Abstract
Background:
Pharmacotherapies for alcohol use disorder have been shown to reduce hazardous drinking and improve overall health. The effect sizes for the effectiveness of these medications, however, are small, underscoring the need to expand the range of therapeutics and develop personalized treatment approaches. Recent studies have suggested that varenicline, an α4β2-nicotinic partial agonist widely used for smoking cessation, can help alcoholics reduce drinking, but the neurocognitive underpinnings of its effectiveness remain largely unexplored.
Methods:
In this double-blind study, 32 heavy drinkers were randomized to receive varenicline (2 mg/d) or placebo. After 2 weeks of dosing, participants underwent functional MRI scans, during which they viewed images of faces with either neutral or fearful expressions at baseline and following an intravenous alcohol infusion to a target breath alcohol concentration of 80 mg%. Blood oxygen level-dependent (BOLD) response was analyzed with Analysis of Functional Neuroimaging software. Linear mixed-effects models were used to examine the effects of facial expression (fearful vs. neutral) and medication (placebo vs. varenicline) on BOLD response. The effect of medication on measures of subjective response to alcohol was also examined.
Results:
Results indicated a significant facial expression-by-medication interaction in the left amygdala. The groups showed equivalent activation to neutral faces, but, whereas the placebo group showed increased activation to fearful faces, the varenicline group showed no change in activation. Amygdala activation to fearful faces correlated with number of drinks in the previous 90 days and Obsessive Compulsive Drinking Scale scores. There was no effect of varenicline on subjective response to alcohol.
Conclusions:
Our results indicate that varenicline may disrupt amygdala response to fearful faces in heavy drinkers. Further, amygdala activation correlated with alcohol consumption, suggesting that the effects of varenicline may be related to aspects of drinking behavior. These results suggest that amygdala response to fearful faces may be developed as a biomarker of the effectiveness of medications being developed for the treatment of alcohol use disorder.
Keywords: Alcohol, Intravenous Infusion, Heavy Drinkers, Faces Task, Amygdala
MORE THAN 1 in 5 Americans will develop an alcohol use disorder over the course of their lifetime (Grant et al., 2015; Hasin et al., 2007), underscoring the continued need for effective treatment. Comprehensive analyses of treatment studies have shown that pharmacotherapies for alcohol dependence can reduce drinking and relapse rates, but the effect sizes of existing medications are small (Jonas et al., 2014; Rosner et al., 2010). This may be due to the multiple etiologic pathways that can lead to alcoholism and suggests that personalized treatment approaches are warranted, along with the development of new medications (Heilig et al., 2011). One potential medication is varenicline, a partial agonist at the α4β2 nicotinic acetylcholine receptor (nAChR) that is widely prescribed to help people quit smoking (Coe et al., 2005; Kuehn, 2006; Tonstad et al., 2006). Laboratory studies in humans and animals have shown that varenicline can reduce alcohol consumption (McKee et al., 2009; Steensland et al., 2007). Further, several clinical trials have tested whether varenicline can reduce drinking, providing preliminary evidence that it may be an effective treatment (Litten et al., 2013; although see Erwin and Slaton, 2014; Plebani et al., 2013). Better understanding of varenicline’s effects in heavy drinkers may help determine how to maximize its benefit as a treatment.
Varenicline putatively reduces smoking through reducing rewarding effects of cigarettes and relieving adverse symptoms such as withdrawal (Jorenby et al., 2006); these effects are thought to arise from varenicline’s partial agonism at the nAChR. Given the high comorbidity between smoking and alcohol use disorders (Dani and Harris, 2005), these drugs presumably affect common neural circuitry. For example, evidence shows that alcohol stimulates the nAChR and repeated alcohol use leads to both tolerance at the nAChR and cross-tolerance to nicotine (Davis and de Fiebre, 2006). Thus, the same mechanisms involved in smoking cessation may also contribute to reduction in drinking. A few studies have shown evidence that varenicline reduces neural correlates of reward in response to smoking cues (Franklin et al., 2011; Ray et al., 2015). In heavy drinkers, varenicline increased self-reported control over alcohol-related thoughts and reduced orbitofrontal cortical response to alcohol cues (Schacht et al., 2014). In smokers, some evidence suggests that varenicline relative to placebo improves participants’speed at recognizing emotions in faces and reduces amygdala activity when viewing images of angry or fearful faces (Loug-head et al., 2013), although there is heterogeneity in this effect (Sutherland et al., 2013). This suggests that varenicline affects neural correlates associated with processing negative emotionality in smokers, which corroborates evidence that directly stimulating nAChRs can reduce anxiety in both animals and humans (Elliott et al., 2004; Kassel and Shiffman, 1997; Turner et al., 2011). This may be particularly relevant for heavy drinkers given the high comorbidity between anxiety and alcohol use disorders (Kushner et al., 1990). To date, however, no study had tested whether varenicline alters neural processing of negative emotional cues in heavy drinkers.
