Abstract
Introduction
The Spanish COPD guideline (GesEPOC) classifies COPD into four clinical phenotypes based on the exacerbation frequency and dominant clinical manifestations. In this study, we compared the disease-specific health-related quality of life (HRQoL) of patients with different clinical phenotypes.
Methods
This was a cross-sectional study of patients with COPD attending the respiratory medicine clinic of University of Malaya Medical Centre from 1 June 2017 to 31 May 2018. Disease-specific HRQoL was assessed by using the COPD Assessment Test (CAT) and St George’s Respiratory Questionnaire for COPD (SGRQ-c).
Results
Of 189 patients, 28.6% were of non-exacerbator phenotype (NON-AE), 18.5% were of exacerbator with emphysema phenotype (AE NON-CB), 39.7% were of exacerbator with chronic bronchitis phenotype (AE CB), and 13.2% had asthma-COPD overlap syndrome phenotype (ACOS). The total CAT and SGRQ-c scores were significantly different between the clinical phenotypes (P<0.001). Patients who were AE CB had significantly higher total CAT score than those with ACOS (P=0.033), AE NON-CB (P=0.001), and NON-AE (P<0.001). Concerning SGRQ-c, patients who were AE CB also had a significantly higher total score than those with AE NON-CB (P=0.001) and NON-AE (P<0.001). However, the total SGRQ-c score of AE CB patients was only marginally higher than those who had ACOS (P=0.187). There was a significant difference in the score of each CAT item (except CAT 7) and SGRQ-c components between clinical phenotypes, with AE CB patients recording the highest score in each of them.
Conclusion
Patients who were AE CB had significantly poorer HRQoL than other clinical phenotypes and recorded the worst score in each of the CAT items and SGRQ-c components. Therefore, AE CB patients may warrant a different treatment approach that focuses on the exacerbation and chronic bronchitis components.
Keywords: chronic obstructive pulmonary disease, chronic bronchitis, asthma, clinical phenotypes, health-related quality of life, emphysema, exacerbation
Introduction
COPD is a common, preventable, and treatable airway disease characterized by persistent respiratory symptoms and airflow limitation, due to abnormal inflammatory response of the airways to noxious particles or gases.1 It is currently the fourth leading cause of death in the world. It is also expected to become the third leading cause of death, and fifth leading cause of disease burden by 2020.2,3 The overall prevalence of COPD in the Asia-Pacific region is 6.2%.4 COPD population represents a substantial socioeconomic burden in these countries as two-fifth of them have work restriction, one-fifth has severe respiratory symptoms, and one-fifth had required hospital admission in the past 12-months because of exacerbation.4
COPD phenotype refers to a single or combination of disease attributes that describe differences between COPD patients based on clinically significant parameters, such as exacerbation, symptoms, response to treatment, rate of disease progression, and mortality.5 In 2012, the Spanish Society of Pulmonology and Thoracic Surgery published the Spanish COPD guideline (GesEPOC), which was the first clinical guideline that phenotypes COPD patients based on their exacerbation frequency and dominant clinical manifestations, such as bronchitis, emphysema, and bronchial asthma (BA).6 Even though many existing data report on the health-related quality of life (HRQoL) of COPD patients, studies that compare HRQoL among different clinical phenotypes simultaneously are lacking.
HRQoL is defined as individual satisfaction or happiness with an aspect of their life that is affected by their health either in physical, mental, emotional, or social functioning.7 Poor HRQoL is associated with increased level of dyspnea, physical impairment, mental health problem, hospital admission, and mortality.8–10 HRQoL of COPD patients can be measured by using generic questionnaires, such as Short Form Survey-36 and Euro-Qol-5D; or disease-specific questionnaires, such as COPD Assessment Test (CAT), St Georges Respiratory Questionnaire for COPD (SGRQ-c), and Clinical COPD Questionnaire (CCQ).11,12
In this study, we aimed to compare the disease-specific HRQoL of patients with COPD attending the University of Malaya Medical Centre (UMMC) based on their clinical phenotype as defined by the GesPOC guideline. UMMC is a tertiary teaching hospital, located in Kuala Lumpur, the capital of Malaysia.
