Abstract
Purpose of review:
The most effective strategies for treating the patient with acute respiratory distress syndrome (ARDS) center on minimizing ventilation-induced lung injury (VILI). Yet, current standard-of-care does little to modify mechanical ventilation to patient-specific risk. This review focuses on evaluation of bedside respiratory mechanics, which when interpreted in patient-specific context, affords opportunity to individualize lung-protective ventilation in patients with ARDS.
Recent findings:
Four biophysical mechanisms of VILI are widely accepted: volutrauma, barotrauma, atelectrauma, and stress concentration. Resulting biotrauma, i.e. local and systemic inflammation and endothelial activation, may be thought of as the final common pathway that propagates VILI-mediated multiorgan failure. Conventional, widely utilized techniques to assess VILI risk rely on airway pressure, flow, and volume changes, and remain essential tools for determining overdistension of aerated lung regions, particularly when interpreted cognizant of their limitations. Emerging bedside tools identify regional differences in mechanics, but further study is required to identify how they might best be incorporated into clinical practice.
Summary:
Quantifying patient-specific risk of VILI requires understanding each patient’s pulmonary mechanics in context of biological predisposition. Tailoring support at bedside according to these factors affords the greatest opportunity to date for mitigating VILI and alleviating associated morbidity.
Keywords: ventilator-induced lung injury, acute respiratory distress syndrome, acute lung injury, mechanical ventilation
Introduction
Injurious forces during mechanical ventilation represent a key modifiable factor influencing the clinical course of patients with acute respiratory distress syndrome (ARDS) [1,2]. Clinical management of ARDS therefore must prioritize optimizing ventilatory support to minimize injury precipitated by mechanical ventilation. Quantifying the patient-specific risk of ventilation-induced lung injury (VILI) requires understanding patient-specific biological predisposition and pulmonary mechanics [3*,4]. Implications of molecular subphenotypes of ARDS are discussed elsewhere in this Issue. This article explores practical strategies for personalizing ventilatory support. Bedside tools to help discern injurious mechanical ventilation are reviewed, including their interpretation, assumptions, strengths, and limitations (Table 1).
Table 1.
Summary of Bedside Techniques
| Bedside Tool | Mechanism(s) of Ventilation-Induced Injury to be Measured | Advantages | Limitations |
|---|---|---|---|
| Tools Widely Available at Bedside Now | |||
| Physical exam |
|
|
|
| Airway plateau pressure |
|
|
|
| Airway driving pressure |
|
|
|
| Exhaled tidal volume |
|
|
|
| Ventilator waveform inspection |
|
|
|
| Emerging Tools | |||
| Esophageal manometry |
|
|
|
| Measures of ‘baby lung’ volume |
|
|
|
| Ventilator waveform computational analysis |
|
|
|
| Electrical impedance tomography |
|
|
|
| Lung & diaphragm ultrasound |
|
|
|
Abbreviations: PL: transpulmonary pressure, defined as airway minus pleural pressure.
Why bedside physiology matters
Clinically overt barotrauma has been recognized as a potential complication of positive pressure ventilation for over half a century [5]. While gross barotrauma of course is an example of VILI, it is not synonymous with VILI. Innumerable experiments have proven mechanical ventilation also can have less overt deleterious effects [6,7]. Endothelial barrier disruption, pulmonary edema formation, and cellular and tissue injury historically were thought due to ARDS per se, but over the last few decades all have been shown to be manifestations of VILI as well [2].
Whether these occult signs of VILI translate affect patient-centered outcomes also has been settled. Three landmark clinical trials together offer definitive proof in humans that mechanical ventilation strategy contributes to morbidity and mortality in patients with ARDS [1,8,9]. They collectively demonstrated that ventilation with lower-than-conventional tidal volumes attenuate lung injury, extrapulmonary organ injury, and systemic inflammation, and improve survival in ARDS. In the largest and most widely cited of these trials, the NHLBI ARDS Network trial observed improved morbidity and mortality with lower tidal volumes despite no difference in gross barotrauma [1]. Otherwise stated, clinically relevant VILI can be pernicious.
Due to lack of well-validated, clinically available, real-time biomarkers of VILI, bedside respiratory physiology represents the primary tool for adapting mechanical ventilation to patient-specific risk. Several groups are pursuing lung-specific molecular markers of injury that can be detected in the blood [10-14], although a well-performing marker specific to lung injury has proven elusive so far. Even if/when a “troponin-equivalent for the lung” is established for clinical use, bedside physiology will be crucial for identifying the patient-specific mechanisms of VILI and guiding appropriate interventions.
VILI mechanisms intended for measurement
Familiarity with VILI mechanisms is important for evaluating the role for various bedside physiologic tools aimed at quantifying lung injury risk. Five classic mechanisms of VILI are widely recognized: volutrauma, barotrauma, atelectrauma, stress concentration, and biotrauma [15].