This study assessed the neural correlates of emotional face processing and examined subjective differences in response to alcohol administration between heavy drinkers treated with either placebo or varenicline. Numerous studies have shown that images of fearful faces increase amygdalar activity (Hariri et al., 2002; Yang et al., 2002). This may be important for heavy drinkers, as evidence suggests that greater attention to threat is associated with more alcohol consumption among healthy individuals (Keough and O’Connor, 2014). Further, amygdala activity in response to emotional faces was recently shown to discriminate which depressed patients were most likely to respond to treatment with medication (Williams et al., 2015), suggesting that amygdala response might signal clinically meaningful information. We hypothesized that varenicline, compared to placebo, would attenuate amygdalar response to threatening cues and would reduce the subjective experience of liking and wanting alcohol.
MATERIALS AND METHODS
Participant Characteristics
Heavy drinkers were recruited for this randomized, double-blind, placebo-controlled experimental medicine study, which occurred over the course of 3 weeks. Part of this study had been reported in a previous manuscript, including use of the same subjects (Vatsalya et al., 2015). The study was conducted at the NIH Clinical Center in Bethesda, MD, and all participants gave informed consent prior to participating in the study. Candidates completed a screening visit that included both a clinical and psychiatric evaluation (Structured Clinical Interview for the DSM-IV; First et al., 2002). We used the Fagerström Test for Nicotine Dependence (Fagerstrom, 1978) and 90-day Timeline Followback (TLFB; Sobell and Sobell, 1992) to assess smoking and drinking history, respectively. Participants also completed the Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001) and the Self-Rating of the Effects of Alcohol (SRE; Schuckit et al., 1997). The AUDIT assesses recent drinking by measuring consumption, dependence symptoms, and harmful drinking. The SRE quantifies level of response to alcohol. Participants were included if they (i) consumed an average of >20 drinks per week for men and 15 drinks per week for women and (ii) were not seeking help for alcohol-related problems.
Participants were excluded if they met any of the following criteria: (i) lifetime history of Axis I mood, anxiety, or substance use disorders (other than alcohol or nicotine use disorders); (ii) recent or regular use of illicit or nonprescribed psychoactive substances; (iii) history of clinically significant alcohol withdrawal; (iv) lifetime history of violence, suicide attempts, or self-injurious behavior; (v) current or chronic medical conditions, including cardiovascular conditions, requiring inpatient treatment or frequent medical visits; (vi) use of medications contraindicated with varenicline in the past 90 days, or those that may affect the hemodynamic response (e.g., antihypertensives) within the past 30 days, or those that may interact with alcohol within 2 weeks prior to the study; or (vii) metal in body, left-handedness, or claustrophobia (MRI exclusion criteria). The complete list of inclusion/exclusion criteria is available at clinicaltrials.gov (NCT00695500).
Forty-nine participants were randomized, but 3 failed to return. Nine participants dropped out or failed to comply with study procedures (3 varenicline participants, 6 placebo participants); 5 participants were removed from the final sample due to excessive motion artifacts during imaging (i.e., >20% of imaging repetition times were censored due to motion; 3 varenicline participants, 2 placebo participants). Thirty-two participants were included in the final dataset (varenicline: N = 17, placebo: N = 15). Participants were given medication in pill bottles, and adherence was monitored by counting the pills remaining at each visit.
Study Procedures
Following enrollment and a baseline visit, participants began medication with varenicline or placebo for 3 weeks. The varenicline dose was titrated during the first week (0.5 mg/d for the first 3 days, 1 mg/d for the next 4 days) to a final regimen of 2 mg/d for the remainder of the study (Vatsalya et al., 2015). After 2 weeks of taking medication, participants completed an fMRI session where they underwent scans prior to and following an IV alcohol infusion. Participants were instructed not to drink alcohol in the 48 hours prior to the study session or smoke after entering the scanning facility.
Self-Report Questionnaires
To examine relationships between brain activation and subjective response, several self-report measures were included.
The Drug Effects Questionnaire (DEQ) is one measure of early response to drug effects, which has been used in studies involving oral alcohol (King et al., 2011). The DEQ prompts participants to answer the following questions on a scale of 0 (not at all) to 100 (extremely): (i) Do you feel any drug effects? (ii) Do you feel high? (iii) Do you like the effects you are feeling now? (iv) Would you like more of what you received, right now? (v) Do you feel intoxicated?
The Obsessive Compulsive Drinking Scale (OCDS) is a 14-item questionnaire of the obsessive and compulsive dimensions of alcohol dependence (Anton et al., 1995). Craving and obsessions have shared characteristics; for example, Modell and colleagues (1992) found that those who craved alcohol also reported high obsessional thinking. The same group also identified correlations between compulsive drinking and craving. These findings indicate that obsessions and compulsions may be indicators of problem drinking.