Methods
Study design and patients
This is a cross-sectional study of patients with COPD attending the respiratory medicine clinic of UMMC from 1 June 2017 to 31 May 2018. All patients studied were aged ≥40 years, with the ratio of post-bronchodilator FEV1 (PB-FEV1) over post-bronchodilator FVC of <0.7. Patients with concomitant BA were included, but patients with other chronic lung diseases, such as bronchiectasis or interstitial lung disease were excluded. The estimated sample size (n) was 155 patients, calculated based on the reported COPD prevalence of 11.4%, Zα of 1.96, and precision of 0.05.13 This study aimed to determine the impact of different COPD clinical phenotypes on patients’ disease-specific HRQoL assessed by using CAT and SGRQ-c. The primary objective was to compare the total CAT and SGRQ-c score of different clinical phenotypes; while the secondary objective was to compare the score of individual items of CAT and each component of SGRQ-c of different clinical phenotypes. The hospital’s ethics committee approved this study. Written informed consent was obtained from all the study patients. This study was conducted in accordance with the Declaration of Helsinki.
Procedure
Eligible patients were consecutively identified from those attending the respiratory medicine clinic. Demographic and clinical data of these patients were obtained from face-to-face interview and case records.
Patients were categorized as never-smokers if they had smoked <100 cigarettes in their lifetime; previous smokers if they had smoked >100 cigarettes in their lifetime and no more smoking; and current smokers if they had smoked >100 cigarettes in their lifetime and still smoking every day or some of the days.14 The patients’ dyspnea was graded according to the modified Medical Research Council scale that consists of five grades: 0 – no dyspnea except on strenuous activity; 1) dyspnea when walking uphill; 2) walk slower than people with same age due to dyspnea; 3) stops for breath due to dyspnea after walking 100 m at level; and 4) too dyspnoeic to leave home or dyspnea when dressing.15
PB-FEV1 was expressed in % of predicted (PB-FEV1 %). Total exacerbation only considered the number of moderate and severe exacerbations. A moderate exacerbation was defined as an exacerbation that required outpatient treatment with corticosteroid and/or antibiotic; while severe exacerbation was defined as an exacerbation that needed hospital admission.16 For patients on COPD treatment, a new episode of exacerbation required at least 4 weeks from the resolution of a previous exacerbation; while for patients not on COPD treatment, a new episode of exacerbation required at least 6 weeks from the last exacerbation.17 Chronic bronchitis (CB) was defined as a cough with sputum for at least 3 months in a year for 2 consecutive years.18 In the presence of COPD, BA was defined as those already currently diagnosed with BA or had very definite PB-FEV1 improvement of >400 mL and 15%, or blood eosinophil of >300 cells/mm.3,19
The COPD clinical phenotypes were defined according to the GesEPOC guideline.20 Non-exacerbator phenotype (NON-AE) was defined by the presence of <2 episodes of moderate exacerbation and without severe exacerbation in the past 1 year. Exacerbator phenotype (AE) was defined by the presence of 2 or more episodes of moderate exacerbation or an episode of severe exacerbation in the past 1 year. This phenotype was further divided into AE with CB phenotype (AE CB) and AE with emphysema phenotype (AE NON-CB). The former phenotype was defined by the presence of CB; while the latter phenotype was defined by the presence of air-trapping on examination or investigations. Asthma-COPD overlap syndrome phenotype (ACOS) was defined by the presence of BA criteria as mentioned previously without the consideration of the number of exacerbation.
In assessing HRQoL, the patients were instructed to answer the CAT and SGRQ-c questionnaires independently with minimal assistance from the investigators. The patients could choose to answer the original English version, or validated Malay or Chinese version. The CAT questionnaire consists of eight items, namely, cough (CAT 1), phlegm (CAT 2), chest tightness (CAT 3), breathlessness (CAT 4), activity limitation (CAT 5), confidence in leaving home (CAT 6), sleep (CAT 7), and energy (CAT 8).21 The score for each of these items ranges from 0 to 5 with the total score ranging from 0 to 40. The total CAT score in a healthy subject is ≤6, with a higher score reflecting a greater or worse impact of COPD on HRQoL.22 The SGRQ-c questionnaire consists of 14 questions, with questions 1–7 interrogating the symptom component, questions 9 and 12 concerning activity component, and questions 8, 10, 11, 13, and 14 relating to impact component.23 The score of each component and the total score range from 0% to 100%, with a higher score reflecting worse HRQoL. In healthy subjects, the score of the total is ≤6%, symptom component is ≤12%, activity component is ≤9%, and impact component is not >2%.24 CAT is a quick assessment tool, while SGRQ-c is more comprehensive but time-consuming.
