Abstract
Recent evidence suggests that trastuzumab, a monoclonal antibody which targets HER2, in combination with chemotherapy is a therapeutic option in patients with HER2-positive gastric or gastroesophageal junction cancer. Widely accepted guidelines for HER2 testing in gastric and gastroesophageal junction cancer have not been established. The purpose of this study was to analyze the incidence and patterns of HER2 expression in gastric and gastroesophageal junction cancer using a tissue microarray approach, which closely simulates small biopsies routinely tested for HER2. One hundred sixty-nine patients, including 99 primary gastric adenocarcinomas and 70 primary gastroesophageal junction carcinomas were analyzed for HER2 overexpression by immunohistochemistry and HER2 gene amplification by fluorescence in situ hybridization using scoring schemes proposed by both American Society of Clinical Oncology/ College of American Pathologists (ASCO/CAP) and the results of the recently published Trastuzumab for Gastric cancer (ToGA) trial. In our analysis, 19 adenocarcinomas were HER2 positive, defined as either a HER2/CEP17 ratio > 2.2 and/or a 3+ HER2 immunohistochemistry score with either the ASCO/CAP or ToGA scoring schemes. Of the 19 HER2-positive adenocarcinomas, 8 (42%) exhibited a characteristic strongly intense basolateral membranous staining pattern which would be interpreted as negative (1+) using the accepted ASCO/CAP scoring scheme for HER2 assessment in breast carcinoma, but were correctly labeled as 3+ positive using the proposed ToGA scoring scheme. Of the 19 HER2-positive adenocarcinomas, 8 (42%) demonstrated heterogeneous HER2 protein expression by immunohistochemistry. Twelve of 99 (12%) gastric carcinomas were positive for HER2. Of these, HER2 was more often identified in intestinal-type adenocarcinomas (10 of 52, 19%) compared with diffuse (2 of 34, 6%) adenocarcinoma. Seven of 70 (10%) gastroesophageal junction carcinomas were positive for HER2 of which all were intestinal type (7 of 58, 12%). HER2 status or primary tumor site did not correlate with patient survival. Gastric and gastroesophageal junction adenocarcinomas typically display a characteristic basolateral membranous pattern of HER2 expression which is often heterogeneous rendering routine evaluation of HER2 status on small tissue samples challenging.
Keywords: HER2, gastric adenocarcinoma, gastroesophageal junction adenocarcinoma, fluorescence in situ hybridization, immunohistochemistry
Gastric and gastroesophageal junction adenocarcinomas constitute a major health problem worldwide. Gastric cancer is the fourth most prevalent malignancy and the second leading cause of cancer death worldwide.1 In the United States, an estimated 2100 cases of gastric cancer will be diagnosed and 10,570 patients will die from this disease in 2010.2 Carcinoma of the esophagus and gastroesophageal junction is overall less common, but the incidence has risen faster than any other malignancy in the last 25 years in the United States and other Western countries.3 For localized disease, adjuvant treatment is multidisciplinary and usually includes a combination of surgery, radiation, and chemotherapy. The INT 00164 and Medical Research Council Adjuvant Gastric Infusional Chemotherapy5 trials established perioperative chemoradiation as the standard of care over surgery alone for resectable gastroesophageal junction cancer. For advanced disease, there is no formal consensus or evidence-based rationale regarding the best chemotherapy regimen. Although treatment for patients with unresectable or metastatic disease remains palliative and survival rates still low, chemotherapy improves survival and quality of life when compared to best supportive care.6 The median time to progression for advanced disease is 4 to 6 months and median overall survival (OS) is 7 to 10 months.
Until recently, targeted agents have not shown a significant survival advantage in advanced gastroesophageal cancers. Phase III clinical trials investigating vascular endothelial growth factor inhibitors and epidermal growth factor receptor inhibitors are ongoing and results with bevacizumab are provocative.7–9 A phase III study investigating trastuzumab was presented by the Trastuzumab for Gastric Cancer (ToGA) investigators first at the 2009 ASCO annual meeting and was then published.10 A total of 3803 patients with advanced gastric or gastroesophageal junction cancer were screened for HER2 by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). Eight hundred ten (21%) were HER2 positive, 594 of these were enrolled in the therapeutic study and randomized to standard therapy with a fluoropyrimidine and cisplatin with or without trastuzumab. The majority of all enrolled patients were male (77%), Asian (51%), had gastric cancer (80%), and of those patients with gastric cancer, the majority had intestinal-type adenocarcinoma. Patients receiving trastuzumab had a statistically longer median OS [13.8 vs. 11.1 mo; hazard ratio, 0.74], progression free survival (6.7 vs. 5.5 mo; hazard ratio, 0.71), and response rate (47% vs. 35%). In an accompanying abstract from the same investigators, Bang et al11 reported the pathologic features of the ToGA population in more detail. Final data showed an overall HER2-positivity rate of 22% evaluated from 3803 patients. The HER2-positivity rate was similar between European (23.6%) and Asian (23.5%) patients. HER2-positivity rates were higher in gastroesophageal junction than gastric cancer (33.2% vs. 20.9%; P <0.001) and in intestinal than diffuse/mixed cancer (32.2% vs. 6.1%/20.4%; P <0.001). The Hofmann et al12 HER2 scoring system for gastric cancer was utilized for analysis of the ToGA samples.
Although the response rate of HER2-positive gastric cancer to trastuzumab-containing regimen is encouraging, it remains unclear how relevant this approach may be to a population in the United States. In addition, as opposed to breast carcinoma, widely accepted and systematic guidelines for HER2 testing in gastric and gastroesophageal junction cancer have not been established. Herein, we (1) analyze the incidence of HER2-positive gastric and gastroesophageal junction cancer in representative cases from our institution using a high-throughput tissue microarray analysis which closely simulates the small tissue biopsies routinely tested for HER2 in clinical practice; (2) evaluate the patterns of HER2 expression in gastric and gastroesophageal junction carcinoma; and (3) propose recommendations and an algorithmic approach for evaluating HER2 status in gastric and gastroesophageal junction carcinoma in routine clinical practice.
MATERIALS AND METHODS
Study Population
The clinicopathologic records and pathology slides of 169 patients with radical resection of primary gastroesophageal junction or gastric adenocarcinoma accessioned at the Department of Pathology, Stanford University Hospital from January 2000 to September 2009 were reviewed. The pathology reports and hospital charts were reviewed and the following information was obtained: type of initial surgical procedure and subsequent therapy, margin status, the extent of tumor invasion at initial presentation, and the presence or absence of lymph node metastases. Demographic and intraoperative data were obtained from hospital and clinic charts under the guidelines of the Stanford University Institutional Review Board. Clinical follow-up data were obtained by reviewing patient records and using the Social Security Death Index.
