Abstract
D-serine, an endogenous coagonist of N-methyl-d-aspartate receptors (NMDARs) at the glycine binding site, is synthesized by serine racemase (SR) through conversion of L-serine. Dysregulation of SR/D-serine and Disrupted-In-Schizophrenia-1 (DISC1) contributes to the pathogenesis of schizophrenia at converging pathways, as perturbation of SR-DISC1 binding in astrocytes elicits schizophrenia-like behaviors in mice. However, an association of neuronal SR with DISC1 remains elusive. Here we report that SR associates with DISC1 and its agglomerates in cortical neurons, which can be modulated by NMDAR activity. Endogenous SR colocalizes with DISC1 large agglomerates in the soma and with smaller puncta in the nucleus and dendrites of cortical neurons. Co-immunoprecipitation assays demonstrate SR interaction with DISC1 in cortical neuronal lysates, suggesting the physiological presence of functional SR-DISC1 complexes in neurons. Moreover, exogenous D-serine application significantly increases the interaction of SR with DISC1, the number of DISC1-SR large agglomerates and the levels of DISC1 agglomerated form along with SR in the triton-insoluble pellet fraction, whereas application of glycine with a glycine transporter inhibitor fails to increase their interactions, abundance of DISC1-SR large agglomerates and levels of DISC1 agglomerated form. This increase by D-serine application is blocked by 7-chlorokynurenic acid, a specific antagonist at the glycine site of NMDARs, suggesting mediation through NMDARs. Our findings thus demonstrate neuronal SR association with DISC1 and its agglomerates, which can be modulated by D-serine, thereby validating a novel neuronal SR-DISC1 complex responsive to NMDAR activation and providing a molecular mechanism by which pathways implicated in schizophrenia converge.
Keywords: D-serine, serine racemase, Disrupted-In-Schizophrenia-1, cortical neurons, NMDA receptor, agglomerates
Introduction
NMDARs are glutamate-gated ionotropic channels that are crucial for many physiological processes including neurotransmission, synaptic plasticity, and learning and memory. In addition to glutamate, NMDAR activation requires the binding of a coagonist, D-serine or glycine, to a specific site of the NR1 subunit of NMDARs [1–8]. In some paradigms, D-serine preferentially gates synaptic NMDARs and glycine preferentially gates extrasynaptic NMDARs [5, 9]. D-serine is synthesized by serine racemase (SR) through conversion of L-serine and degraded by D-amino acid oxidase (DAAO) [10–16]. D-serine is now recognized as an important physiological modulator in many NMDAR-dependent processes and functions, including brain development, synaptic transmission and plasticity, learning and memory, and social interactions [3–8, 17–23].
Abnormally reduced levels of D-serine have been found in the cerebrospinal fluid and postmortem brains of schizophrenia patients [24–26]. D-serine/NMDAR hypofunction have been implicated in the pathogenesis of schizophrenia [27–37]. Targeted deletion of SR in mice reduces D-serine production and glutamatergic transmission in the forebrain and leads to schizophrenia-like behavior [30]. Disruption of D-serine/SR during development has also been associated with schizophrenia. Neonatal disruption of SR and D-serine synthesis in mice leads to schizophrenia-like behavioral abnormalities in adulthood [34]. These findings strongly suggest that SR and D-serine are crucial for maintaining normal cortical functions in healthy individuals and implicate SR/D-serine deficiency in pathogenesis of schizophrenia.
SR is found preferentially in excitatory and inhibitory neurons, and D-serine is predominantly produced and released by neurons in rodent and human brains [8, 20, 38–44]. SR is a highly regulated enzyme that binds several NMDAR- and AMPA receptor (AMPAR)-interacting proteins including DISC1, GRIP1, PICK1, stargazin and PSD-95 [20, 35, 45–49]. Our previous studies have demonstrated that SR associates with PSD-95 in postsynaptic terminals and that D-serine stabilizes glutamatergic synapses during development in cortical neurons [20, 48]. In the current study, we investigate the association of SR with DISC1 in neurons and how D-serine modulates their association.
Materials and Methods
Materials
Timed-pregnant C57BL/6 mice were purchased from Charles River Laboratories. Biochemicals included MK-801, D-serine, glycine and lithium (Sigma), 7-chlorokynurenic acid (7-CK) (Tocris Bioscience). Antibodies included α-PSD-95 (BD Transduction Laboratories, 1:1000 for WB; NeuroMab, 75–028, 1:150 for ICC)[20, 29], α-DISC1 (Millipore, ABN425, 1:250 for ICC; 1:1000 for WB), α-serine racemase (Abcam, ab45434, 1:250 for ICC, 1:1000 for WB, 2.5μg for IP) [20], α-actin (Abcam, ab3280, 1:5000 for WB)[20, 28, 29, 50]
Neuronal cultures and Drug treatment
Primary cortical cultures from E17–19 C57BL/6 were prepared as described [50] in accordance with the protocol approved by The Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee (IACUC). Briefly, the cortex was dissected, gently minced, trypsinized (0.027%, 37 °C; 7% CO2 for 20 min), and then washed with 1× HBSS. Neurons were seeded to a density of 3 × 105 viable cells in 35-mm culture dish with five 12-mm glass coverslips (low-density culture, 3×104/cm2) or a density of 1.6 × 106 viable cells in 60-mm culture dishes (high-density culture, 8×104/cm2). The culture dishes were coated with poly-D-Lysine (100 μg/ml) prior to seeding neurons. Neurons were maintained at 37°C with 5% CO2 in Neurobasal medium with B27 supplement. Cortical cultures contain 5–10% of glia cells and 90–95% cortical neurons. At 15–17 (high-density cultures, 8×104/cm2) or 21–23 (low-density cultures, 3×104/cm2) days in vitro (DIV), cultures were subject to drug treatment, western blotting analysis, co-immunoprecipitation and immunocytochemistry. For drug treatment, the cortical cultures were treated with vehicle, D-serine (50 μM), D-serine (50 μM) + MK-801 (10 μM), MK-801 (10 μM), or glycine (100 μM) for 7 days, or with vehicle, D-serine (50 μM) D-serine (50 μM) + 7-CK (50 μM), glycine (50 μM) + lithium (100 μM) for 24 hrs.
