Abstract
Organoid culture has had a significant impact on in vitro studies of the intestinal epithelium; however, the exquisite architecture, luminal accessibility, and lineage compartmentalization found in vivo has not been recapitulated in the organoid systems. We have used a microengineered platform with suitable extracellular matrix contacts and stiffness to generate a self-renewing mouse colonic epithelium that replicates key architectural and physiological functions found in vivo, including a surface lined with polarized crypts. Chemical gradients applied to the basal–luminal axis compartmentalized the stem/progenitor cells and promoted appropriate lineage differentiation along the in vitro crypt axis so that the tissue possessed a crypt stem cell niche as well as a layer of differentiated cells covering the luminal surface. This new approach combining microengineered scaffolds, native chemical gradients, and biophysical cues to control primary epithelium ex vivo can serve as a highly functional and physiologically relevant in vitro tissue model.
Keywords: intestinal epithelial stem cells, differentiation, intestine-on-a-chip, microfabrication, gradient, tissue mimics
Graphical Abstract

INTRODUCTION
The interior surface of the colon consists of tubular invaginations from the luminal wall that are termed crypts. These microscopic features are organized as an array of individually polarized epithelial tissues in which proliferative stem and progenitor cells are located near the crypt base, while their progeny differentiate as the cells migrate to the luminal surface.1 This polarized architecture is believed to be maintained by biochemical gradients along the crypt axis as well as biophysical cues in the supporting stroma. Gradients of growth signals (Wnt-3A, Notch, BMP), microbial metabolites (e.g., short-chain fatty acids), and other molecules (e.g., oxygen, glucose) are known to span the crypt long axis from the luminal region to the basal region.2 Only relatively recently has a sustainable in vitro culture of primary intestinal epithelium been realized by embedding isolated crypts or stem cells within a soft matrix (Matrigel), which in the presence of supporting growth factors (Wnt-3A, R-spondin 1, and noggin) form spheroids termed organoids.3,4 Organoids form an enclosed lumen surrounded by a monolayer of cells that possess a luminal-to-basal polarity. The organoids contain proliferating stem cells and all of the differentiated cell lineages found in the intestinal epithelium in vivo. As a result of these attributes, organoid culture has had a substantial impact on studies of the intestinal epithelium.5,6
Despite their great potential, organoid cultures have attributes that limit their use as an experimental platform. Organoids spontaneously produce buds that tend to be rich in stem and proliferative cells and show some characteristics of the stem cell niche.3 Nevertheless, these budding structures do not recapitulate the architecture of the crypt epithelium and do not mimic the lineage compartmentalization and cell migration found in vivo.7 There are also limitations on the manner in which organoids can be interrogated under experimental conditions. Because of the properties of the surrounding matrix and the absence of chemical gradients, organoids form with their luminal compartment inside the spheroid. Additionally, as the organoids are cultured embedded within a Matrigel paddy, exposure of the cells requires addition of reagents to the support medium overlying the Matrigel. The agents must then diffuse varying distances through the Matrigel to reach the organoids, which are located at different depths within the paddy. Because of the time required for diffusion and the potential for reagent adsorption by the hydrogel constituents, it is not possible to precisely control the timing or concentration of cell exposure to the reagent. Furthermore, exposure of the organoids to chemical gradients as found in vivo is not possible. It is for these reasons that organoids fail to recapitulate many of the critical features of the in vivo intestinal epithelium, limiting their use as an in vitro model in many investigations.3,5,8–10
Microfabrication technologies provide unique approaches to mimic in vivo tissue architecture and function by virtue of the ability to precisely control the tissue microenvironment both temporally and spatially. A number of microengineered physiological systems or organs-on-chips have been described.11–16 Among these systems, a few gut-on-a-chip models have mimicked the in vivo architecture and peristaltic contractions of the intestine.17–19 Nevertheless, to date these platforms still rely on the use of immortalized tumor cell lines (e.g., Caco-2), with a major limitation being that the cancer phenotype poorly reflects the normal intestinal physiology and the microarchitecture found in vivo. Wang and colleagues combined primary intestinal stem cell culture techniques with microfabricated scaffolds with the goal of building a living colonic epithelium for in vitro studies in a readily accessible and user-controlled microenvironment.20–22 Freshly isolated mouse colonic crypts were captured and embedded in Matrigel pockets in a polydimethylsiloxane (PDMS) microwell array to create a hybrid 2D/3D culture system with cryptlike structures that displayed distinct proliferative and nonproliferative regions.22 However, the luminal surface of the in vitro crypt microstructures remained inaccessible, the geometry of the in vivo crypt was poorly replicated, and the epithelial cells growing on the PDMS did not possess proper luminal-to-basal polarity. The use of the artificial PDMS substrate in this system likely failed to provide the proper extracellular matrix contacts and stiffness needed to replicate all features of in vivo crypts.
In the current work, microdevices with a collagen hydrogel scaffold of the appropriate stiffness and composition were developed to support the proliferation of colonic epithelial stem cells in a monolayer format rather than as an organoid. The collagen scaffold was then microformed into a multiwell array with dimensions similar to those of the murine colon crypt. This platform acted to guide the expansion of both proliferating and differentiating cells, essentially folding the in vitro tissue into an open-lumen, cryptlike architecture. Finally, the crypt mimics were polarized along their long axis by imposing chemical gradients of the principal factors believed to be responsible for the in vivo polarization of the colonic crypt. The in vitro crypts generated in this manner recapitulated key in vivo epithelial attributes not supported by organoid culture systems, including cell compartmentalization, crypt architecture, and luminal accessibility.
EXPERIMENTAL SECTION
Isolation of Crypts from Mouse Colon
Crypts were isolated from wild-type and CAG-DsRed/Sox9-EGFP mice (6–9 weeks old) by soaking the excised colon in a chelating buffer (2.0 mM EDTA and 0.5 mM DTT) for 75 min followed by vigorous shaking.21,23 The crypts were used within 30 min of isolation. The mice were fed with a standard mouse chow (Envigo 2920× Teklad global soy protein-free extruded). Male mice were used. The Sox9-EGFP mice were crossed to CAG-DsRED mice to generate mice with the dual reporter gene CAG-DsRed/Sox9-EGFP. All of the mice were on a C57Bl6 genetic background. All of the experiments were performed in compliance with the relevant laws and institutional guidelines at the University of North Carolina at Chapel Hill (UNC). All of the experiments and animal usage were approved by the Institutional Animal Care and Use Committee (IACUC) at UNC under protocol no. 13-200.
Matrigel-Embedding Organoid Culture
Isolated crypts were embedded in Matrigel for organoid culture according to previous publications.21,23,24 Briefly, 10 000 crypts were suspended in 50 μL of cold Matrigel (Corning, no. 356235), and then a 5 μL suspension was added to each well of a 96-well plate. After gelation of the Matrigel at 37 °C for 15 min, 150 μL of stem medium (SM) was added to each well. The medium was changed every 48 h. SM was prepared from a mixture of advanced DMEM/F12 medium, Wnt-3A-conditioned medium, R-spondin 2-conditioned medium, and noggin-conditioned medium with final Wnt-3A, R-spondin 2, and noggin concentrations of 30, 75, and 71 ng/mL, respectively, supplemented with fetal bovine serum (10%), GlutaMAX (1×), B27 serum-free media supplement (1×), HEPES (10 mM), N-acetylcysteine (1.25 mM), nicotinamide (10 mM), murine EGF (50 ng/mL), gastrin (10 nM), prostaglandin E2 (10 nM), A83-01 (500 ng/mL), penicillin (100 units/mL), streptomycin (100 μg/mL), and gentamicin (50 μg/mL). The detailed protocol to prepare the media and their formulations are described in the Supporting Information (Tables S1 and S2). Y-27632 was used only for the first 48 h. Every 3–4 days, the organoids were dissociated with Accutase (Stemcell Technologies, no. 07920) and passaged to a new 96-well plate at a ratio of 1:3.
