Fish diversity enhances the process of herbivory on coral reefs, with consequent benefits also to ecosystem integrity.
Abstract
There is now a general consensus that biodiversity positively affects ecosystem functioning. This consensus, however, stems largely from small-scale experiments, raising the question of whether diversity effects operate at multiple spatial scales and flow on to affect ecosystem structure in nature. Here, we quantified rates of fish herbivory on algal turf communities across multiple coral reefs spanning >1000 km of coastline in the Dominican Republic. We show that mass-standardized herbivory rates are best predicted by herbivore biomass and herbivore species richness both within (α-diversity) and across sites in the region (β-diversity). Using species-diversity models, we demonstrate that many common grazer species are necessary to maximize the process of herbivory. Last, we link higher herbivory rates to reduced algal turf height and enhanced juvenile coral recruitment throughout the ecosystem. Our results suggest that, in addition to high herbivore biomass, conserving biodiversity at multiple scales is important for sustaining coral reef function.
INTRODUCTION
The idea of biological diversity as both a response to and a driver of ecosystem processes has led to parallel tracks of investigation over the past century. On the one side, macroecological research has focused on the origin and maintenance of diversity, and how communities of species come together across space and time. Integral to this approach is the partitioning of diversity into local α and regional γ components, with a third component—β—that quantifies compositional variation among local communities within a region (1). A second field relating biodiversity to the processes that underpin functioning ecosystems has now unequivocally demonstrated that the loss of species leads to measurable declines in many processes such as biomass accumulation, nutrient cycling, and decomposition (2, 3). A major criticism of these studies, however, is their limited scope: As most studies are limited to analysis of a single location, they often ignore the larger regional pool from which patterns in local diversity arise (4). Recent theoretical (5, 6) and experimental (7–11) studies have linked local- and landscape-scale effects of biodiversity using metacommunity dynamics, and new efforts to “scale-up” biodiversity research in the real world have leveraged observational datasets across large spatial and environmental gradients (3). Still, few analyses have incorporated an explicit regional context to explain local functioning (12–14), although doing so would help to reconcile the demonstrated benefits of biodiversity with the scales at which natural resources are often managed.
Here, we evaluate biodiversity-ecosystem functioning relationships at various scales by relating patterns of tropical fish α- and β-diversity to herbivory rates on Dominican coral reefs. Herbivory is a key ecological process on reefs globally, where intense grazing by herbivores prevents the establishment and accumulation of algae that can suppress coral growth, survival, and reproduction (15). The removal of herbivorous fishes from coral reefs has led to state shifts in many regions, particularly in the Caribbean, where decades of overfishing, temperature-induced coral bleaching, and disease events have fostered algal dominance (15). The role of biodiversity loss in mediating this transition, however, remains unclear, leading to the suggestion that high biomass of only a few select key species are needed to sustain or rebuild reef function (16). At the same time, several recent studies have shown that different herbivores target different algal resources (17–19), creating the potential for strong complementarity in these ecosystems. This new experimental evidence raises questions regarding whether and how diversity effects manifest, whether they are broadly generalizable, whether they rival the importance of high herbivore biomass, and, critically, whether their contributions to herbivory generate measurable consequences for the reef itself (17). Moreover, while some herbivory studies have revealed a dominant effect of herbivore identity at discrete locations (20), such identity effects might combine and give rise to an emergent effect of diversity at larger scales of space and time, as has been shown in terrestrial ecosystems (21, 22). If so, many more species might be needed to maintain ecosystem functioning across large, naturally varied reefscapes than would be expected from existing evidence.
To evaluate the relationship between herbivore diversity and the process of herbivory, we deployed remote video cameras on 10 reefs spanning >1000 km of coastline in the Dominican Republic and quantified herbivore grazing rates on the benthic algal turf community. We then predicted mass-standardized bite rates [i.e., bites multiplied by the biomass of the herbivore, given that per capita feeding impacts scale with fish body size (20)] as a function of total herbivore biomass, local α-diversity and within-region β-diversity, co-occurring bottom-up drivers such as resource availability, and unmeasured site-to-site differences (via random effects). Here, we use the compositional uniqueness of each community relative to the regional species pool as a measure of local contributions to β-diversity (LCBD) (23). This approach provides a unique value of β-diversity for each site—increasing our statistical power—with larger values indicating greater compositional differences relative to other sites. Last, to assess the cascading consequences of changing herbivore biomass and richness at the ecosystem level, we evaluated how observed herbivory rates from our video assays predicted measures of benthic community structure on each reef (derived from independent transect surveys).
