Tropical secondary forests recover quickly (decades) in tree species richness but slowly (centuries) in species composition.
Abstract
Old-growth tropical forests harbor an immense diversity of tree species but are rapidly being cleared, while secondary forests that regrow on abandoned agricultural lands increase in extent. We assess how tree species richness and composition recover during secondary succession across gradients in environmental conditions and anthropogenic disturbance in an unprecedented multisite analysis for the Neotropics. Secondary forests recover remarkably fast in species richness but slowly in species composition. Secondary forests take a median time of five decades to recover the species richness of old-growth forest (80% recovery after 20 years) based on rarefaction analysis. Full recovery of species composition takes centuries (only 34% recovery after 20 years). A dual strategy that maintains both old-growth forests and species-rich secondary forests is therefore crucial for biodiversity conservation in human-modified tropical landscapes.
INTRODUCTION
Tropical forests store the majority of the world’s tree diversity, with an estimated 53,000 tree species (1). Over the past decades, many hyperdiverse old-growth forests and their biodiversity have disappeared because of the conversion of forests into agricultural lands (2). Secondary forests regrowing after abandonment of agricultural lands increase rapidly in extent and may constitute important biodiversity reservoirs (3). It is therefore critical to assess the biodiversity conservation potential of secondary tropical forests (3, 4) by analyzing biodiversity recovery (i.e., the rate of recovery to a predisturbance state) of tropical forests during secondary succession. Biodiversity recovery could be fast because species richness (i.e., the number of species) may recover rapidly to old-growth forest levels over succession (5). Recovery of species composition (i.e., species identity and relative abundance), in contrast, could take centuries (6), particularly if old-growth species go locally extinct or fail to be dispersed into regenerating forest areas.
Recovery rates of tree species richness and composition have been evaluated for individual sites (7–9) and summarized in meta-analyses (5, 10), but how recovery rates vary across large-scale gradients in environmental conditions and anthropogenic disturbance remains unknown. Community assembly during succession depends on the size and the composition of the regional species pool and on local effects of environmental filtering and dispersal limitation that determine which species actually establish. The size and composition of the regional species pool have been shaped by historical effects and vary with water and soil nutrient availability (11). Locally, tree species’ establishment during succession depends on (i) water and nutrient availability that constrain or facilitate seedling establishment, (ii) forest cover and quality in the surrounding landscape matrix that indicate the availability and proximity of seed sources and dispersal agents (12, 13), and (iii) the type and intensity of previous land use. Previous land use modifies environmental conditions, such as soil structure and nutrient availability, and determines the presence of forest legacies (e.g., remnant trees, a soil seed bank, and resprouting tree stumps) that accelerate succession (14).
Here, we assess how tree species richness and composition recover during secondary succession across major gradients in environmental conditions and anthropogenic disturbance in the Neotropics using original data from 56 sites, 1630 plots, and >183,000 trees (Fig. 1 and table S1) (15). We quantify biodiversity recovery as the absolute recovery rate at which tree species richness increases over succession and as the relative recovery of species richness and composition to old-growth forest values to assess if, and when, secondary forests attain the old-growth stage. We hypothesize that biodiversity recovery will (i) increase with water availability and soil fertility in absolute terms, because of larger regional species pools in wetter forests and at high fertility soils (16, 17) and enhanced tree growth and survival under these conditions, but decrease in relative terms because of the larger species pool that needs to be recovered; (ii) increase with forest cover in the landscape matrix because high forest cover tends to indicate greater availability of old-growth forests in the landscape that ensure seed availability of old-growth species (12, 13); and (iii) be higher on abandoned shifting cultivation fields compared to pasture because of the lower levels of disturbance associated with shifting cultivation (18).
Fig. 1. Tree species richness and recovery of Neotropical secondary forests.
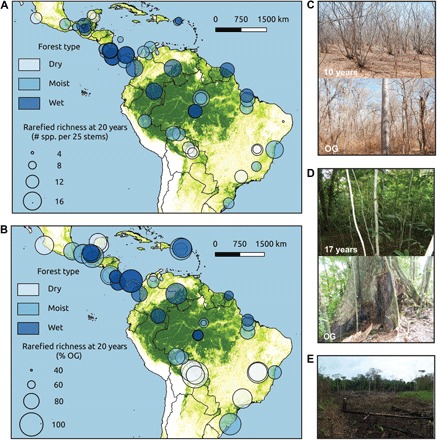
(A) Absolute recovery of species richness (number of species per 25 stems). (B) Relative recovery of species richness [% old-growth (OG)] after 20 years. The 56 study sites (45 sites for relative recovery) are indicated; symbol size scales with predicted recovery at 20 years after abandonment. Green shading indicates forest cover in the year 2000 (39). Dry forests have an annual rainfall of <1500 mm year−1, moist forests have an annual rainfall of 1500 to 2499 mm year−1, and wet forests have an annual rainfall of ≥2500 mm year−1. (C) Forest recovery in dry tropical forests: secondary forest and old-growth forest plot in a dry forest site in the Atlantic forest in Brazil. (D) Forest recovery in wet tropical forests: secondary forest and old-growth forest plot in the wet forest site Sarapiquí in Costa Rica. Stand age (in years) of the secondary forests is indicated. (E) Forest legacies in an agricultural field in Márques de Comillas, Mexico. Photo credit: M.M.E.-S., D.M.A.R., and M.M.-R.
We assessed the biodiversity recovery of Neotropical forests using data from 56 chronosequence sites, where successional change is inferred from plots that vary in time since abandonment (Fig. 1 and table S1). For each site, we calculated absolute recovery of tree species richness per secondary forest plot as the number of species per 25 stems ≥5 cm diameter at breast height (dbh). For 45 sites for which data from old-growth forest plots were available, we calculated relative recovery of species richness as a percentage of the mean number of species per 25 stems of old-growth plots and relative recovery of species composition (the mean pairwise similarity in species composition between secondary and old-growth plots based on the Chao-Jaccard index expressed as a percentage of the mean within-site similarity between old-growth plots). We used linear mixed-effects models to model absolute recovery of species richness and relative recovery of species richness and composition as a function of stand age, the size of the local old-growth forest species pool (for relative recovery; calculated using the Chao 1 estimator), climatic water availability (CWA), soil cation exchange capacity (CEC; an indicator for soil fertility), forest cover in the landscape matrix (based on tree cover in the year 2000 in a 5000-m radius around the plots), previous land use (shifting cultivation, pasture, or a combination of these), and plot size (to account for variation in plot size across sites) as fixed effects, along with a random intercept and slope for stand age per site.
RESULTS
Recovery of species richness and species composition
Absolute recovery of tree species richness and relative recovery of species richness and composition significantly increased with stand age (Fig. 2). After 20 years, predicted species richness was, on average, 11 species per 25 stems but varied fourfold (from 4 to 16 species; Fig. 2A) across sites. Predicted relative recovery of species richness was, on average, 80% of the richness of old-growth forest after 20 years in standardized samples of 25 stems and varied twofold across sites (from 46 to 99%; Fig. 2B). Predicted relative recovery of composition was, on average, 34% after 20 years, ranging from 5 to 102% across sites (Fig. 2C).
Fig. 2. Absolute recovery of species richness and relative recovery of species richness and composition in relation to stand age for Neotropical secondary forests.
