Taphonomic data suggest that early humans in Europe had more variable diet breadths than assumed by current evolutionary models.
Abstract
Investigating diet breadth is critical for understanding how archaic Homo populations, including Neanderthals, competed for seasonally scarce resources. The current consensus in Western Europe is that ungulates formed the bulk of the human diet during the Lower and Middle Paleolithic, while small fast prey taxa were virtually ignored. Here, we present a multisite taphonomic study of leporid assemblages from Southern France that supports frequent exploitation of small fast game during marine isotope stages 11 to 3. Along with recent evidence from Iberia, our results indicate that the consumption of small fast game was more common prior to the Upper Paleolithic than previously thought and that archaic hominins from the northwestern Mediterranean had broader diets than those from adjacent regions. Although likely of secondary importance relative to ungulates, the frequent exploitation of leporids documented here implies that human diet breadths were substantially more variable within Europe than assumed by current evolutionary models.
INTRODUCTION
Past and present human foragers show considerable variation in the animal component of their diets (1, 2). Behavioral ecological studies have demonstrated that much of this dietary variation can effectively be predicted using modeled relationships between the availability, distribution, and profitability of prey taxa and the foraging decisions made to maximize fitness given a number of constraints, including scheduling conflicts and potential failure (3, 4). Several recent studies of human subsistence strategies conducted at multi-millennial time scales have documented early shifts to greater exploitation of small fast game [e.g., leporids, birds; (5)], taxa typically associated with low return rates relative to ungulates, particularly when procured singly (6). The increased inclusion of small fast taxa in the diet, first documented in regions like Spain and the eastern Mediterranean during the Early Upper Paleolithic (EUP) (7, 8), potentially provided greater demographic resilience to groups during periods of low food availability (9, 10).
In Western Europe, the general consensus is that, prior to the Upper Paleolithic, small fast prey taxa were rarely included in the diet of archaic Homo, including Neanderthals (11, 12). The fact that most of the faunal assemblages in this area are dominated by ungulates has generally been interpreted as support for the view that they comprised most of the dietary picture, hereafter referred to as the narrow diet breadth (NDB) hypothesis. Yet, recent studies of Middle Paleolithic sites in the northwestern Mediterranean (Iberia and Southern France) with moderately high proportions (>50%) of human-accumulated leporids (13, 14) and in Iberia, birds (5, 15–17), raise questions concerning the NDB model. Here, we examine the NDB hypothesis by comparing newly studied leporid assemblages with natural control samples to test for early human exploitation of rabbits in the region. Our findings indicate that the NDB hypothesis requires substantial revision.
Hominin diet breadths in a natural rabbit laboratory: The northwestern Mediterranean
The NDB model assumes that small fast game such as rabbits and birds were rarely consumed by archaic hominins because they were ranked lower than larger-bodied ungulates in terms of net energy returns. This view includes rabbits (Oryctolagus cuniculus), a species with high population turnover and low hunting return rates, except when mass collected (18). The low return rate is explained by an elevated risk of acquisition failure and the high costs of pursuing small, elusive prey for small energetic returns, leading to the historical perception of rabbits as a famine food (2). During the Middle and Late Pleistocene, high rabbit abundances in Iberia, Southern France, and Italy are evidenced by the many naturally accumulated rabbit remains at sites such as Baume-Bonne, Orgnac 3, and Lunel-Viel, among others (19–21). Given this high potential encounter rate with rabbits, the northwestern Mediterranean constitutes an ideal natural laboratory to assess changing dietary exploitation of costly animal resources across broad time scales.
To assess whether the NDB model is accurate in the northwestern Mediterranean, we examine 21 newly studied leporid assemblages from eight sites (Fig. 1). The assemblages are associated with Acheulean and Middle Paleolithic occupations and date from marine isotope stages (MISs) 11 to 3. To address the problem of agency, this new sample is compared to several control assemblages, including a rabbit warren (22), and modern assemblages created by raptors and small carnivores (23, 24) (Materials and Methods). Moreover, comparisons are made with published anthropogenic or partly anthropogenic leporid collections that range in time from the Late Middle Pleistocene to Early Holocene to examine small fast game exploitation over a long time scale.
Fig. 1. MP (Acheulean/Middle Paleolithic) sites included in this study (red circles).
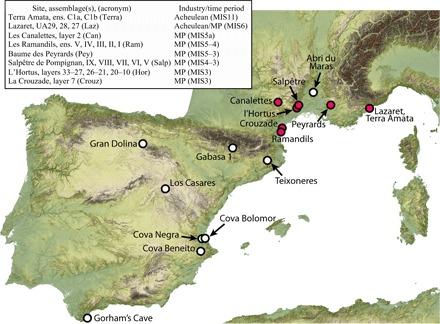
White circles denote published MP sites with evidence of human exploitation of leporids.
RESULTS
All of the newly studied Acheulean and Middle Paleolithic assemblages show strong taxonomic representation of leporids, save for the lower ensemble from l’Hortus (Table 1). This trend is consistent with published assemblages with ≥1 cutmarked leporid remains (Fig. 2A). However, only les Ramandils and le Lazaret have sample sizes reminiscent of many Late Upper Paleolithic/Early Holocene (LUP/H) assemblages (>10,000 leporid specimens). Most of the assemblages that we studied yielded cutmarks (17/21 or 81.0%) at percentages near or above the mean for published collections (Fig. 2B). Cutmarks were frequently observed on meat-bearing bones (e.g., humerus and femur), indicating human processing for consumption (Fig. 3). Terra Amata, the oldest site (MIS11) in the dataset, les Ramandils III-I, and la Crouzade (MIS3) are associated with relatively high percentages of cutmarks (1.9 to 2.4%, 2.2 to 4.8%, and 2.7%, respectively). Conversely, cutmarks are rare at le Salpêtre de Pompignan (Salpêtre; 0 to 0.5%) and l’Hortus (0 to 0.3%).
Table 1. Taxonomic representation, age profile, and taphonomic data for the sites considered in this study.
L.NISP, leporid NISP; U.NISP, ungulate NISP; nd, no data. Abbreviations for the assemblages are as in Fig. 1. The age profile and taphonomic data are for leporids only. Sample sizes are smaller for certain analyses (see data file S1). Percentages were calculated using number of identifiable specimens (NISP) for leporids. See Supplementary Materials for methods of calculation.
