Abstract.
This study was conducted to find the burden of dengue virus (DENV) and Japanese encephalitis virus (JEV) among children under the age of 13, who presented with acute encephalitis syndrome at Mandalay Children Hospital in Myanmar in 2013. Molecular and serological investigations were performed on 123 cerebrospinal fluid (CSF) samples collected from these patients. By neutralization tests and/or virus isolation, four (3.3%) JEV- and one DENV-associated encephalitis cases (0.8%) were confirmed. Antibody titer against JEV Genotype 3 was the highest among the laboratory-confirmed JEV cases. One strain of DENV-1 with Genotype 1 was isolated from the CSF sample of the dengue encephalitis patient; this was similar to the virus circulating in the study area and neighboring countries. This study shows that flaviviruses are important pathogens causing encephalitis in Myanmar. Active disease surveillance, vector control, and vaccination programs should be enforced to reduce the morbidity and mortality caused by flavivirus encephalitis.
INTRODUCTION
Myanmar is one of the countries in Southeast Asia, where tropical and subtropical viral infections particularly Japanese encephalitis (JE) and dengue (DEN) are endemic.1–3 Both Japanese Encephalitis virus (JEV) and Dengue viruses (DENV) which belong to the family Flaviviridae are carried by mosquito vectors that are very prevalent in Myanmar.1,4 Both flaviviruses can cause a wide range of clinical manifestations ranging from asymptomatic infection to severe disease encephalitis, a condition in which morbidity and mortality rates are high.5 Flaviviruses are one of the main etiological agents of acute encephalitis syndrome (AES), which is a public health problem in Asia.
Diagnosis of JE or DEN encephalitis at resource-limited settings is an issue due to the cross-reactivity of antibodies produced by infected patients against other members of the flaviviruses.6 Because Myanmar is one of the countries where both JEV and DENV are endemic, only neutralization test can confirm infection caused by each virus. To date, there has been no report concerning DEN encephalitis cases in Myanmar. This work was the first to confirm JE cases in Myanmar by using the gold standard neutralization tests. In Myanmar, JEV vaccine was added to the Expanded Program on Immunization starting from December 2017. The present study was conducted before the introduction of JEV vaccination program in Myanmar. This study explored the burden of JEV and DENV encephalitis among AES patients in Myanmar and determined the circulating genotype of JEV among children who sought medical treatment in a hospital.
MATERIALS AND METHODS
Samples.
Single cerebrospinal fluid (CSF) samples were obtained from 123 patients under the age of 13 diagnosed to have AES based on the clinical definition of the disease provided by the World Health Organization (WHO)–recommended standards for surveillance of selected vaccine-preventable diseases. The definition of AES included acute onset of fever along with at least one of the following: 1) change in mental status including symptoms such as confusion, disorientation, coma, or inability to talk; and 2) new onset of seizures (excluding simple febrile seizures).7 The patients were those admitted in the 550-bedded Mandalay Children Hospital from January to September 2013. Patients diagnosed with bacterial infection were excluded from this study. The study was approved by the Institutional Ethics Committee on Medical Research Involving Human Subjects under the Department of Medical Research (Upper Myanmar) (12/Ethics/DMRUM/2014).
ELISA and neutralization tests.
Cerebrospinal fluid samples were used to detect for the presence of anti-JEV IgM as well as anti-DENV IgM antibodies by the in-house IgM-capture ELISA (IgM ELISA),8 and for the presence of anti-flavivirus IgG by the in-house IgG indirect ELISA.9 In IgM ELISA, the result was considered positive if the P/N ratio was ≥ 2 at an optical density taken at 492 nm wavelength, where P was either the positive control or the sample and N as the negative control. IgG titers of patient serum samples were determined from a standard curve. Japanese encephalitis virus or DENV serotype-specific antibody titers were determined by focus reduction neutralization test (FRNT) with the following viruses: JEV JaOrS982 strain, 99St12A strain (DENV-1), 00St22A strain (DENV-2), SLMC 50 strain (DENV-3), and SLMC 318 strain (DENV-4).10 To determine the titers of neutralizing antibodies against different genotypes of JEV, plaque reduction neutralization test (PRNT) was carried out by using JaOrS982 strain for Genotype-3, Mie 41/2002 strain for Genotype 1, and Muar strain for Genotype 5, and the ratios of the neutralizing antibody titers of Genotype 3 against the other genotypes were calculated.11
Virus isolation, gene sequencing, and phylogenetic analysis.
