Abstract
Purpose:
Increased rates of toxicity have been described following stereotactic body radiotherapy (SBRT) for central lung tumors within 2 cm of the proximal bronchial tree (PBT). Recent studies have defined a new class of “ultracentral” tumors. We report our experience treating ultracentral, central, and paramediastinal tumors with SBRT and compare toxicity, disease control, and survival.
Methods and Materials:
We reviewed the records of patients with central lung tumors treated with SBRT between September 2009-July 2017. Tumors were classified as central if within 2 cm of the PBT, ultracentral if the planning target volume touched the PBT or esophagus, and paramediastinal if touching mediastinal pleura. Actuarial rates of grade 2+ and 3+ toxicity, local control (LC), and overall survival (OS) were assessed using the Kaplan-Meier method and compared using a log-rank test. Toxicity was scored with CTCAE V4.03.
Results:
We identified 68 patients with 69 central lung tumors, including 14 ultracentral, 15 paramediastinal, and 39 central tumors. Fifty-three patients were treated for early stage lung cancer and 15 for lung metastases. Prescribed dose ranged from 40–60 Gy over 3–8 fractions. Most patients were treated using five-fractions (83%) followed by eight-fractions (10%). Median follow up was 19.7 months (range: 3.3–78.3). Two-year estimates of LC (89%, 85%, and 93%; p=0.72) and OS (76%, 73%, and 72%; p=0.75) for ultracentral, central, and paramediastinal tumors were similar. Ultracentral tumors had increased risk of grade 2+ toxicity (57.6% vs. 14.2% vs. 7.1%, p= 0.007) at 2 years. One ultracentral patient developed grade 5 respiratory failure.
Conclusion:
Oncologic outcomes following SBRT for ultracentral, central, and paramediastinal lung tumors were similar, with LC exceeding 85% at 2 years using predominantly 5-fraction schedules. Ultracentral lung tumors were associated with increased risk of toxicity in our patient cohort. Additional studies are needed to minimize toxicity for ultracentral tumors.
Keywords: SBRT, lung cancer, ultracentral, toxicity
INTRODUCTION
Stereotactic body radiation therapy (SBRT) has emerged as a definitive treatment option for patients with medically inoperable early stage non-small cell lung cancer (NSCLC) and for limited metastatic deposits in the lungs from solid tumors. SBRT relies on the delivery of ablative radiation doses over 1–5 fractions with steep dose gradients using precise tumor localization and motion management. For peripherally located tumors, in-field control is excellent, exceeding 90% in many series with grade 3+ toxicities rare (<5%) [1].
However, despite its millimeter accuracy, centrally located tumors adjacent to mediastinal structures such as the large airways, great vessels, heart, and esophagus remain at risk for radiation injury. Several prior institutional studies have revealed increased treatment toxicity following SBRT for such tumors. A previous phase II lung SBRT study reported an 11-fold increase in risk of severe pulmonary toxicity with treatment of perihilar or central tumors compared to more peripheral locations [2], although with longer follow-up this finding lost statistical significance [3]. A high profile case report of fatal central airway necrosis following SBRT for a central tumor also contributed to concerns about the safety [4]. RTOG 0813, a Phase I/Phase II study evaluating 5 fraction SBRT for centrally located lung tumors has been reported in abstract form only [5, 6]. Preliminary findings from RTOG 0813 identified a Bayesian –based probability of dose limiting (grade 3+) toxicity (DLT) within the first year of 7.2% at the highest dose level, deemed acceptable by the pre-specified study parameters, although additional high-grade toxicities were noted beyond the DLT window. Details on tumor location and critical organ dose volume parameters for patients with high-grade toxicity have not yet been presented. Importantly, RTOG/NRG 0813 enrolled both patients with tumors located within 2 cm of the PTB, as well as those with a planning target volume (PTV) overlapping the mediastinal pleural. This extended the definition of central beyond that of the Indiana University report, which included only those tumors within 2 cm of the PTB. Several smaller retrospective analyses have recently introduced the concept of the “ultracentral” tumor with a planning target volume overlapping the PBT and expected to pose a higher risk than other centrally located tumors. Limited data addresses the safety profile of SBRT for ultracentral tumors.
