Abstract
Aim: It is speculated that statin therapy modulates the synthesis of polyunsaturated fatty acids (PUFA), including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). However, the data available on the effects of statin therapy on the serum levels of PUFA and the subsequent impact on in-stent restenosis (ISR) in patients with acute coronary syndrome (ACS) are limited.
Methods: A total of 120 ACS patients who received emergent coronary stent implantation, follow-up coronary angiography to evaluate ISR, and new statin therapy were enrolled. We measured the serum levels of the PUFA and lipids at the onset of ACS and at the follow-up coronary angiography.
Results: The follow-up coronary angiography revealed 38 ISR cases. New statin therapy significantly reduced the serum levels of DHA and low-density lipoprotein cholesterol (LDL-C), while it did not affect EPA level. Single regression analysis revealed that a decreased serum level of LDL-C was associated with decreased DHA level. The multiple logistic regression analysis revealed that the decreased DHA level after statin therapy and low serum level of EPA on admission were determinants of prevalence of ISR.
Conclusion: Statin therapy decreased the serum level of DHA with a parallel reduction in LDL-C level in patients with ACS. Decreased DHA level after statin therapy and low EPA level on admission are risk factors for ISR, indicating that in patients with ACS, decreased serum levels of DHA may be a residual target for the prevention of ISR.
Keywords: Polyunsaturated fatty acids, Eicosapentaenoic acid, Docosahexaenoic acid, In-stent restenosis
Introduction
Primary percutaneous coronary intervention (PCI) with stent implantation improves outcomes in patients with acute coronary syndrome (ACS)1–4). However, the treatment of in-stent restenosis (ISR) remains a significant clinical challenge, especially in patients with ACS.
Decreased serum levels of n-3 polyunsaturated fatty acids (PUFA), including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are reportedly associated with an increased incidence of cardiovascular (CV) events and mortality5, 6). In addition, PUFA therapy and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) decrease CV events in patients with or without coronary artery diseases7, 8). This may be attributed to the pleiotropic effects of PUFA including vascular anti-inflammatory effects and inhibitory effects on neointimal proliferation9–12), which suggests their possible role in the prevention of ISR. However, the data available on the correlation between PUFA therapy and the risk of developing ISR are limited13).
Statin therapy is established for the primary and secondary prevention of CV events14). In addition, they are speculated to modulate the activity of PUFA synthesis and to decrease the levels of EPA and DHA15). This study aimed to investigate the effects of statin therapy on the serum levels of PUFA and the subsequent impact on ISR in patients with ACS, because decreased serum levels of n-3 PUFA may be used as a target for the prevention of ISR following statin therapy and the data available on the impact of statins on PUFA and ISR in patients with ACS are limited.
Material and Methods
We retrospectively reviewed data from Japanese patients with ACS who underwent successful emergent PCI with stents implantation for de novo lesions at the Department of Cardiovascular Medicine in Tokushima University Hospital between January 2009 and July 2017. A total of 120 patients with ACS who received emergent coronary stent implantation, follow-up scheduled coronary angiography to evaluate ISR at least 3 months following the emergent PCI, and statin therapy, including rosuvastatin, pitavastatin, atorvastatin, pravastatin, fluvastatin, and simvastatin were serially enrolled (Fig. 1). According to the drug information for dyslipidemic Japanese patients, a regular-dose statin regimen is defined as 5 mg of rosuvastatin, 2 mg of pitavastatin, 10 mg of atorvastatin, 10 mg of pravastatin, or 30 mg of fluvastatin. A high dose is defined as a dose higher than the regular dose, and a low dose is defined as a dose lower than the regular dose.
Fig. 1.
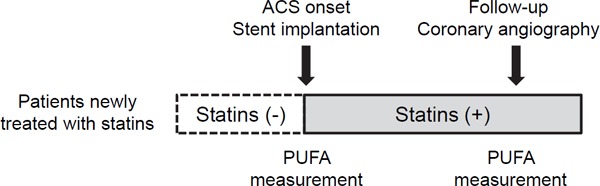
Flowchart of the study
Excluded were patients using fish oil supplements or n-3 PUFA–containing drugs on admission or those who had used fish oil supplements or received n-3 PUFA therapy after ACS onset. In addition, patients with symptomatic, active malignant diseases, liver dysfunction (aspartate aminotransferase levels > 100 IU/L, alanine aminotransferase levels > 100 IU/L), or severe renal dysfunction with hemodialysis were excluded.
ACS included acute myocardial infarction and unstable angina. Acute myocardial infarction was defined as the transient increase of the muscle and brain (MB) fraction of creatine kinase to a threshold of more than 3 times the 99th percentile of the upper reference limit (150 U/L) following PCI in patients with ischemic symptoms and/or typical electrocardiographic findings (ST elevation)7). Unstable angina was defined as angina at rest, accelerated exertional angina combined with typical electrocardiographic changes (ST depression), or an increase in the intensity of anti-ischemic therapy with a transient increase of the MB fraction of creatine kinase to a threshold of less than 3 times the 99th percentile of the upper reference limit, as described previously5).
ISR was defined16) as binary angiographic restenosis when ≥ 50 percent of luminal narrowing at follow-up angiography was detected and as clinical restenosis when both binary angiographic restenosis and clinical symptoms or signs of ischemia (either at rest or with stress) were present OR when a ≥ 70 percent of luminal narrowing at follow-up angiography was detected even in the absence of clinical symptoms or signs.
Blood samples were collected prior to the emergent PCI on admission and prior to the follow-up catheter examination. The serum samples were stored at −30°C until assayed. Gas-liquid chromatography at a commercially available laboratory (SRL, Tokyo, Japan) was used to measure the serum composition of PUFA, including the levels of EPA, DHA, and arachidonic acid (AA)8, 9). The intra- and inter-assay coefficients of variation for the EPA, DHA, and AA measurements were 1.3% and 3.3%, 1.5% and 2.2%, and 1.1% and 2.2%, respectively17). In addition, other biochemical parameters, including low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were measured.