Volutrauma
High tidal volumes cause inflammatory pulmonary edema and histologic evidence of VILI [1,8,16,17] termed volutrauma. The volume of aerated lung is substantially less in ARDS, relative to healthy lung size, due to alveolar edema and atelectasis [18]. This “baby lung,” i.e. the smaller aerated lung volume available for tidal ventilation, requires smaller tidal volumes than tolerated in healthy lungs to prevent overdistension [19]. Targeting 6 mL/kg predicted body weight scales tidal volume to healthy lung size, while an ideal strategy might scale tidal volume to baby lung size [20**-23]. Measuring baby lung volume directly, or developing surrogate bedside measures, enables tailoring ventilatory support to risk of overdistension. Still, differences in regional mechanics within the aerated baby lung can occur, predisposing some lung units to overdistension injury even if global lung volumes appear reasonable [24].
Barotrauma
Pressure-mediated lung injury, including both gross barotrauma and deleterious microscopic lung injury [7,25] is termed barotrauma. Transpulmonary pressure is the pertinent distending pressure of the lung, and defined as the difference in pressure inside versus outside the lung, i.e. airway pressure minus pleural pressure (Figure 1) [26]. High airway and transpulmonary pressures encountered in ARDS may reflect overdistension of the smaller aerated baby lung volume [27]. Aerated lung volume and transpulmonary pressure correlate well in both imaging and pulmonary function studies [20**,23,27]. Like the limitations with volume-based measures, regional differences in mechanics may risk lung injury even if global pressure-based measures appear reasonable [28].
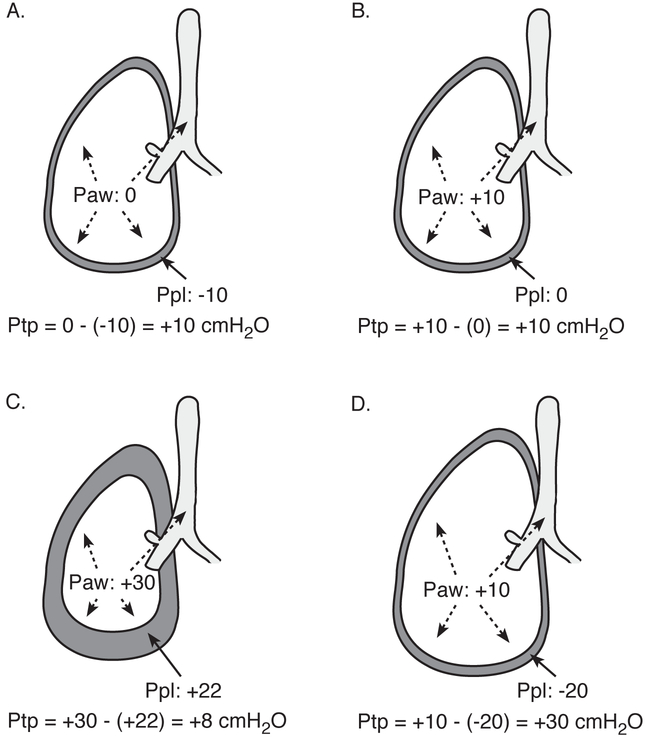
Figure 1. Transpulmonary pressure.
Transpulmonary pressure (Pairway – Ppleural) is the pertinent distending pressure of the lung. At zero flow, airway and alveolar pressure are equal; for example, during an end-inspiratory plateau pres- sure maneuver. (A) Nonintubated patient, normal spontaneous breathing at end inspiration. (B) Intubated patient without respiratory disease, passive on mechanical ventilator at end inspiration. (C) Intubated patient, chest wall stiffness results in lower transpulmonary pressure and lower lung volume at end inspiration despite higher airway pressure. (D) Intubated patient, forceful inspiratory muscle effort, such as from heightened respiratory drive, produces high transpulmonary pressure and lung volume at end inspiration even though airway pressure is reasonably low. Paw, airway pressure; Ppl, pleural pressure; Ptp, transpulmonary pressure. (Reprinted from Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. 2016;37:633-646.)
Atelectrauma
Cyclic collapse and re-expansion of atelectatic but recruitable lung units causes injurious high shear forces, a phenomenon termed atelectrauma [29,30]. The predilection for atelectrauma in the ARDS lung is at least partially explained by surfactant dysfunction with lung inflammation [29,31]. As an air bolus propagates along a collapsed lung unit, the re-expanding airway takes a “zipper-like” conformational shape that produces tremendously high shear forces, contributing to epithelial injury [32]. Higher PEEP often is used to minimize atelectrauma but may increase risk of overdistension [33].
Stress concentration
Lung injury also can be induced by shear forces from regional differences in lung mechanics. Adjacent alveoli share an interalveolar septum and are mechanically interdependent [34]. When one alveolus is collapsed or fluid-filled and an adjacent alveolus is aerated, their shared interalveolar septum stretches to shift toward the non-aerated alveolus, introducing shear force that may cause injury [35]. The atypical conformation the aerated alveolus now takes affects other nearby alveoli because of their mechanical interdependence, with shear forces greatest closest to the collapsed or fluid-filled alveolus, i.e. stress concentration (Figure 2). Greater mechanical heterogeneity and thereby more stress concentration corelates with biomarkers of VILI and increased mortality [36,37**], supporting clinical relevance.