Tissue Microarray Construction
Four tissue microarrays containing a total of 647 tissue cores, each measuring 1.0 mm, were created using a tissue arrayer (Beecher Instruments, Silver Spring, MD) according to a previously described method.13,14 Representative areas from each invasive adenocarcinoma were selected for the microarray from paraffin blocks based on hematoxylin and eosin (H&E)-stained sections. For each adenocarcinoma, 2 distinctly different areas of invasive carcinoma at least 1.0 cm apart were selected for inclusion in the tissue microarray. In cases with mixed tumor morphology, tissue cores were selected from each morphologically distinct area. For gastroesophageal junction adenocarcinomas associated with Barrett esophagus, representative tissue cores from Barrett mucosa and Barrett esophagus-associated dysplastic lesions were also included. Barrett esophagus-associated dysplastic lesions were classified into low and high grade using previously published criteria.15–18 Tissue cores from 4 invasive breast carcinomas with known HER2 gene amplification identified by FISH were also included as positive controls. Negative control tissue cores included normal gastric oxyntic (n = 10) and antral mucosa (n = 10), esophageal squamous mucosa (n = 10), small bowel mucosa (n = 10), normal tonsil (n = 3), and normal skeletal muscle (n = 3).
HER2 IHC
Four-micron sections of the tissue microarray were incubated with primary antibody to HER2 (clone A0485; Dako Hercep Test, Carpinteria, CA) and processed according to the manufacturer’s instructions. HER2 staining was scored by a single pathologist (R.K.P.) using 2 different scoring schemes. HER2 IHC was scored using the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guideline recommendations for HER2 testing in breast cancer as follows: 0, no staining; 1+, weak, incomplete membrane staining in any proportion of tumor cells; 2+, complete membrane staining that is either nonuniform or weak in intensity but with obvious circumferential distribution in at least 10% of cells or intense, complete membrane staining of 30% or fewer tumor cells; and 3+, uniform, intense membrane staining of > 30% of tumor cells.19 HER2 IHC was also scored using the scoring scheme proposed by Hofmann et al12 and employed in the ToGA cohort of gastroesophageal junction and gastric carcinomas as follows (ToGA score): 0, no staining or membranous reactivity in <10% of tumor cells; 1+, weak, barely perceptible membranous reactivity in > 10% of tumor cells; 2+, complete or basolateral membranous reactivity that is either nonuniform or weak in intensity in at least 10% of cells; and 3+, complete or basolateral membranous reactivity of strong intensity in ≥ 10% of cells. Cytoplasmic reactivity without membranous staining was also separately recorded when present. All cases with either AsCo/CAP or ToGA 2+ or 3+ HER2 IHC scores identified on the tissue microarray were analyzed for HER2 IHC expression on full cross-sections of tumor.
HER2 FISH
Four-micron sections of the formalin-fixed, paraffin-embedded tissue microarray were pretreated by standard protocol using the VP2000 slide pretreatment instrument (Abbott Molecular). In brief, slides were deparaffinized with CitroSolv (Fisher Scientific), digested with a 10% pepsin solution at 37°C (Protease I, VP2000 Protease Buffer; Abbott Molecular), pretreated with a sodium thiocyanate solution at 80°C (VP2000 Pretreatment Solution; Abbott Molecular), refixed in 10% buffered formalin, and dehydrated in an ethanol series. Dried, dehydrated tissue microarray slides were hybridized with the dual-color PathVysion HER2/D17Z1 probe set (Abbot Molecular), sealed with rubber cement, denatured with a VysisHYBrite instrument at 73°C for 6 minutes and incubated for 20 hours at 37°C. Coverslips were gently removed, and slides washed with 2 × saline sodium citrate/ 0.3% NP-40 at 73°C for 2 minutes, counterstained with 4’,6-diamidino-2-phenylindole and analyzed with an Olympus BX51 microscope equipped a × 100 oil immersion objective, appropriate fluorescent filters and CytoVision imaging software (Genetix). HER2 and CEP17 signals were enumerated in 25 cells after scanning each tissue core for areas with increased number of HER2 signals. A total HER2/CEP17 was calculated for each tissue core. For each probe, signal counts >10 were considered innumerable and scored as 10.
Cases were assigned a score based on the ASCO/ CAP guideline recommendations for HER2 testing in breast cancer as follows: negative, HER2/CEP17 ratio < 1.8; positive, HER2/CEP17 ratio >2.2; and equivocal, HER2/CEP17 ratio between 1.8 and 2.2.19 Cases were also assigned a score using the ToGA FISH scoring scheme for HER2 testing in gastric and gastroesophageal junction cancer as follows: negative, HER2/CEP17 ratio < 2.0; and positive, HER2/CEP17 ratio ≥ 2.0.
Statistical Analysis
The Kaplan-Meier method was used to calculate rates of OS. Elapsed time between diagnosis and death or last contact was calculated and log-rank tests, calculated with SAS 9.2 TS2M3, were used to compare the survival times for the groups. Multivariate analysis was not done due to the small patient numbers.
RESULTS
Clinicopathologic Characteristics
Of the 169 patients included in the tissue microarray, there were 99 primary gastric carcinomas and 70 primary gastroesophageal junction carcinomas. In addition included were 16 Barrett esophagus-associated high-grade dysplastic lesions, 3 Barrett esophagus-associated low-grade dysplastic lesions, and 27 Barrett esophagus without dysplasia. The mean age was 65 years (range, 23 to 93 y) with a male predominance (116 of 169; 69%). White race was the most common (108 of 169; 64%), followed by the Asian (33 of 169; 19%). These sex and race patterns were different when examined by site. Male patient predominance was more pronounced for gastroesophageal junction carcinomas compared with gastric carcinomas (86% vs. 57%). A larger proportion of patients with gastroesophageal junction carcinomas were white (86%) compared with patients with gastric carcinoma (49%). Gastric carcinomas were more likely to be encountered in the Asians compared with patients with gastroesophageal junction carcinomas (32% vs. 1%).
Most carcinomas were intestinal-type (52 of 99, 53% of gastric tumors; 58 of 70, 83% of gastroesophageal junction tumors) (Table 1). Overall, most tumors were pathologic stage T3 or T4a/T4b (141 of 169, 83%) compared with T1a/T1b or T2 tumors (28 of 169, 17%). The majority of carcinomas were also poorly differentiated (120 of 169, 71%) with fewer numbers of moderately differentiated (46 of 169, 27%) and well-differentiated (3 of 169, 2%) carcinomas. Most tumors were surgically resected with negative microscopic margins (137 of 169, 81%).
TABLE 1.