|
Assemblage, L.NISP |
U.NISP | % Leporids | % Adults | % Infants | % Cut | % Burned | % Tubes | % Pits/gnaw | % Dig |
| Terra C1b, n = 210 | 407 | 66.0 | 88.5 | 4.6 | 1.9 | 1.6 | 21.1 | 0.1 | 0 |
| Terra C1a, n = 790 | 205 | 50.6 | 82.6 | 2.8 | 2.4 | 3.8 | 27.0 | 0 | 0 |
| Laz UA27, n = 10802 | nd | nd | 56.3 | 23.2 | 1.0 | 5.6 | 20.3 | 7.0 | 0.3 |
| Laz UA28, n = 9095 | 2201 | 80.5 | 68.4 | 14.6 | 0.9 | 7.1 | 12.9 | 5.7 | 0.6 |
| Laz UA29, n = 4554 | nd | nd | 47.5 | 17.1 | 0.6 | 2.3 | 12.8 | 5.8 | 1.5 |
| Can 2, n = 353 | 348 | 50.6 | 88.5 | 0 | 1.4 | 7.6 | 12.5 | 0.3 | 0 |
| Ram I, n = 138 | 3 | 97.9 | 100 | 0 | 2.2 | 4.3 | 15.4 | 5.8 | 0.7 |
| Ram II, n = 876 | 55 | 94.1 | 86.2 | 0.8 | 4.8 | 17.1 | 29.0 | 5.6 | 0.3 |
| Ram III, n = 247 | 119 | 67.5 | 88.6 | 1.3 | 2.8 | 28.3 | 19.6 | 2.0 | 0.4 |
| Ram IV, n = 287 | 5 | 98.3 | 98.1 | 1.2 | 0.7 | 39.7 | 15.6 | 1.7 | 0 |
| Ram V, n = 169 | 0 | 100 | 85.7 | 0 | 1.2 | 17.2 | 14.3 | 0.6 | 0 |
| Pey, n = 1080 | nd | nd | 91.5 | 0.2 | 0.7 | 0.6 | 54.2 | 0.7 | 0.1 |
| Salp V, n = 471 | 20 | 96.0 | 75.0 | nd | 0 | 0 | 0 | 0.2 | 0.4 |
| Salp VI, n = 173 | 23 | 88.3 | 86.7 | nd | 0 | 0 | 0 | 1.2 | 0.6 |
| Salp VII, n = 1030 | 71 | 93.6 | 86.0 | 3.2 | 0.1 | 0.1 | 0 | 0.5 | 0.2 |
| Salp VIII, n = 222 | 55 | 80.1 | 92.9 | 0 | 0.5 | 0.5 | 11.1 | 0.5 | 0.5 |
| Salp IX, n = 359 | 242 | 59.7 | 92.5 | 0 | 0 | 0.6 | 0 | 0 | 0.6 |
| Hor 10–20, n = 1180 | 1650 | 41.7 | 76.0 | 2.4 | 0.2 | 0.3 | 9.1 | 2.5 | 0.3 |
| Hor 21–26, n = 1540 | 361 | 81.0 | 29.1 | 36.4 | 0.3 | 0.5 | 7.9 | 1.1 | 1.2 |
| Hor 27–33, n = 228 | 730 | 23.8 | 41.9 | 36.5 | 0 | 0 | 0 | 0.4 | 1.8 |
| Crouz 7, n = 256 | 38 | 87.1 | 69.0 | 8.8 | 2.7 | 1.2 | 11.6 | 0.4 | 13.3 |
Fig. 2. Comparison of leporid abundance and marked and/or altered leporid specimens in modern control assemblages (yellow symbols) and published (green symbols) and newly examined (red symbols) archaeological assemblages.
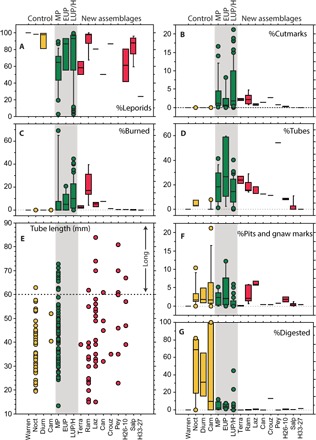
The data include (A) the percentage of leporids, (B) leporid specimens with cutmarks, (C) burned specimens, (D) and long bone diaphysis tubes (out of the total long bone NISP, ulna excluded), (E) the length of tibia tubes in millimeters (green breaks only), and (F) the percentage of specimens with pits and/or gnawing marks, and (G) digested specimens. All percentages are based on NISP counts. Noct, nocturnal raptors; diurn, diurnal raptors; carn, small carnivores; EUP, Early Upper Paleolithic; LUP, Late Upper Paleolithic; LUP/H, Late Upper Paleolithic/early Holocene (see Supplementary Materials). Sample sizes and raw data are provided in data file S1.
Fig. 3. Anthropogenic marks in the newly studied assemblages.
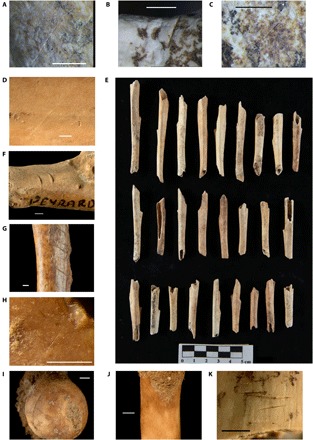
Examples of cutmarked leporid specimens from Terra Amata (A, C1a; B and C, C1b), la Baume des Peyrards (D and F), la Crouzade (G, layer 7b), l’Hortus (H, layer 26), les Ramandils (I and J, N21, NW26), le Salpêtre de Pompignan (K, level VII base), and tibia diaphysis tubes from la Baume des Peyrards (E). Skeletal elements: tibia (A, C to E, and H), ulna (B), calcaneus (F), humerus (G), femur (I), metatarsal II (J), and innominate (K). Scale bars, 1 mm except for (E). Photo credit: E. Morin and J. Meier, Trent University and University of North Florida (A to D and F to J); D. Drainat, Centre Européen de Recherches Préhistoriques, Tautavel (E).
Burned remains are common at Terra Amata (ensemble C1a; 3.8%), le Lazaret (UA29–27, 2.3 to 7.1%), les Canalettes (layer 2, 7.6%), and, especially, les Ramandils (ensembles V–I, 4.3 to 39.7%; Fig. 2C and Table 1), where the values surpass those for most published LUP/H assemblages and differ markedly from control samples where burning is absent. Unlike nonhuman predators, humans frequently produce long bone cylinders by removing articular ends through dental pressure to obtain marrow (25). In the studied assemblages, proportions of cylinders generally exceed those of the control samples and tend to fall within the range of published archaeological assemblages (Fig. 2D and Table 1). However, l’Hortus and Salpêtre have few or no cylinders. It is unclear whether the high percentage of cylinders recorded at Baume des Peyrards (54.2%; Fig. 3E) was inflated by recovery methods (26).
Limited quantitative data suggest that cylinders are longer when produced by humans than by natural agents (14, 25). This inference finds quantitative confirmation in Fig. 2E, which plots tibia cylinder lengths for natural control samples (Bubo bubo: Carry-le-Roux, Hautes-Alpes, and Archiduc; Vulpes vulpes: Rochers de Villeneuve) and for the published anthropogenic Acheulean/Middle Paleolithic (MP) assemblage from les Canalettes [layer 4; (14)]. To circumvent the issues of intraskeletal variation and postdepositional damage, we only considered tibiae with green bone breaks. Our recorded tibia cylinder length distributions are similar to the observed lengths at les Canalettes layer 4, with the exception of les Ramandils, where postdepositional fragmentation is high. We frequently recorded long tibia cylinders [≥60 mm; following (14)] in the new assemblages (n = 11/50 or 22.0%; Fig. 3), which contrasts with their low representation in the control sample [n = 2/49 or 4.1%; t statistic (ts) = 2.83; P < 0.01]. Furthermore, no tibia cylinders (n = 277) yielded evidence of cortical thinning, a significant difference from remains ingested by eagle owl (Archiduc; tibiae with ≥1 thinned end, 66.7%; n = 30; ts = 9.94, P < 0.0001).
In addition to human-inflicted damage, the new assemblages presented here yielded limited evidence of carnivore and raptor activity. Percentages of nonhuman tooth pits and gnaw marks are low (≤2.5%; Table 1), with the exception of les Ramandils II–I and le Lazaret, where they are more frequent (5.6 to 5.8% and 5.7 to 7.0%, respectively; Fig. 2F). However, these values possibly include some human tooth marks, which are difficult to distinguish from those made by small terrestrial carnivores (27). Digested specimens are scarce (<2%; Fig. 2G) in the newly examined assemblages, unlike carnivore scat or raptor pellet accumulations (28). One exception is la Crouzade (layer 7, 13.3%; Table 1). Thus, of the 21 new assemblages, only la Crouzade, les Ramandils II–I, and the assemblages from le Lazaret provide evidence for substantial modification by nonhuman predators.
