Abstract
Fatty acid-binding proteins (FABPs), a family of lipid chaperones, contribute to systemic metabolic regulation via several lipid signaling pathways. Fatty acid-binding protein 4 (FABP4), known as adipocyte FABP (A-FABP) or aP2, is mainly expressed in adipocytes and macrophages and plays important roles in the development of insulin resistance and atherosclerosis in relation to metabolically driven low-grade and chronic inflammation, referred to as ‘metaflammation’. FABP4 is secreted from adipocytes in a non-classical pathway associated with lipolysis and acts as an adipokine for the development of insulin resistance and atherosclerosis. Circulating FABP4 levels are associated with several aspects of metabolic syndrome and cardiovascular disease. Ectopic expression and function of FABP4 in cells and tissues are also related to the pathogenesis of several diseases. Pharmacological modification of FABP4 function by specific inhibitors, neutralizing antibodies or antagonists of unidentified receptors would be novel therapeutic strategies for several diseases, including obesity, diabetes mellitus, atherosclerosis and cardiovascular disease. Significant roles of FABP4 as a lipid chaperone in physiological and pathophysiological conditions and the possibility of FABP4 being a therapeutic target for metabolic and cardiovascular diseases are discussed in this review.
Keywords: Adipocyte, Macrophage, Endothelium, Atherosclerosis, Insulin resistance, Adipokine, aP2, A-FABP, Mal1, E-FABP
Introduction
Several factors, including genetics, behavior and environment, cause visceral fat accumulation, which is associated with obesity-related metabolic disorders1, 2). In the adipose tissue of obesity, adipocytes and several immune cells, especially macrophages, interact with each other and induce insulin resistance, diabetes mellitus, dyslipidemia and hypertension, leading to the development of atherosclerosis2) (Fig. 1). Adipose tissue can secrete several hormonal bioactive molecules called adipokines, including adiponectin, leptin, resistin and fatty acid-binding protein 4 (FABP4)2). It has recently been reported that FABP4, mainly expressed in adipocytes and macrophages, plays significant roles in the development of insulin resistance and atherosclerosis via both intracellular and extracellular effects3–6) (Fig. 1).
Fig. 1.

FABP4-mediated insulin resistance and atherosclerosis in obesity
Several factors, including genetics, stress, excess nutrients and lack of exercise, induce obesity characterized by visceral fat accumulation. In adipose tissue, adipocytes and several immune cells, especially macrophages, interact with each other and induce insulin resistance, diabetes mellitus (DM), dyslipidemia (DL) and hypertension (HT), leading to the development of atherosclerosis. Fatty acid-binding protein 4 (FABP4), which is mainly expressed in adipocytes and macrophages, plays significant roles in the development of insulin resistance and atherosclerosis via both intracellular and extracellular effects. EC, endothelial cell; PVAT, perivascular adipose tissue; SMC, smooth muscle cell.
Fatty Acid-Binding Proteins (FABPs)
Fatty acid trafficking in cells affects many aspects of cellular function3). Fatty acids act both as an energy source and as signals for metabolic regulations including gene expression, inflammatory and metabolic responses, and growth and survival pathways. Fatty acid-binding proteins (FABPs), a family of intracellular lipid chaperones, regulate lipid trafficking and responses in cells and are linked to metabolic and inflammatory pathways3–6). FABPs are abundantly expressed 14-15-kDa proteins that reversibly bind hydrophobic ligands, such as long-chain fatty acids and other lipids. It has been proposed that FABPs actively facilitate the transport of fatty acids to specific organelles in the cell for lipid oxidation in the mitochondrion or peroxisome, transcriptional regulation in the nucleus, signaling, trafficking and membrane synthesis in the endoplasmic reticulum (ER), and regulation of enzyme activity and storage as lipid droplets in the cytoplasm3).
At least 9 different FABP isoforms have been identified. The FABP family includes liver (L-FABP/FABP1), intestinal (I-FABP/FABP2), heart (H-FABP/FABP3), adipocyte (A-FABP/FABP4/aP2), epidermal (E-FABP/FABP5/mal1), ileal (Il-FABP/FABP6), brain (B-FABP/FABP7), myelin (M-FABP/FABP8), and testis (T-FABP/FABP9) isoforms3). FABPs have about 15% to 70% sequence identity between different isoforms and have almost the same three-dimensional structures showing a cap by the helix-loop-helix region and two orthogonal five-stranded β-sheets by a 10-stranded anti-parallel β-barrel structure3, 7). The fatty acid-binding pocket is located inside the β-barrel. The opening of the binding pocket is framed on one side by the N-terminal helix–loop–helix cap domain, and usually one long-chain fatty acid can be bound to the interior cavity of FABPs except for FABP1, which can bind two fatty acids. Each FABP has different ligand selectivity and binding affinity for fatty acids because of structural differences3).
Expression of FABP4
Expression of FABP4 is highly induced during adipocyte differentiation and is transcriptionally controlled by peroxisome proliferator-activated receptor (PPAR) γ agonists, fatty acids, insulin and dexamethasone8–12). Expression of FABP4 is also induced during differentiation from monocytes to macrophages and by treatment with lipopolysaccharide (LPS), phorbol 12-myristate 13-acetate, PPARγ agonists, oxidized low-density lipoprotein and advanced glycation end products13–17). Similar to macrophages, monocytederived dendritic cells express FABP4 during differentiation18). Conversely, treatment with omega-3 fatty acids19) and sitagliptin20) decreases FABP4 expression in 3T3-L1 adipocytes. In macrophages, treatment with atorvastatin21) and metformin22) reduces FABP4 expression. FABP4 also triggers the ubiquitination and subsequent proteasomal degradation of PPARγ and consequently inhibits PPARγ-related functions, thereby providing a negative feedback loop23).
The upstream of the 5′ flanking region of the mouse FABP4 gene contains a direct repeat-1 (DR-1)-type PPAR response element (PPRE) at −5.3 kb24, 25), a glucocorticoid response element (GRE) at −393 to −385 bp9), a CCAAT/enhancer-binding protein (C/EBP) α binding site at −149 to −130 bp26), and an activator protein-1 (AP-1) site at −122 to −116 bp27). A functionally significant genetic variation at the FABP4 locus in humans, T-87C polymorphism, has been reported to result in decreased FABP4 expression in adipose tissue due to alteration of the C/EBP and reduced transcriptional activity of the FABP4 promoter28).
FABP4 is also expressed in capillary and venous, but not arterial, endothelial cells in a normal condition29). Treatment with vascular endothelial growth factor (VEGF)-A via VEGF-receptor-2 or basic fibroblast growth factor (bFGF) induces FABP4 expression in endothelial cells29), and FABP4 in endothelial cells promotes angiogenesis30). Interestingly, cellular senescence and oxidative stress induce FABP4 expression in microvascular endothelial cells31, 32). Furthermore, FABP4 is ectopically induced in injured arterial endothelial cells33, 34).
Fatty Acid Affinity of FABP4
In an assay for fatty acid-binding affinity, FABP4 generally had higher affinity and selectivity for long-chain fatty acids than did albumin35). Linoleic acid and α-linolenic acid, essential polyunsaturated fatty acids, had the highest affinity for FABP4 under a basal condition35), suggesting that transport of linoleic acid and α-linolenic acid is a physiological role of FABP4. Under an oxidative condition, the affinity of FABP4 for most of the fatty acids except for palmitic acid was decreased35), indicating that palmitic acid, a saturated fatty acid, has relatively high affinity for FABP4 under a specific condition such as obesity-induced oxidative stress.
Function of FABP4 in the Cell
Similar to other FABPs, FABP4 is thought to carry fatty acids to several organelles in the cell such as the mitochondrion, peroxisome, ER and nucleus (Fig. 2). The primary sequence of FABP4 does not have a typical nuclear localization signal (NLS) or nuclear export signal (NES) as a potential functional domain3). However, the NLS and NES could be found in the three-dimensional structure of FABP436). The NLS in FABP4 is activated by closure of the portal loop and perturbation of a swinging doorway region37). Non-activating ligands, such as oleic acid and stearic acid, protrude from the portal and prevent its closure, leading to masking of the NLS, while activating ligands, such as linoleic acid, troglitazone and anilinonaphthalene sulphonate, expose the NLS37) (Fig. 2).
Fig. 2.

Possible intracellular and extracellular functions of FABP4
FABP4 is abundant in the cytosolic fraction of adipocytes and can bind one long-chain fatty acid (FA), including palmitic acid (PA), stearic acid (SA), oleic acid (OA), linoleic acid (LA) or α-linolenic acid (ALA). FABP4 facilitates the transport of FAs to specific organelles in the cell such as the mitochondrion, peroxisome, endoplasmic reticulum (ER) and nucleus, regulates enzyme activity, and stores excess FA as lipid droplets in adipocytes. LA-bound, but not SA- or OA-bound, FABP4 can be moved into the nucleus by unmasking of the nuclear localization signal (NLS). The protein-protein interactions of FABP4 with hormone-sensitive lipase (HSL) and comparative gene identification-58 (CGI-58), a potent co-activator of adipose triglyceride lipase (ATGL), regulate intracellular triglyceride hydrolysis, and FABP4 is secreted from adipocytes in a non-classical pathway associated with lipolysis. FABP4 in macrophages inhibits the peroxisome proliferator-activated receptor γ (PPARγ)-liver X receptor α (LXRα)-ATP-binding cassette A1 (ABCA1) pathway and induces inflammatory responses through activation of the inhibitor of nuclear kappa B kinase (IKK)-nuclear factor-kappa B (NF-κB) and c-Jun N-terminal kinase (JNK)-activator protein-1 (AP-1) pathways. FABP4 is ectopically induced in injured arterial endothelial cells (ECs). Macrophages and ECs can also secrete FABP4. Secreted FABP4 may act as a carrier of LA and ALA, essential polyunsaturated FAs, to organs because of high affinities for FABP4 under normal conditions. Circulating FABP4 may affect several responses in target cells, including macrophages, ECs, smooth muscle cells (SMCs), adipocytes and other cells, through unidentified receptor-mediated effects in bound FA-dependent and -independent manners and/or possible internalization of FABP4 into the cell. Obesity and increased visceral fat promote oxidative stress. An oxidative stress condition can induce conformation change of the FABP4 structure and decrease affinity of most of the FAs, except for PA, for FABP4. Under a specific condition such as obesity-induced oxidative stress, PA would have relatively high affinity for FABP4, leading to PA-dependent inflammatory responses through unidentified receptors of PA-bound FABP4 and/or delivery of PA to toll-like receptor 4 (TLR4).
Receptor*, an unidentified receptor of non-specific FA-bound FABP4. Receptor†, an unidentified receptor of specific PA-bound FABP4.
