Abstract
Pathogen-specific biomarkers are secreted in the host during infection. Many important biomarkers are not proteins but rather small molecules that cannot be directly detected by conventional methods. However, these small molecule biomarkers, such as phenolic glycolipid-I (PGL-I) of Mycobacterium leprae and Mycobactin T (MbT) of Mycobacterium tuberculosis, are critical to the pathophysiology of infection, and may be important in the development of diagnostics, vaccines, and novel therapeutic strategies. Methods for the direct detection of these biomarkers may be of significance both for the diagnosis of infectious disease, and also for the laboratory study of such molecules. Herein, we present, for the first time, a transduction approach for the direct and rapid (30 min) detection of small amphiphilic biomarkers in complex samples (e.g. serum) using a single affinity reagent. To our knowledge, this is the first demonstration of an assay for the direct detection of PGL-I, and the first single-reporter assay for the detection of MbT. The assay format exploits the amphiphilic chemistry of the small molecule biomarkers, and is universally applicable to all amphiphiles. The assay is only the first step towards developing a robust system for the detection of amphiphilic biomarkers that are critical to infectious disease pathophysiology.
Keywords: Amphiphiles, Biomarkers, Phenolic glycolipid, Mycobactin, Lipid bilayer, Membrane insertion assay
1. Introduction
Pathogens secrete characteristic molecules that may be useful for their growth, survival, and pathogenesis in an infected host, and are often signatures of infection (aka biomarkers). Many of these biomarkers, such as Lipoarabinomannan (LAM) from Mycobacteriumtuberculosis (Kaur et al., 2009; Mishra et al., 2011), and lipopolysaccharide (LPS) from Escherichia coli (Arenas, 2012) (Fig. 1A), are not proteins, but are lipidated glycans (Ray et al., 2013) that are critical to bacterial virulence. Indeed, many such biomarkers are known innate immune agonists that are secreted and present in the infected host very early in infection, and thus are potential diagnostic targets for infectious diseases. Indeed, the direct detection of both LAM and LPS has been used for the diagnosis of tuberculosis (Minion et al., 2011) and food poisoning (De Boer and Heuvelink, 2000). The detection of said biomarkers directly in blood, however, has been elusive. As suggested by their biochemistry, many amphiphilic biomarkers do not occur in monomeric confirmation in the aqueous host vasculature, but are found in association with host carrier proteins. For instance, it has been demonstrated that LPS (Levine et al., 1993; Van Amersfoort et al., 2003) and LAM (Sakamuri et al., 2013a) associate with host high-density lipoprotein (HDL) in blood, and this interaction may play a critical role in the distribution, recognition, and clearance of these molecules. Understanding the interaction of amphiphilic virulence factors, and studying their distribution and expression in the infected host is therefore critical to efficient prevention, diagnosis, and treatment of infectious diseases.
Fig. 1.
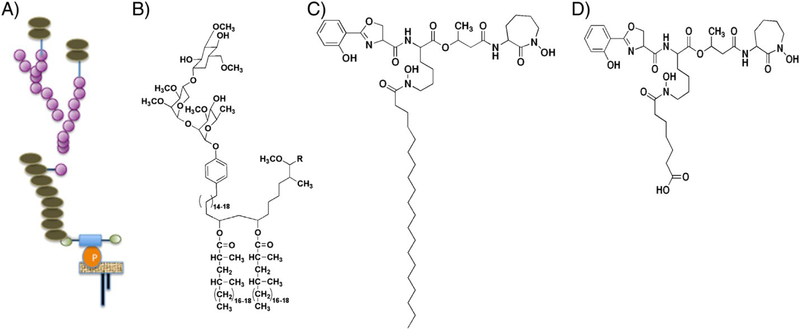
Schematic illustration of the structure of (A) lipoarabinomannan, (C) MbT, (D) carboxymycobactin T from Mycobacterium tuberculosis, and (B) PGL-I from Mycobacterium leprae (not drawn to scale).
