Summary
Background
Few studies have examined the efficacy of drug-eluting stents (DES) for reducing aortocoronary saphenous vein bypass graft (SVG) failure compared with bare-metal stents (BMS) in patients undergoing stenting of de-novo SVG lesions. We assessed the risks and benefits of the use of DES versus BMS in de-novo SVG lesions.
Methods
Patients were recruited to our double-blind, randomised controlled trial from 25 US Department of Veterans Affairs centres. Eligible participants were aged at least 18 years and had at least one significant de-novo SVG lesion (50–99% stenosis of a 2·25–4·5 mm diameter SVG) requiring percutaneous coronary intervention with intent to use embolic protection devices. Enrolled patients were randomly assigned, in a 1:1 ratio, by phone randomisation system to receive a DES or BMS. Randomisation was stratified by presence or absence of diabetes and number of target SVG lesions requiring percutaneous coronary intervention (one or two or more) within each participating site by use of an adaptive scheme intended to balance the two stent type groups on marginal totals for the stratification factors. Patients, referring physicians, study coordinators, and outcome assessors were masked to group allocation. The primary endpoint was the 12-month incidence of target vessel failure, defined as the composite of cardiac death, target vessel myocardial infarction, or target vessel revascularisation. The DIVA trial is registered with ClinicalTrials.gov, number NCT01121224.
Findings
Between Jan 1, 2012, and Dec 31, 2015, 599 patients were randomly assigned to the stent groups, and the data for 597 patients were used. The patients’ mean age was 68·6 (SD 7·6) years, and 595 (>99%) patients were men. The two stent groups were similar for most baseline characteristics. At 12 months, the incidence of target vessel failure was 17% (51 of 292) in the DES group versus 19% (58 of 305) in the BMS group (adjusted hazard ratio 0·92, 95% CI 0·63–1·34, p=0·70). Between-group differences in the components of the primary endpoint, serious adverse events, or stent thrombosis were not significant. Enrolment was stopped before the revised target sample size of 762 patients was reached.
Interpretation
In patients undergoing stenting of de-novo SVG lesions, no significant differences in outcomes between those receiving DES and BMS during 12 months of follow-up were found. The study results have important economic implications in countries with high DES prices such as the USA, because they suggest that the lower-cost BMS can be used in SVG lesions without compromising either safety or efficacy.
Introduction
Coronary artery bypass graft (CABG) surgery is one of the most common surgical procedures in the USA and Europe, with an estimated 400 000 CABG surgeries done in the USA during 2010.1 Patients who undergo CABG often develop bypass graft failure, especially in saphenous vein grafts (SVGs).2–5 Approximately one in five patients undergoing percutaneous coronary intervention (PCI) in the USA has had previous CABG, and 6% of all PCIs in the USA are done in SVGs.4,6 Stents are the current standard of care for PCI. Although drug-eluting stents (DES) improve outcomes compared with bare-metal stents (BMS) in native coronary artery lesions,7 their efficacy and safety in SVG lesions has received little study.8–13 Four randomised studies have been done with conflicting results (three studies showed benefit with DES11–13 and one showed harm9). These studies were limited by small size,9,11,13 absence of blinding,11–13 routine angiographic follow-up,9,11,12 low use of embolic protection devices12 and use of first-generation DES.9,11–13
We, therefore, examined the risks and benefits of DES versus BMS in SVGs in a prospective, double-blind, randomised trial.
Methods
Study design and participants
The DIVA trial14 was a multicentre, randomised, double-blind controlled trial done at 25 US Department of Veterans Affairs centres. The DIVA trial study design has been published.14 A planning committee and an executive committee designed the study, managed the study conduct and data collection, and made the decision to submit the manuscript for publication. The trial was approved by the Department of Veterans Affairs Central Institutional Review Board, and each participating site’s Research and Development Committee.
Patients with previous CABG undergoing cardiac catheterisation at participating sites were evaluated for enrolment. Eligible patients were aged at least 18 years, had at least one significant de-novo SVG lesion (50–99% stenosis of a 2·25–4·5 mm diameter SVG) requiring PCI with intent to use embolic protection devices, and agreed to participate and take medication as prescribed. Patients were excluded if they had planned non-cardiac surgery within 12 months of screening; presented with ST-segment elevation acute myocardial infarction; had a target SVG that was the last remaining vessel or was a left main equivalent; had any previous percutaneous treatment of the target vessel within the previous 12 months; had haemorrhagic diatheses, or refused to receive blood transfusions; required warfarin administration for the following 12 months and were considered to be at high risk of bleeding with triple anticoagulation/antiplatelet therapy; had recent positive pregnancy test, breast feeding, or possibility of a future pregnancy; had coexisting conditions that limited life expectancy to less than 12 months; had a history of allergic reaction or significant sensitivity to any drug or metal included in DES; were allergic to clopidogrel and did not present with acute coronary syndrome at sites that use blinded study medication; or were already participating in another interventional randomised trial. All patients provided written informed consent.
Randomisation and masking
Enrolled patients were randomly assigned, by phone randomisation system in a 1:1 ratio, to receive DES or BMS in the target SVGs. Each patient received as many stents as clinically indicated on the basis of operator judgment. Randomisation was stratified by presence or absence of diabetes and number of target SVG lesions requiring PCI (one vs two or more lesions) within each participating site by use of an adaptive scheme intended to balance the two stent-type groups on marginal totals for the stratification factors.
The cardiac interventionalist was not masked to the group assignment, but patients, referring physicians, primary study coordinators, and outcome assessors (clinical events committee and angiographic and intravascular ultrasonography core laboratories), were masked to group allocation. An independent clinical events committee adjudicated possible target vessel failure events (all deaths, myocardial infarctions not reported as non-target vessel, and target vessel revascularisations), reported by the sites that occurred during the first 12 months of follow-up. In addition, the study’s angiographic and electro cardio graphic core laboratories reviewed the angiograms and electrocardiographs in a blinded manner to verify that events reported by the sites as definite stent thrombosis met the definition used in DIVA. Counts for all other event types are based on cases reported by the sites.
To preserve the study blinding, at sites where standard of care was to treat patients receiving BMS with 1 month of a P2Y12 inhibitor, patients who did not present with an acute coronary syndrome and who did not require at least 12 months of P2Y12 inhibitor for clinical reasons were given clopidogrel or matching placebo after the first month for another 11 months.
