Abstract
BACKGROUND:
Small rodent models are routinely used to evaluate the safety and efficacy of blood transfusions. Limited comprehensive literature exists about effect of different storage solutions in rat red blood cells (RBCs) characteristics. RBCs undergo time dependent biochemical and biophysical changes during storage known as hypothermic storage lesions (HSLs).
OBJECTIVE:
This study evaluates the effects of RBC additive solutions (AS) during storage of rat RBCs.
METHODS:
Blood was leukoreduced and stored as per manufacturer instructions at 4°C up to 42-days. Three solutions, CPDA-1; AS-1; and AS-7 (SOLX), were evaluated. Biochemical parameters measured included extracellular K+, pH, hemolysis, 2,3-diphosphoglycerate (2,3-DPG), oxygen affinity, ATP, and lactate. Mechanical properties measured included RBC deformability, elongation index (EI), RBC membrane shear elastic modulus (SEM), mean corpuscular volume (MCV), viscosity, and aggregability.
RESULTS:
There were no differences in biochemical or mechanical parameters at baseline or after one week of storage. However, after two weeks, AS-7 preserved biochemical and mechanical properties as compared to CPDA-1 and AS-1. Changes were observed to be significant after 14-days of storage. AS-7 prevented extracellular K+ increase, reduced acidosis, showed lower hemolysis, preserved ATP and 2,3-DPG levels (consequently oxygen affinity), and reduced lactate. AS-7, when compared to CPDA-1 and AS-1, prevented the reduction in RBC deformability and was found to preserve the EI at multiple shear stresses, the membrane SEM, the aggregability and viscosity.
DISCUSSION:
Rat RBCs stored with AS-7 presented reduced changes in biochemical and mechanical parameters, when compared with rat RBCs stored in CPDA-1 and AS-1, after as early as two weeks of storage.
Keywords: Banked blood, blood storage, deformability, storage lesions, blood transfusion
1. Introduction
Blood banks are responsible for the collection, screening, and storage of blood, which is used by nearly 5 million Americans, who receive blood transfusions annually [1]. The ability to store blood for transfusions is a key element for medical care and public health. Human red blood cells (RBCs) are typically stored for as long as 42 days at 2° to 6°C with the appropriate additives [2]. Despite the widespread use of transfusions, regulation of blood products only covers the procedures for collection, processing, and storage [3]. Concerns have recently emerged about the safety and efficacy of transfusing stored RBCs. Controversial studies have found correlations between blood transfusions and negative clinical outcomes post transfusion in certain populations [4–8]. Others have shown that stored RBCs are associated with worse outcomes when compared to fresh RBCs [9].
RBCs undergo time dependent biochemical and biophysical changes during storage, known as hypothermic storage lesions (HSLs) [10], which reduce post-transfusion recovery of RBCs and increase hemolysis [11]. These changes include depletion of adenosine triphosphate (ATP), decreased 2,3-diphosphoglycerate acid (2,3 DPG) levels, decreased pH, increased intracellular potassium (K+) release, reduced nitric oxide (NO) levels in the RBC, oxidative damages to lipid and proteins, and changes in cell volume and cell deformability [10]. Since RBCs become less deformable during storage, they are more susceptible to hemolysis and decreased RBC nitric oxide synthase (RBC-NOS) activity [12]. Reduced RBCs deformability and impaired RBC-NOS activity impact RBCs ability to transit through the microcirculation. As such, decreased deformability reduces blood flow, increases vascular resistance, and reduces O2 delivery deliver oxygen (O2) to the tissues [13]. Therefore, while transfusion of RBCs with decreased deformability increases O2 carrying capacity, increase in tissue oxygenation is not necessarily observed [14]. HSLs depend on the storage additive solution (AS) used during RBCs preparation; thus, the effects on tissue oxygenation should be measurable different between RBCs stored with different AS. Clinical researchers have employed FDA approved, noninvasive methods to assess the effectiveness of a RBC transfusion, using sublingual microscopy and near-infrared spectroscopy to measure changes in microcirculatory density and tissue oxygenation, respectively [15]. The robustness of measurements provided by these methods is problematic in clinical research settings. Therefore, the study of microvascular function and tissue oxygen post transfusion remains limited to experimental models for rigorous validation. Animal models are often used as a translational tool to understand the mechanisms behind HSL effects on microvascular function and tissue oxygen [16]. Since not all AS for RBC storage are the same, understanding the differences in HSLs between RBCs from animal model species are the first steps in animal translational studies. Eventually, our goal is to establish a rat RBC production method that closely mimics human RBC techniques that are currently in use. These methods can be applied in rat models of transfusion studying the HSL and evaluate novel RBC preservation strategies.
Early RBC storage solutions included saline-adenine-glucose-mannitol (SAGM), which enabled up to 6 weeks refrigerated storage [17]. Citrate phosphate dextrose adenine (CPDA-1) solution is used as anticoagulant and to preserved RBCs up to 35 days, producing drastic HSL after storage [18]. Other additives, however, have shown to reduce HSLs when compared to CPDA-1, including: Adsol® (AS-1, l; Baxter Healthcare Corp., Deerfield, Il), Nutricel® (AS-3, Medsep Corp., Covina, CA), and Optisol (AS-5, Terumo Corp., Somerset, NJ) and SOLX® (AS-7, Haemonetics Corp., Braintree, MA) [19] SOLX has shown to maintain human RBC 2–3 DPG and ATP levels compared to other additives [20]. SOLX is a mixture of dextrose monohydrate, adenine, dibasic sodium phosphate, mannitol, and sodium bicarbonate [20]. The differences in HSLs between with various AS is documented in the literature for human RBCs, but this information is not available for animal models.