Faces Task
While in the scanner, participants were presented with 45 images of fearful and 45 images of neutral faces obtained from the NimStim set (Tottenham et al., 2009). The faces appeared after a jittered period of time (2 to 14 seconds) in randomized order. Each cue appeared for 2 seconds; participants passively viewed the cues and were not asked to respond. The task took approximately 6 minutes. Following the initial faces run, participants received an IV infusion of alcohol to achieve a target breath alcohol concentration (BrAC) of 80 mg% over 15 minutes, after which the BrAC was clamped at the target level for an additional 15 min (Ramchandani et al., 1999). Five minutes after achieving the target BrAC, participants were scanned again while the faces task was repeated. Data from the second faces task are not reported due to concerns about alcohol’s effects on cerebral blood flow (Marxen et al., 2014; Strang et al., 2015) and order effects because the sober scan always occurred first. Following completion of the scan, the alcohol infusion was stopped, and participants were removed from the scanner. Participants were monitored until their BrAC levels fell below 20 mg% after which they were sent home in a taxi.
Subjective Response to Alcohol Analysis
The subjective response to alcohol was measured via the DEQ. The DEQ was administered immediately before the faces task, and following the conclusion of the alcohol clamp. The difference between baseline and postclamp DEQ scores constituted our measure of the subjective effects of alcohol. Data were analyzed with an analysis of variance (ANOVA) model with medication-type as the between-group variable and DEQ change scores for each question (feeling, wanting more, liking, high, and intoxicated) as the dependent measures. Each model was also tested with age as a covariate as the groups differed on this variable.
fMRI Scanning
The fMRI session was conducted using a General Electric (Fair-field, CT) or Siemens Skyra (Munich, Germany) 3T scanner with a 12 or 20 channel head coil, respectively. Structural scans were also collected for co-registration with functional images using the MPRAGE sequence. The faces task was collected after a modified version of the monetary incentive delay task, which is reported in a separate manuscript (Vatsalya et al., 2015). Two functional runs time-locked to the start of the faces task were acquired using a T2*-EPIRT sequence (T2*-weighted echoplanar imaging; TR = 2,000 ms, TE = 30 ms, FoV = 240 mm, 64 × 64 matrix, 36 axial slices with 0 mm gap, flip angle = 90°, total duration: 6 minute, 20 seconds, 3.75 × 3.75 × 3.8 mm3 voxels) that measured changes in blood oxygen level-dependent (BOLD) contrast.
Imaging Processing
Data were preprocessed using Analysis of Functional Neuro-Images (AFNI) software (Cox, 1996). Echoplanar images were aligned to anatomical images. We inspected data for motion, censoring time points with >0.3 mm motion or 0.3° of rotation relative to the previous time point. Images were spatially smoothed using a 6-mm Gaussian kernel. In the process of aligning the echoplanar images to the anatomical image and warping them to standardized space, voxels were resampled to 3.5 × 3.5 × 3.5 mm3 to generate isotropic voxels of the minimum original dimension, but truncated to 3 significant bits (i.e., 3.75 was truncated to 3.5). Signal for each voxel was scaled by the mean so the average intensity was 100; thus, output could be viewed as percent signal change from baseline. A general linear model fit was performed using AFNI’s 3dDeconvolve function, with regressors for alcohol, food, and neutral cues for each phase (cue, target, hit, miss). Six motion parameters were included in the model as regressors of noninterest.
Brain Imaging Analysis
Linear mixed-effects (LME) analyses were conducted using AFNI’s 3dLME (Chen et al., 2013). Medication (placebo, varenicline) and face type (fear, neutral) were fixed effects in the model and individual participants were treated as random effects. We also controlled for scanner type to account for the use of 2 different scanners (4 placebo and 5 varenicline were scanned with the Siemens scanner). We also examined the results by scanner to confirm that the results were in the same direction (see Figure S1). LME analysis examined main effect of medication, face type, and medication-by-face-type interaction. Analyses were performed voxel-wise across the entire brain. Volume-threshold adjustment based on Monte Carlo simulations (AFNI’s 3dClustSim) was applied to protect family-wise error rate. For a main effect of face type, a more stringent threshold was applied in an attempt to restrict significant clusters to single anatomical regions. Thus, an a priori voxel-wise probability of p < 0.01 in a cluster of 1,158 μl (27 voxels) resulted in an a posteriori probability of p < 0.01. For medication-by-face-type interaction analyses, which have half the power of a main effect analysis, an a priori voxel-wise probability of p < 0.05 in a cluster of 1,402 μl (33 voxels) for the entire brain resulted in an a posteriori probability of p < 0.05. Age was tested as a covariate for significant clusters because the groups differed on this variable.
Exploratory Analyses: Drinking Measures and Brain Activity Relationships
We examined whether drinking-related measures correlated with brain activation in response to fearful faces. Specifically, we examined the total number of drinks over the previous 90 days as assessed by the TLFB and the total score on the OCDS. We performed voxel-wise correlations using AFNI’s 3dttest++ in a region-of-interest mask of the bilateral amygdala based on a priori hypotheses and the results of the main effect of face-type and the medication-by-face-type interaction results. Volume-threshold adjustment based on Monte Carlo simulations (AFNI’s 3dClust-Sim) was applied to protect family-wise error rate. The amygdala mask contained 108 voxels. Based on the simulations, an a priori voxel-wise probability of p < 0.05 in a cluster of 3 voxels resulted in an a posteriori probability of p < 0.05.