Procedures
Stents used in the trial were commercially available in clinical practice during the study period. Operators were allowed to use the DES or BMS of their choice. PCI was done with standard techniques, at the discretion of each operator. In patients with multiple lesions, the same type of stent was used for all lesions whenever possible. All patients were prescribed aspirin as per standard of care.
Patients were followed up every 3 months during the first year and every 6 months thereafter. Quality-of-life questionnaires were administered by the study coordinators at baseline and 1, 6, 12, 18, and 24 months.
Outcomes
The primary endpoint was the 12-month incidence of target vessel failure, defined as the composite of cardiac death, target vessel myocardial infarction, or target vessel revascularisation. All events that were part of the primary endpoint were centrally assessed. Periprocedural myocardial infarction was not included in the primary endpoint. Secondary endpoints included procedural success and complications; all-cause death and cardiac death; follow-up myocardial infarction;15 stent thrombosis;16 target lesion revascularisation; non-target vessel revascularisation; the composite endpoint of any death, any myocardial infarction, or target vessel revascularisation (patient-oriented composite endpoint); the composite endpoint of cardiac death, target vessel myocardial infarction, or target lesion revascularisation (device-oriented composite endpoint for target lesion failure);16 stroke; and incremental cost-effectiveness of DES relative to BMS (not included in this manuscript). The definitions of the study endpoints were published in the DIVA design paper.14 Adverse events that were part of the primary endpoint were adjudicated by a clinical events committee.
Statistical analysis
We estimated a sample size of 519 participants at 25 sites would provide 123 participants with a primary outcome (12-month target vessel failure) and 90% power for detection of the difference between the two groups, assuming a 12-month target vessel failure rate of 30% in BMS and 18% in DES (corresponding hazard ratio [HR] 0·556).9,10,17,18 Because of a lower than anticipated overall target vessel failure rate of 16% after 384 randomisations, the sample size was increased to 762 to provide 86% power to detect a group difference on the basis of 122 participants with target vessel failure, an overall target vessel failure rate of 16%, and 40% relative reduction for the DES group (corresponding HR 0·573). Two interim analyses were planned, at approximately 25% and 60% of target-adjudicated primary outcomes, to allow early stopping for efficacy (on the basis of O’Brien-Fleming boundary) or futility (on the basis of conditional power).
The analyses for all outcomes followed the intention-to-treat principle. The primary analysis was the comparison of time to first target vessel failure between the DES and BMS groups in the initial 12 months following index stent implantation. Cumulative incidence curves for time to target vessel failure were calculated by stent group. Participants lost to follow-up were censored at the time of last contact. Log-rank tests were used to compare the time to target vessel failure. This test was stratified by presence or absence of diabetes and by number of target SVG lesions requiring percutaneous coronary intervention (one vs more than one). Additionally, the Cox proportional hazards model19 was used to assess the effect of stent type and various covariates on time to target vessel failure. Participant and graft characteristics thought to be potentially predictive of SVG patency, including diabetes, number of target SVG lesions (one or more than one), reference vessel diameter, stent length, the SVG recipient vessel (left anterior descending artery, circumflex, right coronary artery), lesion location (aorto-ostial, body, distal anastomotic), and baseline thrombolysis in myocardial infarction flow (three vs less than three), were first entered into the regression model. Stent assignment (DES vs BMS) was then added to the model to see if it was a statistically significant predictor of target vessel failure above and beyond the participant-graft characteristics. Interaction terms between stent type and other significant participant graft characteristics were also assessed. Potential time-varying stent effects were assessed by plotting the scaled Schoenfeld residuals against time with the Grambsch and Therneau test.20 We also assessed piecewise constant HRs for the time period up to 1 year of follow-up (the interval for the primary endpoint) versus after 1 year. A two-sided p value of 0·05 from the stratified log-rank test was used as the level of significance for the primary outcome. As a preplanned secondary analysis, we did similar analyses as above to compare time to target vessel failure on the basis of all follow-up data.
The procedural success rate and the incidence of postprocedural myocardial infarction and postprocedural GUSTO moderate or severe bleeding21,22 were compared between the DES and BMS groups by the difference between two independent proportions. Cumulative incidence curves and stratified log-rank tests were used to compare the two stent groups on the incidence of the secondary clinical outcomes listed above. When appropriate, competing risks analyses with plots of cumulative incidence curves and comparisons of cumulative incidences with Gray’s test23 and Fine and Gray’s24 methods were done. Proportional hazards regression for subdistributions of competing risks were also done. SAS 9.2 (TS2M3; SAS Institute, Cary, NC, USA) and R version 3.4.4 were used for the analyses.
The DIVA trial is registered with ClinicalTrials.gov, number NCT01121224.
Role of the funding source
The funder was involved in study design, data collection, site monitoring, data analysis, data interpretation, and writing of the report, but did not influence the interpretation of the trial results nor the decision to submit the paper for publication. Members of the executive committee had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
Between Jan 1, 2012, and Dec 31, 2015, 3482 patients with previous CABG undergoing cardiac catheterisation at the participating centres were screened for participation in the trial, and 597 (17%) patients were randomly assigned to either the BMS or DES group and were included in the final analysis (figure 1). The first interim analysis was done on Oct 13, 2015, when there were 35 adjudicated primary outcomes (29% information time); at that time, 576 participants had been randomly assigned. The data monitoring committee recommended the study continue. Trial enrolment was terminated on Dec 31, 2015, because of slower than expected enrolment and funding issues; the final number of randomisations was 599. No additional interim analysis was done. We only report data for 597 patients because the consent process was done improperly for two patients. Study follow-up ended on Dec 31, 2016. The minimum length of follow-up was 1 year and the maximum was 5 years. The study with 109 adjudicated primary outcomes has 86% post-hoc power to detect the originally hypothesised HR of 0·556 and 83% power to detect the revised hypothesised HR of 0·573.
Figure 1: Enrolment, randomisation, and follow-up.
The number of patients who were eligible is lower than the number randomly assigned, because some ineligible patients were randomly assigned. SVG=saphenous vein aortocoronary bypass graft. PCI=percutaneous coronary intervention. *Two patients who were screened and randomly assigned without proper consent are not included. †Criteria are not mutually exclusive.