This study presents the time dependent biochemical and biomechanical changes that occur in rat RBCs stored with SOLX, AS-1 and CPDA-1. Therefore, the aim of this study was to evaluate differences between rat RBCs stored with SOLX, AS-1 and CPDA-1, including in vitro quality assays to examine membrane-related, biochemical, and hemorheological parameters. This study applied detailmeasuring techniques to determine the mechanical properties of RBCs, in terms of RBC volume, RBC filterability, RBC deformability, RBC aggregability, and RBC membrane elastic properties. In addition to blood rheology, all these parameters determine the flow properties of RBCs. The information reported in this study allows researchers to develop effective rat experimental models to study transfusion complications and to separate groups into “fresh blood” and “old blood” based on robust observations. These types of studies are only possible in animals, since when humans receive RBCs via blood transfusions, it is not possible to standardize the age of the RBC units received.
2. Methods (supplemental material includes expanded version of the methods)
2.1. Blood collection and storage
Experiments were approved by the Institutional Animal Care and Use Committee (S00149) and conducted accordingly to the Guide for the Care and Use of Laboratory Animals (US National Research Council, 2010). Blood was obtained from Sprague-Dawley rats (Harlan laboratories 300–400 g). Rats were anesthetized with ketamine/xylazine (100/10 mg/kg), and the left femoral artery was exposed. A sterile femoral artery catheter (PE-50) filled with heparin in sterile saline (500 units/mL) was implanted. From the whole blood collection kits of each storage solution, the appropriate volume of anticoagulant for exsanguination of 60% of the animal’s blood volume (estimated as 7% of the body weight) was aseptically transferred into sterile tubes (10 mL, 366441, BD Vacutainer, Franklin Lakes, NJ). After collection, tubes were gently mixed and then centrifuged at 2600 RPM (~1000g) for 7 minutes. The supernatant was removed and centrifuged again at 1875 g for 7 min to obtain platelet-free plasma. SOLX and AS-1 were then aseptically drawn from their whole blood collection kits, added to the RBC pellet, and gently mixed. Leukoreduction was completed using the blood pooled from 15 rats, using a hard-shell WB leukoreduction filter (Haemonetics, part # RCEZ1T), and allowed to flow through the filter into sterile bags (150 mL, S5904–52, B Braun, Irvine, CA). Units were stored in a monitored 4°C refrigerator for up to 8 weeks.
2.2. RBC filterability
Deformability was measured using the cells filtration method [13]. Briefly, cell suspension was passed through filter with 5 μm pores size (Nuclepore, Pleasanton, CA) at various flow rates, and the pressure drop across the filter was measured [21].
2.3. Micropipette aspiration
Cells were subjected to micropipettes aspiration. Aspiration length was measured off line from recorded images [22]. Cell membrane elastic module and cell mean corpuscular volume (MCV) were calculated based on the relationship between pressure and aspiration length, as previously reported [23].
2.4. Elongation index
Cell elongation index (EI) was measured as previously described [22]. Briefly, cell suspension was passed through cylindrical microcapillaries (ID: 20 μm; Polymicro Technologies, Phoenix, AR) at different flow rates, and the extent of cell deformation was determined from the diffraction image created by the laser beam [24, 25].
2.5. Blood rheology and aggregability
Viscosity of cell suspensions was measured at shear rates between 3 and 450 sec−1 (37°C) using a cone-plate rheometer AR-G2 (TA Instruments, New Castle, DE, USA) [16,26]. RBC aggregation was determined using a photometric rheoscope (Myrenne Aggregometer®, Germany) [16, 22, 27].
2.6. Blood-oxygen equilibrium curve
Blood oxygen equilibrium curves were obtained using the Hemox Analyzer (TCS Scientific Corp., New Hope, PA) [28].
2.7. RBC glucose consumption and G6PD activity
RBC glucose consumption was determined using a YSI 2300 STAT Plus (YSI, Yellow Springs, Ohio), and glucose consumption was calculated using glucose concentration and time [29]. G6PD activity was evaluated by adding 0.5 mM methylene blue (MB) to increase pentaphosphate pathway flux.
2.8. Data analysis
Results are presented as mean ± standard deviation. Statistically significant were analyzed using two-way ANOVA, followed by the appropriate post hoc analyses. Statistics were calculated using GraphPad Prism 6 (San Diego, CA). Changes were considered statistically significant if P < 0.05.
3. Results
3.1. Hematocrit and hemoglobin concentration
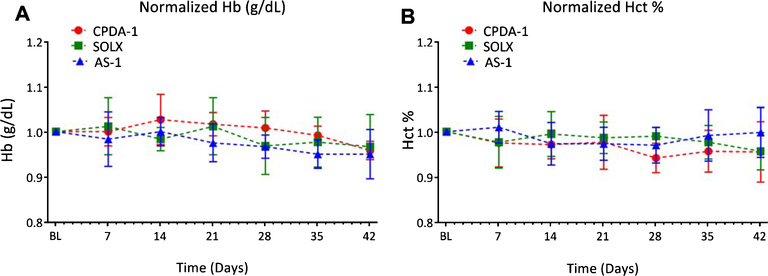
Although storage of RBCs with SOLX adds a larger volume of AS to the RBCs relative to CPDA-1 and AS-1, there were no statistical differences in Hct and Hb concentration at baseline (t = 0) after volume normalization. Additionally, no statistically significant differences in both Hct and Hb concentration between storage solutions during the storage time were observed. A summary of the Hct and Hb results is shown in Fig. 1.
Fig. 1.
Percentage Hematocrit and Hemoglobin concentration for RBCs stored in CPDA-1, AS-1, and SOLX (N = 6). (A). Concentration of Hemoglobin in blood tracked over 35 days for blood stored with CPDA-1, AS-1, and SOLX measured with the Hemocue. (B). Percentage hematocrit in blood tracked over 42 days for blood stored with CPDA-1, AS-1, and SOLX.