Cell lysate preparation and fraction isolation
For cell lysate preparation, cultures were lysed in lysis buffer (150 mM NaCl, 1 mM EDTA, 100 mM Tris-HCl, 1% Triton X-100, and 0.5% sodium deoxycholate, pH 7.4) supplemented the day of use with 1:500 EDTA-free protease inhibitor cocktail III (Calbiochem) for 1 hr at 4°C. The whole cell lysates were harvested and centrifuged at 16,100 × g for 20 mins at 4°C. After the centrifugation, the supernatants were collected as the triton-soluble fraction for co-immunoprecipitation assays and western blot analysis, the pellets were collected as the triton-insoluble fraction. The supernatant and pellets were stored at −80°C until use.
Co-immunoprecipitation and Western blotting analysis
Co-immunoprecipitation and Western blotting were performed as described previously [51]. Protein content of cortical lysates was determined using BCA Protein Assay (Thermo Scientific). Equal amounts of total protein lysates (250 μg) were first added 2 μg primary antibody (α-SR) or normal IgG and incubated at 4°C for 2hrs. Immunocomplexes were then precipitated with protein G-agarose beads shaking overnight at 4°C, washed twice in lysis buffer, eluted by boiling in SDS-PAGE sample buffer, and subjected to Western blot analysis. Equal volumes of eluted buffers for co-immunoprecipitation assay or equal amounts of total protein (15 μg cell lysate) for protein input analysis were subjected to 4–12% NuPAGE Gel for electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked with 3% nonfat milk and incubated with primary antibody overnight at 4 °C. Blots were then incubated with appropriate horseradish peroxidase, HRP-conjugated secondary antibodies (Cell Signaling) for 2 hrs at room temperature and then washed; reaction bands were visualized using a luminol-enhanced chemiluminescence (ECL) HPR substrate (Thermo Scientific). Each blot was then incubated with stripping buffer (2% SDS, 50 mM Tris, pH 6.8, and 100 mM β-mercaptoethanol) for 1 hr at room temperature to remove the signals and reprobed for other proteins. For quantification analysis, reaction product levels were quantified by scanning densitometry and the ratio of co-precipitated protein was normalized by input levels from 3 different cultures and experiments using NIH Image J software.
Immunocytochemistry and fluorescence imaging
Primary cultured cortical neurons were fixed for 20 min at 4°C with 4% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.4), and then subjected to the immunostaining procedure. For immunostaining procedure, after blocking with 5% normal goat serum and 1 % bovine serum albumin in combination with 0.3% (vol/vol) Triton X-100 in PBS at room temperature for 1 hr, the coverslips or slides were incubated with primary antibodies at 4°C overnight and then secondary antibodies conjugated to Alexa Fluor 488 or 568 (Invitrogen) at room temperature for 60–90 mins. Following several washes with PBS, cells or slides were mounted with Vectashield with DAPI (Vector Laboratories).
Fluorescence images were obtained with Leica laser scanning confocal microscope. For cortical cultures, neurons were sequentially stained for SR, DISC1 and PSD-95. For quantification analysis, the confocal images were acquired from three to four 4 neurons for each treatment condition (Control, D-serine, D-serine + 7-CK, or Glycine + Lithium), we quantified the number of large DISC1- and SR-positive agglomerates in the soma and nucleus on one z-plane. The area of the soma (including the nucleus) in the image was measured via NIH Image J software, so the number of agglomerates per neuron could be normalized to the somatic area for the respective neuron. Additionally, the percentage of DISC1 and SR colocalization with DISC1- and SR-positive agglomerates were also quantified from three neurons in three different experiments. Statistical analysis was performed on these normalizations within and across the four treatment conditions.
Statistical analysis
Data were shown as the mean ± S.E.M. Experiments were analyzed using Student’s t-test to compare two conditions or ANOVA followed by planned comparisons of multiple conditions. Significance was set at P < 0.05.
Results
DISC1 distributes as large agglomerates and small puncta in cortical neurons
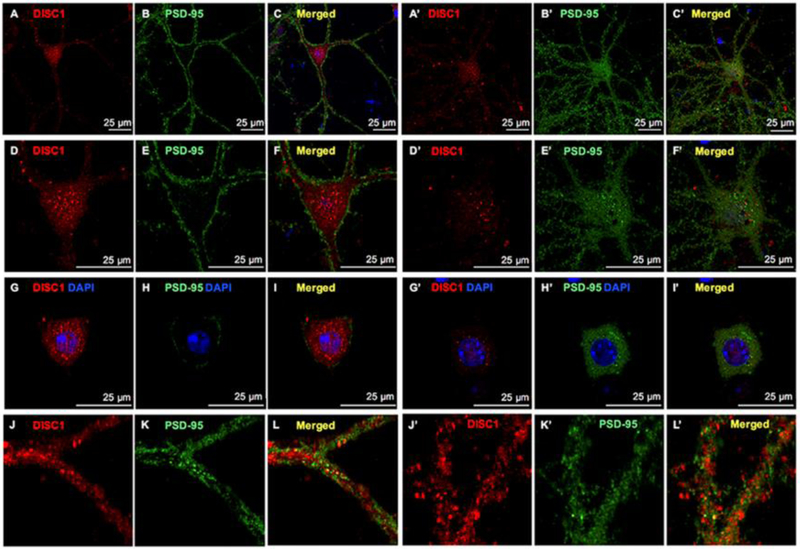
To explore the possible association of DISC1 with SR in cortical neurons, we first examined the expression and localization of DISC1 in primary cortical neuronal cultures at 15–23 days in vitro (DIV) by immunocytochemistry. In GABA-containing cortical interneurons, PSD-95 localizes to the shaft-like synapses on the somatic and dendritic membrane, whereas in cortical glutamatergic neurons, PSD-95 is distributed in the soma and in dendritic spine-like synapses [20]. DISC1 is abundant in both GABAergic (Figs. 1A, 1D, 1G and 1J) and glutamatergic neurons (Figs. 1A’, 1D’, 1G’ and 1J’), marked by PSD-95-positive non-spiny (Figs. 1B, 1E, 1H and 1K) or spiny (Figs. 1B’,1E’, 1H’ and 1K’) morphology, respectively. The intensity of DISC1 immunofluorescence is much higher in GABAergic (Figs.1A and 1D) than in glutamatergic neurons (Figs. 1A’ and 1D’), suggesting higher DISC1 levels in GABAergic neurons. In both types of neurons, DISC1 is more abundant in the soma than in dendrites, nucleus and axons. Interestingly, DISC1 appears as large agglomerates in the soma and small puncta in the nucleus and dendrites. The large somatic DISC1 agglomerates may be high molecular weight multimers of DISC1 as reported [52]. Notably, a few DISC1 puncta colocalize with PSD-95 puncta in the dendrites of cortical neurons (Figs. 1J–1L and 1J’–1L’).