The study groups were well balanced for most clinical and angiographic characteristics (table 1), except for SVG age and race categories. During the index procedure, 77 (13%) of 597 patients had one target SVG with multiple SVG lesions treated, 23 (4%) had multiple SVGs treated, and 95 (16%) had native coronary artery lesions treated. An embolic protection device was used in 409 (69%) patients across both groups. In the group receiving DES, 256 (88%) of 292 participants received second-generation DES (63% everolimus-eluting stents and 26% zotarolimus-eluting stents, with some participants receiving both). Newer generation BMS with thin struts were used in the BMS group. The names and manufacturers of all types of stents that were implanted at baseline are given in the appendix. The median follow-up was 2·7 years (IQR 1·7–3·7).
Table 1:
Baseline clinical and angiographic characteristics of the study patients and lesions, procedural techniques, and outcomes
| Drug-eluting stents (n=292) | Bare-metal stents (n=305) | |
|---|---|---|
| Patient characteristics | ||
| Age, years | 69.0 (7.4) | 68.2 (7.7) |
| Men | 290/292 (99%) | 305/305 (100%) |
| Race | ||
| White | 263/287 (92%) | 254/299 (85%) |
| Black | 17/287 (6%) | 32/299 (11%) |
| Hispanic | 16/286 (6%) | 14/300 (5%) |
| Body-mass index, kg/m2 | 30.6 (5.6), n=288 | 30.4 (5.3), n=301 |
| Waist circumference, inches | 41.8 (6.0), n=240 | 41.7 (5.4), n=254 |
| Time since coronary artery bypass graft surgery, years |
13.9 (6.7), n=291 |
12.8 (6.8), n=303 |
| Number of diseased coronary vessels | ||
| One | 3/291 (1%) | 8/304 (3%) |
| Two | 30/291 (10%) | 25/304 (8%) |
| Three | 258/291 (89%) | 271/304 (89%) |
| Indication for PCI | ||
| Stable angina | 117/289 (40%) | 105/304 (35%) |
| Unstable angina | 89/289 (31%) | 95/304 (31%) |
| Non-ST-segment elevation acute MI |
66/289 (23%) | 74/304 (24%) |
| Other | 17/289 (6%) | 30/304 (10%) |
| Hypertension | 278/292 (95%) | 296/305 (97%) |
| Hyperlipidaemia | 287/292 (98%) | 294/305 (96%) |
| Diabetes | 173/292 (59%) | 187/305 (61%) |
| Current smoker | 61/292 (21%) | 72/305 (24%) |
| Previous MI | 163/292 (56%) | 153/305 (50%) |
| History of atrial fibrillation | 51/292 (17%) | 57/305 (19%) |
| Congestive heart failure | 92/292 (32%) | 118/305 (39%) |
| Ejection fraction | 52.5 (47.0), n=175 | 49.4 (13.3), n=182 |
| Peripheral arterial disease | 51/292 (17%) | 56/305 (18%) |
| Lesion characteristics | ||
| Target graft recipient vessel | 301 | 315 |
| Left anterior descending | 9 (3%) | 13 (4%) |
| Diagonal artery | 53 (18%) | 62 (20%) |
| Circumflex or obtuse marginal | 122 (41%) | 129 (41%) |
| Right coronary artery or posterior descending artery | 117 (39%) | 111 (35%) |
| SVG target lesion location | 330 | 359 |
| Ostial | 68 (21%) | 88 (25%) |
| Body | 234 (71%) | 240 (67%) |
| Distal anastomosis | 28 (8%) | 31 (9%) |
| Prestenting target SVG lesion flow | ||
| 0 | 2 (1%) | 3 (1%) |
| 1 | 10 (3%) | 14 (4%) |
| 2 | 54 (16%) | 42 (12%) |
| 3 | 264 (80%) | 300 (84%) |
| Post-stenting target SVG lesion flow | ||
| 0 | 1 (0%) | 1 (0%) |
| 1 | 1 (0%) | 0 (0%) |
| 2 | 7 (2%) | 4 (1%) |
| 3 | 321 (97%) | 354 (99%) |
| Index procedure characteristics | ||
| Arterial access | ||
| Femoral | 267 (91%) | 279 (91%) |
| Radial | 20 (7%) | 23 (8%) |
| Anticoagulant | ||
| Unfractionated heparin | 166 (57%) | 177 (58%) |
| Bivalirudin | 123 (42%) | 137 (45%) |
| Glycoprotein IIb/IIIa inhibitor | 44 (15%) | 43 (14%) |
| Staged PCI | 30 (10%) | 35 (11%) |
| Haemodynamic support during PCI | 1 (0%) | 3 (1%) |
| Embolic protection device used | 199 (68%) | 210 (69%) |
| Number of target SVGs intervened per patient |
1.0 (0.2) | 1.0 (0.2) |
| Patients who underwent PCI of more than one target SVG lesion |
42 (14%) | 54 (18%) |
| Number of target SVG lesions intervened per patient |
1.2 (0.5) | 1.2 (0.5) |
| Number of stents in target SVG lesions per patient |
1.3 (0.6) | 1.4 (0.8) |
| Number of non-target lesions intervened per patient |
1.4 (0.6) | 1.4 (0.7) |
| Type of drug-eluting stent used in target lesions | ||
| First generation | 21 (7%) | 0 (0%) |
| Second generation | 256 (88%) | 8 (3%) |
| Total length of stents in target lesion per patient, mm | 27.0 (19.0) | 26.6 (18.3) |
| Target lesion stent diameter, mm | 3.38 (0.50) | 3.42 (0.56) |
| Intravascular ultrasound guidance | 64 (22%) | 57 (19%) |
| Angiographic success | 274 (94%) | 291 (95%) |
| Any procedural complication | 15 (5%) | 22 (7%) |
| Periprocedural MI* | 9 (3%) | 23 (8%) |
Data are mean (SD), n/N (%), n, or n (%). Summary statistics in all tables are based on the number of participants who provided a response for each given characteristic. PCI=percutaneous coronary intervention. MI=myocardial infarction. SVG=saphenous vein aortocoronary bypass graft.