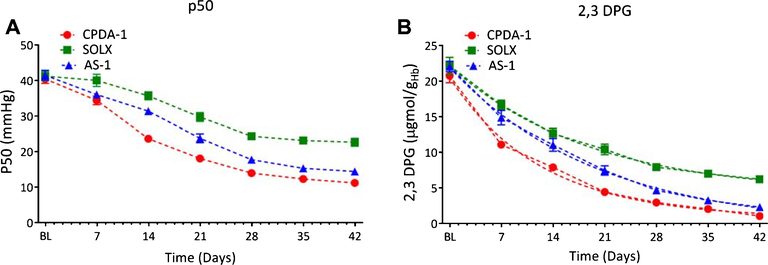
3.2. Oxygen affinity (P50) and 2,3 DPG
P50, the partial pressure of oxygen required for 50% RBC saturation, and 2,3 DPG concentration over long term RBCs storage is presented in Fig. 2. There were no statistically significance differences in P50 or 2,3 DPG among storage solution at baseline. However, after 7 days of storage, 2,3 DPG levels were found to be statistically significantly higher for RBC stored with SOLX when compared to cells stored in AS-1. After 14 days of storage, both P50 and 2,3 DPG levels were found to be statistically significantly greater for RBCs stored with SOLX when compared to cells stored in AS-1 or CPDA-1. This trend continued for the remainder of the storage time for both parameters.
Fig. 2.
p50 (mmHg) and 2,3 DPG Concentration μgmol gHb−1) for RBCs stored in CPDA-1, AS-1, and SOLX (N = 6). (A). p50, partial pressure of oxygen for 50% oxygen saturation of hemoglobin, tracked over 42 days for blood stored CPDA-1, AS-1, and SOLX. (B). Concentration of 2,3 DPG per gram of hemoglobin in blood tracked over 42 days for blood stored with CPDA-1, AS-1, and SOLX. Data was fit with an exponential decay, and time constants were found to be: CPDA-1 (τ = 12.4), SOLX (τ = 18.9), and AS-1 (τ = 20.0).
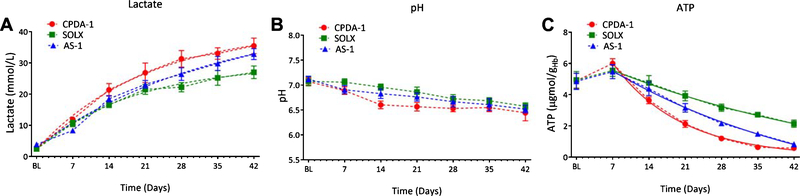
3.3. Metabolic markers: Lactate, pH, and ATP
A summary of metabolic markers (Lactate, pH, and ATP) of RBCs stored with CPDA-1, AS-1, and SOLX is presented in Fig. 3. RBCs stored with AS-1 and CPDA-1 increased their lactate concentration more rapidly than RBCs stored with SOLX. Lactate concentration of RBCs stored with CPDA-1 was statistically higher when compared to RBCs stored with SOLX as early as 21 days. RBCs stored with SOLX for more than 28 days showed statistically lower lactate concentration when compared to RBCs stored with CPDA-1 and AS-1, respectively. RBCs stored with SOLX showed statistically higher pH when compared to RBCs stored with CPDA-1 and AS-1 after 21 days of storage, respectively. RBCs stored with AS-1 and CPDA-1 showed faster rates of ATP det.
Fig. 3.
Metabolic Properties of RBCs: Lactate Concentration, ATP, pH, K+, and free Hb for RBCs stored in CPDA-1, AS-1, and SOLX (N = 6). (A). Concentration of Lactate, a by-product of RBC metabolism, tracked over 42 days for blood stored in CPDA-1, AS-1, and SOLX. Data was fit with an exponential decay, and the time constants were found to be: CPDA-1 (τ = 21.2), SOLX (τ =18.9), and AS-1 (τ = 34.0). (B). pH of blood tracked over 42 days for RBCs stored in CPDA-1, AS-1, and SOLX. (C). Concentration of ATP per gram hemoglobin for RBCs stored in CPDA-1, AS-1, and SOLX tracked over 42 days. Data after 7 days storage time was fit was an exponential decay, and the time constants were found to be: CPDA-1 (τ = 13.2), SOLX (τ = 51.4), and AS-1 (τ = 43.0).
