Abstract
The survival outlook in advanced mycosis fungoides (MF) is poor. Autologous and allogeneic stem cell transplants (SCT) have been shown, in small case series and case reports, to have the potential for long-term remission or to alter disease course. Allogeneic SCT is thought to have a curative potential secondary to a graft-versus-lymphoma (GVL) effect. A patient-level meta-analysis was performed to compare the outcome of allogeneic versus autologous SCT in patients with MF/Sézary syndrome (SS) using 39 cases from the literature. There were a total of 20 allogeneic and 19 autologous transplant cases. The gender, age, and stage distribution was similar between the transplant groups. The allogeneic group received significantly more systemic therapies prior to transplant (P < .0005) and had longer follow-up after transplant. Overall survival (OS) results showed a more favorable outcome of patients who received allogeneic SCT (P = .027). Event-free survival (EFS) demonstrated a more durable response in patients who received allogeneic SCT (P = .002). In the allogeneic group, the majority (70%) of patients experienced persistent graft-versus-host disease (GVHD), mostly with mild to moderate severity, and 2 of 4 deaths were related to GVHD. Meanwhile, the majority of the deaths (8 of 10) in the autologous group were because of progressive disease. These results support the belief that allogeneic SCT offers a better survival and disease-free outcome versus autologous SCT in MF/SS, likely because of a GVL effect.
Keywords: Mycosis fungoides, Cutaneous T cell lymphoma, Autologous stem cell transplant, Allogeneic stem cell transplant, Sézary Syndrome
INTRODUCTION
Mycosis fungoides (MF) and Sézary syndrome (SS) belong to a group of cutaneous T cell lymphomas (CTCL) characterized by a proliferation of CD4+/CLA+/CCR4+/CD7−/CD26− T cells in the skin. MF usually presents with patch and plaque lesions, but in advanced stages may progress to skin tumors with greater risk for lymph node and visceral organ involvement [1]. SS is the erythrodermic or leukemic form of MF with circulating malignant T cells in the peripheral blood and often portends a poorer prognosis [2].
Although it is the most common primary lymphoma of the skin, MF is rare and usually indolent. Conventional treatments include topical nitrogen mustard, topical high potency steroids, electron beam radiotherapy, and phototherapy [3,4]. These treatments usually produce complete or partial remission. For mild MF disease—up to stage IIA—prognosis is generally good. Patients with stage IA disease have similar overall survival (OS) compared to others of the same age, gender, and race, and median survival is 11 years for patients with stage IB-IIA MF [5].
A subset of patients have advanced MF or SS, which is heralded by the presence of skin tumors or erythroderma at presentation. Other unfavorable prognostic factors include large cell transformation on histologic examination, and development of extracutaneous disease. In these patients, prognosis is poor with 5-year survival for stage IIB/III and stage IV MF of 47% and 27%, and 15-year survival of 15% and 10%, respectively [5]. The management of advanced MF includes chemotherapy, photopheresis, radiotherapy, interferon-α, retinoids like bexarotene, as well as targeted therapies such as monoclonal antibodies and denileukin diftitox. These treatments are only expected to be palliative with limited-duration remissions [3,6].
There is hope that stem cell transplants (SCT) have the potential to provide prolonged remissions or possible cure of advanced MF/SS. The collective experience in allogeneic and autologous transplant is limited but optimistic, especially for allogeneic SCT. A proposed gravt-versus-leukemia (GVL) effect is thought to be responsible for higher effectiveness of allogeneic transplants [7]. However, there have been no controlled prospective studies to date, and the literature thus far consists of case reports, small case series, and reviews [8].
PATIENTS AND METHODS
Data Collection and Patient Selection
This retrospective meta-analysis was based on data obtained from several sources. A PubMed search was performed, using the phrases “mycosis fungoides”/“Sézary syndrome”/“cutaneous T cell lymphoma” and “stem cell transplant,” to include data from previously reported cases and case series of MF/SS patients. Patients with CD4+ predominant MF or SS who received peripheral blood or bone marrow-derived SCTs were included in the study. Papers needed to be written in English and published prior to March 2008 for study inclusion. In total, 9 papers on allogeneic SCTs in MF/SS and 7 papers on autologous SCTs were reviewed, and all were used in the data analysis. Staging was based on TNM and B classification of MF/SS described by the Committee on Staging and Classification of MF/SS in 1979 [9]. Although there are revised staging recommendations by the International Society for Cutaneous Lymphomas, the publications from which these patients were gathered were written prior to the recent guidelines [10].