RESULTS
Demographics
The characteristics of participants are shown in Table 1. A t-test showed that the groups differed in age (placebo: μ = 39.1, SD = 13.6; varenicline: μ = 29.5, SD = 8.6; p = 0.02). However, as a Shapiro–Wilks test revealed that age was not normally distributed (W = 0.85, p < 0.01), we also performed a nonparametric analysis. A Mann–Whitney U-test showed that the groups did not differ significantly (p = 0.15). There were no between-group differences in drinking history, smoking, and physical attributes.
Table 1.
Participant Characteristics
| Placebo (n = 15) N (%) |
Varenicline (n = 17) N (%) |
|
|---|---|---|
| Female | 3 (20) | 2 (12) |
| FHPa | 3 (20) | 3 (18) |
| Current abuse | 1 (7) | 5 (29) |
| Current dependence | 1 (7) | 3 (18) |
| Current smoker | 5 (33) | 7 (41) |
| Mean (SD) | Mean (SD) | |
| Ageb (years) | 39.1 (13.1) | 29.5 (8.4) |
| Age at first drink (years) | 15.6 (4.4) | 15.6 (2.0) |
| 90-day Timeline Followback (TLFB) | ||
| Total drinksc | 449.1 (162.2) | 406.4 (201.2) |
| Drinking daysc | 69.4 (15.5) | 67.0 (17.7) |
| Drinks/drinking dayc | 6.5 (2.2) | 6.3 (3.0) |
| Heavy drinking daysc | 50.7 (22.4) | 40.9 (25.2) |
| Obsessive Compulsive Drinking Scale (OCDS)totald | 9.9 (5.9) | 9.8 (5.1) |
| OCDS obsessived | 3.1 (3.5) | 2.9 (2.5) |
| OCDS compulsived | 6.8 (2.8) | 6.9 (3.0) |
| Cigarettes per day (smokers only) | 8.2 (4.5) | 6.7 (6.5) |
The varenicline group was significantly younger than the placebo group as assessed by a t-test, but a Shapiro–Wilks test revealed that age was not normally distributed. Therefore, a Mann–Whitney U-test was performed, which revealed no significant differences between groups. Based on t-tests and chi-square tests, the groups did not differ on any other variable.
Family History Positive for Alcoholism.
Groups were significantly different based on a t-test. As age was not normally distributed, however, groups were also assessed with a Mann–Whitney U-test and did not differ significantly (p = 0.15).
Based on a 90-day TLFB interview.
Obsessive Compulsive Drinking Scale.
Neuroimaging: Effect of Face-Type and Medication by Face-Type Interaction
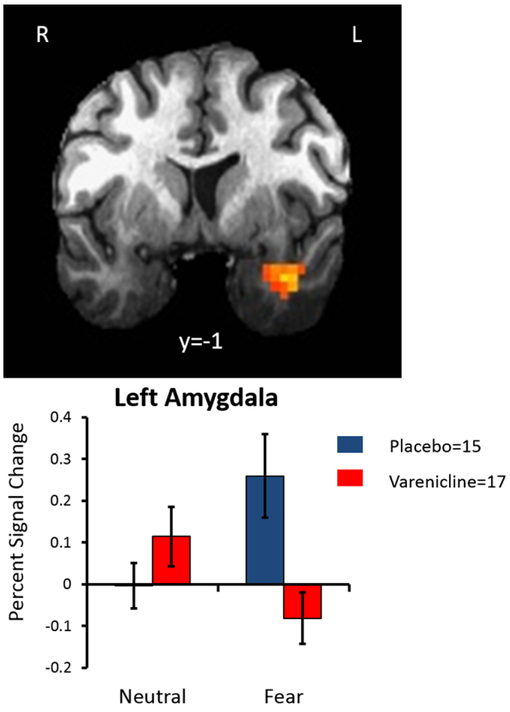
Linear mixed-effects analysis revealed that there was a main effect of face type wherein a cluster centered on the left uncus that contained the left amygdala demonstrated greater activation in response to fearful faces relative to neutral faces. A complete list of significant clusters is available in Table 2. The whole brain analysis examining medication-by-face-type interaction identified a significant cluster containing the left amygdala. As seen in Fig. 1, the placebo group, relative to the varenicline group, showed significantly more amygdalar activation in response to fearful faces, but the groups showed equivalent activation in response to neutral faces. When age was added as a covariate, the medicationby-face-type interaction remained significant, but age was not significantly related to amygdala activity (p = 0.35). A complete list of significant clusters is presented in Table 3. The effect remained significant when covarying for scanner type (GE vs. Siemens), but scanner type was not a significant variable in the model, indicating that it did not significantly influence the results.
Table 2.