Figure 1. DISC1 distributes as large agglomerates and small puncta in cortical neurons.
Confocal images of DISC1 (red immunofluorescence), PSD-95 (green immunoflurorescence) and merged images with DAPI-stained nucleus showing that DISC1 appears as large agglomerates in the soma and small puncta in the nucleus and dendrites of cortical glutamatergic (A-L) and GABAergic neurons (A’-L’). Scale bars as indicated.
SR associates with DISC1 and its agglomerates in cortical neurons
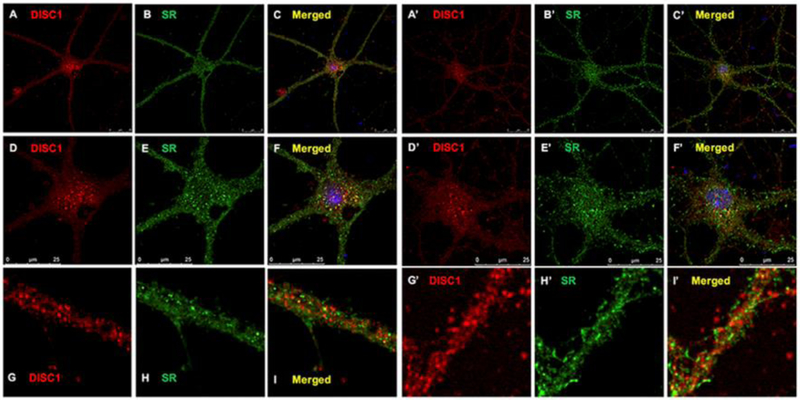
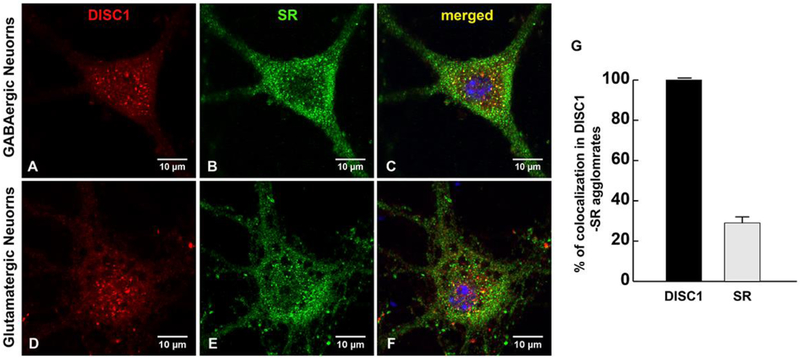
We then examined the association of SR with DISC1 agglomerates by sequentially staining primary cortical neuronal cultures with DISC1 and SR antibodies. While SR appears as puncta in the soma, nucleus and dendrites [20], the large DISC1 agglomerates (Figs. 2A and 2D, 2A’ and 2’) colocalize with SR puncta (Figs. 2B and 2E, 2B’ and 2E’) in the soma of GABAergic (Figs. 2A–2F) and glutamatergic neurons (Figs. 2A’–2F’). SR colocalizes with or are adjacent to DISC1 small puncta in the dendrites and synapses of GABAergic and glutamatergic cortical neurons (Figs. 2G–2I and 2G’–2I’). We also observed colocalization of SR with DISC1 small puncta in the nucleus, especially on the nuclear membrane as distinguished by DAPI staining (Fig. 3A–3F). More intriguingly, almost all of DISC1 puncta colocalize with SR in the soma and nucleus, whereas only 28±3% of SR puncta colocalizes with DISC1 large agglomerates (Fig. 3G), suggesting that DISC1 agglomerates may be modulated by SR. The results thus demonstrate that SR associates with DISC1 agglomerates in cortical neurons.
Figure 2. SR associates with DISC1 large agglomerates and small puncta in cortical neurons.
Confocal images of DISC1 (red immunofluorescence), SR (green immuoflurorescence) and merged images with DAPI-stained nucleus showing colocalization of SR with DISC1 large agglomerates n the soma and small puncta in the nucleus and dendrites of cortical glutamatergic (A-L) and GABAergic neurons (A’-L’). Scale bars as indicated.
Figure 3. A small portion of SR puncta colocalizes with DISC1 agglomrates whereas all DISC1 aggolmerates contain SR in the soma of cortical neurons.
Confocal images of DISC1 (red immunofluorescence), SR (green immuoflurorescence) and merged images with DAPI-stained nucleus showing alomost all DISC1 agglomerates colocalize with SR puncta, whereas only a small portion of SR puncta colocalize with DISC agglomerates in the soma and nucleus of cortical GABAergic (A-C) and glutamatergic neurons (D-F). DISC1-SR puncta are also observed on the nuclear membranes of cortical neurons (C, F). G. Quantification analysis of percentage of DISC1 and SR colocalization with DISC1-SR agglomerates in the soma and nucleus of cortical neurons (n=9 neurons from 3 different experiments). Scale bars as indicated.