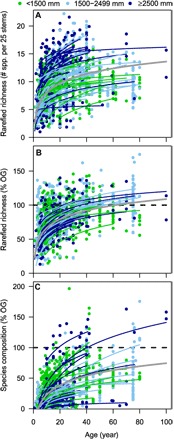
Each line indicates predicted recovery per site based on the site-specific intercept and slope from the mixed-effects models. Lines span the age range of secondary forest per site; symbols indicate the individual plots. Dry forests (annual rainfall of <1500 mm year−1) are indicated in green, moist forests (1500 to 2499 mm year−1) are indicated in light blue, and wet forests (≥2500 mm year−1) are indicated in dark blue. The gray line indicates the average predicted recovery rate for a site that is recovering after shifting cultivation, with all other predictors kept constant at the mean. (A) Rarefied species richness (per 25 stems; n = 56 sites). (B) Relative recovery of rarefied species richness [as a percentage of old-growth (% OG) forest; n = 45 sites]. The black dashed line indicates 100% recovery to the species richness of old-growth forest. (C) Relative recovery of species richness (n = 45 sites) based on the Chao-Jaccard index. The black dashed line indicates 100% recovery to the mean similarity in species composition (0.47 ± 0.040 SE) between old-growth plots in the same site averaged across the 41 sites with at least two old-growth plots to account for within-site variation in composition across old-growth plots.
Strong effects of stand age on biodiversity recovery
Among all predictors, stand age had the strongest effect on all three types of recovery (Fig. 3). Absolute recovery of species richness also significantly increased with CWA and with plot size but was not influenced by CEC, landscape forest cover, and previous land use (Fig. 3A and fig. S2). Relative recovery of species richness significantly decreased with the size of the species pool and increased with forest cover (Fig. 3B). Relative recovery of species composition significantly increased with plot size (Fig. 3C).
Fig. 3. Effects of stand age, the size of the local old-growth forest species pool, CWA, CEC, forest cover, previous land-use type, and plot size on biodiversity recovery in Neotropical secondary forests.
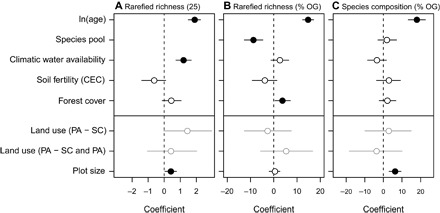
The size of the local old-growth forest species pool was estimated based on the Chao 1 estimator. Standardized coefficients with bootstrapped 95% confidence intervals are indicated. Negative coefficients indicate a negative relation, and positive coefficients indicate a positive relation. Effect sizes of land-use type comparisons are not directly comparable with those of the other predictors. SC, shifting cultivation; SC and PA, some plots shifting cultivation and some plots pasture; PA, pasture. Filled symbols indicate significant responses, and open symbols indicate nonsignificant responses. (A) Absolute recovery of rarefied species richness (number of species per 25 stems; n = 56 sites). Effects of the local species pool on absolute recovery of rarefied richness were not included, as old-growth plots were not available for all sites. (B) Relative recovery of rarefied richness [% old-growth (OG); n = 45 sites]. (C) Relative recovery of species composition [% OG; based on the Chao-Jaccard index (31)], accounting for variation in composition among old-growth plots (n = 45 sites).
Time needed to recover to old-growth forest values
Across sites, the median predicted time span to full recovery of old-growth forest values was 54 years for species richness (range, 11 to 228 years) and 780 years for species composition (range, 19 years to no recovery at all). Recovery to 90% of old-growth values was 31 years for species richness (range, 5 to 134 years) and 487 years for species composition (range, 14 years to no recovery). Given the high median value and tremendous site-to-site variation in relative recovery of species composition (Fig. 2C), it is safest to conclude that recovery to old-growth forest composition may take centuries.
DISCUSSION
Quick recovery of species richness but slow recovery of species composition
Tree species richness increased very rapidly during secondary succession with 80% recovery of old-growth values after only 20 years, which highlights the resilience of tropical forests in terms of species richness. In many secondary forests, tree species richness surpassed that of old-growth forest (Fig. 2B), which is in line with the intermediate disturbance hypothesis (19, 20): Biodiversity peaks in mid-successional forests because of the co-occurrence of persisting pioneer species that established just after disturbance and late-successional, shade-tolerant species that established in the shade of pioneers (7, 9). Fast relative recovery of species richness is likely facilitated by forest legacies (e.g., a soil seed bank, tree stumps, and roots from which trees establish), by remnant trees that attract seed dispersal agents (14), and by propagule availability in the landscape matrix.
Relative recovery of species composition was much slower (5, 10) because of the low dispersal capacity of rare old-growth specialists that may delay their arrival, as well as the often centuries-long lifespan of trees that results in slow species replacement over succession. Secondary forests have a remarkably high tree species richness, but much of their diversity may be accounted for by second-growth specialists (21). Despite the presence of old-growth species in secondary forests (7, 9), full recovery of old-growth forest composition is estimated to take centuries, assuming propagule availability. Hence, secondary forests have a high conservation value in human-modified tropical landscapes, but in the short term, they cannot replace old-growth forests that harbor many old-growth specialists (22).
Effects of climate and landscape forest cover
Absolute recovery of species richness increased with water availability, which might suggest that more species are able to establish under wetter conditions, as a result of weaker environmental filtering (23). Nevertheless, the slow absolute recovery of species richness in sites with low water availability may also result from the smaller species pool in these forests (fig. S3) and mirrors variation in species richness of old-growth forests in the Neotropics.
Relative recovery of species richness decreased with the size of the local species pool and increased with landscape forest cover. Since the size of the species pool is strongly, positively related to CWA (fig. S3), recovery may be faster in drier forests where the lower number of species present allows for faster recovery. High forest cover is generally associated with greater availability of seed trees and dispersal agents and increased landscape connectivity, enhancing relative recovery of species richness. For 45 of our sites, we estimated that 53% (±3.8 SE) of total forest cover around the plots consisted of secondary forest, with the remainder consisting of old-growth forest. Propagule availability of both secondary and old-growth forest species therefore ensured recovery. Nevertheless, the effect of forest cover on relative recovery of species richness was weak and did not influence absolute recovery of species richness and relative recovery of species composition, possibly because we quantified landscape forest cover for the year 2000 only. Ideally, we would have included the surrounding forest cover at the time of abandonment for each plot, but unfortunately, historical forest cover data were not always available. Therefore, we used a remote sensing–based tree cover map for the year 2000 (24), which provided a standardized measure of forest cover for all sites, although local accuracy is not completely verified. Another reason for finding weak effects of landscape forest cover may be that most of our sites had a relatively high landscape forest cover (>50%), although, overall, the range in landscape forest cover across our sites was large (9.4 to 99.9%; table S1). Possibly, biodiversity recovery is only hampered at very low levels of landscape forest cover.
No effects of soil fertility and previous land use
Unexpectedly, we did not find soil fertility effects on biodiversity recovery, possibly because (i) CEC was obtained from a global database rather than locally measured for many sites, (ii) phosphorus and nitrogen may be more important than CEC, and/or (iii) biogeographical history may be driving the observed patterns (i.e., higher diversity in the central and western Amazon than in Central America and Mexico). We neither detected differences in biodiversity recovery among the broad categories of previous land use that we defined, despite known effects of land-use history in some of our sites (13, 25), which could be due to within–land-use type variation in land-use intensity (13). Recovery will likely depend on the number of cycles that a fallow is cultivated or used as pasture and on the use of fire (25), but it proved impossible to obtain detailed information in a standardized way for all sites. Recovery will likely also depend on the extent of additional perturbations during the recovery process (26), but we neither had data on the occurrence of disturbances after secondary forests started regrowing.
Conclusions
We show that species richness recovers remarkably fast in secondary forests across the Neotropics, which highlights their potential for biodiversity conservation in human-modified tropical landscapes. Forest cover in the surrounding landscape should be maintained to safeguard seed sources and dispersers. Average forest cover in our sites was high (76%); recovery may be much slower in severely deforested landscapes. Fast recovery of species richness, along with fast recovery of standing biomass (27), could also promote the provision of other ecosystem services, such as carbon storage and sequestration (15, 28, 29). Secondary forests should be left to grow to advanced age to sustain species pools in the landscape and to enhance landscape connectivity (26), particularly where old-growth forests are nearby (12). Our results indicate that natural regeneration is an effective, nature-based solution for maintaining tree biodiversity. Species composition, in contrast, may take centuries to recover. Conservation policies and restoration efforts should therefore maintain both secondary and old-growth forests in the landscape to enhance the potential for biodiversity conservation of secondary forests (3, 26, 30) and thereby that of the entire landscape.