The Wnt-3A-, R-spondin 2-, and noggin-conditioned media were prepared following a published protocol.21 The conditioned media were produced by growing tissue-cultured cell lines transfected with the genes for Wnt-3A, R-spondin 2, and noggin. The cell lines secrete the growth factors into the media, which are then harvested for use with the primary intestinal cells as described in previous publications.24,25 The factors were used in a Wnt-3A:R-spondin ratio of 1:2.5 (ng/mL:ng/mL) in this work, following accepted practice in the intestinal stem cell community (i.e., in the range of 1:2–1:5 ng/mL:ng/mL when recombinant Wnt-3A and R-spondin proteins are used).24,26–28
Monolayer Culture on a Neutralized Collagen Hydrogel
Neutralized collagen hydrogels (1 mg/mL) were prepared in a six-well plate by neutralizing rat tail collagen (in 0.02 N acetic acid, Corning, no. 356236) with sodium hydroxide, HEPES, sodium bicarbonate, and phosphate-buffered saline (PBS).29 Isolated crypts or monolayer fragments were placed on top of the collagen hydrogel at a density of 1000 crypts/cm2 and cultured in 4 mL of SM. The medium was changed every 48 h. When the cell coverage was greater than 80% (typically after 3–4 days of culture), the monolayers were detached with collagenase (type IV, Worthington Biochemical, no. LS004189), dissociated with EDTA (0.5 mM), and subcultured on a new collagen hydrogel at a passage ratio of 1:3. The detailed protocols for preparation of neutralized collagen hydrogels and passage of monolayers are provided in the Supporting Information.
Lineage Differentiation
Lineage differentiation was performed using a method adapted from that published previously.30 Cells were cultured in monolayers or as organoids in SM for 48–72 h and then exposed to differentiation medium (DM) to initiate cell differentiation for 48–72 h. DM did not possess Wnt-3A or R-spondin, and its formulation is listed in Tables S1 and S2 in the Supporting Information. DM was changed every 24 h. IWP-2 (abbreviated as “I”, 2 μM), sodium butyrate (abbreviated as “B”, 0.5 mM), and γ-secretase inhibitor LY-411575 (abbreviated as “G”, 10 μM) were added as indicated to induce differentiation into either absorptive colonocytes or mucus-producing goblet cells.
Micromolding/Cross-Linking of Collagen Scaffolds on a Modified Transwell Insert
To construct the modified insert, the polycarbonate porous membrane was removed from a commercially available Transwell insert (Corning, no. 3401) using sandpaper. Next, a hydrophilic PTFE porous membrane (Millipore, no. BGCM00010) was attached to the insert using a biocompatible transfer adhesive (3M, no. 1504XL). To reduce the effective area of the porous membrane from 12 mm diameter to 3 mm diameter, the back side of the porous membrane was blocked by attaching a nonpermeable cyclic olefin copolymer (COC) plastic film (TOPAS Advanced Polymers, 2 mil thick).
PDMS stamps were used to micromold collagen scaffolds with an array of microwells (diameter = 75 μm, height = 250 μm). The stamps were fabricated by photolithography and soft lithography. The microfabrication process and surface modification of PDMS stamps are described in the Supporting Information.
Collagen was prepared to form the hydrogel scaffold by EDC/NHS cross-linking chemistry.31–33 A collagen solution (type I, rat tail, Corning, no. 356236) was first lyophilized for 72 h to remove water and acetic acid. The lyophilized collagen was redissolved in 2-(N-morpholino)ethanesulfonic acid (MES) buffer (0.1 M, pH 5) at a concentration of 5 mg/mL. The modified Transwell inserts were placed into a 12-well plate. A mixture of 4 mg/mL collagen (in MES buffer), 60 mM 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride (EDC), and 15 mM N-hydroxysuccinimide (NHS) in MES buffer (0.1 M, pH 5) was prepared in a conical tube on ice, and the mixture was homogenized by repetitive pipetting (40×). The trapped air bubbles in the mixture were removed by centrifugation at 3000g for 1 min. The collagen mixture (50 μL) was added to the center of the insert, and a PDMS stamp was placed on top of the mixture. [Note: The mixture starts to gel within 5 min upon mixing of the collagen with EDC and NHS. Thus, the above steps must be completed within 5 min.] To remove the trapped air bubbles among the microposts of the PDMS stamp, the 12-well plate was placed inside an airtight container (Dental Planet, no. 448PP) and pressurized to 40 psi using nitrogen for 2 h. After removal of the 12-well plate from the container, the PDMS stamp was demolded from the collagen scaffold. The collagen scaffold was incubated in 2 L of deionized water for 3 h to remove unincorporated EDC/NHS. The scaffolds were sterilized with 75% ethanol for 5 min, rinsed with PBS for 5 min three times, and stored in PBS at 4 °C until use. The total thickness of the collagen layer was 310 μm. Thus, the 250 μm-deep microwells possessed a 60 μm-thick layer of gel underneath the wells. This was the thinnest layer of collagen that we could reproducibly mold.
Generating in Vitro Colon Crypts by Expanding Cells on the Scaffolds Followed by Application of a Gradient of Wnt-3A and R-spondin
Prior to plating of cells on the micromolded collagen scaffold, the scaffold was incubated with 2 mL of PBS containing 10 μg/mL rat tail collagen at 37 °C overnight. The scaffold was then rinsed with 2 mL of PBS. Primary cells growing as a monolayer on neutralized collagen were detached from the collagen by incubation with collagenase when the monolayers reached >80% confluence. The monolayer was fragmented by incubation with EDTA (0.5 mM in PBS buffer) combined with mechanical agitation. The cells were then plated on the top surface of the molded, cross-linked collagen in the modified insert. Cells from one well of the six-well plate were dispersed onto four separate inserts. The cells were cultured in 3 mL of SM per insert (1 mL in the top compartment and 2 mL in the bottom compartment), and the medium was exchanged every 24 h. Once the cells covered the entire surface of the scaffolds (typically ~48 h), a gradient of growth factors was applied across the shaped collagen scaffold and cells. DM (0.5 mL) was placed into the top compartment, and SM (1.5 mL) was loaded into the bottom compartment. The DM and SM were replaced every 24 h to maintain a stable gradient across the z axis of the tissue. Typically, polarization of the mouse tissue was observed in 48 h under this chemical gradient condition.
EdU/ALP/Muc2/Nuclei Four-Color Staining Protocol
A four-color fluorescence staining protocol was used to reveal the proliferation/differentiation state of the cells on the same tissue (monolayers or in vitro crypt tissues on shaped collagen scaffolds). The cells were first pulsed with 5-ethynyl-2-deoxyuridine (EdU) and then sequentially stained using fluorescent markers for alkaline phosphatase (ALP), S-phase cells, mucin 2, and DNA. Cells were incubated with EdU (10 μM) in medium for 3 h at 37 °C. The living cells were then rinsed with PBS and incubated for 30 min at 37 °C with an ALP substrate (Vector Laboratories, no. SK-5100) in Tris buffer (0.15 M, pH 8.4). The cells were rinsed with PBS and fixed in 4% paraformaldehyde for 15 min, followed by permeabilization with 0.5% Triton X-100 in PBS for 20 min. The proliferative cells were stained using a Click-iT EdU Alexa Fluor 647 imaging kit (Thermo Fisher, no. C10340). The cells were then rinsed with 0.75% glycine in PBS for 5 min three times, followed by blocking with 10% donkey serum (Jackson Immunoresearch, no. 017-000-121) for 1 h at 20 °C. The cells were incubated in rabbit α-Muc2 (1:200, Santa Cruz, no. sc-15334) at 4 °C overnight and stained with donkey anti-rabbit IgG-conjugated Alexa Fluor 488 (1:500, Jackson Immunoresearch, no. 711-545-152) for 45 min at 20 °C. As the final step, the DNA was stained with Hoechst 33342 (2 μg/mL, Sigma-Aldrich, no. B2261) for 15 min at 20 °C.