The sequence of FABP4 includes a hormone-sensitive lipase (HSL) binding site38). A direct protein-protein interaction between FABP4 and HSL in adipocytes regulates lipolysis, and adipocytes in FABP4-deficient mice have decreased lipolysis in vitro and in vivo39, 40) (Fig. 2). FABP4 also interacts with comparative gene identification-58 (CGI-58), a potent co-activator of adipose triglyceride lipase (ATGL) that catalyzes the initiating step of intracellular triglyceride hydrolysis 41). Interestingly, during experimentally induced lipolysis, FABP4-deficient mice have reduced insulin secretion39). As metabolic crosstalk between host and pathogen, Chlamydia pneumoniae, which needs to obtain nutrients such as ATP and lipids from host cells, infects and proliferates in adipocytes by inducing HSL-mediated lipolysis42). Interestingly, Chlamydia pneumoniae exploits host FABP4 to facilitate fat mobilization and intracellular replication in adipocytes, suggesting that intracellular pathogens acquire energy via hijacking of the host lipid metabolism pathway42).
As another protein-protein interaction, ligand-bound FABP4 binds to Janus kinase 2 (JAK2) and attenuates its signaling43). Phosphatase and tensin homolog on chromosome 10 (PTEN), which negatively regulates the phosphoinositide 3-kinase pathway, interacts with FABP4, possibly for regulation of lipid metabolism and adipocyte differentiation44). Notably, PTEN-null keratinocytes showed an elevated expression of FABP4, suggesting that PTEN plays a role in the transcriptional regulation of FABP4 expression45).
Phenotype of FABP4 Deficiency
FABP4-deficient mice with high-fat diet-induced and genetic obesity show reduced insulin resistance, but there is no effect of FABP4 on insulin sensitivity in lean mice46, 47). Knockdown of the Fabp4 gene by RNA interference in dietary obese mice increases body weight and fat mass without significant changes in glucose and lipid homeostasis48), being similar to the phenotype of FABP4 heterozygous knockout mice on a high-fat diet46). The remaining expression of FABP4 might maintain some parts of FABP4 function.
FABP4 deficiency protects against atherosclerosis in apolipoprotein E (ApoE)-deficient mice13, 49). FABP4 in macrophages increases accumulation of cholesterol ester and foam cell formation via inhibition of the PPARγ-liver X receptor α (LXRα)-ATP-binding cassette A1 (ABCA1) pathway and induces inflammatory responses through activation of the inhibitor of nuclear kappa B kinase (IKK)-nuclear factor-kappa B (NF-κB) and c-Jun N-terminal kinase (JNK)-AP-1 pathways50, 51) (Fig. 2). Furthermore, the lack of FABP4 in macrophages decreases redox signaling and inflammasome activation via upregulation of uncoupling protein 2 (UCP2)52) and sirtuin 3 (SIRT3)53). FABP4 in dendritic cells also regulates the IKK-NF-κB pathway and T cell priming18).
Secretion of FABP4
FABP4 lacks a signal peptide in the N-terminal sequence3), which is necessary for the classical secretory pathway, i.e., ER-Golgi-dependent secretion. However, FABP4 is secreted from adipocytes in a non-classical secretion pathway associated with lipolysis, a series of intracellular triglyceride hydrolysis mediated by ATGL, HSL and monoacylglycerol lipase (MGL)54–56) (Fig. 2). Secretion of FABP4 is also regulated by an intracellular calcium-dependent pathway57). Furthermore, FABP4 is secreted partially by microvesicles derived from adipocytes55, 58, 59), an established mechanism for unconventional secretion from adipocytes60). However, the release of FABP4 via adipocyte-derived microvesicles is a small fraction and conveys a minor activity55, 58). In addition, unconventional secretion of FABP4 by endosomes and secretory lysosomes has recently been reported61). It has also been confirmed that FABP4 is secreted from macrophages35) and vascular endothelial cells34) (Fig. 2), though the predominant contributors of circulating FABP4 are adipocytes rather than macrophages and endothelial cells54, 62).
FABP4 as a Bioactive Molecule
FABP4 secreted from adipocytes, macrophages and endothelial cells may have bioactive effects since direct effects of exogenous FABP4 have been demonstrated in various types of cells. Exogenous FABP4 enhances hepatic glucose production in vivo and in vitro54), induces endoplasmic reticulum stress in HepG2 liver cells63), inhibits activation of endothelial nitric oxide synthase (eNOS) in vascular endothelial cells35), increases proliferation/migration of vascular smooth muscle cells35), decreases cardiomyocyte contraction in vitro58), potentiated glucose-stimulated insulin secretion in pancreatic β cells64), and increases breast cancer cell proliferation65).
Obesity and increased visceral fat have been reported to promote oxidative stress66). FABP4 prefers to bind linoleic acid and α-linolenic acid for transport of essential polyunsaturated fatty acids in a normal condition, but the affinity of FABP4 would be changed to prefer binding palmitic acid, a saturated fatty acid, probably via conformation change of FABP4 structure, in a condition of obesity-induced oxidative stress35) (Fig. 2). Microarray analysis using macrophages treated with recombinant FABP4 in the presence and absence of palmitic acid demonstrated fatty acid-dependent and -independent effects of exogenous FABP435). Notably, treatment of macrophages with recombinant FABP4 in the presence, but not the absence, of palmitic acid significantly increased inflammatory responses, including chemokine signaling and TNFα-NF-κB signaling pathways35). Furthermore, activation of palmitic acid-dependent inflammatory responses by FABP4 was observed in not only in macrophages but also endothelial cells and vascular smooth muscle cells35).
Taken together, circulating FABP4 secreted from adipocytes, macrophages and vascular endothelial cells seems to not only carry fatty acids to organs but also act as a bioactive molecule in several target cells, including macrophages, endothelial cells, smooth muscle cells, adipocytes and other cells (Fig. 2). Because of the presence of fatty acid-dependent and -independent effects in cells, there would be both receptors for non-specific fatty acid-bound FABP4 and receptors for specific fatty acid, especially palmitic acid, -bound FABP4. However, possible receptors of FABP4 have not been identified yet, though there was a report that cytokeratin 1 interacts with FABP4 on the endothelial cell membrane67). Palmitic acid-bound FABP4 may just deliver palmitic acid to its receptor, toll-like receptor 4 (TLR4). Furthermore, it has been reported that a part of exogenous FABP4 internalizes into the cell34), though whether internalized FABP4 has some functions for signaling or whether internalization is a process of degradation remains unclear (Fig. 2). It has recently been reported that cytokeratin 1 facilitates cellular uptake of exogenous FABP4 in endothelial cells, leading to regulation of oxidative and pro-inflammatory effects68). It has also been shown that FABP4 binds to megalin, an endocytic receptor expressed in proximal tubule epithelial cells of the kidney, which plays a major role in reabsorption of proteins filtered through glomeruli69).
Circulating FABP4 Level
The concentration of FABP4 is highest among levels of FABP1∼5 under a physiological condition in a general population without medication70). FABP4 level is significantly higher in females than in males, possibly due to the larger amount of body fat in females than in males since there is an independent and strong correlation between FABP4 level and adiposity70, 71). In addition, androgen may partially contribute to the gender difference in serum FABP4 levels72). Circulating FABP4 levels are significantly correlated with norepinephrine levels during exercise testing73), being consistent with FABP4 secretion from adipocytes via β-adrenergic-mediated lipolytic mechanisms54–56). Loss of body weight by exercise training74) and bariatric surgery75, 76) induces a significant reduction in FABP4 concentrations.
Gene and protein expression levels of FABP4 have been reported to be higher in subcutaneous adipose tissue than in visceral adipose tissue of both lean and obese subjects77), but associations of subcutaneous adipose tissue and visceral adipose tissue assessed by multidetector computed tomography with circulating FABP4 levels were reported to be almost the same78). One possible reason for the discrepancy is that visceral adipose tissue is more metabolically active and sensitive to lipolysis than is subcutaneous adipose tissue79), leading to the predominant contribution of visceral adipose tissue to the lipolysis-mediated secretion and circulating level of FABP4. The main source of circulating FABP4 is adipocytes rather than macrophages and endothelial cells54, 62). However, it has been reported that circulating FABP4 was detected in patients with lipodystrophy despite adipose tissue loss in contrast to other adipokines including leptin and adiponectin80).
Multiplex proteomics demonstrated that FABP4 level was strongly associated with kidney function decline over a period of 5 years81). Serum FABP4 level was shown to be negatively correlated with estimated glomerular filtration rate70), suggesting that FABP4 is eliminated from circulation mainly by renal clearance. It has recently been reported that circulating FABP4 is eliminated by the kidney via glomerular filtration followed by megalin-mediated reabsorption69). FABP4 level in hemodialysis patients with end-stage kidney disease was about 20-times higher than that in controls with normal renal function and was decreased by 57.2% after hemodialysis82).
Increased circulating FABP4 levels have been shown to be associated with obesity71), metabolic syndrome83), insulin resistance70, 84), type 2 diabetes mellitus85), hypertension86), dyslipidemia87), atherosclerosis88), left ventricular diastolic dysfunction89) and heart failure90) (Fig. 3). Xanthine oxidoreductase (XOR), a rate-limiting and catalyzing enzyme of uric acid formation in purine metabolism, is involved in an increase in reactive oxygen species91), and plasma XOR activity has been shown to be a novel biomarker of metabolic disorders92, 93). A cohort study demonstrated that plasma XOR activity is independently associated with levels of adipokines, including FABP494). Circulating FABP4 is independently associated with the level of PCSK9, which binds to and degrades the low-density lipoprotein (LDL) receptor, suggesting associations with hypercholesterolemia and cardiovascular risk95). Furthermore, the basal FABP4 level is independently associated with change in carotid intima-media thickness, a marker of atherosclerosis, per year, indicating that FABP4 level predicts progress of atherosclerosis96). It has also been reported that serum FABP4 level predicts long-term cardiovascular events and mortality82, 97–99).
Fig. 3.