Concerning rabbits, the proportion of infant leporids [<2 weeks old; (22)] can yield further insights about agency, as many natural predators (e.g., raptors, carnivores) target young prey (28, 29). However, the presence of infants may also reflect mass procurement from warrens by humans (18) or attritional accumulations (22), posing interpretive challenges in archaeological contexts (see below). In the new assemblages, sites with clear indicators of human involvement (Terra Amata, les Ramandils, les Canalettes, and Baume des Peyrards) all show very low percentages (0 to 4.6%) of infants (Table 1). Conversely, higher proportions of infants were recorded at le Lazaret (14.6 to 23.2%) and in layers 33 to 21 from l’Hortus (36.4 to 36.5%). The proportion of infants is inversely correlated with the combined percentages for burning and cutmarks (rs = −0.52, P < 0.05; Salpêtre was excluded because of small sample sizes), suggesting that the infant assemblages in le Lazaret and l’Hortus have a mostly natural origin in the newly examined collections, perhaps as a result of attritional in situ mortality. The negative correlation between the relative frequency of anthropic markers and the proportion of infants implies that mass harvesting of rabbits from warrens was not a common practice in the new dataset. Overall, our results indicate that signs of human intervention are least evident at l’Hortus and, perhaps, Salpêtre and are most conspicuous at Terra Amata, les Ramandils (layers V–III), les Canalettes, and Baume des Peyrards. Le Lazaret and la Crouzade both show mixed signs of human and nonhuman predation.
Time-transgressive trends
As shown in Fig. 4A, among the previously published assemblages, the proportion of collections with very high percentages (>85%) of leporids and adults increased significantly between the MP (0/11 or 0%) and LUP/H (25/45 or 55.6%, ts = 5.00, P < 0.0001; EUP sites were excluded because of small sample size). In the newly investigated collections, the proportion of assemblages with high leporid and adult representation (6/18 or 33.3%) is similar to the LUP/H collections and significantly different from previously published MP assemblages (new assemblages versus MP: ts = 3.21, P < 0.01; new assemblages versus LUP/H: ts = 1.62, P = 0.11). Levels 33 to 27 from l’Hortus have low relative abundances of leporids and adults, which is consistent with naturally accumulated assemblages.
Fig. 4. Comparisons of different attributes of leporid assemblages.
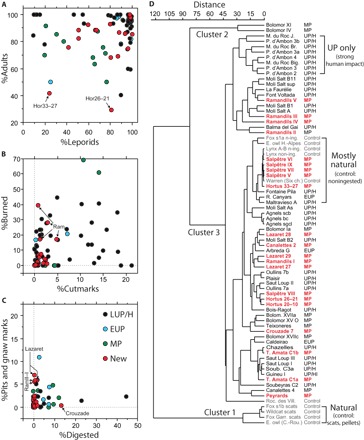
These comparisons include scatterplots of %NISP of (A) leporids versus adults; (B) burned versus cutmarked specimens; and (C) specimens with pits and/or gnaw mark versus digestion damage. (D) Cluster analysis of five taphonomic attributes expressed in percentage (see text). Data points in the scatterplots correspond to assemblages analyzed for this study (red) and published MP (green), EUP (blue), and LUP/H sites (black). In (D), the newly studied assemblages are shown in bold red, whereas modern control assemblages are shown in gray.
In addition, the relative proportions of burned and cutmarked specimens increased in the combined dataset of sites over time (Fig. 4B). Save for two MP outliers [Bolomor Cave layers XI and IV; (5)], LUP/H assemblages show considerably wider interquartile ranges for percentages of specimens with cutmarks and/or burning relative to earlier sites (Fig. 2B). The newly examined assemblages have distributions consistent with MP sites, with the exception of ensembles V–II at les Ramandils, which better match the LUP/H collections in terms of burning damage. All of the newly studied assemblages contain relatively fewer cutmarks than several LUP/H collections, possibly signaling shifts in patterns of site occupation and/or food preparation methods. With respect to nonhuman modifications, the new data suggest a slight decrease in leporid remains with signs of gastric etching from the MP to the LUP/H (Figs. 2, F and G, and 4C). This difference is, however, not statistically significant (means: MP, 4.7%, n = 9; LUP/H, 2.2%, n = 46; ts = 0.38, P = 0.70).
The dendrogram in Fig. 4D synthesizes these data by simultaneously considering five categories of bone modification (percentages of cutmarks, burning, cylinders, pit and gnaw marks, and gastric etching). The analysis revealed three main clusters. Cluster 1 comprises all of the control assemblages with high levels of digestion. Cluster 2 includes only two assemblages with exceptionally high percentages of anthropic damage (Bolomor Cave layers XI and IV). Cluster 3 encompasses the remaining assemblages with further subgroupings, although separated by short branches indicating less-robust divisions. Within Cluster 3, the “mostly natural” subgroup associates modern assemblages of noningested rabbit carcasses accumulated by carnivores or raptors and natural death in a warren with archaeological assemblages with only scarce anthropic evidence (Salpêtre layers IX, VII–V and l’Hortus layers 33 to 27), suggesting that the latter assemblages were mainly deposited by nonhuman predators. At l’Hortus, this is supported by the presence of numerous remains of bats in the bottom and intermediate layers—animals averse to human presence (30). Other subgroupings within cluster 3 do not reveal any clear chronological separation; some are dominated by MP assemblages, others by Upper Paleolithic assemblages, while still others show a combination of the two. However, no MP assemblages are grouped with LUP/H assemblages showing strong human impact (e.g., Bois-Ragot, Moulin du Roc, and “UP only” subgrouping in Fig. 4D). Despite this pattern, variation in the evidence for human agency in leporid exploitation seems to be greater within, than between, time periods in this dataset.
DISCUSSION
The new taphonomic data presented here push back the earliest date for leporid exploitation in the region to the Late Middle Pleistocene at the site of Terra Amata [ca. 400 thousand years ago; (31)]. Considering the evidence above with the spatial overlap of leporid and ungulate remains and stone tools at the site (19) indicates that humans were the main accumulators of leporids at this location. The abundance of leporid remains (50.6 to 66.0%; Table 1) and the presence of several cutmarks on meat-bearing elements (7/20 or 35.0%; marks on humeri, innominates, and mandibles) support this conclusion. Thus, Terra Amata provides one of the oldest unequivocal cases for recurrent exploitation of small fast game in Europe. Other early occurrences include Gran Dolina TD10-1 (MIS9) and Bolomor Cave [MIS9 to MIS5e; (5)], whereas later sites are dated to MIS6 to MIS5 (le Lazaret and Cova Negra), MIS5 to MIS4 (les Ramandils and les Canalettes), and MIS3 [e.g., la Crouzade, Cova Beneito; (20)]. Thus, early anthropogenic leporid exploitation covered a long time span (MIS11 to MIS3) in the study region. Moreover, some anthropogenic assemblages are spread throughout long stratigraphic sequences (e.g., Bolomor Cave, le Lazaret, les Ramandils), indicating that procurement of small elusive prey occurred in the northwestern Mediterranean despite substantial environmental fluctuations.
The phylogeography of rabbits, particularly their origins within Iberia and Southern France (21), makes the northwestern Mediterranean a natural laboratory for the study of small fast game exploitation. Overall, our findings suggest that broader diet breadths persisted across different areas of the northwestern Mediterranean during the Late Middle through the Early Late Pleistocene. However, wider faunal studies indicate that the broader diets recorded during the MP were followed in Southern France by an NDB episode coinciding with the EUP (9), when particularly cool climatic events likely negatively affected local leporid populations. Similar alternations between broader and narrower dietary episodes due to climate were probably common throughout MIS11 to MIS3 in this area. Although poorly documented in Iberia and parts of France, the NDB episode documented during the EUP suggests that diet breadth and exploitation goals (e.g., use of small game parts as ornaments and tools) were far from uniform and unchanging in the northwestern Mediterranean before the Last Glacial Maximum.
