Test; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council Dyspnea Scale; SABA, short-acting beta agonist; SAMA, short-acting muscarinic antagonist.
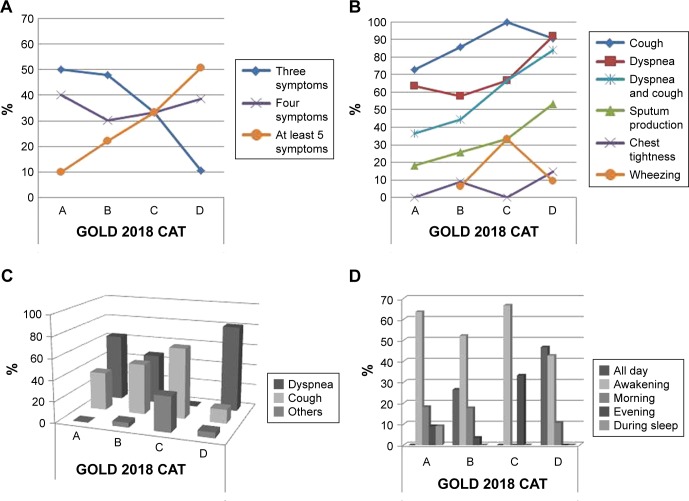
The prevalence (%) of respiratory symptoms as reported by patients within A–D groups is shown in Table 3. Dyspnea and cough were the most important symptoms mentioned, and there was a great variation between the A–D groups. Additionally, dyspnea was reported as the most bothering symptom for Groups A, B, and D, but in Group C the most frequent bothering symptom depended on the tool used (Table 3). For instance, cough was the most prevalent bothersome symptom when CAT was used, while dyspnea was reported as the most frequent bothersome symptom when mMRC was used. Patients in Group D reported symptoms all day long, whereas patients in Groups A–C reported symptoms mainly when they wake up (Figure 4). These symptom trends were similar between CAT and mMRC-based A–D GOLD 2018 classification (Table 3).
Table 3.
Prevalence of respiratory symptoms within GOLD 2018 A–D groups
| GOLD 2018 | GOLD 2018 | |||||||
|---|---|---|---|---|---|---|---|---|
| CAT-based | mMRC-based | |||||||
| A (n=11) | B (n=113) | C (n=3) | D (n=75) | A (n=57) | B (n=70) | C (n=20) | D (n=55) | |
| Cough, n (%) | 8 (72.7) | 97 (85.8) | 3 (100) | 68 (90.7) | 49 (86.0) | 58 (82.9) | 18 (90.0) | 55 (93.2) |
| Dyspnea, n (%) | 7 (63.6) | 65 (57.5) | 2 (66.7) | 69 (92) | 32 (56.1) | 41 (58.6) | 18 (90.0) | 53 (89.8) |
| Sputum production, n (%) | 2 (18.2) | 29 (25.7) | 1 (33.3) | 40 (53.3) | 13 (22.8) | 19 (27.1) | 8 (40.0) | 33 (55.9) |
| Chest tightness, n (%) | 0 | 10 (8.8) | 0 | 11 (14.7) | 3 (5.3) | 7 (10.0) | 2 (10.0) | 8 (13.6) |
| Wheezing, n (%) | 0 | 7 (6.2) | 1 (33.3) | 7 (9.3) | 3 (5.4) | 5 (7.1) | 1 (5.0) | 7 (11.9) |
| Most bothering symptom, n (%) | ||||||||
| Dyspnea | 7 (63.6) | 53 (47.3) | 0 | 61 (81.3) | 27 (48.2) | 34 (48.6) | 14 (70.0) | 47 (79.7) |
| Cough | 4 (36.4) | 54 (48.2) | 2 (66.7) | 10 (13.3) | 28 (50.0) | 31 (44.3) | 4 (20.0) | 9 (15.3) |
| Sputum production | 0 | 4 (3.6) | 0 | 4 (5.3) | 1 (11.1) | 4 (44.4) | 1 (11.1) | 3 (5.1) |
| Others | 0 | 1 (0.9) | 1 (33.3) | 0 | 0 | 1 (1.4) | 1 (5.0) | 0 |
| Time of symptoms, n (%) | ||||||||
| All day | 0 | 30 (26.5) | 0 | 35 (46.7) | 9 (15.8) | 22 (31.4) | 5 (25.0) | 30 (50.8) |
| Awakening | 7 (63.6) | 59 (52.2) | 2 (66.7) | 32 (42.7) | 36 (63.2) | 32 (45.7) | 10 (50) | 25 (42.4) |
| Morning | 2 (18.2) | 20 (17.7) | 0 | 8 (10.7) | 8 (14.0) | 14 (20.0) | 4 (20.0) | 4 (6.8) |
| Evening | 1 (9.1) | 4 (3.5) | 1 (33.3) | 0 | 5 (5.3) | 5 | 1 (5.0) | 0 |
| During sleep | 1 (9.1) | 0 | 0 | 0 | 1 (1.8) | 0 | 0 | 0 |
Abbreviations: CAT, COPD Assessment Test; mMRC, modified Medical Research Council Dyspnea Scale.
Figure 4.
Characteristics of respiratory symptoms within the ABCD groups.
Notes: Prevalence of number of symptoms (A), type of symptoms (B), most bothering symptom (C), and time of symptoms (D) based on GOLD 2018 (CAT-based). CAT (missing n=53).