Statistical analyses
In this study, categorical variables were expressed as percentages; while continuous variables were expressed as the mean ± SD, median or range depending on the normality of the distribution of the variable. For categorical variables, the difference between clinical phenotypes was compared by using the chi-squared test. An adjusted standardized residual of >2 was considered as significant in the post hoc analysis. For continuous variables, the difference between clinical phenotypes was compared by using one-way ANOVA test or Kruskal–Wallis H test, as applicable. The post hoc analysis for the former was the Tukey’s test, while for the latter, was the Dunn’s procedure with a Bonferroni adjustment. A P-value of <0.05 was considered statistically significant. Statistical analyses were performed using the software package, SPSS for Windows version 23.0 (SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinical characteristics
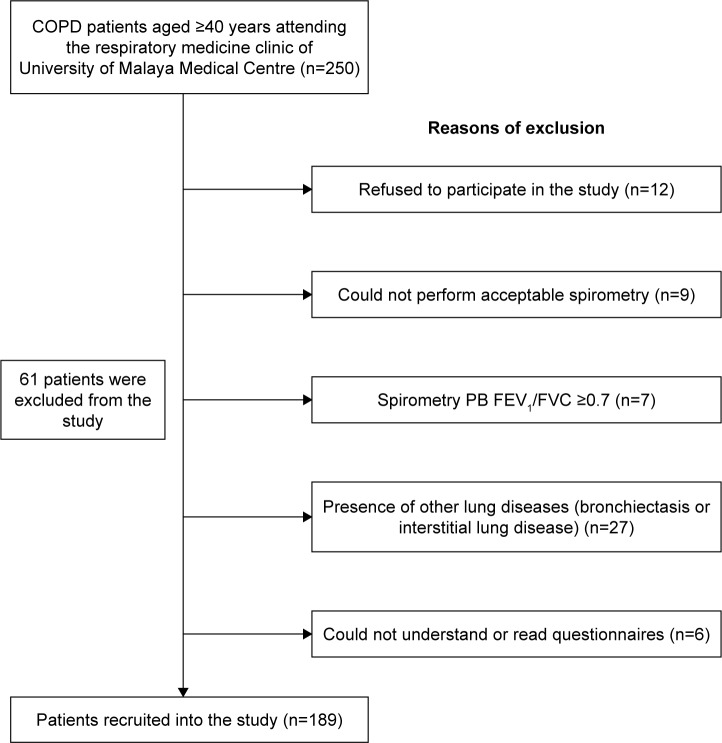
A total of 189 patients who met the study criteria were included in the study (Figure 1). Patients’ demographic and clinical characteristics are shown in Table 1. A total of 28.6% of patients were of NON-AE, 18.5% were of AE NON-CB, 39.7% were of AE CB, and 13.2% had ACOS. Among patients of NON-AE, 46.3% were of CB and 53.7% were of NON-CB. Regardless of the phenotypes, male patients were predominant and the majority of the patients were ex-smokers. The age, gender proportion, ethnicity distribution, smoking status, and smoking quantity difference among the patients with the different clinical phenotypes were not significant.
Figure 1.
Algorithm of patients’ recruitment into the study.
Abbreviation: PB-FEV1/FVC, ratio of post-bronchodilator force expiratory volume in 1 second/forced vital capacity.
Table 1.
Demographic and clinical characteristics of COPD patients according to their clinical phenotypes
| Characteristics | COPD phenotype (n, %)
|
||||
|---|---|---|---|---|---|
| NON-AE 54 (28.6) | AE NON-CB 35 (18.5) | AE CB 75 (39.7) | ACOS 25 (13.2) | P-value | |
|
| |||||
| Age (years, mean ± SD) | 74.1±8.1 | 72.7±8.7 | 70.7±9.2 | 70.0±13.1 | 0.151 |
|
| |||||
| Gender (n, %) | |||||
| Male | 50 (92.6) | 32 (91.4) | 70 (93.3) | 23 (92.0) | 0.986 |
| Female | 4 (7.4) | 3 (8.6) | 5 (6.7) | 2 (8.0) | |
|
| |||||
| Ethnicity (n, %) | |||||
| Malay | 19 (35.2) | 12 (34.3) | 32 (42.7) | 10 (40.0) | 0.734 |
| Chinese | 27 (50.0) | 18 (51.4) | 30 (40.0) | 9 (36.0) | |
| Indian | 8 (14.8) | 5 (14.3) | 13 (17.3) | 6 (24.0) | |
|
| |||||
| Smoking status (n, %) | |||||
| Never smoker | 2 (3.7) | 3 (8.6) | 4 (5.3) | 3 (12.0) | 0.348 |
| Previous | 40 (74.1) | 19 (54.3) | 55 (73.3) | 15 (60.0) | 0.601 |