Clinicopathologic Features of Study Cases
| Gastric Adenocarcinoma |
Gastroesophageal Junction Adenocarcinoma |
||||||
|---|---|---|---|---|---|---|---|
| Overall | Diffuse | Intestinal | Other | Overall | Intestinal | Other | |
| Pathologic Feature | (n = 99) | (n = 34) | (n = 52) | (n = 13) | (n = 70) | (n = 58) | (n = 12) |
| Median tumor size in cm (range) | 4.6 (1.1–19) | 3.6 (1.5–19) | 4.8 (1.1–10.5) | 8.0 (3.4–14.0) | 4.0 (1.3–13.5) | 4.0 (1.5–13.5) | 4.1 (1.3–11.5) |
| T Stage | |||||||
| T1a/T1b | 11 | 4 | 6 | 1 | 12 | 11 | 1 |
| T2 | 3 | 2 | 1 | 0 | 2 | 2 | 0 |
| T3 | 56 | 14 | 32 | 10 | 54 | 44 | 10 |
| T4a/T4b | 29 | 14 | 13 | 2 | 2 | 1 | 1 |
| Grade | |||||||
| Well differentiated | 3 | 0 | 3 | 0 | 0 | 0 | 0 |
| Moderately differentiated | 21 | 0 | 21 | 0 | 25 | 25 | 0 |
| Poorly differentiated | 75 | 34 | 28 | 13 | 45 | 33 | 12 |
| Median number involved lymph nodes (range) | 4 (0–69) | 7 (0–69) | 5 (0–36) | 4 (0–24) | 1 (0–15) | 1 (0–15) | 4 (0–13) |
| Margin status | |||||||
| Negative | 79 | 25 | 42 | 12 | 58 | 50 | 8 |
| Positive | 20 | 9 | 10 | 1 | 12 | 8 | 4 |
Comparison of HER2 IHC Scoring Schemes
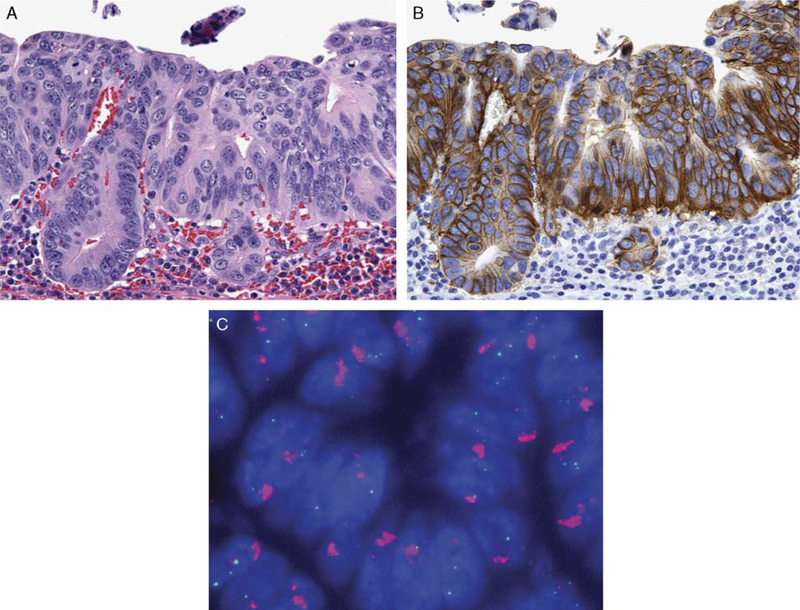
All 169 carcinomas had adequate tumor present in the tissue cores within the tissue microarrays. Using the ASCO/CAP IHC scoring scheme, carcinomas were more often given a score of 1+ (29 cases, 17%) with fewer numbers of carcinomas demonstrating 2+ (5 cases, 3%) or 3+ (5 cases, 3%) immunoreactivity (Table 2). In contrast, using the ToGA scoring scheme, an increased proportion of 3+ positive carcinomas (10 cases, 6%) and 2+ positive carcinomas (12 cases, 7%) was identified (Table 2). The primary reason for the change in score designation between the 2 scoring schemes was the frequent occurrence of intense but incomplete HER2 membrane staining with a basolateral distribution (Fig. 1). Five cases were assigned a 1+ ASCO/CAP score, but were designated 3+ using the ToGA scoring scheme. Similarly, 7 cases were assigned a 1+ ASCO/ CAP score scheme, but were designated 2+ using the ToGA scoring scheme. All 18 cases with a 1+ ToGA score were also assigned 1+ ASCO/CAP score. One tumor had intense cytoplasmic reactivity without membrane staining and was scored as negative (0) using both scoring schemes.
TABLE 2.
Analysis of IHC Staining and HER2 FISH for all Tumor Types and Location
| Immunohistochemical Scoring Scheme | No. Cases (n = 164) | Positive (n = 16) | HER2 FISH (ASCO-CAP) Negative (n = 144) |
Equivocal (n = 5) |
|---|---|---|---|---|
| ASCO/CAP | 5 | 5 | 0 | 0 |
| 3+ | ||||
| 2+ | 5 | 2 | 3 | 0 |
| 1+ | 29 | 6 | 19 | 4 |
| 0 | 126 | 3 | 122* | 1 |
| ToGA | ||||
| 3+ | 10 | 10 | 0 | 0 |
| 2+ | 12 | 3 | 6 | 3 |
| 1+ | 18 | 0 | 18 | 1 |
| 0 | 124 | 3 | 120* | 1 |
Five cases which were negative by HER2 IHC had faint FISH signals which were uninterpretable.
ASCO/CAP indicates American Society of Clinical Oncology/College of American Pathologists; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry.
FIGURE 1.
Intestinal-type adenocarcinoma of the gastroesophageal junction A;hematoxylin and eosin (H&E), × 400) with strong basolateral membranous HER2 immunoreactivity (B, × 400) which would be scored as 1+ using the ASCO/CAP scoring scheme and 3+ using the ToGA scoring scheme. This adenocarcinoma demonstrated HER2 gene amplification by fluorescence in situ hybridization (C, × 100).
Comparison of HER2 FISH Scoring Schemes
Of the 169 carcinomas represented on the tissue microarrays, 164 (97%) had at least 1 tissue core with interpretable signals by FISH analysis, whereas 5 cases had FISH signals too faint to score. Using the ASCO/CAP cut-points, 16 cases (9.5%) were positive by HER2 FISH, whereas 5 cases (3%) demonstrated equivocal FISH results (Table 2). With the ASCO/CAP cut-points, FISH-positive cases had a median HER2/CEP17 ratio of 4.35 (range, 2.24 to 7.14). The different cut-points for a positive HER2 FISH amplification between the ASCO/CAP and ToGA schemes resulted in discrepant results for 3 of 164 (1.8%) cases with all discrepant cases having HER2/CEP17 ratios between 1.8 and 2.2. Two intestinal-type gastric adenocarcinomas demonstrated HER2/CEP17 ratios between 1.8 and 2.0 and were scored as negative with the ToGA FISH scoring scheme and 1 intestinal-type gastric adenocarcinoma with a HER2/CEP17 ratio of 2.05 was interpreted as positive in the ToGA. All 3 cases were designated equivocal in the ASCO/ CAP FISH scoring scheme. In addition, 2 cases of intestinal-type gastroesophageal carcinoma demonstrated FISH amplification characterized by>125 signals per 25 cells and a ratio of HER2/CEP17 ratio of approximately 1.0.
Concordance of HER2 IHC and HER2 FISH: Significance of Heterogeneous HER2 Immunoreactivity
The ToGA IHC scoring scheme demonstrated greater concordance with the HER2 FISH analysis compared with the ASCO/CAP IHC scoring scheme. With the ASCO/CAP IHC scoring scheme, HER2 FISH-positive cases received the following scores: 3+ (5 cases), 2+ (2 cases), 1+ (6 cases), and 0 (3 cases) (Table 2). In contrast, with the ToGA IHC scoring scheme, HER2 FISH-positive cases received the following scores: 3+ (10 cases), 2+ (3 cases), 1+ (no cases), and 0 (3 cases). Thus, the ToGA IHC scoring scheme correctly labeled 5 additional HER2 FISH-positive carcinomas as having 3+ and 1 additional case as having 2+ HER2 expression. No HER2 FISH-positive cases received a 1+ ToGA score as opposed to 6 HER2 FISH-positive cases with a 1+ ASCO/CAP score. Notably, 1 gastric diffuse-type adenocarcinoma exhibited strong cytoplasmic reactivity without membranous staining and demonstrated positive HER2 FISH amplification. In addition, 3 carcinomas had no HER2 protein overexpression by IHC but had positive HER2 gene amplification by FISH with HER2/CEP17 ratios of 2.59, 2.79, and 3.73. In contrast, no cases were negative for HER2 gene amplification by FISH but demonstrated HER2 protein overexpression by IHC.