Abbreviation: CAT, COPD Assessment Test.
Qualitative part
Moreover, we conducted interviews with a random sample (10 primary care physicians) to assess whether they use questionnaires in their daily practice and whether they follow the GOLD guidelines or the national ones, especially concerning the use of ICS and dual bronchodilation. The results of the interviews showed that GPs face many barriers; they think it is difficult to use CAT in daily clinical practice while mMRC was considered easier. They said they still prescribe ICS because they feel insecure about the diagnosis, they have often limited access to diagnostic procedures, they felt that ICS are still the cornerstone of treatment for COPD in the Greek national primary care guidelines that have not been updated since 2014, and they feel insecure with ICS withdrawing. They also mentioned that they are struggling with following so many GOLD updates. Moreover, they mention as obstacles the lack of collaboration with pulmonologists and that undiagnosed patients who had visited an emergency room had an ICS combination therapy as a first prescription. Prescription restrictions by the national health care system have also been mentioned as the most important obstacle as the e-prescription does not allow dual bronchodilators to be prescribed by a primary care physician without spirometry, while ICS are free to be prescribed and PD4 inhibitors are prescribed only by pulmonologists.
Ethics statement
All subjects signed a written informed consent, and ethical approval was provided by the University of Crete Ethics Committee (2015/protocol number 7985). The authors confirm that the study was conducted in accordance with the Declaration of Helsinki.
Discussion
Our study showed that GPs do not follow the current GOLD guidelines regarding the COPD management with the majority of patients using ICS for symptom management in all A–D groups. Physicians prescribed combinations of bronchodilator with ICS and not single or dual bronchodilation as a first choice, and this was independent of the GOLD group. However, although somebody could easily argue that GPs do not meet treatment standards, the interviews of this study showed that there are a lot of important barriers, eg, access to diagnostic procedures, Greek guidelines have not been updated, lack of collaboration with pulmonologists, undiagnosed patients who visit emergency rooms in hospitals have an ICS as a first prescription, prescription-reimbursement issues, and questionnaire/exacerbation assessments are time consuming. We also confirmed a mismatch between CAT and mMRC-based classification in A–D groups. Furthermore, our study showed that some groups frequently present with other symptoms besides dyspnea, and therefore, the use of a health status tool as CAT describes more adequately patients’ general health status.