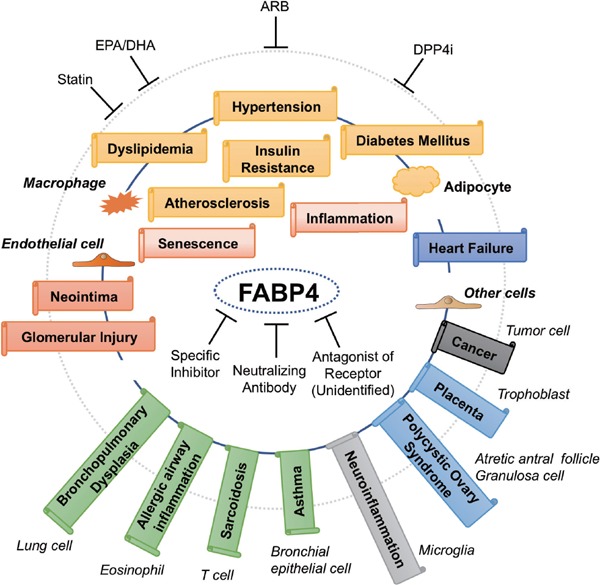
Chain of FABP4-associated pathological conditions
FABP4 is expressed not only in adipocytes and macrophages but also in several types of tissues and cells under physiological and pathophysiological conditions and may contribute to several aspects of metabolic and cardiovascular diseases as well as renal, respiratory, neurological, gynecological and oncological diseases. Several drugs, including a statin, eicosatetraenoic acid (EPA)/docosahexaenoic acid (DHA) agent, angiotensin II receptor blocker (ARB) and dipeptidyl peptidase 4 inhibitor (DPP4i), can decrease FABP4 levels. Specific inhibitors and neutralizing antibodies of FABP4 and antagonists of unidentified FABP4 receptors may be candidates of therapeutic strategies for several FABP4-associated diseases.
Perivascular adipose tissue and epicardial fat have recently been proposed to influence vascular function and the pathogenesis of vascular disease100, 101). FABP4 mRNA expression in epicardial adipose tissue is profoundly increased compared with its expression in paraaortic adipose tissue in patients with metabolic syndrome102). FABP4, mainly derived from epicardial fat, is locally enrich in the pericardial cavity of cardiovascular disease patients103). The coronary veno-arterial difference in FABP4 levels in the aortic root and coronary sinus was shown to be an independent predictor of the severity of coronary stenosis after adjustment of conventional risk factors35). FABP4 levels are significantly elevated during the early hours after onset of acute myocardial infarction and are robustly increased in out-of-hospital cardiac arrest survivors, probably due to rapid lipolytic release of FABP4 from epicardial fat by adrenergic overdrive that accompanies acute cardiovascular disease104).
Several drugs can modify circulating FABP4 levels (Fig. 3). Treatment with atorvastatin105), a hydroxymethylglutaryl-CoA reductase inhibitor, several angiotensin II receptor blockers106), omega-3 fatty acid ethyl esters containing eicosapentaenoic acid and docosahexaenoic acid19), and sitagliptin20), a dipeptidyl peptidase-4 inhibitor, reduces FABP4 concentrations. On the other hand, treatment with pioglitazone, a PPARγ agonist known as an insulin-sensitizing thiazolidinedione, increases FABP4 levels107), presumably due to direct activation of PPARγ since the FABP4 gene promoter includes the PPRE24, 25). Treatment with canagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, paradoxically increased serum FABP4 level in some diabetic patients despite amelioration of glucose metabolism and adiposity reduction, possibly via induction of catecholamine-induced lipolysis in adipocytes, and patients in whom FABP4 level was increased by canagliflozin had significantly smaller improvements of insulin resistance and hemoglobin A1c than did patients with decreased FABP4 level108). The increased FABP4 induced by PPARγ agonists or SGLT2 inhibitors may act as a carrier of linoleic acid and α-linolenic acid, as a physiological function. Therefore, it is important to enforce diet therapy for reducing accumulation of visceral fat and to prevent binding of FABP4 to palmitic acid, especially in the treatment with a PPARγ agonist and/or an SGLT2 inhibitor.
Ectopic Expression of FABP4
FABP4 is expressed in endothelial cells of capillaries and small veins but not arteries under a physiological condition29). FABP4 in capillary endothelial cells is involved in transendothelial fatty acid transport into fatty acid-consuming organs109). FABP4 is ectopically induced in regenerated arterial endothelial cells after endothelial balloon denudation33) and wire-induced vascular injury34). Neointima formation after wire-induced vascular injury is significantly decreased in FABP4-defficient mice compared with that in wildtype mice34). Intermittent hypoxia also increases the expression of FABP4 in human aortic endothelial cells110). FABP4 is expressed in the aortic endothelium of old, but not young, ApoE-deficient atherosclerotic mice, and chronic treatment with BMS309403, a small molecule FABP4 inhibitor, significantly improves endothelial dysfunction in old ApoE-deficient mice111). Both FABP4 and FABP5 are also involved in cellular senescence of vascular endothelial cells31, 32) (Fig. 3). FABP4 secreted from vascular endothelial cells increases gene expression of inflammatory cytokines in cells, promotes proliferation and migration of vascular smooth muscle cells, and decreases phosphorylation of eNOS in vascular endothelial cells, which are attenuated in the presence of an anti-FABP4 antibody34). Ectopic expression of FABP4 under a pathological condition, but not physiological expression of FABP4, in the endothelium may contribute to the pathogenesis of atherosclerosis and vascular injury.
In normal kidneys, FABP4 is expressed in endothelial cells of the tubulointerstitial peritubular capillary and vein in both the cortex and medulla, but not in glomerular or arterial endothelial cells, under a normal physiological condition29). Ectopic expression of FABP4 in endothelial cells and macrophages of the glomerulus is associated with progression of proteinuria and renal dysfunction112) (Fig. 3). It has also been reported that FABP4 is expressed in glomerular mesangial cells of diabetic nephropathy113). Among the FABPs, FABP1 (L-FABP) is expressed in proximal tubular epithelial cells in the kidney, and urinary FABP1 reflects damage of proximal tubular epithelial cells114). Urinary FABP4 has been proposed to be a novel biomarker reflecting glomerular damage115), and determination of both FABP1 and FABP4 in urine may enable better characterization of renal injury.
Evidence for the involvement of FABP4 in several kinds of diseases and conditions has been accumulating3–6) (Fig. 3). FABP4 has been shown to be expressed in lung and bronchoalveolar cells in bronchopulmonary dysplasia116), lung lavage cells in sarcoidosis117), bronchial epithelial cells118) and vascular endothelial cells119) in asthma, eosinophils in allergic airway inflammation120), microglia cells in neuroinflammation121), resident macrophages/microglial cells and endothelial cells of the hyaloid vasculature in the immature retina of proliferative retinopathy122), pulmonary artery endothelial cells in pulmonary thromboembolism123), hepatocytes in liver ischemia/reperfusion injury124), human placental trophoblasts during placental development125), and granulosa cells of the ovary in polycystic ovary syndrome126, 127). Furthermore, FABP4 has been detected in human ovarian cancer cells at the adipocyte-tumor cell interface, and FABP4 deficiency in mice substantially impaired metastatic tumor growth128). FABP4 can substantially increase the metastatic potential of ovarian cancer cells129). FABP4 expression has also been detected in lipoblasts of lipoblastoma and liposarcoma130), glioblastoma131) and urothelial carcinomas132), breast cancer133, 134), prostate cancer135), hepatocytes136, 137) and hepatic stellate cells138) in hepatocellular carcinoma, oral and cervical squamous cell carcinoma139, 140) and non-small cell lung cancer141).
FABP4 as a Therapeutic Target
Several series of FABP4 inhibitors have been synthesized3, 142–144). The specific FABP4 inhibitor BMS309403 is an orally active small molecule that interacts with the fatty acid-binding pocket within the interior of FABP4 to inhibit binding of endogenous fatty acids3, 142, 143). Treatment with BMS309403 has been shown to improve insulin resistance, diabetes mellitus, fatty liver disease and atherosclerosis in experimental models143), indicating that chemical inhibition of FABP4 could be a therapeutic strategy against several aspects of metabolic syndrome (Fig. 3). Recent studies have also demonstrated that neutralization of secreted FABP4 with an antibody to FABP4 could be a feasible approach for treatment of insulin resistance, type 2 diabetes mellitus and vascular injury34, 54, 145, 146) (Fig. 3). Furthermore, treatment with antagonists of receptors for FABP4, especially PA-bound FABP4, would be a novel therapeutic strategy, though receptors for FABP4 have not been identified yet.
FABP5 as an FABP4-Related Lipid Chaperone
FABP5, another FABP known as epidermal FABP (E-FABP), psoriasis-associated FABP (PAFABP) or mal1, is expressed most abundantly in epidermal cells of the skin but is also present in several tissues and cells including adipocytes3). FABP4 and FABP5 have 52% amino acid similarity and bind to several fatty acids with similar affinity and selectivity. The amount of FABP4 in adipocytes is about 100-fold larger than that of FABP5 in adipocytes147). Both FABP4 and FABP5 are also expressed in macrophages and dendritic cells, though the amount of FABP4 in adipocytes is about 10,000-fold larger than that in macrophages118). The stoichiometry of FABP4 and that of FABP5 are nearly equal in macrophages under physiological conditions13). FABP4 deficiency induces a strong compensatory increase of FABP5 in adipose tissue but not in macrophages or dendritic cells13, 18, 46). Other than adipocytes and macrophages, FABP5 is coexpressed with FABP4 in microvascular endothelial cells in the heart and kidney29, 109) and even in endothelial cells of larger blood vessels148). Similar to FABP4, FABP5 facilitates transendothelial transport of fatty acids into fatty acid-consuming organs109).
Expression of FABP5 in macrophages is increased by treatment with toll-like receptor (TLR) agonists: LPS, a TLR4 agonist, and zymosan, a fungal product that activates TLR2149). Expression of FABP5 in endothelial cells is induced by cellular senescence and H2O2-induced oxidative stress31, 32). In contrast to FABP4, FABP5 is not induced by VEGF-A or bFGF in endothelial cells148). FABP5, similar to FABP4, in endothelial cells promotes angiogenic responses, but FABP5 can also exert opposing effects on endothelial survival, indicating that the balance between FABP4 and FABP5 in endothelial cells may be important for the regulation of angiogenic versus quiescent phenotypes in blood vessels148).
FABP5 transgenic mice in adipose tissue on a high-fat diet show enhanced basal and hormone-stimulated lipolysis and reduced insulin sensitivity150, 151). On the other hand, FABP5 deficiency mildly increases systemic insulin sensitivity in dietary and genetic obesity mouse models150). Ablation of FABP5 suppresses atherosclerosis in LDL receptor-deficient mice on a western-style hypercholesterolemic diet, and the antiatherosclerotic effect of FABP5 deletion is associated with reduction of inflammatory response152).
Secretome analyses showed that FABP5 is also secreted from cells62, 111, 153), though the mechanism remains unclear. Transcriptome and metabolome analyses showed that exogenous FABP4 and FABP5 differentially affect transcriptional and metabolic regulation in adipose-derived stem cells near adipocytes153). Circulating FABP5 has been reported to be detected at levels of about one tenth or less of circulating FABP4 concentrations, and FABP5 levels are associated with components of metabolic syndrome, although the correlation is not as strong as that of FABP470, 154). Interestingly, the concentration of FABP5, but not FABP4, is negatively and independently correlated with cholesterol efflux capacity from macrophages, the first step in the reverse cholesterol transport pathway, suggesting a potential biomarker for residual risk of atherosclerosis155).