The Tokushima University Hospital Ethics Committee approved the study protocol (No. 2092), and the study was conducted in accordance with the Declaration of Helsinki.
Statistical Analyses
The continuous variables were expressed as a mean ± standard deviation, while the categorical variables were expressed as percentages. The differences between CV risk factors with and without ISR were evaluated using Student's t-test, the Mann–Whitney U test, or the chi-square test according to their distributions. The paired t-test was used to evaluate the differences in the serum levels and the ratio of PUFA and the levels of LDL-C, HDL-C, and TG on the onset of ACS and on the follow-up coronary angiography. After stratification by dosage or type of statins, the differences in the levels or ratio of PUFA were determined by one-way analysis of variance. Pearson's correlation analysis was used to evaluate the correlation of changes in the serum levels of DHA with that of LDL-C. Single and multivariate logistic regression analysis was used to assess the degree of association between ISR and CV risk factors. JMP software version 10 (SAS, Cary, NC) was used to perform all the statistical analyses. Statistical significance was defined as a P-value of < 0.05.
Results
Clinical Characteristics of Subjects
Tables 1 and 2 show the characteristics of patients newly treated with statins stratified by ISR on admission. Follow-up coronary angiography was performed 7.3 ± 1.9 months following PCI on the onset of ACS. Follow-up coronary angiography revealed 38 ISR cases. Except for those in the levels of EPA and rate of anticoagulants use, no differences between the characteristics of the patients in the ISR and non-ISR groups were noted. In addition, no differences in the prevalence of drug-eluting stent (DES) implantation and the stent diameter or length were noted.
Table 1. Clinical characteristics of patients with/without in-stent restenosis at the onset of acute coronary syndrome.
| Variables | All patients | ISR(−) | ISR(+) | P-value |
|---|---|---|---|---|
| Number of patients | 120 | 82 | 38 | |
| Age (years) | 67 ± 13 | 67 ± 12 | 68 ± 13 | 0.67 |
| Male gender, n (%) | 89 (74%) | 59 (72%) | 30 (79%) | 0.26 |
| Body mass index (kg/m2) | 24 ± 4 | 24 ± 4 | 23 ± 4 | 0.70 |
| HbA1c (%) | 6.3 ± 1.3 | 6.2 ± 1.1 | 6.5 ± 1.6 | 0.31 |
| eGFR (mL/min/1.73 m2) | 70 ± 23 | 70 ± 24 | 71 ± 22 | 0.41 |
| TG (mg/dL) | 122 ± 64 | 125 ± 71 | 116 ± 43 | 0.46 |
| HDL-C (mg/dL) | 51 ± 14 | 50 ± 15 | 51 ± 13 | 0.84 |
| LDL-C (mg/dL) | 121 ± 33 | 120 ± 35 | 122 ± 27 | 0.75 |
| Fatty acid concentrations | ||||
| EPA (µg/mL) | 57 ± 33 | 62 ± 34 | 47 ± 29 | 0.02 |
| DHA (µg/mL) | 134 ± 44 | 139 ± 46 | 122 ± 37 | 0.06 |
| AA (µg/mL) | 174 ± 52 | 171 ± 48 | 181 ± 59 | 0.34 |
| EPA/AA | 0.37 ± 0.35 | 0.41 ± 0.39 | 0.28 ± 0.20 | 0.07 |
| DHA/AA | 0.84 ± 0.44 | 0.89 ± 0.48 | 0.74 ± 0.29 | 0.09 |
| Complications | ||||
| Dyslipidemia, n (%) | 38 (32%) | 27 (32%) | 11 (29%) | 0.66 |
| Hypertension, n (%) | 79 (66%) | 54 (65%) | 25 (65%) | 0.99 |
| Diabetes mellitus, n (%) | 39 (33%) | 27 (33%) | 12 (32%) | 0.88 |
| Current smoking, n (%) | 53 (44%) | 37 (45%) | 16 (42%) | 0.76 |
| Culprit lesion, LAD/RCA/LCX, n | 60/45/15 | 39/32/11 | 21/13/4 | 0.73 |
| Number of stents | 143 | 99 | 44 | |
| Stent diameter, mm | 3.1 ± 0.4 | 3.1 ± 0.4 | 3.0 ± 0.5 | 0.46 |
| Stent length, mm | 18.4 ± 4.4 | 18.8 ± 4.5 | 17.5 ± 4.1 | 0.13 |
| Drug eluting stents, n (%) | 31 (26%) | 23 (28%) | 8 (21%) | 0.42 |
Abbreviations: ISR; in-stent restenosis; HbA1c, glycated hemoglobin; eGFR estimated glemerular filtration rate; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol, LDL-C, low-density lipoprotein cholesterol; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid LAD, left anterior descending artery; RCA, right coronary artery, LCX, left circumflex artery.
Table 2. Administered drugs during the follow-up period in patients newly treated with statins.
| Variables | All patients | ISR(−) | ISR(+) | P-value |
|---|---|---|---|---|
| Number of patients | 120 | 82 | 38 | |
| Statins | ||||
| Rosuvastatin | 58 (48%) | 38 (32%) | 20 (17%) | |
| Pitavastatin | 36 (30%) | 24 (20%) | 12 (10%) | |
| Atorvastatin | 15 (13%) | 11 (9%) | 4 (3%) | 0.67 |
| Pravastatin | 7 (6%) | 5 (4%) | 2 (2%) | |
| Fluvastatin | 4 (3%) | 4 (3%) | 0 (0%) | |
| ACEI/ARB, n (%) | 101 (84%) | 67 (82%) | 34 (89%) | 0.27 |
| β-blockers, n (%) | 32 (27%) | 22 (27%) | 10 (26%) | 0.95 |
| Calcium channel blockers, n (%) | 12 (10%) | 10 (12%) | 2 (5%) | 0.24 |
| Ezetimibe, n (%) | 6 (5%) | 6 (7%) | 0 (0%) | 0.08 |
| Antidiabetics, n (%) | 7 (6%) | 5 (6%) | 2 (5%) | 0.86 |
| Anticoaglants, n (%) | 22 (18%) | 11 (13%) | 11 (28%) | 0.04 |
Abbreviations: ISR; in-stent restenosis; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin Ⅱ receptor blockers
Statin Therapy Decreases Levels of LDL-C in Patients with ACS
New statin therapy significantly decreased serum levels of LDL-C, while it did not change those of the TG and the HDL-C (Table 3).