Health status/symptom exacerbations
Health status was very poor as assessed with two different tools: the CAT and mMRC. Nearly 90% of the patients reported having a poor health status similar to another study performed in Greece.21 DACCORD, a large prospective reallife observational study, also showed that the majority of COPD subjects are symptomatic, with dyspnea and cough to be the prominent symptoms.22,23 Independent of the tool used, dyspnea and cough were reported by the patients in all groups. However, when patients reported their symptoms there was a great variation between the groups, a fact that shows that symptoms are very subjectively reported by patients and that a use of a structured tool such as CAT may capture better different symptoms than mMRC that only captures dyspnea. Similar to our study, studies show that patients with COPD mention morning to be the worst time of the day in terms of symptoms,24,25 but in our study we also specify that this is predominant in Groups A–C. For patients in Group D, the symptoms unfortunately persist throughout the day. The ASSESS study showed that symptoms most frequently occur during daytime and early in the morning and that night-time symptoms were present but were not the most frequent.26 To what extent those symptoms are persistent due to the inappropriate management remains to be considered.
GOLD 2018 A–D group classification and symptoms reported
We found the highest percentage of patients to be in Groups B and D; however, we found more patients in Group B than Group D, as opposed to other studies that showed that the majority of patients are in Group D.17,23,27–29 Group C was the least frequent in our study, a finding consistent with other studies that showed prevalence between 4.2% and 11.3%.17,30 Studies have shown a mismatch in the GOLD classification depending on the health status tool used (CAT or mMRC). Similar to previous studies, we showed that a higher proportion of patients were classified as more symptomatic by CAT than by mMRC score.17,19,23,31 The use of CAT resulted in the reclassification of a substantial number of patients from Group A to Group B (45 out of 55 [81.8%]) and from Group C to Group D (17 out of 20 [85%]). This mismatch is reconfirmed in several studies.4,5,17,31 Clinicians could get confused not knowing which treatment to suggest depending on different methods to assess symptoms, and therefore, there is a clear need to propose tools that measure health status with high agreement. Still, this could probably further highlight the discrepancy between guidelines and actual care in primary health care settings.
Adherence to guidelines
Our study showed a poor adherence to guidelines regarding treatment based on A–D classification. Interestingly, a lower proportion of Group D patients were on ICS compared to that of the other groups, which further highlights the discrepancy between guidelines and actual care. Numerous studies have shown that physicians do not follow the guidelines in the treatment of COPD.14,27,32,33 Adherence to evidence-based guidelines had been reported to be poor also in other studies with physicians mentioning that they do not follow them for several reasons, such as unfamiliarity and disagreement,12,13 difficulties in assessing response to therapy, and failure to recognize improvements with treatment.34–36
Not using medications according to guidelines increases the burden of the disease and exacerbations25,36–41 with respect to quality of life/symptom control and money spent on medication and health care use.41 It seems that this gap does involve not only primary care units but also pulmonologists who do not follow the guidelines42 and overprescribe ICS.43,44 The fact that bronchodilators, especially LAMA, have been underused in our study may have contributed to the symptoms burden.
Treatment
In our population of Group A where there is a GOLD suggestion for use of short-acting bronchodilation, we found an underuse of SABA and SAMA, similar to the study by Safka et al.17
Our study showed an underuse of LABA/LAMA and an overuse of ICS. LABA/LAMA have been underused similarly in a number of studies.14,27,32,33,45 Many studies also show an overprescription of ICS8,10,14,27,32,33,46,47 with worldwide ICS prescription rates ranging from 19.5% to more than 70% and sometimes 80% depending on the country and the population studied. Our study was conducted in a primary care setting but similar nonadherence and overuse of ICS were found in Safka et al’s17 study including patients from tertiary care. Therefore, the overuse of ICS is universal and influences all levels of care. White et al46 mentioned that the mMRC score and the high exacerbation rate were the most important factors for prescribing ICS, which although was beyond the scope of our study may also have influenced the prescribers in our study.