In this study, we report our institutional experience in treating central lung tumors with SBRT, stratifying as central (C), ultracentral (UC) or paramediastinal (P) with a focus on evaluation of treatment toxicity for each cohort. We also analyze rates and patterns of tumor control and overall survival (OS).
METHODS AND MATERIALS
Patients
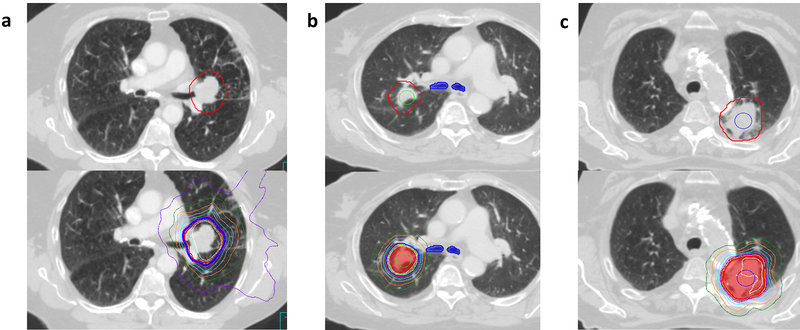
We performed a retrospective review all patients age ≥ 18 years-old treated with SBRT to primary or metastatic lung tumors at XXXX between September 2009 and July 2017. Eligible tumors were located within 2 cm of the proximal bronchial tree (PBT) or the planning target volume (PTV) overlapped the mediastinal pleura. Tumors were classified as UC if the PTV overlapped the PBT or esophagus (Figure 1), C if located within 2 cm of the PBT but not meeting criteria as UC, and PM if the PTV touched or overlapped the mediastinal pleura but did not otherwise meet criteria as UC or C.
Figure 1:
a) Axial slices showing PTV (top) and dose distribution from a) an ultracentral lung case directly abutting the left mainstem bronchus, treated to 56 Gy in 8 fractions with a maximum point dose to the proximal bronchial tree, great vessels, esophagus, and heart of 58.4Gy, 59.6Gy, 30.0Gy, and 52.5 Gy respectively b) a central lung case with the tumor located <2 cm from the proximal bronchial tree with a maximum point dose to the proximal bronchial tree, great vessels, esophagus, and heart of 49.3 Gy, 32.4 Gy, 14.5 Gy, and 10.4 Gy and c) a paramediastinal lung case with the PTV overlapping the aorta with a maximum point dose to the proximal bronchial tree, great vessels, esophagus, and heart of 11.3 Gy, 51.9 Gy, 28.5 Gy, and 1.8 Gy. The planning target volume is shown in red for each case, and the 95% isodose line in royal blue.
Treatment
Patients were simulated with Vac-lok (CIVCO Medical Solutions, Orange City, IA) for immobilization. Abdominal compression was used to limit diaphragmatic excursion to ≤1 cm, verified with fluoroscopy. Planning computed tomography (CT) scans were obtained with 2mm slice thickness. Ten-phase four dimensional CT (4DCT) datasets were routinely obtained at simulation.
Gross tumor volume (GTV) was defined based on the planning CT scan. The maximum intensity projection or a review of all 10 phases from the 4DCT datasets were used to generate the internal target volume (ITV). A 5mm margin was then added to the ITV to generate the planning target volume (PTV). Organs at risk were contoured based on RTOG guidelines [7]. Treatment planning required the prescription isodose line to cover at least 95% of the PTV, and at least 90% of the prescription dose covering 99% of the PTV. All patients were treated using 6 MV photon on Elekta-Synergy linear accelerator (Elekta AB, Stockholm, Sweden). Fluoroscopy and cone beam CT were obtained prior to each treatment for confirmation of tumor excursion and anatomy matching.