Cerebrospinal fluid samples were inoculated in C6/36 E2 mosquito cells to isolate and propagate the virus.12 From the culture fluids of the infected cells, viral RNA was extracted by using QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany). The presence of the virus RNA gene was determined by using Ta Ka Ra one-step reverse transcriptase polymerase chain reaction (RT PCR) Kit (Ver 2), (TAKARA BIO, Shiga, Japan) together with the DENV- or JEV-specific primers.13 Gene sequencing was performed and the data were analyzed by ABI Prism™ Capillary Sequencer 3130-Avant Genetic Analyzer (ThermoFisher Scientific, Waltham, MA). The maximum likelihood method using PHYML 3.0.114 was used to construct phylogenetic trees based on the full E gene region of the DENV strains isolated in this study and of the strains previously isolated in Myanmar, its neighboring countries and other regions of the world. The substitution model was selected by jmodeltest-2.1.10 and general time reversible model + I was chosen as the model. Trees were drawn by FigTree software, version 1.4.3 (FigTree).14 The sequences of E gene region of the DENV were submitted to GenBank (GenBank accession no. MF683399).
Criteria for confirming JEV and DENV infection.
Following the CDC guidelines for the laboratory confirmation of JEV infection, infection was confirmed when a CSF sample was not only positive by JEV IgM capture ELISA, but also by the presence of neutralizing antibodies against JEV at a titer > 1:10 and at a titer 4-fold higher than that against other flaviviruses.15
Laboratory confirmation of DENV infection was determined either by virus isolation or by monotypic antibody response (primary infection), which was defined as FRNT50 > 10 to only one serotype, or FRNT50 > 10 to more than one serotype but ≥ 80 to only one serotype. Multitypic antibody response (secondary infection) was defined as FRNT50 > 10 to more than one serotype without an FRNT50 ≥ 80 to only one serotype.10
RESULTS
ELISA and neutralization tests.
Five (4.1%) of 123 CSF samples from AES patients were positive by JEV IgM ELISA and two (1.6%; CSF-37, CSF-61) by DENV IgM ELISA (Table 1; JEV IgM–positive sample CSF-47 not shown in the table). The two positives by DENV IgM ELISA were also positive by JEV IgM ELISA and part of the five samples mentioned previously. The P/N ratios of these two samples were higher in the JEV IgM ELISA than in the DENV IgM ELISA. Seven CSF samples (5.3%; three samples are included in Table 1) were anti flavivirus IgG positive. All patients survived and were discharged from the hospital without any morbidities.
Table 1.
Identification of DENV and JEV in CSF samples from acute encephalitis syndrome cases in Myanmar by FRNT50, and virus isolation
| Sample ID | Age (year) | Gender | Day of collection | P/N ratio (Cut off = 2) | FRNT50 | Virus isolation | Diagnosis interpretation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JEV IgM | DENV IgM | Flavi IgG titer | JEV | DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||||||
| CSF-35 | 3 | F | 4 | 3.7 | 1.2 | 8 | 40 | < 10 | < 10 | < 10 | < 10 | neg | JEV |
| CSF-37 | 9 | F | 6 | 7.8 | 3 | 13,604 | 20 | < 10 | < 10 | < 10 | < 10 | neg | JEV |
| CSF-42 | 0.6 | M | 7 | 10.4 | 1.8 | 9 | 80 | < 10 | < 10 | < 10 | < 10 | neg | JEV |
| CSF-61 | 10 | M | 7 | 19.2 | 11.4 | 42,470 | 140 | 20 | 40 | < 10 | 20 | neg | JEV |
| CSF-122 | 1.5 | M | 2 | 1.6 | 1.8 | 516 | ND | ND | ND | ND | ND | pos | DENV-1 |
CSF = cerebrospinal fluid; DENV-1 = dengue virus serotype-1; DENV-2 = dengue virus serotype-2; FRNT50 = 50% focus reduction neutralization test; JEV = Japanese encephalitis virus; pos = positive; neg = negative; ND = not done (shortage of CSF amount); F = female; M = male. Day of collection: the interval between disease onset and sample collection.
Confirmation of JEV and DENV encephalitis cases by FRNT method and virus isolation.