The PDE-4 inhibitors have not been prescribed at all by GPs in our study. This is in accordance with the Safka et al’s study that mentioned an underuse of PDE-4 inhibitors in Groups C and D.17 An explanation could be non-adherence to guidelines or the fact that those medications in Greece are not allowed to be prescribed by GPs but only by pulmonologists.
The interviews helped us identify the reasons for poor adherence with underuse of dual bronchodilation and overuse of ICS. Most of those reasons are out of the control of GPs such as the lack of often national guideline updates, the struggle to follow the changing GOLD updates that occur very often, as well as the important barrier of the prescription restrictions.
Strengths and limitations of the study
This is a real-life study enrolling patients from usual consultations in primary care independently of the disease severity, and therefore, findings may be applicable to a wider population. The qualitative part of this study adds knowledge that may help interventions to improve guideline implementation in the future as significant factors have been underlined. As a cross-sectional study, there is risk of bias and lack of ability to show cause and effect. The study had some more limitations. First, the patients were recruited from primary care units and may not reflect the management from secondary–tertiary care. Second, the diagnosis was based on patients’ records only, as spirometry data performed by chest physicians were not linked with the setting of this study, and therefore, introducing risk of misclassification bias. Additionally, the majority of study population were males, and this could probably add some barriers in finding generalizability when discussing symptoms and outcomes in female population groups. This may slightly limit external validity; however, the authors believe that it is a well-representative sample for the Greek COPD population and other rural populations sharing similar characteristics. Furthermore, COPD burden, symptoms, and comorbidities are more prominent in males than in females in Greece.
Implications of the study
The WHO defined rational use of medicines as “patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community”.48 Irrational use of medicines is a major problem worldwide. Inappropriate use leads to adverse effects, sub-optimal outcomes, and waste of resources (money, health professionals, and patients’ time).
WHO advocates 12 key interventions to promote more rational prescribing, which we think have application for COPD as well. Those include establishment of a multidisciplinary national body to coordinate policies on medication use, use of clinical guidelines, development and use of national essential medicines list, establishment of drug and therapeutics committees in districts/hospitals, inclusion of problem-based pharmacotherapy training in undergraduate curricula, continuing in-service medical education as a licensure requirement, supervision, audit, and feedback, use of independent information on medicines, public education about medicines, avoidance of perverse financial incentives, use of appropriate and enforced regulation, and sufficient government expenditure to ensure availability of medicines/staff. Several other approaches to improve implementation have been proposed as creation of shared clinical models at the system evidence level, generation and analysis of underlying evidence from electronic sources, providing infrastructure to support rapid dissemination, validation, and impact analysis of clinical evidence in practice.49
Moreover, although we recognize the need of guidelines, it is also essential to collaborate on a national/international level to reassure implementation and re-evaluate them by also considering the prescribers’ needs. The implementation of guidelines should also involve policy stakeholders as restrictions could cause inappropriate prescriptions. Reducing inappropriate ICS prescribing could be used as a quality indicator. In Greece, despite the continuous pressure from general practice, the e-prescription still does not allow the prescribing of dual bronchodilation unless a spirometry is available. This indicates an urgent need for action.
Conclusion
Our study showed that GPs prescribed ICS combined with long-acting bronchodilation and not single or dual bronchodilation as a first choice independently of the GOLD group. However, many barriers for adhering to guidelines have been reported by GPs. Patients were highly symptomatic with symptoms reported of not only dyspnea but also cough. The latter in combination with a mismatch between CAT and mMRC-based classification in A–D groups shows a clear need for using a health status tool instead of the mMRC that assesses only dyspnea. A multiapproach is needed to achieve a better adherence to guidelines.
Acknowledgments
The Greek UNLOCK team includes Eleftheria Lintovoi, Dimitris Karanassos, Kyriakos Maltezis, Maria Chorti, Evangelos Petrovitsos, Sofia Dimopoulou, Sam Hamind, Ioannis Gialamas, Polyxeni Athanasiou, Vasiliki Bempi, and Irene Lambraki. This study was funded through a research grant from IPCRG to develop a Greek UNLOCK database and to analyze the findings. IPCRG has advised in design.