3.4. Mechanical properties
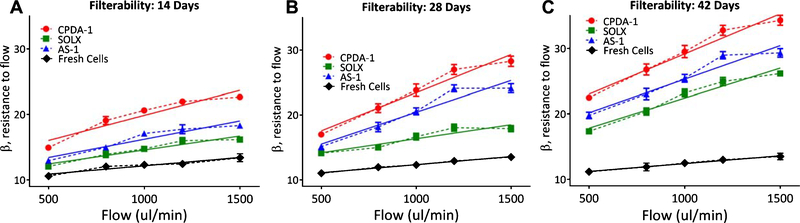
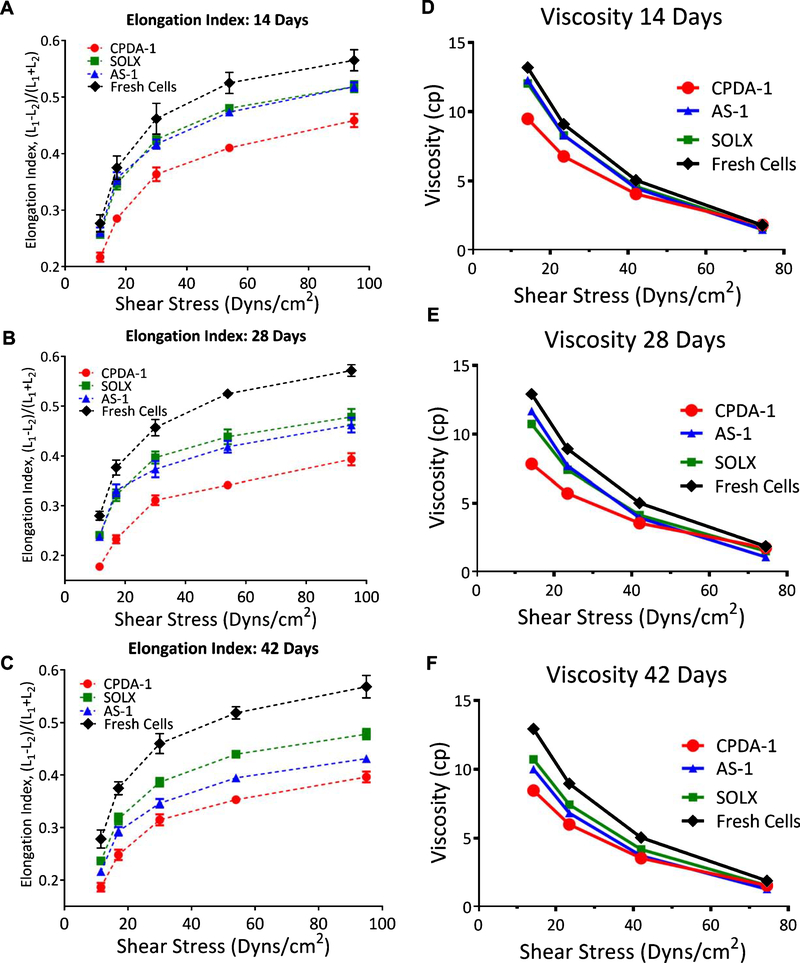
Resistance to filtration and elongation index of RBCs stored with CPDA-1, AS-1, and SOLX are presented in Fig. 4. As a function of flow, resistance increased more rapidly for RBCs stored with CPDA-1 than RBCs stored with either SOLX or AS-1. RBCs stored with SOLX, CPDA-1, and AS-1 had statistically higher resistance to filtration when compared to fresh cells at all flow rates and time points. Additionally, RBCs stored with SOLX had no difference in resistance to flow when compared to fresh cells at all time points. At all-time points, resistance as a function of flow increased more rapidly for AS-1, CPDA-1, and SOLX as compared with fresh cells. Ektacytometry results show statistically significant reduced cell deformability for RBCs stored with all additive solutions compared to fresh cells (Fig. 5). Viscosity was found not to be statistically significant between RBCs stored in AS-1, CPDA-1, and SOLX at high shear stresses; however, at low shear stresses, stored RBCs were statistically different from fresh cells.
Fig. 4.
Filterability of RBCs stored in CPDA-1, AS-1, and SOLX (N = 6). Filterability: Cell deformability measured using the resistance of red cells to pass by pore, normalized by the cell concentration and cell/pore volume, i.e. the β factor. Data was fit with a linear regression analysis and slopes, which indicate deterioration rate, were determined. Data for fresh cells was also recorded and the same analysis was performed. (A) 14 days: Slopes from linear regression were determined to be: CPDA-1 (m = 0.008), AS-1 (m = 0.006), SOLX (m = 0.004), and Fresh Cells (m = 0.003). (B). 28 Days: Slopes from linear regression were determined to be: CPDA-1 (m = 0.012), AS-1 (m = 0.010), SOLX (m = 0.004), and Fresh Cells (m = 0.002). (C). 42 days: Slopes from linear regression were determined to be: CPDA-1 (m = 0.012), AS-1 (m = 0.010), SOLX (m = 0.009), and Fresh Cells (m = 0.002).
Fig. 5.
Elongation Index (N = 6): Changes in red cell elongation index (EI) as a function of shear stress between fresh and stored cells, measured based on the length and width of the diffraction pattern, as (L1-L2)/(L1+L2), where L1 and L2 are the length and width of the diffraction pattern after: (A) 14 days, (B) 28 days, and (C) 42 Days for Fresh RBCs and RBCs stored in CPDA-1, AS-1, and SOLX. Viscosity was determined numerically by applying a second order numerical difference of the EI: (D) 14 days, (E) 28 days, and (F) 42 Days for Fresh RBCs and RBCs stored in CPDA-1, AS-1, and SOLX.
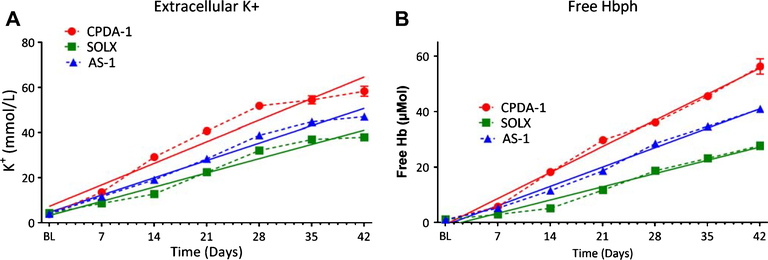
3.5. Potassium and hemolysis
Storage of RBCs resulted in a rise in intracellular Na+ and a fall in intracellular K+ with concomitant opposite changes in Na+(data not shown) and K+ levels in the suspending media (Fig. 6). After 7 days of storage, cells stored with SOLX presented a statistically lower extracellular K+ compared to cells stored with CPDA-1 or AS-1, respectively. This trend continued over the storage period. Similarly, after 14 days of storage, hemolysis for cell store with SOLX was statistically lower compared to cells store with CPDA-1 or AS-1, respectively. Hemolysis remained lower for cell stored with SOLX over the storage period.
Fig. 6.