Information regarding patient age, gender, stage at diagnosis, stage at progression, previous treatments, type of SCT, inductive therapy, preparation/conditioning therapy, use of total-body irradiation (TBI) or total skin electron beam (TSEB) therapy, remission/relapse, graft-versus-host disease (GVHD), infection, mortality, time of transplant, and last follow-up were obtained from published reports. Authors were contacted by email or phone for additional information where the literature was not clear. Complete remission (CR) was defined as resolution of skin, lymph node, viscera, or blood involvement (if applicable) clinically, histologically, radiographically, or molecularly, within 60 days of transplant. GVHD was categorized as acute (occurring <100 days from transplant) or chronic, and presence at last follow-up was noted. If acute, the affected area was noted, if possible, and if chronic, it was described as limited or extensive. Although the terminology has undergone debate and change, the reports described GVHD in those terms.
Statistical Analysis
Event-free survival (EFS), OS, and relapse curves were created from the date of transplant until the date of last follow-up or an event, and calculated by the method of Kaplan and Meier [11]. Both log-rank and Cox models were used for analysis. Competing risk models were created with tests described by Gray [12]. Two-tailed, independent samples t-tests were used to compare means. Analyses were performed with R version 2.2.0 and SPSS version 15.0 [13]. Disease progression, relapse, and death from any cause defined events for EFS.
RESULTS
The clinical characteristics of the allogeneic and autologous SCT groups were similar (Table 1). There were 39 patients total: 20 patients in the allogeneic group, 19 in the autologous with similar gender and age demographics (P, age = .236). Patients with advanced disease, that is, stages IIB and IVA MF, were represented in both groups, with more stage IVA patients in the allogeneic group (15 versus 5). Meanwhile, 6 patients in the allogeneic group and 1 patient in the autologous transplant group had SS. Allogeneic patients had longer follow-up times from transplant. The median number of total therapies prior to SCT was 6.5 in the allogeneic groups (range: 3-12) and 3 (range: 0-8; P<.0005) in the autologous group. When divided into skin-directed versus systemic therapies, the number of skin-directed therapies was similar between the 2 groups, whereas the allogeneic transplant group received significantly more systemic therapies prior to SCT (P<.0005). Thirteen patients in the autologous and 12 in allogeneic group received TBI or electron beam therapy in addition to chemotherapy in their preparatory regimens (Table 2). Nine patients in the allogeneic group received myeloablative preparative regimens, whereas the remainder received reduced-intensity (nonablative) protocols. In the allogeneic group, stem cells were obtained from the bone marrow or peripheral blood of matched siblings or matched unrelated donors.
Table 1.
Patient Characteristics
| Allogeneic | Autologous | ||
|---|---|---|---|
| Total No. pts | 20 | 19 | |
| Age (years) | median | 42 | 47 |
| range | (21-59) | (20-67) | |
| Gender | male | 9 | 10 |
| female | 11 | 9 | |
| Clinical stage (NCI 1979) at transplant | IB | 1 | 1 |
| IIB | 3 | 9 | |
| IIIA | 1 (SS) | 0 | |
| IVA | 15 (5 SS) | 5 | |
| IVB | 0 | 4 | |
| Median No. prior therapies | Skin-directed | 2 | 2 |
| Systemic* | 4.5 | 1 | |
| Total follow-up time (months) | median | 38 | 21 |
| range | (1-108) | (0.5-84) | |
| Follow-up time of surviving patients (months) | median | 45 | 22 |
| range | (15-108) | (10-84) |
Significant (P < .05) differences are marked.
Table 2.