Table 3. Lipid and polyunsaturated fatty acids profiles at the onset and follow-up of acute coronary syndrome.
| Variables | ACS onset | Follow-up | P-value |
|---|---|---|---|
| TG (mg/dL) | 122 ± 64 | 135 ± 60 | 0.05 |
| HDL-C (mg/dL) | 51 ± 14 | 53 ± 12 | 0.06 |
| LDL-C (mg/dL) | 121 ± 33 | 86 ± 23 | < 0.0001 |
| Fatty acid concentrations | |||
| EPA (µg/mL) | 57 ± 33 | 55 ± 31 | 0.39 |
| DHA (µg/mL) | 134 ± 4 | 118 ± 42 | < 0.0001 |
| AA (µg/mL) | 174 ± 52 | 180 ± 50 | 0.11 |
| EPA/AA | 0.37 ± 0.35 | 0.34 ± 0.31 | 0.15 |
| DHA/AA | 0.84 ± 0.44 | 0.71 ± 0.37 | < 0.0001 |
| (EPA+DHA)/AA | 1.21 ± 0.75 | 1.05 ± 0.65 | < 0.0001 |
Abbreviations as in Table 1.
Statin Therapy Decreases Levels of DHA in Patients with ACS
New statin therapy significantly decreased serum levels of DHA, DHA/AA, and (EPA + DHA)/AA but did not change those of EPA, AA, and EPA/AA (Table 3). There were no differences in the change in EPA, AA, EPA/AA, and DHA/AA levels among the statin dosages (Fig. 2). However, statin therapy decreased serum level of DHA in a dose-dependent manner. In addition, there were no differences in the change in EPA, DHA, AA, EPA/AA, and DHA/AA levels among the types of statins including rosuvastatin, pitavastatin, atorvastatin, pravastatin, and fluvastatin/simvastatin (Fig. 3).
Fig. 2.
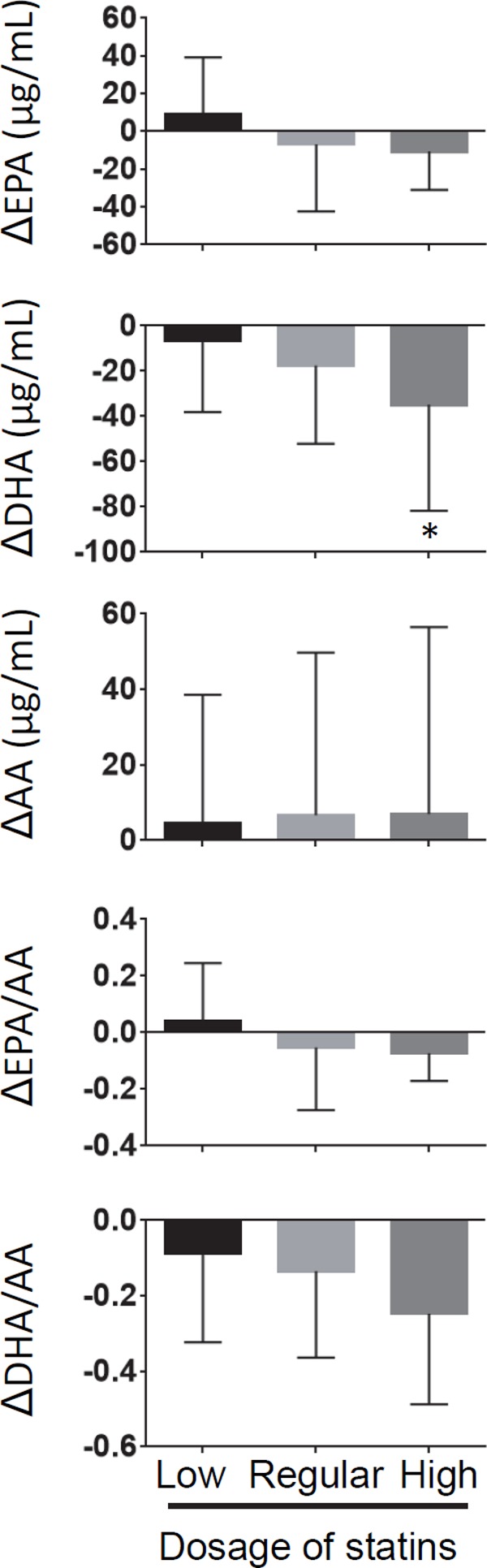
Change in the levels and ratio of PUFA after statin therapy stratified by statin dosage (*p < 0.05 compared with low dosage)
Δ: levels and ratio of PUFA on follow-up–levels and ratio of PUFA on ACS onset
Fig. 3.
