Abstract
Introduction
Chronic diseases of the liver are a global health problem with high morbidity and mortality. Viral hepatitis is the predominant cause for liver disease in low and middle-income countries. Viral hepatitis is also frequent in high-income countries but mainly as a complication to drug abuse or iatrogenic to treatment. In high-income countries non-fatty-liver-disease and complications to alcohol consumption are the most frequent etiology of liver disease. Viral hepatitis B and C is prevalent in Ethiopia but there are only few studies done in relation to chronic liver disease and a relationship between the increasing alcohol consumption and chronic liver disease.
Objective
A The aim of the study was to assess the association between chronic liver disease in Ethiopia and infection with viral hepatitis and possible relation to alcohol consumption.
Methods and Material
An unmatched case control study was conducted in Addis Ababa. Cases were chronic liver disease patients (n = 812) and controls were patients without liver disease (n = 798). Data were collected from the records of patients treated at a specialized clinic of Gastrsoenterology and Hepatology in Addis Ababa from 1st January 2013 – 31st, December 2013.
Results
The odds of having hepatitis infection among chronic liver disease was AOR = 100.96, (95%CI: 62.15 – 164.02) for HBV and AOR = 59.2, (95%CI: 27.23 – 130.9) for HCV. Consumption of alcohol was associated with chronic liver disease (OR: 8.23 95%CI: 3.76 – 12.70). Liver enzymes were elevated significantly in patients with hepatitis and alcohol consumption compared to patients without alcohol consumption.
Conclusion
Viral hepatitis infections are strongly associated with chronic liver disease. Prevention of viral hepatitis infections and control of alcohol consumption need to be strengthened in order to reduce the burden of chronic liver disease in Ethiopia.
Keywords: Chronic Liver Disease, Hepatitis B, Hepatitis C, Alcohol, Ethiopia, Non-Communicable Diseases
Introduction
Chronic liver disease (CLD) is a significant cause of morbidity and mortality worldwide but remains a largely neglected health issue in developing countries [1,2]. The most common liver diseases includes infections (hepatitis A, B, C, D, E), alcohol damage, non-alcoholic fatty liver disease, cirrhosis, cancer, and side effects to conventional and herbal medications [3]. The pathophysiology of CLD is hepatocellular necrosis and a persistent inflammation caused by viral infection or toxic substances leading to a regenerative process with destruction of the normal liver structure and function [4].
Chronic infections with hepatitis B virus (HBV) or hepatitis C virus (HCV) are a global challenge. World Health Organization (WHO, March 2015) estimates that 240 million people live with a chronic infection and 780,000 die each year, 130,000 from acute hepatitis B and 650,000 from complicating cirrhosis and liver cancer. Infection with hepatitis C is generally asymptomatic and a chronic infection occurs in approximately 80% and 130 – 150 million people live with a chronic infection and 350,000 to 500,000 die from cirrhosis or hepatocellular carcinoma.
Chronic liver disease attributes to 2% of the overall deaths in Addis Ababa [5] and a high prevalence of HBsAg and HBV markers have been reported, 6% and 44.8% respectively [6]. Transmission of infection with HBV and HCV can also be a occupational hazard as indicated by increased a prevalence of HBsAg 6–8% among medical waste handler and 1% among non-medical waste handlers [7,8] or a complication to other infectious and non-infectious diseases. A study showed a prevalence of HCV 9.9% in diabetics and 3.3% in non-diabetic controls and 10.3% and 6% in HIV-infected individuals and non-HIV infected individuals respectively [9,10]. All the available data indicates that viral hepatitis is prevalent in Ethiopia [4,6]. However, there are few studies done in relation to chronic liver disease and its etiology. Understanding the etiology of liver disease is needed to provide reliable information to implement cost effective prevention programs and treatment [11]. Therefore, results of this study will facilitate the education of health workers and staff at health facilities to perform an initial screening of patients thereby securing a correct and timely referral to specialized clinics.
Materials and Methods
Material
Study area and period
Patients with CLD and non-CLD controls were selected from patients attending a specialized in Gastroenterology and Hepatology. The clinic serves as a referral center for liver diseases and the majority of patients are from Addis Ababa but a considerable number of patients are referred from all Ethiopia (Table 1). Patients are referred from other health facilities including other clinics and specialized government hospitals. Patients with established or suspected liver disease represents approximately 30% of the patients.