Defined as an increase at least three times the upper limit of normal in patients with normal baseline creatine kinase-muscle/brain (CK-MB) and at least a 50% increase in patients with elevated baseline CK-MB; p=0·0156.
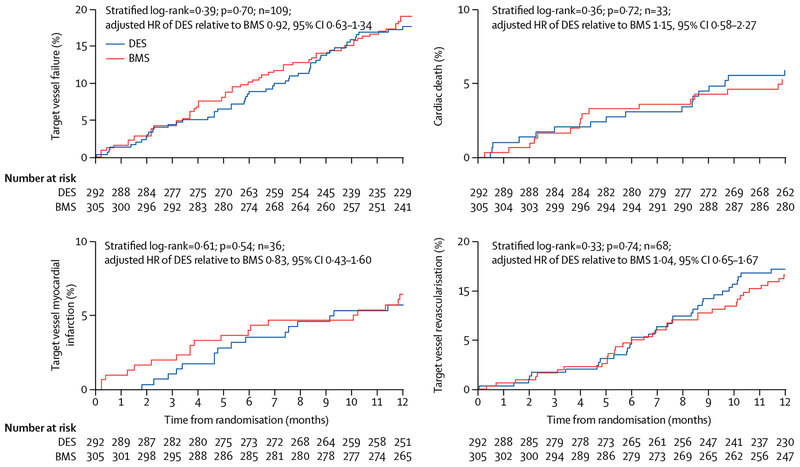
At 12 months, the incidence of target vessel failure was 17% (51 of 292) in the DES group and 19% (58 of 305) in the BMS group (stratified log-rank test; HR adjusted for stratification of randomisation 0·92, 95% CI 0·63–1·34, p=0·70; table 2; figure 2). Log-rank tests unstratified by diabetes status and one versus two or more SVG lesions also were not significant (data not shown). The between-group difference in the primary outcome after adjustment for baseline imbalance in SVG age (median <13·5 years vs ≥13·5 years) was not significant. The results for target vessel failure were consistent in subgroups defined by demo graphic, clinical, lesion, and procedural characteristics (appendix). Adding stent assignment to the proportional hazards model with participant and graft characteristics as covariates did not significantly increase the model’s predictive ability (p=0·83) of target vessel failure at 12 months.
Table 2:
Clinical events during 12-month follow-up and medications administered during follow-up
| Drug-eluting stents (n=292) |
Bare-metal stents (n=305) | Adjusted hazard ratio (95% CI) | p value* | |
|---|---|---|---|---|
| TVF | 51 (17%) | 58 (19%) | 0.92 (0.63–1.34) | 0.70 |
| Death from any cause | 23 (8%) | 21 (7%) | 1.17 (0.65–2.12) | 0.63 |
| Cardiac or unknown cause of death (component of TVF) | 17 (6%) | 16 (5%) | 1.15 (0.58–2.27) | 072 |
| Cardiac | 15 (5%) | 11 (4%) | 1.47 (0.68–3.20) | 0.35 |
| Non-cardiac | 6 (2%) | 5 (2%) | 1.23 (0.37–4.05) | 073 |
| Unknown cause | 2 (1%) | 5 (2%) | 0.43 (0.08–2.24) | 0.30 |
| Any MI during follow-up | 28 (10%) | 31 (10%) | 0.94 (0.56–1.57) | 0 75 |
| Target vessel or indeterminate MI during follow-up (component of TVF) | 16 (5%) | 20 (7%) | 0.83 (0.43–1.60) | 0 54 |
| Non-target vessel MI during follow-up | 13 (4%) | 13 (4%) | 1.05 (0.49–2.27) | 0.96 |
| Any revascularisation | 56 (19%) | 53 (17%) | 1.11 (0.76–1.61) | 0.44 |
| Any PCI | 55 (19%) | 53 (17%) | 1.09 (0.74–1.58) | 0.51 |
| Any CABG | 2 (1%) | 0 (0%) | .. | 0.50 |
| Target vessel revascularisation (component of TVF) | 34 (12%) | 34 (11%) | 1.04 (0.65–1.67) | 0.74 |
| With PCI | 33 (11%) | 34 (11%) | 1.01 (0.62–1.63) | 0.85 |
| With CABG | 2 (1%) | 0 (0%) | .. | 0.50 |
| Non-target vessel revascularisation | 33 (11%) | 26 (9%) | 1.34 (0.80–2.24) | 0.20 |
| With PCI | 32 (11%) | 26 (9%) | 1.30 (0.77–2.18) | 0.25 |
| With CABG | 2 (1%) | 0 (0%) | .. | 0.50 |
| Target lesion revascularisation | 26 (9%) | 25 (8%) | 1.09 (0.63–1.88) | 0.62 |
| With PCI | 25 (9%) | 25 (8%) | 1.04 (0.60–1.82) | 0.73 |
| With CABG | 2 (1%) | 0 (0%) | .. | 0.50 |
| Patient-oriented composite endpoint (composite of all-cause death, any MI, and target lesion revascularisation) | 62 (21%) | 68 (22%) | 0.94 (0.67–1.33) | 0.81 |
| Target lesion failure (composite of cardiac or unknown death, target vessel MI, and target lesion revascularisation) | 45 (15%) | 51 (17%) | 0.93 (0.62–1.38) | 0 76 |
| Definite stent thrombosis | 6 (2%) | 7 (2%) | 0.85 (0.28–2.53) | 0.81 |
| Definite or probable stent thrombosis | 14 (5%) | 17 (6%) | 0.87 (0.43–1.76) | 0.70 |
| Stroke | 2 (1%) | 3 (1%) | 0.72 (0.12–4.32) | 0.71 |
| Post-procedural bleed | 0 (0%) | 2 (1%) | .. | 0.50 |
| TVF or SVG occlusion that did not undergo intervention | 51 (17%) | 59 (19%) | 0.90 (0.62–1.31) | 0.64 |
| Medications during follow-up | ||||
| Patients who had a 12-month visit | 258 | 270 | NA | 0.95 |
| Aspirin at 12 months | 241 (93%) | 249 (92%) | NA | 0.58 |
| P2Y12 inhibitor at 12 months | 230 (89%) | 240 (89%) | NA | 0.92 |
| Clopidogrel at 12 months | 220 (85%) | 217 (80%) | NA | 0.13 |
| Ticlopidine at 12 months | 0 (0%) | 1 (0%) | NA | 1.00 |
| Prasugrel at 12 months | 6 (2%) | 16 (6%) | NA | 0.0385 |
| Ticagrelor at 12 months | 4 (2%) | 7 (3%) | NA | 0.40 |
| Statin at 12 months | 240 (93%) | 251 (93%) | NA | 0.97 |
| Patients who had a 24-month visit | 198 | 211 | NA | 0.72 |
| Aspirin at 24 months | 179 (90%) | 192 (91%) | NA | 0.84 |
| P2Y12 inhibitor at 24 months | 110 (56%) | 131 (62%) | NA | 0.18 |
| Clopidogrel at 24 months | 103 (52%) | 118 (56%) | NA | 0.43 |
| Ticlopidine at 24 months | 0 | 0 | NA | NA |
| Prasugrel at 24 months | 4 (2%) | 6 (3%) | NA | 0.75 |
| Ticagrelor at 24 months | 3 (2%) | 7 (3%) | NA | 0.34 |
| Statin at 24 months | 184 (93%) | 192 (91%) | NA | 0.47 |
| Patients who had a 36-month visit | 130 | 133 | NA | 0.82 |
| Aspirin at 36 months | 110 (85%) | 111 (83%) | NA | 0.73 |
| P2Y12 inhibitor at 36 months | 62 (48%) | 58 (44%) | NA | 0.66 |
| Clopidogrel at 36 months | 51 (39%) | 55 (41%) | NA | 0.57 |
| Ticlopidine at 36 months | 0 | 0 | NA | NA |
