Synopsis
Androgen deprivation therapy (ADT) has been conventional treatment of newly diagnosed metastatic prostate cancer for more than 70 years. However, all patients eventually become castration-resistant and a significant proportion of life span is spent in the castration-resistant state. Prospective randomized control trials have incorporated early chemotherapy along with ADT based on the hypothesis that a significant level of resistance to ADT already exists in newly diagnosed metastatic prostate cancer and ADT exhibits synergistic antitumor activity with taxanes. In this chapter we discuss the changing landscape of management of newly diagnosed metastatic prostate cancer patients based on recently published landmark randomized trials.
Keywords: Prostate cancer (PCa), Hormone sensitive prostate cancer (HSPC), Androgen deprivation therapy (ADT), Docetaxel, Androgen axis inhibitors
Introduction
Metastatic prostate cancer (mPCa) carries a dismal five-year survival rate of 29.3 %1. This is in stark contrast to the nearly 100 % five-year survival for low volume organ-confined disease. The conventional treatment of PCa has been Androgen Deprivation Therapy (ADT) ever since the landmark discovery of androgen ablation for metastatic PCa by Charles Huggins and Clarence Hodges in 19412. In a retrospective review from the National Cancer Center, the median time for progression to metastatic castration-resistant PCa (mCRPC) was found to be 13.1 months and 19.3 months in patients with and without radiological evidence of metastasis at initiation of ADT, respectively.3 A systematic review of 12 studies including 71,179 patients found that 10–20% of mPCa patients develop mCRPC within 5 years of follow-up.4
The survival of PCa in metastatic patients is being more clearly defined and has changed in the modern era. James et al5, reported a median failure-free survival (FFS) of 11 months (2-yr FFS of 29%) for newly diagnosed metastatic PCa patients enrolled in the recent Systemic Therapy in Advancing or Metastatic PCa: Evaluation of Drug Efficacy (STAMPEDE) trial. The median overall survival (OS) was 42 months (2-yr OS was 72%) in this same cohort. The finding that median OS is almost four-times the median FFS demonstrates that the mCRPC now makes up the majority of the survival time rather than being a short terminal phase with limited treatment options. Furthermore, in the same study, it was observed that the median time to the next therapy was 20 months for the control arm and 15.4 months for the experimental arm, emphasizing that important time is lost in waiting for CRPC transition. Median OS times in the SWOG trials cited by Tangen et al6 ranged from 32 months in the oldest trial to 49 months in the more recent one, demonstrating improved survival in more modern studies similar to the results reported in STAMPEDE.7
Metastatic Hormone Sensitive PCa (mHSPC) is a heterogeneous disease that consists of both Androgen Receptor (AR) positive and AR negative cells. ADT eventually selects a clonal population that is capable of surviving without AR mediated signaling.8 The mechanisms of overcoming androgen loss during CRPC transition include autocrine androgen production, amplification of androgen receptor (AR) protein and mechanisms that bypass the AR, such as coactivators and trans activators. Some of the most important of these biologically heterogeneous mechanisms involve cancer stem cells, receptor tyrosine kinases and neuroendocrine differentiation (NE). Cells that have a ‘stem-like’ phenotype are potentially resistant to ADT and can differentiate into androgen independent cells.9,10 The activation of the PI3/Akt tyrosine kinase signaling by deletion, mutation and methylation silencing of PTEN tumor suppressor gene function is thought to be caused by selective pressure caused by ADT.11–13 NE differentiation also occurs in a adenocarcinoma PSA-secreting environment under the selection pressure of ADT. These cells effectively progress to CRPC through the production of neurosecretory peptides in potentially up to 25% of advanced cancers.11,12 AR gene amplification is another important mechanism by which PCa cells acquire resistance to conventional ADT and these cells are a target for second line hormonal therapy14,15 Thus, CRPC is now known to be the consequence of selective pressure exerted by ADT on mHSPC, which induces clonal selection and the growth of androgen independent clones16–21
Docetaxel was initially approved for the treatment of metastatic CRPC in 2004 based on 2 separate studies that for the first time confirmed a survival benefit in that setting.22, 23 Despite the small increase in overall survival (2.4 months in TAX 327 and 1.9 months in SWOG 99-16, respectively), it was approved for the treatment and paved the way to subsequent studies that saw an increasing number of newer agents for CRPC management.24 Subsequently, combining docetaxel with ADT in the hormone-sensitive setting emerged as an appealing strategy in order to delay development of CRPC and prolong survival. The rationale behind this approach was some degree of resistance to ADT is already present at the time of diagnosis, a phenomenon that is thought to be proportional to the tumor burden. Early chemotherapy could potentially eradicate the hormone-resistant subpopulation, thus prolonging the time to CRPC transition. In support of this hypothesis, simultaneous castration and treatment with paclitaxel in mouse models was found to be superior to sequential administration.25,25 Engrafted mice receiving chemohormonal therapy showed delayed median time to progression compared to those treated with sequential castration and chemotherapy. The explanation for the synergistic activity of taxanes and ADT was provided by Zhu et al in 2010,26 when they showed that taxanes blocked the microtubule mediated AR nuclear localization by androgens, thus effectively blocking AR-signaling pathways. In addition to the potential synergistic effect of taxanes to ADT, a proportion of patients might be too frail at the time of development of CRPC and thus might miss the opportunity to receive treatment with a potent chemotherapeutic agent.27
DISCUSSION
To date, three large-scale Phase III studies have examined the role of docetaxel in HSPC:
GETUG-AFU 15 (Groupe d’Etude des Tumeurs Uro-Genital and Association Française d’Urologie)
CHAARTED (Chemo-Hormonal therapy versus Androgen Ablation Randomized Trial for Extensive Disease in PCa)
STAMPEDE (Systemic Therapy in Advancing or Metastatic PCa: Evaluation of Drug Efficacy)
These studies have driven the recent change in paradigm regarding the early treatment with docetaxel and ADT in metastatic HSPC.