The fractionation schedule was at the discretion of the treating radiation oncologist. In general, our preferred fractionation schedule for a centrally located lung tumors is 50 Gy in 5 fractions since early data by Timmerman et al. [2] had demonstrated increased toxicity using 60–66 Gy in 3 fractions. However, for UC tumors with significant PTV overlap with the PBT or esophagus, eight fraction regimens were sometimes selected at the discretion of the treating physician. Several alternative schedules were additionally used early in our institutional SBRT experience.
Follow up
Patients were followed with physical exam and CT chest every 3 months for the first 1–2 years and every 6 months for years 3–5 to assess for treatment response and toxicity. Primary tumor failures were defined as recurrence within or at the margin of the PTV. Local failures were defined as primary tumor failure plus any failures in the same lobe. Locoregional failures include local failure plus any nodal metastasis in the hilum, mediastinum, or supraclavicular fossa. All other failures were classified as distant.
Statistical analysis
Study endpoints included treatment related grade toxicity, Local tumor control (LC), locoregional control (LRC), distant control (DC), and OS. Treatment toxicities were graded based on CTCAE v4.03.
Actuarial estimates of toxicity, LC, LRC, and OS were generated using the Kaplan-Meier method, and were compared between groups using the log-rank method. Dosimetric data were compared using ANOVA after confirming normality of distribution for each category using the Kolmogorov-Smirnov test. Relationship between maximum central airway dose and rate of pulmonary toxicity was examined using logistic regression. Values of p <0.05 were considered statistically significant. For the purpose of dosimetric and toxicity analysis, one central tumor patient who had two synchronous NSCLC primaries which were treated at the same time was counted as one patient, while another paramediastinal patient who had two metachronous lung primaries were counted as two separate patients.
All analyses were performed using Statview version 5.01 (SAS Institute Inc., Cary, NC)
RESULTS
Patient Characteristics
Patient characteristics are outlined in Table 1. We identified 68 total patients with 69 eligible tumors, including 39 C, 15 PM, and 14 UC tumors. Fifty-three patients had early stage NSCLC and another 15 were treated for oligometastatic tumors involving the lungs. The median age at treatment was 73 (range: 31–93). 39 patients (58%) were female and 29 (42%) were male. Median follow up for living patients was 17.5 months.
