Abstract
We aimed to assess the impact of ophthalmology weight-based hydroxychloroquine (HCQ) dosing guidelines on prescribing patterns. We examined initial HCQ prescription dosing between 2007 and 2016 and determined independent predictors for HCQ dosing above the previous (2011) recommended ≤ 6.5 mg/kg of ideal body weight (IBW)/day and the latest (2016) recommended ≤ 5.0 mg/kg of actual body weight (ABW)/day using logistic regression. Among 17,797 patients (82% female), the proportion of 400 mg prescribed daily dosing declined sharply from 80% in 2007–2011 to nearly 40% in 2014, whereas the proportions of 200-and 300-mg daily doses showed the opposite trends during the same periods. Accordingly, the risk of HCQ dosing above the guideline recommendations declined by more than 60%. While 36% of normal body mass index (BMI) individuals were classified as dosing above the IBW-based guideline, 66% would have received dosing above the latest ABW-based guideline. The risk of excess dosing was associated with female patients and dermatology prescribers (adjusted odds ratios ≥ 2 according to IBW- or ABW-based guidelines). There has been a sharp decline in HCQ dosing following ophthalmology weight-based guidelines in recent years. While this trend is likely helpful in reducing the risk of retinopathy, its potential impact on HCQ efficacy remains to be clarified.
Keywords: Epidemiology, Preventative medicine, Rheumatic diseases, Rheumatoid arthritis, Systemic lupus erythematosus
Introduction
Hydroxychloroquine (HCQ) is widely used in the treatment of systemic lupus erythematosus (SLE), with benefits including improved survival, reduced disease activity, and a lower risk of pregnancy complications, venous thrombo-embolism, dyslipidemia, and insulin resistance [1–7]. HCQ is also commonly used as a component of “triple therapy” in the treatment of rheumatoid arthritis (RA) and in other autoimmune and dermatologic conditions [8].
The major long-term risk of this otherwise well tolerated medication is vision-threatening retinopathy [9, 10]. Historically, HCQ retinopathy was considered to be rare [11–13], but a recent study in Kaiser Permanente Northern California (KPNC) using more sensitive screening methods showed a prevalence of 7.5% among long-term HCQ users and reinforced earlier reports that daily dose is a key risk factor in the development of toxicity [14]. To reduce the risk of retinopathy, the 2011 American Academy of Ophthalmology (AAO) guidelines recommended a maximum safe dose of 6.5 mg/kg/day (as determined by ideal body weight [IBW]) [15]. However, after the recent KPNC study suggested that actual body weight (ABW) was a better predictor of HCQ retinopathy than IBW [14], the AAO updated its guidelines in 2016 to recommend a maximum daily dose of 5.0 mg/kg/day by ABW [16]. As these guidelines were developed by ophthalmologists, who are not typical prescribers of HCQ, it remains largely unknown to what extent these guidelines have impacted HCQ prescribing patterns in the USA.
To examine the impact of ophthalmology weight-based HCQ dosing guidelines on prescribing patterns, we assessed HCQ prescriptions over a recent 10-year period among 17,797 HCQ initiators from a US large population-based cohort.
Materials and methods
Data source
Our study data source was KPNC, a large integrated health network which includes a diverse population of approximately 4.1 million patients. The KPNC electronic medical record database includes demographic and pharmacy data for all patients in the KPNC service area. Health care information includes demographics, outpatient and inpatient encounters, practitioner specialty, diagnoses, medication dispensing, imaging, and laboratory results.
Study population and design
We identified subjects age 21 or older with at least 1 or more prior years of KPNC membership with incident HCQ prescriptions between January 1, 2007 and December 31, 2016. Prescriptions for an indication of malaria prophylaxis were excluded. We divided the 10-year study period into five 2-year cohorts based on the date of the first HCQ prescription to examine the prescription trends.
Assessment of HCQ prescriptions
We determined the incident HCQ prescription doses based on the Sig instructions in the medication order. For prescriptions written for initial dose uptitration or a loading dose, we included the intended ongoing prescription dose in the analysis. We classified the weight-based HCQ dose according to the AAO recommended maximum dose per IBW (i.e., > 6.5 mg/kg/day) and ABW (i.e., > 5.0 mg/kg/day) [15, 16].