GETUG-AFU 15
The GETUG-AFU 15 was the first phase III study published that compared ADT alone versus ADT plus docetaxel.28 Three hundred eighty-five men with mHSPC were enrolled in 29 centers in France and 1 in Belgium. Eligible patients were required to have biopsy proven PCa with radiological evidence of metastasis, Karnofsky score above 70, minimum life expectancy of 3 months and adequate hepatic and renal function.
Treatment plan
Patients were 1:1 randomized to ADT vs. ADT in combination with docetaxel. Treatment with ADT included orchiectomy or Luteinizing Hormone Releasing Hormone (LHRH) agonists, alone or in combination with steroidal antiandrogens. In addition, patients in the chemohormonal therapy arm received docetaxel 75mg/m2 every 3 weeks for a maximum of 9 cycles. The primary end point of the study was OS, with clinical progression-free survival (PFS) and biochemical PFS being secondary endpoints. Patients who had started ADT within 2 months of the study enrollment were included and efficacy analysis.
Evaluation
The initial assessment included clinical history, physical examination, weight, and Karnofsky performance status. Imaging (CT scan and bone scan as indicated) electrocardiography, and blood investigation including serum PSA were done within 30 days of treatment initiation. Patients in the chemohormonal arm underwent clinical and laboratory evaluation every 3 weeks while receiving chemotherapy and every 3 months thereafter, whereas patients in the ADT alone arm were evaluated every 3 months. Imaging studies were repeated every 3 months. For patients remaining on study for more than 42 months, clinical, laboratory and radiographic evaluations were spaced out to every 6 months. Biochemical PFS was defined as PSA decrease of at least 50% and an increase of at least 50% above the nadir, with an absolute increase of 5 ng/ml. For patients without PSA nadir <50%, progression was defined as a PSA increase of at least 25% above the nadir and 5ng/ml both confirmed by a confirmatory testing. Clinical progression was defined as progression of pre-existing lesions with Response Evaluation Criteria in Solid Tumors (RECIST) or appearance of new bone lesions, whichever occurred first.
Results
Between October 2004 and December 2008, 192 patients were randomly assigned to receive ADT plus docetaxel and 193 to receive ADT alone. A total of 71% of the patients who participated had metastatic disease at diagnosis. The median number of cycles of treatment with docetaxel was 8, with less than half of all patients receiving all 9 cycles. Median follow-up was 50 months (IQR 39–63). Median overall survival was not significantly different between the two arms (58·9 months for the chemohormonal arm vs. 54·2 months for the ADT alone arm [hazard ratio (HR) 1·01, 95% CI 0·75–1·36]). However, a significantly longer biochemical PFS (22.9 vs. 12.9 months; HR, 0.72 [95% CI, 0.57–0.91]; P = 0.005) and clinical PFS (23.5 vs. 15.4 months; HR, 0.75 [95% CI, 0.59–0.94]; P = 0.015), respectively was observed in the patients receiving chemohormonal therapy.
In a subsequent report of GETUG-AFU 15 with a longer median follow-up of 83.9 months, there was a numerically improved OS for the chemohormonal therapy group, but this did not reach statistical significance (62.1 months vs. 48.6 months; HR, 0.88 [95% CI, 0.68–1.14]; P = 0.3). Of note, the initial design of that study did not include tumor volume as a stratification factor. A subsequent retrospective analysis based on tumor volume was conducted and again failed to reach statistical significance for overall survival for high-volume patients on Docetaxel + ADT compared to high volume disease patients on ADT alone (39.8 months vs. 35.1 months; HR, 0.78 [95% CI, 0.56–1.09]; P = 0.14). The investigators concluded that the addition of chemotherapy to ADT does not improve overall survival compared with ADT alone, although PFS (clinical and biochemical) and PSA control were improved.
E3805 The CHAARTED trial
This landmark trial was the first one to show that the addition of 6 cycles of docetaxel 75 mg/m2 every 3 weeks to standard ADT significantly improved outcomes in men with metastatic HSPC.29 The study was designed in 2005 by the Eastern Cooperative Oncology Group (now ECOG-ACRIN) and enrolled patients through ECOG, the Southwest Oncology Group (SWOG), the Alliance for Clinical Trials in Oncology, and NRG Oncology (a merged group that includes the National Surgical Adjuvant Breast and Bowel Project, the Radiation Therapy Oncology Group, and the Gynecologic Oncology Group) and the Clinical Trials Support Unit. The primary objective of the study was to determine whether the addition of docetaxel to ADT could improve the overall survival in patients with newly diagnosed mHSPC. Eligible patients were required to have either a pathological diagnosis of metastatic PCa or an elevated serum prostate specific antigen (PSA) with clinical features consistent with metastatic disease and an ECOG performance status between 0 and 2. Furthermore, patients that had received ADT in the adjuvant setting were allowed to enroll as long as the duration of treatment was less than 24 months and it had stopped at least 12 months prior to the development of metastatic disease. Patients that had already started ADT had a window period of 120 days prior to randomization.