RESULTS AND DISCUSSION
We found that herbivorous fish community biomass, α-diversity, and β-diversity (LCBD) all significantly predicted mass-standardized grazing rate using a general linear mixed-effects model (Fig. 1 and table S1). The model, which also included the (nonsignificant) influence of coral abundance, turf algae abundance, and sea urchin (Diadema antillarum) abundance (24) on rates of herbivory, explained R2 = 80% of the variance in grazing rate when considering fixed effects only, and 92% when considering both fixed and random effects. Comparison of standardized effect sizes revealed that herbivore biomass was the strongest predictor of herbivory rate (βstd = 0.74). However, α- and β-diversity also had strong, independent impacts beyond that of biomass (βstd = 0.48 and 0.53 for α and β, respectively). Partial effects plots, which isolate the independent effect of each predictor after accounting for the influence of all other predictors in the multiple regression model, demonstrate that, for a given level of herbivore biomass and resource availability, more diverse and more compositionally unique herbivore assemblages are each associated with more intense grazing on the reef (Fig. 1, B and C).
Fig. 1. Herbivore biomass, local α-diversity, and between-community β-diversity significantly predict mass-standardized herbivory rates.
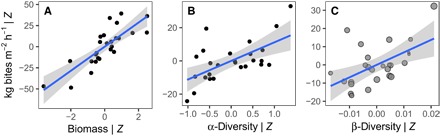
Plotted values are the partial effects, which, having accounted for the influence of all other predictors (Z) in the linear mixed-effects model, thus reflect the statistically independent effect of herbivore (A) biomass, (B) α-diversity, and (C) β-diversity on the response (mass-standardized bite rate). Fitted lines are linear regressions ± 95% confidence intervals. Points in (C) are scaled by local herbivore richness so that larger points reflect sites with more species. The full model results are found in table S1.
The biomass of one or a few functionally important species is often considered the primary determinant of herbivory (20). To elucidate which herbivores significantly contributed to herbivory, we used species-diversity models to quantify the unique contribution of each species to, as well as their average pairwise interactive effects on, the total grazing rate (25). Of the nine species observed in our study, four were found to independently enhance this process (Table 1). Previous studies have interpreted such a result as evidence for species complementarity (25), or the idea that each species contributes differently and additively to a process. Feeding complementarity is the most parsimonious explanation for why grazing rates scale positively with local herbivore richness (Fig. 1B), as recent field studies have shown that herbivorous fish species finely partition the niche by each targeting different components of the algal turf community and/or habitat features when feeding (26, 27). The lack of a significant interaction term suggests that these effects do not arise synergistically—that is, the presence of another species does not modify the contributions of any one species on average (Table 1). However, this interpretation should be met with caution, as these models consider only two-way interactions and linearized relationships (25), whereas actual interactions in nature may manifest between many species and may occur nonlinearly (28). Moreover, species-diversity models are also sensitive to sample size, and while some species appear to contribute substantially to grazing rate when they are present (e.g., Scarus taeniopterus, Scarus vetula, and Sparisoma viride; Fig. 2), they may not have been sufficiently abundant across the study sites (e.g., because of high fishing pressure at some sites) to generate a significant effect in our model (Table 1). Thus, while potentially conservative, these model results suggest that many species underpin the process of herbivory at the local scale, thereby creating a positive effect of herbivore α-diversity on ecosystem function.
Table 1. Results from a species-diversity model regressing mass-standardized bite rate against the proportional biomass of each species at each site, as well as their average pairwise interaction.
The average pairwise interaction was obtained by computing the product of the relative biomass of each species and then summing these products. The model explained R2 = 90% of the variation in local herbivory rate.
| Species | Estimate | SE | t | P |
| Acanthurus bahianus | 2.257 | 0.576 | 3.918 | 0.001 |
| Acanthurus coeruleus | 3.070 | 1.260 | 2.437 | 0.027 |
| Scarus iseri | 1.957 | 0.715 | 2.735 | 0.015 |
| Scarus taeniopterus | 2.608 | 3.372 | 0.773 | 0.451 |
| Scarus vetula | 0.970 | 0.883 | 1.098 | 0.288 |
| Sparisoma aurofrenatum | 1.282 | 0.375 | 3.419 | 0.004 |
| Sparisoma chrysopterum | 0.783 | 2.056 | 0.381 | 0.708 |
| Sparisoma rubripinne | −0.792 | 3.072 | −0.258 | 0.800 |
| Sparisoma viride | 1.164 | 0.828 | 1.406 | 0.179 |
| Average interaction | 0.662 | 0.421 | 1.572 | 0.136 |
Fig. 2. Contributions to total bite rate by each grazer species at each reef.
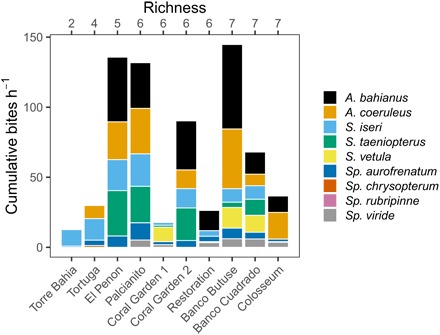
Values are averaged across all cameras at the 10 reef sites (primary x axis). Reef sites are in order of increasing herbivore species richness (secondary x axis).