Overview of the Literature with Transplant Regimens
| Reference | Age/Gender | Stage at Transplant |
Conditioning Regimen | Myelo- ablative? |
Radiation? | Type of Transplant |
Disease relapse | Remission at Last f/u |
Outcome @ Last f/u | GVHD at Last f/u? |
|---|---|---|---|---|---|---|---|---|---|---|
| Molina et al., 1999, 2005 [24, 27] | 22F | SS/IVA | CyP 60mg/kg × 2D | Y | Y; TBI | Allo; MUD BMT | N | Y | alive @ 108 mo | Y; extensive skin |
| 45 | IIB | CyP 60mg/kg × 2D | Y | Y; TBI | Allo; sibling matched BMT | N | Y | alive @ 89 mo | Y; limited skin | |
| 46 | SS/IVA | CyP 60mg/kg × 2D | Y | Y; TBI and TSEB | Allo; sibling matched PBSCT | N | Y | died @ 16 mo from GVHD complications | Y; extensive skin, oral | |
| 21F | SS/IVA | busulfan 16mg/kg, CyP 60mg/kg × 2D | Y | N | Allo; sibling matched PBSCT | N | Y | alive @ 60 mo | Y; limited skin | |
| 59 | SS/IIIA | Flu, 25mg/m2 × 5D then Mel 140mg/m2 × 1D | N | N | Allo; sibling matched PBSCT | N | Y | alive @ 53 mo | Y; extensive skin | |
| 50 | IVA | Flu, 25mg/m2 × 5D then Mel 140mg/m2 × 1D | N | N | Allo; MUD BMT | N | Y | alive @ 45 mo | Y; limited skin | |
| 48 | IVA | Flu, 25mg/m2 × 5D then Mel 140mg/m2 × 1D | N | N | Allo; MUD BMT | N | Y | alive @ 33 mo | Y; limited skin | |
| 35 | IIB | Flu, 25mg/m2 × 5D then Mel 140mg/m2 × 1D | N | N | Allo; MUD PBSCT | N | Y | died @ 1.1 mo from RSV | N | |
| Guitart et al., 2002 [14] | 36M | IIB | CyP 120mg/kg with mesna | Y | Y; TBI | Allo; sibling matched BMT | N | Y | alive @ 54 mo | N |
| 39F | SS/IVA | CyP 120mg/kg with mesna, etopisode 30mg/kg | Y | Y; TBI | Allo; sibling matched BMT | N | Y | alive @ 15 mo | N | |
| Burt et al., 2000 [28] | 27F | IVA | CyP 200mg/kg | Y | Y; TBI | Allo; sibling matched BMT + CD34 PBSC | Y @ 9 mo and 5 yrs | N | alive s/p 1 DLI @ 60 mo | Y; limited skin |
| Masood et al.,2002[29] | 37F | IVA | CyP 120mg/kg × 2 doses | Y | Y; TBI and TSEB | Allo; sibling matched BMT + CD34 PBSC | N | Y | alive @ 24 mo | Y; mild gut |
| Soligo et al., 2003 [30] | 56M | IVA | Flu 30mg/m2 × 3D | N | Y; TBI | Allo; sibling matched BMT + CD34 PBSC | N | Y | alive @ 24 mo | N |
| 47M | IVA | 2 cycles: Flu, 30mg/m2 × 3D + CyP 300mg/kg × 3D | N | Y; TBI | Allo; sibling matched BMT + CD34 PBSC | N | Y | alive @ 18 mo | Y; limited skin | |
| 37M | IVA | 2 cycles: Flu 30mg/m2 × 3D + CyP 300mg/kg × 3D | N | Y; TBI | Allo; sibling matched BMT + CD34 PBSC | N | Y | died @ 2.4 mo of sepsis, myocarditis | Y; grade II cut aGVHD | |
| Koeppl et al., 1994 [31] | 29F | IVA | CyP 60mg/kg × 2D | Y | Y; TBI | Allo; sibling matched BMT | Y @ 70D and 4 yrs | Y | alive @ 72 mo | Y; limited skin |
| Herbert et al., 2004 [6],(personal electronic mail communication2008) | 35M | IIB | Flu, 25mg/m2 × 5D then Mel 140mg/m2 × 1D | N | N | Allo; sibling matched BMT + CD3 | Y @ 4mo, 13mo, 24mo | N | alive s/p 4 DLIs @ 95*mo | Y; extensive skin |
| 49M | SS, IVA | Flu, 25mg/m2 × 5D then Mel 140mg/m2 × 1D | N | N | Allo; sibling matched BMT + CD3 | Y @ D43 and 4mo | N | died s/p 1 DLI @ 11 mo of GVHD complications | Y; extensive skin | |
| 48F | IVA | Flu, 25mg/m2 × 5D then Mel 140mg/m2 × 1D | N | N | Allo; sibling matched PBSCT | Y @ 4 and 6mo | Y | alive @ 17* mo | Y; limited skin | |
| Introcaso et al., 2008 [32] | 53F | IVA | Flu, 25mg/m2, CyP 350mg/m2 | N | Y; TBI | Allo; sibling matched SCT | N | Y | alive @ 43 mo | Y; limited skin |