MATERIALS AND METHODS
Study sites and plot characteristics
Chronosequence data were compiled for 56 Neotropical lowland forest sites, in 10 countries, covering the entire latitudinal gradient in the Neotropics (Fig. 1 and table S1) (15). To reduce the confounding effect of elevation, we included sites that were generally below 1000 m above sea level. Annual rainfall varied from 750 to 6000 mm across sites, topsoil CEC varied from 1.7 to 64.6 cmol(+) kg−1, and percentage of forest cover in the landscape matrix ranged from 9.4 to 99.9% (table S1).
We aimed to assess the rate and extent of biodiversity recovery after abandonment of pastures and shifting cultivation fields. Shifting cultivation is typically performed at a small scale, in which patches of 0.5 to 1 ha are slashed, burned, cultivated, or used as pasture for some years and abandoned, after which they recover (13, 14). We were therefore interested in recovery of alpha diversity at the scale of these local patches. To avoid edge effects of neighboring old-growth forest, secondary forest researchers typically establish small plots (0.1 ha; see below) in abandoned fields. For each chronosequence site, an average of 29.1 plots (range, 4 to 251) were included, with secondary forest plots ranging in stand age from 1 to 100 years across sites (table S1). Plot ages were estimated using landowner interviews (33 sites), satellite images or aerial photographs (6 sites), landowner interviews combined with tree-ring counts (1 site), and satellite images and/or aerial photographs that were combined with information from landowner interviews (16 sites). In general, age estimates for young secondary plots were regarded to be more precise (precise to the year or to 6 months for some sites) than age estimates for older secondary forest plots (error of a few years), and this is exactly what is needed given that initial recovery goes fast (thus exact age estimates are important) and that later in succession recovery rates slow down. Data from old-growth forests were included as a reference for estimating biodiversity recovery for 45 of the 56 sites (table S1). Old-growth forests had no record of previous disturbance for at least 100 years. Plot sizes ranged from 0.01 to 1 ha, with an average of 0.09 ha across all plots. To accurately estimate biodiversity recovery, we assured that, within each chronosequence site, secondary forest plots and old-growth plots had similar sizes, but for 12 sites, old-growth plots were slightly larger or smaller than secondary forest plots (table S1). All stems ≥5 cm dbh of trees, palms, and shrubs were measured for dbh and identified to species, with the exception of six sites for which the minimum dbh was 10 cm. Across chronosequences, on average, 92.8% of the stems were identified to species (range, 58 to 100%) and 99.6% (range, 94 to 100%) were identified to family, genus, species, or morphospecies (table S2).
Recovery of species richness and species composition
To account for differences in stem density among plots within sites and across sites, we calculated rarefied species richness per 25 stems for all secondary (i.e., absolute recovery of species richness) and old-growth plots. Plots with less than 25 stems (only 186 of a total of 1816 plots) were excluded from analyses. Relative recovery of species richness and composition was calculated for 45 sites for which old-growth forest plots were included in the chronosequences. Relative recovery of species richness was expressed as a percentage of the mean rarefied richness (based on 25 stems) of old-growth forest plots in the same site. We used 25 stems as a reference value for comparing absolute and relative recovery in species richness among sites to be able to include as many plots as possible, because plots were generally small and included few stems. Differences in species richness among sites may be compressed in a small sample of 25 stems; therefore, we may underestimate diversity differences across sites. Rates of relative recovery of species richness may depend on the number of stems used for calculating rarefied richness, as rarefied richness based on 25 stems starts to saturate if more than 25 species are present. To assess the influence of the number of stems used for calculation of rarefied richness on estimated rates of relative recovery, we calculated rarefied richness per 25 and per 50 stems for a subset of 697 secondary forest plots (in 36 sites that included old-growth plots) that had at least 50 stems. General ranking in absolute recovery of species richness of plots across gradients in environmental conditions and in anthropogenic disturbance was similar for rarefied richness calculated for 25 and 50 stems, as linear mixed-effects models (as described below) indicated that, for both measures of rarefied richness, the same predictors had an effect on absolute and relative recovery (based on the set of plots with at least 50 stems). Nevertheless, relative recovery of species richness was lower when rarefied richness was calculated based on a sample of 50 stems, with, on average, 81.3 and 77.6% of old-growth species richness recovered after 20 years based on 25 and 50 stems, respectively. We may therefore obtain faster rates of relative recovery of species richness by using the number of species per 25 stems.
Relative recovery of species composition of each secondary forest plot was calculated as the mean pairwise similarity in species composition between the secondary forest plot and the old-growth plots in the same site based on the Chao-Jaccard index, which compares abundances of shared and unshared species between two plots (31). The Chao-Jaccard index reduces undersampling bias by accounting for unseen, shared species, making it suitable for comparing plots of different sizes with many rare species (31). In addition, we accounted for the large variation in species composition across old-growth forest plots within a site that results from strong local species turnover. For the 41 sites with at least two old-growth plots, the overall average within-site similarity of old-growth plots, which is the average of the per-site average similarity between pairs of old-growth plots of the 41 sites, was 0.47 ± 0.040% (mean ± SE). We therefore used a pairwise similarity of 0.47 as the maximum attainable reference value and, thus, as 100% relative recovery of species composition. As such, we estimated recovery in species composition toward a state comparable to old-growth forest. We used the same reference value of 0.47 for all sites, rather than site-specific reference values, as many sites had very few old-growth plots to accurately estimate the within-site average pairwise similarity between old-growth plots. We do recognize that the species composition of old-growth forests may also change over time. For example, species composition may slightly fluctuate in response to short-term drought or other disturbances, but we cannot predict these changes based on our data. By including the current (static) species richness and species composition of old-growth forest as the reference value for assessing secondary forest recovery, we assume that the species richness and species composition of old-growth forests remain stable over time.
The rate of biodiversity recovery may also be influenced by the size of the regional species pool, as forests with more species may take more time to recover. We used the size of the local old-growth forest species pool as a proxy for the regional species pool. As such, we also accounted for differences in biogeographical history across sites, particularly since we also included chronosequence sites on islands (i.e., Providencia Island and Puerto Rico), which may have a totally different species richness compared to forests with the same environmental conditions on the mainland. The local species pool was estimated on the basis of old-growth plots only, as we regarded old-growth forest as the reference for biodiversity recovery. For each site, we estimated the size of the local species pool based on the Chao 1 estimator (32), with bias correction (33), using all stems from old-growth forest plots. Although we did not estimate the exact size of the local old-growth species pool, this approach allows ranking the sites based on the magnitude of their species pool and evaluating the role of the species pool in biodiversity recovery of secondary forests.