Many amphiphilic virulence factors of relevance to bacterial pathogenesis are small molecules that cannot be efficiently studied by conventional methodologies because (1) conventional methods such as ELISA/lateral flow assays are associated with lower specificity and sensitivity, especially in complex biological matrices such as blood, and cannot be used to interrogate the very small circulating concentrations of bacterial biomarkers in the host; (2) for small molecules (e.g. phenolic glycolipids (PGL), of mycobacteria or bacterial siderophores; Ratledge, 2004; Spencer and Brennan, 2011), two recognition ligands that bind orthogonal epitopes are not available, precluding their detection by classic sandwich immunoassays. These biomarkers cannot be conjugated to classical surfaces (ELISA plates, nitrocellulose filter paper) because of their biochemical and solubility properties, making direct detection impossible. Beyond these, the use of conventional platforms (e.g. lateral flow, ELISA, and flow cytometry) developed for protein targets in the detection of lipidated sugars is challenging in itself. Because of these issues, direct detection of amphiphilic pathogen biomarkers, especially small molecules, is not extensively practiced, and biomarker-based detection of infectious diseases has largely relied on serological methods (Herrera et al., 2011). The development of effective strategies for the direct detection of small molecule non-protein biomarkers in a complex background can thus improve our understanding of bacterial pathogenesis, and identify novel diagnostic, vaccine, and therapeutic targets for infectious diseases.
As mentioned earlier, many biomarkers and virulence factors secreted by bacteria (Fig. 1) are amphiphilic, comprising hydrophobic (lipid) and hydrophilic (carbohydrate, peptide, or protein) moieties (Ray et al., 2013). Large amphiphilic biomarkers such as LPS, lipoteichoic acid (LTA, Gram-positive bacteria), and LAM (Fig. 1A) can be detected by sandwich immunoassays because antibodies recognizing orthogonal epitopes of the target are available (De Boer and Heuvelink, 2000; Minion et al., 2011), but such approaches cannot be used for the direct detection of small molecules such as phenolic glycolipid-I (PGL-I) of M. leprae and Mycobactin T (MbT) of M. tuberculosis (Fig. 1B and C). MbT is a siderophore secreted by M. tuberculosis for the sequestration of iron from the host. MbT and carboxy-MbT (Fig. 1C and D) are produced at high concentrations by mycobacteria in vitro during the logarithmic phase of growth under conditions of iron limitation (De Voss et al., 1999; Ratledge, 2004). PGLs are mycobacterial cell wall components and critical virulence factors. PGL in M. tuberculosis is speculated to be an indicator of hypervirulence (Onwueme et al., 2005; Reed et al., 2004). PGL-I in M. leprae is responsible for the neurotropism of the disease and is the basis for the serodiagnosis of leprosy (Cho et al., 1986; Moura et al., 2008; Spencer and Brennan, 2011; Young et al., 1985). This molecule has been found in large concentrations in tissues of experimentally infected nine- banded armadillos (Spencer and Brennan, 2011).
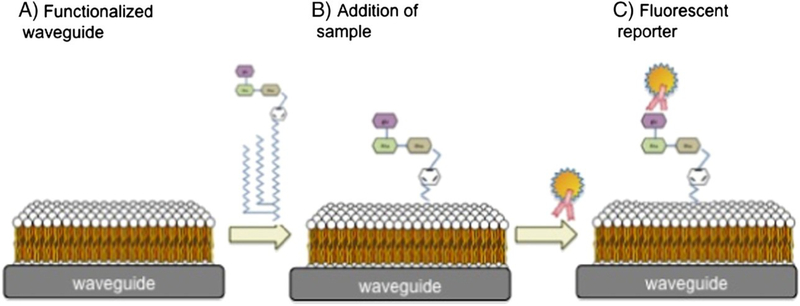
Herein we report a simple method, termed membrane insertion, to detect small molecule amphiphiles with a single recognition ligand by exploiting their association/interaction with a supported lipid bilayer (Fig. 2). We have previously demonstrated the use of this technology for the detection of large molecules (amphiphiles and other), using a waveguide-based biosensor platform that was developed at Los Alamos National Laboratory (Mukundan et al., 2012a, b).The results reported herein represent the first step in translating this technology for the detection of small molecules that have a significant role to play in the pathophysiology of infectious diseases, but yet remain poorly studied to date. This is likely because of their complex biochemistry, and the fact that conventional assay technologies are largely tailored to suit proteins and nucleic acids, and not lipoglycans. The strategy presented in this manuscript is an assay transduction concept, and is not limited to any particular sensor platform for application, that applies to Lipidated sugars of biological significance.
Fig. 2.