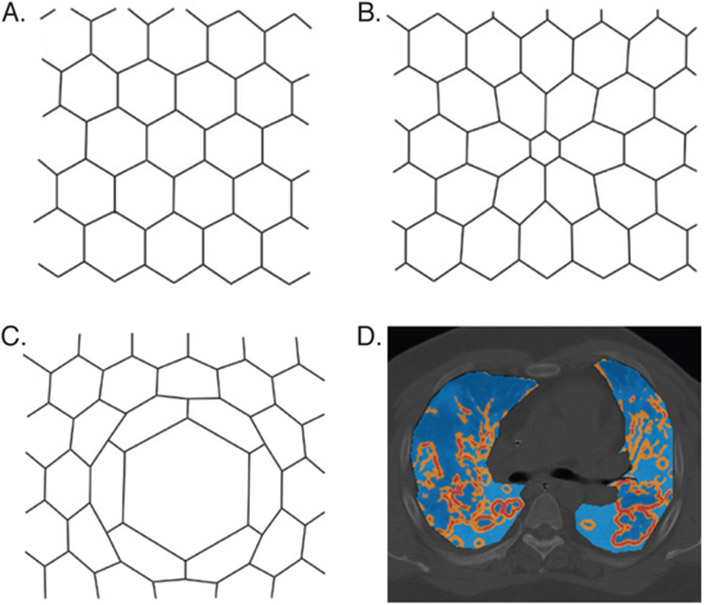
Figure 2. Mechanical alveolar interdependence and shear strain.
Mechanical alveolar interdependence and shear strain. (A–C) Classic model of alveolar interdependence; each hexagon represents an alveolus in cross section. (A) Homogeneous alveolar inflation minimizes strain. (B) Atel- ectasis of center alveolus induces shear strain of neighboring alveoli. (C) Asymmetric inflation of center alveolus in- duces shear strain of neighboring alveoli. (D) CT chest with overlying map of CT-derived regional stress concentration caused by parenchymal heterogeneity in a representative patient with ARDS (light blue indicates low stress; orange indicates moderate stress; red indicates high stress). (Reprinted from [A–C] Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970;28(5):607; and [D] Cressoni M, Cadringher P, Chiurazzi C, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2014;189(2):151.)
Biotrauma
Biotrauma might be thought of as the final common pathway of VILI. Extensive mechanical injury, via the above biophysical mechanisms, precipitates inflammation in the lung that further exacerbates injury [9,38]. What’s more, this biological response in the lungs can trigger a systemic inflammatory response that mediates multiorgan failure [39,40]. Thus, “lung-protective ventilation” is a misnomer. In fact, preventing VILI facilitates more rapid recovery of both the lungs and extrapulmonary organs [1,39].
Tools Available at the Bedside Anywhere
Targets for bedside measurement include quantifying pressures, volumes, and shapes (shear forces), on regional and global scales. Available measures can be categorized further as static, a cross-sectional snapshot at the beginning or end of a respiratory maneuver often used to identify peak exposure; or dynamic, quantifying change during a respiratory maneuver. Both static and dynamic measures are likely to be of value: higher peak stress correlates with worse lung injury [20**,41], and yet for the same peak stress, greater cyclic stress portends worse lung injury [42-44]. Thus, static and dynamic measures should be evaluated together.
To be sure, there is no holy grail technique for guiding mechanical ventilation in ARDS. Newer methods offer intriguing possibilities for filling gaps in VILI detection. However, nothing on the horizon seems can replace the value of the astute clinician carefully interpreting multiple physiologic data points together in patient-specific context. That context necessarily includes (1) measures of gas exchange impairment (PaO2:FiO2 or SpO2:FiO2, dead-space fraction or ventilatory ratio [45]), (2) estimation of the chest wall contribution to gas exchange and respiratory system mechanics, (3) assessment of the patient’s current overall acuity of illness including extrapulmonary organ injury, and (4) assessment of the patient’s clinical trajectory.
Physical exam
Bedside examination affords several opportunities to collect evidence for ascertaining VILI risk. Visual inspection of the patient’s body habitus, fat distribution, and abdominal protuberance, along with palpation for abdominal compliance, color assessment of chest wall mechanics and the extent to which pleural pressure may contribute to observed airway pressures. Visual inspection of the patient’s chest and abdomen paired with listening to the ventilator rhythm can help identify patient-ventilator dyssynchronies [46,47]. Forceful exhalation, which may predispose to atelectrauma, can be appreciated by visualizing “rounding up of the abdomen” or gentle palpation of the abdominal muscles in late expiration [48]. Assessment of airway pressures, inspired and expired tidal volumes, and ventilator waveforms—all detailed below—should be viewed as routine components of the focused physical exam for the ARDS patient.