| Current | 12 (22.2) | 13 (37.1) | 16 (21.3) | 7 (28.0) | |
| Quantity of cigarettes smoked (pack-years, mean) | 42.3±30.4 | 44.6±30.1 | 43.9±27.9 | 39.0±35.4 | |
|
| |||||
| Dyspnea (mean ± SD; 95% CI) | 1.8±1.3; 1.5–2.2 | 2.0±1.2; 1.6–2.4 | 2.7±1.1; 2.4–2.9 | 2.2±1.4; 1.6–2.8 | 0.002 |
|
| |||||
| PB-FEV1 (%, mean ± SD; 95% CI) | 57.8±20.2; 52.3–63.4 | 51.1±23.1; 43.2–59.0 | 48.2±23.6; 42.8–53.7 | 52.8±16.2; 46.1–59.5 | 0.025 |
|
| |||||
| Exacerbations (mean ± SD; 95% CI) | |||||
| Total | 0.5±1.8; 0.1–1.0 | 1.9±1.9; 1.3–2.6 | 4.8±6.8; 3.2–6.4 | 3.4±5.2; 1.3–5.6 | <0.001 |
| Moderate | 0.4±1.3; 0.1–0.8 | 0.7±1.0; 0.4–1.1 | 2.9±5.4; 1.7–4.2 | 1.9±3.2; 0.6–3.3 | <0.001 |
| Severe | 0 | 1.3±1.2; 0.9–1.7 | 1.9±2.1; 1.4–2.4 | 1.5±2.5; 0.5–2.6 | <0.001 |
Abbreviations: ACOS, asthma-COPD overlap syndrome phenotype; AE CB, exacerbator with chronic bronchitis phenotype; AE NON-CB, exacerbator with emphysema phenotype; NON-AE, non-exacerbator phenotype; PB-FEV1, post-bronchodilator forced expiratory volume in 1 second.
The dyspnea symptom (P=0.002), PB-FEV1 % (P=0.025), and total exacerbation (P<0.001) were significantly different between clinical phenotypes. Patients who were of AE CB had significantly worse dyspnea symptom (P=0.002), lower PB-FEV1 % (P=0.018), and higher total number of exacerbations (P<0.01) than patients of NON-AE. The total number of exacerbations was also significantly higher in patients who had ACOS (P<0.001) and AE NON-CB (P<0.001) compared with patients of NON-AE. Despite the total number of exacerbations for patients with ACOS was also higher than that of AE NON-CB (3.4±5.2 vs 1.9±1.9), this difference was not statistically significant (P=0.999).
HRQoL according to clinical phenotypes
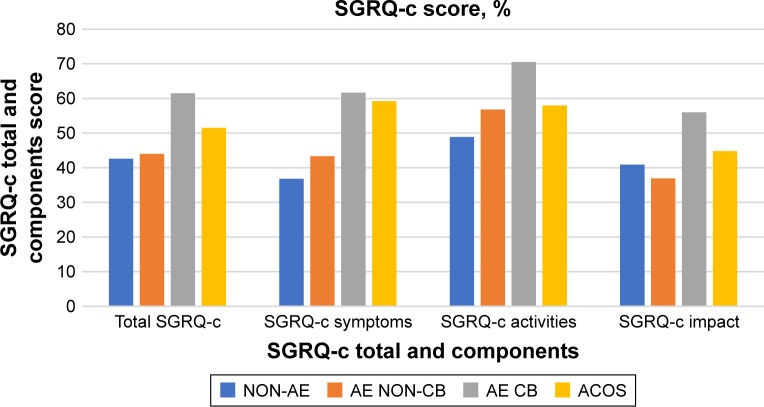
The total CAT and SGRQ-c scores were significantly different between clinical phenotypes (all P-values <0.001) (Table 2). Patients who were of AE CB had significantly higher total CAT score than those with ACOS (23.6±8.0 vs 18.3±10.1, P=0.033), AE NON-CB (23.6±8.0 vs 15.8±8.0, P=0.001), and NON-AE (23.6±8.0 vs 18.0±8.0, P<0.001). In terms of SGRQ-c, patients who were of AE CB also had significant higher total score than those with AE NON-CB (61.5±20.9 vs 44.0±21.4, P=0.001) and NON-AE (61.5±20.9 vs 42.6±20.1, P<0.001). However, the total SGRQ-c score of patients with AE CB was only marginally higher than those who had ACOS (61.5±20.9 vs 51.5±25.9, P=0.187). Although the total CAT score increased from patients who were of AE NON-CB, to NON-AE, and ACOS; while the total SGRQ-c score increased from patients who were of NON-AE, to AE NON-CB, and ACOS, the differences between these clinical phenotypes were not statistically significant.
Table 2.