As stated previously five of 123 CSF samples form AES patients were positive by JEV IgM ELISA; however, only four of the five were confirmed positive by neutralization test (FRNT50). The sample not confirmed was CSF-47 (not shown in Table 1) because it had a neutralizing antibody titer against JEV below the detection limit. Its neutralizing antibody titer against DENV was also below the detection limit. For CSF-122 which gave negative result by DENV IgM ELISA, although its neutralization test was not carried out because of volume limitation, this sample was positive by virus isolation in host cells (Table 1). The virus was identified as DENV-1 by RT-PCR of the RNA extract from the culture fluid of the infected cells. Therefore, a total of four cases of JEV (3.3%) and one case of DENV encephalitis (0.8%) were confirmed among AES patients in this study. The remaining AES cases could be due to other causative agents of AES such as enterovirus, herpes virus, coxsackie virus, etc.
Neutralizing antibody against different genotypes of JEV.
Because of volume limitation of one sample, only three of the four samples confirmed by FRNT were subjected to PRNT for the determination of neutralizing antibody titers against different genotypes of JEV. The neutralizing antibody titers of the three samples against Genotype 3 were higher than those against Genotype 1 or 5 (Table 2), and the ratios of the neutralizing antibody titers between against Genotype 3 (JaOrS982) and Genotype 1 or 5 were four, eight and two times higher for samples CSF-35, CSF-42, and CSF-37, respectively.
Table 2.
Neutralizing antibody titers of CSF samples determined against three different genotypes of JEV
| Sample ID | JEV strains | Genotype 3:1* | Genotype 3:5* | ||
|---|---|---|---|---|---|
| Genotype-1 | Genotype-3 | Genotype-5 | |||
| (Mie) | (JaOrS982) | (Muar) | |||
| CSF-35 | < 10 | 40 | < 10 | 4:1 | 4:1 |
| CSF-37 | < 10 | 20 | < 10 | 2:1 | 2:1 |
| CSF-42 | < 10 | 80 | < 10 | 8:1 | 8:1 |
CSF = cerebrospinal fluid; JEV = Japanese encephalitis virus.
* Ratio was calculated based on JEV JaOrS982 strain (Genotype 3).
Phylogenetic analysis of the isolated DENV.
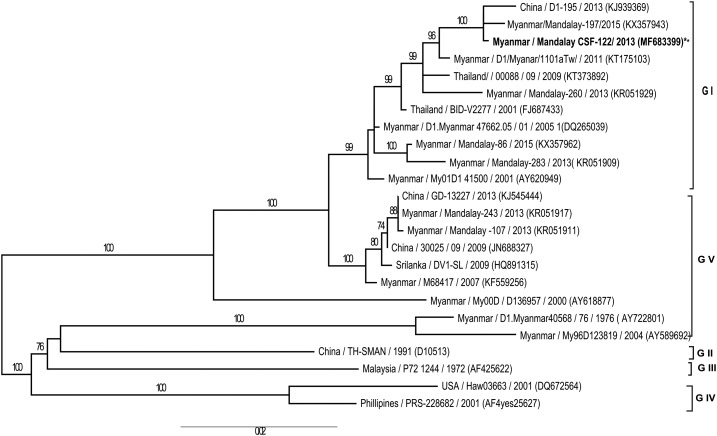
Phylogenetic analysis of the full coding region of E protein gene of the DENV-1 strain isolated in this study together with those of other DENV-1 strains showed that our virus isolate belonged to Genotype 1 (Figure 1) and it was of the same clade of DENV-1 strains circulating in the same area in Myanmar within the same period of our collection of CSF samples. Comparison of amino acids for the full coding region of envelope proteins of the DENV-1 isolated from the AES patient and the DENV-1 isolated from non-AES patient at the same period showed 99–100% amino acid similarity. Moreover, the DENV strain isolated from CSF was closely related to the strains circulating in other countries such as China, Thailand, and Sri Lanka (Figure 1).
Figure 1.
Phylogenetic tree was constructed based on the whole nucleotide sequences of the E protein gene of DENV-1 showing the relationship of 24 strains from different sources, including viral strain of DENV-1 isolated from the cerebrospinal fluid sample of encephalitis patient in this study. The representative strains of each genotype obtained from GenBank are named by country origin, strain name, year of isolation and GenBank accession number. DENV-1 = dengue virus serotype-1.