Main Effect of Face Type. Whole Brain Analysis
| Volume (μl) |
x | y | z | L/R | Region | Brodmann area |
|---|---|---|---|---|---|---|
| 3,216 | −18 | −94 | 13 | L | Cuneus | 18 |
| 2,830 | −53 | 28 | 17 | L | Inferior frontal gyrus | 46 |
| 2,658 | −18 | −5 | −19 | L | Uncus/Amygdala | 34 |
| 2,315 | 38 | 55 | 10 | R | Middle frontal gyrus | 10 |
| 2,187 | 14 | −92 | 18 | R | Cuneus | 18 |
| 1,801 | 31 | 13 | −22 | R | Superior temporal gyrus | 38 |
| 1,629 | −46 | −52 | −18 | L | Fusiform gyrus | 37 |
| 1,629 | 54 | −4 | 2 | R | Superior temporal gyrus | 22 |
| 1,243 | 51 | 28 | 9 | R | Inferior frontal gyrus | 45 |
| 1,157 | −61 | −19 | −8 | L | Middle temporal gyrus | 21 |
| 1,157 | 2 | 39 | 8 | R | Anterior cingulate | 32 |
These clusters had a significant main effect of face type (fearful vs. neutral face) with a voxel level p < 0.01 and a cluster level p < 0.01. Coordinates represent the voxel with the peak activation.
Fig. 1.
There was a significant medication group by face-type interaction on blood oxygen level-dependent (BOLD) response in the left amygdala. The placebo group showed significantly more activity in the left amygdala during fearful faces relative to neutral faces, but this effect was not observed in the varenicline group. Error bars represent SEM.
Table 3.
Medication by Face-Type Interaction. Whole Brain Analysis
| Volume (μl) |
x | y | z | L/R | Region | Brodmann area |
|---|---|---|---|---|---|---|
| 2,615 | −46 | −54 | −17 | L | Fusiform gyrus | 37 |
| 1,544 | −26 | 3 | −24 | L | Uncus/Amygdala | 28 |
| 1,501 | −12 | −96 | 6 | L | Cuneus | 17 |
| 1,458 | 12 | −94 | 12 | R | Cuneus | 18 |
Group by face-type interaction.These clusters had a significant interaction effect of face type (fearful vs. neutral face) and group (placebo, varenicline) with a voxel level p < 0.05 and a cluster level p < 0.05. Coordinates represent the voxel with the peak activation.
Subjective Effects of Alcohol
Mean values for each DEQ item at baseline and during the alcohol infusion are reported in Table 4. An ANOVA model revealed no main effect of medication group on change in DEQ measures of feeling (p = 0.51), wanting more (p = 0.13), liking (p = 0.11), high (p = 0.60), and intoxicated (p = 0.43) from baseline until the end of the alcohol clamp. Age was significantly associated with feeling drug, wanting more, linking, high, and intoxication (all p < 0.05), where older individuals reported greater values for each of these measures.
Table 4.
Subjective Response to Alcohol as Measured by the Drug Effects Questionnaire (DEQ)
| Feel | Like | Want | High | Intoxicated | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Postclamp | Baseline | Postclamp | Baseline | Postclamp | Baseline | Postclamp | Baseline | Postclamp | |
| Placebo Mean (SD) | 0.7 (1.2) | 2.6 (1.5) | 0.8 (1.0) | 1.4 (1.5) | 0.6 (0.8) | 0.9 (1.5) | 0.8 (1.4) | 2.1 (1.8) | 0.7 (1.3) | 1.9 (1.9) |
| Varenicline Mean (SD) | 0.4 (0.6) | 1.5 (1.1) | 0.5 (0.8) | 2.3 (1.2) | 0.5 (0.9) | 1.9 (1.4) | 0 (0) | 1.1 (0.9) | 0.1 (1.3) | 2.1 (1.1) |
The subjective ratings on the 5 DEQ items are shown at 2 time points: baseline and at the end of the second faces run. Analyses focused on the difference between the timepoints (i.e. the change score) and revealed no significant differences between groups for any of the DEQ items.
Exploratory Analyses: Brain Activation and Drinking Measures Relationships
Timeline Followback.
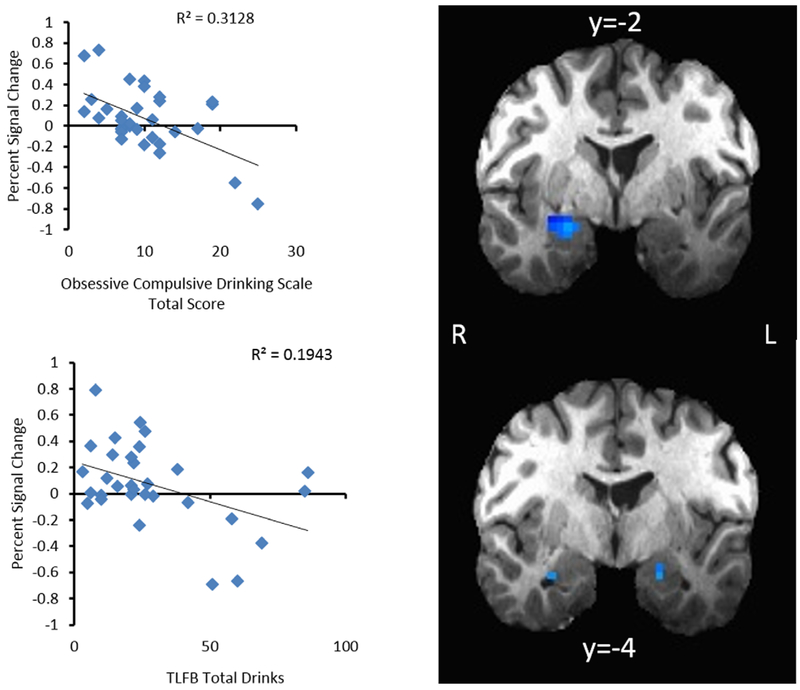
As seen in Fig. 2, total drinks (TLFB) during the week prior to the scan were used to measure the relationship between recent drinking and amygdalar activation in response to fearful faces. The natural log (ln) of total drinks was taken to normalize the distribution of this measure. Limbic masking revealed a significant negative correlation between activation of the amygdala bilaterally (right: Brodmann area: 34; x = 23, y = 2, z = 16, Vol = 472 μl; left: Brodmann area: 28; x =−20, y =−4, z =−16, Vol. = 421 μl) and total drinks consumed (right: R2 = 0.19, p = 0.01; left: R2 = 0.15, p = 0.03). There was no interaction by medication type (p = 0.45).