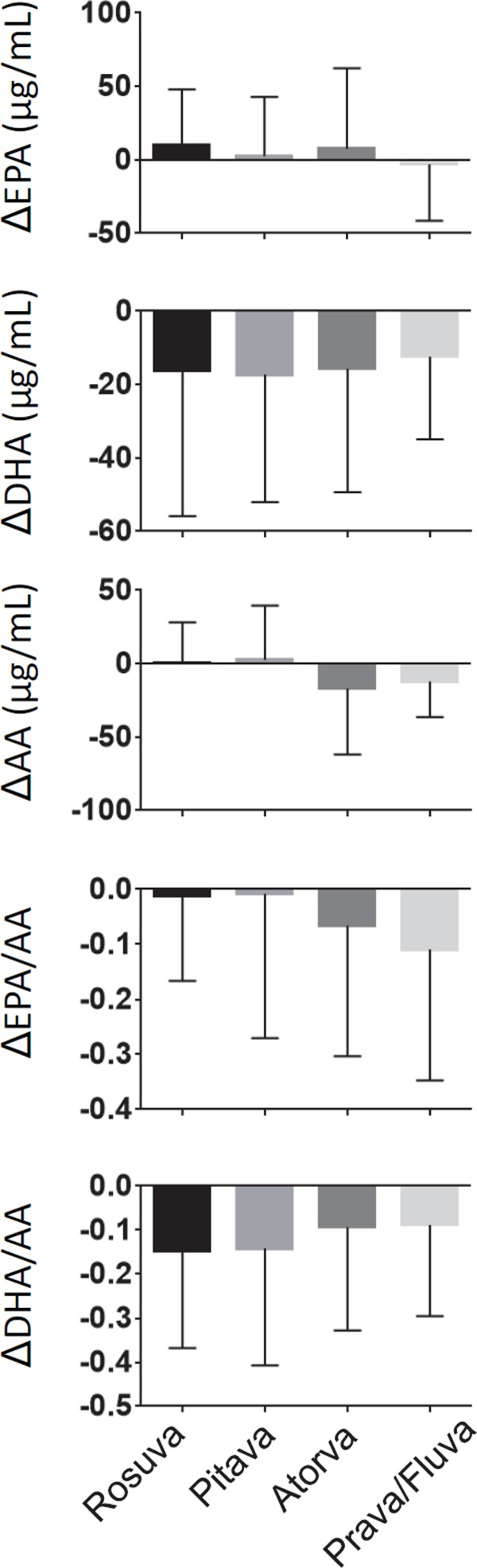
Change in the levels and ratio of PUFA after statin therapy stratified by statin type
Δ: levels of and ratio of PUFA on follow-up–levels of and ratio of PUFA on ACS onset
Rosva, rosuvastatin; Pitava, pitavastatin; Atrova, atorvastatin; Prava, pravastatin; Fluva, fluvastatin.
Correlation between Changes in LDL-C and DHA
We investigated whether the changes in serum levels of DHA were induced by statins by recording the changes in the serum levels of LDL-C. In the patients newly treated with statins, Pearson's correlation analysis revealed that changes in serum levels of LDL-C (LDL-C on follow-up–LDL-C on ACS onset) were positively correlated with changes in serum levels of DHA (DHA on follow-up–DHA on ACS onset) (P < 0.01), indicating that statins decrease DHA, which are reduced in parallel with the reduction of LDL-C levels (Fig. 4).
Fig. 4.
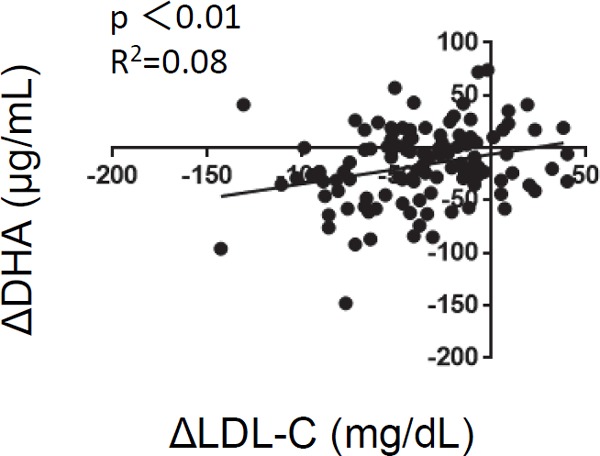
Correlation between changes in low-density lipoprotein cholesterol and changes in docosahexaenoic acid
Changes in the serum levels of DHA (DHA on follow-up–DHA on ACS onset) are correlated with changes in the LDL-C (LDL-C on follow-up–LDL-C on ACS onset).
Statin-Induced Decrease in DHA is a Risk Factor for ISR
We evaluated whether the serum levels of EPA and DHA on admission or on follow-up were risk factors for ISR. The single regression analysis revealed that the levels of EPA and EPA/AA ratios were significantly correlated with an increased prevalence of ISR on admission; however, this correlation was not significant on the follow-up (Table 4). In addition, there was no significant correlation between the prevalence of ISR and age, male gender, body mass index, hypertension, type 2 diabetes, estimate glomerular filtration rate, prevalence of DES implantation, stent diameter and length, and metabolic parameters of TG, HDL-C, LDL-C, and HbA1c both on admission and on follow-up catheter examination (Table 4). In addition, the ISR rate of bare metal stents and second- and third-generation DESs were 34%, 15%, and 32%, respectively; however, there were no significant differences in the prevalence of ISR among the stent types (data not shown). Stent diameter and length, type 2 diabetes, and renal dysfunction are traditional risk factors for ISR18–21). In addition, the serum levels of PUFA are influenced by age17). Multiple logistic regression analysis adjusted for those factors revealed that the decrease in DHA after statin therapy was an independent factor for increased prevalence of ISR. Furthermore, low serum level of EPA on admission was also an independent factor for increased prevalence of ISR (Table 5).
Table 4. Single regression analysis for in-stent restenosis in patients newly treated with statins.