Schematic representation of the membrane-based assay for amphiphilic PAMPs and virulence factors on the waveguide-based optical biosensor platform.
2. Materials and Methods
2.1. Materials
PGL-I and a rabbit polyclonal antibody for the biomarker were obtained from the Leprosy Materials Consortium at the Colorado State University (now BEI Resources). Mycobactin J was obtained from Allied Monitor. Human and bovine serum were purchased from Biomedical Technologies and Hyclone Laboratories, respectively. Alexa Fluor 647 (AF647) Protein Labeling kit was procured from Invitrogen. MbT and anti-mycobactin monoclonal antibodies were generated as previously described (Capek et al., submitted for publication).
2.2. Waveguide-based optical biosensor
Experiments were performed on a waveguide-based biosensor platform, which we have previously applied to the detection of biomarkers associated with breast cancer (Mukundan et al., 2009a,b,c), anthrax (Mukundan et al., 2010), influenza (Kale et al., 2008), and tuberculosis (Mukundan et al., 2012b) using either a sandwich immunoassay or membrane insertion assay as the transduction approach (Mukundan et al., 2012b;Mukundan et al., 2012a; Sakamuri et al., 2013b). Detection of biomarkers within the evanescent field of a planar optical waveguide enhances sensitivity, and minimizes background signal from excitation of fluorescence from impurities in complex biological samples (a result of the short penetration depth of the evanescent field into the sample above the waveguide) (Mukundan et al., 2009a,b,c). All measurements are made on an OceanOptics spectrometer interfaced with the instrument (Mukundan et al., 2009a,b,c).
2.3. Preparation of fluorescently labeled antibodies
Antibodies specific to the biomarkers were labeled with fluorescent dyes (AF647), and characterized by indirect and competition immunoassays using established methods (Mukundan et al., 2009b,c). Antibody concentrations and time of incubation were optimized using immunoassays, and then applied to the waveguide experiment described below.
2.4. Preparation of lipid bilayer as a functional surface on the waveguide
Methods for the functionalization of waveguide surfaces with supported lipid bilayers have been described in detail previously (Martinez et al., 2005; Mukundan et al., 2009b,c). Briefly, waveguide surfaces were cleaned by sonication in chloroform, ethanol and water, followed by UV-ozone cleaning for 40 min. Biotinylated (0.1%) unilamellar vesicles (bilayers) of 1,2- dioleoyl-sn-glycero-3-phosphocholine (DOPC) were prepared, as elsewhere (Martinez et al., 2005; Mukundan et al., 2009b,c). The waveguides were functionalized by vesicle fusion to form supported DOPC bilayers. Biotinylation permits the evaluation of the bilayer integrity at the end of each experiment (see below). In all experiments, the bilayer was allowed to stabilize overnight, and then mounted in a flow cell, as described elsewhere (Martinez et al., 2005). To minimize non-specific interactions, bilayers were blocked for 1 h with phosphate buffered saline (PBS) (pH 7.4) containing 2% bovine serum albumin (BSA).
2.5. Membrane insertion assay for biomarker detection
Because of their extremely hydrophobic nature, MbT and PGL-I were solubilized in dimethyl sulfoxide (DMSO) and ethanol, respectively, and diluted in PBS or serum immediately prior to experimentation. After blocking, the waveguides were washed and laser light was in-coupled through the integrated grating, and the background (an intrinsic measure of impurities associated with the waveguide) was measured before each experiment (Martinez et al., 2005). Non-specific binding was estimated by the addition of fluorescently labeled reporter antibody to the lipid bilayer in the appropriate solvent dilution (DMSO or ethanol, depending on the assay), and the fluorescence signal was measured (Figs. 3 and 4, non-specific binding curves). This provides an accurate measurement of signal associated with interaction of the labeled reporter antibody with the functionalized waveguide surface, in the absence of biomarker (antigen), in each experiment (i.e., no antigen control). At the end of all experiments, 50 pM streptavidin, fluorescently labeled with AF647, was added to the flow cell (data not shown) and the predictable signal was measured as an indicator of surface stability, especially considering exposure to small concentrations of DMSO/ethanol during the experiment. Power coupled into the waveguide was measured before and after each experiment as a metric for the consistency of optical parameters during the measurement. For the measurement of PGL-I, the antigen (in DMSO) was injected into the flow cell at various concentrations in PBS or spiked in bovine serum for 1 hr at room temperature (RT), followed by addition of AF647 labeled anti-PGL-I reporter antibody (20 nM, 10 min, RT). Dilution of the antigen was made immediately before addition. For measurement of MbT, the antigen (in Ethanol) was injected into the flow cell at various concentrations in PBS or spiked in human serum (1:10 dilution, 1 h, RT), followed by AF647-labeled anti-MbT antibody (150 nM, 10 min, RT). Following washing with PBS (2 ml, 60× flow cell volume), the specific fluorescence signal associated with antigen–antibody interaction was measured using the spectrometer interface.