Fig. 2.
Amygdala activity when viewing fearful faces was significantly correlated with obsessive compulsive drinking and total drinks in the past 90 days. Individuals with greater amygdala activity had lower levels on these drinking measures.
Obsessive Compulsive Drinking Scale.
There was a significant, negative correlation between activation in the right amygdala (Brodmann area: 34, x = 24, y = −2, z = −15, Vol = 644 μl) in response to fearful faces and the OCDS (R2 = 0.31, p = .001). There was no interaction by medication type (p = 0.71).
DISCUSSION
This study addressed the hypothesis that chronic varenicline administration would change the neural processing of emotional faces. We show that varenicline administration in heavy drinkers reduces the amygdala response to fearful faces. Further, amygdala activation to fearful faces correlated with drinking measures and indices of obsessive drinking, suggesting that varenicline’s effects may be related to aspects of drinking behavior and anxiety. A second goal of this study was to determine whether varenicline reduced subjective response to the acute effects of alcohol. We did not find evidence to support this hypothesis. The reduced activity in the amygdala in response to fearful faces may offer a mechanism that elucidates previous evidence that varenicline can help heavy drinkers reduce their alcohol consumption.
Consistent with previous studies (Hariri et al., 2000, 2002), there was a main effect for participants in this study to show increased amygdalar activation when viewing fearful relative to neutral faces. There was also a medication-by-cue-type interaction in the left amygdala, where participants in the placebo group showed increased activation for fearful relative to neutral faces. This effect was absent for participants in the varenicline group, as they showed similar activity when viewing neutral and fearful faces. This suggests that one of varenicline’s effects may be to reduce the magnitude of neural response to emotional stimuli. Given the density of nicotinic AChRs in the amygdala (Wada et al., 1989), it is plausible that varenicline would act here. In a previous study, when participants labeled fearful emotions rather than passively viewing them, they showed more activity in cognitive regions such as medial prefrontal cortex, but reduced amygdala activation (Hariri et al., 2000). Given that one effect of nicotinic agonists is to enhance cognitive functions such as working memory (Levin et al., 2006), varenicline may promote a more cognitive appraisal of fearful faces rather than an emotional one. As stress can trigger alcohol consumption in some individuals (Britton and Bell, 2015), a dampened response to anxiogenic stimuli could be a mechanism for reducing desire to consume alcohol. Amygdala activation correlated with several measures of drinking behavior. Across all participants, individuals with lower amygdala activation reported higher levels of obsessive–compulsive drinking and more alcohol consumption in the previous 90 days. There was no evidence of an interaction with varenicline for any of these relationships, suggesting that they are traits rather than an effect of nicotinic modulation. The correlations between drinking measures and amygdala activation suggest that the faces task may be capturing information relevant to alcohol-related behaviors; however, the direction of these correlations is inconsistent with the direction of the medication effect: individuals with less amygdala activity in response to fearful faces drank more, but the varenicline group showed less activity relative to the control group in response to fearful faces. This discrepancy indicates that further work will be needed to determine how varenicline’s effects on neural response to emotional stimuli relate to alcohol consumption.
As previous studies had shown differences between how social and heavy drinking participants responded to fearful faces (Gilman et al., 2012), part of the purpose of this study was to validate that the faces task might be a useful marker of treatment effectiveness for heavy drinkers. The present findings offer mixed support for this hypothesis. The placebo and varenicline groups did not differ on subjective response to alcohol administration, making it difficult to determine varenicline’s effectiveness in the present study. Nonetheless, as previous reports have indicated that varenicline can reduce alcohol consumption and craving (Feduccia et al., 2014; Litten et al., 2013; McKee et al., 2009), the present findings—that (i) varenicline diminished differential amygdalar response to emotional faces and that (ii) amygdala activity correlated with drinking measures—indicate that the faces task may help assess medication utility for heavy drinkers.