| Variables | Univariate |
|
|---|---|---|
| OR (95% CI) | p-value | |
| Age | 1.01 (0.97, 1.04) | 0.64 |
| Male | 1.46 (0.60, 3.84) | 0.42 |
| Body mass index | 1.02 (0.92, 1.14) | 0.70 |
| Hyperetension | 1.00 (0.45, 2.28) | 0.99 |
| Type 2 diabetes eGFR (mL/min/1.73 m2) | 0.94 (0.40, 2.12) | 0.88 |
| Stent | ||
| Drug eluting stents | 0.68 (0.26, 1.66) | 0.42 |
| Diameter | 0.63 (0.25, 1.56) | 0.33 |
| Length | 0.93 (0.84, 1.02) | 0.13 |
| On addmission | ||
| TG (mg/dL) | 1.00 (0.99, 1.00) | 0.46 |
| HDL-C (mg/dL) | 1.00 (0.97, 1.03) | 0.84 |
| LDL-C (mg/dL) | 1.00 (0.99, 1.01) | 0.75 |
| HbA1c (%) | 1.05 (0.87, 1.53) | 0.32 |
| EPA (µg/mL) | 0.98 (0.97, 0.99) | 0.02 |
| DHA (µg/mL) | 0.98 (0.97, 1.00) | 0.06 |
| AA (µg/mL) | 1.00 (0.99, 1.01) | 0.33 |
| EPA/AA | 0.07 (0.01, 0.63) | 0.03 |
| DHA/AA | 0.26 (0.05, 0.97) | 0.09 |
| Follow-up | ||
| TG (mg/dL) | 1.00 (0.99, 1.01) | 0.69 |
| HDL-C (mg/dL) | 0.99 (0.95, 1.02) | 0.46 |
| LDL-C (mg/dL) | 1.00 (0.97, 1.01) | 0.60 |
| HbA1c (%) | 0.98 (0.59, 1.52) | 0.92 |
| EPA (µg/mL) | 1.00 (0.98, 1.01) | 0.71 |
| DHA (µg/mL) | 0.99 (0.98, 1.00) | 0.10 |
| AA (µg/mL) | 1.00 (0.99, 1.00) | 0.39 |
| EPA/AA | 0.83 (0.15, 2.93) | 0.79 |
| DHA/AA | 0.63 (0.16, 1.85) | 0.45 |
Abbreviations as in Table 1.
Table 5. Multivariate regression analysis for in-stent restenosis in patients newly treated with statins.
| Variables | Multivariate |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
Model 6 |
Model 7 |
Model 8 |
|||||||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age (y.o.) | 1.00 (0.98, 1.05) | 0.46 | 1.01 (0.97, 1.05) | 0.58 | 1.02 (0.98, 1.06) | 0.38 | 1.02 (0.98, 1.06) | 0.43 | 1.02 (0.97, 1.05) | 0.60 | 1.02 (0.98, 1.06) | 0.38 | 1.01 (0.97, 1.05) | 0.62 | 1.01 (0.98, 1.05) | 0.47 |
| Hyperetension | 1.14 (0.47, 2.83) | 0.76 | 1.13 (0.47, 2.79) | 0.78 | 0.99 (0.41, 2.43) | 0.97 | 0.94 (0.39, 2.31) | 0.90 | 0.99 (0.41, 2.39) | 0.97 | 1.10 (0.46, 2.70) | 0.83 | 0.96 (0.41, 2.33) | 0.93 | 0.99 (0.41, 2.39) | 0.97 |
| Type 2 diabetes | 0.83 (0.34, 1.98) | 0.68 | 0.81 (0.33, 1.92) | 0.64 | 0.88 (0.36, 2.10) | 0.78 | 0.90 (0.36, 2.13) | 0.90 | 0.83 (0.34, 1.94) | 0.67 | 0.78 (0.31, 1.84) | 0.57 | 0.78 (0.31, 1.84) | 0.72 | 0.86 (0.35, 2.02) | 0.73 |
| eGFR (mL/min/1.73 m2) | 1.00 (0.98, 1.03) | 0.63 | 1.00 (0.98, 1.02) | 0.81 | 1.00 (0.98, 1.03) | 0.70 | 1.00 (0.98, 1.02) | 0.93 | 1.00 (0.98, 1.02) | 0.77 | 1.00 (0.98, 1.02) | 0.17 | 1.00 (0.98, 1.02) | 0.79 | 1.00 (0.98, 1.02) | 0.80 |
| Stents | ||||||||||||||||
| Drug eluting stents | 0.57 (0.20, 1.53) | 0.29 | 0.48 (0.17, 1.30) | 0.17 | 0.61 (0.21, 1.62) | 0.34 | 0.55 (0.19, 1.45) | 0.24 | 0.55 (0.19, 1.44) | 0.24 | 0.54 (0.18, 1.47) | 0.25 | 0.55 (0.19, 1.45) | 0.25 | 0.56 (0.19, 1.46) | 0.25 |
| Diameter (mm) | 0.68 (0.23, 1.91) | 0.41 | 0.62 (0.21, 1.74) | 0.38 | 0.71 (0.24, 1.98) | 0.51 | 0.67 (0.23,1.84) | 0.44 | 0.58 (0.20, 1.62) | 0.31 | 0.50 (0.17, 1.44) | 0.41 | 0.58 (0.20, 1.62) | 0.32 | 0.59 (0.21, 1.63) | 0.32 |
| Length (mm) | 0.93 (0.83, 1.03) | 0.11 | 0.93 (0.84, 1.04) | 0.22 | 0.92 (0.82, 1.02) | 0.14 | 0.93 (0.83, 1.03) | 0.17 | 0.93 (0.98, 1.01) | 0.15 | 0.93 (0.83, 1.02) | 0.11 | 0.93 (0.84, 1.03) | 0.16 | 0.93 (0.83, 1.02) | 0.15 |
| On addmission | ||||||||||||||||
| EPA (µg/mL) | 0.98 (0.96, 0.99) | 0.02 | 1.00 (0.98, 1.01) | 0.51 | ||||||||||||
| DHA (µg/mL) | 0.98 (0.97, 1.00) | 0.05 | 0.98 (0.98, 0.99) | 0.04 | ||||||||||||
| EPA/AA | 0.06 (0.01, 0.56) | 0.03 | 0.71 (0.10, 2.93) | 0.69 | ||||||||||||
| DHA/AA | 0.22 (0.04, 1.00) | 0.07 | 0.46 (0.08, 1.66) | 0.30 | ||||||||||||
Abbreviations as in Table 1.
Discussion
Our results revealed that ACS patients who were newly treated with statins had reduced serum levels of DHA, which were dependent on reduced LDL-C levels, indicating that statins alone can decrease serum levels of DHA but not EPA. In addition, a decreased DHA level after statin therapy and a low EPA level on admission of patients with ACS were risk factors for developing ISR. The results indicated that EPA level should be maintained before the onset of ACS for better outcome of ACS, and DHA should be maintained after statin therapy after onset of ACS.