Assessment of covariates
Covariate information was derived from the most recent available data within 1 year of the index HCQ prescription date for demographic (i.e., age, sex) and anthropometric (i.e., height, weight) characteristics. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. IBW was calculated by the Devine formula: for women, IBW = 45.5 + 2.3 kg for each inch of height over 60 in. and for men, IBW = 50 + 2.3 kg for each inch of height over 60 in. [17]. Patients without a recorded height or a height under 60 in. were excluded from the IBW-related analyses (n = 697). Other covariates of interest were prescriber specialty, chronic kidney disease (CKD; stage ≥ 3), and indication for HCQ therapy.
Statistical analysis
We compared the baseline characteristics of individuals at the index date of their incident HCQ prescriptions according to calendar year categories. We calculated the proportion of different HCQ doses and categorized them into the nearest 100 mg (one half tablet) daily dose (i.e., 200, 300 mg, 400, and over 400 mg) over each of the five 2-year cohorts over the study period. The proportion of incident HCQ prescriptions above the prior IBW-based recommended maximum daily dose [15] or the latest ABW-based recommended maximum daily dose [16] was calculated over the study period. We examined the trends of median and interquartile ranges for initial HCQ dose per IBW and ABW between 2007 and 2016 using quantile regression. We also examined the relation of age, sex, BMI, CKD, provider specialty, and indication for HCQ use to the risk of prescribed HCQ dose above the IBW- or ABW-based recommended dose using logistic regression. The final multivariable model was adjusted for age, sex, BMI, CKD, prescriber specialty, and calendar year.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request and with permission from Kaiser Permanente Northern California Division of Research.
Results
During the 10-year period between 2007 and 2016, 17,797 individuals initiated HCQ therapy (Table 1). The majority were female (82%) with a mean age of 53.2 years and a mean BMI of 28.4 kg/m2. Rheumatologists were the most common prescribers of HCQ (69%), followed by primary care providers (18%) and dermatologists (9%). RA was the most common indication for HCQ use (46%), followed by dermatologic conditions (18%), SLE (15%), and other systemic autoimmune conditions (15%). Dermatologic conditions included cutaneous lupus (together, discoid and subacute cutaneous lupus comprised 33%), as well as alopecia, lichen planus, dermatitis, granuloma annulare, psoriasis, and urticaria. One thousand four hundred and fifty-two (8.2%) prescriptions were written with an initial low starting dose with instructions to raise the dose as tolerated by patient, and 330 (1.9%) were written with an initial loading dose.
Table 1.
Characteristics of hydroxychloroquine incident users by time period of initial prescription (2007–2016)
| Characteristics | 2007–2008 (n = 3793) | 2009–2010 (n = 3388) | 2011–2012 (n = 3600) | 2013–2014 (n = 3120) | 2015–2016 (n = 3896) | All (n = 17,797) |