Phenotype of Combined Deficiency of FABP4 and FABP5
Mice with combined deficiency of FABP4 and FABP5 (Fabp4-/-Fabp5-/-) exhibit protection against type 2 diabetes, fatty liver disease and atherosclerosis more than do FABP4- or FABP5-deficient mice156–158). The effects of FABP4 and FABP5 on atherosclerosis are mainly due to their actions in macrophages13, 152). On the other hand, actions of FABP4 and FABP5 in adipocytes and those in macrophages have distinct roles in regulation of insulin sensitivity through metabolic and inflammatory responses62). Calorie restriction prevents age-related metabolic disease and extends life span159), and it shares many molecular features in combined deficiency of FABP4 and FABP5, but Fabp4-/-Fabp5-/- mice do not have increased longevity160), indicating that extension of a metabolically healthy span in the absence of calorie restriction can be uncoupled from lifespan. It has also been demonstrated that Fabp4-/-Fabp5-/- mice have defective uptake of fatty acid via capillary endothelial cells of the heart and skeletal muscle with compensatory upregulation of glucose consumption in those tissues during fasting161). Furthermore, Fabp4-/-Fabp5-/- mice show impaired thermogenesis after cold exposure during fasting162).
Lipidomic analyses showed increased de novo lipogenesis by induction of stearoyl-CoA desaturase-1 (SCD-1) and fatty acid synthase in adipose tissue of Fabp4-/-Fabp5-/- mice, leading to identification of increased palmitoleate (C16:1n7), an unsaturated free fatty acid, as an adipose tissue-derived lipid hormone, referred to as ‘lipokine’, that can decrease fatty liver and increase glucose uptake in skeletal muscle163). Deletion of FABP4 in macrophages also increases de novo lipogenesis pathways through LXRα-mediated SCD-1 activation, resulting in production of palmitoleate and resistance to ER stress164). Unsaturated fatty acids including palmitoleate modulate histone deacetylation, resulting in decreased basal and LPS-induced expression levels of FABP4 in macrophages165). Treatment with palmitoleate prevents atherosclerosis in ApoE-deficient mice in relation to reduced ER stress and inflammasome activation166). In a human study, palmitoleate level was positively correlated with insulin sensitivity assessed by euglycemic-hyperinsulinemic clamp studies after adjustment of age, gender and adiposity167). The level of the trans isomer of palmitoleate, an exogenous source of C16:1n7, was associated with lower insulin resistance and lower incidence of diabetes mellitus168).
Perspectives and Conclusion
FABP4 is mainly expressed in adipocytes and macrophages and plays important roles in the development of insulin resistance and atherosclerosis in relation to metabolically driven low-grade and chronic inflammation, referred to as ‘metaflammation’ 2) (Fig. 1). FABP4 is involved in the regulation of inflammatory and metabolic processes in target cells (Fig. 2). The presence of FABP4 in cells may be beneficial for storing energy in adipocytes, for acting on an immune response in macrophages against pathogens, and for trafficking of fatty acids in capillary endothelial cells. Additionally, secreted FABP4 in association with lipolysis during fasting may regulate hepatic glucose production for survival in a famine. In the contemporary life-style with excessive caloric intake and decreased energy expenditure, the presence and induction of FABP4 or enhanced secretion of conformation-changed FABP4, which can bind to palmitic acid with a relatively high affinity35), may be rather disadvantageous for regulating inflammatory or metabolic homeostasis. In such conditions, inhibition of FABP4, neutralization/elimination of secreted FABP4 or the use of possible antagonists for unidentified receptors of FABP4 could be an effective therapeutic strategy against metabolic and cardiovascular diseases and possibly other diseases (Fig. 3). Further studies are obviously needed to investigate whether chemical or other types of inhibition/neutralization of FABP4 and blocking receptors of FABP4 can be safely used in humans and to show the efficacy of agents for metabolic and cardiovascular diseases. Furthermore, other than FABP4, several types of FABP inhibitors have been identified169, 170), but there have been few detailed examinations using those inhibitors in in vivo and in vitro studies. Not only FABP4 but also other FABPs, including FABP5, may offer targeting opportunities as a class for prevention or treatment of other diseases. Much work is still needed to determine the precise applications and indications for other isoforms.
Acknowledgements
In relation to this review article, M.F. has been supported by grants from JSPS KAKENHI, MEXT Translational Research Network Program, Uehara Memorial Foundation, SENSHIN Medical Research Foundation, Japan Diabetes Foundation, Takeda Medical Research Foundation, Ono Medical Research Foundation, Takeda Science Foundation, Akiyama Life Science Foundation, Yamaguchi Endocrine Research Foundation, Naito Foundation Natural Science Scholarship, Suhara Memorial Foundation, Kondou Kinen Medical Foundation and Terumo Foundation for Life Science and Arts. The author is grateful to group members of our department for their scientific contribution and is deeply honored to receive Yuichiro Goto Award of the Japan Atherosclerosis Society in 2018. The author also regrets the inadvertent omission of many important references due to space limitations.
Conflict of Interest
None.
References
- 1). Gregor MF, Hotamisligil GS: Inflammatory mechanisms in obesity. Annu Rev Immunol, 2011; 29: 415-445 [DOI] [PubMed] [Google Scholar]
- 2). Hotamisligil GS: Inflammation, metaflammation and immunometabolic disorders. Nature, 2017; 542: 177-185 [DOI] [PubMed] [Google Scholar]
- 3). Furuhashi M, Hotamisligil GS: Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov, 2008; 7: 489-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Furuhashi M, Ishimura S, Ota H, Miura T: Lipid chaperones and metabolic inflammation. Int J Inflam, 2011; 2011: 642612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Furuhashi M, Saitoh S, Shimamoto K, Miura T: Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clin Med Insights Cardiol, 2014; 8: 23-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Hotamisligil GS, Bernlohr DA: Metabolic functions of FABPs--mechanisms and therapeutic implications. Nat Rev Endocrinol, 2015; 11: 592-605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Chmurzynska A: The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet, 2006; 47: 39-48 [DOI] [PubMed] [Google Scholar]
- 8). Amri EZ, Bertrand B, Ailhaud G, Grimaldi P: Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J Lipid Res, 1991; 32: 1449-1456 [PubMed] [Google Scholar]
- 9). Cook JS, Lucas JJ, Sibley E, Bolanowski MA, Christy RJ, Kelly TJ, Lane MD: Expression of the differentiation-induced gene for fatty acid-binding protein is activated by glucocorticoid and cAMP. Proc Natl Acad Sci U S A, 1988; 85: 2949-2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Distel RJ, Robinson GS, Spiegelman BM: Fatty acid regulation of gene expression. Transcriptional and posttranscriptional mechanisms. J Biol Chem, 1992; 267: 5937-5941 [PubMed] [Google Scholar]
- 11). Kletzien RF, Foellmi LA, Harris PK, Wyse BM, Clarke SD: Adipocyte fatty acid-binding protein: regulation of gene expression in vivo and in vitro by an insulin-sensitizing agent. Mol Pharmacol, 1992; 42: 558-562 [PubMed] [Google Scholar]
- 12). Melki SA, Abumrad NA: Expression of the adipocyte fatty acid-binding protein in streptozotocin-diabetes: effects of insulin deficiency and supplementation. J Lipid Res, 1993; 34: 1527-1534 [PubMed] [Google Scholar]
- 13). Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF: Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med, 2001; 7: 699-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Fu Y, Luo N, Lopes-Virella MF, Garvey WT: The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis, 2002; 165: 259-269 [DOI] [PubMed] [Google Scholar]
- 15). Kazemi MR, McDonald CM, Shigenaga JK, Grunfeld C, Feingold KR: Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler Thromb Vasc Biol, 2005; 25: 1220-1224 [DOI] [PubMed] [Google Scholar]
- 16). Pelton PD, Zhou L, Demarest KT, Burris TP: PPARgamma activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem Biophys Res Commun, 1999; 261: 456-458 [DOI] [PubMed] [Google Scholar]
- 17). Fu Y, Luo N, Lopes-Virella MF: Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J Lipid Res, 2000; 41: 2017-2023 [PubMed] [Google Scholar]
- 18). Rolph MS, Young TR, Shum BO, Gorgun CZ, Schmitz-Peiffer C, Ramshaw IA, Hotamisligil GS, Mackay CR: Regulation of dendritic cell function and T cell priming by the fatty acid-binding protein AP2. J Immunol, 2006; 177: 7794-7801 [DOI] [PubMed] [Google Scholar]
- 19). Furuhashi M, Hiramitsu S, Mita T, Omori A, Fuseya T, Ishimura S, Watanabe Y, Hoshina K, Matsumoto M, Tanaka M, Moniwa N, Yoshida H, Ishii J, Miura T: Reduction of circulating FABP4 level by treatment with omega-3 fatty acid ethyl esters. Lipids Health Dis, 2016; 15: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Furuhashi M, Hiramitsu S, Mita T, Fuseya T, Ishimura S, Omori A, Matsumoto M, Watanabe Y, Hoshina K, Tanaka M, Moniwa N, Yoshida H, Ishii J, Miura T: Reduction of serum FABP4 level by sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes mellitus. J Lipid Res, 2015; 56: 2372-2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Llaverias G, Noe V, Penuelas S, Vazquez-Carrera M, Sanchez RM, Laguna JC, Ciudad CJ, Alegret M: Atorvastatin reduces CD68, FABP4, and HBP expression in oxLDL-treated human macrophages. Biochem Biophys Res Commun, 2004; 318: 265-274 [DOI] [PubMed] [Google Scholar]