Treatment plan
Patients were randomly assigned to 2 arms, ADT alone vs ADT plus docetaxel. Docetaxel dose was 75 mg per square meter of body-surface area given every 3 weeks for up to six cycles. Patients developing significant toxicities from docetaxel were allowed two dose reductions to 65 mg and 55 mg per square meter respectively, as clinically indicated. Patient stratification was done according to age (<70 years vs. ≥70 years), ECOG performance status (0 or 1 vs 2), planned use of combined androgen blockade for more than 30 days, prior usage of agents approved for prevention of skeletal-related events in castration resistant disease (Zoledronic acid or denosumab), duration of prior ADT (<12 months vs. ≥12 months) and tumor volume (high vs. low). High volume disease was defined as the presence of visceral metastases or ≥4 bone lesions with at least 1 lesion beyond the vertebral bodies and pelvis. This study design was unique among the contemporary trials in stratifying patients based on tumor volume.
Evaluation
Patients in ADT alone arm were followed every 3 months whereas patients on ADT plus docetaxel arm were followed every 3 weeks for the duration of chemotherapy and every 3 months thereafter. PSA levels were measured at each scheduled visit. Imaging in the form of computed tomography [CT] of the abdomen and pelvis, technetium-99m bone scan and radiography or CT of the chest, as clinically indicated were performed at baseline, as clinically indicated as well as at the time of development of CRPC. Disease progression on imaging was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST). A complete PSA control was defined as a PSA level of less than 0.2 ng per milliliter on two consecutive measurements at least 4 weeks apart. Serologic progression was defined as an increase in the PSA level of more than 50% above the nadir reached after the initiation of ADT, with two consecutive increases at least 2 weeks apart. The date of a first recorded increase of more than 50% above the nadir was deemed the date of progression.
Results
From July 2006 through 2012, a total of 790 patients were enrolled and underwent randomization. The mean follow-up duration was 28.9 months, with 136 deaths in the ADT-alone group and 101 deaths in the combination group. The mean age was 64 years in the combination group and 63 in ADT alone group. In both groups, approximately 85% of the patients were white, approximately 70% had an ECOG performance-status score of 0 and approximately 65% had high-volume disease. A total of 60% had a Gleason score of 8 or higher. The median OS was 57.6 months in chemohormonal arm versus 44 months in the ADT alone arm, thus conferring an improvement of 13.6 months in OS (HR 0.61 [95% CI, 0.47 to 0.80; P<0.001]). The improvement in OS was more pronounced in the high-volume disease subgroup (49.2 months vs 32.2 months; HR 0.60; 95% CI 0.45 to 0.81; P<0.001). In contrary, there was no statistically significant difference in the OS with the addition of docetaxel for low-volume group (64 months vs not reached, HR 1.04; 95% CI 0.70–1.55; P=0.11). In addition to OS, the median time to CRPC was also prolonged in the chemohormonal arm compared to ADT alone arm (20.2 months vs 11.7 months, HR 0.61; 95% CI, 0.51 to 0.72; P<0.001), as well as the median time for clinical progression (33 months vs. 19.8 months; HR 0.61; 95% CI 0.50 to 0.75; P<0.001).
In a more recent report of the CHAARTED trial with a longer follow-up of 53.7 months, the initial results were confirmed (OS of 57.6 months for ADT plus Docetaxel vs. 47.2 months for ADT alone; HR 0.73 [0.59–0.89]; P=0.0018). However, even though patients with high volume disease were clearly found to benefit from the addition of docetaxel (OS 51.2 months Vs 34.4 months; HR 0.63 [0.50–0.79]; P<0.0001), for low-volume patients the addition of docetaxel was not found to confer a survival benefit (OS 63.5 months Vs. NR for ADT alone; HR 1.04 [0.70–1.55]; P=0.86).30 Another subgroup analysis in patients with de novo disease showed a median OS of 48 months in high volume disease treated with chemohormonal therapy compared to high volume disease treated with ADT alone. (48 vs 34.1 months; HR 0.63 0.49 – 0.81, p<0.001).
The investigators concluded that the addition of six cycles of Docetaxel to ADT during the initiation of treatment for high-volume metastatic HSPC was associated with a significant improvement in OS, longer time to development of CRPC, better PSA control at 1 year of follow up, higher cancer specific survival and a substantially longer overall survival.
STAMPEDE
This innovative multiarm, multistage trial incorporated a phase II/III approach to assess the impact of hormonal therapy at the time of initiation of long-term hormonal therapy. This trial added much needed evidence to the findings of CHAARTED trial. It showed a definitive survival benefit from early chemohormonal therapy with docetaxel in HSPC patients31. Newly diagnosed metastatic, node positive, or high-risk locally advanced (with at least two features from T3/4, Gleason score of 8–10, and prostate-specific antigen ≥40 ng/mL); or previously treated with radical surgery, radiotherapy, or both and relapsing with high-risk features were used as inclusion criteria.