| Ferra et al., 1999 [33] | 38F | IVB | CyP, 1500mg/m2 × 2D, carmustine 300mg/m2, etoposide 200mg/m2× 3D | NA | Y; TBI | Auto: PBSCT | Y @ 2mo | N | alive @ 22 mo | NA |
| Sterling et al., 1995 [34] | 48M | IIB | CyP 60mg/kg × 2, prednisone | NA | Y; TBI and local rad to lower leg | Auto: BMT | Y @ 3mo | N | died @ 15 mo of PNA | NA |
| Olavarria, Russell-Jones et al., 2001 [35], [36] | 47M | IIB | melphalan 140mg/m2 | NA | Y; TBI and TSEB | Auto: PBSCT | Y @ 2mo | N | alive @ 38 mo | NA |
| 52F | IVA | BCNU 450mg/m2 × 1D, etoposide 500mg/m2 × 4D, Mel 140mg/m2× 1D | NA | N | Auto: PBSCT | Y @ 12mo | N | alive @ 26 mo | NA | |
| 27M | IVA | etoposide 50mg/kg | NA | Y; TBI | Auto: PBSCT | Y @ 14mo | N | alive @ 30 mo | NA | |
| 49F | IVA | BCNU 450mg/m2 × 1D, etoposide 500mg/m2 × 4D, Mel 140mg/m2 × 1D | NA | N | Auto: BMT | NA | N | died @ 0.5 mo of septicemia | NA | |
| 38M | IVA | BCNU 450mg/m2 × 1D, etoposide 500mg/m2 × 4D, Mel 140mg/m2 × 1D | NA | Y; TSEB | Auto: PBSCT | Y @ 9mo | N | alive @ 21 mo | NA | |
| 67M | IIB | BCNU 450mg/m2 × 1D, etoposide 500mg/m2 × 4D, Mel 140mg/m2 × 1D | NA | Y; TSEB | Auto: PBSCT | Y @ 2mo | N | died @ 11 mo of dz | NA | |
| 42F | IIB | BCNU 450mg/m2 × 1D, etoposide 500mg/m2 × 4D, Mel 140mg/m2 × 1D | NA | N | Auto: PBSCT | Y @ 4mo | N | died @ 8 mo of dz | NA | |
| 38M | IIB | BCNU 450mg/m2 × 1D, etoposide 500mg/m2 × 4D, Mel 140mg/m2 × 1D | NA | N | Auto: PBSCT | N | Y | alive @ 10 mo | NA | |
| 57F | IIB | BCNU 450mg/m2 × 1D, etoposide 500mg/m2 × 4D, Mel 140mg/m2 × 1D | NA | Y; TSEB | Auto: PBSCT | Y @ 1mo | N | died @ 3 mo of dz | NA | |
| Bigler et al., 1991 [37] | 49F | IVB | CyP 40mg/kg × 4D, etoposide 200mg/m2 × 3D, BCNU 300mg/m2 × 1D | NA | Y; TSEB | Auto: BMT | Y @ D64 | N | died @ 9.4 mo of dz | NA |
| 60M | IIB | NA | Y; TBI and TSEB | Auto: BMT | Y @ D70 | N | died @ 55.5 mo of dz | NA | ||
| 49M | IVA | CyP 60mg/kg × 2D | NA | Y; TBI | Auto: BMT | Y @ D93 | N | died @ 12.6 mo of dz | NA | |
| 37F | IIB | CyP 50mg/kg × 4D | NA | Y; TBI and TSEB | Auto: BMT | Y @ D96 | N | died @ 27.5 mo of dz | NA | |
| 26F | IIB | BCNU 200mg/m2 × 3D, etoposide 800mg/m2 × 3D, cisplatin 40mg/m2 × 5D | NA | Y; local rad to mantle and spleen, TSEB | Auto: BMT | N | Y | alive @ 22 mo | NA | |
| 41F | IVB | BCNU 200mg/m2 × 3D, etoposide 800mg/m2 × 3D, cisplatin 40mg/m2 × 5D | NA | Y; local rad to CNS | Auto: BMT | N | Y | alive @ 21 mo | NA | |
| Chen et al., 1986 [38] | 40M | IVB | NA | Y; TBI | Auto: BMT | Y @ D42 | N | died @ 3.7 mo of dz | NA | |
| Ingen-Housz-Oro et al., 2004 [39] | 51M | IB | BCNU, etoposide, aracytine, Mel | NA | N | Auto: PBSCT | Y @ 2.5mo | Y | alive @ 84 mo | NA |
My indicates myeloablative; NMy, nonmyeloablative; N, no; Y, yes; CyP, cyclophosphamide; BMT, bone marrow transplant; PBSCT, peripheral blood stem cell transplant; PBSC, peripheral blood stem cells; SS, Sézary syndrome; Gy, Gray; F, female; M, male; D, days; Flu, fludarabine; Mel, melphalan; Allo, allogeneic; Auto, autologous; NA, not applicable; @, at; mo, months; yrs, years; cut, cutaneous; aGVHD, acute graft-versus-host disease; TBI, total-body irradiation; f/u, follow-up; TSEB, total skin electron beam radiotherapy; BCNU, carmustine; rad, radiation; dz, disease; MUD, matched unrelated donor; s/p, status post; DLI, donor lymphocyte infusions.
Information obtained via author correspondence.