Biochemical Changes of RBCs as a consequence of Increased Rigidity: K+ and free Hb for RBCs store in CPDA-1, AS-1, and SOLX (N = 6). Data was fit via linear regression and slopes, which are indicative of rate of deterioration, are presented. (A). Concentration of K+ in blood released from RBCs tracked over 35 days for RBCs stored in CPDA-1, AS-1, and SOLX. Slopes from linear regression were determined to be: CPDA-1 (m = 1.4), AS-1 (m = 1.1), and SOLX (m = 0.90) (B). Concentration of free hemoglobin not bound to RBCs tracked over 35 days for RBCs stored in AS-1, CPDA-1, and SOLX. Slopes from linear regression were determined to be: CPDA-1 (m = 1.34), AS-1 (m= 1.00), and SOLX (m = 0.68)
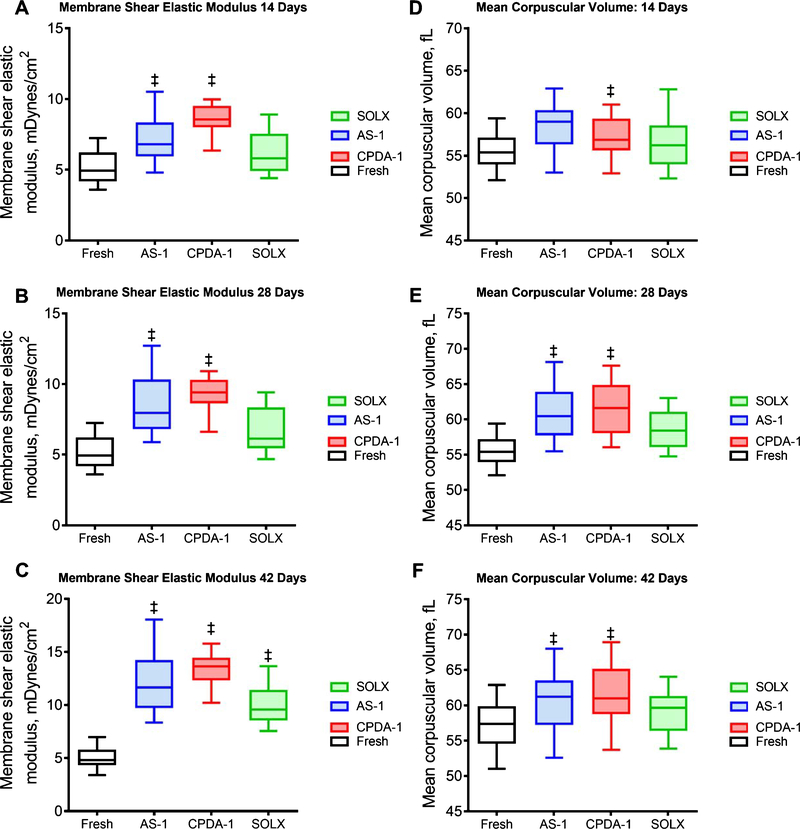
3.6. Membrane mechanics and cell volume
Membrane shear elastic modulus (SEM) and mean corpuscular volume using the micropipette aspiration of stored RBCs after 14, 28 and 42 days presented in Fig. 7. After 14 days of storage, cells stored with CPDA-1 or AS-1 showed statistically higher membrane SEM than fresh RBCs. Similarly, after 28 days of storage, stored cell with CPDA-1 or AS-1 had statistically higher membrane SEM than fresh RBCs. The membrane SEM of cells stored with CPDA-1 was statistically was higher when compared to the membrane SEM of cells stored with SOLX after 14 or 28 days of storage. After 42 days, all stored cells had statistically higher membrane shear elastic moduli when compared to fresh RBCs, and the membrane of cells stored with CPDA-1 was statistically higher than the membrane of cells stored with SOLX. The cell volume of cell stored with CPDA-1 and AS-1 increased as a function of storage time. After 14 days of storage, cells stored with AS-1 had a statistically larger volume compared to the volume of fresh cells. Similarly, after 28 and 42 days of storage, stored cell with CPDA-1 or AS-1 had statistically larger volume than the volume of fresh cells. Surprisingly, the volume of RBCs stored with SOLX was no different from the volume of fresh cells at any time point.
Fig. 7.
Mechanical Properties of RBCs: Membrane Shear Elastic Modulus and Mean Corpuscular Volume for RBCs stored in CPDA-1, AS-1, and SOLX (N = 6). Membrane Shear Elastic Modulus: Red cell membrane SEM based on the relationship pressure and aspiration length for RBCs stored in AS-1, CPDA-1, and SOLX after (A). 14 days, (B). 28 Days, and (C). 42 Days. Mean Corpuscular Volume: Corpuscular volume was measured using the cell aspiration technique for RBCs stored in AS-1, CPDA-1, and SOLX after (D). 14 days, (E). 28 Days, and (F). 42 Days.
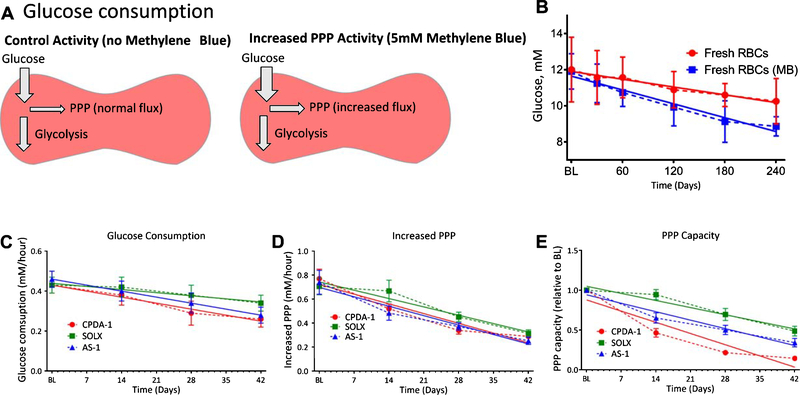
3.7. Glucose utilization and Pentose phosphate pathway (PPP) capacity
Glucose consumption and the PPP capacity are presented in Fig. 8. For all stored cells, stored in SOLX, CPDA-1, and AS-1, statistically significant deterioration of glucose consumption was observed from 28 days of storage and onward and was slower for RBCs stored in SOLX Additionally, no statistical variance was observed in glucose consumption between RBCs stored in SOLX and those in AS-1. Glucose consumption of RBCs stored in SOLX was however found to be statistically different from glucose consumption of RBCs in SOLX after 28 days of storage (Fig. 8A). Storage time dependent deterioration was observed for the PPP Capacity of stored RBCs. Furthermore, statistically significant differences in PPP capacities were observed for RBCs stored in SOLX, AS-1, and CPDA-1 after 14 days of storage (Fig. 8B-C).