- 22). Song J, Ren P, Zhang L, Wang XL, Chen L, Shen YH: Metformin reduces lipid accumulation in macrophages by inhibiting FOXO1-mediated transcription of fatty acid-binding protein 4. Biochem Biophys Res Commun, 2010; 393: 89-94 [DOI] [PubMed] [Google Scholar]
- 23). Garin-Shkolnik T, Rudich A, Hotamisligil GS, Rubinstein M: FABP4 attenuates PPARgamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes, 2014; 63: 900-911 [DOI] [PubMed] [Google Scholar]
- 24). Ross SR, Graves RA, Greenstein A, Platt KA, Shyu HL, Mellovitz B, Spiegelman BM: A fat-specific enhancer is the primary determinant of gene expression for adipocyte P2 in vivo. Proc Natl Acad Sci U S A, 1990; 87: 9590-9594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM: mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev, 1994; 8: 1224-1234 [DOI] [PubMed] [Google Scholar]
- 26). Christy RJ, Yang VW, Ntambi JM, Geiman DE, Landschulz WH, Friedman AD, Nakabeppu Y, Kelly TJ, Lane MD: Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev, 1989; 3: 1323-1335 [DOI] [PubMed] [Google Scholar]
- 27). Rauscher FJ, 3rd, Sambucetti LC, Curran T, Distel RJ, Spiegelman BM: Common DNA binding site for Fos protein complexes and transcription factor AP-1. Cell, 1988; 52: 471-480 [DOI] [PubMed] [Google Scholar]
- 28). Tuncman G, Erbay E, Hom X, De Vivo I, Campos H, Rimm EB, Hotamisligil GS: A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci U S A, 2006; 103: 6970-6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Elmasri H, Karaaslan C, Teper Y, Ghelfi E, Weng M, Ince TA, Kozakewich H, Bischoff J, Cataltepe S: Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J, 2009; 23: 3865-3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Elmasri H, Ghelfi E, Yu CW, Traphagen S, Cernadas M, Cao H, Shi GP, Plutzky J, Sahin M, Hotamisligil G, Cataltepe S: Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: role of stem cell factor/c-kit pathway. Angiogenesis, 2012; 15: 457-468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Ha MK, Soo Cho J, Baik OR, Lee KH, Koo HS, Chung KY: Caenorhabditis elegans as a screening tool for the endothelial cell-derived putative aging-related proteins detected by proteomic analysis. Proteomics, 2006; 6: 3339-3351 [DOI] [PubMed] [Google Scholar]
- 32). Lee MY, Wang Y, Vanhoutte PM: Senescence of cultured porcine coronary arterial endothelial cells is associated with accelerated oxidative stress and activation of NFkB. J Vasc Res, 2010; 47: 287-298 [DOI] [PubMed] [Google Scholar]
- 33). Lee MY, Tse HF, Siu CW, Zhu SG, Man RY, Vanhoutte PM: Genomic changes in regenerated porcine coronary arterial endothelial cells. Arterioscler Thromb Vasc Biol, 2007; 27: 2443-2449 [DOI] [PubMed] [Google Scholar]
- 34). Fuseya T, Furuhashi M, Matsumoto M, Watanabe Y, Hoshina K, Mita T, Ishimura S, Tanaka M, Miura T: Ectopic Fatty Acid-Binding Protein 4 Expression in the Vascular Endothelium is Involved in Neointima Formation After Vascular Injury. J Am Heart Assoc, 2017; 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Furuhashi M, Fuseya T, Murata M, Hoshina K, Ishimura S, Mita T, Watanabe Y, Omori A, Matsumoto M, Sugaya T, Oikawa T, Nishida J, Kokubu N, Tanaka M, Moniwa N, Yoshida H, Sawada N, Shimamoto K, Miura T: Local Production of Fatty Acid-Binding Protein 4 in Epicardial/Perivascular Fat and Macrophages Is Linked to Coronary Atherosclerosis. Arterioscler Thromb Vasc Biol, 2016; 36: 825-834 [DOI] [PubMed] [Google Scholar]
- 36). Ayers SD, Nedrow KL, Gillilan RE, Noy N: Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARgamma by FABP4. Biochemistry, 2007; 46: 6744-6752 [DOI] [PubMed] [Google Scholar]
- 37). Gillilan RE, Ayers SD, Noy N: Structural basis for activation of fatty acid-binding protein 4. J Mol Biol, 2007; 372: 1246-1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Smith AJ, Sanders MA, Juhlmann BE, Hertzel AV, Bernlohr DA: Mapping of the hormone-sensitive lipase binding site on the adipocyte fatty acid-binding protein (AFABP). Identification of the charge quartet on the AFABP/aP2 helix-turn-helix domain. J Biol Chem, 2008; 283: 33536-33543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Scheja L, Makowski L, Uysal KT, Wiesbrock SM, Shimshek DR, Meyers DS, Morgan M, Parker RA, Hotamisligil GS: Altered insulin secretion associated with reduced lipolytic efficiency in aP2-/- mice. Diabetes, 1999; 48: 1987-1994 [DOI] [PubMed] [Google Scholar]
- 40). Shen WJ, Sridhar K, Bernlohr DA, Kraemer FB: Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proc Natl Acad Sci U S A, 1999; 96: 5528-5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Hofer P, Boeszoermenyi A, Jaeger D, Feiler U, Arthanari H, Mayer N, Zehender F, Rechberger G, Oberer M, Zimmermann R, Lass A, Haemmerle G, Breinbauer R, Zechner R, Preiss-Landl K: Fatty Acid-binding Proteins Interact with Comparative Gene Identification-58 Linking Lipolysis with Lipid Ligand Shuttling. J Biol Chem, 2015; 290: 18438-18453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Walenna NF, Kurihara Y, Chou B, Ishii K, Soejima T, Itoh R, Shimizu A, Ichinohe T, Hiromatsu K: Chlamydia pneumoniae exploits adipocyte lipid chaperone FABP4 to facilitate fat mobilization and intracellular growth in murine adipocytes. Biochem Biophys Res Commun, 2018; 495: 353-359 [DOI] [PubMed] [Google Scholar]
- 43). Thompson BR, Mazurkiewicz-Munoz AM, Suttles J, Carter-Su C, Bernlohr DA: Interaction of adipocyte fatty acid-binding protein (AFABP) and JAK2: AFABP/aP2 as a regulator of JAK2 signaling. J Biol Chem, 2009; 284: 13473-13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Gorbenko O, Panayotou G, Zhyvoloup A, Volkova D, Gout I, Filonenko V: Identification of novel PTEN-binding partners: PTEN interaction with fatty acid binding protein FABP4. Mol Cell Biochem, 2010; 337: 299-305 [DOI] [PubMed] [Google Scholar]
- 45). Tsuda M, Inoue-Narita T, Suzuki A, Itami S, Blumenberg M, Manabe M: Induction of gene encoding FABP4 in Pten-null keratinocytes. FEBS Lett, 2009; 583: 1319-1322 [DOI] [PubMed] [Google Scholar]
- 46). Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM: Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science, 1996; 274: 1377-1379 [DOI] [PubMed] [Google Scholar]
- 47). Uysal KT, Scheja L, Wiesbrock SM, Bonner-Weir S, Hotamisligil GS: Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology, 2000; 141: 3388-3396 [DOI] [PubMed] [Google Scholar]
- 48). Yang R, Castriota G, Chen Y, Cleary MA, Ellsworth K, Shin MK, Tran JL, Vogt TF, Wu M, Xu S, Yang X, Zhang BB, Berger JP, Qureshi SA: RNAi-mediated germline knockdown of FABP4 increases body weight but does not improve the deranged nutrient metabolism of diet-induced obese mice. Int J Obes (Lond), 2011; 35: 217-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS: Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler Thromb Vasc Biol, 2002; 22: 1686-1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS: The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem, 2005; 280: 12888-12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Hui X, Li H, Zhou Z, Lam KS, Xiao Y, Wu D, Ding K, Wang Y, Vanhoutte PM, Xu A: Adipocyte fatty acidbinding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein-1. J Biol Chem, 2010; 285: 10273-10280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Steen KA, Xu H, Bernlohr DA: FABP4/aP2 Regulates Macrophage Redox Signaling and Inflammasome Activation via Control of UCP2. Mol Cell Biol, 2017; 37: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Xu H, Hertzel AV, Steen KA, Bernlohr DA: Loss of Fatty Acid Binding Protein 4/aP2 Reduces Macrophage Inflammation Through Activation of SIRT3. Mol Endocrinol, 2016; 30: 325-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, White A, Inouye K, Rickey LM, Ercal BC, Furuhashi M, Tuncman G, Hotamisligil GS: Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab, 2013; 17: 768-778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Mita T, Furuhashi M, Hiramitsu S, Ishii J, Hoshina K, Ishimura S, Fuseya T, Watanabe Y, Tanaka M, Ohno K, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T: FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity (Silver Spring), 2015; 23: 359-367 [DOI] [PubMed] [Google Scholar]
- 56). Ertunc ME, Sikkeland J, Fenaroli F, Griffiths G, Daniels MP, Cao H, Saatcioglu F, Hotamisligil GS: Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J Lipid Res, 2015; 56: 423-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Schlottmann I, Ehrhart-Bornstein M, Wabitsch M, Bornstein SR, Lamounier-Zepter V: Calcium-dependent release of adipocyte fatty acid binding protein from human adipocytes. Int J Obes (Lond), 2014; 38: 1221-1227 [DOI] [PubMed] [Google Scholar]
- 58). Lamounier-Zepter V, Look C, Alvarez J, Christ T, Ravens U, Schunck WH, Ehrhart-Bornstein M, Bornstein SR, Morano I: Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res, 2009; 105: 326-334 [DOI] [PubMed] [Google Scholar]
- 59). Kralisch S, Ebert T, Lossner U, Jessnitzer B, Stumvoll M, Fasshauer M: Adipocyte fatty acid-binding protein is released from adipocytes by a non-conventional mechanism. Int J Obes (Lond), 2014; 38: 1251-1254 [DOI] [PubMed] [Google Scholar]
- 60). Nickel W, Rabouille C: Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol, 2009; 10: 148-155 [DOI] [PubMed] [Google Scholar]
- 61). Villeneuve J, Bassaganyas L, Lepreux S, Chiritoiu M, Costet P, Ripoche J, Malhotra V, Schekman R: Unconventional secretion of FABP4 by endosomes and secretory lysosomes. J Cell Biol, 2018; 217: 649-665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62). Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS: Adipocyte/macrophage fatty acidbinding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest, 2008; 118: 2640-2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Bosquet A, Guaita-Esteruelas S, Saavedra P, Rodriguez-Calvo R, Heras M, Girona J, Masana L: Exogenous FABP4 induces endoplasmic reticulum stress in HepG2 liver cells. Atherosclerosis, 2016; 249: 191-199 [DOI] [PubMed] [Google Scholar]