Airway plateau pressure
Plateau airway pressure, measured absent patient effort during an end-inspiratory breath hold, is among the most widely used markers of VILI risk. Higher plateau pressure may suggest overdistension of aerated baby lung volume. The ARDS Network protocol adopted a maximum permissible plateau pressure of 30 cmH2O [1]. However, patients with lower plateau pressure fared better in that trial [49], tidal hyperinflation can occur even when this plateau pressure threshold is not exceeded [24], and reducing tidal volume to achieve lower plateau pressure in severe ARDS attenuates lung inflammation [41]. Plateau airway pressure cannot be used alone to make inference about baby lung distension for two key reasons. First, plateau pressure measures the contribution of both lung and chest wall recoil [26,50]. Thus, in a patient with morbid obesity or tense abdomen, for example, plateau pressure may be high in part (or entirely) owing to chest wall mechanics and high pleural pressure, with little transmural stress to the lung and low risk of lung injury. Second, plateau pressure values are only meaningful if measured while the patient is passive; inspiratory or expiratory muscle effort will yield misleadingly high or low values, respectively. Still, when measured correctly and interpreted in context with limitations in mind, plateau pressure is a reasonable and readily accessible marker of VILI risk.
Airway driving pressure
Driving pressure, the difference between airway plateau pressure and PEEP [8], may be useful to identify tidal overdistension, particularly when framed as a means for scaling tidal volume to respiratory system compliance (dP = VT/CRS) [51]. Prospective clinical trials scaling tidal volume in this manner are warranted, although the upper limit of “safe” driving pressure remains uncertain and likely varies by biology [3]. In pressure-targeted ventilator modes, preset driving pressure provides false assurance for VILI protection if the patient is making active inspiratory effort, because transpulmonary pressure—the physiologically pertinent distending pressure—and tidal volume are unregulated [52]. Driving pressure also has been used to titrate PEEP [53-55], though its value for such is questionable. A recent trial compared PEEP titrated to minimize driving pressure versus an empiric low-PEEP strategy and demonstrated increased mortality in the driving pressure-guided PEEP arm, although the aggressive recruitment maneuver in this arm also likely contributed to these findings [55].
Exhaled tidal volume
In any assist-control mode (including volume assist-control), the true tidal volume delivered may be higher than intended with breath stacking dyssynchrony, a patient-ventilator interaction in which consecutive machine inspiratory cycles occur with incomplete exhalation between them [56*,57]. On most ventilators, the inspiratory tidal volume reported includes only the volume change during a single machine inspiratory cycle, misrepresenting the true volume change during breath stacking. By contrast, monitoring exhaled tidal volume affords a simple means of detecting occult high volumes from breath stacking, which can be detected by observing high-than-expected exhaled tidal volume immediately after the stacked breath pair. Similarly, in “dual” modes such as volume-targeted pressure-control, tidal volume may fluctuate considerably breath-to-breath with variable patient effort and can be detected with exhaled tidal volume.
Ventilator waveform inspection
Airway pressure and flow waveforms contain a wealth of data that may facilitate insights into respiratory physiology. With respect to VILI risk, inspecting ventilator waveforms may help identify and differentiate mechanisms underlying potentially injurious dyssynchronies, such as double- or reverse-triggering breath stacking with resultant high tidal volumes [56*,58]. Yet, impromptu visual inspection alone is inadequate since dyssynchrony waxes/wanes sufficiently with patient effort over time to escape infrequent inspection [59*], and some dyssynchronies are missed on visual inspection even when present [60].
Emerging Tools
The above conventional metrics fall short at capturing exposure to potentially injurious ventilation in a few key areas. Airway pressure-based measurements do not account for the contribution of pleural pressure to lung stress. Volume-based measurements do not account for the functional baby lung size. Cyclic atelectasis and regional mechanics are not well addressed. Supplementary techniques are emerging in the literature to help close these gaps.
Esophageal manometry
Esophageal pressure-guided ventilation overcomes a key limitation of airway plateau pressure, driving pressure, and PEEP by distinguishing chest wall from lung mechanics to calculate transpulmonary pressure. The chest wall and abdomen contribute unpredictably to pleural pressure [26,50]. Placing the balloon in the retrocardiac mid-thoracic esophagus approximates the center of the chest cavity and affords a reasonable surrogate of average pleural pressure in the chest [50,61-63], although spatial pleural pressure gradients exist [64,65]. Potential uses include to guide PEEP (via end-expiratory transpulmonary pressure) and tidal volume (via transpulmonary driving pressure and transpulmonary plateau pressure) [66]. An ideal PEEP would achieve transpulmonary pressure near zero, sufficiently high to maintain open recruitable airways while low enough to minimize overdistension. A pilot randomized trial found that such esophageal pressure-guided PEEP improved adjusted survival compared to the ARDS Network PEEP-FiO2 strategy, although unadjusted mortality did not achieve statistical significance [67]. Higher transpulmonary plateau pressure is associated with increased mortality in observational studies [20**], but how to use these data to guide clinical management remains uncertain. Esophageal manometry also may be helpful for identifying patient-ventilator dyssynchronies [66], although clinical importance of dyssynchrony remains unclear.