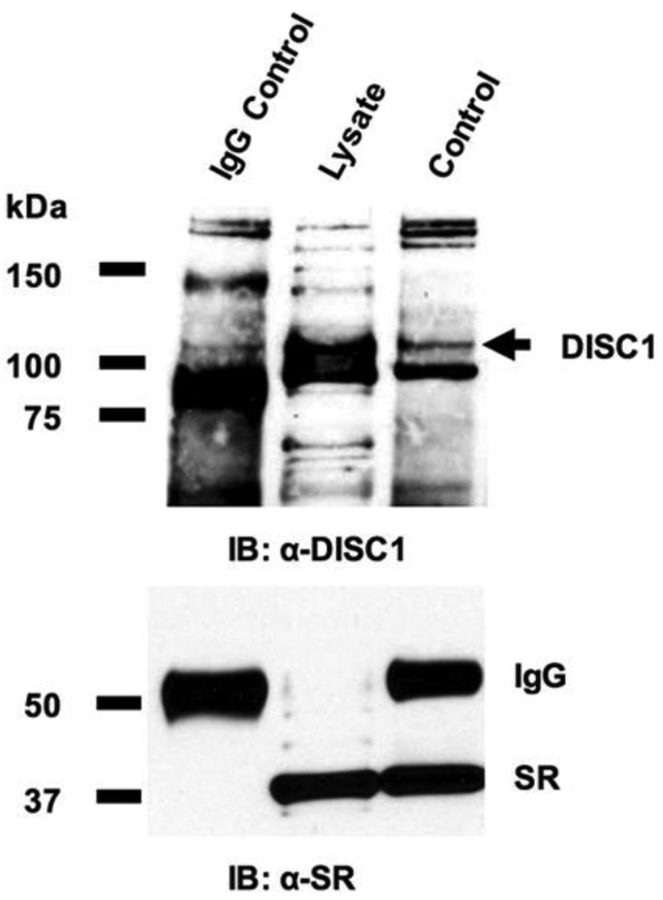
We further examined the in vivo interactions between SR and DISC1 in primary cortical neurons using coimmunoprecipitation assays. In cortical neuronal lysates, α-SR antibody but not control IgG co-precipitates endogenous DISC1, identified at a molecular weight of ~94 kDa as predicted for full-length DISC1 (Fig. 4), demonstrating SR-DISC1 interaction in cortical neurons. Our findings thus suggest the physiological presence of functional SR-DISC1 complexes in neurons.
Figure 4. SR associates with DISC1 in cortical neuronal lysates.
In normal cortical neurons, co-immunoprecipitation (IP) of endogenous DISC1 with SR antibody in cortical neuronal lysates was observed, but co-precipitation with normal IgG was not.
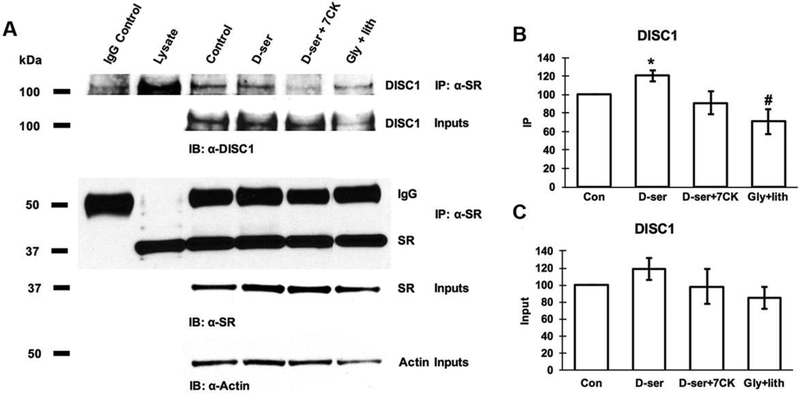
D-serine enhances SR-DISC1 interactions and increases the abundance of large DISC1-SR agglomerates in cortical neurons through NMDA receptors
To explore if D-serine modulate neuronal SR-DISC1 interactions, we examined if D-serine treatment for 24hrs modulates SR-DISC1 interactions using co-immunoprecipitation assays. Exogenous application of D-serine leads to a slight but not significant increase of the DISC1 levels in the lysates compared with control (Figs. 5A and 5B). However, exogenous D-serine application significantly increases the amount of DISC1 that co-precipitates with α-SR (Figs. 5A and 5C), suggesting enhancement of SR-DISC1 interactions. This increase is blocked by co-application of 7-CK. In contrast, exogenous application of glycine along with the glycine transporter inhibitor lithium significantly decreases the amount of DISC1 co-precipitated with α-SR (Figs. 5A and 5C), Our findings suggest that D-serine enhances neuronal SR-DISC1 interaction through NMDA receptors,
Figure 5. D-serine enhances SR-DISC1 interaction in cortical neurons through NMDA receptors.
(A) Coimmunoprecipitation of endogenous DISC1 with SR antibody in cortical neuronal lysates from cultures treated with vehicle, D-serine (50μM), D-serine (50μM) + 7-CK (50μM), glycine (50μM) + lithium (100μM) for 24 hrs. Upper panel: Co-immunoprecipitation assays of cortical lysates (250 μg each lane); Lower panel: Western blot analysis of input levels in cortical lysates (15μg each lane). Graphs show quantification analysis of the inputs (B) and co-precipitated DISC1 with α-SR antibody (C) in the cortical lysates. (* p<0.05 vs. control, # p<0.05 vs. D-serine-treated; t-test, n=4 different experiments).
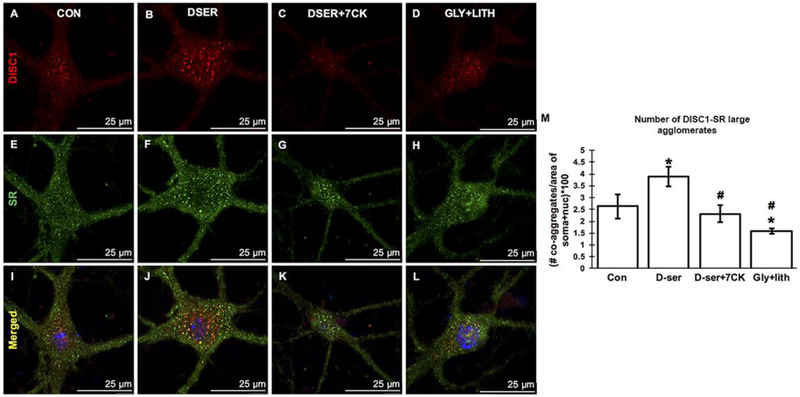
We then examined if D-serine treatment modulates the DISC1-SR agglomerates by immunocytochemistry and confocal microscopy. Exogenous application of D-serine (Figs. 6D–F) markedly increases the number of large DISC1-SR agglomerates in the soma of GABAergic and glutamatergic cortical neurons compared to control (Figs. 6A–C). This increase is blocked by 7-CK, a competitive antagonist at the D-serine/glycine binding site on the NR1 subunit of NMDARs (Figs. 6G–I), but not by exogenous application of glycine with the glycine transporter inhibitor lithium (Figs. 6J–L). Co-treatment of cortical neurons with 7-CK and D-serine blocks the D-serine-induced increase, suggesting increases of DISC1-SR agglomerates through NMDARs. Quantification of the number of large DISC1-SR agglomerates in the soma area (including nucleus) show that exogenous D-serine application significantly increases the number of large DISC1-SR agglomerates (P<0.01 vs. control), whereas exogenous application of glycine and lithium fails to increase the number of large DISC1-SR agglomerates (P<0.001 vs. D-serine-treated). Co-treatment of cortical neurons with 7-CK and D-serine blocks the increase (P<0.01 vs. D-serine-treated). Our findings suggest that D-serine increases the abundance of large DISC1-SR agglomerates through activating NMDA receptors.