Treatment plan
A total of 2962 previously untreated patients with both metastatic and non-metastatic PCa were randomly assigned in a 2:1:1:1 ratio to the following arms.
ADT (n=1184)
ADT plus Zoledronic acid (n=593)
ADT plus Docetaxel (n=592)
ADT plus Zoledronic acid and Docetaxel (n=593)
Patients in ADT arm received LHRH agonist, antagonist or anti androgen, with orchiectomy allowed as an alternative for drug therapy. Six 3 weekly cycles of Zoledronic acid (4 mg) was followed by once a month dosing up to 2 years. Docetaxel (75 mg/m2) was given along with prednisolone for six 3 weekly cycles and trial therapy was discontinued after intolerable side effects or disease progression.
Evaluation
Patients were followed up every 6 weeks for 6 months, every 12 weeks for 2 years and every 6 months for 5 years. PSA was measured at every follow-up visit and other tests were done at the clinician’s discretion. The lowest value of PSA within 24 weeks of starting treatment was considered as the nadir value. The primary end points were overall survival (OS) and failure-free survival (FFS). FFS was defined as time from randomization to onset of: biochemical failure; local or systemic progression; or death from prostate cancer. Biochemical failure was defined as PSA increase of 50% above nadir and absolute increase by 4 ng/mL and confirmed by retesting.
Results
After a median follow-up of 43 months, the primary endpoint of OS was significantly improved for patients who received docetaxel in combination with ADT versus patients who received ADT alone (81 months for the combination arm vs. 71 months for the ADT alone arm; HR, 0.78 [95% CI, 0.66–0.93]; P = 0.006), as well as for the patients who received both Docetaxel and Zoledronic acid versus the patients who received neither docetaxel nor zoledronic acid (76 months Vs. 71 months; HR, 0.82 [95% CI, 0.69–0.97]; P = 0.022). Maximal benefit was seen in the subset of patients with metastasis, with a 15 month improvement in overall survival (60 months vs 45 months (HR, 0.76 [95% CI, 0.62–0.92]; P = 0.005). Median failure-free survival (FFS) and 5 year FFS were better in the chemohormonal arm compared with the ADT arm (37 months and 38% Vs 20 months and 28%)
This study again confirmed that the addition of docetaxel to standard ADT alone was associated with an improvement in median OS, with an HR of 0·78 and a difference in median survival of 10 months. There was statistically significant improvement in the secondary endpoints of PCa specific survival and failure free survival. The time for the onset of first skeletal related event was also significantly prolonged in the chemo hormonal therapy group.
Variations between outcomes in these studies
Even though all three aforementioned trials used a similar treatment design, there were significant differences in the patient populations that could explain the differences in outcomes (Table 2). One possible explanation is regarding to the patient population. In the GETUG trial the control arm that received ADT alone had an overall better survival compared to the patients in the control arms of the CHAARTED and STAMPEDE trials [54 months in GETUG Vs 44 months in CHAARTED and 45 months in STAMPEDE (metastatic subgroup)]. In addition, the 9 cycles of chemotherapy were poorly tolerated by patients in the GETUG trial and consequently, only 48% of the patients completed chemotherapy compared to 74% and 77% in CHAARTED and STAMPEDE respectively.
Table 2.
Phase III chemohormonal trials – Outcome analyses
| Phase III Study | GETUG-AFU15 | CHAARTED | STAMPEDE | |||
|---|---|---|---|---|---|---|
|
|
||||||
| ADT +Doc* | ADT | ADT +Doc* |
ADT | ADT +Doc* | ADT | |
| Median age in years | 63 | 64 | 64 | 63 | 65 | 65 |
| Percentage of metastatic disease | 67 | 75 | 72.8 | 72.8 | N/A | N/A |
| Median OS (mOS) in months | 62.1 | 48.6 | 57.6 | 44 | 60* | 45* |
| Median Biochemical Progression free survival(bPFS) | 22.9 | 12.9 | 20.2 | 11.7 | N/A | N/A |
| Hazard radio for mOS | HR: 0.88 (0.68–1.14) | HR: 0.61 (0.47–0.80) | HR 0·76 (0·62–0·9) | |||
| Hazard ratio for bPFS | 0.72 (0.57–0.91) | HR: 0.61 (0.51–0.72) | HR 0·61 (0·53–0·71) | |||
Doc*- Docetaxel, mOS in STAMPEDE trial is for the mHSPCa cohort.
The timing of chemotherapy could also have played a role in the improved survival seen in CHAARTED and STAMPEDE trials. Patients were enrolled within a window of 120 days before randomization in the CHAARTED and 12 weeks in the STAMPEDE, respectively. In contrary, in the GETUG-AFU15, patients were enrolled within 2 months of starting ADT and 47% actually enrolled within 15 days of starting ADT. There is a transient period of increased hepatic clearance of docetaxel after castration. However, the duration of this altered clearance is not known and could offer an explanation for the observed differences between the trials32. Another reason for increased survival in CHAARTED and STAMPEDE could be due to treatment with newer antiandrogens. The GETUG-AFU 15 was the first of these trials and most participants developed CRPC at a time that neither abiraterone nor enzalutamide were widely available.