Measures of ‘baby lung’ volume
Quantifying ARDS aerated baby lung volume could be useful for scaling tidal volume. Computed tomography (CT) is used extensively in the literature to measure functional residual capacity [27,68-70]. CT has been invaluable for advancing understanding of regional mechanics in ARDS, but safety concerns about transporting high-acuity ARDS patients to the CT scanner and inability to trend over time without repeated CTs have precluded adoption clinically. Nitrogen wash-out/wash-in and helium dilution have been used to quantify end-expiratory lung volumes at bedside [22,23,71], and appear to correlate well with CT-derived measures [71]. The nitrogen wash-out/wash-in technique is available on some new commercially available ventilators. Alternatively, a readily available approach described recently is to measure the baby lung inspiratory capacity by measuring the volume change during a 30-second, high-pressure breath hold at 40 cmH2O [20**]. Regardless of technique for quantifying aerated baby lung volumes, studies are needed to determine how best to use these measures to guide management.
Ventilator waveform computational analysis
Automated analysis of airway pressure and flow waveforms has focused largely on detecting patient-ventilatory dyssynchrony but also could help identify tidal recruitment and hyperinflation. Dyssynchrony detection algorithms are being developed that could easily be incorporated into ventilators via software upgrade [56*,72]. Due to its unintended high tidal volumes [56*,57], breath stacking dyssynchrony in particular seems likely to be injurious if frequent. Whether particular dyssynchronies contribute to injury or are simply epiphenomena remains to be established, and the dose-response between dyssynchrony and injury is unknown. Beyond dyssynchrony, stress index evaluates for tidal recruitment or hyperinflation by calculating the slope of the pressure-time waveform during inspiration. Stress index has performed well in both experimental models and human studies [33,73,74]; however, it requires specialized software at this time and is only validated in ventilator modes with constant inspiratory flow pattern.
Electrical impedance tomography
Lung electrical impedance tomography (EIT) is a fairly new bedside technique that facilitates evaluation of regional lung distension in a single transverse plane. Owing to its novelty for identifying regional differences in mechanics during respirations real-time at bedside, EIT has garnered excitement for use in identifying alveolar collapse, cyclic atelectasis, tidal hyperinflation, and stress concentration [28,75-77]. Major limitations of EIT include its evaluation only of a single cross-sectional plane (which may not accurately reflect activity in other lung regions), nonuniform imaging algorithms or indices, and limited availability [78]. The role for EIT-guided ventilation is being explored [79], but this promising technique likely will be relegated to research applications until wide agreement on relevant indices and their interpretation is established and linked more definitively to lung injury in large human studies.
Lung & diaphragm ultrasound
Lung ultrasound can be useful to aid in diagnosing ARDS [80-82]. Its role for guiding subsequent management to prevent VILI is questionable. Because it can readily detect atelectasis, lung ultrasound may be useful for identifying recruitment real-time in response to changing PEEP [83]. However, inability to detect overdistension or visualize the lung much beyond subpleural zones markedly limit utility for guiding ventilator settings. Mechanical ventilation-associated diaphragm injury can be assessed with ultrasound as well [84,85**], but again the extent to which this information can help guide management that would alter outcome is unproven.
Conclusions
The most effective strategies for managing ARDS center on attenuating biophysical lung injury: low tidal volumes, prone positioning, and arguably neuromuscular blockade [1,8,9,39,86-89]. Even with low tidal volume ventilation targeting 6 mL/kg predicted body weight, the preponderance of evidence indicates some patients still experience VILI [41,90]. Identification of such occult VILI is an essential albeit elusive aspect of managing the ARDS patient. Several widely available metrics are available to evaluate VILI risk and seem particularly useful for determining risk of barotrauma and volutrauma. Emerging tools offer promise for overcoming limitations of conventional metrics and detecting regional mechanics relevant to atelectrauma and stress concentration. Ultimately, strategies will need to be developed that incorporate both bedside mechanics and biological priming for lung injury to tailor mechanical ventilation to patient-specific VILI risk.
Key Points.
Tailoring mechanical ventilation to patient-specific risk of ventilation-induced lung injury (VILI) requires assessing both pulmonary mechanics and biological predisposition to lung injury.
Conventional VILI metrics using airway pressure, flow, and volume changes remain essential bedside tools for assessing lung overdistension when interpreted cognizant of their limitations.
Emerging tools including esophageal manometry, electrical impedance tomography, and ventilator waveform computational analysis overcome key limitations of existing VILI metrics and ultimately may facilitate development of novel personalized ventilation strategies.
Acknowledgements
The author did not receive assistance from unnamed contributors in preparation of this manuscript.