Cryosectioning, Immunofluorescence Staining, Transmission Electron Microscopy, Scanning Electron Microscopy, and Confocal Microscopy
Thin sections (10 μm thickness) of monolayers or in vitro crypts were prepared on a cryostat. Immunofluorescence (IF) staining of the cells was performed using the same protocol as for Muc2 staining described above. The primary antibody was rabbit α-Sox9 (1:500, Millipore, no. AB5535). Actin was stained with Alexa Fluor 488 phalloidin (Thermo Fisher, no. A12379). The ultrastructural details of the monolayer were visualized by transmission electron microscopy (TEM) on a JEOL 100CX II microscope. The topographic features of monolayers were revealed by scanning electron microscopy (SEM) on a FEI Quanta 200 ESEM microscope. Confocal microscopy was performed using a confocal laser scanning microscope (Olympus, Fluoview FV3000) with laser-based excitation and emission wavelengths selected using a holographic transmission diffraction grating.
Modeling of the Chemical Gradient across the Scaffold
COMSOL Multiphysics (COMSOL Inc., Burlington, MA) was used to model the concentration profile of the growth factors Wnt-3a and R-spondin across the microwell scaffold using Fick’s law. The previously reported diffusion coefficient of 40 kDa fluorescein–dextran through Matrigel (7.4 × 10−11 m2/s) was used in the simulation since the molecular weight is similar to those of these growth factors (Wnt-3A, 39.7 kDa; R-spondin, 40.0 kDa).34 The reservoirs were assumed to act as an infinite, well-mixed source and sink. The COMSOL simulation was validated by experimentally measuring the fluorescein–dextran concentrations in both the luminal and basal reservoirs at 24, 48, and 72 h and comparing the experimental data to the model’s output.
Image Acquisition, Analysis, and Statistics
Specimens were imaged using a Nikon Eclipse TE300 inverted epifluorescence microscope equipped with DAPI/FITC/Texas Red/CY5 filter sets. Images were acquired at randomly selected locations within a specimen using a 10× (N.A. = 0.3) or 4× (N.A. = 0.13) objective lens. S-phase cells stained with the Click-iT EdU stain were imaged using a CY5 filter (excitation filter 604–644 nm, emission 672–712 nm). ALP staining was imaged employing a Texas Red filter (excitation filter 542–582 nm, emission 604–644 nm). Muc2 immunofluorescence was visualized with a fluorescein filter (excitation filter 450–490 nm, emission 520 nm long-pass). DNA stained by Hoechst 33342 was identified using a DAPI filter (excitation filter 352–402 nm, emission 417–477 nm). Images were empirically thresholded using ImageJ (https://imagej.nih.gov/ij/). ImageJ was also used to quantitatively measure the percentage of the surface area of each image displaying suprathreshold fluorescence from the EdU, ALP, or Muc2 stains. The identified surface area was then normalized to the total cell area by dividing by the area of the Hoechst fluorescence (nuclei stain). All data utilized four randomly selected locations of the same monolayer (n = 4), and the average with standard deviation is shown unless otherwise specified.
To assess the polarity of the in vitro crypts, side views of the crypts were obtained by scraping the crypts from the scaffold using a tungsten dissecting needle (Roboz Surgical Instrument, no. RS-6063). Horizontally lying crypts were then imaged using standard fluorescence microscopy. The positions of proliferating cells (EdU+) along the basal–luminal crypt axis were obtained by evenly dividing the crypt into six regions. The EdU/nuclei fluorescence intensity ratio from each region was obtained by ImageJ. The EdU/Hoechst fluorescence ratio was then plotted against position along the basal–luminal crypt axis. Quantification was performed on 20 crypts.
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by a multiple comparison test with Tukey’s honestly significant difference procedure conducted at the 5% significance level. Multiple comparison testing was performed on all pairwise comparisons between experimental groups. ANOVA analyses and subsequent multiple comparisons were performed using MATLAB (MATLAB 2014b, The MathWorks, Inc., Natick, MA). In all figures, * denotes p < 0.05 and ** denotes p < 0.005.
Unless otherwise specified, the data shown for a single experiment utilized crypts obtained from or monolayers derived from a single mouse. Multiple experiments from different mice for all data were performed, and the results were consistent over time without outliers. Data from representative experiments are presented.
RESULTS AND DISCUSSION
Mouse Colon Crypts Lose Their Tissue-Level Characteristics in Organoid Culture
Mouse colon crypts possess three key architectural attributes: distinctive shape, cell compartmentalization, and luminal accessibility, i.e., an open rather than an enclosed lumen. In this highly polarized tissue, the proliferative cells (stem cells, progenitors, and transit-amplifying cells) are located at the blind-end or basal aspect of the crypts, while the differentiated cells (absorptive colonocytes, secretive goblet cells, and other nondividing cell types) are located predominantly at the open-end or luminal aspect of the crypts. This tissue polarity is demonstrated by immunofluorescence and enzymatic staining of the colon crypts freshly isolated from in vivo tissues: proliferative cells are marked by enhanced green fluorescent protein (EGFP) expression (Sox9-EGFP), while differentiated cells are marked by mucin 2 (Muc2) immunostaining (goblet cells) and alkaline phosphatase (ALP) activity (absorptive colonocytes) (Figure 1a). The highly polarized structure of colon crypts, including the segregated proliferation and differentiation zones, is readily visualized in freshly obtained tissue.
Figure 1.