Figure 6. D-serine increases the abundance of DISC1-SR large agglomerates in cortical neurons through NMDA receptors.
Confocal images of DISC1 (red immunofluorescence), SR (green immunofluorescence) and merged mages with DAPI-stained nucleus in cortical neurons from control (A-C), D-serine-treated (D-F), D-serine+7-CK-treated (G-I) and glycine+lithium-treated (J-L) cortical cultures. Scale bars as indicated. M, Quantification of the number of large somatic DISC1-SR agglomerates in cortical neurons from control, D-serine-treated, D-serine+7-CK-treated and glycine+lithium-treated cortical cultures (**P<0.01 vs. control, ## P<0.01 ### P<0.001 vs. D-serine-treated; t-test, n=10–12 neurons from 3 different experiments).
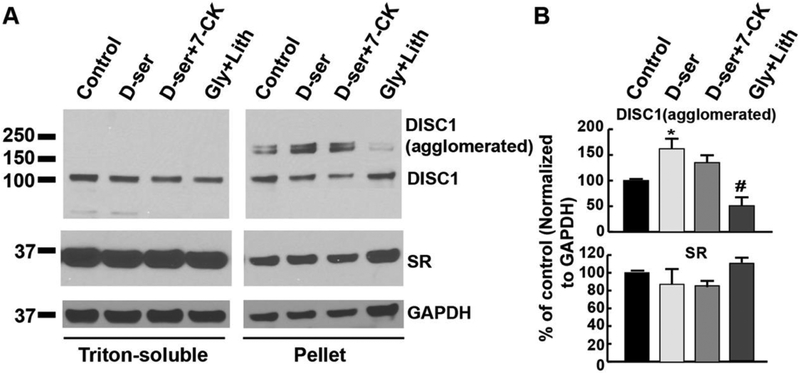
To further analyze the association of DISC1 and SR in large agglomerates, we fractionized the triton-soluble supernatant and insoluble pellets from cortical neuronal lysates and examined the levels of DISC1 and SR in both fractions. Western blot analyses show the presence of SR and DISC1 in the insoluble pellet fraction of neuronal lysates (Fig. 7A). In addition, beside the normal form of DISC1, the agglomerated form of DISC1 (~180–200 kDa, most likely dimer) is indeed found in the insoluble pellet fraction, confirming the physiological presence of DISC1-SR agglomerates in neurons (Fig. 7A). Intriguingly, exogenous D-serine application significantly increases the agglomerated form of DISC1 in the insoluble pellet fraction of cortical neuronal lysates (Figs. 7A and 7B, P<0.05 vs. control), whereas exogenous application of glycine and lithium fails to increase the agglomerated from of DISC1 (Figs. 7A and 7B, # P<0.05 vs. D-serine-treated). Co-treatment of cortical neurons with 7-CK and D-serine blocks the increase (Fig. 7A and 7B). This further supports our findings that D-serine increases SR-DISC1 interaction and DISC1-SR agglomerates through activating NMDA receptors.
Figure 7. D-serine increases the levels of DISC1 agglomerated form in the insoluble pellet fraction of cortical neuronal lysates.
(A) Western blot analysis of DISC1 and SR levels in the fractions of triton-soluble supernatant and insoluble pellet from cortical neuronal cultures treated with vehicle, D-serine (50μM), D-serine (50μM) + AP5 (50μM), D-serine (50μM) + 7-CK (50μM), glycine (50μM) + lithium (100μM) for 24 hrs. Upper panel: DISC1 levels in the fractions of triton-soluble supernatant and insoluble pellet (15 μg each lane); Lower panel: SR levels in the fractions of triton-soluble supernatant and insoluble pellet (15μg each lane). (B) Graphs show quantification analysis of the agglomerated form of DISC1 (upper panel) and SR (lower panel) in the triton-insoluble pellet fraction of cortical neuronal lysates (* P<0.05 vs. control, # P<0.05 vs. D-serine-treated, t-test, n=3 different experiments).
Discussion
The present study uses in vitro cultured cortical neurons to show that neuronal SR interacts and associates with DISC1 agglomerates, which can be modulated by NMDAR activity. Endogenous SR interacts with DISC1 and colocalizes with DISC1 large agglomerates in the soma of cortical neurons and small puncta in the nucleus and dendrites, suggesting the physiological presence of functional SR-DISC1 complexes in cortical neurons. Exogenous D-serine application enhances the interactions of SR with DISC1 and increases the number of large DISC1-SR agglomerates in cortical neurons. Biochemical fractionation assays further confirm the presence of agglomerated form of DISC1 along with SR in the triton-insoluble pellet fraction, which is increased by exogenous D-serine application. Exogenous application of glycine with a glycine transporter inhibitor fails to increase their interactions, large DISC1-SR agglomerates and DISC1 agglomerated form in cortical neurons. This increase by D-serine application is blocked by 7-CK, a competitive inhibitor of NMDARs at the glycine binding site, suggesting mediation by NMDAR activation. Our findings thus demonstrate that D-serine enhances neuronal SR association with DISC1 and its agglomerates, which can be modulated by NMDAR activation, validating a novel neuronal SR-DISC1 complex responsive to NMDAR activation and providing a molecular mechanism at which pathways implicated in schizophrenia converge.