Our finding that herbivory is positively associated with β-diversity (Fig. 1C) has important implications for understanding diversity effects at scales beyond local observations. Such a finding is consistent with the “spatial insurance hypothesis” (5), in which regional biodiversity becomes important across a varied landscape because the dominant contributors differ from site to site (21). This mechanism has recently been revealed at similar spatial scales in analyses of insect pollinator (29) and grassland diversity (22). Our findings also contextualize previous studies of herbivory on coral reefs, which often find different herbivores to be the dominant agents of herbivory on individual reefs [e.g., (20)], by identifying a regional biodiversity effect (i.e., “spatial dominance”) as the underlying driver of that pattern. Our findings therefore indicate that, in addition to high herbivore richness at the local scale (Fig. 1B), a diverse assemblage at the regional level may also be required to sustain ecosystem functioning across the heterogeneous reefscape, in part because different species come to dominate herbivory at different sites.
To evaluate whether the top-down biodiversity effects we observed in nature are associated with enhanced ecosystem structure—a question rarely explored in biodiversity-function research—we statistically compared each estimate of herbivory to the benthic composition of the surrounding reef (derived from independent reef-scale transect surveys). Here, we focused on the components of the benthos most closely linked to the process of grazing, namely, the degree to which algal turf communities were cropped, and the knock-on effects of algal turf canopy height to juvenile coral density (30). We found that, after accounting for the influence of coral cover and sea urchin (D. antillarum) abundance, the mean grazing rate was negatively correlated with the canopy height of the algal turf community (P = 0.03) (Fig. 3A). In turn, algal turf canopy height was negatively associated with the density of juvenile corals on the reef (P = 0.03) (Fig. 3B), a link (i.e., competitive interaction) that has been causally demonstrated elsewhere in the Caribbean (30, 31). A doubling of turf height from 2 to 4 mm predicted 10% lower coral recruitment, whereas a quadrupling to 8-mm canopy height was predicted to result in 30% fewer recruits (Fig. 3B). Thus, our findings imply a diversity-mediated cascade, wherein diverse herbivore assemblages more effectively crop the reef, in turn creating a more hospitable environment for coral settlement and survival, ultimately enhancing reef integrity. Although the components of this cascade are well established (15) and were recently corroborated at similar spatial scales (32), previous studies have generally focused only on fish biomass and did not consider the instigating role of biodiversity in this process. The importance of herbivore diversity in this cascade has clear implications for fisheries management and reef conservation (32, 33).
Fig. 3. Higher herbivore bite rates were associated with more finely cropped turfs, which would otherwise reduce the recruitment of corals to the reef.
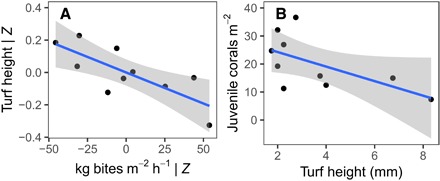
Plotted values in (A) are the partial effects of bite rate, which, having accounted for the influence of all other predictors (Z) in the multiple regression model, thus reflect the statistically independent effect of mass-standardized bite rate (kg bites per m2 per hour) on algal turf canopy height (mm) (R2 = 0.80). (B) Bivariate correlation between algal turf canopy height and the number of juvenile corals per m2 (R2 = 0.48). Fitted lines are linear regressions ± 95% confidence intervals.
Our finding that local and regional biodiversity appears to affect ecosystem functioning is timely, as several global syntheses have revealed a net loss of local species richness in tropical ecosystems over recent timescales (34, 35). While all of the species in our study occur throughout the Caribbean basin—thus forming a common regional pool of species from which local diversity can arise—stochastic processes and human activities have altered species richness on both a regional and reef-to-reef basis, with many harvested species [e.g., the large-bodied parrotfishes, which exert particularly strong impacts (31)] now being rare or absent from heavily fished reefs (Fig. 2). Such differences in local-scale richness were easy to detect using remote video assays and were a strong predictor of local herbivory rate (in addition to fish biomass). One synthesis further noted that in locations where α-diversity has not changed in recent time, this stasis was often countered by a significant shift in species composition, i.e., β-diversity. Recent compositional changes in the Caribbean are striking: The largest parrotfishes (Scarus guacamaia, Scarus coelestinus, and Scarus coeruleus) were extirpated from most locales over the past century (and were thus absent from our study), and other large-bodied species (e.g., Sparisoma viride and Scarus vetula) are now rare on heavily fished reefs (36). Ultimately, while our study provides new insight into the ecological consequences of reduced consumer richness and altered community composition on Caribbean reefs, it was conducted on a “shifted baseline” and is therefore likely to underestimate the true impact of historical biodiversity loss in the region.
Note that, although our study was replicated over >1000 km of coastline, our observations of herbivory still occurred in 1-m2 plots. However, emerging evidence indicates that an area this size is one of the scales at which turf-cropping herbivores partition the niche on coral reefs. The algal turf community considered here, while superficially homogenous, is actually a consortium of filamentous algae, crustose coralline algae, seaweed germlings, microorganisms, detritus, and a variety of endolithic resources. As a result, each 1-m2 area of this community can represent a diverse suite of resources with differing nutritional and defensive properties. Different grazer species consume these resources in a complementary fashion, through targeting different taxa (37) and spatially partitioning their feeding across microtopographic features [the millimeter to centimeter scale (27)] and among vertical versus horizontal surfaces [the centimeter to meter scale (26)]. These, too, are the small scales at which algal-coral competition and coral settlement occur on the reef (30). Thus, small plots are one of several scales at which to investigate herbivore effects in this ecosystem, and may be conservative when considering the additional axes of resource partitioning that are known to arise among herbivores at larger scales (26).