Table 1.
Demographic characteristics of patients with Chronic Liver Diseases and the control patients.
| Variables | CLD Frequency (n = 812) |
Percentage (%) | Control Frequency (n = 798) |
Percentage (%) |
|---|---|---|---|---|
| Age | ||||
| ≤ 19 | 32 | 4.0 | 48 | 6.0 |
| 20 – 29 | 192 | 23.6 | 200 | 25.1 |
| 30 – 39 | 206 | 25.4 | 218 | 27.3 |
| 40 – 49 | 156 | 19.2 | 132 | 16.5 |
| 50 – 59 | 112 | 13.8 | 103 | 12.9 |
| ≥ 60 | 114 | 14.0 | 97 | 12.2 |
| Sex | ||||
| Male | 470 | 57.9 | 420 | 52.6 |
| Female | 342 | 42.1 | 378 | 47.4 |
| Residence | ||||
| Addis Ababa | 489 | 61.0 | 519 | 64.2 |
| Outside AA | 312 | 39.0 | 290 | 35.8 |
Study Design
A retrospective review of patient records was used to register demographic data, known etiological factor for CLD, results of laboratory tests and imaging techniques. Unmatched case control study was employed to compare patients with CLD and a control group of patients with no apparent hepatic disease.
Study Population and Sample size justification
The source population was all patients attending the clinic from 1st January 2013– 31st December 2013. A total sample size of 1554 (minimum 777 cases and 777 controls) was calculated using a formula for double population proportion with the assumption of estimated level of HBV exposure among controls (5.2%) and HCV exposure (3.5%), 80% power, 95% confidence interval, and minimum odds ratio of 2.
Diagnostic Criteria
The clinical feature of patients defined a diagnosis of CLD by the attending clinician, results of laboratory and serological tests, imaging techniques and when available histological assessment of tissues samples.
Cases
Patients diagnosed with CLD and being treated in the Clinic.
Controls
Non- CLD patients who are being treated in the Clinic and without signs of chronic liver disease.
Inclusion criteria
Patients who fulfill the case/control criteria and has complete records.
Sampling Procedures
From 01st January 2013 to 31st December 2013 a total of 6500 patients were seen in the clinic and all medical records were reviewed to prepare the sampling frame. After review 812 CLD cases fulfilled the inclusion criteria and were selected for the study. Non-CLD controls were selected from the sampling frame by simple random sampling methods.
Data Quality Assurance
A pretested structured questionnaire was used for data collection. Two trained clinical nurses did the data collection. To ascertain the validity, 15 records were reviewed by the data collector prior to the actual data collection and compared for any discrepancies between the two of them. The principal investigator supervised the process.
Statistical Analysis
The data was coded and entered to Epi-Info 3.5.3 using a double data entry procedure, a comparison report was run and the data were exported and analyzed using Statistical Program for Social Sciences Version 15.0. Descriptive statistics were used to explain the socio- demographic analysis and causes of liver disease. For categorical variables, Chi-square was used. For continuous variables, analyses of variance and Students t-test were used. Odds ratio and 95% confidence interval (CI) were calculated by univariate and bivariate logistic regression analysis. Variables with P < 0.2 were further evaluated by multivariate analysis. The adjusted OR (AOR) and 95% CI based on the final model were used to interpret the results. Statistical significance was defined as p value of < 0.05.
Ethical Consideration
Ethical clearance was obtained from Addis Continental Institute of Public Health/Haramaya University institutional ethical board and permission to conduct the study was obtained from the Clinic
Results and Discussion
Results
Demographic Characteristics
E total of 812 CLD cases and 798 non-CLD controls were identified from the source population. Table 1 summarizes the demographic characteristics of persons with CLD and the controls. Sixty percent of CLD patients and 65% of non-CLD controls were from Addis Ababa. The male/female ratio was 1,4 for cases and 1,1 of controls. The mean age was 40.7 ± 15.4 years and 38.7 ± 15.3 years for cases and controls respectively (p = 0.011). The age distribution showed that 202 (25.2%) of the cases and 222 (27.4%) of the controls subjects were between 30–39 years (Table 1).