Data on environmental conditions and previous land use
Average annual rainfall (in mm year−1) was obtained from the nearest weather station for each site. As seasonality in water availability is a stronger determinant of species richness and composition than annual rainfall (16), we obtained CWA (in mm year−1) from http://chave.ups-tlse.fr/pantropical_allometry.htm (where CWA is referred to as “climatic water deficit”). CWA indicates the amount of water lost by the environment during dry months, that is, the months in which evapotranspiration is larger than rainfall. CWA is, by definition, negative, and sites with a maximum CWA of 0 do not experience seasonal drought stress. For one site for which CWA was not available (Providencia Island; table S1), we estimated CWA from a linear regression between CWA and rainfall based on the other chronosequence sites (CWA = −822 + 0.203 × rainfall; n = 55, P < 0.0001, R2 = 0.49). Topsoil CEC [in cmol(+) kg−1] over the first 30 cm of the soil was used as an indicator of soil nutrient availability. We preferably included data from old-growth forest plots because soil fertility is expected to recover over the course of succession. CEC represents the amount of exchangeable cations [Ca2+, Mg2+, K+, Na+, Al3+, and H+ in cmol(+) kg−1]. A high CEC can therefore also result from high acidity or aluminum toxicity and may not only reflect soil fertility. For 39 sites for which no local CEC data were available, CEC was obtained from the SoilGrids database (34). SoilGrids did not contain data on soil nitrogen and phosphorus. Phosphorus is thought to limit plant growth in highly weathered tropical soils and may therefore be strongly correlated with the biodiversity recovery of tropical forests. We obtained total exchangeable bases (TEB) from the World Harmonized Soil Database (35), as this variable was not included in SoilGrids, for 55 sites for which data were available (Providencia Island was not included in the database). CEC was significantly, positively correlated with TEB (Pearson’s r = 0.67, P < 0.0001, n = 55), which indicates that, for our dataset, CEC likely reflected soil fertility rather than the degree of aluminum toxicity or acidity. Therefore, we included CEC in the analyses, as for part of the sites, locally measured values were available, while no local data were available for TEB.
Biodiversity recovery will likely be highest when seed sources and seed dispersal agents are nearby, thus with high forest cover and forest quality in the landscape. For each site, percentage of forest cover was calculated for each of the plots within circular buffers with radii of 500, 1000, and 5000 m using a remote sensing–based tree cover map for the year 2000 (24). For 11 chronosequence sites, (part of) the fieldwork was conducted in the 1990s, and for the other sites, the fieldwork was conducted from the year 2000 onward. This does mean that landscape forest cover in the year 2000 generally reflects the landscape matrix for the younger secondary forest plots. Therefore, our estimate of landscape forest cover is ecologically relevant, as it reflects the landscape conditions experienced by younger secondary forests (<20 years), when most of the recovery of species richness and species composition occurs (Fig. 2).
Tree cover data were available at a resolution of 30 m by 30 m (24) and included any type of tree cover (e.g., old-growth forest, secondary forest, and plantations). A threshold of 30% tree cover was applied per pixel to distinguish between forest and nonforest land cover types, and forest cover was calculated on the basis of the number of pixels covered by forest versus nonforest land cover types. For eight sites without individual plot-level coordinates, we similarly calculated percentage of forest cover in circular buffers with radii of 500, 1000, and 5000 m based on just the average coordinates of the site. Landscape-scale forest cover was estimated as percentage of forest cover in the total area covered by a union of circular buffers with radii of 500, 1000, or 5000 m of all individual plots within a chronosequence site. Thus, areas in which circular buffers overlapped were included only once in the calculation of percentage of forest cover in the landscape. In addition, we estimated percentage of old-growth forest and secondary forest cover in the landscape matrix (i.e., in a radius of 1 km around the area that comprises all plots of a chronosequence site) for 45 of our sites (15).
Biodiversity recovery depends on forest legacies that accelerate secondary forest succession, such as the presence of a soil seed bank, resprouts from tree roots or stumps, or remnant forest trees. Both remaining legacies and environmental conditions that influence regeneration, such as soil nutrient availability and soil structure, are partly driven by previous land use (13, 18). We distinguished three types of land use before abandonment (shifting cultivation, pasture, and a combination of these in the landscape) based on interviews with local landowners. Land-use intensity is generally lowest under shifting cultivation, resulting in faster forest recovery in abandoned agricultural fields than abandoned pastures.
Statistical analysis
We modeled absolute recovery of species richness and relative recovery of species richness and composition as a function of stand age, CWA, CEC, forest cover in the landscape matrix, previous land-use type, and plot size using linear mixed-effects models. For relative recovery of species richness and composition, we also included the size of the local old-growth species pool. We did not include the size of the species pool for absolute recovery of species richness, as data from old-growth plots were not available for all sites. Before analysis, stand age was ln-transformed to account for nonlinear recovery responses over time. Stand age, the size of the species pool (for relative recovery), CWA, CEC, landscape-scale forest cover, land-use type, and plot size were included as fixed effects. To account for the nonindependence of plots within a site and for site-specific recovery rates, we included a random intercept and a random slope for stand age per site. In some sites (table S2), plots had a nested design, where large trees were measured in the entire plot and smaller trees were measured in a subplot only. To account for the possibly slightly lower absolute richness of nested sites, we also included a random intercept for nested versus non-nested sites for absolute recovery of species richness. For relative recovery of species richness and composition, inclusion of a random intercept for nested versus non-nested sites did not improve model fits based on likelihood ratio tests. Similarly, likelihood ratio tests indicated that a random intercept for the general region (South America versus Central America and Mexico) to account for the overall higher soil fertility in Central America and Mexico did not improve model fits for absolute recovery of species richness and relative recovery of species richness and composition. We therefore did not include a random intercept for the general region in the models. We compared models with forest cover in circular buffers with radii of 500, 1000, and 5000 m around the plots based on Akaike’s information criterion. Models that included forest cover based on a 5000-m radius were best supported; thus, we included forest cover within a 5000-m radius in the final models. Including an interaction between stand age and forest cover to account for possibly stronger effects of forest cover early in succession did not improve model fits.
Predictors were not strongly correlated (tables S3 and S4). To be able to compare effect sizes among predictors, we standardized all continuous predictors by subtracting the mean and dividing the difference by 1 SD. To assess the significance of the fixed effects, we obtained 95% confidence intervals of the model coefficients using parametric bootstrapping. Fixed effects were considered significant if the confidence interval of the coefficients did not overlap with zero.
For each site, we estimated the recovery time as the time needed to recover to old-growth species richness and composition based on the estimated model coefficients, including a site-specific (random) intercept and slope for stand age. Estimated recovery times may be extrapolated beyond the maximum stand age of included secondary forest plots for sites where secondary forests have not fully recovered to old-growth values yet. All analyses were performed in R 3.1.2 (36). Rarefied species richness, the Chao-Jaccard index, and the Chao 1 estimator were calculated using the “vegan” package (37). Mixed-effects models were performed using the “lme4” package (38).