Among the three phase III trials, only the CHAARTED trial used stratification based on tumor volume and reported a statistically significant improvement in OS for patients with high volume disease, as well as a numerically but non-statistically significant improvement in OS for the low volume group (Table 1). One of the reasons for difference in GETUG trial could be due to the fact that more than half of the patients (52%) had low volume disease. No comparisons based on tumor volume have been done so far in STAMPEDE trial, however 61% of patients had metastatic disease at the time of enrollment. Newer imaging techniques in development, such as sodium fluoride–positron emission tomography/computed tomography (NaF-PET/CT),33,34 have emerged as very useful clinical tools in detecting bone metastasis and defining functional tumor burden, which could prove valuable in selecting patients with high tumor burden for chemohormonal therapy in the future.
Table1.
Phase III chemohormonal Trials- Design and Toxicities.
| Phase III trial | GETUG-AFU15 | CHAARTED | STAMPEDE |
|---|---|---|---|
|
|
|||
| Disease category | M1 | M1 | High risk N0M0, N1M0, M1 |
| Performance status | KPS* ≥70 | ECOG* 0–2 | WHO* 0–2 |
| Median follow-up in months | 50 | 28.9 | 43 |
| No of chemotherapy cycles | 9 | 6 | 6 |
| Adverse events | 72 (38%) | 114 (29%) | 288 (52%) |
| Adverse events type | Neutropenia 40(21%) | Neutropenia 47(12.1%) | Febrile neutropenia |
| Febrile neutropenia 6(3%) | Febrile neutropenia | 84(15%) | |
| Abnormal LFT* 3(2%) | 24(6.1%) | Neutropenia 66(12%) | |
| Fatigue 16(4.1%) | GI* symptoms 45(8%) | ||
| Treatment related deaths | 4 (2%) | 1(0.2%) | 2 (0.3%) |
KPS* – Karnofsky Performance Status, ECOG* - Eastern Cooperative Oncology Group performance status, WHO* - World Health Organization Performance Status. The three most common Grade 3 and above adverse events are compared between the trials.
The application of other androgen axis agents in mHSPC
The development of several newer oral agents to disrupt the androgen axis has resulted in their routine use in CRPC. These include Enzalutamide, an androgen axis inhibitor with multiple sequential actions in the androgen receptor pathway, including competitive inhibition of androgen binding to receptors and inhibition of androgen receptor nuclear translocation and DNA interaction.36 Abiraterone is another androgen axis inhibitor, which blocks androgen biosynthesis by inhibiting steroidal enzyme 17 α-hydroxylase/C17, 20-lyase (CYP17). This causes suppression of androgen synthesis in testicular, adrenal, and prostatic tumor tissues.39 Apalutamide is structurally and pharmacologically similar to enzalutamide, acting as a selective competitive antagonist of the androgen receptor (AR), but has greater potency and reduced central nervous system permeation with improved adverse events profile.35 Orteronel is functionally similar to abiraterone as a steroidal biosynthesis inhibitor, but it is selective for 17,20-lyase relative to 17α-hydroxylase, which reduces need for concomitant corticosteroids unlike Abiraterone.42
ENZALUTAMIDE
Enzalutamide has shown significant survival benefits in patients with mCRPC, both before and after treatment with docetaxel. In a randomized placebo controlled phase III trial (AFFIRM) involving 1199 chemotherapy progressed mCRPC patients, enzalutamide treated patients had an improved mOS (18.4 months Vs 13.6 months HR 0.63; P<0.001).36 In another phase III trial involving 1717 chemotherapy naïve mCRPC (PREVAIL), enzalutamide treated patients had a 65% radiographic progression-free survival (rPFS) at 12 months, as compared with 14% among patients receiving placebo (81% risk reduction; HR 0.19; [CI], 0.15 to 0.23; P<0.001). There was also a 29% risk reduction of death, which was the coprimary end point studied (HR 0.71; 95% CI, 0.60 to 0.84; P<0.001).37
A phase II single-arm study of enzalutamide as monotherapy in mHSPC showed PSA declines to a similar degree as GnRH agonists.38 A Phase III study comparing enzalutamide to conventional anti-androgen + LHRH agonists or surgical castration as first-line treatment for mHSPC (ENZAMET, NCT02446405) is currently recruiting participants. The primary endpoint is OS and secondary endpoints are PFS (Clinical and PSA), adverse events, and Health related Quality of Life and cost effectiveness. Another study comparing enzalutamide +ADT vs placebo + ADT (NCT02677896) is currently recruiting and primary endpoints of this trial include rPFS, with multiple secondary end points including OS, time to CRPC and time to first skeletal-related event. Both are expected to be completed by 2020.