Clinical and biochemical tests
The medical history of patients with regard to TB, Diabetes Mellitus, Hypertension and cardio-vascular disease did not differ from the control group. However, HIV positivity was more frequent in patients with CLD (N: 43; Non-CLD: 10; Chi-sq.: 21.38 p < 0.0001) as were other infectious diseases (CLD: N: 34; Non-CLD: 3; Chi-sq.: 27.15 p < 0.0001). The plasma concentrations of liver enzymes in patients with CLD were increased compared to control patients (Table 2).
Table 2.
Liver enzymes (serum concentrations) in patients with chronic liver disease and control patients. (Mean value and 95% confidence interval)
| CLD | Non-CLD | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean | CI | N | Mean | CI | ||
| WBC | 809 | 7,6 | 7,2 – 8,1 | 781 | 6,8 | 6,6 – 7,1 | * |
| Hgb | 782 | 14,1 | 13,8 – 14,3 | 751 | 15,2 | 15,0 – 15,5 | ** |
| GPT | 774 | 69,4 | 63,1 – 75,1 | 712 | 36,5 | 33,9 – 39,2 | ** |
| GOT | 774 | 77,2 | 71,5 – 82,8 | 712 | 33,5 | 23,0 – 37,0 | ** |
| GGT | 740 | 129,2 | 113,0 – 145,4 | 692 | 45,8 | 39,2 – 52,3 | ** |
p < 0.001;
p < 0.0001 (CLD vs. non-CLD; Students t-test).
Hgb: Hemoglobin, WBC: White blood cells.
Factors Predisposing for Chronic Liver Disease
Hepatitis B virus s-antigen was found in 467 (57.5%) of the cases. Total antibodies to HCV were present in 138 (17.1%) cases. A non-alcohol fatty liver disease was found in 163 (20%) patients with CLD and 192 (24.1%) of the control group (n.s.). Alcohol consumption was recorded in 117 (14.6%) patients with CLD and 16 (1.9%) in the control group (Chi-sq. 84.7 p < 0.00001). Parasitic infection (mainly Schistosomiasis) was noted in 39 (4.8%) cases with CLD and 3 (0.3%) in the control group. Eliminating viral hepatitis, alcohol consumption, Non-alcoholic Fatty Liver Disease and parasitic diseases left 119 (14.8%) cases without apparent predisposing factor.
Role of Viral Hepatitis B
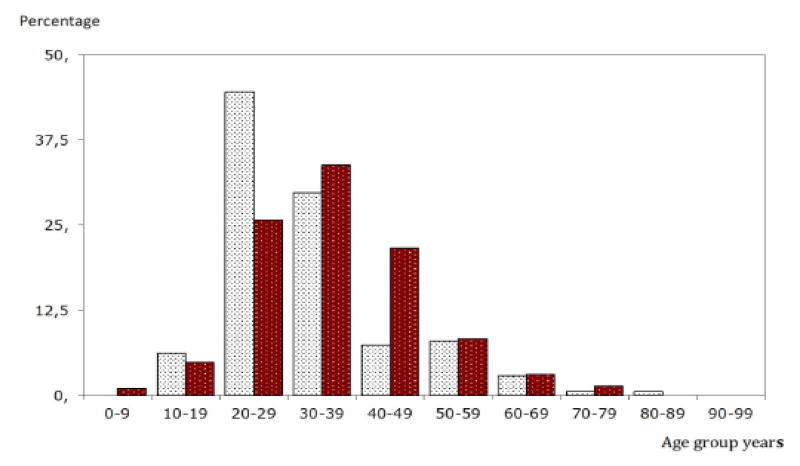
Among the CLD patients the prevalence of HBsAg was higher in male 282 (61.7%) than female 175 (38.3%) [COR = 1.468; 95% CI: 1.106–1.949; P = 0.008]. Figure 1 summarizes and shows the prevalence of HBsAg in cases with CLD stratified for gender and age. The prevalence of HBV increased with age until 30 – 39th year of age and diminishes with older age. The occurrence of HBV was highest (N:149; 32.4%) in the age group of 20 – 29 years and age group 30 – 39 years (N: 148;32.2%). In the age group 4 – 18 years was found 23 cases, the youngest being 8 years.
Figure 1.