The Kaplan-Meier estimates demonstrate significantly superior OS in the allogeneic transplant group with P-value = .027 (Figure 1). OS rates at 1 and 5 years (with 95% confidence intervals [CI]) were 68% (46%-90%) and 23% (0%-58%) in the autologous group, and 85% (69%-100%) and 80% (62%-98%), respectively, in the allogeneic group. EFS was also significantly better in patients receiving an allogeneic versus an autologous transplant (P = .002; Figure 2). EFS rates at 1 and 5 years (with 95% CIs) were 20% (1%-39%) and 0, respectively, in the autologous group. In the allogeneic group, 1-year EFS was 65% (44%-86%) and was 60% (37%-82%) at 5 years.
Figure 1.
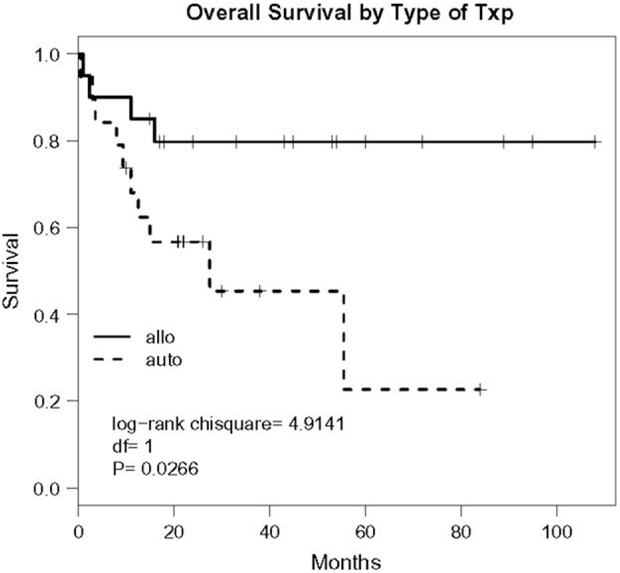
OS of patients with MF receiving an allogeneic transplant (allo) or an autologous transplant (auto), P =.027.
Figure 2.

EFS of patients with MF receiving an allogeneic transplant (allo) or an autologous transplant (auto), P =.0021.
Overall, 4 patients in the allogeneic and 10 in the autologous SCT group died in follow-up. Two of the allogeneic deaths were secondary to infection shortly after transplant. The remaining allogeneic transplant recipients died from GVHD complications, although 1 of those patients had residual MF disease at the time of death. In contrast, of the 10 deaths in the autologous group, 8 were of disease, 1 was of infection in a patient with relapsed disease following transplant, and the remaining patient died of infection shortly after transplant.
Disease relapse accounted for the large difference in EFS and mortality between the groups. The cumulative incidence of cancer death was significantly higher after autologous transplant than allogeneic transplant (Figure 3, test statistic 10.2 on 1 degree of freedom, P = .0014 by the competing risk test), whereas there was no significant difference between the groups on noncancer deaths (test statistic = 0.90, P = .35).
Figure 3.

Estimated cumulative incidences of cancer (CA) and noncancer (nonCA) mortality in allogeneic (allo) and (auto) autologous transplant groups.
The total number of events was small, rendering multivariate analysis unreliable and of low power. Nevertheless, we performed a number of multivariate analyses to check for confounding variables by age, gender, and stage, to see if the results could be accounted for by imbalances in those factors. Age alone had a nonsignificant effect on mortality (each year of age multiplied the hazard of death by 1.04, P = .058 by the deviance test in the Cox model) as well as on the event hazard (factor of 1.03 per year, P=.17). After adjusting for age, the treatment group effect was slightly mitigated. Unadjusted hazards ratio (HR) for treatment group effect on mortality was 3.5, adjusted HR was 2.9, P-value for adjusted effect .061; for events the corresponding HR was 3.6 unadjusted, 3.8 adjusted, P-value for adjusted effect .003. In the multivariate competing risk regression analysis, cancer death was increased significantly by both age (P = .022) and treatment type (P=.03), whereas noncancer death was not associated with either age (P = .89) or treatment type (P = .36). Thus, age did not appear to confound the effects of treatment type.
The analysis of confounding by gender was complicated by missing data on gender in 6 patients. However, gender by itself had no significant effect on OS or EFS and adjustment for it did not appreciably change either the significance or size of the treatment group effect on either mortality or events.
As we started analyzing the possibility of confounding by stage, we observed a difference in the distribution of stage between the 2 treatment groups, with a preponderance of IVA in allogeneic transplant and of IIB in autologous transplant. Of note, in stage IVA, 3/15 allogeneic patients died versus 2/5 in the autologous group, and in stage IIB the death rates were 1/3 for allogeneic and 6/9 for autologous. The raw death rates were higher in the autologous group in both stages. Other stages were so sparse they could not be compared to the treatment groups within the other stages. Within the subgroup of 20 IVA patients and 12 IIB patients, the mortality HR for IIB versus IVA was 1.6, P = .084, and the adjusted HR for autologous treatment was 2.7 (P = .17), indicating some attenuation of effect but not strong confounding. In summary, the results are that stage has no impact, and the effect of transplant type is unchanged after adjustment.