Table 1.
Patient characteristics
| Total | Central | Ultracentral | Paramediastinal | |
|---|---|---|---|---|
| Patients | 68 | 39 | 14 | 15 |
| Male | 29 | 14 | 9 | 6 |
| Female | 39 | 25 | 5 | 9 |
| Median age (years) | 73 (31–93) | 73 (31–92) | 66 (41–87) | 82 (49–93) |
| Median follow-up (months) | 17.5 (1.5–78.2) | 15.3 (1.5–78.2) | 16.2 (4.2–72.5) | 21.3 (3.2–62.1) |
| Tumor type | ||||
| Early stage NSCLC | 53 | 27 | 13 | 13 |
| Metastatic disease | 15 | 12 | 1 | 2 |
| NSCLC Histology | ||||
| Adenocarcinoma | 28 | 18 | 5 | 5 |
| Squamous | 14 | 4 | 4 | 6 |
| NSCLC (NOS) | 11 | 5 | 4 | 2 |
Abbreviations: NSCLC: Non-small cell lung cancer; NOS: Not otherwise specified
Treatment related toxicities
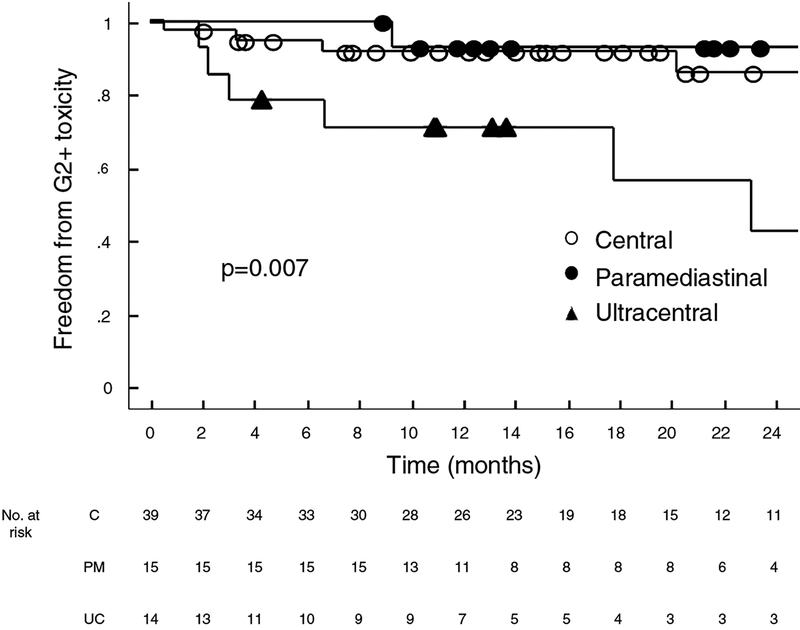
Two-year rate of grade 2+ toxicities were 57.6%, 14.2%, and 7.1% (p=0.007) for ultracentral, central, and paramediastinal tumors respectively (Figure 2). A total of 11 grade 2+ adverse events were recorded, eight of which were pulmonary related toxicities (radiation pneumonitis, post-obstructive pneumonia, and pleural effusion, respiratory failure); two other patients experienced chest wall pain and one patient experienced hoarseness which was attributed to recurrent laryngeal neuropathy. Two UC patients (14%) developed grade 3+ toxicity (one case of post-obstructive pneumonia and one case of grade 5 respiratory failure). One patient with a C tumor developed grade 3 toxicity. No patient with a PM tumor developed grade 3+ toxicity.
Figure 2:
Kaplan Meier estimates of freedom from Grade 2+ toxicities for central, paramediastinal, and ultracentral tumors in the entire cohort
Disease control and survival
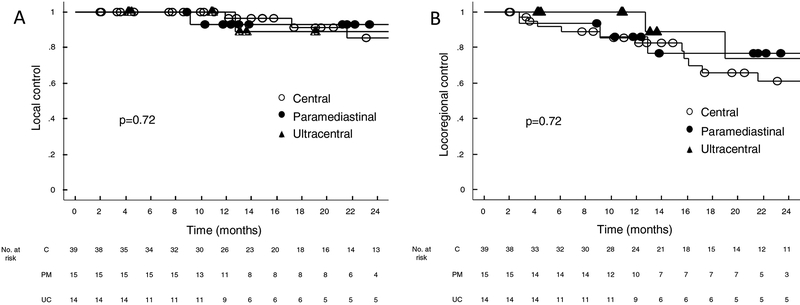
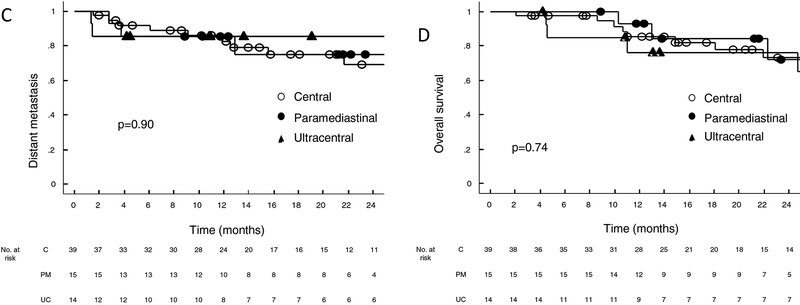
Two-year estimates of LC (89%, 85%, and 93%; p=0.72), LRC (74%, 61%, and 77%; p=0.72), and DC (86%, 69%, and 75%; p=0.90) were similar for the UC, C, and PM cohorts (Figure 3). Two-year and five-year estimates of OS were also similar (2-year: 76%, 73%, and 72%; 5-year: 33%, 22%, and 36%; p=0.74). Twenty-four patients had died at the time of analysis, six of whom were due to cancer progression. When analyzing outcomes for the early stage NSCLC subset separately, we also saw no difference in 2 year LC (88%, 80%, 92%; p=0.86), LRC (73%, 64%, 92%; p=0.46), DC (92%, 81%, 92%; p=0.65), and OS (74%, 72%, 76%, p=0.58) for the UC, C, and PM cohorts respectively.