Supplementary Material
Acknowledgments
This paper is a product of the 2ndFOR collaborative research network on secondary forests. We are grateful to numerous field assistants for help with fieldwork, local institutions for logistical support, and local communities for hospitality. We thank H. de Foresta, J.-F. Molino, and D. Sabatier for the use of plot data. We thank R. B. Foster, S. Lao, and R. Perez for the use of plot data, managed under the Center for Tropical Forest Science and the Smithsonian Tropical Research Institute in Panama. We thank two anonymous reviewers for insightful comments. This is publication #718 in the Technical Series of the Biological Dynamics of Forest Fragments Project (BDFFP-INPA). This is publication #4 from 2ndFOR. Funding: We acknowledge the following agencies for financial support: the Australian Department of Foreign Affairs and Trade-DFAT, the Biological Dynamics of Forest Fragments Project (BDFFP), the Blue Moon Foundation, CGIAR-FTA, CIFOR, COLCIENCIAS (grant no. PRE00503026837, 521, 2010), COLCIENCIAS (grant no. 1243-13-16640), Consejo Nacional de Ciencia y Tecnología (SEP-CONACYT 2009-129740 and SEP-CONACYT 2015-255544 for ReSerBos, SEP-CONACYT CB-2005-01-51043 and CB-2009-128136, CONACYT 33851-B, and SEMARNAT-CONACYT 2002 C01-0597), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 481576/2009-6, 563304/2010-3, 562955/2010-0, 574008/2008-0, 308778/2017-0, PQ 306375/2016-8, PQ 307422/2012-7, and PQ 309874/2015-7), FOMIX-Yucatan (YUC-2008-C06-108863), ForestGEO, Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG CRA APQ-00001-11, PPM-00627-16), Fundación Ecológica de Cuixmala, the Global Environment Facility (GEF-grant VEN/SGP/2010-2015), the Heising-Simons Foundation, HSBC, the IAI Nitrogen Initiative, Investissement d’Avenir grant of the Agence Nationale de la Recherche (CEBA: ANR-10-LABX-25-01), ICETEX, Instituto Internacional de Educação do Brasil (IEB), Instituto Nacional de Ciência e Tecnologia dos Serviços Ambientais da Amazonia (INCT/Servamb), the Inter-American Institute for Global Change Research (Tropi-dry network CRN3-025) via a grant from the U.S. National Science Foundation (grant no. GEO-1128040), Intercolombia, the International Climate Initiative (IKI) of the German Federal Ministry for the Environment, the NASA Terrestrial Ecology Program, the National Science Foundation [NSF-CNH-RCN grant 1313788 for Tropical Reforestation Network: Building a Socioecological Understanding of Tropical Reforestation (PARTNERS), NSF DEB-0129104, NSF DEB-9972116, NSF BCS-1349952, NSF Career Grant DEB-1053237, NSF DEB-1050957, 0639393, 1147429, 0639114, and 1147434], Nature Conservation, Building and Nuclear Safety (BMUB), Netherlands Organisation for Scientific Research (NWO; grant no. NWO-ALWOP.241), the Norwegian Agency for Development Cooperation (Norad), NUFFIC, PAPIIT-DGAPA-UNAM IN213714 and IN218416, Science without Borders Program (CAPES/CNPq) (grant no. 88881.064976/2014-01), Stanley Motta, the Grantham Foundation for the Protection of the Environment, the São Paulo Research Foundation (FAPESP) (grant nos. 2011/06782-5 and 2014/14503-7), the United Nations Development Programme (Venezuela), Instituto Nacional de Investigaciones Agrícolas (INIA-Amazonas), the Silicon Valley Community Foundation, Stichting Het Kronendak, the Tropenbos Foundation, the University of Connecticut Research Foundation, USAID (BOLFOR), Wageningen University (INREF Terra Preta programme and FOREFRONT programme), and Yale-NUS College (grant no. R-607-265-054-121). This study was partly funded by the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 283093 [Role Of Biodiversity In climate change mitigatioN (ROBIN)]. Author contributions: D.M.A.R., L.P., and F.B. conceived the study and coordinated data compilation. D.M.A.R. analyzed the data. J.S.D. and L.P.D. contributed to analytical tools used in the analysis. D.M.A.R., L.P., and F.B. wrote the paper, and all other authors performed fieldwork, provided suggestions for data analyses, discussed the results, and commented on the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Plot-level data of 54 sites are available from DNA (https://doi.org/10.17026/dans-xh8-gh92) and data for two sites can be requested from D.M.A.R. (danae.rozendaal@wur.nl). Additional data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaau3114/DC1
Fig. S1. Relative recovery of species composition [% old-growth (OG)] for Neotropical secondary forests after 20 years.
Fig. S2. Absolute recovery of species richness, and relative recovery of species richness and species composition, in relation to CWA, CEC, and forest cover in the landscape matrix for Neotropical secondary forests.
Fig. S3. Effects of CWA and soil fertility (CEC) on the local old-growth species pool (based on the Chao 1 estimator) for 45 Neotropical secondary forest sites.
Table S1. Characteristics of the included Neotropical secondary forest sites.
Table S2. Characteristics of the dataset for 56 Neotropical secondary forest sites.
Table S3. Correlations between predictors for 56 Neotropical secondary forest sites.
Table S4. Correlations between predictors for 45 Neotropical secondary forest sites for which data from old-growth plots were included.
REFERENCES AND NOTES
- 1.Slik J. W. F., Arroyo-Rodríguez V., Aiba S.-I., Alvarez-Loayza P., Alves L. F., Ashton P., Balvanera P., Bastian M. L., Bellingham P. J., van den Berg E., Bernacci L., da Conceição Bispo P., Blanc L., Böhning-Gaese K., Boeckx P., Bongers F., Boyle B., Bradford M., Brearley F. Q., Hockemba M. B.-N., Bunyavejchewin S., Matos D. C. L., Castillo-Santiago M., Catharino E. L. M., Chai S.-L., Chen Y., Colwell R. K., Chazdon R. L., Clark C., Clark D. B., Clark D. A., Culmsee H., Damas K., Dattaraja H. S., Dauby G., Davidar P., DeWalt S. J., Doucet J.-L., Duque A., Durigan G., Eichhorn K. A. O., Eisenlohr P. V., Eler E., Ewango C., Farwig N., Feeley K. J., Ferreira L., Field R., de Oliveira Filho A. T., Fletcher C., Forshed O., Franco G., Fredriksson G., Gillespie T., Gillet J.-F., Amarnath G., Griffith D. M., Grogan J., Gunatilleke N., Harris D., Harrison R., Hector A., Homeier J., Imai N., Itoh A., Jansen P. A., Joly C. A., de Jong B. H. J., Kartawinata K., Kearsley E., Kelly D. L., Kenfack D., Kessler M., Kitayama K., Kooyman R., Larney E., Laumonier Y., Laurance S., Laurance W. F., Lawes M. J., do Amaral I. L., Letche S. G., Lindsell J., Lu X. H., Mansor A., Marjokorpi A., Martin E. H., Meilby H., Melo F. P. L., Metcalfe D. J., Medjibe V. P., Metzger J. P., Millet J., Mohandass D., Montero J. C., Valeriano M. D., Mugerwa B., Nagamasu H., Nilus R., Ochoa-Gaona S., Onrizal N. P., Parolin P., Parren M., Parthasarathy N., Paudel E., Permana A., Piedade M. T. F., Pitman N. C. A., Poorter L., Poulsen A. D., Poulsen J., Powers J., Prasad R. C., Puyravaud J. P., Razafimahaimodiso J. C., Reitsma J., dos Santos J. R., Spironello W. R., Romero-Saltos H., Rovero F., Rozak A. H., Ruokolainen K., Rutishauser E., Saiter F., Saner P., Santos B. A., Santos F., Sarker S. K., Satdichanh M., Schmitt C. B., Schongart J., Schulze M., Suganuma M. S., Sheil D., Pinheiro E. D., Sist P., Stevart T., Sukumar R., Sun I. F., Sunderand T., Suresh H. S., Suzuki E., Tabarelli M., Tang J. W., Targhetta N., Theilade I., Thomas D. W., Tchouto P., Hurtado J., Valencia R., van Valkenburg J., Do T. V., Vasquez R., Verbeeck H., Adekunle V., Vieira S. A., Webb C. O., Whitfeld T., Wich S. A., Williams J., Wittmann F., Woll H., Yang X. B., Yao C. Y. A., Yap S. L., Yoneda T., Zahawi R. A., Zakaria R., Zang R. G., de Assis R. L., Luize B. G., Venticinque E. M., An estimate of the number of tropical tree species. Proc. Natl. Acad. Sci. U.S.A. 112, 7472–7477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ter Steege H., Pitman N. C. A., Killeen T. J., Laurance W. F., Peres C. A., Guevara J. E., Salomão R. P., Castilho C. V., Amaral I. L., de Almeida Matos F. D., de Souza Coelho L., Magnusson W. E., Phillips O. L., de Andrade Lima Filho D., de Jesus Veiga Carim M., Irume M. V., Martins M. P., Molino J.