The Caribbean appears more susceptible to state shifts from coral to algal dominance than do other tropical regions (38). It is also among the most species-poor regions of the tropics in terms of herbivorous fish richness, raising the question of whether Caribbean reefs are particularly sensitive to species loss. Our analysis indicates that most common grazer species in the Caribbean are critical to the process of herbivory. This result reinforces the notion that herbivore communities exhibit little functional redundancy if examined at sufficient resolution (19, 26, 27, 37). Therefore, measures that foster both herbivore biomass and species diversity at local and regional spatial scales are likely to be more effective in rebuilding Caribbean reef resilience than will approaches focused solely on herbivore abundance, biomass, or identity.
MATERIALS AND METHODS
Experimental design
In May 2017, we studied 10 reefs among six locations that span >1000 km of coastline in the Dominican Republic (fig. S1). We selected these reefs because they vary in herbivore richness and biomass (likely owing to variation in fishing pressure), but otherwise were of the same depth and reef type and are composed of species from the same regional pool of flora and fauna. Reef-to-reef differences in herbivore community structure were randomly distributed across the study range (i.e., they did not follow some underlying oceanographic gradient). Moreover, any potential site-to-site differences in benthic community structure or environmental condition, while small, were accounted for in our model (see below). At each location (except La Caleta and Pedernales, where we only studied one reef), reefs were separated by ~1.5 to 7 km. Locations were separated by ~50 to 250 km (fig. S1). At each reef, we deployed video cameras to capture the process of herbivory and conducted SCUBA-based visual transect surveys to characterize benthic community structure. Camera assays provide a number of benefits beyond traditional approaches: They are less intrusive than diver surveys; allow one to directly quantify the ecological process of interest (herbivory rate) rather than infer it from community attributes (e.g., standing stock proxies such as algal or herbivore abundance); and allow more accurate estimates of herbivory compared to diver follows, because each foraging bout can be slowed down or reviewed during playback to ensure correct scoring. We studied all reefs within a 2-week period to limit confounding variation in abiotic factors (e.g., season) that may affect rates of herbivory or benthic condition. Last, we used a general linear mixed-effects model to quantify the independent effects of herbivore community biomass, herbivore diversity, and habitat characteristics on herbivory.
Quantifying benthic community structure
We used a modified Atlantic and Gulf Rapid Reef Assessment (AGRRA) protocol to quantify sessile benthic community structure on each reef. These surveys were performed to characterize the reef as well as to gauge whether variation in herbivore feeding is associated with notable differences in reef condition. At each site, four replicate 10-m transect lines were deployed randomly on the reef (minimum of 10-m spacing between each). The number of centimeters on the tape intercepted by live coral (measured for each species), sponges, gorgonians, and benthic algae [measured by functional group: filamentous turf algae, encrusting coralline algae (Corallinales), non-coralline (peyssonnelid) chip-like crusts, articulated coralline algae, and upright fleshy macroalgae] was recorded. For each species/functional group found on a given transect, we counted the number of centimeters in which it was intercepted and divided that number by the total transect length to calculate percent cover. On each transect, we also measured the canopy height (to the nearest millimeter) of the algal turf community using a ruler (n = 5 to 10 measurements per transect); these measurements were then averaged to produce a transect-level estimate (n = 4 per reef). Coral species were aggregated into total coral cover for analysis. Filamentous turf algae and crustose coralline algae were combined as the total algal turf cover in our analysis, since both (as well as the detritus, seaweed germlings, and cyanobacteria that reside in the turf) are part of the “epilithic algal matrix” targeted by scraping and cropping herbivores. All upright fleshy macroalgae, articulated coralline algae, and peyssonnelids were aggregated as total macroalgal cover in our analysis.
We quantified the density of juvenile corals on the reef by deploying a 25 cm × 25 cm quadrat at five intervals (0-, 2.5-, 5-, 7.5-, and 10-m marks) along each benthic transect. At each interval, the quadrat was placed on the nearest hard reef substratum largely devoid of adult coral (i.e., <25% cover of live coral). Operationally, we defined juvenile corals as those 10 to 40 mm in diameter; individuals of this size have already “run the recruitment gauntlet” and therefore may, with time, contribute to the adult population (30). Juvenile corals 10 to 40 mm in size are not, however, large enough to influence herbivore biomass or richness. Each juvenile coral found in the quadrat was identified to species and measured to the nearest millimeter. Site-level means were generated from the 20 quadrats performed on the reef.
Last, the density and sizes of the sea urchin D. antillarum were quantified within two belt transects (each 1 m × 10 m) on either side of the transect tape. Thus, within each transect, we surveyed a 20-m2 area (n = 4 per reef). We note that this urchin was functionally absent from all but two sites in our study.