|---|---|---|---|---|---|---|
| Sex (% female) | 3044 (80.3) | 2744 (81.0) | 2940 (81.7) | 2570 (82.4) | 3214 (82.5) | 14,512 (81.5) |
| Age, mean (SD) | 53.3 (15.0) | 53.5 (15.1) | 53.8 (15.7) | 53.1 (15.7) | 52.5 (15.8) | 53.2 (15.5) |
| CKD (≥ stage 3) | 120 (3.2) | 151 (4.5) | 206 (5.7) | 194 (6.2) | 195 (5.0) | 866 (4.9) |
| BMI (kg/m2), mean (SD) | 28.4 (6.7) | 28.6 (6.7) | 28.3 (6.7) | 28.4 (6.7) | 28.3 (6.6) | 28.4 (6.7) |
| BMI Category | ||||||
| Underweight (< 18.5) | 46(1.2) | 63 (1.9) | 51 (1.4) | 45 (1.5) | 43 (1.1) | 248 (1.4) |
| Normal (18.5–24.9) | 1280 (33.8) | 1062 (31.4) | 1226 (34.1) | 1068 (34.3) | 1289 (33.2) | 5925 (33.4) |
| Overweight (25–29.9) | 1197 (31.6) | 1097 (32.4) | 1134 (31.5) | 968 (31.1) | 1279 (32.9) | 5675 (31.9) |
| Obese (≥ 30) | 1261 (33.3) | 1163 (34.36) | 1186 (33.0) | 1033 (33.2) | 1274 (32.8) | 5917 (33.3) |
| Prescriber specialty (%) | ||||||
| Rheumatologist | 2449 (64.6) | 2394 (70.7) | 2513 (69.8) | 2164 (69.4) | 2785 (71.5) | 12,305 (69.1) |
| Primary care | 976 (25.7) | 638 (18.8) | 638 (18.8) | 505 (16.2) | 541 (13.9) | 3255 (18.3) |
| Dermatologist | 242 (6.4) | 253 (7.5) | 340 (9.4) | 340 (9.4) | 340 (9.4) | 1517 (8.5) |
| Other | 126 (3.3) | 103 (3.0) | 152 (4.2) | 135 (4.3) | 204 (5.2) | 720 (4.1) |
| Indication (%) | ||||||
| Rheumatoid arthritis | 1669 (44.0) | 1577 (46.6) | 1705 (47.4) | 1431 (45.9) | 1853 (47.6) | 8235 (46.3) |
| Systemic lupus erythematosus | 536 (14.1) | 472 (13.9) | 574 (15.9) | 537 (17.2) | 620 (15.9) | 2739 (15.4) |
| Dermatologic conditions‡ | 793 (20.9) | 602 (17.8) | 643 (17.9) | 585 (18.6) | 633 (16.3) | 3256 (18.3) |
| Systemic autoimmune condition† | 438 (11.6) | 548 (16.2) | 541 (15.0) | 442 (14.2) | 651 (16.7) | 2620 (14.7) |
| Other | 357 (9.4) | 189 (5.6) | 137 (3.8) | 125 (4.0) | 139 (3.6) | 947 (5.3) |
BMI body mass index, CKD chronic kidney disease
Unless otherwise specified, results are reported as n (%)
Includes alopecia, dermatitis, discoid lupus, granuloma annulare, lichen planus, subacute cutaneous lupus, psoriasis, and urticarial
Excludes systemic lupus erythematosus and rheumatoid arthritis
During the study period, the proportion of 400 mg prescribed daily dosing declined sharply from 80% in 2011 to nearly 40% in 2014 (Fig. 1) and rose back to 60% in 2016, whereas the proportions of 200- and 300-mg daily doses showed the opposite trends during the same periods. Correspondingly, daily HCQ dosing per either IBW or ABW began to decline in 2012 and reached its lowest level in 2014, before rising slightly again in 2015 and 2016. The median HCQ dose per IBW declined from 6.75 to 5.99 mg/kg during the study period, and the median dose per ABW declined from 5.03 to 4.46 mg/kg HCQ (both p values < 0.001), with a nadir of 5.29 mg/kg IBW and 4.06 mg/kg ABW in 2014 (Figs. 2 and 3).
Fig. 1.
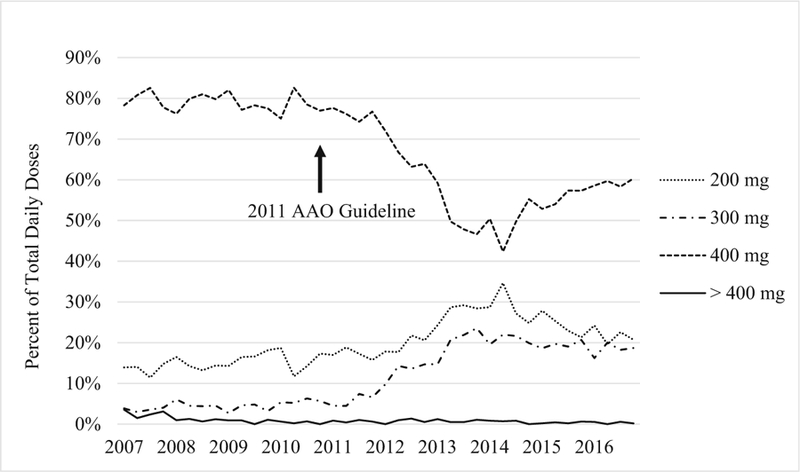
Change in daily dosing trends. After 2011, the number of prescriptions for 400 mg per day dropped markedly, with a concomitant increase in the number prescriptions for 200 mg and 300 mg per day. AAO the American Academy of Ophthalmology
Fig. 2.