-F., Sabatier D., Wittmann F., López D. C., da Silva Guimarães J. R., Mendoza A. M., Vargas P. N., Manzatto A. G., Reis N. F. C., Terborgh J., Casula K. R., Montero J. C., Feldpausch T. R., Coronado E. N. H., Montoya A. J. D., Zartman C. E., Mostacedo B., Vasquez R., Assis R. L., Medeiros M. B., Simon M. F., Andrade A., Camargo J. L., Laurance S. G. W., Nascimento H. E. M., Marimon B. S., Marimon B.-H., Costa F., Targhetta N., Vieira I. C. G., Brienen R., Castellanos H., Duivenvoorden J. F., Mogollón H. F., Piedade M. T. F., Aymard G. A., Comiskey J. A., Damasco G., Dávila N., García-Villacorta R., Diaz P. R. S., Vincentini A., Emilio T., Levis C., Schietti J., Souza P., Alonso A., Dallmeier F., Ferreira L. V., Neill D., Araujo-Murakami A., Arroyo L., Carvalho F. A., Souza F. C., Amaral D. D., Gribel R., Luize B. G., Pansonato M. P., Venticinque E., Fine P., Toledo M., Baraloto C., Cerón C., Engel J., Henkel T. W., Jimenez E. M., Maas P., Mora M. C. P., Petronelli P., Revilla J. D. C., Silveira M., Stropp J., Thomas-Caesar R., Baker T. R., Daly D., Paredes M. R., da Silva N. F., Fuentes A., Jørgensen P. M., Schöngart J., Silman M. R., Arboleda N. C., Cintra B. B. L., Valverde F. C., Di Fiore A., Phillips J. F., van Andel T. R., von Hildebrand P., Barbosa E. M., de Matos Bonates L. C., de Castro D., de Sousa Farias E., Gonzales T., Guillaumet J.-L., Hoffman B., Malhi Y., de Andrade Miranda I. P., Prieto A., Rudas A., Ruschell A. R., Silva N., Vela C. I. A., Vos V. A., Zent E. L., Zent S., Cano A., Nascimento M. T., Oliveira A. A., Ramirez-Angulo H., Ramos J. F., Sierra R., Tirado M., Medina M. N. U., van der Heijden G., Torre E. V., Vriesendorp C., Wang O., Young K. R., Baider C., Balslev H., de Castro N., Farfan-Rios W., Ferreira C., Mendoza C., Mesones I., Torres-Lezama A., Giraldo L. E. U., Villarroel D., Zagt R., Alexiades M. N., Garcia-Cabrera K., Hernandez L., Huamantupa-Chuquimaco I., Milliken W., Cuenca W. P., Pansini S., Pauletto D., Arevalo F. R., Sampaio A. F., Valderrama Sandoval E. H., Gamarra L. V., Estimating the global conservation status of more than 15,000 Amazonian tree species. Sci. Adv. 1, e1500936 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chazdon R. L., Peres C. A., Dent D., Sheil D., Lugo A. E., Lamb D., Stork N. E., Miller S. E., The potential for species conservation in tropical secondary forests. Conserv. Biol. 23, 1406–1417 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Gardner T. A., Barlow J., Chazdon R., Ewers R. M., Harvey C. A., Peres C. A., Sodhi N. S., Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 12, 561–582 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Martin P. A., Newton A. C., Bullock J. M., Carbon pools recover more quickly than plant biodiversity in tropical secondary forests. Proc. Biol. Sci. 280, 20132236 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finegan B., Pattern and process in neotropical secondary rain forests: The first 100 years of succession. Trends Ecol. Evol. 11, 119–124 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Norden N., Chazdon R. L., Chao A., Jiang Y.-H., Vilchez-Alvarado B., Resilience of tropical rain forests: Tree community reassembly in secondary forests. Ecol. Lett. 12, 385–394 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Dent D. H., DeWalt S. J., Denslow J. S., Secondary forests of central Panama increase in similarity to old-growth forest over time in shade tolerance but not species composition. J. Veg. Sci. 24, 530–542 (2013). [Google Scholar]
- 9.van Breugel M., Hall J. S., Craven D., Bailon M., Hernandez A., Abbene M., van Breugel P., Succession of ephemeral secondary forests and their limited role for the conservation of floristic diversity in a human-modified tropical landscape. PLOS ONE 8, e82433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derroire G., Balvanera P., Castellanos-Castro C., Decocq G., Kennard D. K., Lebrija-Trejos E., Leiva J. A., Odén P.-C., Powers J. S., Rico-Gray V., Tigabu M., Healey J. R., Resilience of tropical dry forests—A meta-analysis of changes in species diversity and composition during secondary succession. Oikos 125, 1386–1397 (2016). [Google Scholar]
- 11.Hoorn C., Wesselingh F. P., ter Steege H., Bermudez M. A., Mora A., Sevink J., Sanmartin I., Sanchez-Meseguer A., Anderson C. L., Figueiredo J. P., Jaramillo C., Riff D., Negri F. R., Hooghiemstra H., Lundberg J., Stadler T., Sarkinen T., Antonelli A., Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330, 927–931 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Barlow J., Lennox G. D., Ferreira J., Berenguer E., Lees A. C., Nally R. M., Thomson J. R., de Barros Ferraz S. F., Louzada J., Oliveira V. H. F., Parry L., de Castro Solar R. R., Vieira I. C. G., Aragão L. E. O. C., Begotti R. A., Braga R. F., Cardoso T. M., de Oliveira R. C., Souza C. M. Jr, Moura N. G., Nunes S. S., Siqueira J. V., Pardini R., Silveira J. M., Vaz-de-Mello F. Z., Veiga R. C. S., Venturieri A., Gardner T. A., Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535, 144–147 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Jakovac C. C., Peña-Claros M., Kuyper T. W., Bongers F., Loss of secondary-forest resilience by land-use intensification in the Amazon. J. Ecol. 103, 67–77 (2015). [Google Scholar]
- 14.R. L. Chazdon, Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation (University of Chicago Press, 2014). [Google Scholar]
- 15.Poorter L., Bongers F., Aide T. M., Zambrano A. M. A., Balvanera P., Becknell J. M., Boukili V., Brancalion P. H. S., Broadbent E. N., Chazdon R. L., Craven D., de Almeida-Cortez J. S., Cabral G. A. L., de Jong B. H. J., Denslow J. S., Dent D. H., DeWalt S. J., Dupuy J. M., Durán S. M., Espírito-Santo M. M., Fandino M. C., César R. G., Hall J. S., Hernandez-Stefanoni J. L., Jakovac C. C., Junqueira A. B., Kennard D., Letcher S. G., Licona J.-C., Lohbeck M., Marín-Spiotta E., Martínez-Ramos M., Massoca P., Meave J. A., Mesquita R., Mora F., Muñoz R., Muscarella R., Nunes Y. R. F., Ochoa-Gaona S., de Oliveira A. A., Orihuela-Belmonte E., Peña-Claros M., Pérez-García E. A., Piotto D., Powers J. S., Rodríguez-Velázquez J., Romero-Pérez I. E., Ruíz J., Saldarriaga J. G., Sanchez-Azofeifa A., Schwartz N. B., Steininger M. K., Swenson N. G., Toledo M., Uriarte M., van Breugel M., van der Wal H., Veloso M. D. M., Vester H. F. M., Vicentini A., Vieira I. C. G., Bentos T. V., Williamson G. B., Rozendaal D. M. A., Biomass resilience of Neotropical secondary forests. Nature 530, 211–214 (2016). [DOI] [PubMed] [Google Scholar]