Quantifying herbivory rate
We used video cameras to quantify rates of herbivore grazing on the algal turf community at each site. We deployed cameras (Hero 3 and Hero 4, GoPro Inc.) at three haphazardly selected locations at the same depth (8 to 10 m) around each reef. Cameras were distributed at least 30 to 50 m apart (i.e., comparable in total spread to that of a traditional fish transect survey); this distribution likely exceeded the home range size of some, but not all, herbivorous fish species. However, given that we were specifically interested in quantifying the aggregate effects of grazing observed in each plot through time, any individuals with large home ranges that visited multiple plots (or a single plot multiple times) do not confound our results, but rather intentionally reflect the natural, cumulative impacts of the herbivore community in each plot of reef over time. We positioned each camera so that its field of view was focused on a flat, 1-m2 area of hard (calcium carbonate) continuous reef substrate. In each case, the algal turf community occupied at least 75% of the plot. Patches of sand or rubble, while rare, were avoided. At the beginning of the video, the diver indicated the 1-m2 area in the frame with a meter stick or tape, after which point the plots were left alone for an average of 43 ± 7 min (mean ± SD), the timing of which varied depending on daily logistical constraints. Several replicates were discarded because of poor video quality, resulting in n = 26 cameras capturing 1049 min or ~17.5 hours of observations across all reef sites.
To ensure that our videos were of sufficient duration to rigorously estimate rates of herbivory at each site (and to investigate the potential biases associated with using video footage of varying length), we constructed accumulation curves for both the number of bites and the total species richness observed within each video. We then applied a change point analysis to identify the exact time point at which the curves saturated (see supplementary code). Both the number of bites and the number of species present in a video saturated after an average of 18 and 17 min, respectively (figs. S2 and S3), indicating that our assays (mean, 43 min) were of more than sufficient duration to capture the process of herbivory.
When scoring each video on the computer, the 1-m2 plot in the field of view was traced onto transparency paper placed over the computer screen and marked in 10-cm increments. Scoring of herbivory began 30 s after all human activity ceased in the field of view and fishes resumed their normal swimming and feeding behavior, a period usually lasting 1 to 5 min. No humans were diving in the vicinity of the assay, other than for deployment and retrieval. Each time a nominally herbivorous fish—here, parrotfishes (family Labridae) and surgeonfishes (family Acanthuridae)—entered the plot and first took a bite, we identified the individual to species and estimated its length to the nearest 5-cm size increment. We then enumerated the total number of bites each individual took while in the frame. A bite was counted as any time a fish struck the benthos with its jaw open.
We ignored all observations of juvenile fishes <10 cm, as they could not be accurately identified to species. We also ignored the feeding activities of the sea urchin D. antillarum, as it was not observed in any of the videos and we later accounted for its effects in our models (using urchin density from our benthic survey; see above). We also noted 25 instances where damselfish (family Pomacentridae) interfered with grazing. We removed these feeding events from our analysis. We removed a further 213 data points where fish spent <4 s in the plot and did not feed, and thus were obviously only passing through the plot and not interested in feeding (thereby not contributing to the process of interest). Four seconds reflected a natural breakpoint in the data for fishes that did not take a bite, and was well below the average length of time that individuals who did feed spent in the plot (21.9 s). These procedures left a total of n = 759 observations (feeding bouts) in our final dataset.
Data preparation
We aggregated the benthic survey data at the reef level to produce an average site-level abundance (% cover) for corals, macroalgae, and the algal turf community and to produce an average estimate of algal turf canopy height, urchin density, and juvenile coral density for each site. For each fish observed in our video assay, we estimated its biomass using an established, species-specific length to weight relationship. Total herbivore abundance (i.e., density) and biomass observed in each assay were computed by summing the individual counts and biomasses of each species observed in the assay plot, respectively. To compute the mass-standardized bite rate [i.e., a measure of herbivory that incorporates the known positive influence of herbivore size on feeding impact (20)], we multiplied every bite taken by an individual fish by its biomass (kg × bites) before totaling all bites for each species and dividing by the duration of the video footage. Bite rates were then multiplied by 60 min to yield mass-standardized bites per m2 per hour.
Local α-diversity was calculated as the total number of unique species that grazed in each video assay. We chose to compute diversity from the camera footage rather than using SCUBA-based visual surveys because these values (as well as associated abundance and biomass estimates) reflect the diversity of fishes that actually interacted with each plot and contributed to the process of herbivory. To calculate LCBD, we first constructed a community distance matrix based on presence-absence data from each assay using the Jaccard dissimilarity index. We then partitioned the dissimilarity matrix into the “richness difference” component, which quantifies the differences in community composition that arise from depauperate sites being nested subsets of more speciose sites, and the “replacement” component, which identifies species or sets of species that turn over along a predefined gradient (39). We analyzed both components in our model, but only the richness difference component was significant (table S1), confirming our initial supposition that we had not inadvertently captured some underlying gradient (i.e., less speciose sites were nested subsets of the most diverse sites rather than wholly different sets of species). We then computed indices of LCBD as our index of β-diversity using the square root of the dissimilarity matrices for the richness and replacement components (23, 39). The benefits of the LCBD approach are that it (i) provides a unique measure of β-diversity for each site, increasing statistical power, and (ii) is not derived from, and therefore is statistically independent of, α-diversity (a common issue in macroecological studies). Local α-diversity was only moderately correlated with the richness component of LCBD (r = −0.55) and showed little correlation with the replacement component of LCBD (r = −0.06), indicating that having all three in the model did not introduce strong collinearity.