ABIRATERONE
Abiraterone as a CYP 17 inhibitor has shown survival benefit in the mCRPC setting, both before and after treatment with docetaxel. In a phase III randomized placebo controlled trial (COU-AA-301) involving 1195 mCRPC patients that had progressed on docetaxel, overall survival was longer in the abiraterone acetate-prednisone group than in the placebo-prednisone group (14.8 vs. 10.9 months; HR, 0.65; 0.54 to 0.77; P<0.001). All secondary end points, including PFS, time to PSA progression and PSA response rate were significantly better in the Abiraterone group.39 In another randomized phase III trial (NCT00887198) to assess coprimary end points of radiographic PFS and OS in chemotherapy naïve patients, Abiraterone showed an improved radiographic PFS of 16.5 compared to 8.3 months with prednisone alone (HR 0.53; 0.45 to 0.62; P<0.001) and an improved OS (median not reached, vs. 27.2 months; HR, 0.75; 95% CI, 0.61 to 0.93; P=0.01).40
A phase III trial examining OS as a primary end-point in mHSPC treated with abiraterone acetate with low-dose prednisone plus ADT compared with ADT alone (NCT01715285) is ongoing. Coprimary end points are OS and radiographic PFS and the results are expected by 2018. Similarly, a multi-center phase III study (PEACE1, NCT01957436) is comparing PFS and OS in patients with mHSPC with four treatment arms: (i) ADT; (ii) ADT with abiraterone acetate; (iii) ADT with local radiotherapy; and (iv) ADT with abiraterone acetate and local radiotherapy. The coprimary end points are OS and PFS, and results are expected in 2018.
APALUTAMIDE
This potent antiandrogen demonstrated durable PSA response and safety in a phase II trial in mHSPC. 89% of patients had ≥50% PSA decline at 12 weeks, which was the primary end point and median Time to PSA progression was 24.0 months.41 A phase III trial comparing Apalutamide +ADT to ADT (TITAN; NCT02489318) in the setting of mHSPC is underway. It is estimated to enroll 1000 patients and the coprimary end points are radiographic PFS and OS, with results expected by 2020.
ORTERONEL
This selective adrenal androgen synthesis inhibitor (17 20 lyase inhibitor) demonstrated marked and durable PSA declines in phase II trials in mHSPC. In this study involving 39 nonmetastatic PCa patients with rising PSA, 35 patients had a PSA decrease of >30% and 6 (16%) achieved PSA ≤ 0.2 ng/mL at 3 months.42 In a phase III randomized placebo controlled multi-institutional trial (ELM-PC 4) 2353 patients were randomized to receive Orteronel + prednisolone or placebo + prednisolone, with primary end points of rPFS and OS, determined in the intention to treat population. Both radiographic and progression-free survival were superior in Orteronel arm; median rPFS 13·8 months Vs 8·7 months (hazard ratio [HR] 0·71, 95% CI 0·63–0·80; p<0·0001) and median OS was 31·4 months Vs 29·5 months (HR 0·92, 95% CI 0·79–1·08; p=0·31).43 A Phase III trial comparing Orteronel + ADT with Bicalutamide +ADT (S1216; NCT01809691) is currently recruiting an estimated 1300 participants. The primary end point is OS and the results are expected by 2022.
The results of the above mentioned phase III trials are expected to elucidate the role of newer antiandrogens in the setting of mHSPC. However at present their role in the early management of mHSPC is considered exploratory.
FUTURE DIRECTIONS
Given data that docetaxel has demonstrated efficacy in mHSPC in randomized phase III trials and the proven efficacy of Cabazitaxel in advanced PCa, addition of Cabazitaxel with ADT for chemohormonal therapy is being examined in clinical trials. A randomized phase III trial comparing Cabazitaxel and ADT to ADT alone in mHSPC for high risk disease is currently recruiting participants (SensiCab; NCT01978873). The primary end point is OS, with PFS and PSA control as secondary endpoints. Results are expected in 2018. Other validated chemotherapy agents active in the CRPC will likely be applied to earlier disease in the future based on the findings of the CHAARTED and other trials.
An issue that has arisen is control of the primary tumor in the face of metastatic disease, an approach that is being utilized successfully for other diseases including renal cancer44. In a feasibility study to look at effect of radical prostatectomy (RP) after ADT, patients treated with ADT+ RP combination showed a delayed time to CRPC transition and had a significantly better PFS and CSS.45 Two recent population-based database analyses from the US and Europe have suggested a beneficial role of surgery/radiation in mPCa46,47. With lengthening time of survival in patients with CRPC this is likely to demonstrate improvements for a subset of patients. Several Phase II trials examining this issues are ongoing (NCT01751438 and NCT02716974) with results expected in 2018.