- 16.ter Steege H., Pitman N. C. A., Phillips O. L., Chave J., Sabatier D., Duque A., Molino J.-F., Prévost M.-F., Spichiger R., Castellanos H., von Hildebrand P., Vásquez R., Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 443, 444–447 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Clinebell R. R. II, Phillips O. L., Gentry A. H., Stark N., Zuuring H., Prediction of neotropical tree and liana species richness from soil and climatic data. Biodivers. Conserv. 4, 56–90 (1995). [Google Scholar]
- 18.Guariguata M. R., Ostertag R., Neotropical secondary forest succession: Changes in structural and functional characteristics. For. Ecol. Manage. 148, 185–206 (2001). [Google Scholar]
- 19.Connell J. H., Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310 (1978). [DOI] [PubMed] [Google Scholar]
- 20.Bongers F., Poorter L., Hawthorne W. D., Sheil D., The intermediate disturbance hypothesis applies to tropical forests, but disturbance contributes little to tree diversity. Ecol. Lett. 12, 798–805 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Letcher S. G., Lasky J. R., Chazdon R. L., Norden N., Wright S. J., Meave J. A., Pérez-García E. A., Muñoz R., Romero-Pérez E., Andrade A., Andrade J. L., Balvanera P., Becknell J. M., Bentos T. V., Bhaskar R., Bongers F., Boukili V., Brancalion P. H. S., César R. G., Clark D. A., Clark D. B., Craven D., DeFrancesco A., Dupuy J. M., Finegan B., González-Jiménez E., Hall J. S., Harms K. E., Hernandez-Stefanoni J. L., Hietz P., Kennard D., Killeen T. J., Laurance S. G., Lebrija-Trejos E. E., Lohbeck M., Martínez-Ramos M., Massoca P. E. S., Mesquita R. C. G., Mora F., Muscarella R., Paz H., Pineda-García F., Powers J. S., Quesada-Monge R., Rodrigues R. R., Sandor M. E., Sanaphre-Villanueva L., Schüller E., Swenson N. G., Tauro A., Uriarte M., van Breugel M., Vargas-Ramírez O., Viani R. A. G., Wendt A. L., Williamson G. B., Environmental gradients and the evolution of successional habitat specialization: A test case with 14 Neotropical forest sites. J. Ecol. 103, 1276–1290 (2015). [Google Scholar]
- 22.Gibson L., Lee T. M., Koh L. P., Brook B. W., Gardner T. A., Barlow J., Peres C. A., Bradshaw C. J. A., Laurance W. F., Lovejoy T. E., Sodhi N. S., Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478, 378–381 (2011). [DOI] [PubMed] [Google Scholar]
- 23.M. A. Huston, Biological Diversity: The Coexistence of Species (Cambridge Univ. Press, 1994). [Google Scholar]
- 24.Hansen M. C., Potapov P. V., Moore R., Hancher M., Turubanova S. A., Tyukavina A., Thau D., Stehman S. V., Goetz S. J., Loveland T. R., Kommareddy A., Egorov A., Chini L., Justice C. O., Townshend J. R. G., High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Mesquita R. d. C. G., dos Santos Massoca P. E., Jakovac C. C., Bentos T. V., Williamson G. B., Amazon rain forest succession: Stochasticity or land-use legacy? Bioscience 65, 849–861 (2015). [Google Scholar]
- 26.Arroyo-Rodríguez V., Melo F. P. L., Martínez-Ramos M., Bongers F., Chazdon R. L., Meave J. A., Norden N., Santos B. A., Leal I. R., Tabarelli M., Multiple successional pathways in human-modified tropical landscapes: New insights from forest succession, forest fragmentation and landscape ecology research. Biol. Rev. 92, 326–340 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Lohbeck M., Poorter L., Martínez-Ramos M., Bongers F., Biomass is the main driver of changes in ecosystem process rates during tropical forest succession. Ecology 96, 1242–1252 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Poorter L., van der Sande M. T., Thompson J., Arets E. J. M. M., Alarcón A., Álvarez-Sánchez J., Ascarrunz N., Balvanera P., Barajas-Guzmán G., Boit A., Bongers F., Carvalho F. A., Casanoves F., Cornejo-Tenorio G., Costa F. R. C., de Castilho C. V., Duivenvoorden J. F., Dutrieux L. P., Enquist B. J., Fernández-Méndez F., Finegan B., Gormley L. H. L., Healey J. R., Hoosbeek M. R., Ibarra-Manríquez G., Junqueira A. B., Levis C., Licona J. C., Lisboa L. S., Magnusson W. E., Martínez-Ramos M., Martínez-Yrizar A., Martorano L. G., Maskell L. C., Mazzei L., Meave J. A., Mora F., Muñoz R., Nytch C., Pansonato M. P., Parr T. W., Paz H., Pérez-García E. A., Rentería L. Y., Rodríguez-Velazquez J., Rozendaal D. M. A., Ruschel A. R., Sakschewski B., Salgado-Negret B., Schietti J., Simões M., Sinclair F. L., Souza P. F., Souza F. C., Stropp J., ter Steege H., Swenson N. G., Thonicke K., Toledo M., Uriarte M., van der Hout P., Walker P., Zamora N., Peña-Claros M., Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 24, 1314–1328 (2015). [Google Scholar]
- 29.Chazdon R. L., Broadbent E. N., Rozendaal D. M. A., Bongers F., Zambrano A. M. A., Aide T. M., Balvanera P., Becknell J. M., Boukili V., Brancalion P. H. S., Craven D., Almeida-Cortez J. S., Cabral G. A. L., de Jong B., Denslow J. S., Dent D. H., DeWalt S. J., Dupuy J. M., Durán S. M., Espírito-Santo M. M., Fandino M. C., César R. G., Hall J. S., Hernández-Stefanoni J. L., Jakovac C. C., Junqueira A. B., Kennard D., Letcher S. G., Lohbeck M., Martínez-Ramos M., Massoca P., Meave J. A., Mesquita R., Mora F., Muñoz R., Muscarella R., Nunes Y. R. F., Ochoa-Gaona S., Orihuela-Belmonte E., Peña-Claros M., Pérez-García E. A., Piotto D., Powers J. S., Rodríguez-Velazquez J., Romero-Pérez I. E., Ruíz J., Saldarriaga J. G., Sanchez-Azofeifa A., Schwartz N. B., Steininger M. K., Swenson N. G., Uriarte M., van Breugel M., van der Wal H., Veloso M. D. M., Vester H., Vieira I. C. G., Bentos T. V., Williamson G. B., Poorter L., Carbon sequestration potential of second-growth forest regeneration in the Latin American tropics. Sci. Adv. 2, e1501639 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melo F. P. L., Arroyo-Rodríguez V., Fahrig L., Martínez-Ramos M., Tabarelli M., On the hope for biodiversity-friendly tropical landscapes. Trends Ecol. Evol. 28, 462–468 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Chao A., Chazdon R. L., Colwell R. K., Shen T.-J., A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 8, 148–159 (2005). [Google Scholar]
- 32.Chao A., Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43, 783–791 (1987). [PubMed] [Google Scholar]
- 33.Chiu C.-H., Wang Y.-T., Walther B. A., Chao A. N., An improved nonparametric lower bound of species richness via a modified good-turing frequency formula. Biometrics 70, 671–682 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Hengl T., Mendes de Jesus J., Heuvelink G. B. M., Gonzalez M. R., Kilibarda M., Blagotíc A., Shangguan W., Wright M. N., Geng X., Bauer-Marschallinger B., Guevara M. A., Vargas R., MacMillan R. A., Batjes N. H., Leenaars J. G. B., Ribeiro E., Wheeler I., Mantel S., Kempen B., SoilGrids250m: Global gridded soil information based on machine learning. PLOS ONE 12, e0169748 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FAO/IIASA/ISRIC/ISS-CAS/JRC, “Harmonized World Soil Database (version 1.2)” (FAO, Rome, Italy and IIASA, Laxenburg, Austria, 2012).
- 36.R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2014).
- 37.J. Oksanen, F. G. Blanchett, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, H. H. Wagner, vegan: Community Ecology Package. R package version 2.2-1 (2015).
- 38.D. Bates, M. Maechler, B. Bolker, lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-39 (2014).