Fig. 8.
Glucose Consumption and PPP Capacity for RBCs stored in AS-1, SOLX, and CPDA_1 (N = 6A). (A) Glucose consumption as a function of storage time for RBCs stored in CPDA-1, AS-1, and SOLX. Linear regression analysis was performed to better understand deterioration rates, and the slopes of the regression were found to be: CPDA-1 (m = −0.004), AS-1 (m = −0.004), and SOLX (m = −0.002). (C). Rate of PPP Increase as a function of storage time for RBCs stored in CPDA-1, AS-1, and SOLX. Linear regression analysis was performed, and the slopes from the analysis were determined to be: CPDA-1 (m = −0.016), AS-1 (m = −0.01), and SOLX (m = −0.009). (D). PPP Capacity (PPP relative to baseline) for RBCs stored in CPDA-1, AS-1, and SOLX. Slopes from linear regression were determined to be: CPDA-1 (m = −0.020), AS-1 (m = −0.015), and SOLX (m = −0.013).
4. Discussion
The principle finding of the study is that all biochemical (pH, lactate, ATP) and biomechanical (membrane SEM, viscosity, resistance to filtration, and MCV) characteristics of rat RBCs undergo different time dependent changes during storage, depending on the AS utilized. RBCs stored in SOLX had slower deterioration rates compared to AS-1 or CPDA-1. HSLs of RBCs stored with AS-1 or CPDA-1 did not occur until 14 days for RBCs stored in SOLX. RBCs stored with SOLX were no different from fresh RBCs during the early phase of storage, specifically, no statistically significant differences in both resistance to filtration and MCV were observed. These results confirm rat RBCs decrease of 2,3-DPG over time, which induce a leftward shift in the O2-Hb dissociation curve and would prevent the release of O2 to tissues. After transfusion, RBCs replenish 2,3-DPG within 24 hours as long as the glycolytic pathways allows for [30]. Thus, RBCs stored with AS-1 or CPDA would take longer to restore their 2,3-DPG pools after transfusion compared to SOLX, since they showed reduced glucose utilization and limited pentose phosphate pathway (PPP) flux when compared to RBCs stored with SOLX. Lastly, this study fills the gaps in the existing current literature by evaluating the biochemical and hemorheological changes in rat RBCs when store with various AS.
Extracellular K+ and hemolysis increased during storage, faster deterioration rates were observed for cells stored in CPDA-1, whereas cells stored in SOLX exhibited lower rates of deterioration. Mature RBCs maintain stable volumes when placed in isotonic solution and rapidly adjust their volumes to new stable levels; thus, it is interesting that the rate of increase in MCV was not as pronounced as the rates of increase in extracellular K+. As RBCs swell, their membrane surface area remains constant, as the biconcave cells gains water and become spherical in shape; the critical hemolytic volume is around 1.65 times the original volume [31]. Thus, storage did not increase MCV close to the critical hemolytic volume. The rate of phosphate ester, 2,3 DPG and ATP decrease was correlated with the changes in pH. However, reaction of adenine, present in all storage solutions, with the enzyme adenosine deaminase results in the release of ammonia. As result, the rate of pH decrease was slowed in comparison to the decrease in phosphate ester concentration and the increase in lactate. As 2,3 DPG decreases and lactate increases, whereas for ATP, the presence of adenine in all storage solutions caused an increase in the concentration of ATP during the first 7 days of storage. The slower changes in pH for RBCs stored with SOLX are likely due to the presence of sodium bicarbonate and dibasic sodium phosphate in SOLX.
Resistance to filtration correlated with the compliance (rate of change of elongation index/shear stress curve). Resistance to filtration was found to be greatest for RBCs stored with CPDA-1 and less affected for RBCs stored with SOLX. Thus, RBCs stored with CPDA-1 were found to be the least compliant, while those RBCs stored with SOLX were found to be the most compliant. This can be explained by the increased MCV and membrane SEM in CPDA-1 stored RBCs compared to SOLX. Cells stored with SOLX had minor changes in MCV and membrane SEM. Thus, RBCs stored with SOLX are less rigid when compared with RBCs stored with CPDA-1 and AS-1. Preservation of mechanical properties by SOLX is likely due to the lack of chloride, while containing more bicarbonate, adenine, glucose, mannitol, and phosphate as compared to CPDA-1 and AS-1 storage solutions. In addition to the alkaline components of SOLX, the lack of chloride results in an efflux of chloride from the RBC, maintaining the pH with decreased extracellular sodium bicarbonate. The higher phosphate and carbonate concentrations in SOLX also increase strong ion differences, preserve pH, and provide key substrates for the salvage pathway of ATP biosynthesis. Lastly, SOLX includes also mannitol and phosphate, both of which are designed to improve RBC metabolism during storage by increasing the range and capacity of pH buffering.