Fig. 3.
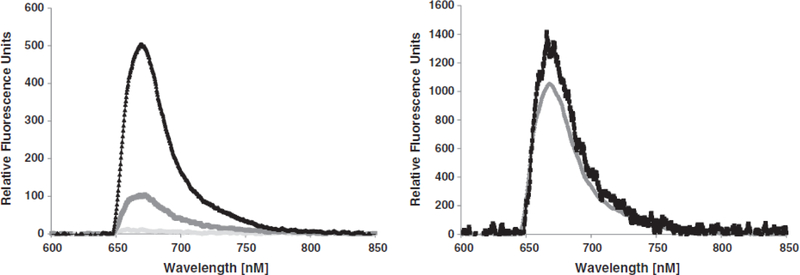
Detection of PGL-I in bovine or human serum using the insertion assay on the optical biosensor. Left: 6 μM of PGL-I detection in bovine serum. Black triangles indicate positive signal for detection of PGL-I spiked in bovine serum. Dark gray line indicates measure of non-specific binding of the reporter antibody with control serum and light gray line indicates the waveguide-associated background. Right: Specific signals with different concentrations of PGL-I (13 μm [black lines] and 20 μm [gray line]) spiked in human serum with the insertion assay. The non- specific binding associated with the reporter antibody was measured and subtracted from the specific data before plotting.
Fig. 4.
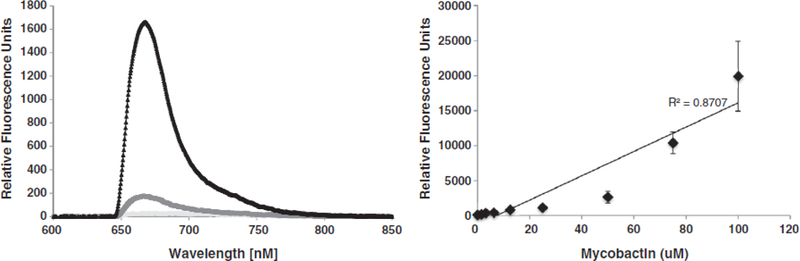
Concentration dependence of the insertion of MbT. Left: Prototype measurement of 50 μM of MbT in human serum using the membrane insertion assay. Light gray line indicates the waveguide-associated background. Dark gray circles indicate non-specific background and black triangles show specific detection of MbT. Right: Standard curve showing concentration dependent increase in signal for the detection of MbT in PBS.
3. Results and Discussion
Detection of 6 μM of PGL-I of M. leprae spiked into bovine serum (limit of detection, 500 nM) is shown in Fig. 3, with the measurements performed within 15 min of spiking of the biomarker into serum. The specific signal was measured in six experiments, and signal intensity that is≥ measured signal+3 (standard deviation) was considered positive. The limit of detection was also calculated using the same metric. We observed a concentration-dependent increase in the signal associated with the association of PGL-I with the lipid bilayer (Fig. 3), with no concomitant increase in non-specific binding. The non-specific binding did not change in the presence of the solvent or control serum (Fig. 3), neither did the streptavidin AF647 signal.
The limit of detection of MbT is 1 μM in spiked serum, with a concentration-dependent increase in signal associated with insertion of the biomarker into the supported lipid bilayer in Fig. 4. We obtain an r2 value of 0.7 over a concentration range of 1 to 100 μM, which indicates an acceptable, albeit imperfect, linearity. We also evaluated the ability of the antibodies to bind carboxy-MbT, and demonstrate an equivalent binding affinity in membrane insertion assays (limit of detection is 1 μM). The antibodies are specific to MbT and carboxy MbT and do not bind mycobactin J, demonstrating specificity of detection for M. tuberculosis (data not shown).