Abbreviations
- CAT
COPD Assessment Test
- GP
general practitioner
- LABA
long-acting beta agonist
- LAMA
long-acting muscarinic antagonist
- mMRC
Modified Medical Research Council Dyspnea Scale
- SABA
short-acting beta agonist
- SAMA
short-acting muscarinic antagonist
- UNLOCK
Uncovering and Noting Long-term Outcomes in COPD and asthma to enhance Knowledge
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.GOLD Global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung Disease (Gold 2018 report) 2018. [Accessed October 10, 2018]. Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf.
- 2.Levy ML, Quanjer PH, Booker R, et al. Diagnostic spirometry in primary care: Proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations: a General Practice Airways Group (GPIAG)1 document, in association with the Association for Respiratory Technology & Physiology (ARTP)2 and Education for Health3 1 www.gpiag.org 2 www.artp.org 3 www.educationforhealth.org.uk. Prim Care Respir J. 2009;18(3):130–147. doi: 10.4104/pcrj.2009.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajiro T, Nishimura K, Tsukino M, et al. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(4):1185–1189. doi: 10.1164/ajrccm.158.4.9802091. [DOI] [PubMed] [Google Scholar]
- 4.Holt S, Sheahan D, Helm C, et al. Little agreement in gold category using cat and mMRC in 450 primary care COPD patients in New Zealand. NPJ Prim Care Respir Med. 2014;24(1):14025. doi: 10.1038/npjpcrm.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Oh J, Kim YI, et al. Differences in classification of COPD group using COPD assessment test (cat) or modified medical Research Council (mMRC) dyspnea scores: a cross-sectional analyses. BMC Pulm Med. 2013;13(1):35. doi: 10.1186/1471-2466-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 7.Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 8.Gruffydd-Jones K, Brusselle G, Jones R, et al. Erratum: changes in initial COPD treatment choice over time and factors influencing prescribing decisions in UK primary care: A real-world study. NPJ Prim Care Respir Med. 2017;27:17004. doi: 10.1038/npjpcrm.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roche N, Lepage T, Bourcereau J, Terrioux P. Guidelines versus clinical practice in the treatment of chronic obstructive pulmonary disease. Eur Respir J. 2001;18(6):903–908. doi: 10.1183/09031936.01.00213701. [DOI] [PubMed] [Google Scholar]
- 10.Corrado A, Rossi A. How far is real life from COPD therapy guidelines? an Italian observational study. Respir Med. 2012;106(7):989–997. doi: 10.1016/j.rmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 11.IPCRG Mapping of national guidelines used by primary care. [Accessed January 30, 2019]. Available from: https://www.theipcrg.org/display/ResMapping/Mapping+of+national+guidelines+used+by+primary+care.