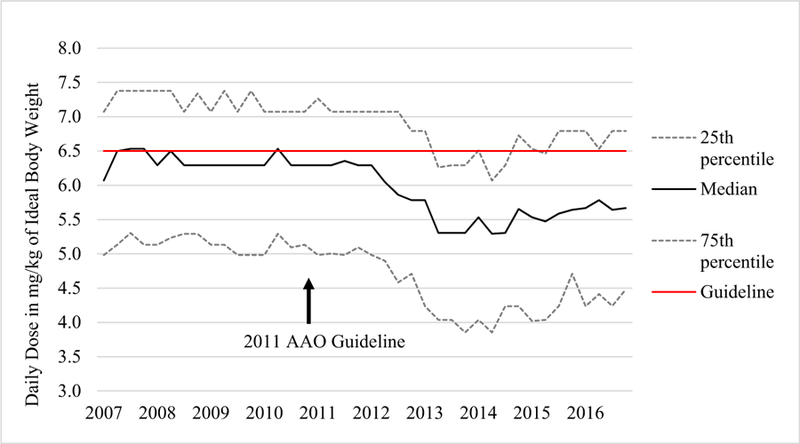
Change in hydroxychloroquine dosing by ideal body weight, 2007 to 2016. Individual points represent quarterly values. The median daily dose declined after 2011 reaching its lowest point in 2014 and rebounding slightly in 2015 and 2016. AAO the American Academy of Ophthalmology
Fig. 3.
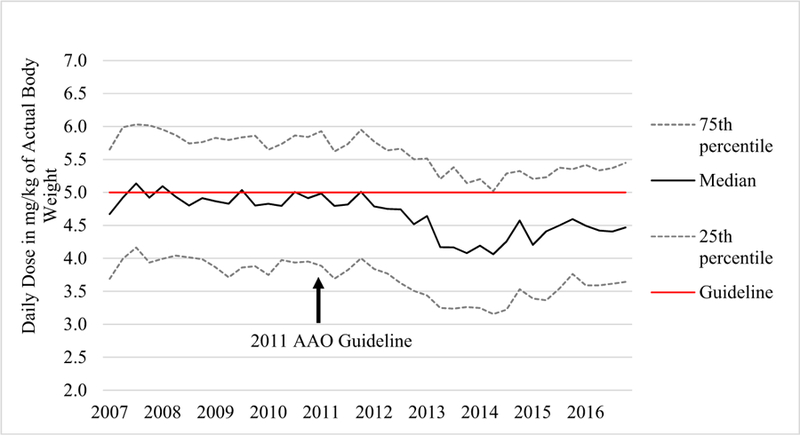
Change in hydroxychloroquine dosing by actual body weight, 2007 to 2016. Individual points represent quarterly values. The median daily dose declined after 2011, reaching its lowest point in 2014 and rebounding slightly in 2015 and 2016. AAO the American Academy of Ophthalmology
The proportion of dosing above the AAO recommenda-tions declined from 53% in the first period (2007–2008) to 33% in the most recent period (2015–2016) per IBW and from 49 to 34% per ABW (Table 2). This corresponds with adjusted odds ratios (ORs) of 0.34 (95% confidence interval [CI], 0.30–0.37) for IBW and 0.38 (95% CI, 0.34–0.43) for ABW in the latest period relative to the first period. At the nadir between 2013 and 2014, the proportions of dosing above AAO recommendations were 26% for IBW and 31% for ABW, corresponding to an adjusted OR of 0.24 (95% CI, 0.21–0.26) and 0.33 (95% CI, 0.30–0.38) relative to the first period, respectively (Table 2).
Table 2.
Hydroxychloroquine prescription dose in relation to dosing recommendations
| Characteristics | >6.5 mg/kg/day, actual body weight (prior recommendation) |
>5.0 mg/kg/day, ideal body weight (current recommendation) |
||||
|---|---|---|---|---|---|---|
| N (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | N (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Year of incident prescription | ||||||
| 2007–2008 | 1930 (53.2) | 1.00 (Reference) | 1.00 (Reference) | 1873 (49.4) | 1.00 (Reference) | 1.00 (Reference) |
| 2009–2010 | 1643 (50.6) | 0.90 (0.82–0.99) | 0.86 (0.77–0.95) | 1579 (46.6) | 0.89 (0.82–0.98) | 0.88 (0.79–0.98) |
| 2011–2012 | 1618 (46.7) | 0.77 (0.70–0.84) | 0.69 (0.62–0.76) | 1622 (45.1) | 0.84 (0.77–0.92) | 0.75 (0.67–0.83) |
| 2013–2014 | 777 (25.9) | 0.31 (0.28–0.34) | 0.24 (0.21–0.26) | 971 (31.1) | 0.46 (0.42–0.51) | 0.33 (0.30–0.38) |
| 2015–2016 | 1229 (32.7) | 0.43 (0.39–0.47) | 0.34 (0.30–0.37) | 1309 (33.6) | 0.52 (0.47–0.57) | 0.38 (0.34–0.43) |
| Sex | ||||||
| Female | 6971 (50.4) | 13.74 (11.96–15.79) | 17.91 (15.51–20.67) | 6352 (43.8) | 1.77 (1.64–1.92) | 2.20 (2.00–2.41) |
| Male | 226 (6.9) | 1.00 (Reference) | 1.00 (Reference) | 1002 (30.5) | 1.00 (Reference) | 1.00 (Reference) |
| Age | ||||||
| < 55 years | 4020 (43.6) | 1.00 (Reference) | 1.00 (Reference) | 3929 (41.3) | 1.00 (Reference) | 1.00 (Reference) |
| > 55 years | 3177 (40.3) | 0.87 (0.82–0.93) | 1.06 (0.99–1.14) | 3425 (41.4) | 1.00 (0.94–1.06) | 0.98 (0.91–1.05) |
| CKD stage | ||||||