Dr. Beitler receives funding from the National Institutes of Health (NIH K23-HL133489) and American Thoracic Society Foundation for his research on mechanical ventilation in acute respiratory distress syndrome.
Funding:
Dr. Beitler receives funding from the National Institutes of Health (NIH K23-HL133489) and American Thoracic Society Foundation for his research on mechanical ventilation in acute respiratory distress syndrome.
Footnotes
Dr. Beitler has no potential conflicts of interest to declare.
REFERENCES
- 1.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000; 342(18):1301–8. [DOI] [PubMed] [Google Scholar]
- 2.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013; 369(22):2126–36. [DOI] [PubMed] [Google Scholar]
- 3.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014; 2(8):611–20.*This secondary analysis of ARDS Network trials identified molecular subtypes of ARDS that appeared to have dissimilar responses to treatment.
- 4.Beitler JR, Goligher EC, Schmidt M, et al. Personalized medicine for ARDS: the 2035 research agenda. Intensive Care Med. 2016; 42(5):756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine (Baltimore). 1944; 23:281–358. [Google Scholar]
- 6.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998; 157(1):294–323. [DOI] [PubMed] [Google Scholar]
- 7.Vlahakis NE, Hubmayr RD. Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med. 2005; 171(12):1328–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998; 338(6):347–54. [DOI] [PubMed] [Google Scholar]
- 9.Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999; 282(1):54–61. [DOI] [PubMed] [Google Scholar]
- 10.Jabaudon M, Blondonnet R, Roszyk L, et al. Soluble receptor for advanced glycation end-products predicts impaired alveolar fluid clearance in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2015; 192(2):191–9. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal A, Zhuo H, Brady S, et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: results from two clinical trials. Am J Physiol Lung Cell Mol Physiol. 2012; 303(8):L634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware LB, Koyama T, Billheimer DD, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010; 137(2):288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ware LB, Koyama T, Zhao Z, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013; 17(5):R253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Laorden MI, Lorente JA, Flores C, et al. Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Ann Transl Med. 2017; 5(14):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med. 2016; 37(4):633–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974; 110(5):556–65. [DOI] [PubMed] [Google Scholar]
- 17.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988; 137(5):1159–64. [DOI] [PubMed] [Google Scholar]
- 18.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006; 354(17):1775–86. [DOI] [PubMed] [Google Scholar]
- 19.Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005; 31(6):776–84. [DOI] [PubMed] [Google Scholar]
- 20.Beitler JR, Majumdar R, Hubmayr RD, et al. Volume delivered during recruitment maneuver predicts lung stress in acute respiratory distress syndrome. Crit Care Med. 2016; 44(1):91–9.**This study validated a simple, novel bedside approach to measuring the inspiratory capacity of the ARDS ‘baby lung.’
- 21.MacIntyre N Lung protective ventilator strategies: beyond scaling tidal volumes to ideal lung size. Crit Care Med. 2016; 44(1):244–5. [DOI] [PubMed] [Google Scholar]
- 22.Mattingley JS, Holets SR, Oeckler RA, et al. Sizing the lung of mechanically ventilated patients. Crit Care. 2011; 15(1):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008; 178(4):346–55. [DOI] [PubMed] [Google Scholar]
- 24.Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007; 175(2):160–6. [DOI] [PubMed] [Google Scholar]
- 25.Kolobow T, Moretti MP, Fumagalli R, et al. Severe impairment in lung function induced by high peak airway pressure during mechanical ventilation. An experimental study. Am Rev Respir Dis. 1987; 135(2):312–5. [DOI] [PubMed] [Google Scholar]
- 26.Talmor D, Sarge T, O’Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med. 2006; 34(5):1389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L, Pesenti A, Avalli L, et al. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis. 1987; 136(3):730–6. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013; 188(12):1420–7. [DOI] [PubMed] [Google Scholar]
- 29.Taskar V, John J, Evander E, et al. Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am J Respir Crit Care Med. 1997; 155(1):313–20. [DOI] [PubMed] [Google Scholar]
- 30.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994; 149(5):1327–34. [DOI] [PubMed] [Google Scholar]
- 31.Albert RK. The role of ventilation-induced surfactant dysfunction and atelectasis in causing acute respiratory distress syndrome. Am J Respir Crit Care Med. 2012; 185(7):702–8. [DOI] [PubMed] [Google Scholar]
- 32.Ghadiali SN, Gaver DP. Biomechanics of liquid-epithelium interactions in pulmonary airways. Respir Physiol Neurobiol. 2008; 163(1-3):232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grasso S, Stripoli T, De Michele M, et al. ARDSnet ventilatory protocol and alveolar hyperinflation: role of positive end-expiratory pressure. Am J Respir Crit Care Med. 2007; 176(8):761–7. [DOI] [PubMed] [Google Scholar]
- 34.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970; 28(5):596–608. [DOI] [PubMed] [Google Scholar]
- 35.Perlman CE, Lederer DJ, Bhattacharya J. Micromechanics of alveolar edema. Am J Respir Cell Mol Biol. 2011; 44(1):34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cressoni M, Cadringher P, Chiurazzi C, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014; 189(2):149–58. [DOI] [PubMed] [Google Scholar]
- 37.Mrozek S, Jabaudon M, Jaber S, et al. Elevated plasma levels of sRAGE are associated with nonfocal CT-based lung imaging in patients with ARDS: a prospective multicenter study. Chest. 2016; 150(5):998–1007.**This study linked sRAGE, a plasma marker of alveolar epithelial injury, to imaging evidence suggesting mechanical heterogeneity.