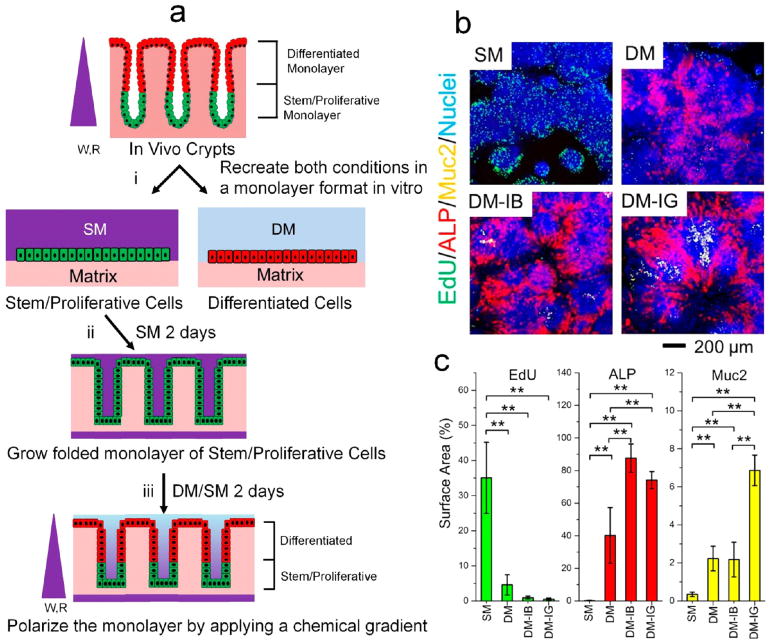
Culture of freshly isolated mouse colon crypts results in rapid loss of crypt shape, crypt polarity, and luminal accessibility. (a) Properties of a freshly isolated crypt. Shown in the left two panels are a schematic and an SEM image. The middle panel is a fluorescence image of a crypt from a Sox9-EGFP/CAG-DsRed mouse. The right two panels are a fluorescence image of mucin 2 (Muc2) immunostaining (red) and a bright-field image following incubation with a colorimetric substrate for alkaline phosphatase (ALP) (red). DNA within nuclei was stained with Hoechst 33342 (blue) in the Muc2 image. (b) A crypt embedded in Matrigel rapidly converts into an organoid when cultured in a growth-factor-rich medium (stem medium (SM)). The crypt lumen closes off, and Sox9-EGFP+ progenitor cells expand across the organoids. Colon crypts from CAG-DsRed/Sox9-EGFP mouse were used in this panel. (c, d) Forced lineage differentiation of mouse colon organoids by incubation in medium altering Wnt and Notch signaling intensity. Abbreviations: SM, stem medium; DM, differentiation medium; I, IWP-2; B, sodium butyrate; G, γ-secretase inhibitor LY-411575. (c) Schematic of the differentiation steps. Green, red, and blue indicate stem/proliferative cells, goblet cells, and absorptive colonocytes, respectively. (d) Fluorescence (left two columns) and bright-field (right column) images of organoids cultured under SM (for 3 days), DM-IB (3 days), or DM-IG (3 days). In the left two columns, EdU incorporation, Hoechst 33342 labeling, and Muc2 immunostaining are marked as green, blue, and red, respectively. In the right column, a colorimetric assay marks ALP in red. Scale bars = 100 μm.
The crypt properties are rapidly lost when crypts are embedded in Matrigel and cultured in a growth-factor-rich medium. The expansion of the basal proliferative cells and death of the luminal differentiated cell types from the isolated crypt transforms the structure into a hollow organoid with an enclosed luminal compartment surrounded by a single layer of cells (Figure 1b). Under these conditions, the organoids are predominantly composed of proliferative cells (EdU+) with a paucity of differentiated goblet cells (Muc2+) and colonocytes (ALP+) (Figure 1c,d, top panels). Cells within the organoids can be forced to differentiate by inhibition of Wnt signaling and titration of Notch signaling.30 To inhibit Wnt signaling, the cells are placed into a medium with minimal growth factors (differentiation medium (DM)) containing IWP-2 (abbreviated “I”), a Wnt inhibitor. Addition of butyrate (abbreviated “B”) to this medium induced cell differentiation toward absorptive colonocytes (ALP+), most likely through maintenance of Notch signaling (Figure 1c,d).35 In contrast, addition of a γ-secretase inhibitor (GSI, abbreviated “G”) increased the formation of goblet cells (Muc2+) by suppressing Notch signaling (Figure 1c,d).36 Notably, all of the initial crypt polarity with respect to stem-differentiated cells is lost in organoids under these conditions. Thus, the Matrigel-embedded organoids cultured failed to recapitulate the exquisite architectural features of shape, polarity, and open lumen of in vivo mouse colonic crypts.
Proliferative Monolayers of Murine Colonic Epithelium Can Be Grown on Both Neutralized and Cross-Linked Collagen Hydrogels
Since the in vivo crypt epithelium can be envisioned as an in-folded monolayer of cells, we initially sought to identify a matrix that could support a monolayer culture of stem and differentiated epithelial monolayers while having the potential to be shaped into an array of cryptlike features (Figure 2a). Wang et al.29 cultured mouse colon crypts from freshly obtained tissue on the surface of matrices possessing a range of stiffness and ECM properties that were suitable for microfabrication, including Matrigel, neutralized collagen hydrogen, PDMS, and polystyrene (Figure S1). With the exception of collagen, all of these surfaces were unable to support a self-renewing monolayer of murine colonic epithelium. A neutralized collagen hydrogel did support the proliferation of colonic epithelial cells as a monolayer possessing both stem and differentiated cells, likely because of the similar stiffness, porosity, and protein composition of collagen compared with the intestinal mucosa (Figures S1 and S2).29,37 However, long-term culture of cells on a soft material such as collagen (112 ± 37 Pa, n = 3) leads to collagen contraction due to mechanical forces imparted by the cells.38 Thus, microstructures fabricated from unmodified collagen are not suitable as a cell scaffold because of collagen deformation over time. For this reason, we also cultured crypts on a stiffer collagen surface, a cross-linked collagen hydrogel (8640 ± 77 Pa, n = 3).39 Cells cultured on the cross-linked collagen grew as a monolayer with similar numbers of proliferative cells as those grown on neutralized collagen (Figure S1c,d).
Figure 2.
Development of a monolayer from primary mouse colonic epithelial cells. (a) Schematic showing the strategy for in vitro crypt construction. (i) In vivo crypts were isolated and used to recreate in vitro monolayers of both stem/proliferative and differentiated cells. SM, stem medium; DM, differentiation medium. (ii) Stem/proliferative cells were grown into a folded monolayer by culturing the cells on scaffolds with an array of microwells. (iii) The in vitro-formed crypts were polarized to create differentiated-cell and stem/proliferative-cell regions by application of a gradient of Wnt-3A (W) and R-spondin (R). (b) Fluorescence images of 1.5 mm-sized regions of the monolayers cultured under different media. Cells were cultured in stem medium (SM) for 2 days followed by either SM or differentiation medium (DM, DM-IB, or DM-IG) for 2 days. The monolayers were assayed for incorporation of EdU (green, 3 h pulse), alkaline phosphatase (red, ALP), mucin 2 (yellow, Muc2), and DNA (blue, Hoechst 33342). Patterning of the various cells as described previously is apparent.29 (c) Percentages of the Hoechst-positive surface area displaying fluorescence from the EdU, ALP, or Muc2 stains. **, p < 0.005; *, p < 0.05.