Figure 3a–d:
Kaplan Meier estimates of a) local control, b) locoregional control c) distant control and d) overall survival for central, paramediastinal, and ultracentral tumors in the entire cohort
Dosimetry
Prescription doses and fractionation schedules for each cohort are shown in Table 2. UC tumors consistently received higher maximal point doses to centrally located critical structures. The median maximum point dose to the PBT was 55.9 Gy (range: 46.5–67.9) for UC tumors as compared to 29.0 (range: 2.1–51.2) for central and 12.5 (range: 2.1–28.6) for PM tumors (p<0.001). Point doses to the great vessels and esophagus were also significantly higher for UC tumors despite similar prescription doses to the targets. Maximal point doses for each cohort to the PBT, great vessels, esophagus, and heart are shown in Table 3. Dosimetric data were confirmed to follow a normal distribution with Kolmogorov-Smirnov test. Higher maximal central airway dose was correlated with increased risk of grade 2+ pulmonary toxicity (p = 0.03).
Table 2.
Fractionation schedules used for each cohort
| Total dose/fractions | Central | Ultracentral | Paramediastinal |
|---|---|---|---|
| 40 Gy/8 | 1 | 0 | 0 |
| 40 Gy/5 | 0 | 1 | 0 |
| 48 Gy/4 | 0 | 0 | 1 |
| 50 Gy/5 | 28 | 9 | 10 |
| 50 Gy/4 | 2 | 0 | 0 |
| 54 Gy/3 | 0 | 0 | 1 |
| 55 Gy/5 | 4 | 0 | 1 |
| 56 Gy/8 | 1 | 3 | 0 |
| 60 Gy/8 | 1 | 1 | 0 |
| 60Gy/5 | 2 | 0 | 2 |
Table 3.
Dosimetric summary
| Airway MPD (Gy) | Great vessel MPD (Gy) | Esophagus MPD (Gy) | Heart MPD (Gy) | |
|---|---|---|---|---|
| Central | 28.95 | 38.12 | 18.20 | 21.22 |
| Ultracentral | 55.91 | 54.33 | 26.96 | 31.81 |
| Paramediastinal | 12.46 | 40.61 | 20.53 | 29.13 |
| p-value | <0.001 | 0.0074 | 0.0053 | 0.1194 |
We compared dosimetric data from our patients against the dose constraints from the RTOG/NRG 0813 protocol, which limited the maximal point dose to the PBT, heart, esophagus, and great vessels to 105% of PTV prescription dose. Ten UC patients in our cohort (71%) exceeded the RTOG/NRG 0813 PBT constraint with a mean difference of 4.6 Gy above the 105% specification. The number of patients exceeding great vessels, esophagus, and heart constraints are 21, 0, and 4 respectively.
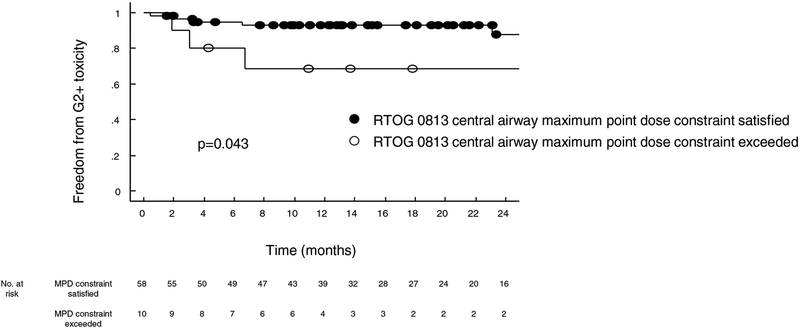
We then compared patient’s risk of pulmonary toxicity based on whether the dose volume histogram complied with the RTOG/NRG 0813 PBT constraint. All patients were separated into two groups: those who satisfied and those who did not. Among eight cases of grade 2+ pulmonary toxicities, 3 (38%) did not comply with the PBT constraint. Analysis of these two groups revealed increased rate of pulmonary toxicity at 3 years (31.4% versus 12.4%, p=0.04) in patients whose maximal point dose to the PBT exceeded 105% PTV prescription dose (Figure 4).