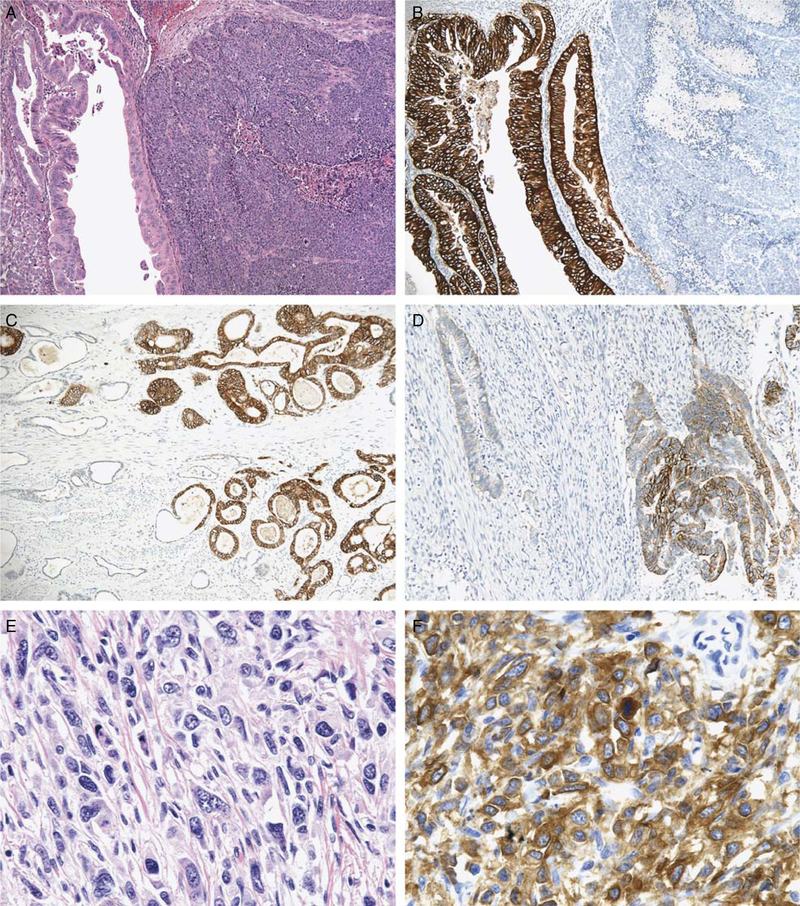
Heterogeneous HER2 immunoreactivity was the most frequent cause of discrepant results between FISH and HER2 IHC (Table 3). In total, 8 of 169 (4.7%) carcinomas demonstrated heterogeneous HER2 immunoreactivity with some areas of tumor displaying strong HER2 staining adjacent to areas lacking HER2 reactivity. Heterogenous HER2 staining was observed in following instances1: 2 gastroesophageal junction adenocarcinomas displayed separate and morphologically distinct tumor growth with areas demonstrating solid and/or complex glandular growth pattern admixed with areas demonstrating a simple tubular growth pattern. HER2 IHC and HER2 FISH analysis demonstrated differences in HER2 expression and gene amplification in the morphologically distinct areas of tumor, confirmed on analysis of full paraffin cross-sections of tumor (Fig. 2).2 Three gastric intestinal-type adenocarcinomas were positive for HER2 gene amplification by FISH in areas of the tumor which were negative for HER2 overexpression by IHC (Table 3). Full-section HER2 IHC analysis in these tumors demonstrated heterogeneous basolateral membranous HER2 staining in 10% to 30% of the tumor cells with the HER2-positive tumor cells localized to 1 area of the tumor; such cases would be assigned a 3+ ToGA score (Table 3).3 Two gastric intestinal-type adenocarcinomas demonstrated equivocal FISH results with HER2/CEP17 ratios between 1.8 and 2.2, and 2+ HER2 expression in the tissue microarray analysis (Table 3). On full-section HER2 IHC analysis, both cases demonstrated basolateral membranous reactivity in > 30% of tumor cells; however, the reactivity was heterogeneous with some areas of tumor negative for HER2 overexpression by IHC and other areas showing an intermixture of HER2-positive and HER2-negative tumor cells (Fig. 2).4 One diffuse-type gastric adenocarcinoma displayed only cytoplasmic HER2 IHC staining and was positive for HER2 gene amplification by FISH (Fig. 3). On full-section HER2 IHC analysis, this gastric carcinoma demonstrated heterogeneous mixed cytoplasmic and circumferential membranous staining in >30% (3+) of tumor cells.
TABLE 3.
Discrepant HER2 FISH and HER2 IIHC Cases
| TMA HER2 FISH | TMA HER2 IHC* | No. Cases | Full-Section HER2 IHC |
|---|---|---|---|
| Positive | Negative | 3 | 3+ |
| Equivocal | 2+ | 2 | 3+ |
| Equivocal | Negative | 1 | 3+ |
IHC scores are based on the ToGA HER2 scoring scheme.
FISH indicates fluorescence in situ hybridization; IHC, immunohistochemistry; TMA, tissue microarray.
FIGURE 2.
Gastroesophageal intestinal-type adenocarcinoma demonstrating heterogeneous morphology with intermixed glandular (left half of image) and solid (right half of image) growth patterns [A;hematoxylin and eosin (H&E, × 100)]. The glandular component displayed 3+ HER2 protein overexpression, whereas the solid component lacks HER2 protein expression (B, × 100). Gastric intestinal-type adenocarcinoma demonstrating heterogeneous HER2 protein overexpression with areas exhibiting 3+ expression (right half of image) adjacent to tumor glands lacking HER2 expression (left half of image) (C, × 100). This gastric intestinal-type adenocarcinoma with HER2 gene amplification (not shown) demonstrated strong HER2 protein expression in only 10% to 30% of tumor cells (D, × 200). Gastric diffuse-type adenocarcinoma (E, H&E, × 400) demonstrating cytoplasmic HER2 protein expression in the tissue microarray analysis (F, × 400) and HER2 gene amplification by fluorescence in situ hybridization (not shown).