Monolayers of Murine Colonic Epithelium Grown on Collagen Hydrogels Can Be Chemically Differentiated
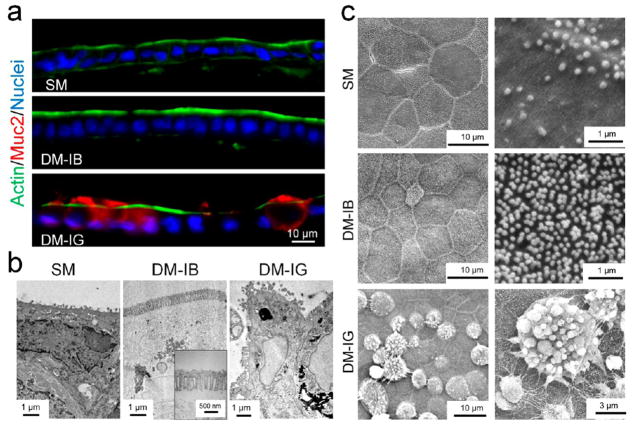
To reconstruct an array of crypts mimicking the colonic epithelium, the scaffold must support the growth of stem cells as well as the formation of differentiated cells. The most common differentiated cell types in the in vivo epithelium are absorptive colonocytes and mucous-secreting goblet cells.1 Absorptive cells express elevated levels of alkaline phosphatase with respect to other intestinal cells and possess a high density of microvilli on their luminal surface to enhance absorption of water/salt.40 In contrast, goblet cells express mucins, packaging them into vesicles for secretion onto the cell’s luminal surface.41 The monolayers grown under different culture conditions were assessed for the features of differentiated and undifferentiated cells: microvilli or their constituent proteins, alkaline phosphatase, mucins and secretory granules, Sox9 expression, and EdU incorporation. Monolayers cultured on collagen hydrogels in the presence of growth-factor-rich medium (SM) were composed predominantly of proliferative cells (EdU+) with an apical luminal surface having a low density of microvilli (5 ± 3 microvilli/μm2, n = 5 images) suggesting that they might be composed predominantly of stem or progenitor cells (Figures 2, 3b,c, and S3). This was supported by the presence of Sox9+ cells in the monolayers (Figure S2). Cross sections of the monolayer grown in SM demonstrated a single layer of polarized cells with actin highly concentrated at the luminal surface, suggesting that the cells also possessed a proper luminal-to-basal polarity (Figure 3a). When cells were cultured in medium with a paucity of growth factors (DM), EdU incorporation or cell proliferation was largely eliminated. Cells differentiated primarily into absorptive cells (increased ALP activity) and mucus-secreting goblet cells (increased mucin 2 expression) (Figures 2 and S3). Forced lineage allocation could be further guided by titrating Wnt and Notch signaling by addition of IWP-2 (I) and either butyrate (B) or the γ-secretase inhibitor LY-411575 (G), so that the monolayer differentiated toward predominately colonocytes in DM-IB medium and goblet cells in DM-IG medium (Figures 2, 3b,c, and S3). Cells in DM-IB possessed a high ALP activity and microvilli density (26 ± 2 microvilli/μm2) on their apical surfaces, suggesting that these were indeed colonocytes or absorptive cells (Figures 2b,c, 3b,c, and S3). In contrast, cells in DM-IG produced Muc2 and displayed secretory granules lining their apical surfaces, demonstrating the presence of goblet cells (Figures 2b,c, 3b,c, and S3). Cross sections of these monolayers also revealed appropriately polarized cells with actin (a component of the microvilli) concentrated along their luminal aspect (Figure 3a). Additionally, goblet cells displayed mucin 2 localized to the intracellular region (secretory granules) or luminal space (Figure 3a,c). The forced differentiation of the monolayers was similar to that observed in the organoids, suggesting that cells in the monolayer behaved in a similar fashion as those in the organoids in response to differentiation stimuli. These data supported that a collagen hydrogel in the presence of factor-supplemented media provided the essential physical and chemical cues to sustain a self-renewing epithelial monolayer that could be induced to differentiate in response to external cues.
Figure 3.
Properties of the chemically differentiated monolayer. (a) Fluorescence images of a 10 μm-thick cross section through the monolayer. Actin was stained using phalloidin (green), mucin was stained with anti-Muc2 antibody (red), and DNA within nuclei was labeled with Hoechst 33342 (blue). The monolayers were cultured in SM for 2 days, followed by either SM, DM-IB, or DM-IG for an additional 2 days. (b) Cross sections of the monolayers visualized by TEM. The monolayers were cultured under the same conditions as in (a). (c) Apical surface topography of mouse colonic monolayer inspected by SEM. The upper two panels show low- and high-magnification images of a monolayer cultured under SM for 4 days. Sparse microvilli are present. The middle two panels show low- and high-magnification images of a monolayer cultured under SM for 3 days followed by DM-IB for 3 days. The cells differentiated into enterocytes with a high density of microvilli on their apical surface. The bottom two panels show low- and high-magnification images of a monolayer cultured under SM for 3 days followed by DM-IG for 3 days. The cells possessed large secretory goblets characteristic of goblet cells.
A Patterned Microfabricated Collagen Hydrogel Enables Gradients To Be Applied across a Folded Monolayer To Create in Vitro Crypts
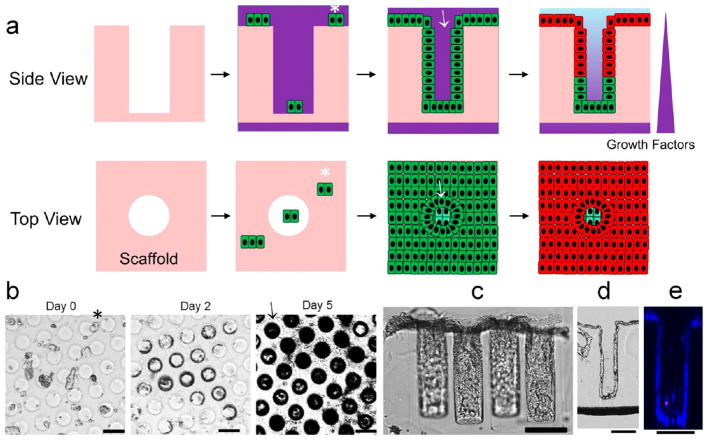
As collagen hydrogels were the most suitable matrices for culture of proliferative monolayers or differentiated mouse colonic epithelial cells, collagen hydrogels were molded on a porous membrane to form an array of high-aspect-ratio microwells with a size and shape similar to that of the colon crypts of mice (Figure 4a–g). The platform was composed of a micromolded collagen (possessing 530 microwells on an area of 7 mm2) in a modified 12-well Transwell insert (Figure 4a–f). The Transwell platform was chosen because it is user-friendly and widely used in biology laboratories. The platform also permits a gradient of chemicals to be formed across the collagen scaffold by placing media with different constituents in the basal and luminal reservoirs (Figure 4g–i). To determine the time span over which the basal and luminal reservoirs could act as an infinite source and sink to support a stable growth-factor gradient across the collagen scaffold, the movement of fluorescein–dextran across the scaffold/membrane was measured (Figure 4h). Fluorescein–dextran (40 kDa) was chosen since it is of similar molecular weight as Wnt-3A (39.7 kDa) and R-spondin (40.0 kDa). Additionally, a plastic film was used to reduce the available area for transport across the membrane from 113 mm2 (standard Transwell size) to 7 mm2 to minimize the area available for diffusion. At 0 h, the fluorescein–dextran concentrations were [luminal] = 0 μg/mL and [basal] = 100 μg/mL, and at 24 h, the concentrations were [luminal] = 3.4 ± 0.3 μg/mL and [basal] = 95.8 ± 2.7 μg/mL (n = 3). The experimental data were similar to those predicted by COMSOL simulation when the scaffold was modeled as a homogeneous collagen slab (Figure 4h). The model predicted that when 30 ng/mL Wnt-3A is loaded into the basal reservoir, [Wnt-3A] should be 17 ng/mL at the bottom of the microwells and then decrease linearly to 0 ng/mL at the top surface of the microwells (Figure 4i). These data suggested that a stable, linear chemical gradient was established across the collagen scaffold when the fluids in the basal and luminal reservoirs were replenished every 24 h.
Figure 4.
Micromolded collagen scaffold to support in vitro crypt formation. (a) Schematic of the components of the modified insert. The drawing is not to scale and is for illustrative purposes only. A nonpermeable COC film was attached to the back side of the porous membrane to reduce the transport area from 12 mm to 3 mm diameter. (b) Schematic of the micromolding process used to generate a shaped cross-linked collagen scaffold on a modified insert. (c) Image of the insert with shaped scaffolding. To demonstrate that trans-scaffold transport occurred only at the central area of the 3 mm diameter, the insert was placed on a 12-well plate and briefly exposed to 0.1% toluidine blue placed in the bottom compartment while PBS was loaded into the top compartment. The toluidine blue stained only the collagen within the central 3 mm-diameter area in contact with the basal compartment. (d) Top view of an array of microwells created in the collagen scaffold. (e) Geometry of the PDMS stamp that was used to micromold collagen. (f) SEM image of the stamp (left) and fluorescence microscopy image showing a side view of a fluorescein-labeled collagen scaffold (right). (g) Simulated model of the concentration of Wnt-3A (40 kDa molecule) across the scaffold when 0 and 30 ng/mL of the molecule are placed into the luminal and basal reservoirs, respectively. (h) Concentration of fluorescein–dextran (MW = 40 kDa) measured experimentally or predicted by the COMSOL model in the luminal (▲, experimental; △, simulation) and basal (■, experimental; □, simulation) reservoirs at 24, 48, and 72 h. At 0 h, 0.5 mL of PBS was added to the luminal reservoir and 1.5 mL of fluorescein–dextran (100 μg/mL) was added to the basal reservoir. (i) Concentration profile of Wnt-3A along the z axis of the scaffold predicted by the COMSOL model using the conditions in (h). On the x axis, the zero point marks the bottom of the microwell or crypt while the 250 μm point marks the luminal surface of the crypt.