- 64). Wu LE, Samocha-Bonet D, Whitworth PT, Fazakerley DJ, Turner N, Biden TJ, James DE, Cantley J: Identification of fatty acid binding protein 4 as an adipokine that regulates insulin secretion during obesity. Mol Metab, 2014; 3: 465-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). Guaita-Esteruelas S, Bosquet A, Saavedra P, Guma J, Girona J, Lam EW, Amillano K, Borras J, Masana L: Exogenous FABP4 increases breast cancer cell proliferation and activates the expression of fatty acid transport proteins. Mol Carcinog, 2017; 56: 208-217 [DOI] [PubMed] [Google Scholar]
- 66). Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I: Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest, 2004; 114: 1752-1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67). Saavedra P, Girona J, Bosquet A, Guaita S, Canela N, Aragones G, Heras M, Masana L: New insights into circulating FABP4: Interaction with cytokeratin 1 on endothelial cell membranes. Biochim Biophys Acta, 2015; 1853: 2966-2974 [DOI] [PubMed] [Google Scholar]
- 68). Martinez-Micaelo N, Rodriguez-Calvo R, Guaita-Esteruelas S, Heras M, Girona J, Masana L: Extracellular FABP4 uptake by endothelial cells is dependent on cytokeratin 1 expression. Biochim Biophys Acta Mol Cell Biol Lipids, 2018; [DOI] [PubMed] [Google Scholar]
- 69). Shrestha S, Sunaga H, Hanaoka H, Yamaguchi A, Kuwahara S, Umbarawan Y, Nakajima K, Machida T, Murakami M, Saito A, Tsushima Y, Kurabayashi M, Iso T: Circulating FABP4 is eliminated by the kidney via glomerular filtration followed by megalin-mediated reabsorption. Sci Rep, 2018; 8: 16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70). Ishimura S, Furuhashi M, Watanabe Y, Hoshina K, Fuseya T, Mita T, Okazaki Y, Koyama M, Tanaka M, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Miura T: Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One, 2013; 8: e81318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NM, Wong WK, Lam KS: Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem, 2006; 52: 405-413 [DOI] [PubMed] [Google Scholar]
- 72). Hu X, Ma X, Pan X, Luo Y, Xu Y, Xiong Q, Bao Y, Jia W: Association of androgen with gender difference in serum adipocyte fatty acid binding protein levels. Sci Rep, 2016; 6: 27762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73). Iso T, Sunaga H, Matsui H, Kasama S, Oshima N, Haruyama H, Furukawa N, Nakajima K, Machida T, Murakami M, Yokoyama T, Kurabayashi M: Serum levels of fatty acid binding protein 4 and fat metabolic markers in relation to catecholamines following exercise. Clin Biochem, 2017; 50: 896-902 [DOI] [PubMed] [Google Scholar]
- 74). Choi KM, Kim TN, Yoo HJ, Lee KW, Cho GJ, Hwang TG, Baik SH, Choi DS, Kim SM: Effect of exercise training on A-FABP, lipocalin-2 and RBP4 levels in obese women. Clin Endocrinol (Oxf), 2009; 70: 569-574 [DOI] [PubMed] [Google Scholar]
- 75). Simon I, Escote X, Vilarrasa N, Gomez J, Fernandez-Real JM, Megia A, Gutierrez C, Gallart L, Masdevall C, Vendrell J: Adipocyte fatty acid-binding protein as a determinant of insulin sensitivity in morbid-obese women. Obesity (Silver Spring), 2009; 17: 1124-1128 [DOI] [PubMed] [Google Scholar]
- 76). Terra X, Quintero Y, Auguet T, Porras JA, Hernandez M, Sabench F, Aguilar C, Luna AM, Del Castillo D, Richart C: FABP 4 is associated with inflammatory markers and metabolic syndrome in morbidly obese women. Eur J Endocrinol, 2011; 164: 539-547 [DOI] [PubMed] [Google Scholar]
- 77). Fisher RM, Eriksson P, Hoffstedt J, Hotamisligil GS, Thorne A, Ryden M, Hamsten A, Arner P: Fatty acid binding protein expression in different adipose tissue depots from lean and obese individuals. Diabetologia, 2001; 44: 1268-1273 [DOI] [PubMed] [Google Scholar]
- 78). Lee JJ, Britton KA, Pedley A, Massaro JM, Speliotes EK, Murabito JM, Hoffmann U, Ingram C, Keaney JF, Jr., Vasan RS, Fox CS: Adipose Tissue Depots and Their Cross-Sectional Associations With Circulating Biomarkers of Metabolic Regulation. J Am Heart Assoc, 2016;. 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79). Wajchenberg BL: Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev, 2000; 21: 697-738 [DOI] [PubMed] [Google Scholar]
- 80). Miehle K, Ebert T, Kralisch S, Hoffmann A, Kratzsch J, Schlogl H, Stumvoll M, Fasshauer M: Adipocyte and epidermal fatty acid-binding protein serum concentrations in patients with lipodystrophy. Cytokine, 2017; 92: 20-23 [DOI] [PubMed] [Google Scholar]
- 81). Carlsson AC, Ingelsson E, Sundstrom J, Carrero JJ, Gustafsson S, Feldreich T, Stenemo M, Larsson A, Lind L, Arnlov J: Use of Proteomics To Investigate Kidney Function Decline over 5 Years. Clin J Am Soc Nephrol, 2017; 12: 1226-1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82). Furuhashi M, Ishimura S, Ota H, Hayashi M, Nishitani T, Tanaka M, Yoshida H, Shimamoto K, Hotamisligil GS, Miura T: Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS One, 2011; 6: e27356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83). Xu A, Tso AW, Cheung BM, Wang Y, Wat NM, Fong CH, Yeung DC, Janus ED, Sham PC, Lam KS: Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation, 2007; 115: 1537-1543 [DOI] [PubMed] [Google Scholar]
- 84). Nakamura R, Okura T, Fujioka Y, Sumi K, Matsuzawa K, Izawa S, Ueta E, Kato M, Taniguchi SI, Yamamoto K: Serum fatty acid-binding protein 4 (FABP4) concentration is associated with insulin resistance in peripheral tissues, A clinical study. PLoS One, 2017; 12: e0179737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85). Tso AW, Xu A, Sham PC, Wat NM, Wang Y, Fong CH, Cheung BM, Janus ED, Lam KS: Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care, 2007; 30: 2667-2672 [DOI] [PubMed] [Google Scholar]
- 86). Ota H, Furuhashi M, Ishimura S, Koyama M, Okazaki Y, Mita T, Fuseya T, Yamashita T, Tanaka M, Yoshida H, Shimamoto K, Miura T: Elevation of fatty acid-binding protein 4 is predisposed by family history of hypertension and contributes to blood pressure elevation. Am J Hypertens, 2012; 25: 1124-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87). Cabre A, Lazaro I, Girona J, Manzanares JM, Marimon F, Plana N, Heras M, Masana L: Plasma fatty acid binding protein 4 is associated with atherogenic dyslipidemia in diabetes. J Lipid Res, 2008; 49: 1746-1751 [DOI] [PubMed] [Google Scholar]
- 88). Yeung DC, Xu A, Cheung CW, Wat NM, Yau MH, Fong CH, Chau MT, Lam KS: Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler Thromb Vasc Biol, 2007; 27: 1796-1802 [DOI] [PubMed] [Google Scholar]
- 89). Fuseya T, Furuhashi M, Yuda S, Muranaka A, Kawamukai M, Mita T, Ishimura S, Watanabe Y, Hoshina K, Tanaka M, Ohno K, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T: Elevation of circulating fatty acid-binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovasc Diabetol, 2014; 13: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90). Rodriguez-Calvo R, Girona J, Alegret JM, Bosquet A, Ibarretxe D, Masana L: Role of the fatty acid-binding protein 4 in heart failure and cardiovascular disease. J Endocrinol, 2017; 233: R173-R184 [DOI] [PubMed] [Google Scholar]
- 91). Nishino T, Okamoto K: Mechanistic insights into xanthine oxidoreductase from development studies of candidate drugs to treat hyperuricemia and gout. J Biol Inorg Chem, 2015; 20: 195-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92). Furuhashi M, Matsumoto M, Tanaka M, Moniwa N, Murase T, Nakamura T, Ohnishi H, Saitoh S, Shimamoto K, Miura T: Plasma Xanthine Oxidoreductase Activity as a Novel Biomarker of Metabolic Disorders in a General Population. Circ J, 2018; 82: 1892-1899 [DOI] [PubMed] [Google Scholar]
- 93). Furuhashi M, Mori K, Tanaka M, Maeda T, Matsumoto M, Murase T, Nakamura T, Koyama M, Moniwa N, Ohnishi H, Saitoh S, Shimamoto K, Miura T: Unexpected high plasma xanthine oxidoreductase activity in female subjects with low levels of uric acid. Endocr J, 2018; 65: 1083-1092 [DOI] [PubMed] [Google Scholar]
- 94). Furuhashi M, Matsumoto M, Murase T, Nakamura T, Higashiura Y, Koyama M, Tanaka M, Moniwa N, Ohnishi H, Saitoh S, Shimamoto K, Miura T: Independent links between plasma xanthine oxidoreductase activity and levels of adipokines. J Diabetes Investig, 2018. December 5. 10.1111/jdi.12982. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95). Furuhashi M, Omori A, Matsumoto M, Kataoka Y, Tanaka M, Moniwa N, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T: Independent Link Between Levels of Proprotein Convertase Subtilisin/Kexin Type 9 and FABP4 in a General Population Without Medication. Am J Cardiol, 2016; 118: 198-203 [DOI] [PubMed] [Google Scholar]
- 96). Furuhashi M, Yuda S, Muranaka A, Kawamukai M, Matsumoto M, Tanaka M, Moniwa N, Ohnishi H, Saitoh S, Shimamoto K, Miura T: Circulating Fatty Acid-Binding Protein 4 Concentration Predicts the Progression of Carotid Atherosclerosis in a General Population Without Medication. Circ J, 2018; 82: 1121-1129 [DOI] [PubMed] [Google Scholar]
- 97). von Eynatten M, Breitling LP, Roos M, Baumann M, Rothenbacher D, Brenner H: Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler Thromb Vasc Biol, 2012; 32: 2327-2335 [DOI] [PubMed] [Google Scholar]
- 98). Chow WS, Tso AW, Xu A, Yuen MM, Fong CH, Lam TH, Lo SV, Tse HF, Woo YC, Yeung CY, Cheung BM, Lam KS: Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: a 12-year prospective study. J Am Heart Assoc, 2013; 2: e004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99). Liu G, Ding M, Chiuve SE, Rimm EB, Franks PW, Meigs JB, Hu FB, Sun Q: Plasma Levels of Fatty Acid-Binding Protein 4, Retinol-Binding Protein 4, High-Molecular-Weight Adiponectin, and Cardiovascular Mortality Among Men With Type 2 Diabetes: A 22-Year Prospective Study. Arterioscler Thromb Vasc Biol, 2016; 36: 2259-2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100). Szasz T, Webb RC: Perivascular adipose tissue: more than just structural support. Clin Sci (Lond), 2012; 122: 1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101). Numaguchi R, Furuhashi M, Matsumoto M, Sato H, Yanase Y, Kuroda Y, Harada R, Ito T, Higashiura Y, Koyama M, Tanaka M, Moniwa N, Nakamura M, Doi H, Miura T, Kawaharada N: Differential phenotypes in perivascular adipose tissue surrounding the internal thoracic artery and diseased coronary artery. J Am Heart Assoc, 2019; 8: e011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102). Vural B, Atalar F, Ciftci C, Demirkan A, Susleyici-Duman B, Gunay D, Akpinar B, Sagbas E, Ozbek U, Buyukdevrim AS: Presence of fatty-acid-binding protein 4 expression in human epicardial adipose tissue in metabolic syndrome. Cardiovasc Pathol, 2008; 17: 392-398 [DOI] [PubMed] [Google Scholar]