CAT and SGRQ-c scores of COPD patients according to their clinical phenotypes
| Parameters | COPD phenotype
|
P-value | |||
|---|---|---|---|---|---|
| NON-AE | AE NON-CB | AE CB | ACOS | ||
|
| |||||
| CAT score (mean ± SD; 95% CI) | |||||
| Total | 18.0 ± 8.0; 15.8-20.2 | 15.8 ± 8.0; 13.1-18.6 | 23.6 ± 8.0; 21.8-25.4 | 18.3 ± 10.1; 14.0-22.7 | <0.001 |
| Cough (CAT 1) | 2.5 ± 1.2; 2.1-2.8 | 2.1 ± 1.3; 1.6-2.5 | 3.2 ± 1.2; 2.9-3.5 | 2.6 ± 1.6; 2.0-3.3 | <0.001 |
| Phlegm (cat 2) | 2.6 ± 1.8; 2.1-3.0 | 1.6 ± 1.6; 1.0-2.1 | 3.3 ± 1.7; 2.9-3.7 | 2.4 ± 1.9; 1.6-3.2 | <0.001 |
| Chest tightness (CAT 3) | 1.4 ± 1.7; 0.9-1.8 | 1.7 ± 1.5; 1.2-2.2 | 2.6 ± 1.6; 2.2-2.9 | 2.2 ± 1.7; 1.5-2.9 | <0.001 |
| Breathlessness (CAT 4) | 3.4 ± 1.7; 3.0-3.9 | 3.2 ± 1.5; 2.7-3.7 | 4.0 ± 1.3; 3.7-4.2 | 3.0 ± 1.7; 2.3-3.7 | 0.020 |
| Activity limitation (CAT 5) | 2.5 ± 1.8; 2.0-3.0 | 2.0 ± 1.7; 1.4-2.6 | 3.0 ± 1.6; 2.6-3.4 | 2.3 ± 1.9; 1.5-3.1 | 0.038 |
| Confidence in leaving home (CAT 6) | 1.7 ± 1.9; 1.2-2.2 | 1.6 ± 1.8; 1.0-2.2 | 2.4 ± 1.6; 2.1-2.8 | 1.9 ± 1.9; 1.1-2.7 | 0.022 |
| Sleep (CAT 7) | 1.6 ± 1.6; 1.2-2.1 | 1.6 ± 1.6; 1.0-2.1 | 2.2 ± 1.6; 1.8-2.5 | 1.8 ± 1.8; 1.1-2.5 | 0.127 |
| Energy (CAT 8) | 2.4 ± 1.1; 2.1-2.7 | 2.2 ± 1.5; 1.7-2.7 | 3.0 ± 1.4; 2.7-3.4 | 2.2 ± 1.6; 1.5-2.8 | 0.004 |
|
| |||||
| SGRQ-c score, % (mean ± SD; 95% CI) | |||||
| Total | 42.6 ± 20.1; 37.1-48.1 | 44.0 ± 21.4; 36.6-51.4 | 61.5 ± 20.9; 56.7-66.3 | 51.5 ± 25.9; 40.8-62.2 | <0.001 |
| Symptoms | 36.8 ± 19.9; 31.4-42.3 | 43.3 ± 23.6; 35.3-51.5 | 61.7 ± 20.4; 57.0-66.4 | 59.2 ± 25.1; 48.9-69.6 | <0.001 |
| Activities | 48.9 ± 32.5; 40.1-57.8 | 56.8 ± 27.8; 47.3-66.4 | 70.5 ± 25.1; 64.7-76.2 | 58.0 ± 34.6; 43.8-72.3 | 0.001 |
| Impact | 40.9 ± 20.1; 35.4-46.3 | 36.9 ± 24.8; 28.4-45.5 | 56.0 ± 27.2; 49.8-62.3 | 44.8 ± 32.1; 31.2-58.1 | 0.001 |
Abbreviations: ACOS, asthma-COPD overlap syndrome phenotype; AE CB, exacerbator with chronic bronchitis phenotype; AE NON-CB, exacerbator with emphysema phenotype; CAT, COPD Assessment Test; NON-AE, non-exacerbator phenotype; SGRQ-c, St Georges Respiratory Questionnaire for COPD.
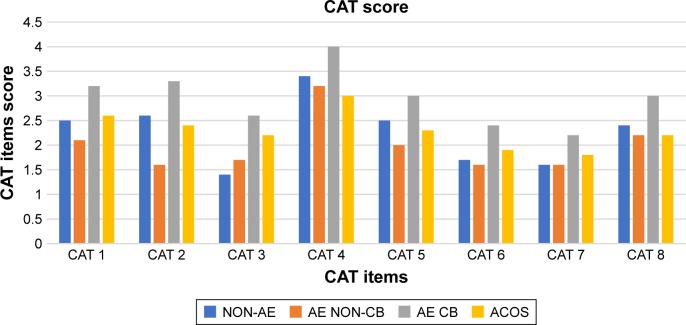
There was a significant difference in the score of each CAT items (except CAT 7) and SGRQ-c components between clinical phenotypes (Table 2). Patients with AE CB had the highest score in each of the CAT items and SGRQ-c components (Figures 2 and 3). Patients who had ACOS had the second highest score in each of the SGRQ-c components and CAT 1, CAT 3, CAT 6, and CAT 7. CAT 4 had the highest score regardless of the patients’ clinical phenotype.