The age and gender of patients with positive HBsAg. Diagram shows the distribution of patients with CLD. (Female vs. Male Chi Square 30.27; p = 0.001)
 Female HBsAg positive
Female HBsAg positive
 Male HBsAg positive
Male HBsAg positive
Role of Viral Hepatitis C
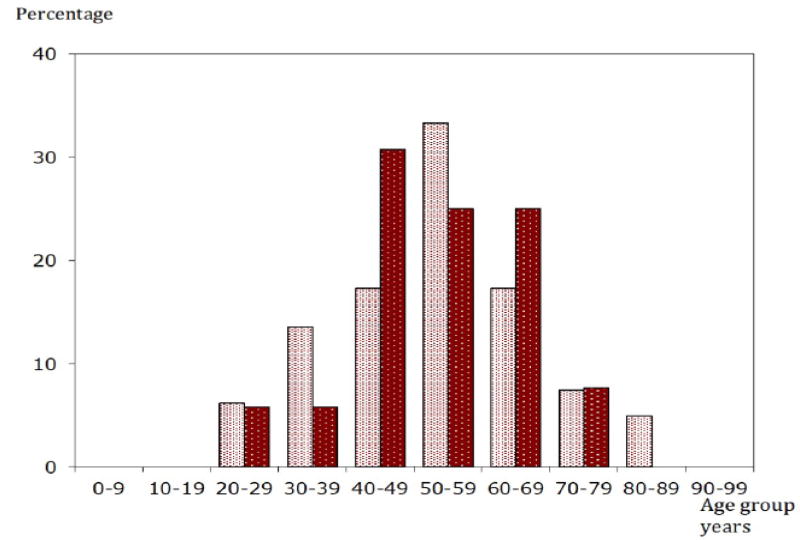
The distribution of HCV showed differences according to gender and age of patients. Figure 2 show that HCV prevalence was higher in women (N: 82; 59.9%) than in men (N: 55; 40.1%) [COR = 2.361; 95% CI: 1.622 – 3.43; P < 0.0001]. HCV prevalence increased with increasing age and there were no cases below 19 years, (N:42; 30.4%) in the age groups 50 – 59 years and above 60 years.
Figure 2.
The Age and gender of patients with positive HCV antibodies. (Female vs. Male Chi-square 8,63; 0,3 < p <= 0.2).
 Female HCV positive
Female HCV positive
 Male HCV positive
Male HCV positive
HCV genotype was measured in a small number of patients. Genotype 4 was found in 25 cases, genotype 2 in 3 and 2 patients with genotype 1 and 5 each.
Other factors than Viral hepatitis
Parasitic infection
Schistosomiasis was demonstrated in 39 patients with CLD. Schistosomiasis and HBsAg positivity was found 13 of 39 cases, and 5 cases were positive for HCV antibodies. In the control group were 3 cases with schistosomiasis, all hepatitis negative.
Alcohol
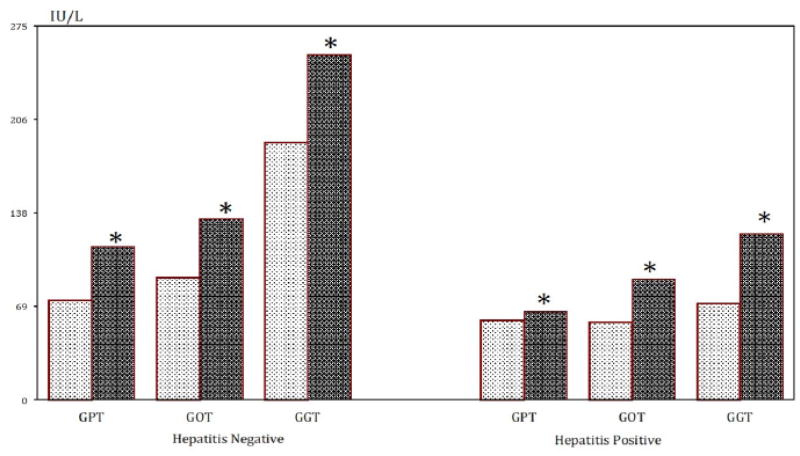
Alcohol consumption was registered in 133 patients, 117 cases with CLD and 16 in the control group (Chi-sq. 84.7 d.f.1 p < 0.0001). Consumption of alcohol was associated with chronic liver disease (OR: 8.23 95%CI: 3.76 – 12.70; p < 0.001). Thirty-two cases with CLD were HBsAg positive, and 36 positive for HCV antibodies. Results of blood liver enzymes are depicted in figure 3. The concentrations of serum-GPT, GOT and GGT were increased in patients with CLD, and HBsAg and/or antibodies to HCV and drinking alcohol compared to patients not drinking alcohol.