The placebo and varenicline groups did not differ on subjective response to alcohol administration. Although previous research has shown that nAChR antagonists reduce the stimulating and rewarding effects of alcohol (Gilman et al., 2008, 2012), there has been mixed evidence for the effect nAChR agonists on alcohol response. Some studies have shown that nAChR agonism increases alcohol self-administration and subjective high (Barrett et al., 2006; Kouri et al., 2004), while others have shown that it leads participants to wait longer before taking a drink (McKee et al., 2008) and reduces subjective high (Ralevski et al., 2012). The absence of a difference in the present study may reflect several factors. First, previous studies showing that nAChR modulation can alter the subjective effects of alcohol used either an antagonist (mecamylamine) or a full agonist (nicotine), whereas varenicline is a partial agonist, and, thus, the effect size may be too small to detect with the sample size in this study. Second, there have been mixed results in the previous studies, suggesting several possibilities: nAChR modulation may not alter the subjective response to alcohol or it may only alter it for select samples. For example, most of the previous research has examined smoking populations, but the present study contained a mix of smokers and nonsmokers. Further, several previous studies have found that nicotine only modulated the subjective response to alcohol in males (Acheson et al., 2006; Kouri et al., 2004), so gender may moderate the effects of nAChR modulation on alcohol response. The absence of an effect of varenicline on the subjective response to alcohol may have resulted because partial agonism of the nAChR does not significantly affect response to alcohol, or it may be due to our sample selection, which included a mix of smoking, nonsmoking, male, and female heavy drinkers. Future studies will need to address whether varenicline modulates subjective response to alcohol in different samples.
This study had several limitations. Participants were scanned at only 1 time point, after 2 weeks on medication, so we cannot assess whether the groups’ neural responses during the faces task differed at baseline, that is, prior to varenicline administration. However, the groups were similar on key demographic variables, including drinking history, and because medication was randomized and double-blind, we believe the lack of a baseline scan did not impact our results. Future longitudinal studies are needed help determine how varenicline alters neural processing of emotion across the course of treatment. Further, as our study assessed non-treatment-seeking heavy drinkers, the findings may not generalize to individuals seeking treatment for alcohol use disorder. Lastly, we used pill counts to assess medication adherence, which is a weak measure and introduces the possibility of deception, which cannot be directly assessed without measuring blood levels of varenicline. Future studies should address the generalizability of the present results.
The findings from this study provide initial evidence that a short-term regimen of varenicline can diminish amygdala activity in response to fearful faces in heavy drinkers. This amygdala activity in response to fearful faces may be related to drinking behavior, suggesting that the findings have bearing on varenicline’s utility as a therapy for alcohol consumption. Although several considerations apply, these findings support the development of neural markers for the assessment of pharmacothera-pies for alcohol use disorders.
Supplementary Material
Fig. S1. There was a significant medication-group by face-type interaction in the GE participants.
ACKNOWLEDGMENT
The authors gratefully acknowledge the NIH Clinical Center Alcohol Clinic (Tom Lionetti and Dave Spero in particular), 5SWS Day Hospital staff for clinical support, and NMR Center staff for technological support. The authors also acknowledge David Herion, MD (NIH Clinical Center), for critical input during the design and development of the study protocol, Ashley Smith, PhD, and Jodi Gilman, PhD (NIAAA), for fMRI task programming support, David T. George, MD (NIAAA), for medical support, Julnar Issa, Marion Coe, and Megan Cooke for data collection support, and the study participants for their participation in the study. Finally, we thank Daniel W. Hommer for his lasting influence over the work in our laboratory.
Dr. Ramchandani had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Authors Ramchandani, Vatsalya, Bartlett, and Heilig contributed to the study concept and design. Authors Bartlett and Ramchandani obtained funding for the study. Authors Vatsalya and Ramchandani contributed to data collection and validation as well as study supervision. Authors Gowin, West-man, Schwandt, and Ramchandani analyzed and interpreted data. Authors Gowin and Westman drafted the manuscript. All authors critically revised the manuscript for intellectual content and have approved the final version. The authors declare that they have no conflicts of interest.
This work was presented in part at the 53rd Annual Meeting of American College of Neuropsychopharmacology (December 10, 2014, Scottsdale, Arizona) and the Research Society on Alcoholism Meeting (June 22, 2015, San Antonio, Texas).
FUNDING
This study was supported by the NIAAA Division of Intramural Clinical and Biological Research (Z1A AA000466). Medication supply was provided by Pfizer Inc. under agreement with Dr. Bartlett. Development of the CAIS software used for the IV-ASA session was supported by Sean O’Connor, MD, at the Indiana Alcohol Research Center (NIH P60 AA007611).