Statistical analysis
All analyses were conducted in R version 3.4.3 (see supplementary code) (40). We initially fit a generalized linear mixed-effects model predicting mass-standardized bite rate using total herbivore biomass, herbivore α-diversity, the LCBD reflecting both components of β-diversity, percent coral cover, percent algal turf cover, percent macroalgal cover, and the abundance of D. antillarum as fixed effects. As our random structure, we nested camera within reef within location to account for potential nonindependence of observations, and to account for the impact of unmeasured factors on herbivory rate. Subsequent exploration of variance inflation factors (VIFs) revealed strong multicollinearity between coral cover, macroalgal cover, and turf cover (VIF ≫ 2). We therefore removed macroalgal cover from the model, focusing on coral cover (which concentrates or diffuses herbivore grazing) and algal turf cover (a direct indicator of resource availability to grazers). We additionally tested for the presence of significant interactions between α-diversity and either the richness or replacement components of β-diversity, but in all cases, the interaction was not significant (P = 0.20, 0.10, 0.39, and 0.88 for richness, replacement, and both interactions in the same model, respectively). Thus, we chose to present only the main effects of diversity. Our data met the assumptions of normality and equal variance for the above tests, as indicated by graphical analysis of residuals.
To test whether the effect of species richness in our model was due to the presence of one or a few strongly interacting species versus many species, we fit a general linear model regressing the proportional biomass of each species against the corresponding community-wide mass-standardized bite rate. We further investigated the potential for synergies by including an “average interaction” term, calculated by taking the product of the relative biomass of each pair of species, and summing across all pairs present in a community (25). This approach limits us to having to evaluate each pairwise interaction, which is prohibitive at low sample sizes. However, this construction assumes that functioning is maximized when all species are even in terms of their biomass, and thus, the lack of a significant interaction may not reflect a true lack of synergy, but instead this simplifying assumption (25). The presence of significant main effects (i.e., species’ identities) captures significant and independent contributions to functioning, and thus may indicate complementarity among multiple species or dominance by one of few species, depending on the community composition. We log10-transformed both the response and the average interaction term not only to satisfy the assumptions of constant variance and normality of errors but also to approximate any potentially nonlinear (e.g., power) relationships (28). We also fixed the intercept to 0, as herbivory cannot occur in the absence of all species (25).
Last, to test the degree to which herbivory rate predicts biologically related aspects of reef community structure, we averaged mass-standardized bite rate per reef (n = 10) and used it to predict average algal turf canopy height at the reef scale. We included two additional covariates: coral cover (which concentrates or diffuses herbivore grazing) and the abundance of the sea urchin D. antillarum [which strongly reduces algal cover when at high densities, and thus directly competes with herbivorous fishes for algal resources when at high density; (24)]. We further predicted the effect of algal turf canopy height on the density of juvenile corals—given its known negative impact on coral recruitment (30)—using simple linear regression. Both responses were log10-transformed to meet assumptions of constant variance and normality of errors.
Supplementary Material
Acknowledgments
This work was conducted under research permissions granted to R.E.T. for ecological monitoring work in 2017. We thank R. de Leon for assistance in the field. Funding: D.B.R. and J.S.L. were supported by Bigelow Laboratory for Ocean Sciences. Benthic surveys were carried out with funds awarded to R.E.T. and R.S.S. from Propogas DR, Incorporated. A.A.I.-G. was supported by a National Science Foundation Research Experience for Undergraduates program at Bigelow Laboratory (NSF EAR 1460861). Author contributions: D.B.R., S.J.B., and J.S.L. conceived the study; D.B.R., R.E.T., and R.S.S. collected the data; A.A.I.-G. analyzed the video footage; J.S.L. conducted the analyses; and J.S.L. and D.B.R. wrote the manuscript with input from all coauthors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav6420/DC1
Fig. S1. Locations of our 10 study sites distributed across the Dominican Republic.
Fig. S2. Accumulation curves for the number of bites observed in each video assay.
Fig. S3. Species accumulation curves for each video assay.
Table S1. Output from a linear mixed-effects model predicting mass-standardized bite rate (kg bites per m2 per hour) on benthic turf algae.
Data file S1. Metadata information.
Data file S2. Benthic community structure data.
Data file S3. Herbivore identity, biomass, richness, and bite rate data.
Data file S4. R code script for reproducing all analyses.