Figure 3.
Liver enzymes (serum concentrations) in patients with CLD in relation to alcohol consumption and HBsAg positivity.
Mean value. * p < 0.01 (ANOVA)
No history of alcohol consumption Alcohol consumption
 Female HCV positive
Female HCV positive
 Male HCV positive
Male HCV positive
Non-Alcoholic Fatty Liver Disease
NAFLD was found in 163 cases with CLD and in 192 cases from the control group (n.s.). In CLD 95 cases were positive for HBsAg and HCV in 32. In the control group none were positive for HBsAg or HCV. Blood enzymes in cases with NAFLD and presence of hepatitis markers were not different from those without NAFLD.
Discussion
This retrospective, case-control study records was done by reviewing records of patients treated during 2013 in a Clinic for Gastroenterology and Hepatology in Addis Ababa and extracting patients with chronic liver disease (CLD). Records of patients with a clinical presentation, biochemical and imaging results compatible with a chronic liver disease were reviewed and possible factors disposing or known to cause CLD were recorded. The most likely etiology of chronic liver disease was hepatitis B infection (57.5%) followed by hepatitis C infection (17.1%). Factors that aggravate the effect of hepatitis virus or by itself cause liver damage were present as a non-alcoholic fatty liver disease (19.9%), alcohol consumption (14.6%) and parasitic infection (4.8%). Rare conditions that results in CLD like autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, inborn errors of the carbohydrate metabolism, alfa-1-antitrypsin deficiency play a significant role in high-income countries but are uncommon in Ethiopia.
A previous or a persistent infection with hepatitis virus B and C is the most frequent finding in CLD. There is a difference in the clinical course of hepatitis B and C. Hepatitis B infection results in acute disease with a lifelong persistence of virus. Hepatitis C is initially asymptomatic and approximately 20% clears the virus spontaneously but a high frequency of patients with develops a chronic infection and complicating cirrhosis and hepatocellular carcinoma.
In our study, the prevalence of HBV and HCV among CLD patients was 57.5% and 17.1% respectively and in the control group 1.4% and 0.7% respectively. Previous studies from Addis Ababa have found a high prevalence of HBV and HCV. In 1992 a study from Addis Ababa reported 28% of patients were positive for HBV and 63% for HCV [12] and in a recent study from Addis Ababa showed a prevalence of 35.8% and 22.5% for HBV and HCV respectively [13]. It is noteworthy that the prevalence is unchanged during the past 20 years [6,12,13].
The gender of CLD patients in the present study showed that the prevalence of HBV was 1.4 times higher in male patients than in female. In contrast, the female patients had a 2.3 times higher risk of HCV compared to male patients. This is in accordance with a previous study from Addis Ababa that reported the prevalence of HBV was higher in men (38%) than women (31.8%) and that women have a higher prevalence of HCV (29.5%) than men (18.4%) [12]. However, it is at variance with a study from Pakistan [13] that found a male to female ratio of 2:1.
Age distribution of HBV among CLD patients in our study showed that 90% of cases were below 50 years, and 23 under 18 years The highest proportion was observed in the age group 20–29 years. A comparable high age distribution of HBV among CLD patients has been reported from Addis Ababa in the age group below 50 [12]. Chronic liver disease patients positive for HCV showed that more than half the cases were 50 years or more. This is in accordance with a previous study of chronic hepatitis C virus infection in sub-Saharan Africa that found the highest HCV prevalence in patients more than 50 years [14].
The age distribution in the present study may reflect different risk factors and behavior is important for transmission of HBV and HCV. The prevalence of HBV is high among the young people and low in high age. This supports the concept that transmission of HBV primarily occurs through sexual contact. A vertical transmission has not been found in Ethiopia but finding cases as young as 8 years suggest that it occurs. In contrast, no cases of HCV positivity were found below 18 years of age and prevalence increased with age. HCV transmission is most often through blood transfusions, surgical procedures, tooth extraction and contaminated utensils. Other routes of transmission may include asymptomatic person with occult viremia [15] or through sexual transmission though the magnitude is uncertain [16].