ISR is the result of arterial damage with subsequent neointimal proliferation/migration of vascular smooth muscle cells from the bone marrow22) and inflammatory changes in the form of macrophage accumulation, extensive neovascularization in response to stent-associated injuries16, 23, 24), and platelet aggregation25). Previous experimental studies have shown that both EPA and DHA inhibit the proliferation/migration of vascular smooth muscle cells26–28), vascular inflammation29), neovascularization30, 31), and collagen-induced platelet aggregation25, 32). Thus, n-3 PUFA, including EPA and DHA, could reduce the incidence of ISR. However, because the effects of EPA and DHA on atherosclerosis are overlapping, the differential effects between DHA and EPA have been less well established. In an animal study, we showed that when combined with EPA, DHA treatment has additional anti-inflammatory and anti-atherosclerotic effects on Apoe-/- mice fed with a Western-type diet29). The result indicates that DHA can potentially prevent coronary atherosclerosis or ISR even when the EPA level is not low. Thus, EPA and/or DHA supplementation can potentially prevent ISR. However, no clinical studies have evaluated the effects of PUFA, including EPA and DHA on ISR. Prior to the stent-era, the accumulated evidence showed no beneficial effect of fish oils, including PUFA on coronary restenosis treated with balloon angioplasty in patients with stable angina; however, previous meta-analyses revealed a positive effect of fish oils on coronary restenosis33, 34). The CART study reported that initiating treatment with PUFA (2.7 g/d of EPA and 2.3 g/d of DHA) at least 2 weeks prior to an elective coronary balloon angioplasty for 6 months after did not prevent restenosis35). Similarly, the EMPAR Study and another study reported that fish oils (3.2 g/d of EPA and 2.2 g/d of DHA) and high doses of fish oils (4.1 g/d of EPA and 2.8 g/d of DHA), respectively, starting at least 1 or 2 weeks prior to an elective coronary balloon angioplasty did not prevent restenosis36, 37). This indicated that 1–2 weeks of PUFA therapy prior to coronary balloon angioplasties may not be long enough to prevent restenosis. Thus, further research is needed to evaluate the effects of PUFA on ISR especially in ACS patients at high risk of ISR.
In this study, follow-up coronary angiographies revealed that the serum levels of DHA were reduced in patients on statin therapy with a parallel reduction in LDL-C levels. In contrast, this was not observed with EPA. Statin therapy and diet reportedly modulate PUFA composition. Jula et al. reported that simvastatin significantly reduced the serum levels of DHA in patients with hyperlipidemia when compared to placebo; however it did not reduce the EPA levels15). Additionally, Nozue et al. reported that pitavastatin decreased serum DHA/AA ratio in patients with CV diseases, whereas it did not decrease EPA/AA ratio38). Although the mechanism by which statins reduce serum levels of DHA has not been fully elucidated, it is speculated that statins can modulate the enzyme activity of PUFA synthesis, including fatty acid desaturases 1 and 2 and elongation of very long chain fatty acids protein 539). Compared to EPA, DHA predominantly circulates in the blood. In addition, statins reduce LDL-C, a lipoprotein that includes EPA and DHA. Thus, higher concentrations of DHA in the serum may be more susceptible to a statin-induced LDL-C lowering.
Several studies reported that, in patients with myocardial infarctions, PUFA intake can decrease CV events. The DART study reported that, when compared to reducing fat intake and increasing cereal fiber intake in patients with myocardial infarction, eating fatty fish reduced the 2-year all-cause mortality by 29%40). The GISSI-Prevenzione study reported that PUFA intake resulted in a significant reduction in the total and CV-related mortality in addition to sudden cardiac death in patients following myocardial infarctions41). This suggests that administration of PUFA may prevent CV events, including ISR in patients who started statin therapy following the onset of ACS.
This study has several limitations. First, we used a retrospective design with a small sample size at a single center. Second, we did not evaluate the dietary intake of PUFA. Third, we excluded patients who did not receive follow-up catheter examinations, which may have led to a patient selection bias. Therefore, larger, prospective studies are needed to validate the effects of PUFA on ISR.
In conclusion, statin therapy decreased the serum levels of DHA in parallel with a reduction in LDL-C levels in patients with ACS. Decreased DHA level after statin therapy and low eicosapentaenoic acid level on admission are risk factors for ISR, which indicated that decreased serum level of DHA may be a residual target for the prevention of ISR in patients with ACS.
Acknowledgments
This work was partially supported by JSPS Kakenhi Grants (grant numbers 18K08040, 16H05299, and 26248050), the Fugaku Trust for Medical Research, and Takeda Science Foundation. We thank the staff of the Hospital Information Center at Tokushima University Hospital for extracting clinical data from medical records. We would like to thank Editage (www.editage.jp) for the English language editing.
Conflicts of Interest
M. Sata received research funding from Tanabe-Mitsubishi, Takeda, Astellas, Bayer Healthcare, Daiichi-Sankyo, MSD, and Ono, and lecture fees from Astellas, Boehringer Ingelheim, Bayer Healthcare, Mochida, Takeda, Tanabe-Mitsubishi, Novartis, AstraZeneca, MSD, and Shionogi. The Department of Cardio-Diabetes Medicine, Tokushima University Graduate School, is supported in part by unrestricted research grants from Actelion, Boehringer Ingelheim, Kowa, and Tanabe-Mitsubishi. The others declare no conflict of interest.