- 39.Hansen M. C., DeFries R. S., Townshend J. R. G., Carroll M. L., Dimiceli C., Sohlberg R. A., Global percent tree cover at a spatial resolution of 500 meters: First results of the MODIS vegetation continuous fields algorithm. Earth Interact. 7, 1–15 (2003). [Google Scholar]
- 40.Peña-Claros M., Changes in forest structure and species composition during secondary forest succession in the Bolivian Amazon. Biotropica 35, 450–461 (2003). [Google Scholar]
- 41.Toledo M., Salick J., Secondary succession and indigenous management in semideciduous forest fallows of the Amazon Basin. Biotropica 38, 161–170 (2006). [Google Scholar]
- 42.Kennard D. K., Secondary forest succession in a tropical dry forest: Patterns of development across a 50-year chronosequence in lowland Bolivia. J. Trop. Ecol. 18, 53–66 (2002). [Google Scholar]
- 43.D. Piotto, “Spatial dynamics of forest recovery after swidden cultivation in the Atlantic forest of Southern Bahia, Brazil,” PhD thesis (Yale University, New Haven, USA, 2011). [Google Scholar]
- 44.Vieira I. C. G., de Almeida A. S., Davidson E. A., Stone T. A., de Carvalho C. J. R., Guerrero J. B., Classifying successional forests using Landsat spectral properties and ecological characteristics in eastern Amazônia. Remote Sens. Environ. 87, 470–481 (2003). [Google Scholar]
- 45.Williamson G. B., Bentos T. V., Longworth J. B., Mesquita R. C. G., Convergence and divergence in alternative successional pathways in Central Amazonia. Plant Ecol. Divers. 7, 341–348 (2014). [Google Scholar]
- 46.Zanini K. J., Bergamin R. S., Machado R. E., Pillar V. D., Müller S. C., Atlantic rain forest recovery: Successional drivers of floristic and structural patterns of secondary forest in Southern Brazil. J. Veg. Sci. 25, 1056–1068 (2014). [Google Scholar]
- 47.Madeira B. G., Espírito-Santo M. M., D’Ângelo Neto S., Nunes Y. R. F., Azofeifa G. A. S., Fernandes G. W., Quesada M., Changes in tree and liana communities along a successional gradient in a tropical dry forest in south-eastern Brazil. Plant Ecol. 201, 291–304 (2009). [Google Scholar]
- 48.Junqueira A. B., Shepard G. H. Jr., Clement C. R., Secondary forests on anthropogenic soils in Brazilian Amazonia conserve agrobiodiversity. Biodivers. Conserv. 19, 1933–1961 (2010). [Google Scholar]
- 49.de Lima Cabral G. A., de Sá Barreto Sampaio E. V., de Almeida-Cortez J. S., Spatial structure and aboveground biomass in different Caatinga Succession Stages, in Santa Terezinha, Paraiba. Rev. Bras. Geogr. Fís. 6, 566–574 (2013). [Google Scholar]
- 50.Vester H. F. M., Cleef A. M., Tree architecture and secondary tropical rain forest development: A case study in Araracuara, Colombian Amazonia. Flora 193, 75–97 (1998). [Google Scholar]
- 51.Ruiz J., Fandiño M. C., Chazdon R. L., Vegetation structure, composition, and species richness across a 56-year chronosequence of dry tropical forest on Providencia island, Colombia. Biotropica 37, 520–530 (2005). [Google Scholar]
- 52.Morales-Salazar M., Vílchez-Alvarado B., Chazdon R. L., Ortega-Gutiérrez M., Ortiz-Malavassi E., Guevara-Bonilla M., Diversidad y estructura horizontal en los bosques tropicales del Corredor Biológico de Osa, Costa Rica. Rev. For. Mesoamericana Kurú 9, 19–28 (2012). [Google Scholar]
- 53.Powers J. S., Becknell J. M., Irving J., Pèrez-Aviles D., Diversity and structure of regenerating tropical dry forests in Costa Rica: Geographic patterns and environmental drivers. For. Ecol. Manage. 258, 959–970 (2009). [Google Scholar]
- 54.Hilje B., Calvo-Alvarado J., Jiménez-Rodríguez C., Sánchez-Azofeifa A., Tree species composition, breeding systems, and pollination and dispersal syndromes in three forest successional stages in a tropical dry forest in Mesoamerica. Trop. Conserv. Sci. 8, 76–94 (2015). [Google Scholar]
- 55.Chazdon R. L., Brenes A. R., Alvarado B. V., Effects of climate and stand age on annual tree dynamics in tropical second-growth rain forests. Ecology 86, 1808–1815 (2005). [Google Scholar]
- 56.Letcher S. G., Chazdon R. L., Rapid recovery of biomass, species richness, and species composition in a forest chronosequence in northeastern Costa Rica. Biotropica 41, 608–617 (2009). [Google Scholar]
- 57.Maury-Lechon G., Régénération forestière en Guyane Française: Recrû sur 25 ha de coupe papetière de forêt dense humide (ARBOCEL). Revue Bois et Forêts des Tropiques 197, 3–21 (1982). [Google Scholar]
- 58.van Breugel M., Martínez-Ramos M., Bongers F., Community dynamics during early secondary succession in Mexican tropical rain forests. J. Trop. Ecol. 22, 663–674 (2006). [Google Scholar]
- 59.Mora F., Martínez-Ramos M., Ibarra-Manríquez G., Pérez-Jiménez A., Trilleras J., Balvanera P., Testing chronosequences through dynamic approaches: Time and site effects on tropical dry forest succession. Biotropica 47, 38–48 (2015). [Google Scholar]
- 60.Orihuela-Belmonte D. E., de Jong B. H. J., Mendoza-Vega J., Van der Wal J., Paz-Pellat F., Soto-Pinto L., Flamenco-Sandoval A., Carbon stocks and accumulation rates in tropical secondary forests at the scale of community, landscape and forest type. Agric. Ecosyst. Environ. 171, 72–84 (2013). [Google Scholar]
- 61.Dupuy J. M., Hernández-Stefanoni J. L., Hernández-Juárez R. A., Tetetla-Rangel E., López-Martínez J. O., Leyequién-Abarca E., Tun-Dzul F. J., May-Pat F., Patterns and correlates of tropical dry forest structure and composition in a highly replicated chronosequence in Yucatan, Mexico. Biotropica 44, 151–162 (2012). [Google Scholar]
- 62.Lebrija-Trejos E., Bongers F., Pérez-García E. A., Meave J. A., Successional change and resilience of a very dry tropical deciduous forest following shifting agriculture. Biotropica 40, 422–431 (2008). [Google Scholar]
- 63.S. P. Hubbell, R. Condit, R. B. Foster, Barro Colorado Forest Census Plot Data (2005); http://ctfs.si.edu/webatlas/datasets/bci.
- 64.Marín-Spiotta E., Silver W. L., Ostertag R., Long-term patterns in tropical reforestation: Plant community composition and aboveground biomass accumulation. Ecol. Appl. 17, 828–839 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Aide T. M., Zimmerman J. K., Pascarella J. B., Rivera L., Marcano-Vega H., Forest regeneration in a chronosequence of tropical abandoned pastures: Implications for restoration ecology. Restor. Ecol. 8, 328–338 (2000). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaau3114/DC1
Fig. S1. Relative recovery of species composition [% old-growth (OG)] for Neotropical secondary forests after 20 years.
Fig. S2. Absolute recovery of species richness, and relative recovery of species richness and species composition, in relation to CWA, CEC, and forest cover in the landscape matrix for Neotropical secondary forests.
Fig. S3. Effects of CWA and soil fertility (CEC) on the local old-growth species pool (based on the Chao 1 estimator) for 45 Neotropical secondary forest sites.
Table S1. Characteristics of the included Neotropical secondary forest sites.
Table S2. Characteristics of the dataset for 56 Neotropical secondary forest sites.
Table S3. Correlations between predictors for 56 Neotropical secondary forest sites.
Table S4. Correlations between predictors for 45 Neotropical secondary forest sites for which data from old-growth plots were included.