In patients who relapsed and died, the median time to death was 9.3 months, and of the 5 allogeneic patients who relapsed only 1 died, compared to 9 of 15 relapsed autologous patients (P = .15). Of the 5 relapses in the allogeneic group, 2 occurred in the myeloablative group and 3 in the nonmyeloablative group. In 3 of the 5 allogeneic recurrences (1 myeloablative, 2 nonmyeloablative), donor lymphocyte infusions were used with some success [6,14].
The incidence of GVHD was high and nearly equal in the myeloablative and nonmyeloablative allogeneic transplant groups. Six of 9 (67%) patients in the myeloablative group and 9 of 11 (81%) patients in the nonmyeloablative group experienced acute GVHD (aGVHD), ranging from grades I-III and with conjunctival, skin, oral and gastrointestinal (GI) manifestations. Seven and 8 patients in the myeloablative and nonmyeloablative sets, respectively, experienced chronic GVHD (cGVHD). Ninety percent of the allogeneic SCT patients experienced aGVHD or cGVHD; however, GVHD was only present in 70% of patients in the final follow-up. In both transplant groups, there were approximately equal numbers of patients with limited or extensive cGVHD. As previously mentioned, 2 of the 4 allogeneic deaths were attributed to GVHD complications. None of the patients in the autologous SCT group experienced GVHD.
DISCUSSION
Currently, there are a lack of therapies that will achieve long-term remissions or cures in patients with advanced stage MF or SS. Primary and salvage treatments for advanced stages of MF include total skin electron beam therapy, oral bexarotene, photopheresis, interferons, denileukin diftitox, alemtuzumab, and chemotherapeutic agents, many of which were tried and failed in the patients included in our meta-analysis [3,4]. Newer therapies have emerged, such as histone deacetylase inhibitors including vorinostat, which was FDA-approved in 2006 for CTCL [15]. The efficacy and optimal role of these therapies as monotherapy, synergistic combinations, or adjuvants are still being developed. Several promising novel or improved therapies are currently undergoing clinical trials. Of the available therapies, hematopoietic SCT may have curative potential; however, currently no large trials exist. This report represents the first meta-analysis of allogeneic and autologous SCTs in MF and SS, the major types of CTCL.
Our univariate and multivariate analysis showed a statistically significantly improvement in relapse, OS and EFS in allogeneic versus autologous SCTs in MF/SS. Patients undergoing autologous SCT rely only on the cytoreductive preparatory regimens prior to stem cell rescue for treatment effect. In some of our study patients, autologous SCT was unable to produce a complete remission, and autologous SCT deaths were usually secondary to disease progression.
The decreased relapse rate and increased OS and EFS in the allogeneic SCT may be consistent with the theorized GVL/tumor effect. The graft-versus-tumor effect was noted by Weiden et al. [16], when his group described significantly decreased relapse rate in leukemia patients with GVHD following allogeneic SCT. Subsequent studies of the GVHD/GVL effect after allogeneic SCT for leukemia and lymphoma have substantiated a decreased relapse rate and increased OS and EFS with cGVHD [17,18]. It is the GVL effect that is thought to be responsible for producing cancer remission following nonmyeloablative regimens [19]. Although the association between GVHD and decreased relapse rates is felt to be indicative of GVL, data suggests there may be methods to separate the GVL and GVHD reactions. Possible approaches include using specific preparative regimens [20], choice of immunosuppressive agents, or other manipulations of specific regulatory and suppressor cell populations [21-23]. The morbidity of allogeneic SCT for MF/SS and other malignancies may be lessened in the future if these methods can be perfected to allow the skewing of the immune system in the GVL direction.
Allogeneic SCTs are traditionally associated with higher risk of morbidity and mortality; however, in this study, the number of deaths within the first year was actually greater in the autologous group. Deaths within the first year in patients who received an autologous transplant were largely secondary to progressive disease (5 out of 6). In the allogeneic group, 3 of the 4 recorded deaths occurred in the first year and were secondary to infection (2) or complications of GVHD (1). Overall there was a 20% transplant-related mortality rate in allogeneic patients, which is comparable to published data for other diseases.