REFERENCES AND NOTES
- 1.Whittaker R. H., Vegetation of the Siskiyou mountains, Oregon and California. Ecol. Monogr. 30, 279–338 (1960). [Google Scholar]
- 2.Cardinale B. J., Duffy J. E., Gonzalez A., Hooper D. U., Perrings C., Venail P., Narwani A., Mace G. M., Tilman D., Wardle D. A., Kinzig A. P., Daily G. C., Loreau M., Grace J. B., Larigauderie A., Srivastava D. S., Naeem S., Biodiversity loss and its impact on humanity. Nature 486, 59–67 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Duffy J. E., Godwin C. M., Cardinale B. J., Biodiversity effects in the wild are common and as strong as key drivers of productivity. Nature 549, 261–264 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Srivastava D. S., Vellend M., Biodiversity-ecosystem function research: Is it relevant to conservation? Annu. Rev. Ecol. Evol. Syst. 36, 267–294 (2005). [Google Scholar]
- 5.Loreau M., Mouquet N., Gonzalez A., Biodiversity as spatial insurance in heterogeneous landscapes. Proc. Natl. Acad. Sci. U.S.A. 100, 12765–12770 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S., Loreau M., Biodiversity and ecosystem stability across scales in metacommunities. Ecol. Lett. 19, 510–518 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.France K. E., Duffy J. E., Diversity and dispersal interactively affect predictability of ecosystem function. Nature 441, 1139–1143 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Venail P. A., MacLean R. C., Bouvier T., Brockhurst M. A., Hochberg M. E., Mouquet N., Diversity and productivity peak at intermediate dispersal rate in evolving metacommunities. Nature 452, 210–214 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Staddon P., Lindo Z., Crittenden P. D., Gilbert F., Gonzalez A., Connectivity, non-random extinction and ecosystem function in experimental metacommunities. Ecol. Lett. 13, 543–552 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Pasari J. R., Levi T., Zavaleta E. S., Tilman D., Several scales of biodiversity affect ecosystem multifunctionality. Proc. Natl. Acad. Sci. U.S.A. 110, 10219–10222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hautier Y., Isbell F., Borer E. T., Seabloom E. W., Harpole W. S., Lind E. M., MacDougall A. S., Stevens C. J., Adler P. B., Alberti J., Bakker J. D., Brudvig L. A., Buckley Y. M., Cadotte M., Caldeira M. C., Chaneton E. J., Chu C., Daleo P., Dickman C. R., Dwyer J. M., Eskelinen A., Fay P. A., Firn J., Hagenah N., Hillebrand H., Iribarne O., Kirkman K. P., Knops J. M. H., la Pierre K. J., McCulley R. L., Morgan J. W., Pärtel M., Pascual J., Price J. N., Prober S. M., Risch A. C., Sankaran M., Schuetz M., Standish R. J., Virtanen R., Wardle G. M., Yahdjian L., Hector A., Local loss and spatial homogenization of plant diversity reduce ecosystem multifunctionality. Nat. Ecol. Evol. 2, 50–56 (2018). [DOI] [PubMed] [Google Scholar]
- 12.van der Plas F., Manning P., Soliveres S., Allan E., Scherer-Lorenzen M., Verheyen K., Wirth C., Zavala M. A., Ampoorter E., Baeten L., Barbaro L., Bauhus J., Benavides R., Benneter A., Bonal D., Bouriaud O., Bruelheide H., Bussotti F., Carnol M., Castagneyrol B., Charbonnier Y., Coomes D. A., Coppi A., Bastias C. C., Dawud S. M., de Wandeler H., Domisch T., Finér L., Gessler A., Granier A., Grossiord C., Guyot V., Hättenschwiler S., Jactel H., Jaroszewicz B., Joly F.-x., Jucker T., Koricheva J., Milligan H., Mueller S., Muys B., Nguyen D., Pollastrini M., Ratcliffe S., Raulund-Rasmussen K., Selvi F., Stenlid J., Valladares F., Vesterdal L., Zielínski D., Fischer M., Biotic homogenization can decrease landscape-scale forest multifunctionality. Proc. Natl. Acad. Sci. U.S.A. 113, 3557–3562 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes A. D., Weigelt P., Jochum M., Ott D., Hodapp D., Haneda N. F., Brose U., Species richness and biomass explain spatial turnover in ecosystem functioning across tropical and temperate ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150279 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burley H. M., Mokany K., Ferrier S., Laffan S. W., Williams K. J., Harwood T. D., Primary productivity is weakly related to floristic alpha and beta diversity across Australia. Glob. Ecol. Biogeogr. 25, 1294–1307 (2016). [Google Scholar]
- 15.Hughes T. P., Graham N. A. J., Jackson J. B. C., Mumby P. J., Steneck R. S., Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 25, 633–642 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Hughes T. P., Barnes M. L., Bellwood D. R., Cinner J. E., Cumming G. S., Jackson J. B. C., Kleypas J., van de Leemput I. A., Lough J. M., Morrison T. H., Palumbi S. R., van Nes E. H., Scheffer M., Coral reefs in the Anthropocene. Nature 546, 82–90 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Burkepile D. E., Hay M. E., Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc. Natl. Acad. Sci. U.S.A. 105, 16201–16206 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burkepile D. E., Hay M. E., Feeding complementarity versus redundancy among herbivorous fishes on a Caribbean reef. Coral Reefs 30, 351–362 (2011). [Google Scholar]