Co-infection with HBV and HCV results in more severe liver disease than either infection alone, with an increased risk of liver cancer [17]. In the present study the prevalence of HBV and HCV dual infection was low. Low prevalence of dual infection was also reported in a study from in Addis Ababa [12]. High prevalence of dual infection occurs in patients exposed to blood products i.e. hemodialysis (3.7%), in patients undergoing organ transplantation (8%), and among injection drug users (42.5%) [18,19]. The difference in the magnitude of co-infection in studies from outside Ethiopia and the present study most likely reflects differences in the study population and geographical variation.
Non-infectious factors for CLD that are known to be important in high-income countries are NAFLD, Diabetes, metabolic syndromes and alcohol consumption. Alcohol consumption has increased In Ethiopia during the last 10 – 15 years and surveys from 2013 describe that alcohol consumption has increased from an average of 0.5 liters/year to more than 10 liters/year [19]. Alcohol drinking may aggravate the course of viral hepatitis or per se cause CLD. In the present study, a history of consuming alcohol was associated with elevated concentrations of liver enzymes GPT, GOT and GGT in patients with hepatitis markers compared to patient’s not drinking alcohol. Alcohol and the hepatitis virus (in particular HCV) act synergistically and promote a rapid progression of liver disease [22].
Chronic Liver diseases are a major health challenge in all countries and viral hepatitis B and C is the most important etiology of chronic hepatitis, cirrhosis and hepatocellular carcinoma. Prevention of viral hepatitis is possible by vaccination for Hepatitis B, control of blood donations and ensuring clean utensils for vaccinations, injections and invasive procedures. The infection and complications are a challenge to the health system due to loss of lives, income and tax revenue, a high cost of the available effective medical treatment and possible liver transplantation.
The changes of society is accompanied by emerging non-communicable diseases like nutritional deficiency, diabetes mellitus, metabolic syndromes and complications to use and abuse of alcohol. Factors that all dispose for chronic liver disease and can aggravate infection with hepatitis virus. Recognizing the need to prevent hepatitis and manage non-infectious factors is of paramount importance with a rapid increase of non-communicable diseases in low and middle-income countries [23].
Conclusion
This is the first reported case control study of Chronic Liver Diseases in Addis Ababa, Ethiopia. It is concluded that, while a direct cause and effect relationship is difficult to demonstrate, the high prevalence of HBV and HCV infection among CLD patients strongly suggests a possible etiological relationship. This study also points to the importance of other predisposing factors like alcohol consumption, metabolic syndromes and non-fatty liver diseases.
Acknowledgments
Supported by The Infectious Disease Fund, Department of Medicine, Hospital of Southern Jutland, Denmark.
Abbreviations
- CLD
Chronic Liver Disease
- HBV
Hepatitis B virus
- HBsAg
Hepatitis B Surface Antigen
- HCV
Hepatitis C Virus
- NAFLD
Non-Alcoholic Fatty Liver Disease
- GPT
Glutamic Pyruvic Transaminase
- GOT
Glutamic Oxaloacetic Transaminase
- GGT
Gamma Glutamyl Transaminase
- OR
Odds Ratio
- CI
Confidence Interval
- WHO
World’s Health Organization
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article letter exists.