Both SR and DISC1 play important roles in synaptic and dendritic development in neurons. For example, SR/D-serine modulates glutamatergic synapse stability through interactions with PSD-95 in postsynaptic neurons [20] and are important for dendritic spine formation in neurons. DISC1 regulates glutamatergic synapse formation on GABAergic interneurons through NRG1-ErbB4 signaling in the mature cortex [53] and regulates spine formation through its interactions with Kal7 in glutamatergic neurons [54]. In addition, DISC1 controls dendrite morphology by regulating mitochondria dynamics in a complex with Miro, TRAK and mitofusin proteins in neurons [55]. Our findings show that neuronal SR interacts and colocalizes with DISC1 large agglomerates in the soma and small puncta in the nucleus and dendrites, suggesting distinctive physiological functions of SR-DISC1 complex in neurons. One hypothesis about how DISC1 modulates its ability to interact with different proteins and carry out many functions points to DISC1’s potential for self multimerization. For example, DISC1 can form large species and octameric states of DISC1 constructs are necessary for functional interactions with NDEL1. However, the presence of functional DISC1 multimers has yet to be verified in vivo [56]. Our results indeed show the presence of agglomerated form of DISC1 (likely dimer) along with SR in the triton-insoluble pellet fraction of cortical lysates. More intriguingly, we observed that almost all of DISC1 puncta colocalize with SR in the soma and nucleus, whereas only about one-third of SR puncta colocalizes with DISC1 agglomerates, suggesting that DISC1 agglomerates may be modulated by SR. Our findings thus suggest that SR-DISC1 interactions may modulate DISC1 and SR signaling and physiological functions in synaptic and dendritic development. In addition, we also observe co-localization of small SR-DISC1 puncta on the nuclear membrane and within the nucleus of neurons. Putative nuclear export signal (NES) sequences have been identified within SR, but a nuclear localization signal (NLS) sequence has not been found [57]. Thus, how SR translocates to the nucleus from the postsynaptic density remains a mystery. DISC1, however, contains possible NLS sequences within its N-terminal domain. Therefore, SR may translocate to and from the nucleus through a SR-DISC protein complex.
SR/D-serine deficiency and mutant DISC1 have been implicated in the pathogenesis of schizophrenia [27–37]. Our findings show that exogenous application of D-serine enhances the interaction of SR with DISC1 and association with DISC1 agglomerates in cortical neurons. This identifies a novel neuronal SR-DISC1 complex responsive to NMDAR activation and provides a molecular mechanism at which pathways implicated in schizophrenia converge. Indeed, D-serine treatment or increasing D-serine production can rescue the schizophrenic symptoms in patients and animal models [33, 35, 58]. SR null mutant mice, which have less than 10% of normal brain D-serine, have schizophrenia-like symptoms and display reduced dendritic spine density that can be partially rescued by chronic D-serine treatment [32, 36, 43]. Mice with genetic mutations of the glycine binding site on NR1 subunit show negative and cognitive symptoms resembling schizophrenia that can be normalized by D-serine treatment [4]. Genetic loss of DAAO activity increases D-serine production and reverses schizophrenia-like phenotypes in mice [27, 59]. Clinical trials show that oral administration of D-serine improves positive, negative, and cognitive symptoms of schizophrenia correlated with elevated plasma D-serine levels as add-on therapy to typical and atypical antipsychotics [60, 61]. D-serine treatment improves the negative symptoms in individualsat clinically high risk of schizophrenia, supporting D-serine treatment for the prodromal symptoms of schizophrenia [58]. Both SR and DISC1 play an important physiological role in dendritic spine density, which is impaired in schizophrenia patients and animal models [20, 32, 33, 54, 62–71]. Our results indicate that D-serine enhancement of SR-DISC1 interactions may improve the schizophrenic symptoms in patients and animal models partially through modulating dendritic spine formation, providing a rationale and molecular mechanism for D-serine treatment in schizophrenia.
Synaptic and extrasynaptic NMDARs are associated with differential gene expression and have different roles in synaptic plasticity and cell death [5, 72–77]. Some studies have shown that D-serine preferentially gates synaptic NMDARs, whereas glycine preferentially gates extrasynaptic NMDARs at the hippocampal Schaffer Collateral-CA1 region [5, 9]. However, other studies have shown that D-serine and glycine activate synaptic NMDA receptors in a synapse-specific and developmental-regulated manner in the hippocampus [7, 8, 23]. D-serine is the preferred coagonist at hippocampal Schaffer Collateral-CA1 (SC-CA1) mature synapses, whereas glycine is mainly involved at medial perforant path-dentate gyrus (mPP–DG) synapses [23]. Our studies show that D-serine, rather than glycine, enhances the interaction of neuronal SR with DISC1 in cortical neurons, supporting a preferential regulatory role of D-serine in controlling neuronal SR-DISC1 signaling as well as synaptic and dendritic development in cortical neurons. This is likely mediated by synaptic, rather than extrasynaptic, NMDARs. Synaptic NMDARs play crucial roles in many forms of synaptic plasticity, such as LTP and LTD. Therefore, control of synaptic NMDARs may provide a mechanism by which D-serine regulates neuronal DISC1 and SR function.
Highlights.
Serine racemase (SR) interacts with Disrupted-In-Schizophrenia-1 (DISC1) in cortical neurons.
SR colocalizes DISC1 in the soma, dendrites and nucleus of cortical neurons as large agglomerates and small puncta.
D-serine enhances SR interaction with DISC1 through NMDAR activation in cortical neurons.
D-serine increases the abundance of DISC1-SR large agglomerates in the soma of cortical neurons through NMDAR activation.
D-serine increases the levels of DISC1 agglomerated form in the triton-insoluble pellet fraction of cortical neurons through NMDAR activation.
These findings validate a novel neuronal SR-DISC1 complex responsive to NMDAR activation and may provide a molecular mechanism by which pathways implicated in schizophrenia converge.
Acknowledgments
This work was supported by CHOP Foerderer Grant for Excellence (HL) and NIH grants R21NS072842, R01NS45986 and U54 HD086984 (DL). We thank Ms. Margaret Maronski for cortical neuronal cultures, Dr. Hajime Takano for aid in confocal imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no competing or financial interests.