In general, the conditions under which a candidate may be approved for a myeloablative allogeneic SCT are limited to patients under the age of 60. Unfortunately, MF tends to occur at an average age of 60 years, which, until recently, may have decreased the availability of allogeneic transplant to these patients [24]. However, the use of reduced-intensity regimens (RICs) should allow more MF patients, including patients in their seventies, the opportunity to proceed to an allogeneic transplant. There was not enough data to perform meaningful comparisons between myeloablative and RICs; however, the frequency of GVHD was similar between the 2 types of preparatory regimens in this patient population. TBI and TSEB have been associated with an increased risk of GVHD, perhaps through injury of thymic epithelial cells or the GI tract [25,26]. In our study, a greater percentage of patients who did not receive TBI therapy had GVHD at last follow-up (6 of 8 patients who did not receive TBI versus 8 of 12 patients who did). In contrast, the percentage of patients with GVHD at last follow-up was higher in patients who received electron beam therapy at some point in their MF/SS therapy (7 or 8 patients who received TSEB versus 7 of 12 patients who did not). However, the statistical significance was not determined because of the small sample size.
With new advances in transplantation and the data presented here, hematopoietic SCT for advanced MF should be considered in patients who have refractory disease or short-lived responses with standard therapies. In this meta-analysis, allogeneic SCT was associated with statistically superior clinical outcomes compared to autologous SCT, which supports a potentially significant GVL effect in MF and SS. It is unclear whether donor leukocyte infusions at the time of relapse or in the case of persistent disease following transplant may also have an impact on disease outcome. The encouraging results from this study further validate the selection of allogeneic over autologous hematopoietic SCT in the management of advanced MF or SS [4], and support the need for further investigation of optimal strategies for allogeneic hematopoietic HSCT in MF and SS.
Acknowledgments
Financial disclosure: The authors have nothing to disclose.
REFERENCES
- 1.Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978–1988. [DOI] [PubMed] [Google Scholar]
- 2.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz SM, Olsen EA, Duvic M, Porcu P, Kim YH. Review of the treatment of mycosis fungoides and Sézary syndrome: a stage-based approach. J Natl Comp Cancer Netw. 2008;6:436–442. [DOI] [PubMed] [Google Scholar]
- 4.Mycosis Fungoides/Sezary syndrome of the cutaneous T-cell lymphomas. NCCN Practice Guidelines in Oncology v12008. NCCN Practice Guidelines Oncol. 2008. [Google Scholar]
- 5.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857–866. [DOI] [PubMed] [Google Scholar]
- 6.Herbert K, Spencer A, Grigg A, et al. Graft-versus-lymphoma effect in refractory cutaneous T-cell lymphoma. Bone Marrow Transplant. 2004;34:521–525. [DOI] [PubMed] [Google Scholar]
- 7.Jones RJ, Ambinder RF, Piantadosi S, Santos GW. Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood. 1991;77:1383–1388. [PubMed] [Google Scholar]
- 8.Duarte R, Schmitz N, Servitje O, Sureda A. Haematopoietic stem cell transplantation for patients with primary cutaneous T-cell lymphoma. Bone Marrow Transplant. 2008;41:597–604. [DOI] [PubMed] [Google Scholar]
- 9.Bunn P, Lamberg S. Report of the committee on staging and classification of cutaneous T-cell lymphomas. Cancer Treat Rep. 1979;63:725–728. [PubMed] [Google Scholar]
- 10.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713–1722. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan E, Meier P. Non-parametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 12.Gray R A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 13.Team RDC. R: A language and environmentforstatistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2005. [Google Scholar]
- 14.Guitart J, Wickless SC, Oyama Y, et al. Long-term remission after allogeneic hematopoietic stem cell transplantation for refractory cutaneous T-cell lymphoma. Arch Dermatol. 2002;138:1359–1365. [DOI] [PubMed] [Google Scholar]
- 15.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. [DOI] [PubMed] [Google Scholar]
- 16.Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Cho BS, Kim SY, et al. Allogeneic stem cell transplantation in first complete remission enhances graft-versus-leukemia effect in adults with acute lymphoblastic leukemia: antileukemic activity of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1083–1094. [DOI] [PubMed] [Google Scholar]