| No CKD or CKD stage < 3 | 6926 (42.6) | 1.00 (Reference) | 1.00 (Reference) | 7058 (41.7) | 1.00 (Reference) | 1.00 (Reference) |
| CKD stage ≥ 3 | 271 (32.9) | 0.66 (0.57–0.77) | 0.80 (0.68–0.95) | 296 (34.2) | 0.73 (0.63–0.84) | 0.82 (0.69–0.97) |
| BMI | ||||||
| Underweight | 52 (21.9) | 0.50 (0.36–0.68) | 0.40 (0.29–0.55) | 146 (58.9) | 0.73 (0.57–0.95) | 0.67 (0.51–0.87) |
| Normal | 2053 (36.1) | 1.00 (Reference) | 1.00 (Reference) | 3916 (66.1) | 1.00 (Reference) | 1.00 (Reference) |
| Overweight | 2285 (41.9) | 1.28 (1.18–1.38) | 1.64 (1.51–1.79) | 2752 (48.5) | 0.48 (0.45–0.52) | 0.49 (0.45–0.53) |
| Obese | 2801 (49.1) | 1.71 (1.59–1.84) | 1.99 (1.83–2.16) | 526 (8.9) | 0.05 (0.05–0.06) | 0.04 (0.04–0.05) |
| Prescriber specialty | ||||||
| Rheumatologist | 4729 (40.0) | 1.00 (Reference) | 1.00 (Reference) | 4929 (40.1) | 1.00 (Reference) | 1.00 (Reference) |
| Primary care | 1395 (44.8) | 1.22 (1.13–1.32) | 1.15 (1.05–1.26) | 1334 (41.0) | 1.04 (0.96–1.12) | 0.97 (0.88–1.06) |
| Dermatologist | 747 (50.8) | 1.55 (1.39–1.72) | 2.03 (1.79–2.30) | 764 (50.4) | 1.52 (1.36–1.69) | 1.96 (1.72–2.23) |
| Other | 326 (47.5) | 1.36 (1.17–1.58) | 1.78 (1.49–2.13) | 327 (45.4) | 1.25 (1.07–1.45) | 1.46 (1.22–1.75) |
CKD chronic kidney disease, BMI body mass index
Using the IBW-based recommendation, 50% of women and 7% of men had dosing over 6.5 mg/kg, with an adjusted OR of 17.91 (95% CI, 15.51–20.67) for women (Table 2). Using the ABW-based recommendation, women also had a higher proportion (44%) of initial prescription dosing over 5.0 mg/kg than men (31%), with an adjusted OR of 2.20 (95% CI, 2.00–2.41). Patients with CKD had a lower risk of excess dosing by IBW (OR 0.80 [95% CI, 0.68–0.95]) as well as by ABW (OR 0.82 [95% CI, 0.69–0.97]).
BMI had opposite effects on the proportions of excess prescribing by IBW- versus ABW-based dosing. Proportions of initial HCQ dosing above the IBW-based recommendation increased with higher BMI (22, 36, 42, and 49% in underweight, normal, overweight, and obese categories, respectively) (Table 2). These resulted in adjusted ORs of 1.64 (95% CI, 1.51–1.79) for overweight and 1.99 (95% CI, 1.83–2.16) for obese individuals. In contrast, proportions of initial HCQ dosing over the ABW-based recommendation increased with lower BMI, with only 9% excess dosing among obese individuals but 66% excess dosing among normal body weight individuals and 49% of overweight individuals. The corresponding adjusted ORs for dosing above the ABW-based recommendations were 0.49 (95% CI, 0.45–0.53) among overweight and 0.04 (95% CI, 0.04–0.05) among obese individuals, as compared with the normal BMI group. Compared with rheumatologists, dermatologists were more likely to prescribe higher doses of HCQ according to either dosing guideline (adjusted ORs 2.03 [95% CI, 1.79–2.30] and 1.96 [95% CI, 1.72–2.23] per IBW- and ABW-based recommendations, respectively).