Mouse colonic epithelial cells were expected to grow into a monolayer following the cryptlike topography of the hydrogel when the cells were cultured on the surfaces in SM (Figure 5a). As expected, the neutralized collagen scaffold did not possess adequate strength to withstand cell remodeling and tensile forces (Figure S4). The cross-linked collagen microwell scaffolds, on the other hand, had sufficient strength to support cell proliferation and to guide the monolayer without cell-induced deformation of the scaffolds (Figure 5b–e). The folded monolayer (hereinafter called an in vitro crypt) possessed crypt-shaped structures with an accessible luminal surface mimicking the tissue-level geometry of in vivo crypts (Figure 5c–e).
Figure 5.
Strategy to fold and polarize the monolayer of colonic epithelial cells. (a) Schematic showing how the microwell scaffold guides the folding of the cell monolayer into a cryptlike geometry, followed by application of a chemical gradient to polarize the crypt. Top row: side views of a slice through the array. Bottom row: top views looking down onto the array and into the crypts. Fragments of a precultured monolayer (indicated with *) were allowed to settle onto the scaffold, attach, proliferate, and spread to cover the entire scaffold surface, including lining the walls of the microwells (indicated by →). (b) Time-lapse images showing the propagation of cells across the surface of the microwell array. Microwells appeared darker in these bright-field images as their walls were lined with cells. (c) Side view of in vitro crypts. (d) Bright-field image of a 10 μm-thick cryosection through an in vitro crypt demonstrating the open lumen and a monolayer of cells covering the microwell wall. (e) A z slice through an in vitro crypt obtained by confocal fluoresecence imaging. The cells were stained with Hoechst 33342 (blue) and propidium iodide (red). Scale bars = 100 μm.
Polarization of in Vitro Colon Crypts Can Be Induced under the Influence of a Biochemical Gradient
When cultured in SM, the proliferative cells (EdU+) on the folded monolayer were randomly distributed along the axis of the microwells, i.e., the average position along the basal–luminal axis was at half the height of the microwells (Figure 6a,b). To induce polarity, a gradient of Wnt-3A and R-spondin was applied along the basal–luminal axis by adding DM to the luminal compartment and SM to the basal compartment. Under this Wnt-3A/R-spondin gradient, proliferative cells (EdU+) were suppressed on the luminal aspect of the in vitro crypts and EdU+ cells were localized to the crypt base (Figure 6a,b). Thus, a simple linear gradient of Wnt-3A and R-spondin was sufficient to polarize the in vitro crypts, creating basal proliferative and luminal non-proliferative cell compartments.
Figure 6.
Formation of polarized in vitro colon crypts. (a) Crypts were not polarized in the absence of a gradient of Wnt-3A and R-spondin (top row), while crypts were polarized in the presence of a gradient of Wnt-3A (0 ng/mL on the luminal side and 30 ng/mL on the basal side) and R-spondin (0 ng/mL on the luminal side and 75 ng/mL on the basal side) for 2 days (bottom row). Left column: projected views of confocal slices through in vitro crypts so that the entirety of each crypt is superimposed onto a plane in the microscopy image (see the schematic of the top view in Figure 5a). Right column: side views of crypts that were detached from the scaffold and then placed on their sides for imaging. L, luminal; B, basal. Green = EdU, blue = DNA. (b) Box plots depicting the relative positions of EdU+ cells along the basal–luminal axis of the crypts. Zero represents the basal end of the crypt, while 1 marks the luminal end of the crypt. (c–e) Effect of gradient steepness on the location of EdU+ cells within the crypt. On the luminal side, [Wnt] = 0 ng/mL and [R-spondin] = 0 ng/mL. On the basal side, varying [Wnt-3a] and [R-spondin] were used: [Wnt-3a]:[R-spondin] = 60:150, 30:75, 15:37, and 7:18 (ng/mL:ng/mL). (c) Side views of crypts removed from the scaffold and imaged lying on their sides. Shown are representative crypts under different gradient steepness conditions. (d) Box plots showing the relative positions of EdU+ cells along the basal–luminal axis of the crypts. On the y axis, the zero point marks the bottom of the microwell or crypt while the 1.0 point marks the luminal surface of the crypt. (e) Box plots representing the number of EdU+ cells per crypt. n ≥ 20 crypts were quantified for (b), (d), and (e). **, p < 0.005; *, p < 0.05. Scale bars = 100 μm.
To determine the optimal slope of the gradient required to polarize the in vitro crypts, the cells were cultured with the luminal concentrations of Wnt-3A and R-spondin set to 0 ng/mL but with Wnt-3A:R-spondin concentrations in the basal reservoir of 60:150, 30:75, 15:37, or 7:18 (ng/mL:ng/mL) (Figure 6c–e). At low Wnt-3A:R-spondin concentrations of 15:37 and 7:18 (ng/mL:ng/mL) in the basal reservoir, very few EdU+ cells were observed in the cells within or above the microwells, suggesting that there was insufficient Wnt-3A and/or R-spondin at any location along the crypt to support cell proliferation. When the Wnt-3A:R-spondin concentrations were 60:150 (ng/mL:ng/mL) in the basal reservoir, EdU+ cells were observed at both basal and luminal regions of the in vitro crypts, suggesting that there was sufficient Wnt-3A and R-spondin throughout the tissue to support proliferation. However, Wnt-3A:R-spondin concentrations of 30:75 (ng/mL:ng/mL) in the basal reservoir supported proliferation of EdU+ cells in the basal crypt but not the luminal region. A COMSOL simulation of this chemical gradient suggested that the Wnt-3A:R-spondin concentrations were 17:43 (ng/mL:ng/mL) at the base of the microwells supporting the crypts (Figure 4i). Thus, a simple linear gradient of Wnt-3A and R-spondin was sufficient to polarize the in vitro crypts, creating basal proliferative and luminal nonproliferative cell compartments.