- 12.Make B, Dutro MP, Paulose-Ram R, Marton JP, Mapel DW. Under-treatment of COPD: a retrospective analysis of US managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis. 2012;7:1–9. doi: 10.2147/COPD.S27032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473–526. doi: 10.1016/S2213-2600(16)00094-1. [DOI] [PubMed] [Google Scholar]
- 14.Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–904. doi: 10.2147/COPD.S62750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Miguel-Díez J, Carrasco-Garrido P, Rejas-Gutierrez J, et al. Inappropriate overuse of inhaled corticosteroids for COPD patients: impact on health costs and health status. Lung. 2011;189(3):199–206. doi: 10.1007/s00408-011-9289-0. [DOI] [PubMed] [Google Scholar]
- 16.Laniado-Laborín R, Rendón A, Alcantar-Schramm JM, Cazares-Adame R, Bauerle O. Subutilization of COPD guidelines in primary care: a pilot study. J Prim Care Community Health. 2013;4(3):172–176. doi: 10.1177/2150131913475817. [DOI] [PubMed] [Google Scholar]
- 17.Safka KA, Wald J, Wang H, Mcivor L, Mcivor A. Gold stage and treatment in COPD: a 500 patient point prevalence study. Chronic Obstr Pulm Dis. 2016;4(1):45–55. doi: 10.15326/jcopdf.4.1.2016.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavannes N, Ställberg B, Lisspers K, et al. Unlock: uncovering and Noting long-term outcomes in COPD to enhance knowledge. Prim Care Respir J. 2010;19(4):408. doi: 10.4104/pcrj.2010.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 20.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the medical Research Council (MRC) Dyspnoea Scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsiligianni IG, van der Molen T, Moraitaki D, et al. Assessing health status in COPD. A head-to-head comparison between the COPD assessment test (cat) and the clinical COPD questionnaire (CCQ) BMC Pulm Med. 2012;12(1):20. doi: 10.1186/1471-2466-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buhl R, Criée CP, Kardos P, et al. A year in the life of German patients with COPD: the DACCORD observational study. Int J Chron Obstruct Pulmon Dis. 2016;11:1639–1646. doi: 10.2147/COPD.S112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worth H, Buhl R, Criée CP, et al. The ‘real-life’ COPD patient in Germany: The DACCORD study. Respir Med. 2016;111:64–71. doi: 10.1016/j.rmed.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an Internet survey. Curr Med Res Opin. 2009;25(8):2043–2048. doi: 10.1185/03007990903103006. [DOI] [PubMed] [Google Scholar]
- 25.Roche N, Small M, Broomfield S, Higgins V, Pollard R. Real world COPD: association of morning symptoms with clinical and patient reported outcomes. COPD. 2013;10(6):679–686. doi: 10.3109/15412555.2013.844784. [DOI] [PubMed] [Google Scholar]
- 26.Miravitlles M, Worth H, Soler Cataluña JJ, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the assess study. Respir Res. 2014;15(1):122. doi: 10.1186/s12931-014-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arkhipov V, Arkhipova D, Miravitlles M, Lazarev A, Stukalina E. Characteristics of COPD patients according to gold classification and clinical phenotypes in the Russian Federation: the support trial. Int J Chron Obstruct Pulmon Dis. 2017;12:3255–3262. doi: 10.2147/COPD.S142997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casanova C, Marin JM, Martinez-Gonzalez C, et al. Differential effect of modified medical Research Council dyspnea, COPD assessment test, and clinical COPD questionnaire for symptoms evaluation within the new gold staging and mortality in COPD. Chest. 2015;148(1):159–168. doi: 10.1378/chest.14-2449. [DOI] [PubMed] [Google Scholar]
- 29.Zbozinkova Z, Barczyk A, Tkacova R, et al. Pope study: rationale and methodology of a study to phenotype patients with COPD in central and eastern Europe. Int J Chron Obstruct Pulmon Dis. 2016;11:611–622. doi: 10.2147/COPD.S88846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrera López C, Casanova Macario C, Marín Trigo JM, et al. Comparison of the 2017 and 2015 global initiative for chronic obstructive lung disease reports. Impact on grouping and outcomes. Am J Respir Crit Care Med. 2018;197(4):463–469. doi: 10.1164/rccm.201707-1363OC. [DOI] [PubMed] [Google Scholar]
- 31.Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J. 2013;42(3):647–654. doi: 10.1183/09031936.00125612. [DOI] [PubMed] [Google Scholar]