- 103). Elie A, Bloksgaard M, Sun WY, Yang K, Man AWC, Xu A, Irmukhamedov A, Riber LP, Wang Y, De Mey JGR: Local enrichment of fatty acid-binding protein 4 in the pericardial cavity of cardiovascular disease patients. PLoS One, 2018; 13: e0206802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104). Obokata M, Iso T, Ohyama Y, Sunaga H, Kawaguchi T, Matsui H, Iizuka T, Fukuda N, Takamatsu H, Koitabashi N, Funada R, Takama N, Kasama S, Kaneko Y, Yokoyama T, Murakami M, Kurabayashi M: Early increase in serum fatty acid binding protein 4 levels in patients with acute myocardial infarction. Eur Heart J Acute Cardiovasc Care, 2018; 7: 561-569 [DOI] [PubMed] [Google Scholar]
- 105). Karpisek M, Stejskal D, Kotolova H, Kollar P, Janoutova G, Ochmanova R, Cizek L, Horakova D, Yahia RB, Lichnovska R, Janout V: Treatment with atorvastatin reduces serum adipocyte-fatty acid binding protein value in patients with hyperlipidaemia. Eur J Clin Invest, 2007; 37: 637-642 [DOI] [PubMed] [Google Scholar]
- 106). Furuhashi M, Mita T, Moniwa N, Hoshina K, Ishimura S, Fuseya T, Watanabe Y, Yoshida H, Shimamoto K, Miura T: Angiotensin II receptor blockers decrease serum concentration of fatty acid-binding protein 4 in patients with hypertension. Hypertens Res, 2015; 38: 252-259 [DOI] [PubMed] [Google Scholar]
- 107). Cabre A, Lazaro I, Girona J, Manzanares JM, Marimon F, Plana N, Heras M, Masana L: Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis, 2007; 195: e150-158 [DOI] [PubMed] [Google Scholar]
- 108). Furuhashi M, Matsumoto M, Hiramitsu S, Omori A, Tanaka M, Moniwa N, Yoshida H, Ishii J, Miura T: Possible Increase in Serum FABP4 Level Despite Adiposity Reduction by Canagliflozin, an SGLT2 Inhibitor. PLoS One, 2016; 11: e0154482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109). Iso T, Maeda K, Hanaoka H, Suga T, Goto K, Syamsunarno MR, Hishiki T, Nagahata Y, Matsui H, Arai M, Yamaguchi A, Abumrad NA, Sano M, Suematsu M, Endo K, Hotamisligil GS, Kurabayashi M: Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler Thromb Vasc Biol, 2013; 33: 2549-2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110). Han Q, Yeung SC, Ip MSM, Mak JCW: Effects of intermittent hypoxia on A-/E-FABP expression in human aortic endothelial cells. Int J Cardiol, 2010; 145: 396-398 [DOI] [PubMed] [Google Scholar]
- 111). Hwang HH, Moon PG, Lee JE, Kim JG, Lee W, Ryu SH, Baek MC: Identification of the target proteins of rosiglitazone in 3T3-L1 adipocytes through proteomic analysis of cytosolic and secreted proteins. Mol Cells, 2011; 31: 239-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112). Tanaka M, Furuhashi M, Okazaki Y, Mita T, Fuseya T, Ohno K, Ishimura S, Yoshida H, Miura T: Ectopic expression of fatty acid-binding protein 4 in the glomerulus is associated with proteinuria and renal dysfunction. Nephron Clin Pract, 2014; 128: 345-351 [DOI] [PubMed] [Google Scholar]
- 113). Yao F, Li Z, Ehara T, Yang L, Wang D, Feng L, Zhang Y, Wang K, Shi Y, Duan H, Zhang L: Fatty Acid-Binding Protein 4 mediates apoptosis via endoplasmic reticulum stress in mesangial cells of diabetic nephropathy. Mol Cell Endocrinol, 2015; 411: 232-242 [DOI] [PubMed] [Google Scholar]
- 114). Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hikawa A, Hirano N, Hirata Y, Goto A, Omata M: Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med, 2004; 143: 23-30 [DOI] [PubMed] [Google Scholar]
- 115). Okazaki Y, Furuhashi M, Tanaka M, Mita T, Fuseya T, Ishimura S, Watanabe Y, Hoshina K, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T: Urinary excretion of fatty acid-binding protein 4 is associated with albuminuria and renal dysfunction. PLoS One, 2014; 9: e115429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116). Ghelfi E, Karaaslan C, Berkelhamer S, Akar S, Kozakewich H, Cataltepe S: Fatty acid-binding proteins and peribronchial angiogenesis in bronchopulmonary dysplasia. Am J Respir Cell Mol Biol, 2011; 45: 550-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117). Maver A, Medica I, Peterlin B: Search for sarcoidosis candidate genes by integration of data from genomic, transcriptomic and proteomic studies. Med Sci Monit, 2009; 15: SR22-28 [PubMed] [Google Scholar]
- 118). Shum BO, Mackay CR, Gorgun CZ, Frost MJ, Kumar RK, Hotamisligil GS, Rolph MS: The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J Clin Invest, 2006; 116: 2183-2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119). Ghelfi E, Yu CW, Elmasri H, Terwelp M, Lee CG, Bhandari V, Comhair SA, Erzurum SC, Hotamisligil GS, Elias JA, Cataltepe S: Fatty acid binding protein 4 regulates VEGF-induced airway angiogenesis and inflammation in a transgenic mouse model: implications for asthma. Am J Pathol, 2013; 182: 1425-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120). Ge XN, Bastan I, Dileepan M, Greenberg Y, Ha SG, Steen KA, Bernlohr DA, Rao SP, Sriramarao P: FABP4 regulates eosinophil recruitment and activation in allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol, 2018; 315: L227-L240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121). Duffy CM, Xu H, Nixon JP, Bernlohr DA, Butterick TA: Identification of a fatty acid binding protein4-UCP2 axis regulating microglial mediated neuroinflammation. Mol Cell Neurosci, 2017; 80: 52-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122). Saint-Geniez M, Ghelfi E, Liang X, Yu C, Spencer C, Abend S, Hotamisligil G, Cataltepe S: Fatty acid binding protein 4 deficiency protects against oxygen-induced retinopathy in mice. PLoS One, 2014; 9: e96253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123). Wang Q, Shi G, Teng Y, Li X, Xie J, Shen Q, Zhang C, Ni S, Tang Z: Successful reduction of inflammatory responses and arachidonic acid-cyclooxygenase 2 pathway in human pulmonary artery endothelial cells by silencing adipocyte fatty acid-binding protein. J Inflamm (Lond), 2017; 14: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124). Hu B, Guo Y, Garbacz WG, Jiang M, Xu M, Huang H, Tsung A, Billiar TR, Ramakrishnan SK, Shah YM, Lam KS, Huang M, Xie W: Fatty acid binding protein-4 (FABP4) is a hypoxia inducible gene that sensitizes mice to liver ischemia/reperfusion injury. J Hepatol, 2015; 63: 855-862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125). Scifres CM, Chen B, Nelson DM, Sadovsky Y: Fatty acid binding protein 4 regulates intracellular lipid accumulation in human trophoblasts. J Clin Endocrinol Metab, 2011; 96: E1083-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126). Nourani MR, Owada Y, Kitanaka N, Sakagami H, Hoshi H, Iwasa H, Spener F, Kondo H: Occurrence of immunoreactivity for adipocyte-type fatty acid binding protein in degenerating granulosa cells in atretic antral follicles of mouse ovary. J Mol Histol, 2005; 36: 491-497 [DOI] [PubMed] [Google Scholar]
- 127). Hu W, Qiao J: Expression and regulation of adipocyte fatty acid binding protein in granulosa cells and its relation with clinical characteristics of polycystic ovary syndrome. Endocrine, 2011; 40: 196-202 [DOI] [PubMed] [Google Scholar]
- 128). Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E: Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med, 2011; 17: 1498-1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129). Gharpure KM, Pradeep S, Sans M, Rupaimoole R, Ivan C, Wu SY, Bayraktar E, Nagaraja AS, Mangala LS, Zhang X, Haemmerle M, Hu W, Rodriguez-Aguayo C, McGuire M, Mak CSL, Chen X, Tran MA, Villar-Prados A, Pena GA, Kondetimmanahalli R, Nini R, Koppula P, Ram P, Liu J, Lopez-Berestein G, Baggerly K, L SE, Sood AK: FABP4 as a key determinant of metastatic potential of ovarian cancer. Nat Commun, 2018; 9: 2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130). Bennett JH, Shousha S, Puddle B, Athanasou NA: Immunohistochemical identification of tumours of adipocytic differentiation using an antibody to aP2 protein. J Clin Pathol, 1995; 48: 950-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131). Cataltepe O, Arikan MC, Ghelfi E, Karaaslan C, Ozsurekci Y, Dresser K, Li Y, Smith TW, Cataltepe S: Fatty acid binding protein 4 is expressed in distinct endothelial and non-endothelial cell populations in glioblastoma. Neuropathol Appl Neurobiol, 2012; 38: 400-410 [DOI] [PubMed] [Google Scholar]
- 132). Ohlsson G, Moreira JM, Gromov P, Sauter G, Celis JE: Loss of expression of the adipocyte-type fatty acid-binding protein (A-FABP) is associated with progression of human urothelial carcinomas. Mol Cell Proteomics, 2005; 4: 570-581 [DOI] [PubMed] [Google Scholar]
- 133). Kim S, Lee Y, Koo JS: Differential expression of lipid metabolism-related proteins in different breast cancer subtypes. PLoS One, 2015; 10: e0119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134). Cha YJ, Kim HM, Koo JS: Expression of Lipid Metabolism-Related Proteins Differs between Invasive Lobular Carcinoma and Invasive Ductal Carcinoma. Int J Mol Sci, 2017; 18: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135). Huang M, Narita S, Inoue T, Koizumi A, Saito M, Tsuruta H, Numakura K, Satoh S, Nanjo H, Sasaki T, Habuchi T: Fatty acid binding protein 4 enhances prostate cancer progression by upregulating matrix metalloproteinases and stromal cell cytokine production. Oncotarget, 2017; 8: 111780-111794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136). Thompson KJ, Austin RG, Nazari SS, Gersin KS, Iannitti DA, McKillop IH: Altered fatty acid-binding protein 4 (FABP4) expression and function in human and animal models of hepatocellular carcinoma. Liver Int, 2018; 38: 1074-1083 [DOI] [PubMed] [Google Scholar]