Discussion
In this population-based cohort from a large integrated health plan, we found that the daily dose for new HCQ prescriptions declined considerably starting in 2011, coinciding with the timing of published AAO HCQ dosing guidelines, and this reduction reached its nadir in 2014 with less than half of all new HCQ prescriptions written for 400 mg per day. This decline in prescribed daily dose corresponded with a 76% decline in the risk of HCQ dosing above the 2011 AAO guideline until 2014. Although there appears to have been a slight rebound to higher average dosing in 2015 and 2016, these data overall suggest a swift response to the AAO recommendations.
Two previous, small-scale, single-center studies (n = 554 and n = 1681) have reported conflicting findings of HCQ dosing trends [18, 19]. The first study captured all rheumatology patients (n = 554) who initiated HCQ between 2009 and 2016 and were seen by an ophthalmologist in a university health care system and reported no change in HCQ trend [19]. While this may reflect the local practice pattern, it is also conceivable that the requirement to be seen by an ophthalmologist may have selected patients with higher initial dosing more frequently even in the recent years. In contrast, another university center study captured all patients (n = 1681) who received any HCQ prescription between 2012 and 2016 and reported a substantial decline in excess dosing, similar to ours [18]. Our study is over ten times larger than this study and spans twice its duration, and we were able to address initial dosing trends.
The vision-threatening nature of HCQ retinopathy likely explains the fast guideline adoption by prescribers. In contrast, concerns regarding patient non-adherence to HCQ, as seen in up to half of all patients with SLE [14, 20], may prevent some providers from lowering the prescription dose of this important medication. To that end, it remains to be clarified whether the renewed concern about HCQ retinopathy may worsen HCQ compliance. While the dosing trend may reduce the risk of retinopathy, its potential impact on HCQ’s wide-ranging benefits (particularly in SLE) remains a concern. To that effect, the recent increase in dosing (2015–2016) is intriguing, as it might indicate a response by prescribers to a loss of clinical efficacy at lower doses of HCQ. Further studies are needed to clarify whether reducing HCQ dose, per the ophthalmology guidelines, will retain efficacy for the treatment of SLE and other rheumatic diseases. Such data would enable us to assess the net benefit of the observed HCQ dosing trend.
In terms of patient factors associated with a higher risk of HCQ dosing above the weight-based recommendations, we found that excess dosing was more prominent among women, occurring in a large proportion (i.e., 50% of women using the IBW criteria and 44% using the ABW criteria). This is explained in part because women are shorter on average and height is included in the IBW calculation, systematically leading to a lower IBW among women; women also have a lower average ABW than men. Therefore, the commonly prescribed HCQ dose of 400 mg daily exceeds the recommended maximum daily dose range according to both 2011 IBW-based and 2016 ABW-based guidelines for the average US female [21].
Dermatologists showed a higher risk of excess HCQ dosing (50%) compared with rheumatologists (40%) and primary care physicians (41%). This may suggest potential differences in awareness of AAO dosing guidelines or perception of the risk of toxic retinopathy among specialties. Alternatively, this could indicate higher treatment doses used for some cutaneous indications despite discordance with the ophthalmology dosing guidelines, as two prospective studies found greater efficacy in treating cutaneous lupus with HCQ dosing > 5 mg/kg/ day ABW [22, 23]. The presence of concomitant CKD was associated with a reduced risk of HCQ excess dosing, which reflects HCQ dose adjustment in patients with renal disease. Although there has not been a clear consensus on appropriate dose reduction in renal insufficiency [24], CKD has been associated with a more than twofold increase of the prevalence of HCQ retinopathy [14]. Nevertheless, more than 30% of CKD patients’ prescriptions still exceeded the recommended doses by either guideline, suggesting potential room for improvement even in this subpopulation.