Early leporid exploitation raises issues concerning the foraging behaviors of archaic Homo groups, given that rabbits can be costly to capture and are typically associated with high risk of failure when procured singly but may provide high return rates when mass harvested from warrens (18). The negative relationship observed between the proportion of infant rabbits and anthropic damage in our data suggests that this hunting technique was infrequently practiced. Nonetheless, the breeding season for rabbits is typically shorter than conventional wisdom has it—between 96 and 192 days in France and between 90 and 270 days in Iberia (32)—which implies that mass harvesting might have been practiced at these sites in the absence of evidence for kit (infant rabbit) procurement. However, rabbit ecology renders mass harvesting an unlikely explanation, as gestation is attuned to local, poorly predictable, variations in temperature and rainfall, leading to highly variable breeding seasons in the Mediterranean (32). For example, one study in Alicante (Spain) reported that the breeding season spanned from October to February in one year, from March to May in the next, and from December to April in the following year (33). These wide interannual fluctuations in breeding season and the average litter size for rabbits [four to five kits; (32)] mean that the possibility that mass-harvesting episodes rarely resulted in the capture of infant rabbits is remote. Unless kits were ignored—a practice inconsistent with the extensive use of animal carcasses during the MP—their low representation in the anthropogenic assemblages that we examined suggests infrequent mass harvesting of rabbits and, thus, high capture costs.
The results presented here conflict with the NDB hypothesis. Contrary to the widely accepted assumption that archaic Homo populations from Europe subsisted almost exclusively on ungulates across their entire geographical range, our findings, along with mounting archaeozoological evidence (5, 14, 15, 34), indicate that this model is inadequate for the northwestern Mediterranean. In this region, there are now at least five sites (Terra Amata, Bolomor Cave, les Ramandils, les Canalettes, and Baume des Peyrards) with strong taxonomic representation of leporids relative to ungulates and clear signs that humans were the dominant agents of accumulation. Moreover, several assemblages [Gran Dolina TD10-1; Bolomor Cave layers XVIIc, XVIIa, XVe, XII, and Ia; le Lazaret UA29–27, 25 (35); and la Crouzade layer 7] show substantial evidence of human processing of leporids, despite hints that they were formed by mixed agents of accumulation. Together, these occurrences—which range from Southern Spain to the France/Italy border and include numerous leporid assemblages fully or at least partly formed by humans—suggest that the dietary exploitation of small fast game by archaic Homo groups was frequent throughout the northwestern Mediterranean. This point is supported by a regional survey of studies of pre–Upper Paleolithic sites that included taphonomic analysis of leporids (n = 26; see Supplementary Materials), a prey type that is commonly reported in the study region. In this sample, sites with multiple lines of evidence that strongly indicate rabbit exploitation are moderately well represented (5/26 or 19.2%). In addition, the survey data reveal that a majority of the taphonomically studied rabbit assemblages include at least one cutmarked rabbit specimen (17/26 or 65.4%). These findings imply that hominins frequently contributed in some part to the leporid accumulations in the study region. However, note that the lack of taphonomic analysis of small game assemblages for many MP sites in the region renders these values somewhat tentative at this time. Nonetheless, evidence for common bird consumption by humans at Bolomor Cave, Gran Dolina, Gorham’s Cave, Vanguard Cave, Ibex Cave, and Cova Negra in Iberia (5, 15, 36), and, to a lesser extent, Pié Lombard in France (37) provides further support for the argument that small fast game were commonly exploited in the northwestern Mediterranean. Although the newly available information indicates that the consumption of leporids and birds has been considerably underestimated in the study region, evidence from many sites from the same area reflects limited procurement of these prey types prior to the Upper Paleolithic, suggesting that their economic significance was largely inferior to that of ungulates.
The new data reported here indicate that small game associated with high capture costs and low returns were selected moderately often as dietary items in the northwestern Mediterranean. This pattern contrasts with the patterns in the Northern European plains and the eastern Mediterranean where hominin populations seem to have closely followed the NDB model (7, 9). However, these regional differences should not be overstated given that the overall picture for the study area suggests that small fast game played a subsidiary role in hominin diets relative to ungulates. For instance, with the possible exception of les Ramandils, none of the sites that we examined show the extremely dense concentrations of rabbit elements observed at several Upper Paleolithic sites (18). Moreover, although the leporid NISP counts are high in most of the assemblages that we studied, rabbits would have provided smaller food packages than much larger-bodied ungulates. Nonetheless, despite their presumed secondary dietary importance, our results indicate that small fast game were more frequently procured by hominins in the study region than envisioned by current models of Middle and Late Pleistocene subsistence.
These results raise larger issues over the potential drivers of dietary change over time. One hypothesis is that, in the northwestern Mediterranean, occasional depressed encounter rates with ungulates more frequently led pre–Upper Paleolithic hominins to expand their diet breadth by including costly resources such as singly captured rabbits than in other regions. Moreover, sites in the Northern European plains show rare evidence of leporid exploitation but more frequent procurement of species living in large herds (e.g., Bos/Bison, Rangifer tarandus) in comparison with the northwestern Mediterranean region, where the main hunted species often consist of individuals that tend to forage alone or in small- to moderate-sized groups (e.g., Cervus elaphus, Dama dama, Capra ibex) (11). Thus, interregional differences in ungulate taxonomic composition could have fueled corresponding disparities in small game use. Likewise, the dietary differences that we report here may have been influenced by variations in anticipated payoffs for rabbits versus hares—two taxa that differ substantially in terms of behavioral characteristics and geography (18)—as hares were probably more frequently encountered than rabbits in the Northern European plains. Climate change probably further affected exploitation of small fast game over broad time scales by altering the relative abundances of high-ranked prey types, a phenomenon hinted at by genetic data documenting considerable fluctuations in the demographic history and range size of several species of large ungulates (38). Another possibility is that archaic Homo groups in the northwestern Mediterranean more frequently adopted risk-minimizing subsistence strategies in comparison to other areas. Although it is premature at this point to determine which of these factors prevailed, the data presented here imply that archaic Homo populations, including Neanderthals, had more variable diet breadths across these regions than generally acknowledged.
The evidence for broader diets than previously estimated in the northwestern Mediterranean during the Lower/Middle Paleolithic has important ramifications concerning the trajectory of subsistence change leading up to even broader diets just prior to the terminal Pleistocene and early Holocene agricultural transition, termed the “broad-spectrum revolution” (BSR) (39). Our results indicate that at least one broader dietary episode preceded the BSR, from which it differed in at least four ways. The earlier MP episode reported here (i) remained mostly land based, lacking evidence for fish consumption; (ii) was restricted to a region where encounter rates with leporids were likely high; (iii) is associated with small game samples that are generally smaller and are more often preyed upon by nonhuman predators than those dated to the LUP/H; and (iv) shows a different range of exploited small game. This last point requires additional explanation. Prior to the LUP/H, small fast prey taxa, characterized by mostly solitary behavior (e.g., hares, small carnivores), were apparently largely ignored, the premium being on gregarious species (i.e., rabbits). In contrast, outside of Iberia, some LUP/H sites show moderate to strong taxonomic representation of hares, such as Bois-Ragot and Gazel in France, Champréveyres and Kesslerloch in Switzerland, Pavlov I in Czechia, and Kostenki 14 in Russia (40–43). More rarely, some LUP/H assemblages strongly suggest human consumption of fox remains [Grotta Romanelli, Italy (44); Dolní Věstonice, Czechia (45)]. Thus, high local rabbit population densities and ubiquity—mediated by climatic conditions—might have been decisive factors driving variations in diet breadth during the Lower and Middle Paleolithic. Nonetheless, the range of evidence for the inclusion of costly resources in diets over long time spans in the northwestern Mediterranean highlights greater intraregional dietary variation in the periods preceding the BSR than previously thought.