- 19.Rasher D. B., Hoey A. S., Hay M. E., Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology 94, 1347–1358 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoey A. S., Bellwood D. R., Limited functional redundancy in a high diversity system: Single species dominates key ecological process on coral reefs. Ecosystems 12, 1316–1328 (2009). [Google Scholar]
- 21.Cardinale B. J., Ives A. R., Inchausti P., Effects of species diversity on the primary productivity of ecosystems: Extending our spatial and temporal scales of inference. Oikos 104, 437–450 (2004). [Google Scholar]
- 22.Isbell F., Cowles J., Dee L. E., Loreau M., Reich P. B., Gonzalez A., Hector A., Schmid B., Quantifying effects of biodiversity on ecosystem functioning across times and places. Ecol. Lett. 21, 763–778 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legendre P., De Cáceres M., Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 16, 951–963 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Carpenter R. C., Mass mortality of a Caribbean sea urchin: Immediate effects on community metabolism and other herbivores. Proc. Natl. Acad. Sci. U.S.A. 85, 511–514 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirwan L., Connolly J., Finn J. A., Brophy C., Lüscher A., Nyfeler D., Sebastià M. T., Diversity-interaction modeling: Estimating contributions of species identities and interactions to ecosystem function. Ecology 90, 2032–2038 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Adam T. C., Kelley M., Ruttenberg B. I., Burkepile D. E., Resource partitioning along multiple niche axes drives functional diversity in parrotfishes on Caribbean coral reefs. Oecologia 179, 1173–1185 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Brandl S. J., Bellwood D. R., Microtopographic refuges shape consumer-producer dynamics by mediating consumer functional diversity. Oecologia 182, 203–217 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Connolly J., Bell T., Bolger T., Brophy C., Carnus T., Finn J. A., Kirwan L., Isbell F., Levine J., Lüscher A., Picasso V., Roscher C., Sebastia M. T., Suter M., Weigelt A., An improved model to predict the effects of changing biodiversity levels on ecosystem function. J. Ecol. 101, 344–355 (2013). [Google Scholar]
- 29.Winfree R., Reilly J. R., Bartomeus I., Cariveau D. P., Williams N. M., Gibbs J., Species turnover promotes the importance of bee diversity for crop pollination at regional scales. Science 359, 791–793 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Arnold S. N., Steneck R. S., Mumby P. J., Running the gauntlet: Inhibitory effects of algal turfs on the processes of coral recruitment. Mar. Ecol. Prog. Ser. 414, 91–105 (2010). [Google Scholar]
- 31.Steneck R. S., Arnold S. N., Mumby P. J., Experiment mimics fishing on parrotfish: Insights on coral reef recovery and alternative attractors. Mar. Ecol. Prog. Ser. 506, 115–127 (2014). [Google Scholar]
- 32.Steneck R. S., Mumby P. J., Macdonald C., Rasher D. B., Stoyle G., Attenuating effects of ecosystem management on coral reefs. Sci. Adv. 4, eaao5493 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozec Y.-M., O’Farrell S., Bruggemann J. H., Luckhurst B. E., Mumby P. J., Tradeoffs between fisheries harvest and the resilience of coral reefs. Proc. Natl. Acad. Sci. U.S.A. 113, 4536–4541 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vellend M., Baeten L., Myers-Smith I. H., Elmendorf S. C., Beauséjour R., Brown C. D., De Frenne P., Verheyen K., Wipf S., Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl. Acad. Sci. U.S.A. 110, 19456–19459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dornelas M., Gotelli N. J., McGill B., Shimadzu H., Moyes F., Sievers C., Magurran A. E., Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Hawkins J. P., Roberts C. M., Effects of artisanal fishing on Caribbean coral reefs. Conserv. Biol. 18, 215–226 (2004). [Google Scholar]
- 37.Kelly E. L. A., Eynaud Y., Clements S. M., Gleason M., Sparks R. T., Williams I. D., Smith J. E., Investigating functional redundancy versus complementarity in Hawaiian herbivorous coral reef fishes. Oecologia 182, 1151–1163 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Roff G., Mumby P. J., Global disparity in the resilience of coral reefs. Trends Ecol. Evol. 27, 404–413 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Legendre P., Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 23, 1324–1334 (2014). [Google Scholar]
- 40.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav6420/DC1
Fig. S1. Locations of our 10 study sites distributed across the Dominican Republic.
Fig. S2. Accumulation curves for the number of bites observed in each video assay.
Fig. S3. Species accumulation curves for each video assay.
Table S1. Output from a linear mixed-effects model predicting mass-standardized bite rate (kg bites per m2 per hour) on benthic turf algae.
Data file S1. Metadata information.
Data file S2. Benthic community structure data.
Data file S3. Herbivore identity, biomass, richness, and bite rate data.
Data file S4. R code script for reproducing all analyses.