Previous studies have shown that human RBCs stored with SOLX maintain ATP and 2,3 DPG, and lower increase in lactate and extracellular K+ compared to other preservation solutions [20, 32]. Our results confirm that rat RBCs closely mimics human RBC changes. This study also demonstrates that RBCs stored with SOLX had minor changes in RBC mechanical characteristics when compared with RBCs stored in AS-1 or CPDA-1. The mechanical characteristics of RBCs stored with SOLX suggest that there may be improvements to RBC quality over AS-1 storage, which could be beneficial for RBC survival post transfusion. Clinical studies have shown that the one day post-transfusion RBC recoveries of human RBCs stored for 42 and 56 days with SOLX in healthy individuals showed recovery of 89 and 86%, respectively [33, 34].
Under similar hemolytic conditions, previous studies have also demonstrated that rat RBCs are less deformable and have increased membrane rigidity when compared with human RBCs [35]. To develop new AS, it is important to know how the rat RBCs, a species that is often used as a model in storage studies, respond to existing not only AS, but also the individual components of those As. These results, in addition to providing information about rat RBCs, demonstrate that HSLs are independent of the absolute magnitude of the rigidity and dependent on the storage time of the cells. This can be concluded primarily because both rat RBCs and human RBCs undergo similar deterioration after storage in CPDA-1 and SAGM [35].
This study shows that rat RBCs stored in SOLX undergo HSL at slower deterioration rates, relative to rat RBCs stored in CPDA-1 and AS-1. Additionally, these results demonstrate that there were no statistically significant changes in MCV and filterability for RBCs stored in SOLX relative to fresh cells over 35 days of storage. Changes in these parameters, however, were significant for RBCs stored in CPDA-1 and AS-1. These results therefore demonstrate fewer storage lesions for RBCs stored in SOLX. The present study of the effects of RBC storage solution have in rat RBCs is primarily designed to understand the differences in storage lesions in rat RBCs and does not directly translate to human erythrocytes. There are noticeable interspecies differences on mechanical stability between rat and human erythrocytes, which are dependent on the osmolality, and the magnitude and duration of the applied stress [36]. These results are expected to be used in the design of rat experimental transfusion models to study the implication of storage lesions and to evaluate novel storage additive and preservation strategies for RBCs. Additionally, future experiments should identify the effects of SOLX increased buffer capacity, in terms of the metabolic pathways involved in the production of HSLs and the microvascular function and O2 delivery to tissues post transfusion.
Acknowledgments
This work was supported by NIH grants from the Heart Lung and Blood Institute, P01-HL110900, R01-HL52684, R01-HL126945 and R56-HL123015.
Funding
This work was supported by NIH grants from the Heart Lung and Blood Institute, P01-HL110900, R01-HL52684, and R56-HL123015.
Footnotes
Competing interests
Authors declare no competing financial interests by the results presented in this manuscript. No financial support was received from Haemanetics Coorporation for the completion of the study. Haemanetics Corporation did not participate in the design of the experimental study, it only provided the SOLX solution.
Conflict of interest
Authors declare no competing financial interests. Haemanetics Corp. donated SOLX solution.
References
- [1].National Blood Collection and Utilization Survey Report In: Services RotUDoHaH, editor. Washington, DC: US Department of Health and Human Services, the Assistant Secretary for Health; 2011. [Google Scholar]
- [2].D’Alessandro A, Liumbruno G, Grazzini G and Zolla L, Red blood cell storage: The story so far, Blood Transfus 8(2) (2010), 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sullivan MT, Cotten R, Read EJ and Wallace EL, Blood collection and transfusion in the United States in 2001, Transfusion 47(3) (2007), 385–394. [DOI] [PubMed] [Google Scholar]
- [4].Hayes MA, Timmins AC, Yau E, Palazzo M, Hinds CJ and Watson D, Elevation of systemic oxygen delivery in the treatment of critically ill patients, New England Journal of Medicine 330(24) (1994), 1717–1722. [DOI] [PubMed] [Google Scholar]
- [5].Marik PE and Sibbald WJ, Effect of stored-blood transfusion on oxygen delivery in patients with sepsis, JAMA 269(23) (1993), 3024–3029. [PubMed] [Google Scholar]
- [6].Marik PE and Corwin HL, Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature, Crit Care Med 36(9) (2008), 2667–2674. [DOI] [PubMed] [Google Scholar]
- [7].Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, et al. , Age of transfused blood is an independent risk factor for postinjury multiple organ failure, Am J Surg 178(6) (1999), 570–572. [DOI] [PubMed] [Google Scholar]
- [8].Leal-Noval SR, Munoz-Gomez M, Arellano-Orden V, Marin-Caballos A, Amaya-Villar R, Marin A, et al. , Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury, Crit Care Med 36(4) (2008), 1290–1296. [DOI] [PubMed] [Google Scholar]
- [9].Shah A, McKechnie S, Brunskill SJ and Stanworth SJ, Fresh versus old red cell transfusions: What have the recent clinical trials found? Curr Opin Hematol 23(6) (2016), 550–556. [DOI] [PubMed] [Google Scholar]
- [10].Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, et al. , Evolution of adverse changes in stored RBCs, Proceedings of the National Academy of Sciences 104(43) (2007), 17063–17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, et al. , Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron, Blood 118(25) (2011), 6675–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grau M, Friederichs P, Krehan S, Koliamitra C, Suhr F and Bloch W, Decrease in red blood cell deformability is associated with a reduction in RBC-NOS activation during storage, Clinical hemorheology and microcirculation 60(2) (2015), 215–229. [DOI] [PubMed] [Google Scholar]