Clinical Trials Registration: NCT00695500.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
REFERENCES
- Acheson A, Mahler SV, Chi H, de Wit H (2006) Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology 186:54–63. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P (1995) The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res 19:92–99. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG (2001) Audit. The Alcohol Use Disorders Identification Test (AUDIT): Guidelines for Use in Primary Care. World Health Organization, Department of Mental Health and Substance Dependence, Geneva, Switzerland. [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO (2006) Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend 81:197–204. [DOI] [PubMed] [Google Scholar]
- Britton A, Bell S (2015) Reasons why people change their alcohol consumption in later life: findings from the Whitehall II cohort study. PloS One 10: e0119421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS, Cox RW (2013) Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage 73:176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD 3rd, O’Neill BT (2005) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA (2005) Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci 8:1465–1470. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM (2006) Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health 29:179–185. [PMC free article] [PubMed] [Google Scholar]
- Elliott BM, Faraday MM, Phillips JM, Grunberg NE (2004) Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav 77:21–28. [DOI] [PubMed] [Google Scholar]
- Erwin BL, Slaton RM (2014) Varenicline in the treatment of alcohol use disorders. Ann Pharmacother 48:1445–1455. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO (1978) Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 3:235–241. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Simms JA, Mill D, Yi HY, Bartlett SE (2014) Varenicline decreases ethanol intake and increases dopamine release via neuronal nicotinic acetylcholine receptors in the nucleus accumbens. Br J Pharmacol 171:3420–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute, New York, NY. [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR (2011) Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry 68:516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW (2012) Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology 37:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW (2008) Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci 28:4583–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on alcohol and related conditions III. JAMA Psychiatry 72: 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC (2000) Modulating emotional responses: effects of a neocortical network on the limbic system. Neurore-port 11:43–48. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR (2002) The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage 17:317–323. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF (2007) Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 64:830–842. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP (2011) Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci 12:670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC (2014) Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311:1889–1900. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays J, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR (2006) Efficacy of varenicline, an a4b2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296:56–63. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Shiffman S (1997) Attentional mediation of cigarette smoking’s effect on anxiety. Health Psychol 16:359. [DOI] [PubMed] [Google Scholar]
- Keough MT, O’Connor RM (2014) Clarifying the measurement and the role of the behavioral inhibition system in alcohol misuse. Alcohol Clin Exp Res 38:1470–1479. [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE (2004) Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug Alcohol Depend 75:55–65. [DOI] [PubMed] [Google Scholar]
- Kuehn BM (2006) FDA speeds smoking cessation drug review. JAMA 295:614. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Beitman BD (1990) The relation between alcohol problems and the anxiety disorders. Am J Psychiatry 147:685–695. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology 184:523–539. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R (2013) A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med 7:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, O’Donnell GP, Senecal N, Siegel S, Gur RC, Lerman C (2013) Brain activity and emotional processing in smokers treated with varenicline. Addict Biol 18:732–738. [DOI] [PubMed] [Google Scholar]
- Marxen M, Gan G, Schwarz D, Mennigen E, Pilhatsch M, Zimmermann US, Guenther M, Smolka MN (2014) Acute effects of alcohol on brain perfusion monitored with arterial spin labeling magnetic resonance imaging in young adults. J Cereb Blood Flow Metab 34:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E (2009) Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O’Malley SS, Shi J, Mase T, Krishnan-Sarin S (2008) Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology 196:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell JG, Glaser FB, Cyr L, Mountz JM (1992) Obsessive and compulsive characteristics of craving for alcohol in alcohol abuse and dependence. Alcohol Clin Exp Res 16:272–274. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Lynch KG, Rennert L, Pettinati HM, O’Brien CP, Kampman KM (2013) Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug Alcohol Depend 133:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevski E, Perry EB Jr, D’Souza DC, Bufis V, Elander J, Limoncelli D, Vendetti M, Dean E, Cooper TB, McKee S, Petrakis I (2012) Preliminary findings on the interactive effects of IV ethanol and IV nicotine on human behavior and cognition: a laboratory study. Nicotine Tob Res 14:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S (1999) A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res 23:617–623. [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED (2015) Varenicline, naltrexone, and their combination for heavy-drinking smokers: preliminary neuroimaging findings. Am J Drug Alcohol Abuse 41:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M (2010) Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev 12:CD001867. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H (2014) Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology 231:3799–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE (1997) The self-rating of the effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction 92:979–988. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back, in Measuring Alcohol Consumption (Litten RZ, Allen JP, National Institute on Alcohol Abuse and Alcoholism, eds), pp 41–72. Springer, New York, NY. [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE (2007) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA 104:12518–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang NM, Claus ED, Ramchandani VA, Graff-Guerrero A, Boileau I, Hendershot CS (2015) Dose-dependent effects of intravenous alcohol administration on cerebral blood flow in young adults. Psychopharmacology 232:733–744. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA (2013) Individual differences in amygdala reactivity following nicotinic receptor stimulation in abstinent smokers. NeuroImage 66:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR (2006) Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 296:64–71. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey B, Nelson C (2009) The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA (2011) Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res 13:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsalya V, Gowin JL, Schwandt ML, Momenan R, Coe MA, Cooke ME, Hommer DW, Bartlett S, Heilig M, Ramchandani VA (2015) Effects of varenicline on neural correlates of alcohol salience in heavy drinkers. Int J Neuropsychopharmacol 18. doi: 10.1093/ijnp/pyv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter JI, Deneris E, Heinemann S, Patrick JI, Swanson LW (1989) Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol 284:314–335. [DOI] [PubMed] [Google Scholar]
- Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, Grieve SM, Harris AW, Usherwood T, Etkin A (2015) Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology 40:2398–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, Gotlib IH, Reiss AL (2002) Amygdalar activation associated with positive and negative facial expressions. NeuroReport 13:1737–1741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. There was a significant medication-group by face-type interaction in the GE participants.