- 32.Wurst KE, Shukla A, Muellerova H, et al. Respiratory pharmacotherapy use in patients newly diagnosed with chronic obstructive pulmonary disease in a primary care setting in the UK: a retrospective cohort study. COPD. 2014;11(5):521–530. doi: 10.3109/15412555.2014.922064. [DOI] [PubMed] [Google Scholar]
- 33.Barrecheguren M, Monteagudo M, Ferrer J, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med. 2016;111:47–53. doi: 10.1016/j.rmed.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Perez X, Wisnivesky JP, Lurslurchachai L, et al. Barriers to adherence to COPD guidelines among primary care providers. Respir Med. 2012;106(3):374–381. doi: 10.1016/j.rmed.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salinas G, Williamson C, Kalhan R, et al. Barriers to adherence to chronic obstructive pulmonary disease guidelines by primary care physicians. Int J Chron Obstruct Pulmon Dis. 2011;6:171–179. doi: 10.2147/COPD.S16396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foda H, Brehm A, Goldsteen K, et al. Inverse relationship between nonadherence to original GOLD treatment guidelines and exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:209–214. doi: 10.2147/COPD.S119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miravitlles M, Mayordomo C, Artes M, et al. Treatment of chronic obstructive pulmonary disease and its exacerbations in general practice. EOLO Group. Estudio Observacional de la Limitacion Obstructiva al Flujo aEreo. Respir Med. 1999;93(3):173–179. doi: 10.1016/s0954-6111(99)90004-5. [DOI] [PubMed] [Google Scholar]
- 38.Esteban C, Moraza J, Quintana JM, et al. Use of medication and quality of life among patients with COPD. Respir Med. 2006;100(3):487–495. doi: 10.1016/j.rmed.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 39.López Varela MV, Muiño A, Padilla RP, et al. [Treatment of chronic obstructive pulmonary disease in 5 Latin American cities: the PLATINO study] Arch Bronconeumol. 2008;44(2):58–64. doi: 10.1016/s1579-2129(08)60016-6. Spanish. [DOI] [PubMed] [Google Scholar]
- 40.Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of confronting COPD international survey. Eur Respir J. 2002;20(4):799–805. doi: 10.1183/09031936.02.03242002. [DOI] [PubMed] [Google Scholar]
- 41.Asche CVC, Leader S, Plauschinat C, et al. Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis. 2012;7:201–209. doi: 10.2147/COPD.S25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glaab T, Banik N, Rutschmann OT, et al. National survey of guideline-compliant COPD management among pneumologists and primary care physicians. COPD. 2006;3(3):141–148. doi: 10.1080/15412550600829299. [DOI] [PubMed] [Google Scholar]
- 43.Decramer M, Bartsch P, Pauwels R, et al. Management of COPD according to guidelines. A national survey among Belgian physicians. Monaldi Arch Chest Dis. 2003;59(1):62–80. [PubMed] [Google Scholar]
- 44.Izquierdo-Alonso JL, de Miguel-Diez J. Economic impact of pulmonary drugs on direct costs of stable chronic obstructive pulmonary disease. COPD. 2004;1(2):215–223. doi: 10.1081/copd-120039809. [DOI] [PubMed] [Google Scholar]
- 45.Chalmers JD, Tebboth A, Gayle A, et al. Determinants of initial inhaled corticosteroid use in patients with GOLD A/B COPD: a retrospective study of UK general practice. NPJ Prim Care Respir Med. 2017;27(1):43. doi: 10.1038/s41533-017-0040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White P, Thornton H, Pinnock H, et al. Overtreatment of COPD with inhaled corticosteroids – implications for safety and costs: cross-sectional observational study. PLoS One. 2013;8(10):e75221. doi: 10.1371/journal.pone.0075221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drivenes E, Østrem A, Melbye H. Predictors of ICS/LABA prescribing in COPD patients: a study from general practice. BMC Family Pract. 2014;15(1):42. doi: 10.1186/1471-2296-15-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WHO The pursuit of responsible use of medicines: sharing and learning from country experiences. [Accessed January 30, 2019]. Available from: http://www.who.int/medicines/areas/rational_use/en/
- 49.Lang ES, Wyer PC, Haynes RB. Knowledge translation: closing the evidence-to-practice gap. Ann Emerg Med. 2007;49(3):355–363. doi: 10.1016/j.annemergmed.2006.08.022. [DOI] [PubMed] [Google Scholar]