Figure 4:
Freedom from grade 2+ toxicity stratified by central airway maximum point dose for those cases that did and did not meet the RTOG/NRG 0813 constraint of 105% of the prescription dose
Discussion
SBRT is a standard and effective treatment modality for patients with medically inoperable, early stage NSCLC. Past studies have shown that SBRT is well tolerated in this generally frail patient population. Recorded rate of grade 3 and 4 toxicities on RTOG 0236 were 12.7% and 3.6% respectively and comparable results were reported in several other studies [1, 8–10]. However, these results were primarily derived from treatment of peripheral tumors, and optimizing the risk-benefit ratio for SBRT to more centrally located tumors remains an area of active investigation.
Different definitions of centrality between studies have made direct comparisons between published studies challenging. Concerns surrounding the toxicity of SBRT for centrally located tumors was first noted in the widely cited Indiana University phase II study, which identified an eleven-fold increased risk of severe toxicity for central tumors, defined as those located within 2 cm of the PTB, with use of a three-fraction SBRT schedule to 60–66 Gy [6]. Subsequent prospective efforts in central tumors have focused on more protracted schedules [11–15]. The largest prospective trial to evaluate the safety of SBRT for centrally located early stage NSCLC is RTOG/NRG 0813, which employed a 5-fraction regimen with a dose escalation up to 60 Gy in 5 fractions. Results have been presented only in abstract form, with a Bayesian-based probability of dose limiting toxicity (grade 3+ within 12 months of treatment) of 7.2% for the highest dose arm, with grade 3+toxicity beyond one-year noted in an additional 5 of 33 patients [5, 6]. Importantly, RTOG/NRG 0813 included patients with tumors defined as central by virtue of PTV abutment or overlap of the mediastinal pleural, and it is uncertain how the inclusion of these patients affected rates of observed toxicity.
The Nordic HILUS trial, presented in abstract form only, enrolled 74 patients with tumors located within 1 cm of the PBT to a phase II study delivering 56 Gy over 8 fractions. Twenty-one patients (28%) developed grade 3–5 toxicity, including 7 (9.5%) with fatal events, primarily hemoptysis [16]. A detailed dosimetric analysis has not been presented, but the results contrast with the relatively favorable toxicity profile noted in RTOG/NRG 0813, suggesting the more stringent criteria for centrality may have played a role.
More protracted regimens using hypofractionated radiation therapy for non-surgical candidates instead of SBRT have been tested with relatively promising results. Examples include CALGB 39904 and NCIC CTG BR.25. [19–20]. Both trials demonstrated superior outcome to historical standard fractionation data. The CALGB 39904 regimen delivered 70 Gy in 17–29 fractions and reported a 77% major response rate (31% complete response and 46% partial response), 38.5 months median overall survival, 28.6 months median progression-free survival, and 3 cases of grade 3 toxicity. Similarly, NCIC CTG BR.25 identified favorable results with 82.7% primary tumor response rate at 3 years and 41.4 months median overall survival. Toxicities rates appeared higher in the NCIC trial which found 26 cases of grade 3, five grade 4, and one grade 5 (pulmonary hemorrhage) adverse event. These trials, however, used 3D-CRT as the radiation planning approach, and did not specifically evaluate centrally located tumors. To date, no completed randomized studies have compared modestly hypofractionated regimens using highly conformal techniques to SBRT for early stage NSCLC. A drawback to modestly hypofractionated approaches is that the BED10 is typically less than 100 Gy, and data have suggested that regimens with a BED below this threshold may lead to lower rates of in-field control [8].
The concept of the UC tumor has recently gained additional attention with the understanding that the risk profile among centrally located lesions varies greatly. UC patients, with PTVs overlapping the PBT or esophagus, constitute a unique subset among those with centrally located lung tumors. Song et al. [17] reported increased toxicities in patients with tumors located in the bronchus. Among six patients with bronchial tumors in this study, three patients developed partial bronchial strictures and three developed complete strictures. There were three events of grade 3 or higher toxicity; one patient with complete bronchial obstruction had associated airway bleeding and from secondary aspiration pneumonia following pneumonectomy performed to control the bleeding.