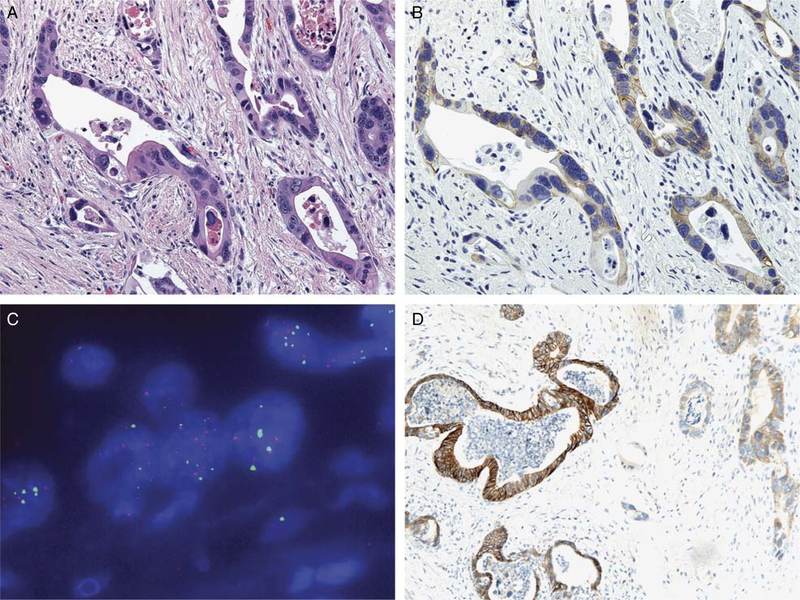
FIGURE 3.
Tissue microarray analysis of a gastroesophageal junction intestinal-type adenocarcinoma resected after neoadjuvant chemotherapy and radiation therapy [A;hematoxylin and eosin (H&E, × 400)], which demonstrated nonuniform and weak membranous HER2 staining (2+) (B, × 400). This adenocarcinoma displayed very high copy numbers of both the chromosome 17 centromere (CEP17) control signal (green) and HER2 signal (red) by fluorescence in situ hybridization (C, × 100). Full-section immunohistochemistry demonstrated 3+ heterogeneous HER2 immunohistochemical expression in this adenocarcinoma (D, × 100).
Very high copy numbers of the chromosome 17 centromeric control signal (CEP17) complicated FISH analysis in 2 cases of intestinal-type gastroesophageal junction carcinoma. Both cases demonstrated equivocal FISH amplification characterized by >125 signals per 25 cells and a ratio of HER2/CEP17 ratio of approximately 1.0. These 2 cases demonstrated very high copy numbers of both the CEP17 control signal and HER2 signal (Fig. 3). For 1 of these cases, 2+ HER2 staining was identified on the tissue microarray analysis; however, on full-section IHC, 3+ heterogeneous IHC staining was observed (Fig. 3, Table 3). The other case was entirely negative for HER2 by IHC. Of note, both cases with high copy numbers of both HER2 and CEP17 had received neoadjuvant chemotherapy and radiation.
HER2 Status and Tumor Type
For gastric carcinomas, HER2 overexpression was identified in 12 of 99 (12%) of cases (Table 4). Of these, HER2 FISH was more often identified in intestinal-type adenocarcinomas (10 of 52, 19%) compared with diffuse (2 of 34, 6%), mixed (0 of 7, 0%), or undifferentiated (0 of 6, 0%) carcinomas. For gastroesophageal junction carcinomas, HER2 overexpression was identified in 7 of 70 (10%) cases of which all were intestinal type (7 of 58, 12%) (Table 5). Carcinomas with diffuse (1 case), mixed intestinal and diffuse (9 cases), or neuroendocrine (2 cases) were negative for HER2 by both FISH and IHC.
TABLE 4.
HER2 FISH and IIHC by Gastric Carcinoma Histologic Subtypes
| ToGA HER2 IHC Score |
||||||
|---|---|---|---|---|---|---|
| Histologic Subtype | No. HER2 Positive (%)* | HER2 FISH | 3+ IHC | 2+ IHC | 1+ IHC | 0 IHC |
| All tumors (n = 99) | 12 (12) | Positive | 5 | 5 | 0 | 0 |
| Negative | 0 | 2 | 8 | 76 | ||
| Equivocal | 2 | 0 | 1 | 0 | ||
| Diffuse (n = 34) | 2(6) | Positive | 2 | 0 | 0 | 0 |
| Negative | 0 | 0 | 2 | 30 | ||
| Intestinal (n = 52) | 10 (19) | Positive | 3 | 5 | 0 | 0 |
| Negative | 0 | 2 | 5 | 34 | ||
| Equivocal | 2 | 0 | 1 | 0 | ||
| Mixed or undifferentiated (n = 13) | 0(0) | Positive | 0 | 0 | 0 | 0 |
| Negative | 0 | 0 | 1 | 12 | ||
Defined as cases demonstrating HER2 FISH amplification and/or 3+ HER2 IHC staining.
FISH indicates fluorescence in situ hybridization; IHC, immunohistochemistry.
TABLE 5.
HER2 FISH and IHC by Gastroesophageal Junction Carcinoma Histologic Subtypes
| ToGA HER2 IHC Score |
||||||
|---|---|---|---|---|---|---|
| Histologic Subtype | No. HER2 Positive (%)* | HER2 FISH | 3+ IHC | 2+ IHC | 1+ IHC | 0 IHC |
| All tumors (n = 70) | 7(10) | Positive | 6 | 0 | 0 | 0 |
| Negative | 0 | 5 | 9 | 42† | ||
| Equivocal | 1 | 1 | 0 | 1 | ||
| Intestinal (n = 58) | 7(12) | Positive | 6 | 0 | 0 | 0 |
| Negative | 0 | 5 | 9 | 31† | ||
| Equivocal | 1 | 1 | 0 | 1 | ||
| Mixed or Diffuse (n = 10) | 0 (0) | Positive | 0 | 0 | 0 | 0 |
| Negative | 0 | 0 | 0 | 9† | ||
| Neuroendocrine (n = 2) | 0 (0) | Positive | 0 | 0 | 0 | 0 |
| Negative | 0 | 0 | 0 | 2 | ||
Defined as cases demonstrating HER2 FISH amplification and/or 3+ HER2 IHC staining.
Four intestinal and 1 diffuse adenocarcinoma case exhibited no HER2 staining by IHC, but had faint FISH signals which were uninterpretable.
FISH indicates fluorescence in situ hybridization; IHC, immunohistochemistry.
Twenty-eight (39%) gastroesophageal junction carcinomas were associated with Barrett esophagus or Barrett-related dysplastic lesions. Three Barrett-associated intestinal-type adenocarcinomas demonstrated HER2 FISH amplification and 3+ HER2 staining by IHC. Overall, high-grade dysplastic lesions (16 cases), low-grade dysplastic lesion (3 cases), and Barrett mucosa without dysplasia (27 cases) were negative for HER2 FISH amplification. One high-grade dysplastic lesion adjacent to a HER2 positive Barrett-associated intestinal-type adenocarcinoma (HER2/CEP17 ratio, 7.14) demonstrated 2+ HER2 immunoreactivity, but was negative for HER2 amplification by FISH (HER2/CEP17 ratio, 1.08).