References
- 1). Feinberg J, Nielsen EE, Greenhalgh J, Hounsome J, Sethi NJ, Safi S, Gluud C, Jakobsen JC: Drug-eluting stents versus bare-metal stents for acute coronary syndrome. The Cochrane database of systematic reviews, 2017; 8: CD012481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Raber L, Kelbaek H, Ostojic M, Baumbach A, Heg D, Tuller D, von Birgelen C, Roffi M, Moschovitis A, Khattab AA, Wenaweser P, Bonvini R, Pedrazzini G, Kornowski R, Weber K, Trelle S, Luscher TF, Taniwaki M, Matter CM, Meier B, Juni P, Windecker S, Investigators CAT : Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA, 2012; 308: 777-787 [DOI] [PubMed] [Google Scholar]
- 3). Sabate M, Cequier A, Iniguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A, Vazquez N, Gomez-Hospital JA, Baz JA, Martin-Yuste V, van Geuns RJ, Alfonso F, Bordes P, Tebaldi M, Masotti M, Silvestro A, Backx B, Brugaletta S, van Es GA, Serruys PW: Everolimus-eluting stent versus baremetal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet, 2012; 380: 1482-1490 [DOI] [PubMed] [Google Scholar]
- 4). Kocka V, Maly M, Tousek P, Budesinsky T, Lisa L, Prodanov P, Jarkovsky J, Widimsky P: Bioresorbable vascular scaffolds in acute ST-segment elevation myocardial infarction: a prospective multicentre study ‘Prague 19’. Eur Heart J, 2014; 35: 787-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Amano T, Matsubara T, Uetani T, Kato M, Kato B, Yoshida T, Harada K, Kumagai S, Kunimura A, Shinbo Y, Kitagawa K, Ishii H, Murohara T: Impact of omega-3 polyunsaturated fatty acids on coronary plaque instability: an integrated backscatter intravascular ultrasound study. Atherosclerosis, 2011; 218: 110-116 [DOI] [PubMed] [Google Scholar]
- 6). Hara M, Sakata Y, Nakatani D, Suna S, Usami M, Matsumoto S, Hamasaki T, Doi Y, Nishino M, Sato H, Kitamura T, Nanto S, Hori M, Komuro I, Osaka Acute Coronary Insufficiency Study I : Low levels of serum n-3 polyunsaturated fatty acids are associated with worse heart failure-free survival in patients after acute myocardial infarction. Circulation journal: official journal of the Japanese Circulation Society, 2013; 77: 153-162 [DOI] [PubMed] [Google Scholar]
- 7). Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Japan EPAlisI : Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]
- 8). Matsuzaki M, Yokoyama M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Matsuzawa Y, Japan JI : Incremental Effects of Eicosapentaenoic Acid on Cardiovascular Events in Statin-Treated Patients With Coronary Artery Disease - Secondary Prevention Analysis From JELIS. Circulation Journal, 2009; 73: 1283-1290 [DOI] [PubMed] [Google Scholar]
- 9). Ross R: Atherosclerosis--an inflammatory disease. The New England journal of medicine, 1999; 340: 115-126 [DOI] [PubMed] [Google Scholar]
- 10). Yagi S, Fukuda D, Aihara KI, Akaike M, Shimabukuro M, Sata M: n-3 Polyunsaturated Fatty Acids: Promising Nutrients for Preventing Cardiovascular Disease. Journal of atherosclerosis and thrombosis, 2017; 24: 999-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Yagi S, Aihara K, Ikeda Y, Sumitomo Y, Yoshida S, Ise T, Iwase T, Ishikawa K, Azuma H, Akaike M, Matsumoto T: Pitavastatin, an HMG-CoA reductase inhibitor, exerts eNOS-independent protective actions against angiotensin II induced cardiovascular remodeling and renal insufficiency. Circulation research, 2008; 102: 68-76 [DOI] [PubMed] [Google Scholar]
- 12). Yagi S, Akaike M, Aihara K, Ishikawa K, Iwase T, Ikeda Y, Soeki T, Yoshida S, Sumitomo-Ueda Y, Matsumoto T, Sata M: Endothelial nitric oxide synthase-independent protective action of statin against angiotensin II-induced atrial remodeling via reduced oxidant injury. Hypertension, 2010; 55: 918-923 [DOI] [PubMed] [Google Scholar]
- 13). Filion KB, El Khoury F, Bielinski M, Schiller I, Dendukuri N, Brophy JM: Omega-3 fatty acids in high-risk cardiovascular patients: a meta-analysis of randomized controlled trials. BMC cardiovascular disorders, 2010; 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Yagi S, Aihara K, Ikeda Y, Akaike M, Sata M, Matsumoto T: Effects of statins on cardiorenal syndrome. International journal of vascular medicine, 2012; 2012: 162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Jula A, Marniemi J, Ronnemaa T, Virtanen A, Huupponen R: Effects of diet and simvastatin on fatty acid composition in hypercholesterolemic men: a randomized controlled trial. Arteriosclerosis, thrombosis, and vascular biology, 2005; 25: 1952-1959 [DOI] [PubMed] [Google Scholar]
- 16). Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R: In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol, 2010; 56: 1897-1907 [DOI] [PubMed] [Google Scholar]
- 17). Yagi S, Aihara K, Fukuda D, Takashima A, Bando M, Hara T, Nishimoto S, Ise T, Kusunose K, Yamaguchi K, Tobiume T, Iwase T, Yamada H, Soeki T, Wakatsuki T, Shimabukuro M, Akaike M, Sata M: Reduced ratio of eicosapentaenoic acid and docosahexaenoic acid to arachidonic acid is associated with early onset of acute coronary syndrome. Nutr J, 2015; 14: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Kastrati A, Schomig A, Elezi S, Schuhlen H, Dirschinger J, Hadamitzky M, Wehinger A, Hausleiter J, Walter H, Neumann FJ: Predictive factors of restenosis after coronary stent placement. J Am Coll Cardiol, 1997; 30: 1428-1436 [DOI] [PubMed] [Google Scholar]
- 19). Kobayashi Y, De Gregorio J, Kobayashi N, Akiyama T, Reimers B, Finci L, Di Mario C, Colombo A: Stented segment length as an independent predictor of restenosis. J Am Coll Cardiol, 1999; 34: 651-659 [DOI] [PubMed] [Google Scholar]