MATERIALS AND METHODS
Experimental design
The 21 new assemblages we examine here were analyzed using a comprehensive taphonomic approach. Except for the open-air site of Terra Amata, the assemblages are all from cave sites, including a new layer from the previously analyzed site of les Canalettes (14). To determine the agent of accumulation, we compared these assemblages to modern control assemblages accumulated by known species of small carnivores (carn), such as fox (V. vulpes), wildcat (Felis silvestris), and the Iberian lynx (Lynx pardinus), diurnal (diurn) and nocturnal (noct) raptors, and a rabbit warren (Supplementary Materials). Furthermore, the assemblages were compared to 90 published leporid assemblages with ≥1 cutmarked leporid specimens from the Acheulean/Middle Paleolithic (MP; MIS9 to MIS3), EUP (Aurignacian to Early Solutrean; MIS3 to MIS2), and LUP/H (MIS2 to MIS1). Note that although Lepus specimens were occasionally observed, the overwhelming majority of the leporids reported here were rabbits (O. cuniculus).
In our sample, human involvement in the leporid accumulations was assessed through the analysis of bone surface modifications and fragmentation patterns [e.g., (23, 27)]. All coded specimens in our samples were examined under a stereomicroscope at magnifications ranging from ×10 to ×45. The percentage of cutmarked or burned specimens was calculated by dividing the number of specimens with each type of damage by the leporid NISP count × 100. However, at l’Hortus, les Ramandils, and le Lazaret, the identification of cutmarks was not always straightforward because scalpels and other metal tools were sometimes used by excavation staff to clean the specimens and remove calcareous concretions from the bone surface. All potential scalpel marks—identified by the lighter color of the recent marks—were eliminated from the cutmark sample. We also used the presence of manganese coating and concretion overlapping marks to assess the mark antiquity. Additional information about recording methods can be found in the Supplementary Materials.
Statistical analysis
Difference in proportions was tested in pairwise comparisons after arcsine transformation of the data using Sokal and Rohlf’s (46, pp. 607–610) ts. We used the Spearman rank order correlation to assess correlations between two variables. The cluster analysis was generated with the PAST (Paleontological Statistics) software (47) using a Manhattan pairwise similarity matrix. We used the Manhattan index because its formulation is very similar to the Brainerd-Robinson index familiar to archaeologists. The dendrogram was calculated using an UPGMA (unweighted pair group method with arithmetic mean) algorithm.
Supplementary Material
Acknowledgments
We thank L. Lloveras for comments on the draft of this article and J. D. Speth for the simulating discussions on leporid processing. H. de Lumley and T. Saos provided access to the study material presented here. Funding: This research was funded by the Social Sciences and Humanities Research Council of Canada (grant #435-2013-0993). Author contributions: E.M. and J.M. wrote the paper; J.M., E.M., K.E.G., D.C., and L.L. compiled the leporid data; A.-M.M., P.V., and L.R. collected the ungulate data; and E.M., J.M., and J.C. performed the statistical analyses. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav9106/DC1
Supplementary Materials and Methods
Fig. S1. Anatomical refits for various taxa at the site of l’Hortus and definition of the three ensembles used in this study.
Data file S1. Leporid data for the new assemblages and the comparative sample.
REFERENCES AND NOTES
- 1.L. R. Binford, Constructing Frames of Reference: an Analytical Method for Archaeological Theory Building Using Ethnographic and Environmental Data Sets (University of California Press, 2001). [Google Scholar]
- 2.J. D. Speth, The Paleoanthropology and Archaeology of Big-Game Hunting: Protein, Fat, or Politics? (Springer, New York, 2010). [Google Scholar]
- 3.E. A. Smith, Inujjuamiut Foraging Strategies. Evolutionary Ecology of an Arctic Hunting Economy (Aldine de Gruyter, New York, 1991). [Google Scholar]
- 4.Bird D. W., Bird R. B., Codding B. F., In pursuit of mobile prey: Martu hunting strategies and archaeofaunal interpretation. Am. Antiq. 74, 3–29 (2009). [Google Scholar]
- 5.Blasco R., Rosell J., Peris J. F., Arsuaga J. L., de Castro J. M. B., Carbonell E., Environmental availability, behavioural diversity and diet: A zooarchaeological approach from the TD10-1 sublevel of Gran Dolina (Sierra de Atapuerca, Burgos, Spain) and Bolomor Cave (Valencia, Spain). Quat. Sci. Rev. 70, 124–144 (2013). [Google Scholar]
- 6.Ugan A., Does size matter? Body size, mass collecting, and their implications for understanding prehistoric foraging behavior. Am. Antiq. 70, 75–89 (2005). [Google Scholar]
- 7.Stiner M. C., Prey choice, site occupation intensity and economic diversity across the Middle to early Upper Palaeolithic at Üçağızlı Caves I and II (Hatay, Turkey). Before Farming 3, 1–20 (2009). [Google Scholar]
- 8.Starkovich B. M., Intensification of small game resources at Klissoura Cave 1 (Peloponnese, Greece) from the Middle Paleolithic to Mesolithic. Quat. Int. 264, 17–31 (2012). [Google Scholar]
- 9.E. Morin, Reassessing Paleolithic Subsistence: The Neandertal and Modern Human Foragers of Saint-Césaire (Cambridge Univ. Press, New York, 2012). [Google Scholar]
- 10.Stiner M. C., Munro N. D., Surovell T. A., Tchernov E., Bar-Yosef O., Paleolithic population growth pulses evidenced by small animal exploitation. Science 283, 190–194 (1999). [DOI] [PubMed] [Google Scholar]
- 11.E. Morin, J. D. Speth, J. Lee-Thorp, Middle Palaeolithic diets: A critical examination of the evidence, in The Oxford Handbook of the Archaeology of Diet, J. Lee-Thorp, M. A. Katzenberg, Eds. (Oxford Univ. Press, 2016), pp. 1–33. [Google Scholar]
- 12.Stiner M. C., An unshakable Middle Paleolithic? Trends versus conservatism in the predatory niche and their social ramifications. Curr. Anthropol. 54, S288–S304 (2013). [Google Scholar]
- 13.Blasco R., Fernández Peris J., A uniquely broad spectrum diet during the Middle Pleistocene at Bolomor Cave (Valencia, Spain). Quat. Int. 252, 16–31 (2012). [Google Scholar]
- 14.Cochard D., Brugal J.-P., Morin E., Meignen L., Evidence of small fast game exploitation in the Middle Paleolithic of Les Canalettes Aveyron, France. Quat. Int. 264, 32–51 (2012). [Google Scholar]
- 15.Martínez Valle R., Guillem Calatayud P. M., Villaverde Bonilla V., Bird consumption in the final stage of Cova Negra (Xátiva, Valencia). Quat. Int. 421, 85–102 (2016). [Google Scholar]