HER2 Status and Clinical Outcome
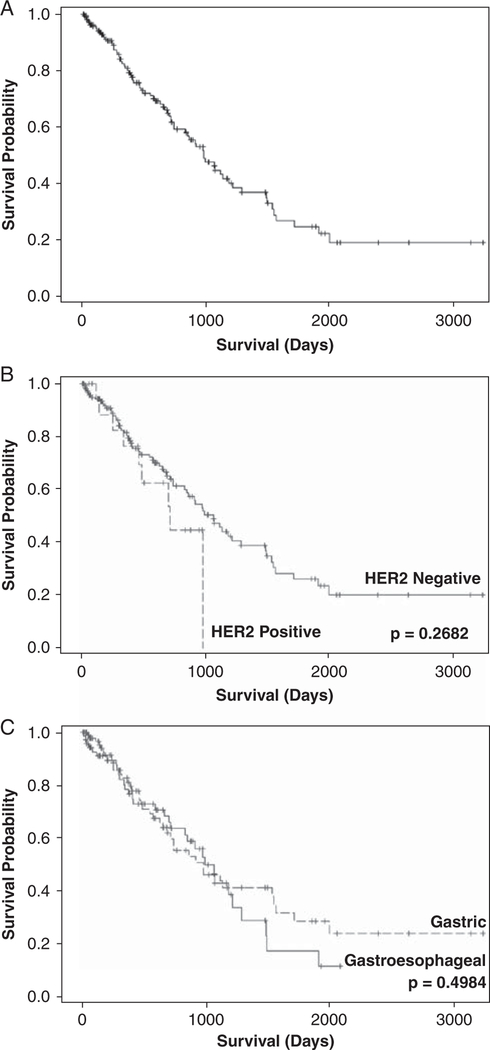
Kaplan-Meier curves for OS are shown in Figure 4. Median OS for the study population is 34 months and 5-year survival is 25%. There were no differences in survival by site or HER2 status. The total number of cases with HER2 gene overexpression was too small to do allow further subset analysis by specific patient or tumor characteristics.
FIGURE 4.
Kaplan-Meier survival curves: (A) all patients, (B) survival by HER2 status (positive vs. negative), (C) survival by site (gastric vs. gastroesophageal junction). Median overall survival for the study population is 34 months and 5-year survival is 25%. There were no differences in survival by site or HER2 status.
DISCUSSION
The prognostic and predictive power of HER2 gene overexpression in breast cancer has been well established with published guidelines for evaluating HER2 status. Recent evidence suggests that trastuzumab, a monoclonal antibody that targets HER2, in combination with chemotherapy is a therapeutic option in patients with HER2-positive advanced gastric or gastroesophageal junction cancers.10 However, relatively few studies have rigorously analyzed the currently available methods of testing and evaluating HER2 gene overexpression in gastric and gastroesophageal junction carcinomas.12 In addition, as the therapy regimen including trastuzumab for gastric and gastroesophageal junction cancer is typically reserved for those patients with inoperable locally advanced or metastatic disease, HER2 assessment is typically performed on small endoscopic biopsies. Our tissue microarray analysis closely simulates the small tissue biopsies of upper gastrointestinal tract carcinomas routinely tested for HER2. The frequent occurrence of heterogeneous HER2 expression, up to 4.7% in our series, has important implications when assessing for HER2 overexpression in small tissue biopsies. The potential for false-negative results is increased if HER2 assessment is limited to small biopsy samples. In our tissue microarray analysis, HER2 FISH was the more robust assay as it more often identified HER2-positive adenocarcinomas due to frequent occurrence of heterogeneous HER2 protein IHC expression in gastric and gastroesophageal junction adenocarcinomas. Importantly, tumors with heterogeneous HER2 IHC staining often exhibited HER2 gene amplification in the areas lacking HER2 IHC overexpression. In addition, heterogeneous HER2 expression was observed in adenocarcinomas lacking morphologic heterogeneity, that is, the neoplastic glands demonstrating HER2 overexpression appeared histologically identical to those lacking HER2 overexpression and were often intermixed with each other. Interestingly, in the ToGA cohort, patients who had tumors demonstrating HER2 gene amplification by FISH but lacking HER2 protein overexpression by IHC did not appear to benefit from the addition of trastuzumab to their chemotherapy regimen.10 This suggests that the level of HER2 expression, irrespective of the presence of gene amplification, predicts response to trastuzumab therapy. However, it is not clearly stated in the ToGA study what proportion of the HER2 FISH-positive/ IHC-negative cases was from surgically resected tumors versus biopsy samples. Results of our tissue microarray analysis suggest that small tumor samples which demonstrate HER2 FISH-positivity but lack HER2 protein expression when more thoroughly examined with whole sections of the tumor typically demonstrated areas with unequivocal HER2 protein overexpression, albeit focally and in reduced proportion (10% to 30% of tumor cells). Whether such patients would benefit from the addition of trastuzumab therapy to their chemotherapy regimen is not entirely clear.
Our results confirm that there are significant differences in the pattern of HER2 expression in gastric and gastroesophageal junction adenocarcinomas compared with breast carcinoma with important clinical implications for the assessment of HER2 status in gastric and gastroesophageal junction carcinoma. Importantly, our findings emphasize that the ASCO/CAP HER2 IHC scoring scheme is not applicable to gastric and gastroesophageal junction adenocarcinomas. First, gastric and gastroesophageal junction adenocarcinomas frequently lack complete membranous HER2 immunoreactivity and instead often exhibit a distinct basolateral membranous staining pattern, as observed by Hofmann et al.12 Our results confirm that the basolateral staining pattern for HER2 is commonly identified in gastric and gastroesophageal junction adenocarcinomas and when observed should be scored as 3+ positive as it is always associated with HER2 gene amplification. Second, the appropriate threshold for extent of tumor staining to be scored as 3+ positive staining is controversial. The ASCO/CAP HER2 scoring scheme for breast carcinoma set a threshold of >30% of tumor showing strong membrane staining for a positive result.20 One reason stated for increasing the threshold to 30% included the goal of decreasing the incidence of false-positive 3+ cases with cases demonstrating strong membranous staining in <30% of the tumor scored as equivocal (2+). However, we observed HER2 FISH-positive gastric carcinomas that exhibited strong, basolateral staining in 10% to 30% of tumor cells. Requiring a 30% cut-point for a 3+ HER2 result is likely too restrictive and may lead to false-negative results in gastric and gastroesophageal junction adenocarcinomas. Rather, a 10% threshold for 3+ positive HER2 result may be more appropriate for surgically-resected gastric and gastroesophageal junction adenocarcinomas. Similarly, the appropriate thresholds for FISH analysis are controversial. The ASCO/CAP guideline recommendations include an FISH equivocal category for cases with HER2/CEP17 ratios between 1.8 and 2.2. To our knowledge, all literature reports, including the ToGA cohort, did not include the equivocal FISH category and set a cut-point of 2.0 for positive HER2 amplification by FISH. In our study, 2 gastric carcinomas demonstrated HER2/ CEP17 ratios between 1.8 and 2.2 and 3+ but heterogeneous HER2 IHC staining. This suggests that the FISH equivocal category including HER2/CEP17 ratios between 1.8 and 2.2 is useful in gastric and gastroesophageal junction adenocarcinomas as it identifies those patients who are potentially eligible for trastuzumab therapy but require more analysis, either by HER2 IHC or repeat HER2 FISH testing.