References
- 1.Johnson JW and Ascher P, Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature, 1987. 325(6104): p. 529–31. [DOI] [PubMed] [Google Scholar]
- 2.Kleckner NW and Dingledine R, Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science, 1988. 241(4867): p. 835–7. [DOI] [PubMed] [Google Scholar]
- 3.Mothet JP, et al. , D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A, 2000. 97(9): p. 4926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labrie V, Lipina T, and Roder JC, Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacology (Berl), 2008. 200(2): p. 217–30. [DOI] [PubMed] [Google Scholar]
- 5.Papouin T, et al. , Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell, 2012. 150(3): p. 633–46. [DOI] [PubMed] [Google Scholar]
- 6.Li YH and Wang J, Membrane insertion of new AMPA receptors and LTP induced by glycine is prevented by blocking NR2A-containing NMDA receptors in the rat visual cortex in vitro. Curr Neurovasc Res, 2013. 10(1): p. 70–5. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg D, et al. , Neuronal D-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J Neurosci, 2013. 33(8): p. 3533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mothet JP, Le Bail M, and Billard JM, Time and space profiling of NMDA receptor co-agonist functions. J Neurochem, 2015. 135(2): p. 210–25. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, et al. , Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A, 2003. 100(25): p. 15194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martineau M, Baux G, and Mothet JP, D-serine signalling in the brain: friend and foe. Trends Neurosci, 2006. 29(8): p. 481–91. [DOI] [PubMed] [Google Scholar]
- 11.Wolosker H, D-serine regulation of NMDA receptor activity. Sci STKE, 2006. 2006(356): p. pe41. [DOI] [PubMed] [Google Scholar]
- 12.Wolosker H, Serine racemase and the serine shuttle between neurons and astrocytes. Biochim Biophys Acta, 2011. 1814(11): p. 1558–66. [DOI] [PubMed] [Google Scholar]
- 13.Labrie V, Clapcote SJ, and Roder JC, Mutant mice with reduced NMDA-NR1 glycine affinity or lack of D-amino acid oxidase function exhibit altered anxiety-like behaviors. Pharmacol Biochem Behav, 2009. 91(4): p. 610–20. [DOI] [PubMed] [Google Scholar]
- 14.Billard JM, D-Amino acids in brain neurotransmission and synaptic plasticity. Amino Acids, 2012. 43(5): p. 1851–60. [DOI] [PubMed] [Google Scholar]
- 15.Campanini B, et al. , Serine racemase: a key player in neuron activity and in neuropathologies. Front Biosci (Landmark Ed), 2013. 18: p. 1112–28. [DOI] [PubMed] [Google Scholar]
- 16.Van Horn MR, Sild M, and Ruthazer ES, D-serine as a gliotransmitter and its roles in brain development and disease. Front Cell Neurosci, 2013. 7: p. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, et al. , Reduced brain infarct volume and improved neurological outcome by inhibition of the NR2B subunit of NMDA receptors by using CP101,606–27 alone and in combination with rt-PA in a thromboembolic stroke model in rats. J Neurosurg, 2003. 98(2): p. 397–403. [DOI] [PubMed] [Google Scholar]
- 18.Kim MJ, et al. , Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron, 2005. 46(5): p. 745–60. [DOI] [PubMed] [Google Scholar]
- 19.DeVito LM, et al. , Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes Brain Behav, 2011. 10(2): p. 210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H, et al. , D-Serine and Serine Racemase Are Associated with PSD-95 and Glutamatergic Synapse Stability. Front Cell Neurosci, 2016. 10: p. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balu DT, et al. , Serine Racemase and D-serine in the Amygdala Are Dynamically Involved in Fear Learning. Biol Psychiatry, 2018. 83(3): p. 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sason H, et al. , Asc-1 Transporter Regulation of Synaptic Activity via the Tonic Release of d-Serine in the Forebrain. Cereb Cortex, 2017. 27(2): p. 1573–1587. [DOI] [PubMed] [Google Scholar]
- 23.Le Bail M, et al. , Identity of the NMDA receptor coagonist is synapse specific and developmentally regulated in the hippocampus. Proc Natl Acad Sci U S A, 2015. 112(2): p. E204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada K, et al. , Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and D-serine levels. Biol Psychiatry, 2005. 57(12): p. 1493–503. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K, et al. , Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry, 2003. 60(6): p. 572–6. [DOI] [PubMed] [Google Scholar]
- 26.Bendikov I, et al. , A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res, 2007. 90(1–3): p. 41–51. [DOI] [PubMed] [Google Scholar]
- 27.Labrie V, Wong AH, and Roder JC, Contributions of the D-serine pathway to schizophrenia. Neuropharmacology, 2012. 62(3): p. 1484–503. [DOI] [PubMed] [Google Scholar]
- 28.Lin H, et al. , Cortical parvalbumin GABAergic deficits with alpha7 nicotinic acetylcholine receptor deletion: implications for schizophrenia. Mol Cell Neurosci, 2014. 61: p. 163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin H, et al. , Cortical synaptic NMDA receptor deficits in alpha7 nicotinic acetylcholine receptor gene deletion models: implications for neuropsychiatric diseases. Neurobiol Dis, 2014. 63: p. 129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu AC, et al. , Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry, 2009. 14(7): p. 719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balu DT and Coyle JT, Glutamate receptor composition of the post-synaptic density is altered in genetic mouse models of NMDA receptor hypo- and hyperfunction. Brain Res, 2011. 1392: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balu DT and Coyle JT, Neuronal D-serine regulates dendritic architecture in the somatosensory cortex. Neurosci Lett, 2012. 517(2): p. 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balu DT and Coyle JT, Chronic D-serine reverses arc expression and partially rescues dendritic abnormalities in a mouse model of NMDA receptor hypofunction. Neurochem Int, 2014. 75: p. 76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagiwara H, Iyo M, and Hashimoto K, Neonatal disruption of serine racemase causes schizophrenia-like behavioral abnormalities in adulthood: clinical rescue by d-serine. PLoS One, 2013. 8(4): p. e62438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma TM, et al. , Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry, 2013. 18(5): p. 557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balu DT, et al. , The NMDA receptor co-agonists, D-serine and glycine, regulate neuronal dendritic architecture in the somatosensory cortex. Neurobiol Dis, 2012. 45(2): p. 671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balu DT and Coyle JT, The NMDA receptor ‘glycine modulatory site’ in schizophrenia: D-serine, glycine, and beyond. Curr Opin Pharmacol, 2015. 20: p. 109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kartvelishvily E, et al. , Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem, 2006. 281(20): p. 14151–62. [DOI] [PubMed] [Google Scholar]