DISCUSSION
In Myanmar, there is an average of 25 JE cases reported per year.3 Based on this routine surveillance, the total number of cases was 17 in 2013, which did not include the findings of our study conducted in the Mandalay Region.3 Thus, active surveillance of JEV infection should be conducted so as not to miss JE cases in the national AES surveillance system. Previous studies in Myanmar reported the isolation of JEV from piglets16,17 and the presence of the vector Culex mosquitoes.18 In this country, JEV vaccination program was introduced to children under 15 years starting from December 2017. Therefore, JEV vaccination must be scaled up to reduce the morbidity and mortality caused by JEV.
According to the JEV surveillance of piglets in Myanmar, JEV Genotypes 3 and 1 were isolated from samples collected in 2009 and 2010.16,17 These studies proved that both Genotypes 1 and 3 were circulating in Myanmar. Therefore, in this study, the neutralizing antibody data against different genotypes of JEV supported the findings of previous studies17 and the PRNT against different genotypes of JEV was useful in identifying the circulating genotypes of JEV.
Among the laboratory-confirmed JE cases in this study, some cases showed positive results by both JEV IgM ELISA and DENV IgM ELISA. It could be due to the cross reactivity of the antibodies of the patient CSF to the ELISA assay antigens (either from the JEV or DENV), which could react against antibodies from clinical samples of a patient infected by any flavivirus. The results of JEV and DENV IgM ELISAs did not create a problem in establishing the infecting pathogens of the patients in this study because the results gave a higher antibody titer against the infecting virus and the neutralization test provided the confirmation. According to WHO AES surveillance guideline, if anti JEV IgM antibody level is detected from a single CSF sample, it is considered as laboratory-confirmed JE case.19 Therefore, this study supported the use of IgM capture ELISA as the diagnostic test for JEV infection in this study area. Furthermore, the detection of IgM against JEV in the CSF indicated recent viral infection of the patient.20
Only one case of DEN encephalitis (0.8%) was detected in this study. A strain of DENV-1 was isolated from the CSF sample in this case; however, anti-DENV IgM antibodies were not detected in the sample because the sample was collected on day 2 after the onset of symptoms. The absence of the DENV IgM antibodies during the acute phase of the illness at which period the patient was still at the viremic phase made the virus isolation possible.21 DEN encephalopathy is a well-recognized and common entity with the incidence ranging from 0.5 to 6.2 %.22 Moreover, one study in mice reported that mutant DENV-1 infection can cause extensive encephalitis.23
In this study, the number of positive cases was small. We collected CSF samples within 7 days after the onset of symptoms but we failed to isolate JEV from these samples. Infected culture fluid (ICF) of cells inoculated with the patient CSF samples gave negative results by JEV RT-PCR. It may probably be because of the short transient viremia phase of JEV in human beings and we failed to collect samples during this phase.24 Moreover, because we collected samples at the early phase, it was possible that some patients had not yet produced detectable levels of IgM antibodies at the time of sample collection. On the other hand, one patient (with sample CSF-47) showed positive result by JEV IgM ELISA but the antibody neutralization titer was below the detection limit (< 1:10). We diagnosed JEV infection for this patient as probable and did not consider it as laboratory confirmed. A limitation of this study was that only single CSF samples were collected and that we did not check the virus gene directly from these samples by molecular techniques because of limited sample volume.
In conclusion, this study described the incidence of laboratory-confirmed JEV and DENV encephalitis cases among patients presenting with AES in Mandalay, Myanmar. Not only JEV, but also DENV was an important pathogen for causing AES in Myanmar. Therefore, early diagnosis, effective treatment, and prevention strategies should be emphasized to reduce the burden of this disease.
Acknowledgment:
We would like to thank all of the staff at the Department of Virology, Institute of Tropical Medicine, Nagasaki University, Virology Research Division of the Department of Medical Research (Pyin Oo Lwin Branch) for all of the help extended.