In contrast, Chaudhuri et al. [14] reported similar rate of toxicity between patients with peripheral, central, and ultracentral (defined as GTV abutting the central airway) lung tumors. Specifically, they did not observe any grade 2 or higher toxicities for seven patients with ultracentral tumors using 50 Gy in 4 to 5 fractions. Two years overall survival and local control were equal between ultracentral, central, and peripheral tumors.
Similarly, Princess Margaret Cancer Center recently reported their results on treating 26 ultracentral lung tumors, which were defined as lesions with PTV overlapping the PBT, trachea, pulmonary vein/artery, and esophagus [18]. SBRT to UC lung tumors were found to be safe and effective with similar rates of grade 2+ toxicity, LC, and OS compared to C lung tumors. No grade 4–5 toxicities were recorded.
In our single-institution experience, we identified an increased rate of grade 2 and higher toxicities associated with SBRT for UC tumors, including 1 case of fatal respiratory failure. The majority of recorded toxicities were pulmonary (radiation pneumonitis, post-obstructive pneumonia, pleural effusion, respiratory failure). Differences between rates of toxicities are likely attributed to the higher radiation dose to the PBT in the UC patients as 71% of these patients exceeded RTOG/NRG 0813 maximal point dose constraint. However, the rate of grade 3+ toxicity for UC tumors remained relatively low at 14% in this challenging patient population.
In conclusion, our study indicates that SBRT for UC lung tumors results in similar tumor control and OS compared to more peripheral lesions. However, our results also suggest a greater risk of toxicity, including fatal toxicity, for these patients which warrants careful patient counseling and shared decision making. Several limitations of our study include its retrospective nature, small sample size of the UC and PM cohorts, and heterogeneous fractionation schedules. Given the small sample size, comparisons between groups should be validated in larger, preferably prospective cohorts. Further investigations are needed to establish the optimal treatment approach and fractionation schedules for UC lung tumors.
Acknowledgements:
Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number K12CA138464 (M.E.D). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in part at the 2016 World Conference on Lung Cancer in Vienna, Austria
Disclosures/Conflicts of Interest:
M. Daly: Research Funding, EMD Serono
A. Monjazeb: Research Funding, Incyte, Transgene, Genentech,
REFERENCES
- 1.Timmerman R, et al. , Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA : the journal of the American Medical Association, 2010. 303(11): p. 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmerman R, et al. , Excessive Toxicity When Treating Central Tumors in a Phase II Study of Stereotactic Body Radiation Therapy for Medically Inoperable Early-Stage Lung Cancer. Journal of Clinical Oncology, 2006. 24(30): p. 4833–4839. [DOI] [PubMed] [Google Scholar]
- 3.Fakiris AJ, et al. , Stereotactic Body Radiation Therapy for Early-Stage Non-Small-Cell Lung Carcinoma: Four-Year Results of a Prospective Phase II Study. International Journal of Radiation Oncology, Biology, Physics. 75(3): p. 677–682. [DOI] [PubMed] [Google Scholar]
- 4.Corradetti MN, Haas AR, and Rengan R, Central-Airway Necrosis after Stereotactic Body-Radiation Therapy. New England Journal of Medicine, 2012. 366(24): p. 2327–2329. [DOI] [PubMed] [Google Scholar]
- 5.Bezjak A, et al. , Efficacy and Toxicity Analysis of NRG Oncology/RTOG 0813 Trial of Stereotactic Body Radiation Therapy (SBRT) for Centrally Located Non-Small Cell Lung Cancer (NSCLC). International Journal of Radiation Oncology, Biology, Physics. 96(2): p. S8. [Google Scholar]