- 16.Blasco R., Finlayson C., Rosell J., Marco A. S., Finlayson S., Finlayson G., Negro J. J., Pacheco F. G., Vidal J. R., The earliest pigeon fanciers. Sci. Rep. 4, 5971 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blasco R., Rosell J., Rufà A., Sánchez Marco A., Finlayson C., Pigeons and choughs, a usual resource for the Neanderthals in Gibraltar. Quat. Int. 421, 62–77 (2016). [Google Scholar]
- 18.Jones E. L., Prey choice, mass collecting, and the wild European rabbit (Oryctolagus cuniculus). J. Anthropol. Archaeol. 25, 275–289 (2006). [Google Scholar]
- 19.K. El Guennouni, thesis, Muséum National d’Histoire Naturelle, Paris (2001). [Google Scholar]
- 20.Sanchis Serra A., Fernández Peris J., Procesado y consumo antrópico de conejo en la Cova de Bolomor (Tavernes de la Valldigna, Valencia): el nivel XVIIc (ca 350 ka). Complutum 19, 25–46 (2008). [Google Scholar]
- 21.E. Donard, thesis, Université de Bordeaux I (1982). [Google Scholar]
- 22.Pelletier M., Brugal J.-P., Cochard D., Lenoble A., Mallye J.-B., Royer A., Identifying fossil rabbit warrens: Insights from a taphonomical analysis of a modern warren. J. Archaeol. Sci. Rep. 10, 331–344 (2016). [Google Scholar]
- 23.Rodríguez-Hidalgo A., Saladié P., Marín J., Canals A., Expansion of the referential framework for the rabbit fossil accumulations generated by Iberian lynx. Palaeogeogr. Palaeoclimatol. Palaeoecol. 418, 1–11 (2015). [Google Scholar]
- 24.Hockett B. S., Corroded, thinned and polished bones created by golden eagles (Aquila chrysaetos): Taphonomic implications for archaeological interpretations. J. Archaeol. Sci. 23, 587–591 (1996). [Google Scholar]
- 25.Hockett B. S., Toward distinguishing human and raptor patterning on leporid bones. Am. Antiq. 56, 667–679 (1991). [Google Scholar]
- 26.C. Daujeard, thesis, Université de Lyon (2008). [Google Scholar]
- 27.Lloveras L., Moreno-García M., Nadal J., Butchery, cooking and human consumption marks on rabbit (Oryctolagus cuniculus) bones: An experimental study. J. Taphon. 7, 179–201 (2009). [Google Scholar]
- 28.Lloveras L., Cosso A., Solé J., Claramunt-López B., Nadal J., Taphonomic signature of golden eagles (Aquila chrysaetos) on bone prey remains. Hist. Biol., 1–20 (2017). [Google Scholar]
- 29.Rodríguez-Hidalgo A., Lloveras L., Moreno-García M., Saladié P., Canals A., Nadal J., Feeding behaviour and taphonomic characterization of non-ingested rabbit remains produced by the iberian lynx (Lynx pardinus). J. Archaeol. Sci. 40, 3031–3045 (2013). [Google Scholar]
- 30.H. de Lumley, La grotte de l’Hortus (Valflaunès, Hérault). Etudes Quaternaires et Géologie, Paléontologie, Préhistoire (Études Quaternaire Mémoire 1, Marseille, 1972). [Google Scholar]
- 31.H. de Lumley, Terra Amata: Nice, Alpes-Maritimes, France. Tome I. Cadre Géographique, Historique, Contexte Géologique, Stratigraphie, Sédimentologie, Datation (CNRS Editions, Paris, 2009). [Google Scholar]
- 32.P. M. Rogers, C. P. Arthur, R. C. Soriguer, The rabbit in continental Europe, in The European Wild Rabbit: The History of A Succesful Colonizer, H. Thompson, C. M. King, Eds. (Oxford Univ. Press, 1994), pp. 22–63. [Google Scholar]
- 33.Tablado Z., Revilla E., Palomares F., Breeding like rabbits: Global patterns of variability and determinants of European wild rabbit reproduction. Ecography 32, 310–320 (2009). [Google Scholar]
- 34.Blasco R., Fernández Peris J., Small and large game: human use of diverse faunal resources at level IV of Bolomor Cave (Valencia, Spain). Comptes Rendus Palevol 11, 265–282 (2012). [Google Scholar]
- 35.H. De Lumley, A. Echassoux, S. Bailon, S. Abdessadok, E. Fauquembergue, Le sol d’occupation Acheuléen de l’unité Archéostratigraphique UA 25 de La Grotte du Lazaret (Edisud, France, 2004). [Google Scholar]
- 36.Finlayson C., Brown K., Blasco R., Rosell J., Negro J. J., Bortolotti G. R., Finlayson G., Sánchez Marco A., Giles Pacheco F., Rodríguez Vidal J., Carrión J. S., Fa D. A., Rodríguez Llanes J. M., Birds of a feather: Neanderthal exploitation of raptors and corvids. PLOS ONE 7, e45927 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero A., Díez J., Brugal J., Aves de caza. Estudio tafonómico y zooarqueológico de los restos avianos de los niveles musterienses de Pié Lombard (Alpes-Maritimes, Francia). Munibe, 73–84 (2017). [Google Scholar]
- 38.Lorenzen E. D., Nogués-Bravo D., Orlando L., Weinstock J., Binladen J., Marske K. A., Ugan A., Borregaard M. K., Gilbert M. T. P., Nielsen R., Ho S. Y. W., Goebel T., Graf K. E., Byers D., Stenderup J. T., Rasmussen M., Campos P. F., Leonard J. A., Koepfli K.-P., Froese D., Zazula G., Stafford T. W. Jr., Aaris-Sørensen K., Batra P., Haywood A. M., Singarayer J. S., Valdes P. J., Boeskorov G., Burns J. A., Davydov S. P., Haile J., Jenkins D. L., Kosintsev P., Kuznetsova T., Lai X., Martin L. D., McDonald H. G., Mol D., Meldgaard M., Munch K., Stephan E., Sablin M., Sommer R. S., Sipko T., Scott E., Suchard M. A., Tikhonov A., Willerslev R., Wayne R. K., Cooper A., Hofreiter M., Sher A., Shapiro B., Rahbek C., Willerslev E., Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359–364 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flannery K. V., Origins and ecological effects of early domestication in Iran and the Near East. Domest. Exploit. plants Anim., 73–100 (1969). [Google Scholar]
- 40.D. Cochard, J.-P. Brugal, Importance des fonctions de site dans les accumulations paléolithiques de léporidés, in Petits animaux et sociétés humaines: du complément alimentaire aux ressources utilitaires, J. P. Brugal, J. Desse, Eds. (Éditions APDCA, Antibes, 2004), pp. 283–296. [Google Scholar]
- 41.Fontana L., Chauvière F.-X., L’exploitation du lièvre variable à la Madeleine (Dordogne, France) et le statut d’un petit gibier au Dryas ancien. Paléo 19, 303–336 (2007). [Google Scholar]
- 42.Wojtal P., Wilczyński J., Bocheński Z. M., Svoboda J. A., The scene of spectacular feasts: Animal remains from Pavlov I south-east, the Czech Republic. Quat. Int. 252, 122–141 (2012). [Google Scholar]
- 43.Hoffecker J. F., Kuz’mina I. E., Syromyatnikova E. V., Anikovich M. V., Sinitsyn A. A., Popov V. V., Holliday V. T., Evidence for kill-butchery events of early Upper Paleolithic age at Kostenki, Russia. J. Archaeol. Sci. 37, 1073–1089 (2010). [Google Scholar]
- 44.A. Tagliacozzo, I. Fiore, in Economie Préhistorique: Les comportements de subsistance au Paléolithique, J.-P. Brugal, M.-H. Pathou-Mathis, L. Meignen, Eds. (Éditions APDCA, Antibes, 1998), pp. 413–423. [Google Scholar]
- 45.Wilczyński J., Wojtal P., Robličková M., Oliva M., Dolní Věstonice I (Pavlovian, the Czech Republic) – Results of zooarchaeological studies of the animal remains discovered on the campsite (excavation 1924–52). Quat. Int. 379, 58–70 (2015). [Google Scholar]
- 46.R. R. Sokal, F. J. Rohlf, Biometry (Freeman and Company, San Francisco, 1969). [Google Scholar]
- 47.Ø. Hammer, PAST. Paleontological Statistics. Version 2.07. Reference manual (2011).