- 39.Wolosker H, et al. , D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J, 2008. 275(14): p. 3514–26. [DOI] [PubMed] [Google Scholar]
- 40.Miya K, et al. , Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol, 2008. 510(6): p. 641–54. [DOI] [PubMed] [Google Scholar]
- 41.Benneyworth MA, et al. , Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol, 2012. 32(4): p. 613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehmsen JT, et al. , D-serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J Neurosci, 2013. 33(30): p. 12464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balu DT, et al. , D-serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cell Mol Neurobiol, 2014. 34(3): p. 419–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martineau M, Parpura V, and Mothet JP, Cell-type specific mechanisms of D-serine uptake and release in the brain. Front Synaptic Neurosci, 2014. 6: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim PM, et al. , Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci U S A, 2005. 102(6): p. 2105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujii K, et al. , Serine racemase binds to PICK1: potential relevance to schizophrenia. Mol Psychiatry, 2006. 11(2): p. 150–7. [DOI] [PubMed] [Google Scholar]
- 47.Hikida T, et al. , Modulation of D-serine levels in brains of mice lacking PICK1. Biol Psychiatry, 2008. 63(10): p. 997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma TM, et al. , Serine racemase regulated by binding to stargazin and PSD-95: potential N-methyl-D-aspartate-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (NMDA-AMPA) glutamate neurotransmission cross-talk. J Biol Chem, 2014. 289(43): p. 29631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia M, et al. , DISC1, astrocytes and neuronal maturation: a possible mechanistic link with implications for mental disorders. J Neurochem, 2016. 138(4): p. 518–24. [DOI] [PubMed] [Google Scholar]
- 50.Lin H, et al. , Axonal alpha7 nicotinic ACh receptors modulate presynaptic NMDA receptor expression and structural plasticity of glutamatergic presynaptic boutons. Proc Natl Acad Sci U S A, 2010. 107(38): p. 16661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhai J, et al. , HoxB2 binds mutant SOD1 and is altered in transgenic model of ALS. Hum Mol Genet, 2005. 14(18): p. 2629–40. [DOI] [PubMed] [Google Scholar]
- 52.Leliveld SR, et al. , Insolubility of disrupted-in-schizophrenia 1 disrupts oligomer-dependent interactions with nuclear distribution element 1 and is associated with sporadic mental disease. J Neurosci, 2008. 28(15): p. 3839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seshadri S, et al. , Interneuronal DISC1 regulates NRG1-ErbB4 signalling and excitatory-inhibitory synapse formation in the mature cortex. Nat Commun, 2015. 6: p. 10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi-Takagi A, et al. , Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci, 2010. 13(3): p. 327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norkett R, et al. , DISC1-dependent Regulation of Mitochondrial Dynamics Controls the Morphogenesis of Complex Neuronal Dendrites. J Biol Chem, 2016. 291(2): p. 613–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soares DC, et al. , DISC1: Structure, Function, and Therapeutic Potential for Major Mental Illness. ACS Chem Neurosci, 2011. 2(11): p. 609–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolodney G, et al. , Nuclear compartmentalization of serine racemase regulates d-serine production. IMPLICATIONS FOR N-METHYL-d-ASPARTATE (NMDA) RECEPTOR ACTIVATION. J Biol Chem, 2016. 291(6): p. 2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kantrowitz JT, et al. , D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry, 2015. 2(5): p. 403–12. [DOI] [PubMed] [Google Scholar]
- 59.Labrie V, et al. , Genetic loss of D-amino acid oxidase activity reverses schizophrenia-like phenotypes in mice. Genes Brain Behav, 2010. 9(1): p. 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sacchi S, et al. , D-amino acid oxidase inhibitors as a novel class of drugs for schizophrenia therapy. Curr Pharm Des, 2013. 19(14): p. 2499–511. [DOI] [PubMed] [Google Scholar]
- 61.Kantrowitz JT, et al. , High dose D-serine in the treatment of schizophrenia. Schizophr Res, 2010. 121(1–3): p. 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa E, et al. , Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiol Dis, 2001. 8(5): p. 723–42. [DOI] [PubMed] [Google Scholar]
- 63.Garey LJ, et al. , Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry, 1998. 65(4): p. 446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glantz LA and Lewis DA, Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry, 2000. 57(1): p. 65–73. [DOI] [PubMed] [Google Scholar]
- 65.Glantz LA and Lewis DA, Dendritic spine density in schizophrenia and depression. Arch Gen Psychiatry, 2001. 58(2): p. 203. [DOI] [PubMed] [Google Scholar]
- 66.Glausier JR and Lewis DA, Dendritic spine pathology in schizophrenia. Neuroscience, 2013. 251: p. 90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hill JJ, Hashimoto T, and Lewis DA, Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry, 2006. 11(6): p. 557–66. [DOI] [PubMed] [Google Scholar]
- 68.Kolluri N, et al. , Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry, 2005. 162(6): p. 1200–2. [DOI] [PubMed] [Google Scholar]
- 69.Konopaske GT, et al. , Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry, 2014. 71(12): p. 1323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moyer CE, Shelton MA, and Sweet RA, Dendritic spine alterations in schizophrenia. Neurosci Lett, 2015. 601: p. 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sweet RA, et al. , Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology, 2009. 34(2): p. 374–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parsons MP and Raymond LA, Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron, 2014. 82(2): p. 279–93. [DOI] [PubMed] [Google Scholar]
- 73.Gladding CM and Raymond LA, Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci, 2011. 48(4): p. 308–20. [DOI] [PubMed] [Google Scholar]
- 74.Hardingham GE and Bading H, Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci, 2010. 11(10): p. 682–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karpova A, et al. , Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell, 2013. 152(5): p. 1119–33. [DOI] [PubMed] [Google Scholar]
- 76.Kaufman AM, et al. , Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. J Neurosci, 2012. 32(12): p. 3992–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meunier CN, et al. , D-Serine and Glycine Differentially Control Neurotransmission during Visual Cortex Critical Period. PLoS One, 2016. 11(3): p. e0151233. [DOI] [PMC free article] [PubMed] [Google Scholar]