| Prasugrel at 36 months | 7 (5%) | 1 (1%) | NA | 0.07 |
| Ticagrelor at 36 months | 4 (3%) | 2 (2%) | NA | 0.68 |
| Statin at 36 months | 115 (88%) | 118 (89%) | NA | 0.38 |
Data are n (%) or n. TVF=target vessel failure. MI=myocardial infarction. PCI=percutaneous coronary intervention. CABG=coronary artery bypass graft surgery. SVG=saphenous vein aortocoronary bypass graft. NA=not applicable.
The p value has been calculated from the stratified log-rank test.
Figure 2: Clinical outcomes at 12 months.
Kaplan-Meier plot of cumulative incidence curves for patients who received drug-eluting stents (DES) or bare-metal stents (BMS) for the primary outcome of target vessel failure (composite outcome of cardiac death, target vessel myocardial infarction, or target vessel revascularisation), cardiac death, target vessel myocardial infarction, and target vessel revascularisation. HR=hazard ratio.
Analyses of the 12-month incidence of target vessel failure with non-cardiac death as a competing risk, target vessel or indeterminate myocardial infarction with any death as a competing risk, and target vessel revascularisation with any death as a competing risk showed no significant effects from stent assignment on any cause-specific hazard.
The between-group difference in the 12-month incidence of all-cause death, any myocardial infarction, definite or probable stent thrombosis, stroke, or other secondary outcomes was not significant (table 2; appendix).
Median follow-up was 2·7 years, with 406 (68%) of 597 participants followed up for at least 2 years and 235 (39%) followed up for at least 3 years. Over the entire length of follow-up, the incidence of target vessel failure was 37% (108 of 292) in the DES group and 34% (105 of 305) in the BMS group (adjusted HR 1·10, 95% CI 0·84 to 1·43, p=0·44; table 3; appendix). The results for target vessel failure over the entire follow-up were consistent in subgroups defined by demographic, clinical, lesion, and procedural characteristics (appendix). There was no significant evidence of non-proportional hazards (Grambsch-Therneau test p=0·08). In a post-hoc analysis, the between-group difference in the primary outcome after adjustment for baseline imbalance in SVG age (<13·5 years vs ≥13·5 years) was not significant.
Table 3:
Clinical events during entire duration of follow-up (median 2·7 years)
| Drug-eluting stents (n=292) |
Bare-metal stents (n=305) | Adjusted hazard ratio (95% CI) | p value* | |
|---|---|---|---|---|
| TVF | 108 (37%) | 105 (34%) | 1.10 (0.84–1.43) | 0.44 |
| Death from any cause | 55 (19%) | 51 (17%) | 1.14 (0.78–1.67) | 0.51 |
| Cardiac or unknown cause of death (component of TVF) | 41 (14%) | 41 (13%) | 1.08 (0.70–1-66) | 0.75 |
| Cardiac | 25 (9%) | 21 (7%) | 1.28 (0.72–2.29) | 0.41 |
| Non-cardiac | 14 (5%) | 10 (3%) | 1.39 (0.61–3.14) | 0.42 |
| Unknown cause | 16 (5%) | 20 (7%) | 0.86 (0.44–1.66) | 0.65 |
| Any MI during follow-up | 53 (18%) | 62 (20%) | 0.89 (0.61–1.28) | 0.52 |
| Target vessel or indeterminate MI during follow-up (component of TVF) | 35 (12%) | 40 (13%) | 0.90 (0.57–1.42) | 0.66 |
| Non-target vessel MI during follow-up | 25 (9%) | 31 (10%) | 0.85 (0.50–1.43) | 0.53 |
| Any revascularisation | 107 (37%) | 96 (31%) | 1.20 (0.91–1.59) | 0.11 |
| Any PCI | 102 (35%) | 94 (31%) | 1.16 (0.87–1.53) | 0.20 |
| Any CABG | 6 (2%) | 4 (1%) | 1.57 (0.44–5.59) | 0.37 |
| Target vessel revascularisation (component of TVF) | 68 (23%) | 58 (19%) | 1.23 (0.87–1.75) | 0.15 |
| With PCI | 64 (22%) | 56 (18%) | 1.19 (0.83–1.71) | 0.24 |
| With CABG | 5 (2%) | 4 (1%) | 1.27 (0.34–4.78) | 0.56 |
| Non-target vessel revascularisation | 69 (24%) | 60 (20%) | 1.25 (0.89–1.77) | 0.14 |
| With PCI | 64 (22%) | 59 (19%) | 1.17 (0.82–1.67) | 0.30 |
| With CABG | 6 (2%) | 1 (0%) | 6.33 (0.76–52.71) | 0.0355 |
| Target lesion revascularisation | 47 (16%) | 40 (13%) | 1.23 (0.80–1.87) | 0.22 |
| With PCI | 44 (15%) | 39 (13%) | 1.17 (0.76–1.81) | 0.33 |
| With CABG | 4 (1%) | 1 (0%) | 4.20 (0.47–37.78) | 0.13 |
| Patient-oriented composite endpoint (composite of all-cause death, any MI, and target lesion revascularisation) | 127 (43%) | 127 (42%) | 1.06 (0.83–1.36) | 0.55 |
| Target lesion failure (composite of cardiac or unknown death, target vessel MI, and target lesion revascularisation) | 94 (32%) | 95 (31%) | 1.05 (0.79–1.39) | 0.65 |
| Definite stent thrombosis | 9 (3%) | 10 (3%) | 0.90 (0.36–2.22) | 0.88 |
| Definite or probable stent thrombosis | 26 (9%) | 30 (10%) | 0.90 (0.53–1.52) | 0.74 |
| Stroke | 10 (3%) | 12 (4%) | 0.88 (0.38–2.03) | 0.84 |
| TVF or SVG occlusion that did not undergo intervention | 112 (38%) | 110 (36%) | 1.09 (0.84–1.42) | 0.44 |
Data are n (%). TVF=target vessel failure. MI=myocardial infarction. PCI=percutaneous coronary intervention. CABG=coronary artery bypass graft surgery. SVG=saphenous vein aortocoronary bypass graft.