- 48.L. Meignen, L’abri des Canalettes: Un Habitat Moustérien sur les Grands Causses (Nant, Aveyron) Fouilles 1980–1986 (CNRS Éditions, 1993). [Google Scholar]
- 49.L. Meignen, J.-P. Brugal, in Settlement dynamics of the Middle Paleolithic and Middle Stone Age, N. J. Conard, Ed. (Kerns Verlag, Tübingen, Germany, 2001), pp. 463–483. [Google Scholar]
- 50.Lebegue F., Le Paléolithique moyen récent entre Rhône et Pyrénées: Approche de l’organisation techno-économique des productions lithiques, schémas de mobilité et organisation du territoire (Les Canalettes, L’Hortus, Bize-Tournal, La Crouzade et La Roquette II). Bull. Soc. Préh. Fr. 109, 579–580 (2012). [Google Scholar]
- 51.H. Valladas, J. L. Joron, in L’abri des Canalettes: Un habitat moustérien sur les grands Causses (Nant, Aveyron). Fouilles 1980–1986, L. Meignen, Ed. (CNRS Éditions, 1993), pp. 141–146. [Google Scholar]
- 52.J. P. Brugal, M. Patou-Mathis, in L’abri des Canalettes. Un habitat moustérien sur les grands Causses (Nant, Aveyron). Fouilles 1980–1986, L. Meignen, Ed. (CNRS Éditions, 1993), pp. 77–87. [Google Scholar]
- 53.M. Patou-Mathis, in L’Abri des Canalettes. Un habitat moustérien sur les grands Causses (Nant, Aveyron). Fouilles 1980–1986, L. Meignen, Ed. (CNRS Éditions, 1993), pp. 199–235. [Google Scholar]
- 54.D. Cochard, thesis, Université de Bordeaux I (2004). [Google Scholar]
- 55.Boutié P., Ajaja O., Banes L., Moles V., Kabiri L., Grégoire S., Port-la-Nouvelle. Le gisement moustérien des Ramandils. ADLFI 2004, 2–26 (2004). [Google Scholar]
- 56.Moles V., Boutié P., Contribution à la reconnaissance d’une microproduction au Paléolithique moyen: les industries de la grotte des Ramandils (Port-La Nouvelle, Aude, France). Anthropologie 113, 356–380 (2009). [Google Scholar]
- 57.L. Banes, A. Dorigny, in Peuplements humains et variations environnementales au Quaternaire, A. Tuffreau, Ed. (British Archaeological Reports 1352, 2005), pp 95–104. [Google Scholar]
- 58.J.-P. Gerber, thesis, Université de Provence (1973). [Google Scholar]
- 59.C. Percie du Sert, thesis, Université Paul Valéry, Montpellier (1992). [Google Scholar]
- 60.Magniez P., Incidence des fluctuations climatiques sur la taille du renne (Rangifer tarandus) au Pléistocène supérieur. Quat. Rev. 21, 259–279 (2010). [Google Scholar]
- 61.A. Testu, thesis, Université de Perpignan (2006). [Google Scholar]
- 62.T. Saos, Le Paléolithique moyen de la grotte de la Crouzade: actualisation des données et contexte chronostratigraphique, paper presented at the 18th Annual Meeting of the International Union of Prehistoric and Protohistoric Sciences, Paris, France, 4 to June 2018. [Google Scholar]
- 63.J. Quilès, thesis, Paris, Muséum National d’Histoire Naturelle (2003). [Google Scholar]
- 64.Farbos-Texier S., Miskovsky J.-C., Marquet J.-C., Meignen L., Coularou J., Etude paléoclimatique en Languedoc oriental d'après les niveaux paléolithiques de la Grotte du Salpêtre de Pompignan (Pompignan, Gard). Bull. de l’Association Française pour l’Étude du Quat. 18, 129–147 (1981). [Google Scholar]
- 65.C. Bergès, thesis, Centre Européen de Recherches Préhistoriques, Tautavel, France (1997). [Google Scholar]
- 66.F. Lebègue, N. Boulbes, S. Gregoire, A.-M. Moigne, Exploitation des ressources et mobilité des Néandertaliens de l’Hortus, in Settlement Dynamics, N. Conard, Ed. (Kerns Verlag, Tübingen, 2010), vol. 3, pp. 455–483. [Google Scholar]
- 67.F. Rivals, thesis, Université de Perpignan (2002). [Google Scholar]
- 68.B. Pillard, in La grotte de l’Hortus (Valflaunès, Hérault). Les chasseurs néandertaliens et leur milieu de vie. Élaboration d’une chronologie du Würmien II dans le Midi méditerranéen, H. de Lumley, J. P. Bard, Eds. (Études Quaternaires 1, Marseille, 1972), pp. 163–205 (1972). [Google Scholar]
- 69.Bordes F., Sur la notion de sol d’habitat en préhistoire paléolithique. Bull. Soc. Préh. Fr. 72, 139–144 (1975). [Google Scholar]
- 70.H. de Lumley, Le Paléolithique inférieur et moyen du Midi méditerranéen dans son cadre géologique (CNRS Éditions, Paris, 1969). [Google Scholar]
- 71.Daujeard C., Vettese D., Britton K., Béarez P., Boulbes N., Crégut-Bonnoure E., Desclaux E., Lateur N., Pike-Tay A., Rivals F., Allué E., Chacón M. G., Puaud S., Richard M., Courty M.-A., Gallotti R., Hardy B., Bahain J. J., Falguères C., Pons-Branchu E., Valladas H., Moncel M.-H., Neanderthal selective hunting of reindeer? The case study of Abri du Maras (south-eastern France). Archaeol. Anthropol. Sci., 1–27 (2017). [Google Scholar]
- 72.Porraz G., Les pièces amincies de la Baume des Peyrards (Massif du Luberon, Vaucluse): Analyse des procédés de réalisation. Préhistoires Méditerranéennes 10–11, 27–38 (2002). [Google Scholar]
- 73.H. de Lumley, Terra Amata: Nice, Alpes-Maritimes, France. Tome II. Palynologie, Anthracologie, Faunes, Mollusques, Écologie et Biogéomorphologie, Paléoanthropologie, Empreinte de pied humain, Coprolithes (CNRS Editions, Paris, 2011). [Google Scholar]
- 74.Villa P., Conjoinable pieces and site formation processes. Am. Antiq. 47, 276–290 (1982). [Google Scholar]
- 75.P. Valensi, H. de Lumley, M. Beden, L. Jourdan, F. Serre, in Terra Amata, Nice, Alpes-Maritimes, France Tome II, H. de Lumley, Ed. (CNRS Editions, Paris, 2011), pp. 41–290. [Google Scholar]
- 76.S. Bailon, et al, in Terra Amata: Nice, Alpes-Maritimes, France. Tome II, H. de Lumley, Ed. (CNRS Editions, Paris, 2011), pp. 298–378. [Google Scholar]
- 77.Michel V., Shen G., Valensi P., de Lumley H., ESR dating of dental enamel from Middle Palaeolithic levels at Lazaret Cave, France France. Quat. Geochronol. 4, 233–240 (2009). [Google Scholar]
- 78.Valensi P., Evolution of large mammal populations in West Mediterranian Europe during middle and upper Pleistocene. A regional example: French and Italian Southern Alps. Quaternaire 20, 551–567 (2009). [Google Scholar]
- 79.C. V. Sharada, thesis, Universita di Ferrara, Italy (2013). [Google Scholar]
- 80.Jullien R., Pillard B., Les lagomorphes découverts sur le sol de la cabane acheuléenne du Lazaret. Mémoire de la Société Préhistorique Française 7, 75–83 (1969). [Google Scholar]
- 81.C. Callou, De la garenne au clapier. Étude archéozoologique du Lapin en Europe occidentale (Muséum National d’Histoire Naturelle, Paris, 2003). [Google Scholar]
- 82.Brugal J.-P., Petit gibier et fonction de sites au Paléolithique supérieur. Les ensembles fauniques de la grotte d’Anecrial (Porto de Mos, Estremadure, Portugal). Paléo 18, 45–68 (2006). [Google Scholar]
- 83.Caparrós M., Ruíz C. B., Moigne A. M., Monclova A., Did Neanderthals and carnivores compete for animal nutritional resources in the surroundings of the cave of Zafarraya? J. Taphon. 10, 395–415 (2012). [Google Scholar]
- 84.X. Terradas, J.-M. Rueda, in Économie préhistorique: les comportements de subsistance au Paléolithique, J.-P. Brugal, L. Meignen, M. Patou-Mathis, Eds. (APDCA, Antibes, France, 1998), pp. 349–361. [Google Scholar]