The sensitivity of both assays is ultimately limited by the binding affinity of the reporter antibodies to the respective biomarker(s). The sensitivities for the detection ofMbT (1 μM) and PGL-I (500 nM) can potentially be enhanced by using higher-affinity antibodies. It is possible that different phospholipids, and consequently different displays of the hydrophilic portion of these small amphiphiles to the affinity reagents, may affect insertion kinetics and binding affinities. The specificity of the assay is also limited by the specificity of the antibody. For the PGL-I assay, the antibody used in the current assay is highly specific. Although the lipid moiety and the two rhamnose sugars (attached to the phenol ring) of PGL of Mycobacterium leprae (PGL-1) and that of M. tuberculosis are very similar, they differ in the terminal sugar, which is glucose in PGL-I and fucose in case of PGL-TB, and also the methylation and the linkage of the sugars are different. The antibody in the current assay binds to the two sugars rhamnose-glucose moeity of the PGL-I, but not to rhamnose-fucose of PGL-TB (Cho et al., 1986). For the MbT assay, the antibody used in the current assay is very specific to Mycobactin T but not to Mycobactin J and Mycobactin S (Capek et al., submitted for publication).
3.1. Detection of PGL-1
Currently, serological approaches are used to measure the host immune response to PGL-I for the diagnosis of leprosy (Moura et al., 2008; Spencer and Brennan, 2011). These assays have limited diagnostic value because of their inability to distinguish between subclinical infection, active disease, and inactive infection. Furthermore, it has been reported that there is no significant decrease of PGL-I antibodies following multi-drug treatment, the reduction rate of the antibodies being 50% after 2 years (Cho et al., 2001). Therefore, measuring antibodies to PGL-I is not a viable strategy for monitoring prognosis or relapse. However, PGL-I biomarker levels in the serum dropped rapidly by about 50% in 2 weeks of chemotherapy (Cho et al., 2001), indicating that measurement of the biomarker itself can help monitor prognosis and relapse. Furthermore, direct detection of PGL-I signifies active disease, but could not be performed routinely with previously available strategies.
Earlier work on direct detection of PGL-I involved tedious sample preparation processes, i.e., lipid extraction from serum, followed by immunoblot-based detection (Cho et al., 2001; Young et al., 1985). In fact, detection is mainly based on the color intensity (visual observation) of the spots on the immunoblot and then comparing with the PGL-I standards spotted on the same immunoblot. However, this approach was labor-intensive, had poor sensitivity, and was not useful in case of low bacillary index leprosy patients (Cho et al., 2001). Yet, PGL-I remains the most reliable biomarker for leprosy. It therefore follows that the development of methods for direct detection of PGL-1, and tracking in host serum will be of immense benefit to the understanding of the pathophysiology of leprosy, and to the development of diagnostics and therapeutics for the disease.
The approach described herein could provide a reliable and simple strategy for the direct detection of PGL-I in serum without intensive sample processing. It is noted that the sensitivity of the assay should be further improved, likely by the development of more specific and sensitive recognition ligands, or clinical samples should be concentrated before use in this assay format. Indeed, membrane insertion is in itself a viable strategy for the concentration of amphiphilic biomarkers like PGL-1 from serum. Adaptation of this technology to the detection of PGL from M. tuberculosis may allow for the rapid identification of hypervirulent strains, thereby guiding appropriate intervention strategies.
We note that the direct use of membrane insertion for the detection of amphiphiles may be compromised in samples that contain lipoproteins or other lipid assemblies (e.g., blood and serum). Indeed, previous work from our laboratory and others has suggested that amphiphilic biomarkers like LPS and LAM associate with carrier molecules like HDL in host serum (Sakamuri et al., 2013a). Such interactions are possible for PGL-1 as well, given its amphiphilic biochemistry, but have not been reported. To address the potential interaction of PGL-I with lipid carriers in serum, our assays were performed immediately after the antigen was added to the PBS and/or in serum to minimize time required for the association with lipid carriers. The fact that the association was minimal or absent was confirmed by the fact that the specific signal measured for a given concentration of the antigen (in the linear range of assay performance) was not significantly lower than that observed in lipid-free buffer. Membrane insertion studies can be used to understand the physiological confirmation of amphiphiles like PGL-1, and their association with carrier molecules, in infected serum. Without this understanding, direct detection strategies, and targeted countermeasures are less likely to succeed when translated to the human host from in vitro studies. The different confirmation and presentation of the biomarker in vitro compared with in physiological systems can also account for the difficulty in generating robust and sensitive recognition ligands for amphiphilic targets.