In our analysis, 19 of 169 (11.2%) of gastric and gastroesophageal junction carcinomas were HER2 positive by either IHC or FISH, one of the largest US studies to date. The reported frequency of HER2 overexpression in the literature varies widely with a range of HER2-positivity rate by IHC of 6.8% to 26.8% (median, 13.6%) (Table 6). Similarly, the reported frequency of HER2 gene amplification by FISH in the literature varies widely from 7.1% to 27.1% (median, 15.2%) (Table 6). Comparing the results of our analysis to other literature reports is complicated by the various scoring schemes with different cut-points employed in previously published studies, the various tumor types included in earlier analyses, and the inclusion of both 2+ and 3+ as indicative as being positive for HER2 overexpression in many studies. In our analysis, HER2-positivity rates were higher in gastric adenocarcinomas than gastroesophageal junction adenocarcinomas (12 of 99, 12% vs. 7 of 70, 10%; P = 0.80). In contrast in the ToGA population, HER2-positivity rates were higher in gastroesophageal junction cancer than gastric cancer (33.2% vs. 20.9%; P <0.001). In our analysis, HER2-positivity rates were higher in intestinal-type adenocarcinoma than diffuse-type adenocarcinoma (17 of 157, 10.8% vs. 2 of 44, 4.5%; P = 0.25), which was also observed in the ToGA population.
TABLE 6.
Selected Literature Reports of HER2-Positivity Rate in Gastric, Gastroesophageal junction, and Esophageal Carcinoma by IHC and FISH
| Reference | No. Cases | Tumor Type(s) | HER2 IHC Positive (%) | HER2 FISH Positive (%) |
|---|---|---|---|---|
| Barros-Silva et al21 | 463 | Gastric | 9.3* | 8.2 |
| Ooi22 | 396 | Gastric | 10.1* | 10.1 |
| Yano23 | 200 | Gastric | 17.0 | 27.1 |
| Hofmann et al12 | 178 | Gastric, GEJ, and esophageal | 10.7 | 17.4 |
| Takehana24 | 352 | Gastric | 6.8 | 7.1 |
| Marx et al25 | 166 | Gastric | 14.5 | 16 |
| Reichelt26 | 110 | Esophageal | 12.7 | 15 |
| Risio27 | 72 | Gastric | 17.0 | 15.3 |
| Grabsch et al28 | 924 | Gastric | 6.0 | Not done |
| Lee29 | 841 | Gastric | 17.0* | Not done |
| Ruschoff et al30 | 547 | Gastric | 22.8* | Not done |
| Allgayer31 | 189 | Gastric | 11.6* | Not done |
| Pinto-de-Sousa32 | 157 | Gastric | 15.3* | Not done |
| Koeppen33 | 62 | Gastric | 8.0 | Not done |
| Ougolkov34 | 56 | Gastric | 26.8 | Not done |
Positive HER2 immunohistochemistry included both 2+ and 3+ cases in this study.
FISH indicates fluorescence in situ hybridization; GEJ, gastroesophageal junction; IHC, immunohistochemistry.
Our study has several limitations. First, the reason for the relatively low rate of HER2 protein overexpression and gene amplification identified in our analysis is not entirely clear but may be related to our tissue microarray analysis. Although our tissue microarray approach closely simulates the small tissue fragments obtained by endoscopic biopsies of carcinomas in routine clinical practice and allowed for high-throughput testing of a large number of carcinoma cases, only a limited area of each tumor was present in the tissue microarray for HER2 IHC and FISH analyses. Given the possibility of heterogenous HER2 overexpression and gene amplification within a tumor, we cannot entirely exclude the possibility of false-negative HER2 results given the limited volume of each tumor included in the tissue microarrays. Second, geographic and ethnic heterogeneity of tumor-associated aberrations exists in solid tumors and may help to explain the difference in HER2 overexpression and amplification in various studies.35–38 However, the HER2-positivity rate in the ToGA population was similar between European (23.6%) and Asia (23.5%) patients. In addition, the differences in HER2 positivity in our study (11.2%) compared with the ToGA study may be related to underlying differences in race or other population characteristic.
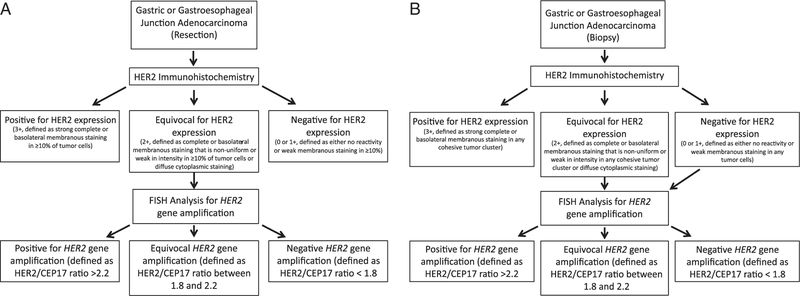
On the basis of the reported experience in the literature and the results of our analysis, we propose the following algorithmic approach for the assessment of HER2 protein overexpression and gene amplification in gastric and gastroesophageal junction adenocarcinoma, as depicted in Figure 5. HER2 IHC in large tissue sections obtained from surgical resections is able to correctly identify adenocarcinomas with HER2 overexpression even if there is a heterogeneous HER2 expression, and we propose routine assessment of HER2 status by IHC in surgical resection specimens. One benefit of HER2 assessment by IHC over HER2 FISH gene amplification analysis is the apparent correlation between protein expression and response to trastuzumab therapy identified in the ToGA population. Importantly, tumors displaying morphologic heterogeneity with distinct tumor growth patterns should have IHC analysis performed on all morphologically distinct areas of tumor, which may entail IHC analysis on sections of tumor from >1 paraffin block. Cases with equivocal HER2 IHC results should be evaluated by HER2 FISH analysis. We propose an equivocal FISH category be employed when evaluating for HER2 gene amplification in gastric and gastroesophageal junction cancer, as our analysis identified adenocarcinomas with equivocal HER2 gene amplification, but unequivocal HER2 protein overexpression suggesting that these patients may benefit from trastuzumab. In small biopsy samples, to reduce the potential of false-negative HER2 results by IHC, all gastric and gastroesophageal junction adenocarcinomas with initial negative or equivocal HER2 IHC results in a small tissue biopsy sample should be evaluated for HER2 gene amplification by FISH (Fig. 5). Our tissue microarray analysis indicates that the HER2 IHC analysis of small samples of large tumors will result in an underestimation of the level of HER2 protein expression, as analysis of whole-tumor sections often display areas with unequivocal HER2 tumor expression. Such adenocarcinomas typically display HER2 gene amplification by FISH in areas of tumor lacking HER2 protein expression. In addition, adenocarcinomas demonstrating diffuse strong cytoplasmic HER2 immunoreactivity should also be evaluated by HER2 FISH, as 1 gastric diffuse-type adenocarcinoma in our analysis demonstrated this pattern of immunoreactivity and concurrent HER2 gene amplification. We agree with Hofmann et al12 that in biopsy samples, tumors with strong, membranous staining should be scored as positive irrespective of the extent of immunopositive cells, as tumors may display only focal areas with strong membranous staining in a limited biopsy sample. However, recently Ruschoff et al30 reasonably proposed a minimum of at least 5 cohesive tumor cells demonstrating strong complete or basolateral membranous staining before classifying a tumor as HER2 positive on small tissue biopsy samples.
FIGURE 5.
Proposed algorithm for HER2 testing in surgical resection specimens (A) and biopsy specimens (B).