Bibliography
- 1.Cainelli F. Liver diseases in developing countries. World Journal of Hepatology. 2012;4.3:66–67. doi: 10.4254/wjh.v4.i3.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan R, et al. Accuracy of ultrasound to identify chronic liver disease. World Journal of Gastroenterology. 2010;16.28:3510. doi: 10.3748/wjg.v16.i28.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bane A, et al. An outbreak of veno-occlusive liver disease in northern Ethiopia, clinical findings. Ethiopian Medical Journal. 2012;50.2:9–16. [PubMed] [Google Scholar]
- 4.Tabassum F, et al. Identification of Marker Protein, In Patient with Chronic Liver Disease. Pakistan Journal of Biological Sciences. 2000;13.9:1509–1510. [Google Scholar]
- 5.Misganaw A, et al. Patterns of mortality in public and private hospitals of Addis Ababa, Ethiopia. BMC Public Health. 2012;12.1:1007. doi: 10.1186/1471-2458-12-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abebe A, et al. Sero- epidemiology of hepatitis B virus in Addis Ababa, Ethiopia: transmission patterns and vaccine control. Epidemiology and Infection. 2003;131.1:757–770. doi: 10.1017/s0950268803008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anagaw B, et al. Sero-prevalence of hepatitis B and C viruses among medical waste handlers at Gondar town Health institutions, Northwest Ethiopia. BMC Research Notes. 2012;5.1:55. doi: 10.1186/1756-0500-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiferaw Y, et al. Hepatitis B virus infection among medical waste handlers in Addis Ababa, Ethiopia. BMC Research Notes. 2011;4.1:479. doi: 10.1186/1756-0500-4-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali S, et al. Association of Hepatitis C Virus Infection with Type II Diabetes in Ethiopia: A Hospital-Based Case-Control Study. Interdisciplinary Perspectives on Infectious Diseases. 2012 doi: 10.1155/2012/354656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alemayehu A, et al. Prevalence and risk factors of Hepatitis C among individuals presenting to HIV testing centers, Hawassa city, Southern Ethiopia. BMC Research Notes. 2011;4.1:193–197. doi: 10.1186/1756-0500-4-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blachier M, et al. The burden of liver disease in Europe: a review of available epidemiological data. Journal of Hepatology. 2013;58.3:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Tsega E, et al. Chronic liver disease in Ethiopia: a clinical study with emphasis on identifying common causes. Ethiopian Medical Journal. 1992;30.2:1–33. [PubMed] [Google Scholar]
- 13.Ayele AG, Gebre-Selassie S. Prevalence and Risk Factors of Hepatitis B and Hepatitis C Virus Infections among Patients with Chronic Liver Diseases in Public Hospitals in Addis Ababa, Ethiopia. ISRN Tropical Medicine. 2013 [Google Scholar]
- 14.Khan TS, Rizvi F. Hepatitis B seropositivity among chronic liver disease patients in Hazara division Pakistan. Journal of Ayub Medical College Abbottabad. 2003;15.3:54–55. [PubMed] [Google Scholar]
- 15.Emmanuel E Ekanem, et al. Asymptomatic Hepatitis C Infection in Nigerian Adolescents. EC Gastroenterology and Digestive System. 2017;4.4:113–118. [Google Scholar]
- 16.Paez Jimenez A, et al. HCV iatrogenic and intrafamilial transmission in Greater Cairo, Egypt. Gut. 2010;59.11:1554–1560. doi: 10.1136/gut.2009.194266. [DOI] [PubMed] [Google Scholar]
- 17.Madhava V, et al. Epidemiology of chronic hepatitis C virus infection in sub-Saharan Africa. Lancet Infectious Diseases. 2002;2.5:293–302. doi: 10.1016/s1473-3099(02)00264-5. [DOI] [PubMed] [Google Scholar]
- 18.Zarski JP, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. Journal of Hepatology. 1998;28.1:27–33. doi: 10.1016/s0168-8278(98)80198-0. [DOI] [PubMed] [Google Scholar]
- 19.Reddy GA, et al. Prevalence of HBV and HCV dual infection in patients on haemodialysis. Indian Journal of Medical Microbiology. 2005;23.1:41–43. doi: 10.4103/0255-0857.13872. [DOI] [PubMed] [Google Scholar]
- 20.Aroldi A, et al. Natural history of hepatitis B and C in renal allograft recipients. Transplantation. 2005;79.9:1132–1136. doi: 10.1097/01.tp.0000161250.83392.73. [DOI] [PubMed] [Google Scholar]
- 21.Literature Review - Alcohol Consumption in Ethiopia. John Hopkins University Center for Communication Programs 2013 (JHU·CCP); [Google Scholar]
- 22.Punzalan CS, et al. Alcoholic hepatitis and HCV interactions in the modulation of liver disease. Journal of Viral Hepatitis. 2015;22.10:769–776. doi: 10.1111/jvh.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UN Task Force on NCDs: Non-communicable diseases and their risk factors. The Government of Ethiopia is working jointly with the UN System to strengthen the national NCD response [Google Scholar]