Figure 2.
Score of CAT items according to COPD clinical phenotypes.
Abbreviations: ACOS, asthma-COPD overlap syndrome phenotype; AE CB, exacerbator with chronic bronchitis phenotype; AE NON-CB, exacerbator with emphysema phenotype; CAT, COPD Assessment Test; NON-AE, non-exacerbator phenotype.
Figure 3.
Total and component scores of SGRQ-c according to COPD clinical phenotypes.
Abbreviations: ACOS, asthma-COPD overlap syndrome phenotype; AE CB, exacerbator with chronic bronchitis phenotype; AE NON-CB, exacerbator with emphysema phenotype; NON-AE, non-exacerbator phenotype; SGRQ-c, St Georges Respiratory Questionnaire for COPD.
Discussion
Regardless of the clinical phenotypes, the HRQoL of our patients was markedly impaired compared with healthy individuals. AE CB was the only clinical phenotype associated with significantly poorer HRQoL. The poor HRQoL was not solely attributable to cough and phlegm symptoms (CAT 1 and 2), which could be confounded by the definition of CB. The sub-analysis of CAT items demonstrated that AE CB was associated with more chest tightness, breathlessness, activity limitation, fear of leaving home, sleep disturbance, and lack of energy (CAT 3–8). Moreover, sub-analysis of SGRQ-c components also demonstrated that AE CB was associated with more respiratory symptoms and disturbance to daily physical activities as well as psychosocial function. Patients with ACOS appeared to have poorer HRQoL than those with AE NON-CB or NON-AE although the difference was not statistically significant, which could be due to the smaller number of subjects with ACOS. ACOS was associated with more core symptoms of BA, such as a cough, chest tightness, and sleep disturbance, while daily activities and energy were less affected. Surprisingly, the HRQoL of patients with AE NON-CB was not markedly worse than those with NON-AE. Such a finding suggests that higher exacerbation frequency alone without an unfavorable clinical manifestations might not be sufficient to have a detrimental effect on patients’ HRQoL. The clinical manifestation that was associated with the poorest HRQoL was CB, followed by BA and NON-CB.
From this study, we can conclude that HRQoL is significantly worse in patients with AE CB and numerically worse in patients with ACOS compared with patients with AE NON-CB, and least affected in patients with NON-AE. These findings agree with the result of earlier studies. In the COPD History Assessment in Spain study, Cosio et al reported a significant higher CAT score in patients with AE CB (P<0.001), and the CAT score was numerically higher in those with ACOS followed by those with AE NON-CB and NON-AE.25 In another multicenter observational study in Spain, Miravitlles et al also reported a significant higher CAT score in patients with AE CB (P<0.001).26 Those with ACOS and AE NON-CB had an intermediate score, while patients of NON-AE had the lowest score. A study by Corlateanu et al, however, reported that the CAT, SRGQ, and CCQ scores were significantly higher in frequent exacerbators; and those with AE NON-CB recording the highest values in each of the parameters.27 In short, the present study and available data consistently highlight that patients with NON-AE had the best HRQoL, while patients with frequent exacerbations, particularly of the AE CB phenotype had the worst HRQoL. Our study further complements these existing works by demonstrating that AE CB phenotype is indeed associated with the worst score in each of the CAT items and SGRQ-c components.