- 20). de Feyter PJ, Kay P, Disco C, Serruys PW: Reference chart derived from post-stent-implantation intravascular ultrasound predictors of 6-month expected restenosis on quantitative coronary angiography. Circulation, 1999; 100: 1777-1783 [DOI] [PubMed] [Google Scholar]
- 21). Latif F, Kleiman NS, Cohen DJ, Pencina MJ, Yen CH, Cutlip DE, Moliterno DJ, Nassif D, Lopez JJ, Saucedo JF, Investigators E : In-hospital and 1-year outcomes among percutaneous coronary intervention patients with chronic kidney disease in the era of drug-eluting stents: a report from the EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) registry. JACC Cardiovasc Interv, 2009; 2: 37-45 [DOI] [PubMed] [Google Scholar]
- 22). Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R: Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med, 2002; 8: 403-409 [DOI] [PubMed] [Google Scholar]
- 23). Goto K, Zhao Z, Matsumura M, Dohi T, Kobayashi N, Kirtane AJ, Rabbani LE, Collins MB, Parikh MA, Kodali SK, Leon MB, Moses JW, Mintz GS, Maehara A: Mechanisms and Patterns of Intravascular Ultrasound In-Stent Restenosis Among Bare Metal Stents and First- and Second-Generation Drug-Eluting Stents. The American journal of cardiology, 2015; 116: 1351-1357 [DOI] [PubMed] [Google Scholar]
- 24). Hoffmann R, Mintz GS, Dussaillant GR, Popma JJ, Pichard AD, Satler LF, Kent KM, Griffin J, Leon MB: Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation, 1996; 94: 1247-1254 [DOI] [PubMed] [Google Scholar]
- 25). Takada K, Ishikawa S, Yokoyama N, Hosogoe N, Isshiki T: Effects of eicosapentaenoic acid on platelet function in patients taking long-term aspirin following coronary stent implantation. International heart journal, 2014; 55: 228-233 [DOI] [PubMed] [Google Scholar]
- 26). Shiina T, Terano T, Saito J, Tamura Y, Yoshida S: Eicosapentaenoic acid and docosahexaenoic acid suppress the proliferation of vascular smooth muscle cells. Atherosclerosis, 1993; 104: 95-103 [DOI] [PubMed] [Google Scholar]
- 27). Terano T, Shiina T, Tamura Y: Eicosapentaenoic acid suppressed the proliferation of vascular smooth muscle cells through modulation of various steps of growth signals. Lipids, 1996; 31: S301-S304 [DOI] [PubMed] [Google Scholar]
- 28). Mizutani M, Asano M, Roy S, Nakajima T, Soma M, Yamashita K, Okuda Y: omega-3 polyunsaturated fatty acids inhibit migration of human vascular smooth muscle cells in vitro. Life Sci, 1997; 61: Pl269-Pl274 [DOI] [PubMed] [Google Scholar]
- 29). Takashima A, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Nishimoto S, Yagi S, Yamada H, Soeki T, Wakatsuki T, Taketani Y, Shimabukuro M, Sata M: Combination of n-3 polyunsaturated fatty acids reduces atherogenesis in apolipoprotein E-deficient mice by inhibiting macrophage activation. Atherosclerosis, 2016; 254: 142-150 [DOI] [PubMed] [Google Scholar]
- 30). Kanayasu T, Morita I, Nakao-Hayashi J, Asuwa N, Fujisawa C, Ishii T, Ito H, Murota S: Eicosapentaenoic acid inhibits tube formation of vascular endothelial cells in vitro. Lipids, 1991; 26: 271-276 [DOI] [PubMed] [Google Scholar]
- 31). Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD: Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A, 2013; 110: 6530-6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Nelson GJ, Schmidt PS, Bartolini GL, Kelley DS, Kyle D: The effect of dietary docosahexaenoic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids, 1997; 32: 1129-1136 [DOI] [PubMed] [Google Scholar]
- 33). O'Connor GT, Malenka DJ, Olmstead EM, Johnson PS, Hennekens CH: A meta-analysis of randomized trials of fish oil in prevention of restenosis following coronary angioplasty. American journal of preventive medicine, 1992; 8: 186-192 [PubMed] [Google Scholar]
- 34). Gapinski JP, VanRuiswyk JV, Heudebert GR, Schectman GS: Preventing restenosis with fish oils following coronary angioplasty. A meta-analysis. Archives of internal medicine, 1993; 153: 1595-1601 [PubMed] [Google Scholar]
- 35). Johansen O, Brekke M, Seljeflot I, Abdelnoor M, Arnesen H: n-3 fatty acids do not prevent restenosis after coronary angioplasty: Results from the CART study. J Am Coll Cardiol, 1999; 33: 1619-1626 [DOI] [PubMed] [Google Scholar]
- 36). Cairns JA, Gill J, Morton B, Roberts R, Gent M, Hirsh J, Holder D, Finnie K, Marquis JF, Naqvi S, Cohen E: Fish oils and low-molecular-weight heparin for the reduction of restenosis after percutaneous transluminal coronary angioplasty. The EMPAR Study. Circulation, 1996; 94: 1553-1560 [DOI] [PubMed] [Google Scholar]
- 37). Leaf A, Jorgensen MB, Jacobs AK, Cote G, Schoenfeld DA, Scheer J, Weiner BH, Slack JD, Kellett MA, Raizner AE, et al. : Do fish oils prevent restenosis after coronary angioplasty? Circulation, 1994; 90: 2248-2257 [DOI] [PubMed] [Google Scholar]
- 38). Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Nozato T, Miyake S, Takeyama Y, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Michishita I: Effects of statins on serum n-3 to n-6 polyunsaturated fatty acid ratios in patients with coronary artery disease. Journal of cardiovascular pharmacology and therapeutics, 2013; 18: 320-326 [DOI] [PubMed] [Google Scholar]
- 39). Ishihara N, Suzuki S, Tanaka S, Watanabe Y, Nagayama D, Saiki A, Tanaka T, Tatsuno I: Atorvastatin increases Fads1, Fads2 and Elovl5 gene expression via the geranylgeranyl pyrophosphate-dependent Rho kinase pathway in 3T3-L1 cells. Mol Med Rep, 2017; 16: 4756-4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM: Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet, 1989; 2: 757-761 [DOI] [PubMed] [Google Scholar]
- 41). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet, 1999; 354: 447-455 [PubMed] [Google Scholar]