- 18.Gassas A, Sung L, Saunders E, Doyle J. Graft-versus-leukemia effect in hematopoietic stem cell transplantation for pediatric acute lymphoblastic leukemia: significantly lower relapse rate in unrelated transplantation. Bone Marrow Transplant. 2007;40:951–955. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama D, Fukuda T, Kato R, et al. Comparable antileukemia/lymphoma effects in nonremission patients undergoing allogeneic hematopoietic cell transplantation with a conventional cytoreductive or reduced-intensity regimen. Biol Blood Marrow Transplant. 2007;13:932–941. [DOI] [PubMed] [Google Scholar]
- 20.Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353:1321–1331. [DOI] [PubMed] [Google Scholar]
- 21.Soiffer R Immune modulation and chronic graft-versus-host disease. Bone Marrow Transplant. 2008;42:S66–S69. [DOI] [PubMed] [Google Scholar]
- 22.Sprangers B, Van Wijmeersch B, Fevery S, Waer M, Billiau AD. Experimental and clinical approaches for optimization of the graft-versus-leukemia effect.Nat Clin Pract Oncol.2007;4:404–414. [DOI] [PubMed] [Google Scholar]
- 23.Liang Y, Liu C, Djeu JY, et al. Beta2 integrins separate graft-versus-host disease and graft-versus-leukemia effects. Blood. 2008;111:954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina A, Zain J, Arber DA, et al. Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sezary syndrome and mycosis fungoides. J Clin Oncol. 2005;23:6163–6171. [DOI] [PubMed] [Google Scholar]
- 25.Zecca M, Prete A, Rondelli R, et al. Chronic graft-versus-host disease in children: incidence, risk factors and impact on outcome. Blood. 2002;100:1192–1200. [DOI] [PubMed] [Google Scholar]
- 26.Alyea E, Neuberg D, Mauch P, et al. Effect of total body irradiation dose escalation on outcome following T-cell-depleted allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2002;8:139–144. [DOI] [PubMed] [Google Scholar]
- 27.Molina A, Nademanee A, Arber DA, Forman SJ. Remission of refractory Sezary syndrome after bone marrow transplantation from a matched unrelated donor. Biol Blood Marrow Transplant. 1999;5:400–404. [DOI] [PubMed] [Google Scholar]
- 28.Burt RK, Guitart J, Traynor A, et al. Allogeneic hematopoietic stem cell transplantation for advanced mycosis fungoides: evidence of a graft-versus-tumor effect. Bone Marrow Transplant. 2000;25:111–113. [DOI] [PubMed] [Google Scholar]
- 29.Masood N, Russell KJ, Olerud JE, et al. Induction of complete remission of advanced stage mycosis fungoides by allogeneic hematopoietic stem cell transplantation. J Am Acad Dermatol. 2002;47:140–145. [DOI] [PubMed] [Google Scholar]
- 30.Soligo D, Ibatici A, Morandi P, et al. Treatment of advanced mycosis fungoides by allogeneic stem-cell transplantation with a non-myeloablative regimen. Bone Marrow Transplant. 2003;31:663–666. [DOI] [PubMed] [Google Scholar]
- 31.Koeppel M-C, Stoppa A-M, Resbeut M, et al. Mycosis fungoides and allogeneic bone marrow transplantation. Acta Derm Venereol. 1994;74:331–332. [DOI] [PubMed] [Google Scholar]
- 32.Introcaso CE, Leber B, Greene K, et al. Stem cell transplantation in advanced cutaneous T-cell lymphoma. J Am Acad Dermatol. 2008;58:645–649. [DOI] [PubMed] [Google Scholar]
- 33.Ferra C, Servitje O, Petriz L, et al. Autologous haematopoietic progenitor transplantation in advanced mycosis fungoides. Br J Dermatol. 1999;140:1188–1189. [PubMed] [Google Scholar]
- 34.Sterling JC, Marcus R, Burrows NP, Roberts SO. Erythrodermic mycosis fungoides treated with total body irradiation and autologous bone marrow transplantation. Clin Exp Dermatol. 1995;20:73–75. [DOI] [PubMed] [Google Scholar]
- 35.Olavarria E, Child F, Woolford A, et al. T-cell depletion and autologous stem cell transplantation in the management of tumour stage mycosis fungoides with peripheral blood involvement. Br J Haematol. 2001;114:624–631. [DOI] [PubMed] [Google Scholar]
- 36.Russell-Jones R, Child F, Olavarria E, et al. Autologous peripheral blood stem cell transplantation in tumor-stage mycosis fungoides: predictors of disease-free survival. Ann N Y Acad Sci. 2001;941:147–154. [DOI] [PubMed] [Google Scholar]
- 37.Bigler RD, Crilley P, Micaily B, et al. Autologous bone marrow transplantation for advanced stage mycosis fungoides. Bone Marrow Transplant. 1991;7:133–137. [PubMed] [Google Scholar]
- 38.Chen YC, Wang CH, Huang SC, et al. Autologous bone marrow transplantation after supralethal dose of total body irradiation in a case of mycosis fungoides. Taiwan Yi Xue Hui Za Zhi. 1986;85:304–314. [PubMed] [Google Scholar]
- 39.Ingen-Housz-Oro S, Bachelez H, Verola O, et al. High-dose therapy and autologous stem cell transplantation in relapsing cutaneous lymphoma. Bone Marrow Transplant. 2004;33:629–934. [DOI] [PubMed] [Google Scholar]