- 38.Tremblay L, Valenza F, Ribeiro SP, et al. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997; 99(5):944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranieri VM, Giunta F, Suter PM, Slutsky AS. Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA. 2000; 284(1):43–4. [DOI] [PubMed] [Google Scholar]
- 40.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005; 33(1):1–6. [DOI] [PubMed] [Google Scholar]
- 41.Terragni PP, Del Sorbo L, Mascia L, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009; 111(4):826–35. [DOI] [PubMed] [Google Scholar]
- 42.Protti A, Andreis DT, Monti M, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med. 2013; 41(4):1046–55. [DOI] [PubMed] [Google Scholar]
- 43.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000; 162(2 Pt 1):357–62. [DOI] [PubMed] [Google Scholar]
- 44.Ye H, Gao Z, Ren Y, et al. Cyclic deformation-induced injury and differentiation of rat alveolar epithelial type II cells. Respir Physiol Neurobiol. 2012; 180(2-3):237–46. [DOI] [PubMed] [Google Scholar]
- 45.Sinha P, Calfee CS, Beitler JR, et al. Physiological analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mellott KG, Jones DJ, Hanneman SK. Continuous biobehavioral measures to identify patient ventilator asynchrony: methodological and practice challenges. Am J Respir Crit Care Med. 2016; 193:A5274. [Google Scholar]
- 47.Mellott KG, Grap MJ, Munro CL, et al. Patient ventilator asynchrony: associated patient behaviors. Am J Respir Crit Care Med. 2015; 191:A3897. [Google Scholar]
- 48.Loring SH, Townsend SR, Gallagher DC, et al. Expiratory abdominal rounding in acute dyspnea suggests congestive heart failure. Lung. 2006; 184(6):324–9. [DOI] [PubMed] [Google Scholar]
- 49.Hager DN, Krishnan JA, Hayden DL, et al. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005; 172(10):1241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loring SH, O’Donnell CR, Behazin N, et al. Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol. 2010; 108(3):515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amato MBP, Meade MO, Slutsky AS, et al. Driving pressure and survival in acute respiratory distress syndrome. N Engl J Med. 2015; 372(8):747–55. [DOI] [PubMed] [Google Scholar]
- 52.Richard JCM, Lyazidi A, Akoumianaki E, et al. Potentially harmful effects of inspiratory synchronization during pressure preset ventilation. Intensive Care Med. 2013; 39(11):2003–10. [DOI] [PubMed] [Google Scholar]
- 53.Suter PM, Fairley B, Isenberg MD. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med. 1975; 292(6):284–9. [DOI] [PubMed] [Google Scholar]
- 54.Pintado MC, de Pablo R, Trascasa M, et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013; 58(9):1416–23. [DOI] [PubMed] [Google Scholar]
- 55.Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017; 318(14):1335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beitler JR, Sands SA, Loring SH, et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the BREATHE criteria. Intensive Care Med. 2016; 42(9):1427–36.*This study validated objective criteria to identify occult high tidal volumes from breath stacking dyssynchrony.
- 57.Pohlman MC, McCallister KE, Schweickert WD, et al. Excessive tidal volume from breath stacking during lung-protective ventilation for acute lung injury. Crit Care Med. 2008; 36(11):3019–23. [DOI] [PubMed] [Google Scholar]
- 58.Akoumianaki E, Lyazidi A, Rey N, et al. Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest. 2013; 143(4):927–38. [DOI] [PubMed] [Google Scholar]
- 59.Vaporidi K, Babalis D, Chytas A, et al. Clusters of ineffective efforts during mechanical ventilation: impact on outcome. Intensive Care Med. 2016; 43(2):184–91.*A novel method for identifying clusters of dyssynchrony was established in this study of failed triggering dyssynchrony.