CONCLUSIONS
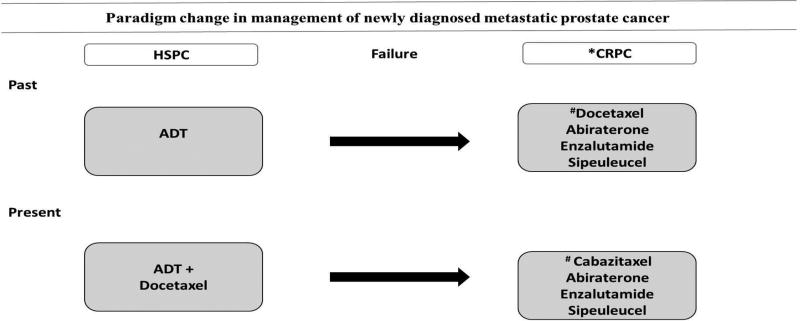
Metastatic HSPC is a heterogeneous disease and recent studies have shown that patients with metastatic disease spend the majority of their remaining life in the castration resistant state. There has been a paradigm change in the therapeutic approach to mHSPC with recently published phase III studies confirming a survival advantage with chemohormonal therapy (Figure 1). A recent abstract on the analysis of use indicates that chemohormonal therapy is being employed for 65% of high volume and 35 % of low volume mHSPC. The updated National Comprehensive Cancer Network (NCCN) guidelines recommend chemohormonal therapy for all adequately fit men with newly diagnosed mHSPC, regardless of the disease burden.48
Figure 1. Schematic representation of the paradigm shift of treatment for newly diagnosed metastatic HSPC.
Randomized mature trials suggest the addition of Docetaxel chemotherapy at the initiation of ADT results in a cancer-specific improvement in survival. After failure of ADT, other options are available for metastatic CRPC.
*First line treatment options for mCRPC
#Docetaxel and Cabazitaxel preferred for symptomatic CRPC.
Key points.
Median Overall Survival is almost four times the Failure-Free Survival and metastatic Castration Resistant Prostate Cancer (CRPC) makes up most of the survival time in patients with metastatic prostate cancer. Three major phase III studies combining ADT with docetaxel in patients with newly diagnosed metastatic prostate cancer have been recently reported.
The GETUG AFU 15 trial failed to show a survival advantage for chemohormonal therapy over ADT alone, although Progression-Free Survival (clinical and biochemical) and PSA control were improved.
The role of surgery and newer androgen-receptor pathway inhibitors in metastatic hormone-sensitive prostate cancer is currently being studied.
Early chemohormonal therapy for hormone-sensitive metastatic prostate cancer leads to improved overall survival and should be utilized for good performance patients with moderate and high volume metastatic disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have no disclosures, nor any commercial or financial conflicts of interest nor funding sources.
Contributor Information
Shivashankar Damodaran, Dept. of Urology, University of Wisconsin School of Medicine and Public Health, Phone: 608.262.0759 | FAX: 608.262.6453 | sdamodaran@urology.wisc.edu.
Christos E. Kyriakopoulos, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison WI 53705.
David F Jarrard, Dept. of Urology, University of Wisconsin School of Medicine and Public Health, University of Wisconsin Carbone Cancer Center, University of Wisconsin–Madison. Phone: 608.262.0759 | FAX: 608.262.6453 | jarrard@urology.wisc.edu.
References
- 1.https://seer.cancer.gov/statfacts/html/prost.htm
- 2.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nature reviews Cancer. 2002;2(5):389–396. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifi N, Dahut WL, Steinberg SM, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005 Nov;96(7):985–9. doi: 10.1111/j.1464-410X.2005.05798.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirby M, Hirst C, Crawford ED. Characterizing the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011 Nov;65(11):1180–92. doi: 10.1111/j.1742-1241.2011.02799x. Review. [DOI] [PubMed] [Google Scholar]
- 5.James ND, Spears MR, Clarke NW, et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the "Docetaxel Era": Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019) Eur Urol. 2015 Jun;67(6):1028–38. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Tangen CM, Hussain MH, Higano CS, et al. Improved overall survival trends of men with newly diagnosed M1 prostate cancer: a SWOG phase III trial experience (S8494, S8894 and S9346) J Urol. 2012;188:1164–1169. doi: 10.1016/j.juro.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangen CM, Hussain MH, Higano CS, et al. Improved overall survival trends of men with newly diagnosed M1 prostate cancer: a SWOG phase III trial experience (S8494, S8894 and S9346) M. Ahmed, L.C. Li Adaptation and clonal selection models of castration-resistant prostate cancer: current perspective. Int J Urol. 2013;20:362–371. [Google Scholar]
- 8.Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res. 2009;15:3251–5. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 9.Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 10.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 11.Craft N, Chhor C, Tran C, et al. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 1999;59:5030–6. [PubMed] [Google Scholar]
- 12.Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841–9. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whang YE, Wu X, Suzuki H, et al. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci U S A. 1998;95:5246–50. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–9. [PubMed] [Google Scholar]
- 15.Palmberg C, Koivisto P, Kakkola L, et al. Androgen receptor gene amplification at primary progression predicts response to combined androgen blockade as second line therapy for advanced prostate cancer. J Urol. 2000;164:1992–5. doi: 10.1016/S0022-5347(05)66935-2. [DOI] [PubMed] [Google Scholar]
- 16.Scher HI, Sawyers CK. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 17.Nelson PS. Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signalling in prostate cancer. J Clin Oncol. 2012;30:644–646. doi: 10.1200/JCO.2011.39.1300. [DOI] [PubMed] [Google Scholar]