3.2. Detection of MbT
Production of MbT and carboxy-MbT is regulated by the concentration of iron available in the host during infection, and biosynthesis of the siderophore is known to play an essential role in bacterial virulence (Krithika et al., 2006). Previously, mycobactin has been measured primarily in bioassays that determine the inhibition of growth in an iron-starved environment, or by lipidomic profiling studies using mass spectrometry (Madigan et al., 2012). Herein, we have shown a novel and simple approach for the direct detection of MbT in spiked serum. This approach can be extended to other amphiphilic siderophores, and may allow for the rapid and quantitative extrapolation of activity in biological assays. Again, the interaction of MbT with carrier molecules and other proteins (e.g. siderocalins) should be considered before this assay is applied to physiological systems. To address this, MbT insertion in serum was also studied immediately after addition of the biomarker into serum, and the observed results were not significantly different from those in lipid-free buffers.
Thus, we did not observe any significant effects of incubation in serum on the results. Indeed, when the biomarkers were incubated in serum overnight, we observed that although the signal was slightly lower than before, the results were not significantly different for both antigens. This is very different from what we have seen before for other amphiphiles (e.g. Lipoarabinomannan (Sakamuri et al., 2013a,b) and Lipopolysacharide (unpublished data)). This could be because of many reasons: (1) PGL-1 and mycobactin do not bind to carrier molecules in serum; (2) the interaction of small amphiphiles with carrier molecules is transient, such that at any given time there is a significant concentration of the biomarker in free-form, capable of inserting into the supported lipid bilayer. It is definitely possible that the transient nature of this interaction is responsible for the non-linearity observed in Fig. 4. Further studies are required to assess the validity of each possibility. However, both in the case of PGL-1 and MbT, simple sonication can dissociate host associations, and release monomeric or assembled lipidic biomarkers, making them available to insert in the supported bilayers, as required for membrane insertion.
4. Conclusion
The results presented in this communication demonstrate, for the first time, a rapid and universal strategy for the sensitive and specific detection of small amphiphilic biomarkers of relevance to infectious disease. It is noted that the sensitivity and specificity of the assay, in current format, are dependent on the antibody used. However, using reagent free assay platforms (e.g. impedance-based or interferometric measurements), the need for antibodies can potentially be eliminated, making this assay more widely usable. Small amphiphiles constitute a significant population of pathogenic biomarkers of infectious disease. Examples include, but are not limited to, trehalose dimycolates (Hunter et al., 2006) and glycopeptidolipids (Schorey and Sweet, 2008) of mycobacteria, rhamnolipids of Pseudonomas spp., quorum-sensing molecules like acyl homoserine lactones, sophorolipids of Candida spp., amphiphilic siderophores, and others. Indeed, all these molecules are structurally diverse: LAM and PGL-I (Fig. 1A and B) are glycolipids, whereas MbT (Fig. 1C) has a hydroxyphenyloxazoline moiety linked to acylated hydroxylysine via an amide bond (De Voss et al., 1999; Ratledge, 2004). However, all of them share a common amphiphilic nature, which may be critical in their in host biochemistry. It is noteworthy that many bacterial biomarkers recognized by the host innate immune response receptors (e.g. toll-like receptors) very early in infection are amphiphilic in nature (Kawai and Akira, 2010). Developing methods to detect such small molecule amphiphiles is therefore critical to our ability to understand disease pathophysiology, developing effective methods for detection and countermeasure design. To conclude, we have exploited the common amphiphilic feature of small molecule biomarkers to develop a novel transduction strategy to rapidly capture and detect them. The results reported in this manuscript are only the first step towards developing a robust system for the detection and characterization of critical small molecule amphipiles in clinical samples.