In this study, the higher exacerbation frequency and active inflammation of CB led to poorer HRQoL in patients with the AE CB phenotype. Frequent exacerbation has been known to have a profound impact on HRQoL. Seemungal et al reported that patients with three or more exacerbations per year had significant worse SGRQ score (P<0.001), while Mackay et al reported that patients with two or more exacerbations per year had significantly worse CAT score (P=0.025).28,29 Similarly, CB was reported to be independently associated with poorer HRQoL compared with without CB in several recent studies. In the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints and Genetic Epidemiology of COPD (COPDGene) studies, Agusti et al and Kim et al reported a significantly worse SGRQ score in CB patients (all P<0.001); while in the Korea COPD Subgroup Study Team, Choi et al reported significantly worse CAT (P<0.001) and SGRQ (P<0.001) scores in the CB group.30–32 de Oca et al also reported significantly poorer respiratory symptoms, including dyspnea (P=0.002) and wheezing (P=0.003) as well as general HRQoL (P=0.049) measured by Short Form-12 among CB patients of the COPD in five Latin America Cities study.33 In comparison with NON-CB, the airway inflammation of COPD that is mediated by neutrophil, macrophage, and CD8+ lymphocyte is more aggressive in CB leading to more mucus production and small airway obstruction, which explains the worse HRQoL.34 In the COPDGene and Epidemiologic Study of COPD in Spain study, Hardin et al and Miravitlles et al, reported significantly worse SGRQ in patients of ACOS (P=0.009 and P<0.001, respectively).35,36 The number of subjects of ACOS in both of these studies were much more than ours (n=119 and n=67) and, therefore, both these studies were able to show significantly worse HRQoL in patients of ACOS. In ACOS, the dual inflammatory processes of COPD and BA mediated by eosinophils, mast cells, and CD4+ lymphocytes led to poorer HRQoL.37
The present study findings support the importance of phenotyping COPD based on exacerbation frequency and dominant clinical manifestations according to GesEPOC guideline to allow a more personalized treatment. Patients with AE CB may warrant a different treatment approach that focuses on the exacerbation and CB components because they have the poorest HRQoL. Treatment with azithromycin to reduce exacerbation frequency and roflumilast to control CB symptoms should be added early in patients with this phenotype, especially when long-acting beta 2-agonist and long-acting muscarinic antagonist fail to control the COPD symptoms.20 In order to preserve the HRQoL in ACOS, inhaled corticosteroid that controls the BA component should be initiated upon diagnosis. Besides, other non-pharmacological therapy, such as smoking cessation, pulmonary rehabilitation, and nutritional support should be offered early to improve respiratory symptoms and HRQoL.
To our knowledge, this is the first study in the Asia– Pacific region that compares the HRQoL of all the COPD clinical phenotypes simultaneously. Different disease-specific HRQoL assessment tools were utilized to obtain more representative results. The impact of COPD clinical phenotypes on each of the individual CAT items and SGRQ-c components was also analyzed.
This study has several limitations. First, it was performed in a single center, thus limiting the generalizability of the results. Second, there is the possibility of misclassification of the clinical phenotypes because the exacerbation frequency was subjected to the recall error of the patients. We tried to minimize this error by double checking the exacerbation history from patients’ available medical records. Finally, the number of ACOS patients was disproportionately small for statistically significant differences to be achieved when comparison of HRQoL was made with the other phenotypes.
Conclusion
This study concluded that patients who were AE CB had significant poorer HRQoL than other clinical phenotypes. It further complements existing studies by demonstrating that patients of AE CB also had the worst score in each of the CAT items and SGRQ-c components. Therefore, patients of AE CB may warrant a different treatment approach that focuses on the exacerbation and CB components. Phenotyping COPD patients based on exacerbation frequency and dominant clinical manifestations are essential to achieving a more directed treatment.
Ethics approval and informed consent
The UMMC Hospital’s ethics committee approved this study (reference MECID.No 2017814-5496). Informed consent was obtained from all the study patients.
Data availability
The study data will be shared on request due to patients’ confidentiality and can be obtained from the corresponding author.
Acknowledgments
We want to express our gratitude to all the patients who participated in the study. This study was fully supported by the Research Acculturation Grant Scheme of the Ministry of Education, Malaysia (RAGS/1/2015/SKK02/UNIMAS/03/2 or FA052000-0708-0029).
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global initiative for obstructive lung disease report. 2018. [Accessed October 18, 2018]. Available from: https://goldcopd.org/wp-content/uploads/2018/02/WMS-GOLD-2018-Feb-Final-to-print-v2.pdf.