- 18.Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenton MA, Shuster TD, Feting Am, et al. Functional characterization of mutant androgen receptors from androgen-independent prostate cancer. Clin Cancer Res. 1997;3:1383–1388. [PubMed] [Google Scholar]
- 20.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 22.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 23.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 24.Suzman DL, Antonarakis ES. Castration-resistant prostate cancer: latest evidence and therapeutic implications. Ther Adv Med Oncol. 2014;6:167–179. doi: 10.1177/1758834014529176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eigl BJ, Eggener SE, Baybik J, et al. Timing is everything: preclinical evidence supporting simultaneous rather than sequential chemo hormonal therapy for prostate cancer. Clin Cancer Res. 2005;11:4905–4911. doi: 10.1158/1078-0432.CCR-04-2140. [DOI] [PubMed] [Google Scholar]
- 26.Zhu ML, Horbinski CM, Garzotto M, et al. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyriakopoulos CE, Liu G. Chemohormonal Therapy for Hormone-Sensitive Prostate Cancer: A Review. Cancer J. 2016 Sep-Oct;22(5):322–325. doi: 10.1097/PPO.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 28.Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with Docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–158. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 29.Sweeney CJ, Chen YH, Carducci M, et al. Chemo hormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.http://oncologypro.esmo.org/Meeting-Resources/ESMO-2016/Long-term-efficacy-and-QOLdata-of-chemohormonal-therapy-C-HT-in-low-and-high-volume-hormone-naive-metastaticprostate-cancer-PrCa-E3805-CHAARTED-trial
- 31.James ND, Sydes MR, Clarke NW, et al. Addition of Docetaxel, Zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franke RM, Carducci MA, Rudek MA, et al. Castration-dependent pharmacokinetics of Docetaxel in patients with prostate cancer. J Clin Oncol. 2010 Oct 20;28(30):4562–7. doi: 10.1200/JCO.2010.30.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apolo AB, Lindenberg L, Shih JH, et al. Prospective study evaluating Na18F-positron emission tomography/computed tomography (NaF-PET/ CT) in predicting clinical outcomes and survival in advanced prostate cancer. J Nucl Med. 2016;57:886–892. doi: 10.2967/jnumed.115.166512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu G, Perlman S, Perk T, et al. Quantitative total bone imaging (QTBI) in patients with metastatic castration-resistant prostate cancer (CRPC) using NaF PET/CT. J Clin Oncol. 2015;33(suppl 7) abstr 180. [Google Scholar]
- 35.Ya-Xiong Tao. Elsevier Science. Jun 11, 2014. Pharmacology and Therapeutics of Constitutively Active Receptors; p. 351. ARN-509 is related structurally to enzalutamide with greater in vivo activity in CRPC xenograft models (Clegg et al., 2012). [Google Scholar]
- 36.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012 Sep 27;367(13):1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 37.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014 Jul 31;371(5):424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tombal B, Borre M, Rathenborg P, et al. Enzalutamide monotherapy in hormone-naive prostate cancer: primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol. 2014 May;15(6):592–600. doi: 10.1016/S1470-2045(14)70129-9. [DOI] [PubMed] [Google Scholar]
- 39.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith MR, Antonarakis ES, Ryan CJ, et al. Phase 2 Study of the Safety and Antitumor Activity of Apalutamide (ARN-509), a Potent Androgen Receptor Antagonist, in the High-risk Nonmetastatic Castration-resistant Prostate Cancer Cohort. Eur Urol. 2016 Dec;70(6):963–970. doi: 10.1016/j.eururo.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussain M, Corn PG, Michaelson MD, et al. Prostate Cancer Clinical Trials Consortium, a program of the Department of Defense Prostate Cancer Research Program and the Prostate Cancer Foundation. Phase II study of single-agent orteronel (TAK-700) in patients with nonmetastatic castration-resistant prostate cancer and rising prostate-specific antigen. Clin Cancer Res. 2014 Aug 15;20(16):4218–27. doi: 10.1158/1078-0432.CCR-14-0356. [DOI] [PubMed] [Google Scholar]
- 43.Saad F, Fizazi K, Jinga V, et al. Orteronel plus prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (ELM-PC4): a double-blind, multicentre, phase 3, randomised, placebo-controlled trial. Lancet Oncol. 2015 Mar;16(3):338–48. doi: 10.1016/S1470-2045(15)70027-6. [DOI] [PubMed] [Google Scholar]
- 44.Bamias A, Tzannis K, Papatsoris A, et al. Prognostic significance of cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma treated with first-line sunitinib: a European multiinstitutional study. Clin Genitourin Cancer. 2014 Oct;12(5):373–83. doi: 10.1016/j.clgc.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015;193(3):832–838. doi: 10.1016/j.juro.2014.09.089. [DOI] [PubMed] [Google Scholar]
- 46.Stephen H Culp, Schellhammer Paul F, et al. Might Men Diagnosed with Metastatic Prostate Cancer Benefit from Definitive Treatment of the Primary Tumor? A SEER-Based Study, European Urology. 2014 Jun;65(6):1058–1066. doi: 10.1016/j.eururo.2013.11.012. ISSN 0302-2838, [DOI] [PubMed] [Google Scholar]
- 47.Gratzke C, Engel J, Stief CG. Role of radical prostatectomy in metastatic prostate cancer: data from the Munich Cancer Registry. Eur Urol. 2014 Sep;66(3):602–3. doi: 10.1016/j.eururo.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 48.National Comprehensive Cancer Network. [Accessed March 11, 2016];Clinical practice guidelines in oncology version 2.2016: prostate cancer. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.