Acknowledgments
The authors thank Dr. L. Via at the TB Research Section of the NIAID, for technical discussions and suggestions. We thank K.W. Grace, A.S. Anderson, and Felicia Archuleta for technical help and helpful consultation. This work was supported by a LANL LDRD Directed Research Award to Drs. B.I. Swanson and B.T. Korber, LDRD Exploratory Research Awardto Dr. H.Mukundan, and the Bill and Melinda Gates Foundation through the Tuberculosis Drug Accelerator Program (C.E.B. and T.J.D.). The authors thank BEI Resources (Colorado State Materials Consortium) for the antigens and antibodies used for the PGL-1 assay.
References
- Arenas J, 2012. The role of bacterial lipopolysaccharides as immunemodulator in vaccine and drug development. Endocr. Metab. Immune Disord. Drug Targets 12, 221–235. [DOI] [PubMed] [Google Scholar]
- Capek P,McBride NS, Dona V, Angrish D, Tahlan K, Saccavini C, Jiang B, Boshoff H, Barry CEI, Dickerson TJ, 2014. Total synthesis of Mycobactin S and T: synthetic revision and biological properties. J. Am. Chem. Soc (Submitted for publication).
- Cho SN, Hunter SW, Gelber RH, Rea TH, Brennan PJ, 1986. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis 153, 560–569. [DOI] [PubMed] [Google Scholar]
- Cho SN, Cellona RV, Villahermosa LG, Fajardo TT Jr., Balagon MV, Abalos RM, Tan EV,Walsh GP, Kim JD, Brennan PJ, 2001. Detection of phenolic glycolipid I of Mycobacterium leprae in sera from leprosy patients before and after start of multidrug therapy. Clin. Diagn. Lab. Immunol 8, 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer E, Heuvelink AE, 2000. Methods for the detection and isolation of Shiga toxin producing Escherichia coli. Symp. Ser. Soc. Appl. Microbiol 133S–143S. [DOI] [PubMed]
- De Voss JJ, Rutter K, Schroeder BG, Barry CE III, 1999. Iron acquisition and metabolism by mycobacteria. J. Bacteriol 181, 4443–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera V, Perry S, Parsonnet J, Banaei N, 2011. Clinical application and limitations of interferon-gamma release assays for the diagnosis of latent tuberculosis infection. Clin. Infect. Dis 52, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Hunter RL, Olsen MR, Jagannath C, Actor JK, 2006. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann. Clin. Lab. Sci 36, 371–386. [PubMed] [Google Scholar]
- Kale RR, Mukundan H, Price DN, Harris JF, Lewallen DM, Swanson BI, Schmidt JG, Iyer SS, 2008. Detection of intact influenza viruses using biotinylated biantennary Ssialosides. J. Am. Chem. Soc 130, 8169–8171. [DOI] [PubMed] [Google Scholar]
- Kaur D, Guerin ME, Skovierova H, Brennan PJ, Jackson M, 2009. Chapter 2: Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Adv. Appl. Microbiol 69, 23–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S, 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol 11, 373–384. [DOI] [PubMed] [Google Scholar]
- Krithika R, Marathe U, Saxena P, Ansari MZ, Mohanty D, Gokhale RS, 2006. A genetic locus required for iron acquisition in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A 103, 2069–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL, 1993. In vivo protection against endotoxin by plasma high density lipoprotein. Proc. Natl. Acad. Sci. U. S. A 90, 12040–12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan CA, Cheng TY, Layre E, Young DC, McConnell MJ, Debono CA, Murry JP, Wei JR, Barry CE 3rd, Rodriguez GM, Matsunaga I, Rubin EJ, Moody DB, 2012Lipidomic discovery of deoxysiderophores reveals a revised mycobactin biosynthesis pathway in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A 109, 1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JS, Grace WK, Grace KM, Hartman N, Swanson BI, 2005. Pathogen detection using single mode planar optical waveguides. J. Mater. Chem 15, 4639–4647. [Google Scholar]