- 6.Bezjak A, et al. , Primary Study Endpoint Analysis for NRG Oncology/RTOG 0813 Trial of Stereotactic Body Radiation Therapy (SBRT) for Centrally Located Non-Small Cell Lung Cancer (NSCLC). International Journal of Radiation Oncology, Biology, Physics. 94(1): p. 5–6. [Google Scholar]
- 7.Kong F-M, et al. , Consideration of Dose Limits for Organs at Risk of Thoracic Radiotherapy: Atlas for Lung, Proximal Bronchial Tree, Esophagus, Spinal Cord, Ribs, and Brachial Plexus. International journal of radiation oncology, biology, physics, 2011. 81(5): p. 1442–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onishi H, et al. , Hypofractionated Stereotactic Radiotherapy (HypoFXSRT) for Stage I Non-small Cell Lung Cancer: Updated Results of 257 Patients in a Japanese Multi-institutional Study. Journal of Thoracic Oncology, 2007. 2(7, Supplement 3): p. S94–S100. [DOI] [PubMed] [Google Scholar]
- 9.Taremi M, et al. , Stereotactic Body Radiotherapy for Medically Inoperable Lung Cancer: Prospective, Single-Center Study of 108 Consecutive Patients. International Journal of Radiation Oncology*Biology*Physics, 2012. 82(2): p. 967–973. [DOI] [PubMed] [Google Scholar]
- 10.Videtic GMM, et al. , Stereotactic Body Radiation Therapy-Based Treatment Model for Stage I Medically Inoperable Small Cell Lung Cancer. Practical Radiation Oncology, 2013. 3(4): p. 301–306. [DOI] [PubMed] [Google Scholar]
- 11.Xia T, et al. , Promising Clinical Outcome of Stereotactic Body Radiation Therapy for Patients with Inoperable Stage I/II Non–Small-Cell Lung Cancer. International Journal of Radiation Oncology, Biology, Physics, 2006. 66(1): p. 117–125. [DOI] [PubMed] [Google Scholar]
- 12.Lagerwaard FJ, et al. , Outcomes of Risk-Adapted Fractionated Stereotactic Radiotherapy for Stage I Non–Small-Cell Lung Cancer. International Journal of Radiation Oncology*Biology*Physics, 2008. 70(3): p. 685–692. [DOI] [PubMed] [Google Scholar]
- 13.Haasbeek CJA, et al. , Outcomes of Stereotactic Ablative Radiotherapy for Centrally Located Early-Stage Lung Cancer. Journal of Thoracic Oncology, 2011. 6(12): p. 2036–2043. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhuri AA, et al. , Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer, 2015. 89(1): p. 50–56. [DOI] [PubMed] [Google Scholar]
- 15.Chang JY, et al. , Stereotactic Body Radiation Therapy in Centrally and Superiorly Located Stage I or Isolated Recurrent Non–Small-Cell Lung Cancer. International journal of radiation oncology, biology, physics, 2008. 72(4): p. 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindberg K, et al. , OA24.05 The Nordic HILUS-Trial - First Report of a Phase II Trial of SBRT of Centrally Located Lung Tumors. Journal of Thoracic Oncology. 12(1): p. S340. [Google Scholar]
- 17.Song SY, et al. , Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer, 2009. 66(1): p. 89–93. [DOI] [PubMed] [Google Scholar]
- 18.Yau V, et al. , MA 09.02 Ultra-Central Tumors Treated with Stereotactic Body Radiotherapy: A Single Institutional Experience. Journal of Thoracic Oncology. 12(11): p. S1835. [Google Scholar]
- 19.Bogart JA, et al. (2010). “Phase I Study of Accelerated Conformal Radiotherapy for Stage I Non–Small-Cell Lung Cancer in Patients With Pulmonary Dysfunction: CALGB 39904.” Journal of Clinical Oncology. 28(2): 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung P, et al. (2014). “Phase II Study of Accelerated Hypofractionated Three-Dimensional Conformal Radiotherapy for Stage T1–3 N0 M0 Non–Small Cell Lung Cancer: NCIC CTG BR.25.” JNCI: Journal of the National Cancer Institute. 106(8): dju164–dju164. [DOI] [PubMed] [Google Scholar]