- 137). Zhong CQ, Zhang XP, Ma N, Zhang EB, Li JJ, Jiang YB, Gao YZ, Yuan YM, Lan SQ, Xie D, Cheng SQ: FABP4 suppresses proliferation and invasion of hepatocellular carcinoma cells and predicts a poor prognosis for hepatocellular carcinoma. Cancer Med, 2018; 7: 2629-2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138). Chiyonobu N, Shimada S, Akiyama Y, Mogushi K, Itoh M, Akahoshi K, Matsumura S, Ogawa K, Ono H, Mitsunori Y, Ban D, Kudo A, Arii S, Suganami T, Yamaoka S, Ogawa Y, Tanabe M, Tanaka S: Fatty Acid Binding Protein 4 (FABP4) Overexpression in Intratumoral Hepatic Stellate Cells within Hepatocellular Carcinoma with Metabolic Risk Factors. Am J Pathol, 2018; 188: 1213-1224 [DOI] [PubMed] [Google Scholar]
- 139). Lee D, Wada K, Taniguchi Y, Al-Shareef H, Masuda T, Usami Y, Aikawa T, Okura M, Kamisaki Y, Kogo M: Expression of fatty acid binding protein 4 is involved in the cell growth of oral squamous cell carcinoma. Oncol Rep, 2014; 31: 1116-1120 [DOI] [PubMed] [Google Scholar]
- 140). Jin J, Zhang Z, Zhang S, Chen X, Chen Z, Hu P, Wang J, Xie C: Fatty acid binding protein 4 promotes epithelial-mesenchymal transition in cervical squamous cell carcinoma through AKT/GSK3beta/Snail signaling pathway. Mol Cell Endocrinol, 2018; 461: 155-164 [DOI] [PubMed] [Google Scholar]
- 141). Tang Z, Shen Q, Xie H, Zhou X, Li J, Feng J, Liu H, Wang W, Zhang S, Ni S: Elevated expression of FABP3 and FABP4 cooperatively correlates with poor prognosis in non-small cell lung cancer (NSCLC). Oncotarget, 2016; 7: 46253-46262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142). Sulsky R, Magnin DR, Huang Y, Simpkins L, Taunk P, Patel M, Zhu Y, Stouch TR, Bassolino-Klimas D, Parker R, Harrity T, Stoffel R, Taylor DS, Lavoie TB, Kish K, Jacobson BL, Sheriff S, Adam LP, Ewing WR, Robl JA: Potent and selective biphenyl azole inhibitors of adipocyte fatty acid binding protein (aFABP). Bioorg Med Chem Lett, 2007; 17: 3511-3515 [DOI] [PubMed] [Google Scholar]
- 143). Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS: Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature, 2007; 447: 959-965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144). Hertzel AV, Hellberg K, Reynolds JM, Kruse AC, Juhlmann BE, Smith AJ, Sanders MA, Ohlendorf DH, Suttles J, Bernlohr DA: Identification and characterization of a small molecule inhibitor of Fatty Acid binding proteins. J Med Chem, 2009; 52: 6024-6031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145). Miao X, Wang Y, Wang W, Lv X, Wang M, Yin H: The mAb against adipocyte fatty acid-binding protein 2E4 attenuates the inflammation in the mouse model of high-fat diet-induced obesity via toll-like receptor 4 pathway. Mol Cell Endocrinol, 2015; 403: 1-9 [DOI] [PubMed] [Google Scholar]
- 146). Burak MF, Inouye KE, White A, Lee A, Tuncman G, Calay ES, Sekiya M, Tirosh A, Eguchi K, Birrane G, Lightwood D, Howells L, Odede G, Hailu H, West S, Garlish R, Neale H, Doyle C, Moore A, Hotamisligil GS: Development of a therapeutic monoclonal antibody that targets secreted fatty acid-binding protein aP2 to treat type 2 diabetes. Sci Transl Med, 2015; 7: 319ra205. [DOI] [PubMed] [Google Scholar]
- 147). Simpson MA, LiCata VJ, Ribarik Coe N, Bernlohr DA: Biochemical and biophysical analysis of the intracellular lipid binding proteins of adipocytes. Mol Cell Biochem, 1999; 192: 33-40 [PubMed] [Google Scholar]
- 148). Yu CW, Liang X, Lipsky S, Karaaslan C, Kozakewich H, Hotamisligil GS, Bischoff J, Cataltepe S: Dual role of fatty acid-binding protein 5 on endothelial cell fate: a potential link between lipid metabolism and angiogenic responses. Angiogenesis, 2016; 19: 95-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149). Feingold KR, Kazemi MR, Magra AL, McDonald CM, Chui LG, Shigenaga JK, Patzek SM, Chan ZW, Londos C, Grunfeld C: ADRP/ADFP and Mal1 expression are increased in macrophages treated with TLR agonists. Atherosclerosis, 2010; 209: 81-88 [DOI] [PubMed] [Google Scholar]
- 150). Maeda K, Uysal KT, Makowski L, Gorgun CZ, Atsumi G, Parker RA, Bruning J, Hertzel AV, Bernlohr DA, Hotamisligil GS: Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes, 2003; 52: 300- 307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151). Hertzel AV, Bennaars-Eiden A, Bernlohr DA: Increased lipolysis in transgenic animals overexpressing the epithelial fatty acid binding protein in adipose cells. J Lipid Res, 2002; 43: 2105-2111 [DOI] [PubMed] [Google Scholar]
- 152). Babaev VR, Runner RP, Fan D, Ding L, Zhang Y, Tao H, Erbay E, Gorgun CZ, Fazio S, Hotamisligil GS, Linton MF: Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptorgamma-regulated genes. Arterioscler Thromb Vasc Biol, 2011; 31: 1283-1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153). Yamamoto T, Furuhashi M, Sugaya T, Oikawa T, Matsumoto M, Funahashi Y, Matsukawa Y, Gotoh M, Miura T: Transcriptome and Metabolome Analyses in Exogenous FABP4- and FABP5-Treated Adipose-Derived Stem Cells. PLoS One, 2016; 11: e0167825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154). Yeung DC, Wang Y, Xu A, Cheung SC, Wat NM, Fong DY, Fong CH, Chau MT, Sham PC, Lam KS: Epidermal fatty-acid-binding protein: a new circulating biomarker associated with cardio-metabolic risk factors and carotid atherosclerosis. Eur Heart J, 2008; 29: 2156-2163 [DOI] [PubMed] [Google Scholar]
- 155). Furuhashi M, Ogura M, Matsumoto M, Yuda S, Muranaka A, Kawamukai M, Omori A, Tanaka M, Moniwa N, Ohnishi H, Saitoh S, Harada-Shiba M, Shimamoto K, Miura T: Serum FABP5 concentration is a potential biomarker for residual risk of atherosclerosis in relation to cholesterol efflux from macrophages. Sci Rep, 2017; 7: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156). Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, Minokoshi Y, Kahn BB, Parker RA, Hotamisligil GS: Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab, 2005; 1: 107-119 [DOI] [PubMed] [Google Scholar]
- 157). Cao H, Maeda K, Gorgun CZ, Kim HJ, Park SY, Shulman GI, Kim JK, Hotamisligil GS: Regulation of metabolic responses by adipocyte/macrophage Fatty Acidbinding proteins in leptin-deficient mice. Diabetes, 2006; 55: 1915-1922 [DOI] [PubMed] [Google Scholar]
- 158). Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS: Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation, 2004; 110: 1492-1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159). Anderson RM, Weindruch R: Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab, 2010; 21: 134-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160). Charles KN, Li MD, Engin F, Arruda AP, Inouye K, Hotamisligil GS: Uncoupling of Metabolic Health from Longevity through Genetic Alteration of Adipose Tissue Lipid-Binding Proteins. Cell Rep, 2017; 21: 393-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161). Syamsunarno MR, Iso T, Hanaoka H, Yamaguchi A, Obokata M, Koitabashi N, Goto K, Hishiki T, Nagahata Y, Matsui H, Sano M, Kobayashi M, Kikuchi O, Sasaki T, Maeda K, Murakami M, Kitamura T, Suematsu M, Tsushima Y, Endo K, Hotamisligil GS, Kurabayashi M: A critical role of fatty acid binding protein 4 and 5 (FABP4/5) in the systemic response to fasting. PLoS One, 2013; 8: e79386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162). Syamsunarno MR, Iso T, Yamaguchi A, Hanaoka H, Putri M, Obokata M, Sunaga H, Koitabashi N, Matsui H, Maeda K, Endo K, Tsushima Y, Yokoyama T, Kurabayashi M: Fatty acid binding protein 4 and 5 play a crucial role in thermogenesis under the conditions of fasting and cold stress. PLoS One, 2014; 9: e90825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163). Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS: Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell, 2008; 134: 933-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164). Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS: Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med, 2009; 15: 1383-1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165). Coleman SL, Park YK, Lee JY: Unsaturated fatty acids repress the expression of adipocyte fatty acid binding protein via the modulation of histone deacetylation in RAW 264.7 macrophages. Eur J Nutr, 2011; 50: 323-330 [DOI] [PubMed] [Google Scholar]
- 166). Cimen I, Kocaturk B, Koyuncu S, Tufanli O, Onat UI, Yildirim AD, Apaydin O, Demirsoy S, Aykut ZG, Nguyen UT, Watkins SM, Hotamisligil GS, Erbay E: Prevention of atherosclerosis by bioactive palmitoleate through suppression of organelle stress and inflammasome activation. Sci Transl Med, 2016; 8: 358ra126. [DOI] [PubMed] [Google Scholar]
- 167). Stefan N, Kantartzis K, Celebi N, Staiger H, Machann J, Schick F, Cegan A, Elcnerova M, Schleicher E, Fritsche A, Haring HU: Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care, 2010; 33: 405-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168). Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS: Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med, 2010; 153: 790-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169). Berger WT, Ralph BP, Kaczocha M, Sun J, Balius TE, Rizzo RC, Haj-Dahmane S, Ojima I, Deutsch DG: Targeting fatty acid binding protein (FABP) anandamide transporters - a novel strategy for development of antiinflammatory and anti-nociceptive drugs. PLoS One, 2012; 7: e50968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70). Wang YT, Liu CH, Zhu HL: Fatty acid binding protein (FABP) inhibitors: a patent review (2012–2015). Expert Opin Ther Pat, 2016; 26: 767-776 [DOI] [PubMed] [Google Scholar]