- Minion J, Leung E, Talbot E, Dheda K, Pai M, Menzies D, 2011. Diagnosing tuberculosis with urine lipoarabinomannan: systematic review and meta-analysis. Eur. Respir. J 38, 1398–1405. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Driessen NN, Appelmelk BJ, Besra GS, 2011. Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host–pathogen interaction. FEMS Microbiol. Rev 35, 1126–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura RS, Calado KL, Oliveira ML, Buhrer-Sekula S, 2008. Leprosy serology using PGL-I: a systematic review. Rev. Soc. Bras. Med. Trop 41 (Suppl. 2), 11–18. [DOI] [PubMed] [Google Scholar]
- Mukundan H, Anderson AS, Grace WK, Grace KM, Hartman N, Martinez JS, Swanson BI, 2009a. Waveguide-based sensors for pathogen detection. Sensors 9, 5783–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundan H, Holt A, Shively JE, Martinez JS, Grace K, Grace WK, Swanson BI, 2009b. Planar optical waveguides for the quantitative detection of tumor markers. Sensors Actuators B 138, 453–460. [Google Scholar]
- Mukundan H, Xie H, Anderson AS, Grace WK, Shively JE, Swanson BI, 2009c. Optimizing a waveguide- based sandwich immunoassay for tumor biomarkers: evaluating fluorescent labels and functional surfaces. Bioconjug. Chem 20, 222–230. [DOI] [PubMed] [Google Scholar]
- Mukundan H, Xie H, Price D, Kubicek-Sutherland JZ, Grace WK, Anderson AS, Martinez JS, Hartman N, Swanson BI, 2010. Quantitative multiplex detection of pathogen biomarkers on multichannel waveguides. Anal. Chem 82, 136–144. [DOI] [PubMed] [Google Scholar]
- Mukundan H, Kumar S, Price DN, Ray SM, Lee YJ, Min S, Eum S, Kubicek- Sutherland J, Resnick JM, Grace WK, Anderson AS, Hwang SH, Cho SN, Via LE, Barry C 3rd, Sakamuri R, Swanson BI, 2012a. Rapid detection of Mycobacterium tuberculosis biomarkers in a sandwich immunoassay format using a wave guide based optical biosensor. Tuberculosis 92, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundan H, Price DN, Goertz M, Parthasarathi R, Montano GA, Kumar S, Scholfield MR, Anderson AS, Gnanakaran S, Iyer S, Schmidt J, Swanson BI, 2012b. Understanding the interaction of Lipoarabinomannan with membrane mimetic architectures. Tuberculosis 92, 38–47. [DOI] [PubMed] [Google Scholar]
- Onwueme KC, Vos CJ, Zurita J, Ferreras JA, Quadri LE, 2005. The dimycocerosate ester polyketide virulence factors of mycobacteria. Prog. Lipid Res 44, 259–302. [DOI] [PubMed] [Google Scholar]
- Ratledge C, 2004. Iron, mycobacteria and tuberculosis. Tuberculosis 84, 110–130. [DOI] [PubMed] [Google Scholar]
- Ray A, Cot M, Puzo G, Gilleron M, Nigou J, 2013. Bacterial cell wall macroamphiphiles: pathogen-/microbe- associated molecular patterns detected by mammalian innate immune system. Biochimie 95, 33–42. [DOI] [PubMed] [Google Scholar]
- Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE III, 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431, 84–87. [DOI] [PubMed] [Google Scholar]
- Sakamuri RM, Price DN, Lee M, Cho SN, Barry CE III, Via LE, Swanson BI, Mukundan H, 2013a. Association of lipoarabinomannan with high density lipoprotein in blood: implications for diagnostics. Tuberculosis 93, 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamuri RM, Wolfenden MS, Anderson AS, Swanson BI, Schmidt JS, Mukundan H, 2013b. Novel optical strategies for biodetection. In: Mohseni H, Agahi MH, Razeghi M (Eds.), Biosensing and Nanomedicine VI, Vol. 812, In SPIE NanoScience + Engineering, International Society for Optics and Photonics., San Diego, California, United States: 881209-881209. [Google Scholar]
- Schorey JS, Sweet L, 2008. The mycobacterial glycopeptidolipids: structure, function, and their role in pathogenesis. Glycobiology 18, 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JS, Brennan PJ, 2011. The role of Mycobacterium leprae phenolic glycolipid I (PGL-I) in serodiagnosis and in the pathogenesis of leprosy. Lepr. Rev 82, 344–357. [PubMed] [Google Scholar]
- Van Amersfoort ES, Van Berkel TJ, Kuiper J, 2003. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev 16, 379–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DB, Harnisch JP, Knight J, Buchanan TM, 1985. Detection of phenolic glycolipid I in sera from patients with lepromatous leprosy. J. Infect. Dis 152, 1078–1081. [DOI] [PubMed] [Google Scholar]