- 85.Zilhão J., Ajas A., Badal E., Burow C., Kehl M., López-Sáez J. A., Pimenta C., Preece R. C., Sanchis A., Sanz M., Weniger G. C., White D., Wood R., Angelucci D. E., Villaverde V., Zapata J., Cueva Antón: A multi-proxy MIS 3 to MIS 5a paleoenvironmental record for SE Iberia. Quat. Sci. Rev. 146, 251–273 (2016). [Google Scholar]
- 86.Fagoaga A., Ruiz-Sánchez F. J., Laplana C., Blain H. A., Marquina R., Marin-Monfort M. D., Galván B., Palaeoecological implications of Neanderthal occupation at Unit Xb of El Salt (Alcoi, eastern Spain) during MIS 3 using small mammals proxy. Quat. Int. 481, 101–112 (2018). [Google Scholar]
- 87.Marks A. E., Brugal J.-P., Chabai V. P., Monigal K., Goldberg P., Hockett B., Peman E., Elorza M., Mallol C., Le gisement pléistocène moyen de Galeria Pesada (Estrémadure, Portugal): Premiers résultats. Paléo 14, 77–100 (2002). [Google Scholar]
- 88.Arriaza M. C., Huguet R., Laplana C., Pérez-González A., Márquez B., Arsuaga J. L., Baquedano E., Lagomorph predation represented in a middle Palaeolithic level of the Navalmaíllo Rock Shelter site (Pinilla del Valle, Spain), as inferred via a new use of classical taphonomic criteria. Quat. Int. 436, 294–306 (2017). [Google Scholar]
- 89.Moncel M.-H., Moigne A.-M., Sam Y., Combier J., The emergence of neanderthal technical behavior: New evidence from Orgnac 3 (Level 1, MIS 8), Southeastern France. Curr. Anthropol. 52, 37–75 (2011). [Google Scholar]
- 90.Lloveras L., Moreno-García M., Nadal J., Zilhão J., Who brought in the rabbits? Taphonomical analysis of Mousterian and Solutrean leporid accumulations from Gruta do Caldeirão (Tomar, Portugal). J. Archaeol. Sci. 38, 2434–2449 (2011). [Google Scholar]
- 91.Lloveras L., Moreno-García M., Moreno-García M., Nadal J., Maroto J., Maroto J., Soler J., Soler J., Nadal J., Soler N., Soler N., The application of actualistic studies to assess the taphonomic origin of Musterian rabbit accumulations from Arbreda Cave (North-East Iberia). Archaeofauna 19, 99–119 (2010). [Google Scholar]
- 92.Lloveras L., Nadal J., Moreno-García M., Thomas R., Anglada J., Baucells J., Martorell C., Vilasís D., The role of the Egyptian Vulture (Neophron percnopterus) as a bone accumulator in cliff rock shelters: An analysis of modern bone nest assemblages from North-eastern Iberia. J. Archaeol. Sci. 44, 76–90 (2014). [Google Scholar]
- 93.Lloveras L., Moreno-García M., Nadal J., Feeding the foxes: An experimental study to assess their taphonomic signature on leporid remains. Int. J. Osteoarchaeol. 22, 577–590 (2012). [Google Scholar]
- 94.Lloveras L., Moreno-García M., Nadal J., Taphonomic analysis of leporid remains obtained from modern Iberian lynx (Lynx pardinus) scats. J. Archaeol. Sci. 35, 1–13 (2008). [Google Scholar]
- 95.Hockett B., Haws J. A., Taphonomic and methodological perspectives of leporid hunting during the Upper Paleolithic of the western Mediterranean Basin. J. Archaeol. Method Theory 9, 269–302 (2002). [Google Scholar]
- 96.Lloveras L., Thomas R., Cosso A., Pinyol C., Nadal J., When wildcats feed on rabbits: An experimental study to understand the taphonomic signature of European wildcats (Felis silvestris silvestris). Archaeol. Anthropol. Sci. 10, 449–464 (2018). [Google Scholar]
- 97.Lloveras L., Moreno-García M., Nadal J., The eagle owl (Bubo bubo) as a leporid remains accumulator: Taphonomic analysis of modern rabbit remains recovered from nests of this predator. Int. J. Osteoarchaeol. 19, 573–592 (2009). [Google Scholar]
- 98.Lloveras L., Thomas R., Lourenço R., Caro J., Dias A., Understanding the taphonomic signature of Bonelli’s Eagle (Aquila fasciata). J. Archaeol. Sci. 49, 455–471 (2014). [Google Scholar]
- 99.Lloveras L., Moreno-García M., Nadal J., Assessing the variability in taphonomic studies of modern leporid remains from Eagle Owl (Bubo bubo) nest assemblages: the importance of age of prey. J. Archaeol. Sci. 39, 3754–3764 (2012). [Google Scholar]
- 100.Lloveras L., Moreno-Garcia M., Nadal J., Taphonomic study of leporid remains accumulated by the Spanish Imperial Eagle (Aquila adalberti). Geobios 41, 91–100 (2008). [Google Scholar]
- 101.Grayson D. K., On the quantification of vertebrate archaeofaunas. Adv. Archaeol. Method Theory 2, 199–237 (1979). [Google Scholar]
- 102.D. K. Grayson, Quantitative Zooarchaeology (Academic Press, 1984). [Google Scholar]
- 103.E. Jones, thesis, University of Washington (2004). [Google Scholar]
- 104.A. Gardeisen, S. Valenzuela-Lamas, À propos de la présence de lapins en contexte gallo-romain à Lattara, in Petits animaux et sociétés humaines, J. P. Brugal, J. Desse, Eds. (APDCA, Antibes, France, 2004), pp. 235–354. [Google Scholar]
- 105.A. Bournery, J. D. Vigne, J. Vaquer, in Petits animaux et sociétés humaines, J. P. Brugal, J. Desse, Eds. (Éditions APDCA, Antibes, 2004), pp. 207–222. [Google Scholar]
- 106.Pelletier M., Royer A., Holliday T., Maureille B., Lièvre et lapin à Regourdou (Montignac-sur-Vézère, Dordogne, France): Études paléontologique et taphonomique de deux accumulations osseuses d'origine naturelle. Paléo 26, 161–183 (2015). [Google Scholar]
- 107.Fernández-Jalvo Y., Andrews P., When humans chew bones. J. Hum. Evol. 60, 117–123 (2011). [DOI] [PubMed] [Google Scholar]
- 108.Saladié P., Rodríguez-Hidalgo A., Díez C., Martín-Rodríguez P., Carbonell E., Range of bone modifications by human chewing. J. Archaeol. Sci. 40, 380–397 (2013). [Google Scholar]
- 109.K. T. Jones, in Carnivores, Human Scavengers & Predators: A Question of Bone Technology, G. M. Lemoine, A. S. MacEachern, Eds. (The University of Calgary, Archaeological Association, Calgary, 1983), pp. 171–191. [Google Scholar]
- 110.Hockett B. S., Bicho N. F., The rabbits of Picareiro Cave: Small mammal hunting during the Late Upper Palaeolithic in the Portuguese Estremadura. J. Archaeol. Sci. 27, 715–723 (2000). [Google Scholar]
- 111.Bicho N., Haws J., Hockett B., Two sides of the same coin—rocks, bones and site function of Picareiro Cave, central Portugal. J. Anthropol. Archaeol. 25, 485–499 (2006). [Google Scholar]
- 112.Y. Fernández-Jalvo, P. Andrews, Atlas of Taphonomic Identifications (Springer, 2016). [Google Scholar]
- 113.R. Jullien, in La grotte de l’Hortus (Valflaunès, Hérault). Les chasseurs néandertaliens et leur milieu de vie. Élaboration d’une chronologie du Würmien II dans le Midi méditerranéen, H. de Lumley, J. P. Bard, Eds. (Études Quaternaires 1, Marseille, 1972), pp. 247–265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav9106/DC1
Supplementary Materials and Methods
Fig. S1. Anatomical refits for various taxa at the site of l’Hortus and definition of the three ensembles used in this study.
Data file S1. Leporid data for the new assemblages and the comparative sample.