The p value has been calculated from the stratified log-rank test.
Between-group differences in the incidence of all-cause death, myocardial infarction during follow-up, definite or probable stent thrombosis, stroke, or other secondary outcomes were not significant (table 3; appendix). The proportion of participants reporting one or more serious adverse events overall, and by classification by use of the Medical Dictionary for Regulatory Activities (MedDRA), was not significant (table 4). Most repeat revascularisations were done in patients who presented with an acute coronary syndrome (70% [133 of 191]) or stable angina (24% [46 of 191]).
Table 4:
Serious adverse events
| DES(n=292) | BMS (n=305) | p value | |
|---|---|---|---|
| Number of participants with serious adverse events | 231 (79%) | 244 (80%) | 079 |
| Number of serious adverse events | 1066 | 1110 | NA |
| Serious adverse events attributed to stent | NA | NA | 045 |
| Not attributed | 906 | 959 | NA |
| Possibly attributed | 102 | 90 | NA |
| Yes, attributed | 56 | 54 | NA |
| Serious adverse events attributed to thienopyridine | NA | NA | 0.33 |
| Not attributed | 1014 | 1049 | NA |
| Possibly attributed | 49 | 53 | NA |
| Yes, attributed | 1 | 5 | NA |
| MedDRA higher level term group | |||
| Cardiac arrhythmias | 36 (12%) | 38 (12%) | 0.96 |
| Coronary artery disorders | 134 (46%) | 140 (46%) | 1.00 |
| Heart failure | 52 (18%) | 58 (19%) | 0.70 |
| Complications associated with device | 25 (9%) | 28 (9%) | 0.79 |
| General system disorders NEC | 35 (12%) | 34 (11%) | 0.75 |
| Bacterial infectious disorders | 19 (7%) | 13 (4%) | 0.22 |
| Infections—pathogen unspecified | 51 (17%) | 51 (17%) | 0.81 |
| Procedural related injuries and complications NEC | 15 (5%) | 14 (5%) | 0.76 |
| Electrolyte and fluid balance conditions | 14 (5%) | 18 (6%) | 0.55 |
| CNS vascular disorders | 20 (7%) | 23 (8%) | 0.74 |
| Renal disorders (excluding nephropathies) | 28 (10%) | 30 (10%) | 0.92 |
| Bronchial disorders (excluding neoplasms) | 15 (5%) | 8 (3%) | 0.11 |
| Decreased and non-specific blood pressure disorders and shock | 11 (4%) | 16 (5%) | 0.38 |
Data are n or n (%). Tallies of participants and events, including participants with at least one serious adverse event by MedDRA classification experienced by at least 5% of participants in one or both treatment arms. DES=drug-eluting stent. BMS=bare metal stent. NA=not applicable. NEC=not elsewhere classified.
A post-hoc analysis for the combination of target vessel failure or SVG occlusion that was intervened on, or both, showed no difference between treatment groups at 12 months (table 2) and during the entire duration of follow-up (table 3).
Discussion
We did not find a significant difference between DES and BMS in the incidence of the combined endpoint of cardiac death, target vessel myocardial infarction, or target vessel revascularisation during the first 12 months and over the entire length of follow-up (median 2·7 years) among patients undergoing stenting of de-novo SVG lesions. There was also no difference in the risk for stent thrombosis or bleeding. DIVA did not mandate angiographic follow-up, which is known to increase the rates of repeat revascularisation in favour of DES.25 DIVA had higher use of embolic protection devices (69% [409 of 597 patients]) than any previous SVG stenting trial.