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: gold executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 4.Lim S, Lam DC, Muttalif AR, et al. Impact of chronic obstructive pulmonary disease (COPD) in the Asia-Pacific region: the EPIC Asia population-based survey. Asia Pac Fam Med. 2015;14(1):4. doi: 10.1186/s12930-015-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish Society of Pulmonology and Thoracic Surgery Spanish COPD guidelines (GesEPOC): pharmacological treatment of stable COPD. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2012;48(7):247–257. doi: 10.1016/j.arbres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Ware JE, Jr, Sherbourne CD. The mos 36-item short-form health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 8.Hu J, Meek P. Health-related quality of life in individuals with chronic obstructive pulmonary disease. Heart Lung. 2005;34(6):415–422. doi: 10.1016/j.hrtlng.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Osman IM, Godden DJ, Friend JA, Legge JS, Douglas JG. Quality of life and hospital re-admission in patients with chronic obstructive pulmonary disease. Thorax. 1997;52(1):67–71. doi: 10.1136/thx.52.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domingo-Salvany A, Lamarca R, Ferrer M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(5):680–685. doi: 10.1164/rccm.2112043. [DOI] [PubMed] [Google Scholar]
- 11.Cave AJ, Atkinson L, Tsiligianni IG, Kaplan AG. Assessment of COPD wellness tools for use in primary care: an IPCRG initiative. Int J Chron Obstruct Pulmon Dis. 2012;7:447–456. doi: 10.2147/COPD.S29868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickard AS, Yang Y, Lee TA. Comparison of health-related quality of life measures in chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2011;9(1):26. doi: 10.1186/1477-7525-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adeloye D, Chua S, Lee C, et al. Global Health Epidemiology Reference Group (GHERG) Global and regional estimates of COPD prevalence: systematic review and meta–analysis. J Glob Health. 2015;5(2):020415. doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults – United States, 1992, and changes in the definition of current cigarette smoking. MMWR Morb Mortal Wkly Rep. 1994;43(19):342–346. [PubMed] [Google Scholar]
- 15.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(4):1185–1189. doi: 10.1164/ajrccm.158.4.9802091. [DOI] [PubMed] [Google Scholar]
- 16.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 17.Soler-Cataluña JJ, Martínez García MA, Catalán P. The frequent exacerbator. A new phenotype in COPD? Hot Topics Respir Med. 2011;19:7–12. [Google Scholar]
- 18.Burgel PR. Chronic cough and sputum production: a clinical COPD phenotype? Eur Respir J. 2012;40(1):4–6. doi: 10.1183/09031936.00022412. [DOI] [PubMed] [Google Scholar]
- 19.Miravitlles M, Alvarez-Gutierrez FJ, Calle M, et al. Algorithm for identification of asthma-COPD overlap: consensus between the Spanish COPD and asthma guidelines. Eur Respir J. 2017;49(5):1700068. doi: 10.1183/13993003.00068-2017. [DOI] [PubMed] [Google Scholar]
- 20.Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53(6):324–335. doi: 10.1016/j.arbres.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 22.Pinto LM, Gupta N, Tan W, et al. Derivation of normative data for the COPD assessment test (cat) Respir Res. 2014;15(1):68. doi: 10.1186/1465-9921-15-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George respiratory questionnaire. Chest. 2007;132(2):456–463. doi: 10.1378/chest.06-0702. [DOI] [PubMed] [Google Scholar]
- 24.Ferrer M, Villasante C, Alonso J, et al. Interpretation of quality of life scores from the St George’s respiratory questionnaire. Eur Respir J. 2002;19(3):405–413. doi: 10.1183/09031936.02.00213202. [DOI] [PubMed] [Google Scholar]
- 25.Cosio BG, Soriano JB, López-Campos JL, et al. CHAIN study Distribution and outcomes of a phenotype-based approach to guide COPD management: results from the chain cohort. PLoS One. 2016;11(9):e0160770. doi: 10.1371/journal.pone.0160770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miravitlles M, Barrecheguren M, Román-Rodríguez M. Frequency and characteristics of different clinical phenotypes of chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2015;19(8):992–998. doi: 10.5588/ijtld.15.0021. [DOI] [PubMed] [Google Scholar]
- 27.Corlateanu A, Botnaru V, Rusu D, Eugenia S. Assessment of health-related quality of life in different phenotypes of COPD. Curr Respir Med Rev. 2017;13(2):105–109. [Google Scholar]
- 28.Seemungal TAR, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 29.Mackay AJ, Donaldson GC, Patel ARC, Jones PW, Hurst JR, Wedzicha JA. Usefulness of the chronic obstructive pulmonary disease assessment test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med. 2012;185(11):1218–1224. doi: 10.1164/rccm.201110-1843OC. [DOI] [PubMed] [Google Scholar]
- 30.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the eclipse cohort. Respir Res. 2010;11(1):122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim V, Davey A, Comellas AP, et al. COPDGene® Investigators Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res. 2014;15(1):52. doi: 10.1186/1465-9921-15-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JY, Yoon HK, Park SJ, et al. Chronic bronchitis is an independently associated factor for more symptom and high-risk groups. Int J Chron Obstruct Pulmon Dis. 2016;11:1335–1341. doi: 10.2147/COPD.S105516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Oca MM, Halbert RJ, Lopez MV, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012;40(1):28–36. doi: 10.1183/09031936.00141611. [DOI] [PubMed] [Google Scholar]
- 34.King PT. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Transl Med. 2015;4(1):68. doi: 10.1186/s40169-015-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardin M, Silverman EK, Barr RG, et al. COPDGene Investigators The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12(1):127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053–1060. doi: 10.1016/j.rmed.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54(8):577–589. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data will be shared on request due to patients’ confidentiality and can be obtained from the corresponding author.