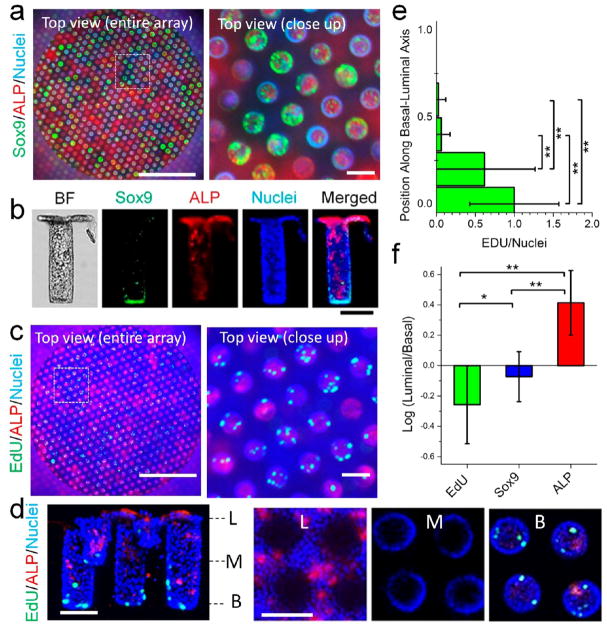
To determine whether stem/progenitor and differentiated cell compartments were also created by the gradient of Wnt-3A:R-spondin, the in vitro crypts were assayed for stem/progenitor and differentiated cell markers. An array of in vitro mouse crypts was generated on the platform under the optimized gradient and stained for ALP and either Sox9 (Figures 7a and S5) or EdU incorporation (Figures 7c and S6). For the EdU-pulsed scaffolds, 57.0 ± 7.8% (n = 3 arrays) of the wells possessed EdU+ cells. The other wells did not possess cells that were in S phase during the 3 h EdU pulse. Of the wells with EdU+ cells, 89.5 ± 5.3% (n = 3) were polarized, i.e., the EdU fluorescence in the lower 50% of the well was greater than that in the top portion of the well. Of these wells, the majority possessed no EdU+ cells in the upper portion of the well (Figure 7d), and the majority of EdU+ cells were located in the most basal one-sixth of the crypt (Figure 7e). In addition to possessing a proliferative cell compartment, the in vitro crypts were also polarized with stem/progenitor cells (Sox9+) located predominantly on the basal aspect of the tissue and suppressed on the luminal side (Figure 7b). The differentiated absorptive colonocytes (ALP+) were also primarily concentrated on the luminal crypt end (Figures 7b,d and S7). When the polarity of the crypts was quantified by measuring the ratios of the EdU, Sox9, and ALP fluorescence intensities in the luminal and basal halves of the structures, the in vitro crypts were clearly biased to have differentiated cells in the upper crypt and stem/progenitor cells localized to the crypt base (Figure 7f). Thus, these in vitro crypts possessed a basal stem cell niche and a layer of differentiated cells covering the luminal tissue surface. The in vitro crypts formed under these conditions bore a striking resemblance to in vivo crypts in their geometry, segregation of stem and differentiated cells, and luminal accessibility.
Figure 7.
Arrays of polarized colon crypts. (a) Wide-field (left) and close-up (right) top views of a 3 mm-diameter array stained for ALP (red), Sox9 (green), and DNA (blue). The images were obtained with a low-magnification, large-depth-of-field objective so that the crypt regions are superimposed into a single plane (see the schematic of the top view in Figure 5a). (b) Side views of a crypt removed from the scaffold and imaged lying on its side. Shown is a representative in vitro crypt that was stained for Sox9, ALP, and DNA. The image labeled “BF” is a bright-field image. Polarity is demonstrated in the merged image. (c) Wide-field (left) and close-up (right) top views of a 3 mm-diameter array stained for ALP (red), EdU (green), and DNA (blue). The images were obtained as in panel (a). (d) Confocal fluorescence images of crypts stained for ALP (red), EdU (green), and DNA (blue). Left: side view of a 3D reconstructed image. Right: images of x–y slices through the crypts at three locations in the crypt (L, luminal; M, middle; B, basal). (e) Distribution of EdU incorporation along the crypt axis. The y-axis value of 0 marks the crypt base, while 1 denotes 250 μm from the base. (f) Quantification of polarity of in vitro crypts. Shown are the ratios of the fluorescence intensities of the stains for EdU, Sox9, and ALP in the luminal half of the crypt to those in the basal half. n ≥ 20 crypts were quantified for (e) and (f). **, p < 0.005; *, p < 0.05. Scale bars = 100 μm for all images except the wide-field views in (a) and (c) (scale bars = 1 mm).
CONCLUSION
An array of in vitro mouse crypts was created by combining a self-sustaining epithelial monolayer with a shaped scaffold and simple linear gradients of growth factors. The collagen scaffolding enabled the prolonged adhesion, growth, and migration of the cell monolayer by supplying appropriate stiffness as well as suitable ECM contacts. Properly scaled chemical gradients supported the establishment and maintenance of basal stem/proliferative cell zones as well as luminal differentiated cell regions within the shaped monolayer. The in vitro crypts recapitulated key in vivo epithelial attributes not supported by organoid culture systems, such as crypt shape, stem-differentiated cell polarity, and luminal accessibility. Provision of a combination of biophysical and biochemical cues to an in vitro culture reconstructed tissues from stem cells to mimic the in vivo tissues at an unprecedented level. Such experiments can be used to estimate in vivo concentrations and gradient profiles of soluble growth and morphogenic factors. We expect that this platform will enable detailed studies of compounds such as nutrients, prebiotics, and microbiota on primary intestinal tissue under precisely controlled conditions.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health under Award R01DK109559 to N.L.A., S.B., and S.M. The authors thank Ian Williamson for coordinating the procurement of mouse colons. This work was performed in part at the Chapel Hill Analytical and Nanofabrication Laboratory (CHANL), a member of the North Carolina Research Triangle Nanotechnology Network (RTNN), which is supported by the National Science Foundation (Grant ECCS-1542015) as part of the National Nanotechnology Coordinated Infrastructure (NNCI).
Footnotes
Notes
The authors declare the following competing financial interest(s): N.L.A., Y.W., C.E.S., S.T.M., and S.J.B. have a financial interest in Altis Biosystems LLC.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.7b00368.
Culture medium, preparation of neutralized collagen hydrogel for proliferative 2D monolayer culture, cross-linking of collagen hydrogel by EDC/NHS chemistry, microfabrication and surface modification of PDMS stamps, measurement of hydrogel stiffness using AFM, Figures S1–S7, and Tables S1 and S2 (PDF)
Author Contributions
Y.W., M.D., S.J.B., C.E.S., S.T.M., and N.L.A. designed the experiments; Y.W., M.D., D.B.G., P.J.A., M.I.R., J.S.D., M.S.L., and D.L.N. performed the experiments and/or provided technical support; Y.W., M.D., and N.L.A. analyzed the data; Y.W. and N.L.A. administered the experiments; Y.W., C.E.S., and N.L.A. wrote the paper.