BMI categories posed opposing risks of excess dosing between the two weight-based dosing guidelines. While 36% of normal BMI individuals were exposed to dosing above the IBW-based guidelines, 66% would have been exposed to dosing above the latest ABW-based guidelines. In contrast, 44% of obese individuals were exposed to dosing above the IBW-based guideline, compared with only 9% using the ABW-based guideline. While the latest evidence, based on a sample of nearly 2500 long-term HCQ users, suggests that ABW-based dosing is a better predictor of toxicity [14], confirming these findings in a prospective study would be valuable. In the meantime, some authors advocate using the lower of the two methods to minimize retinopathy risk [25]. Nevertheless, the impact of IBW- or ABW-based dosing treatment efficacy of HCQ remains unknown, calling for future investigations.
Our study has several strengths and limitations which warrant recognition. Our source population KPNC is generally representative of the residents of that region [26]. As such, our study captured a diverse patient population as well as prescription dosing by multiple providers and provider specialties and provides population-level data on HCQ prescribing patterns in relation to HCQ dosing guidelines. Furthermore, our study was substantially longer and larger than previous single-center studies, providing higher precision in our results [18, 19]. However, our data lacked information regarding disease activity for patients with SLE, RA, and other conditions, limiting our ability to assess its potential relationship with initial HCQ dosing. It would be valuable for future studies to address this issue.
In conclusion, in this large population-based cohort, we found that there has been a sharp decline in HCQ dosing, corresponding with 2011 AAO guidelines on weight-based HCQ dosing. While this trend is likely helpful in reducing the risk of retinopathy, its potential impact on HCQ’s anti-rheumatic efficacy remains to be clarified to allow assessment of the net impact of this dramatic dosing trend.
Acknowledgments
Funding information Dr. Jorge is supported in part by the T32 Ruth L. Kirschstein Institutional National Research Service Award from the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (T32-AR-007258).
Dr. Marmor is supported in part by Research to Prevent Blindness.
Dr. Choi is supported by the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (P50-AR-AR060772).
Footnotes
Compliance with ethical standards
The study was performed under KPNC Institutional Review Board approval and complied with tenets of the Declaration of Helsinki. Informed consent was waived.
Disclosures None.
References
- 1.Ruiz-Irastorza G, Khamashta M (2008) Hydroxychloroquine: the cornerstone of lupus therapy. Lupus 17:271–273 [DOI] [PubMed] [Google Scholar]
- 2.Bertsias G, Ioannidis JP, Boletis J, Bombardieri S, Cervera R, Dostal C, Font J, Gilboe IM, Houssiau F, Huizinga T, Isenberg D, Kallenberg CG, Khamashta M, Piette JC, Schneider M, Smolen J, Sturfelt G, Tincani A, van Vollenhoven R, Gordon C, Boumpas DT (2008) Task force of the ESCfICSITEULAR recommendations for the management of systemic lupus erythematosus Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis 67(2):195–205. 10.1136/ard.2007.070367 [DOI] [PubMed] [Google Scholar]
- 3.Petri M (2011) Use of hydroxychloroquine to prevent thrombosis in systemic lupus erythematosus and in antiphospholipid antibody-positive patients. Curr Rheumatol Rep 13:77–80 [DOI] [PubMed] [Google Scholar]
- 4.Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM, Vila LM, Reveille JD, Group LS (2007) Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 66(9):1168–1172. 10.1136/ard.2006.068676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, Urowitz M, Fortin PR, Petri M, Barr S, Gordon C, Bae SC, Isenberg D, Zoma A, Aranow C, Dooley MA, Nived O, Sturfelt G, Steinsson K, Alarcon G, Senecal JL, Zummer M, Hanly J, Ensworth S, Pope J, Edworthy S, Rahman A, Sibley J, El-Gabalawy H, McCarthy T, St Pierre Y, Clarke A, Ramsey-Goldman R (2006) Mortality in systemic lupus erythematosus. Arthritis Rheum 54(8):2550–2557. 10.1002/art.21955 [DOI] [PubMed] [Google Scholar]
- 6.Clowse ME, Magder L, Witter F, Petri M (2006) Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 54(11):3640–3647. 10.1002/art.22159 [DOI] [PubMed] [Google Scholar]
- 7.Cairoli E, Rebella M, Danese N, Garra V, Borba EF (2012) Hydroxychloroquine reduces low-density lipoprotein cholesterol levels in systemic lupus erythematosus: a longitudinal evaluation of the lipid-lowering effect. Lupus 21:1178–1182 [DOI] [PubMed] [Google Scholar]