Lastly, our survival analysis showed a median OS of approximately 32 months and 5-year survival of approximately 25%. Our data also confirm that HER2 overexpression is of no prognostic significance for gastric and gastroesophageal junction cancers, as no significant survival difference was identified between HER2-positive and HER2-negative patients. Previous studies have shown conflicting results when evaluating HER2 as a predictive biomarker. Some have shown that HER2 overexpression is associated with worse OS39–41 whereas others, like our own study, have shown that HER2 overexpression is of no prognostic significance.21,25,28,42 These conflicting data may be due to low prevalence of HER2 in gastric and gastroesophageal junction carcinomas and differences in HER2 assessment. Perhaps with more consistent guidelines on HER2 assessment in this disease, future studies will provide more clarity around this issue.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893–1907. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- 3.Parfitt JR, Miladinovic Z, Driman DK. Increasing incidence of adenocarcinoma of the gastroesophageal junction and distal stomach in Canada: an epidemiological study from 1964–2002. Can J Gastroenterol 2006;20:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345: 725–730. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 6.Wagner A Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2005;3:CD004064. [DOI] [PubMed] [Google Scholar]
- 7.Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 2006;24:5201–5206. [DOI] [PubMed] [Google Scholar]
- 8.Kang Y, Ohtsu A, Van Cutsem E, et al. AVAGAST: a randomized, double-blind, placebo-controlled phase III study of first-line capecitabine and cisplatin plus bevacizumab or placebo in patients with advanced gastric cancer. J Clin Oncol 2010;28:LBA4007. [Google Scholar]
- 9.El-Rayes BF, Zalupski M, Bekai-Saab T, et al. A phase II study of bevacizumab, oxaliplatin, and docetaxel in locally advanced and metastatic gastric and gastroesophageal junction cancers. Ann Oncol 2010; 21:1999–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–697. [DOI] [PubMed] [Google Scholar]
- 11.Bang Y, Chung H, Xu J, et al. Pathological features of advanced gastric cancer (GC): relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening programme of the ToGA trial. J Clin Oncol 2009;27:15s (suppl; abstr 4556). [Google Scholar]
- 12.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797–805. [DOI] [PubMed] [Google Scholar]
- 13.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998;4:844–847. [DOI] [PubMed] [Google Scholar]
- 14.Nocito A, Kononen J, Kallioniemi OP, et al. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer 2001;94:1–5. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol 2001;32:368–378. [DOI] [PubMed] [Google Scholar]
- 16.Reid BJ, Haggitt RC, Rubin CE, et al. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol 1988;19: 166–178. [DOI] [PubMed] [Google Scholar]
- 17.Downs-Kelly E, Mendelin JE, Bennett AE, et al. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett’s esophagus biopsies. Am J Gastroenterol 2008;103:2333–2340. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery E, Goldblum JR, Greenson JK, et al. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol 2001;32:379–388. [DOI] [PubMed] [Google Scholar]
- 19.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118–145. [DOI] [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007;131:18–43. [DOI] [PubMed] [Google Scholar]
- 21.Barros-Silva JD, Leitao D, Afonso L, et al. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer 2009;100: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ooi A, Kobayashi M, Mai M, et al. Amplification of c-erbB-2 in gastric cancer: detection in formalin-fixed, paraffin-embedded tissue by fluorescence in situ hybridization. Lab Invest 1998;78: 345–351. [PubMed] [Google Scholar]
- 23.Yano T, Doi T, Ohtsu A, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep 2006;15:65–71. [PubMed] [Google Scholar]
- 24.Takehana T, Kunitomo K, Kono K, et al. Status of c-erbB-2 in gastric adenocarcinoma: a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immunosorbent assay. Int J Cancer 2002;98:833–837. [DOI] [PubMed] [Google Scholar]
- 25.Marx AH, Tharun L, Muth J, et al. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol 2009;40:769–777. [DOI] [PubMed] [Google Scholar]
- 26.Reichelt U, Duesedau P, Tsourlakis M, et al. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol 2007;20:120–129. [DOI] [PubMed] [Google Scholar]
- 27.Risio M, De Rosa G, Sarotto I, et al. HER2 testing in gastric cancer: molecular morphology and storage time-related changes in archival samples. Int J Oncol 2003;23:1381–1387. [PubMed] [Google Scholar]
- 28.Grabsch H, Sivakumar S, Gray S, et al. HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value-conclusions from 924 cases of two independent series. Cell Oncol 2010;32:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KE, Lee HJ, Kim YH, et al. Prognostic significance of p53, nm23, PCNA and c-erbB-2 in gastric cancer. Jpn J Clin Oncol 2003;33:173–179. [DOI] [PubMed] [Google Scholar]
- 30.Ruschoff J, Dietel M, Baretton G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch 2010;457:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allgayer H, Babic R, Gruetzner KU, et al. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol 2000;18:2201–2209. [DOI] [PubMed] [Google Scholar]
- 32.Pinto-de-Sousa J, David L, Almeida R, et al. c-erb B-2 expression is associated with tumor location and venous invasion and influences survival of patients with gastric carcinoma. Int J Surg Pathol 2002; 10:247–256. [DOI] [PubMed] [Google Scholar]
- 33.Koeppen HK, Wright BD, Burt AD, et al. Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology 2001;38:96–104. [DOI] [PubMed] [Google Scholar]
- 34.Ougolkov A, Yamashita K, Bilim V, et al. Abnormal expression of E-cadherin, beta-catenin, and c-erbB-2 in advanced gastric cancer: its association with liver metastasis. Int J Colorectal Dis 2003;18: 160–166. [DOI] [PubMed] [Google Scholar]
- 35.Johansson B, Mertens F, Mitelman F. Geographic heterogeneity of neoplasia-associated chromosome aberrations. Genes Chromosomes Cancer 1991;3:1–7. [DOI] [PubMed] [Google Scholar]
- 36.Symvoulakis EK, Zaravinos A, Panutsopulos D, et al. Highly conserved sequence of exon 15 BRAF gene and KRAS codon 12 mutation among Greek patients with colorectal cancer. Int J Biol Markers 2007;22:12–18. [DOI] [PubMed] [Google Scholar]
- 37.House MG, Wistuba II, Argani P, et al. Progression of gene hypermethylation in gallstone disease leading to gallbladder cancer. Ann Surg Oncol 2003;10:882–889. [DOI] [PubMed] [Google Scholar]
- 38.Nagahashi M, Ajioka Y, Lang I, et al. Genetic changes of p53, Kras, and microsatellite instability in gallbladder carcinoma in high-incidence areas of Japan and Hungary. World J Gastroenterol 2008; 14:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia I, Vizoso F, Martin A, et al. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol 2003;10:234–241. [DOI] [PubMed] [Google Scholar]
- 40.Yan B, Yau EX, Bte Omar SS, et al. A study of HER2 gene amplification and protein expression in gastric cancer. J Clin Pathol 2010;63:839–842. [DOI] [PubMed] [Google Scholar]
- 41.Zhang XL, Yang YS, Xu DP, et al. Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J Surg 2009;33:2112–2118. [DOI] [PubMed] [Google Scholar]
- 42.Yu GZ, Chen Y, Wang JJ. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol 2009;135:1331–1339. [DOI] [PubMed] [Google Scholar]