References
- 1.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15(1):19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 2.Kosinski C, Li VSW, Chan ASY, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A. 2007;104(39):15418–15423. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 4.Jung P, Sato T, Merlos-Suarez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, Clevers H, Batlle E. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17(10):1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 5.Date S, Sato T. Mini-Gut Organoids: Reconstitution of Stem Cell Niche. Annu Rev Cell Dev Biol. 2015;31:269–289. doi: 10.1146/annurev-cellbio-100814-125218. [DOI] [PubMed] [Google Scholar]
- 6.Yin XL, Mead BE, Safaee H, Langer R, Karp JM, Levy O. Engineering Stem Cell Organoids. Cell Stem Cell. 2016;18(1):25–38. doi: 10.1016/j.stem.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneeberger K, Spee B, Costa P, Sachs N, Clevers H, Malda J. Converging biofabrication and organoid technologies: the next frontier in hepatic and intestinal tissue engineering? Biofabrication. 2017;9(1):013001. doi: 10.1088/1758-5090/aa6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustata RC, Vasile G, Fernandez-Vallone V, Strollo S, Lefort A, Libert F, Monteyne D, Perez-Morga D, Vassart G, Garcia MI. Identification of Lgr5-Independent Spheroid-Generating Progenitors of the Mouse Fetal Intestinal Epithelium. Cell Rep. 2013;5(2):421–432. doi: 10.1016/j.celrep.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Wells JM, Spence JR. How to make an intestine. Development. 2014;141(4):752–760. doi: 10.1242/dev.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol. 2014;15(10):647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Choi J-H, Kim HJ. Human gut-on-a-chip technology: will this revolutionize our understanding of IBD and future treatments? Expert Rev Gastroenterol Hepatol. 2016;10(8):883–885. doi: 10.1080/17474124.2016.1200466. [DOI] [PubMed] [Google Scholar]
- 12.Kilic O, Pamies D, Lavell E, Schiapparelli P, Feng Y, Hartung T, Bal-Price A, Hogberg HT, Quinones-Hinojosa A, Guerrero-Cazares H, Levchenko A. Brain-on-a-chip model enables analysis of human neuronal differentiation and chemotaxis. Lab Chip. 2016;16(21):4152–4162. doi: 10.1039/c6lc00946h. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13(18):3599–3608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasotharan S, Pinto S, Sled JG, Bolz SS, Gunther A. Artery-on-a-chip platform for automated, multimodal assessment of cerebral blood vessel structure and function. Lab Chip. 2015;15(12):2660–2669. doi: 10.1039/c5lc00021a. [DOI] [PubMed] [Google Scholar]
- 15.Stucki AO, Stucki JD, Hall SRR, Felder M, Mermoud Y, Schmid RA, Geiser T, Guenat OT. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip. 2015;15(5):1302–1310. doi: 10.1039/c4lc01252f. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, LesherPerez SC, Kim BCC, Yamanishi C, Labuz JM, Leung B, Takayama S. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication. 2016;8(1):015021. doi: 10.1088/1758-5090/8/1/015021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12(12):2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 18.Ramadan Q, Jafarpoorchekab H, Huang CB, Silacci P, Carrara S, Koklu G, Ghaye J, Ramsden J, Ruffert C, Vergeres G, Gijs MAM. NutriChip: nutrition analysis meets microfluidics. Lab Chip. 2013;13(2):196–203. doi: 10.1039/c2lc40845g. [DOI] [PubMed] [Google Scholar]
- 19.Sung JH, Yu JJ, Luo D, Shuler ML, March JC. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip. 2011;11(3):389–392. doi: 10.1039/c0lc00273a. [DOI] [PubMed] [Google Scholar]
- 20.Wang YL, Dhopeshwarkar R, Najdi R, Waterman ML, Sims CE, Allbritton N. Microdevice to capture colon crypts for in vitro studies. Lab Chip. 2010;10(12):1596–1603. doi: 10.1039/b927316f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Ahmad AA, Shah PK, Sims CE, Magness ST, Allbritton NL. Capture and 3D culture of colonic crypts and colonoids in a microarray platform. Lab Chip. 2013;13(23):4625–4634. doi: 10.1039/c3lc50813g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YL, Ahmad AA, Sims CE, Magness ST, Allbritton NL. In vitro generation of colonic epithelium from primary cells guided by microstructures. Lab Chip. 2014;14(9):1622–1631. doi: 10.1039/c3lc51353j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141(5):1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 24.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc. 2013;8(12):2471–2482. doi: 10.1038/nprot.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujii M, Matano M, Nanki K, Sato T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat Protoc. 2015;10(10):1474–1485. doi: 10.1038/nprot.2015.088. [DOI] [PubMed] [Google Scholar]
- 26.Guezguez A, Pare F, Benoit YD, Basora N, Beaulieu JF. Modulation of stemness in a human normal intestinal epithelial crypt cell line by activation of the WNT signaling pathway. Exp Cell Res. 2014;322(2):355–364. doi: 10.1016/j.yexcr.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 27.O’Rourke KP, Ackerman S, Dow LE, Lowe SW. Isolation, Culture, and Maintenance of Mouse Intestinal Stem Cells. Bio-Protoc. 2016;6(4):e1733. doi: 10.21769/bioprotoc.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujii S, Suzuki K, Kawamoto A, Ishibashi F, Nakata T, Murano T, Ito G, Shimizu H, Mizutani T, Oshima S, Tsuchiya K, Nakamura T, Araki A, Ohtsuka K, Okamoto R, Watanabe M. PGE(2) is a direct and robust mediator of anion/fluid secretion by human intestinal epithelial cells. Sci Rep. 2016;6:36795. doi: 10.1038/srep36795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, DiSalvo M, Gunasekara DB, Dutton J, Proctor A, Lebhar MS, Williamson IA, Speer J, Howard RL, Smiddy NM, Bultman SJ, Sims CE, Magness ST, Allbritton NL. Self-renewing monolayer of primary colonic or rectal epithelial cells. Cell Mol Gastroenterol Hepatol. 2017;4(1):165–182. doi: 10.1016/j.jcmgh.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin XL, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5(+) intestinal stem cells and their progeny. Nat Methods. 2014;11(1):106–112. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrana NE, Builles N, Kocak H, Gulay P, Justin V, Malbouyres M, Ruggiero F, Damour O, Hasirci V. EDC/NHS cross-linked collagen foams as scaffolds for artificial corneal stroma. J Biomater Sci, Polym Ed. 2007;18(12):1527–1545. [PubMed] [Google Scholar]
- 32.Liu YW, Gan LH, Carlsson DJ, Fagerholm P, Lagali N, Watsky MA, Munger R, Hodge WG, Priest D, Griffith M. A simple, cross-linked collagen tissue substitute for corneal implantation. Invest Ophthalmol Visual Sci. 2006;47(5):1869–1875. doi: 10.1167/iovs.05-1339. [DOI] [PubMed] [Google Scholar]
- 33.Yang CR. Enhanced physicochemical properties of collagen by using EDC/NHS-crosslinking. Bull Mater Sci. 2012;35(5):913–918. [Google Scholar]
- 34.Ahmad AA, Wang YL, Sims CE, Magness ST, Allbritton NL. Optimizing Wnt-3a and R-spondin1 concentrations for stem cell renewal and differentiation in intestinal organoids using a gradient-forming microdevice. RSC Adv. 2015;5(91):74881–74891. [Google Scholar]
- 35.Cayo MA, Cayo AK, Jarjour SM, Chen H. Sodium butyrate activates Notch1 signaling, reduces tumor markers, and induces cell cycle arrest and apoptosis in pheochromocytoma. Am J Transl Res. 2009;1(2):178–183. [PMC free article] [PubMed] [Google Scholar]
- 36.Olsauskas-Kuprys R, Zlobin A, Osipo C. Gamma secretase inhibitors of Notch signaling. OncoTargets Ther. 2013;6:943–955. doi: 10.2147/OTT.S33766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egorov VI, Schastlivtsev IV, Prut EV, Baranov AO, Turusov RA. Mechanical properties of the human gastrointestinal tract. J Biomech. 2002;35(10):1417–1425. doi: 10.1016/s0021-9290(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 38.Olson AD. Contraction of collagen gels by intestinal epithelial cells depends on microfilament function. Dig Dis Sci. 1993;38(3):388–395. doi: 10.1007/BF01316489. [DOI] [PubMed] [Google Scholar]
- 39.Ali MY, Chuang CY, Saif MTA. Reprogramming cellular phenotype by soft collagen gels. Soft Matter. 2014;10(44):8829–8837. doi: 10.1039/c4sm01602e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinnebusch BF, Siddique A, Henderson JW, Malo MS, Zhang WY, Athaide CP, Abedrapo MA, Chen XM, Yang VW, Hodin RA. Enterocyte differentiation marker intestinal alkaline phosphatase is a target gene of the gut-enriched Kruppel-like factor. Am J Physiol: Gastrointest Liver Physiol. 2004;286(1):G23–G30. doi: 10.1152/ajpgi.00203.2003. [DOI] [PubMed] [Google Scholar]
- 41.Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol: Cell Physiol. 1991;260(2):C183–C193. doi: 10.1152/ajpcell.1991.260.2.C183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.