- 8.O’Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, Lew RA, Cannella AC, Kunkel G, Phibbs CS, Anis AH, Leatherman S, Keystone E (2013) Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med 369(4):307–318. 10.1056/NEJMoa1303006 [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA (2010) Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 69(1): 20–28. 10.1136/ard.2008.101766 [DOI] [PubMed] [Google Scholar]
- 10.Costedoat-Chalumeau N, Dunogue B, Leroux G, Morel N, Jallouli M, Le Guern V, Piette JC, Brezin AP, Melles RB, Marmor MF (2015) A critical review of the effects of hydroxychloroquine and chloroquine on the eye. Clin Rev Allergy Immunol 49(3):317–326. 10.1007/s12016-015-8469-8 [DOI] [PubMed] [Google Scholar]
- 11.LG D, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T (1997) Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis Rheum 40(8):1482–1486 [DOI] [PubMed] [Google Scholar]
- 12.Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, Mavrikakis M (2003) The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine. Ophthalmology 110(7):1321–1326. 10.1016/s0161-6420(03)00409-3 [DOI] [PubMed] [Google Scholar]
- 13.Wolfe F, Marmor MF (2010) Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 62(6):775–784. 10.1002/acr.20133 [DOI] [PubMed] [Google Scholar]
- 14.Melles RB, Marmor MF (2014) The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol 132(12):1453–1460. 10.1001/jamaophthalmol.2014.3459 [DOI] [PubMed] [Google Scholar]
- 15.Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF (2011) Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 118(2):415–422. 10.1016/j.ophtha.2010.11.017 [DOI] [PubMed] [Google Scholar]
- 16.Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, American Academy of O (2016) Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology 123(6):1386–1394. 10.1016/j.ophtha.2016.01.058 [DOI] [PubMed] [Google Scholar]
- 17.Devine B (1974) Case number 25: gentamicin therapy. Drug Intelligence and Clin Pharm 8:650–655 [Google Scholar]
- 18.Gianfrancesco MA, Schmajuk G, Haserodt S, Trupin L, Izadi Z, Jafri K, Shiboski S, Sirota M, Adams Dudley R, Yazdany J (2017) Hydroxychloroquine dosing in immune-mediated diseases: implications for patient safety. Rheumatol Int 37:1611–1618. 10.1007/s00296-017-3782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braslow RA, Shiloach M, Macsai MS (2017) Adherence to hydroxychloroquine dosing guidelines by rheumatologists: an electronic medical record-based study in an integrated health care system. Ophthalmology 124:604–608. 10.1016/j.ophtha.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 20.Feldman CH, Collins J, Zhang Z, Subramanian SV, Solomon DH, Kawachi I, Costenbader KH (2018) Dynamic patterns and predic-tors of hydroxychloroquine nonadherence among Medicaid benefi-ciaries with systemic lupus erythematosus. Semin Arthritis Rheum 10.1016/j.semarthrit.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anthropometric Reference Data for Children and Adults: United States, 2007–2010 (2012. October). US Department of Health and Human Services, CDC 11 (252) [PubMed] [Google Scholar]
- 22.Chasset F, Arnaud L, Costedoat-Chalumeau N, Zahr N, Bessis D, Frances C (2016) The effect of increasing the dose of hydroxychloroquine (HCQ) in patients with refractory cutaneous lupus erythematosus (CLE): an open-label prospective pilot study. J Am Acad Dermatol 74(4):693–699.e693. 10.1016/j.jaad.2015.09.064 [DOI] [PubMed] [Google Scholar]
- 23.Frances C, Cosnes A, Duhaut P, Zahr N, Soutou B, Ingen-Housz-Oro S, Bessis D, Chevrant-Breton J, Cordel N, Lipsker D, Costedoat-Chalumeau N (2012) Low blood concentration of hydroxychloroquine in patients with refractory cutaneous lupus erythematosus: a French multicenter prospective study. Arch Dermatol 148(4):479–484. 10.1001/archdermatol.2011.2558 [DOI] [PubMed] [Google Scholar]
- 24.Durcan L, Clarke WA, Magder LS, Petri M (2015) Hydroxychloroquine blood levels in systemic lupus erythematosus: clarifying dosing controversies and improving adherence. J Rheumatol 42(11):2092–2097. 10.3899/jrheum.150379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browning DJ, Lee C, Rotberg D (2014) The impact of different algorithms for ideal body weight on screening for hydroxychloroquine retinopathy in women. Clin Ophthalmol(Auckland, NZ) 8:1401–1407. doi: 10.2147/opth.s66531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger N (1992) Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health 82(5):703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request and with permission from Kaiser Permanente Northern California Division of Research.