Our findings are in contrast with those of three11–13 of the four randomised controlled trials done to date that showed benefit with DES over BMS in SVGs. The ISAR-CABG trial12 randomly assigned 610 participants to a first-generation DES or a BMS, and reported lower 12-month incidence of target vessel revascularisation in the DES group (7% vs 13%, p=0·01), and no significant differences in all-cause mortality, myocardial infarction, and definite or probable stent thrombosis as compared with BMS. ISAR-CABG had planned angiographic follow-up as did two smaller studies.9–11 The first study9 showed harm (higher mortality with DES compared with BMS at 3 years [29% vs 0%, p=0·001], although most deaths were due to non-cardiac events) and similar target vessel revascularisation in both groups. The second study10,11 showed lower risk for myocardial infarction and target lesion revascularisation with DES than BMS. The BASKET-SAVAGE trial13 randomly assigned 173 patients to receive either DES or BMS, had no planned angiographic follow-up, and had lower incidence of target vessel revascularisation in the DES group (4·5% vs 19·1% at 3 years, p<0·001). The absence of improved outcomes with DES in DIVA was observed despite the use of second-generation DES in 256 (88%) of 292 patients in the DES group, whereas first-generation DES were used in all previous studies.9,11–13
The absence of benefit with DES in DIVA could be related to the different pathophysiology of SVG atherosclerosis (more concentric and diffuse with less well defined fibrous cap) compared with native coronary artery atherosclerosis.26,27 The absence of benefit with DES could also be because of the large burden of comorbidities among patients with a previous CABG,14 or the use of thin-strut bare-metal stents that might have lower risk for restenosis as compared with thicker strut stents that were used in previous SVG PCI studies; 9,11–13 it is unlikely to be because of differences in stenting technique and concomitant medications, since those characteristics were similar in both groups. Unknown confounders could have played a role, but are unlikely given randomisation. The study results have important economic implications in countries with high DES prices such as the USA, because they suggest that the lower cost BMS can be used in SVG lesions without compromising either safety or efficacy. The financial implications of the study might differ between countries, depending on local stent pricing. An alternative treatment approach would be to recanalise the native coronary artery instead of the diseased SVG,4,28 although such interventions can be technically challenging.29,30
Our study has limitations. As is typical in Veterans Affairs studies, nearly all study participants were men, which limits the extrapolation of the results to women, although most patients undergoing PCI after CABG are men.31 The interventionalists doing the index SVG PCI were not masked to the type of stent used, although the patients, clinicians, and event adjudicators were masked. The study was stopped before completion of the revised enrolment target, yet the number of patients recruited was greater than originally planned and the post-hoc power (86%) was similar to the pre-hoc power (90%) to detect the originally hypothesised HR of 0·556.
In summary, in our evaluation of the clinical outcomes of 597 patients undergoing PCI of de-novo SVG lesions, we found no significant difference in the 12-month and long-term (median 2·7 years) incidence of cardiac death, target vessel myocardial infarction, or target vessel revascularisation.
Supplementary Material
Research in context.
Evidence before this study
Saphenous vein graft failure can be challenging to treat because of high rates of periprocedural complications and in-stent restenosis. We searched PubMed for all published randomised controlled trials of drug-eluting versus bare-metal stents in saphenous vein graft lesions. Drug-eluting stents appeared to provide improved outcomes in three of the four randomised controlled trials done to date, driven by lower rates of target lesion revascularisation compared with bare-metal stents. However, these studies were limited by small size, absence of blinding, routine angiographic follow-up, low use of embolic protection devices, and use of first-generation drug-eluting stents.
Added value of this study
The DIVA trial investigated the efficacy and safety of drug-eluting stents versus bare-metal stents in patients presenting with de-novo saphenous vein graft lesions and did not mandate routine angiographic follow-up. At 12 months, the incidence of target vessel failure (composite of cardiac death, target vessel myocardial infarction, or target vessel revascularisation) was not significantly different between groups. Similarly, during a median follow-up of 2·7 years, target vessel failure occurred in approximately one in three patients, with no difference between bare-metal and drug-eluting stents.
Implications of all the available evidence
The common clinical perception is that patients who need stenting of saphenous vein graft lesions derive benefit from drug-eluting stent implantation. The results of the DIVA trial show that drug-eluting stents and bare-metal stents are associated with similar clinical outcomes. Given their lower cost compared with drug-eluting stents, bare-metal stents might be preferred in saphenous vein graft lesions.
Acknowledgments
This study was funded by the US Department of Veterans Affairs Cooperative Studies Program (125). The views expressed in this Article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or any US Government agency.
Funding US Department of Veterans Affairs Cooperative Studies Program.
Footnotes
Declaration of interests
ESB consults for and receives speaker honoraria from Abbott Vascular, Amgen, Asahi, Boston Scientific, Cardinal Health, CSI, Elsevier, GE Healthcare, Medicure, Medtronic, and Nitiloop, and receives research support from Boston Scientific, InfraRedx and Osprey. ESB’s spouse was an employee of Medtronic. DLB is an advisory board member of Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; is on the board of directors of Boston VA Research Institute and Society of Cardiovascular Patient Care; is chair of the American Heart Association Quality Oversight Committee; is on the data monitoring committees of Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, and Population Health Research Institute; receives honoraria from the American College of Cardiology, Belvoir Publications, Duke Clinical Research Institute, Harvard Clinical Research Institute, HMP Communications, Journal of the American College of Cardiology, Population Health Research Institute, Slack Publications, Society of Cardiovascular Patient Care, and WebMD; is deputy editor of Clinical Cardiology, and chair of the NCDR-ACTION registry steering committee and VA CART research and publications committee; receives research funding from Abbott, Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, and The Medicines Company; receives royalties from Elsevier; is a site co-investigator for Biotronik, Boston Scientific, and St Jude Medical (now Abbott); is a trustee of the American College of Cardiology; and does unfunded research for FlowCo, Merck, PLx Pharma, and Takeda. SG receives research support from the National Institutes of Health, the VA Cooperative Studies Program, the Arizona Biomedical Research Commission, and Merck Pharmaceuticals, and is the co-founder of Avery Therapeutic. SVR consults for Medtronic. KS consults for Medeon Bio Inc, TransAortic Medical, and Terumo, and receives research support from Siemens Medical Systems and Medinol. AAB consults for the American College of Cardiology. SG receives research grants from Edwards Lifesciences and VA Office of Research and Development, and consults for Medtronic, Boston Scientific, Osprey Medical, and Surmodics. FL is a speaker for Abbott Vascular, and consults for Medicure. EA consults for Abbott Vascular, Boston Scientific, Cardiovascular Systems, Medtronic, and Spectranetics. BVR receives a research grant from InfraRedx and the Spectranetics Corporation, and salary support from the VA Cooperative Studies Program. YL consults for PTC Therapeutics and PaxVax. SB receives speaker honoraria from AstraZeneca, CSI, Gore, and Medtronic, and institutional research grants from Boston Scientific Corporation, and Merck. All other authors declare no competing interests.