- 60.Colombo D, Cammarota G, Alemani M, et al. Efficacy of ventilator waveforms observation in detecting patient-ventilator asynchrony. Crit Care Med. 2011; 39(11):2452–7. [DOI] [PubMed] [Google Scholar]
- 61.Terragni P, Mascia L, Fanelli V, et al. Accuracy of esophageal pressure to assess transpulmonary pressure during mechanical ventilation. Intensive Care Med. 2016; 43(1):142–3. [DOI] [PubMed] [Google Scholar]
- 62.Washko GR, O’Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol. 2006; 100(3):753–8. [DOI] [PubMed] [Google Scholar]
- 63.Pelosi P, Goldner M, McKibben A, et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med. 2001; 164(1):122–30. [DOI] [PubMed] [Google Scholar]
- 64.Lai-Fook SJ, Rodarte JR. Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J Appl Physiol. 1991; 70(3):967–78. [DOI] [PubMed] [Google Scholar]
- 65.Mutoh T, Guest RJ, Lamm WJ, Albert RK. Prone position alters the effect of volume overload on regional pleural pressures and improves hypoxemia in pigs in vivo. Am Rev Respir Dis. 1992; 146(2):300–6. [DOI] [PubMed] [Google Scholar]
- 66.Akoumianaki E, Maggiore SM, Valenza F, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014; 189(5):520–31. [DOI] [PubMed] [Google Scholar]
- 67.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008; 359(20):2095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gattinoni L, D’Andrea L, Pelosi P, et al. Regional effects and mechanism of positive end-expiratory pressure in early adult respiratory distress syndrome. JAMA. 1993; 269(16):2122–7. [PubMed] [Google Scholar]
- 69.Pelosi P, D’Andrea L, Vitale G, et al. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994; 149(1):8–13. [DOI] [PubMed] [Google Scholar]
- 70.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001; 164(9):1701–11. [DOI] [PubMed] [Google Scholar]
- 71.Chiumello D, Cressoni M, Chierichetti M, et al. Nitrogen washout/washin, helium dilution and computed tomography in the assessment of end expiratory lung volume. Crit Care. 2008; 12(6):R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blanch L, Villagrá A, Sales B, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 2015; 41(4):633–41. [DOI] [PubMed] [Google Scholar]
- 73.Grasso S, Terragni P, Mascia L, et al. Airway pressure-time curve profile (stress index) detects tidal recruitment/hyperinflation in experimental acute lung injury. Crit Care Med. 2004; 32(4):1018–27. [DOI] [PubMed] [Google Scholar]
- 74.Terragni PP, Filippini C, Slutsky AS, et al. Accuracy of plateau pressure and stress index to identify injurious ventilation in patients with acute respiratory distress syndrome. Anesthesiology. 2013; 119(4):880–9. [DOI] [PubMed] [Google Scholar]
- 75.Costa E, Borges JB, Melo A, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009; 35(6):1132–7. [DOI] [PubMed] [Google Scholar]
- 76.Victorino JA, Borges JB, Okamoto VN, et al. Imbalances in regional lung ventilation: a validation study on electrical impedance tomography. Am J Respir Crit Care Med. 2004; 169(7):791–800. [DOI] [PubMed] [Google Scholar]
- 77.Zhao Z, Möller K, Steinmann D, et al. Evaluation of an electrical impedance tomography-based global inhomogeneity index for pulmonary ventilation distribution. Intensive Care Med. 2009; 35(11):1900–6. [DOI] [PubMed] [Google Scholar]
- 78.Leonhardt S, Lachmann B. Electrical impedance tomography: the holy grail of ventilation and perfusion monitoring? Intensive Care Med. 2012; 38(12):1917–29. [DOI] [PubMed] [Google Scholar]
- 79.Wolf GK, Gómez-Laberge C, Rettig JS, et al. Mechanical ventilation guided by electrical impedance tomography in experimental acute lung injury. Crit Care Med. 2013; 41(5):1296–304. [DOI] [PubMed] [Google Scholar]
- 80.Riviello ED, Kiviri W, Twagirumugabe T, et al. Hospital incidence and outcomes of the acute respiratory distress syndrome using the Kigali modification of the Berlin definition. Am J Respir Crit Care Med. 2016; 193(1):52–9. [DOI] [PubMed] [Google Scholar]
- 81.Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008; 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lichtenstein D, Goldstein I, Mourgeon E, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004; 100(1):9–15. [DOI] [PubMed] [Google Scholar]
- 83.Bouhemad B, Brisson H, Le-Guen M, et al. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011; 183(3):341–7. [DOI] [PubMed] [Google Scholar]
- 84.Goligher EC, Fan E, Herridge MS, et al. Evolution of diaphragm thickness during mechanical ventilation: impact of inspiratory effort. Am J Respir Crit Care Med. 2015; 192(9):1080–8. [DOI] [PubMed] [Google Scholar]
- 85.Goligher EC, Dres M, Fan E, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018; 197(2):204–13.**This prospective cohort study established a link between diaphragm thickness, as measured at bedside by ultrasound, and subsequent prolonged mechanical ventilation with difficulty weaning.
- 86.Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013; 368(23):2159–68. [DOI] [PubMed] [Google Scholar]
- 87.Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med. 2014; 40(3):332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010; 363(12):1107–16. [DOI] [PubMed] [Google Scholar]
- 89.Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med. 2010; 363(12):1176–80. [DOI] [PubMed] [Google Scholar]
- 90.Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus “conventional” protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013; 39(5):847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]