- [13].Cabrales P, Effects of erythrocyte flexibility on microvascular perfusion and oxygenation during acute anemia, American Journal of Physiology-Heart and Circulatory Physiology 293(2) (2007), H1206–H15. [DOI] [PubMed] [Google Scholar]
- [14].Zimrin A and Hess J, Current issues relating to the transfusion of stored red blood cells, Vox sanguinis 96(2) (2008), 93–103. [DOI] [PubMed] [Google Scholar]
- [15].Yuruk K, Bartels SA, Milstein DM, Bezemer R, Biemond BJ and Ince C, Red blood cell transfusions and tissue oxygenation in anemic hematology outpatients, Transfusion 52(3) (2012), 641–646. [DOI] [PubMed] [Google Scholar]
- [16].Yalcin O, Ortiz D, Tsai AG, Johnson PC and Cabrales P, Microhemodynamic aberrations created by transfusion of stored blood, Transfusion 54(4) (2014), 1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hogman CF, Akerblom O, Hedlund K, Rosen I and Wiklund L, Red cell suspensions in SAGM medium, Further experience of in vivo survival of red cells, clinical usefulness and plasma-saving effects, Vox Sang 45(3) (1983), 217–223. [DOI] [PubMed] [Google Scholar]
- [18].Adams F, Bellairs G, Bird AR and Oguntibeju OO, Biochemical storage lesions occurring in nonirradiated and irradiated red blood cells: A brief review, Biomed Res Int 2015 (2015), 968302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mukherjee S, Marwaha N, Prasad R, Sharma RR and Thakral B, Serial assessment of biochemical parameters of red cell preparations to evaluate safety for neonatal transfusions, Indian J Med Res 132 (2010), 715–720. [PMC free article] [PubMed] [Google Scholar]
- [20].Cancelas JA, Dumont LJ, Maes LA, Rugg N, Herschel L, Whitley PH, et al. , Additive solution-7 reduces the red blood cell cold storage lesion, Transfusion 55(3) (2015), 491–498. [DOI] [PubMed] [Google Scholar]
- [21].Skalak R, Impelluso T, Schmalzer EA and Chien S, Theoretical modeling of filtration of blood cell suspensions, Biorheology 20(1) (1983), 41–56. [DOI] [PubMed] [Google Scholar]
- [22].Buono MJ, Krippes T, Kolkhorst FW, Williams AT and Cabrales P, Increases in core temperature counterbalance effects of hemoconcentration on blood viscosity during prolonged exercise in the heat, Experimental Physiology (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Evans EA, Bending elastic modulus of red blood cell membrane derived from buckling instability in micropipet aspiration tests, Biophys J 43(1) (1983), 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Johnson RM, Ektacytometry of red blood cells, Methods Enzymol 173 (1989), 35–54. [DOI] [PubMed] [Google Scholar]
- [25].Baskurt OK, Hardeman MR, Uyuklu M, Ulker P, Cengiz M, Nemeth N, et al. , Parameterization of red blood cell elongation index-shear stress curves obtained by ektacytometry, Scand J Clin Lab Invest 69(7) (2009), 777–788. [DOI] [PubMed] [Google Scholar]
- [26].Buono MJ, Krippes T, Kolkhorst FW, Williams AT and Cabrales P, Increases in core temperature counterbalance effects of haemoconcentration on blood viscosity during prolonged exercise in the heat, Exp Physiol 101(2) (2016), 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee BK, Alexy T, Wenby RB and Meiselman HJ, Red blood cell aggregation quantitated via Myrenne aggregometer and yield shear stress, Biorheology 44(1) (2007), 29–35. [PubMed] [Google Scholar]
- [28].Guarnone R, Centenara E and Barosi G, Performance characteristics of Hemox-Analyzer for assessment of the hemoglobin dissociation curve, Haematologica 80(5) (1995), 426–430. [PubMed] [Google Scholar]
- [29].Yalcin O, Oronsky B, Carvalho LJ, Kuypers FA, Scicinski J and Cabrales P, From METS to malaria: RRx-001, a multi-faceted anticancer agent with activity in cerebral malaria, Malar J 14 (2015), 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Flatt JF, Bawazir WM and Bruce LJ, The involvement of cation leaks in the storage lesion of red blood cells, Front Physiol 5 (2014), 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hoffman JF, On red blood cells, hemolysis and resealed ghosts The Use of Resealed Erythrocytes as Carriers and Bioreactors: Springer; 1992, pp. 1–15. [DOI] [PubMed] [Google Scholar]
- [32].Proffitt S, Thomas S, Swann I, Popovsky MA, Smith DJ, Roberts DJ, et al. , Storage of washed or irradiated red cells in AS-7 improves their in vitro characteristics, Vox Sang 109(3) (2015), 203–213. [DOI] [PubMed] [Google Scholar]
- [33].Maes L, Whitley P, Sawyer S, Wellington M, Ritzler P, Cancelas J, et al. , editors. Red Cells Stored in SOLX (R) For 42 Days Demonstrated Reduced Hemolysis, Improved Morphology and Exceptional In Vivo Recovery. Transfusion; 2011: WILEY-BLACKWELL COMMERCE PLACE, 350 MAIN ST, MALDEN 02148, MA USA. [Google Scholar]
- [34].Cancelas J, Dumont L, Maes L, Rugg N, Herschel L, Whitley P, et al. , editors. RBC Storage in SOLX (R) Additive Solution Ameliorated the Storage Lesion and Demonstrated Extended Storage up to 56 Days: Results of a Multicenter Trial. Transfusion; 2012: WILEY-BLACKWELL 111 RIVER ST, HOBOKEN 07030–5774, NJ USA. [Google Scholar]
- [35].da SilveiraCavalcante L, Acker JP and Holovati JL, Differences in rat and human erythrocytes following blood component manufacturing: The effect of additive solutions, Transfusion Medicine and Hemotherapy 42(3) (2015), 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nemeth N, Sogor V, Kiss F and Ulker P, Interspecies diversity of erythrocyte mechanical stability at various combinations in magnitude and duration of shear stress, and osmolality, Clinical Hemorheology and Microcirculation (2016) (Preprint), 1–19. [DOI] [PubMed] [Google Scholar]