Listed in the appendix
References
- 1.Hall MJ, Levant S, DeFrances CJ. Trends in inpatient hospital deaths: National Hospital Discharge Survey, 2000–2010. NCHS Data Brief 2013; 2013: 1–8. [PubMed] [Google Scholar]
- 2.Widimsky P, Straka Z, Stros P, et al. One-year coronary bypass graft patency: a randomized comparison between off-pump and on-pump surgery angiographic results of the PRAGUE-4 trial. Circulation 2004; 110: 3418–23. [DOI] [PubMed] [Google Scholar]
- 3.Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 2005; 294: 2446–54. [DOI] [PubMed] [Google Scholar]
- 4.Brilakis ES, Rao SV, Banerjee S, et al. Percutaneous coronary intervention in native arteries versus bypass grafts in prior coronary artery bypass grafting patients a report from the national cardiovascular data registry. JACC Cardiovasc Interv 2011; 4: 844–50. [DOI] [PubMed] [Google Scholar]
- 5.Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery results from a Department of Veterans Affairs cooperative study. J Am Coll Cardiol 2004; 44: 2149–56. [DOI] [PubMed] [Google Scholar]
- 6.Dehmer GJ, Weaver D, Roe MT, et al. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol 2012; 60: 2017–31. [DOI] [PubMed] [Google Scholar]
- 7.Bonaa KH, Mannsverk J, Wiseth R, et al. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med 2016; 375: 1242–52. [DOI] [PubMed] [Google Scholar]
- 8.Vermeersch P, Agostoni P, Verheye S, et al. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in diseased saphenous vein grafts: six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC Trial. J Am Coll Cardiol 2006; 48: 2423–31. [DOI] [PubMed] [Google Scholar]
- 9.Vermeersch P, Agostoni P, Verheye S, et al. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC trial. J Am Coll Cardiol 2007; 50: 261–67. [DOI] [PubMed] [Google Scholar]
- 10.Brilakis ES, Lichtenwalter C, de Lemos JA, et al. A randomized controlled trial of a paclitaxel-eluting stent versus a similar bare-metal stent in saphenous vein graft lesions the SOS (stenting of saphenous vein grafts) trial. J Am Coll Cardiol 2009; 53: 919–28. [DOI] [PubMed] [Google Scholar]
- 11.Brilakis ES, Lichtenwalter C, Abdel-karim AR, et al. Continued benefit from paclitaxel-eluting compared with bare-metal stent implantation in saphenous vein graft lesions during long-term follow-up of the SOS (stenting of saphenous vein grafts) trial. JACC Cardiovasc Interv 2011; 4: 176–82. [DOI] [PubMed] [Google Scholar]
- 12.Mehilli J, Pache J, Abdel-Wahab M, et al. Drug-eluting versus bare-metal stents in saphenous vein graft lesions (ISAR-CABG): a randomised controlled superiority trial. Lancet 2011; 378: 1071–78. [DOI] [PubMed] [Google Scholar]
- 13.Jeger R Study to test the efficacy and safety of drug eluting vs bare-metal stents for saphenous vein graft interventions (BASKET-SAVAGE). European Society of Cardiology Meeting; Rome, Italy; Aug, 27–31, 2016. 5025. [Google Scholar]
- 14.Brilakis ES, Banerjee S, Edson R, et al. Rationale and design of the drug-eluting stents vs bare-metal stents in saphenous vein graft angioplasty (DIVA) trial. Clin Cardiol 2017; 40: 946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007; 116: 2634–53. [DOI] [PubMed] [Google Scholar]
- 16.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007; 115: 2344–51. [DOI] [PubMed] [Google Scholar]
- 17.Keeley EC, Velez CA, O’Neill WW, Safian RD. Long-term clinical outcome and predictors of major adverse cardiac events after percutaneous interventions on saphenous vein grafts. J Am Coll Cardiol 2001; 38: 659–65. [DOI] [PubMed] [Google Scholar]
- 18.Bansal D, Muppidi R, Singla S, et al. Percutaneous intervention on the saphenous vein bypass grafts-Long-term outcomes. Catheter Cardiovasc Interv 2008; 71: 58–61. [DOI] [PubMed] [Google Scholar]
- 19.Cox DR. Regression models and life-tables. J R Stat Soc Series B 1972; 34: 187–220. [Google Scholar]
- 20.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81: 515–26. [Google Scholar]
- 21.Investigators GUSTO. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329: 673–82. [DOI] [PubMed] [Google Scholar]
- 22.Rao SV, O’Grady K, Pieper KS, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol 2006; 47: 809–16. [DOI] [PubMed] [Google Scholar]
- 23.Gray R A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–54. [Google Scholar]
- 24.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. JASA 1999; 94: 496–509. [Google Scholar]
- 25.Uchida T, Popma J, Stone GW, et al. The clinical impact of routine angiographic follow-up in randomized trials of drug-eluting stents: a critical assessment of “oculostenotic” reintervention in patients with intermediate lesions. JACC Cardiovasc Interv 2010; 3: 403–11. [DOI] [PubMed] [Google Scholar]
- 26.Yahagi K, Kolodgie FD, Otsuka F, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol 2016; 13: 79–98. [DOI] [PubMed] [Google Scholar]
- 27.Yazdani SK, Farb A, Nakano M, et al. Pathology of drug-eluting versus bare-metal stents in saphenous vein bypass graft lesions. JACC Cardiovasc Interv 2012; 5: 666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brilakis ES, O’Donnell CI, Penny W, et al. Percutaneous coronary intervention in native coronary arteries versus bypass grafts in patients with prior coronary artery bypass graft surgery: insights from the Veterans Affairs clinical assessment, reporting, and tracking program. JACC Cardiovasc Interv 2016; 9: 884–93. [DOI] [PubMed] [Google Scholar]
- 29.Dautov R, Manh Nguyen C, Altisent O, Gibrat C, Rinfret S. Recanalization of chronic total occlusions in patients with previous coronary bypass surgery and consideration of retrograde access via saphenous vein grafts. Circ Cardiovasc Interv 2016; 9: e003515. [DOI] [PubMed] [Google Scholar]
- 30.Brilakis ES, Banerjee S, Lombardi WL. Retrograde recanalization of native coronary artery chronic occlusions via acutely occluded vein grafts. Catheter Cardiovasc Interv 2010; 75: 109–13. [DOI] [PubMed] [Google Scholar]
- 31.Brilakis ES, Wang TY, Rao SV, et al. Frequency and predictors of drug-eluting stent use in saphenous vein bypass graft percutaneous coronary interventions: a report from the American College of Cardiology National Cardiovascular Data CathPCI registry. JACC Cardiovasc Interv 2010; 3: 1068–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.