REFERENCES
- 1.Swe T, Thein S, Myint MS, 1979. Pilot sero-epidemiological survey on Japanese encephalitis in north-western Burma. Biken J 22: 125–129. [PubMed] [Google Scholar]
- 2.Thu HM, Lowry K, Myint TT, Shwe TN, Han AM, Khin KK, Thant KZ, Thein S, Aaskov J, 2004. Myanmar dengue outbreak associated with displacement of serotypes 2, 3, and 4 by dengue 1. Emerg Infect Dis 10: 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mon Oo P, Hlaing T, Lwin S, Pittyawonganon C, Srichasinthop J, Khine SK, 2016. A large outbreak of Japanese encephalitis in Rakhine state, Myanmar: implication for vaccine policy. Outbreak, Surveill Invest Rep 9: 8–15. [Google Scholar]
- 4.Thein S, Aung H, Sebastian AA, 1988. Study of vector, amplifier, and human infection with Japanese encephalitis virus in a Rangoon community. Am J Epidemiol 128: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 5.Thisyakorn U, Thisyakorn C, Limpitkul W, Nisalak A, 1999. Dengue infection with central nervous system manifestation. Southeast Asian J Trop Med Public Health 30: 504–506. [PubMed] [Google Scholar]
- 6.A-Nuegoonpipat A, Panthuyosri N, Anantapreecha S, Chanama S, Sa-Ngasang A, Sawanpanyalert P, Kurane I, 2008. Cross-reactive IgM responses in patients with dengue or Japanese encephalitis. J Clin Virol 42: 75–77. [DOI] [PubMed] [Google Scholar]
- 7.Biologicals DoVa , 2008. WHO-Recommended Standards for Surveillance of Selected Vaccine-Preventable Diseases Guideline. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 8.Bundo K, Igarashi A, 1985. Antibody-capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients. J Virol Methods 11: 15–22. [DOI] [PubMed] [Google Scholar]
- 9.Inoue S, et al. 2010. Evaluation of a dengue IgG indirect enzyme-linked immunosorbent assay and a Japanese encephalitis IgG indirect enzyme-linked immunosorbent assay for diagnosis of secondary dengue virus infection. Vector Borne Zoonotic Dis 10: 143–150. [DOI] [PubMed] [Google Scholar]
- 10.Ngwe Tun MM, et al. 2013. Serological characterization of dengue virus infections observed among dengue hemorrhagic fever/dengue shock syndrome cases in upper Myanmar. J Med Virol 85: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 11.Maeki T, et al. 2018. Comparison of neutralizing antibody titers against Japanese encephalitis virus genotype V strain with those against genotype I and III strains in the sera of Japanese encephalitis patients in Japan in 2016. Jpn J Infect Dis 71: 360–364. [DOI] [PubMed] [Google Scholar]
- 12.Igarashi A, 1978. Isolation of a Singh’s Aedes albopictus cell clone sensitive to dengue and chikungunya viruses. J Gen Virol 40: 531–544. [DOI] [PubMed] [Google Scholar]
- 13.Morita K, Tanaka M, Igarashi A, 1991. Rapid identification of dengue virus serotypes by using polymerase chain reaction. J Clin Microbiol 29: 2107–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O, 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59: 307–321. [DOI] [PubMed] [Google Scholar]
- 15.Robinson JS, Featherstone D, Vasanthapuram R, Biggerstaff BJ, Desai A, Ramamurty N, Chowdhury AH, Sandhu HS, Cavallaro KF, Johnson BW, 2010. Evaluation of three commercially available Japanese encephalitis virus IgM enzyme-linked immunosorbent assays. Am J Trop Med Hyg 83: 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latt AZ, Thu HM, Aye KT, Aye KS, Oo KK, Thu HM, Yoksan S, 2011. Isolation and identification of Japanese encephalitis virus from piglets in Dike Oo pig farm. Myanmar Health Sci Res J 23: 21–25. [Google Scholar]
- 17.Latt AZ, Aye KM, Win HM, Aung KM, Oo KK, Thu HM, Win MM, Thu HM, Yoksan S, 2015. Isolation and identification of Japanese encephalitis virus from piglets in Thakata township, Yangon. Myanmar Health Sci Res J 27: 1–6. [Google Scholar]
- 18.Kyaw Kyaw A, et al. 2018. Isolation and genomic characterization of Culex flaviviruses from mosquitoes in Myanmar. Virus Res 247: 120–124. [DOI] [PubMed] [Google Scholar]
- 19.Hills S, Dabbagh A, Jacobson J, Marfin A, Featherstone D, Hombach J, Namgyal P, Rani M, Solomon T, 2009. Evidence and rationale for the World Health Organization recommended standards for Japanese encephalitis surveillance. BMC Infect Dis 9: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharief MK, Thompson EJ, 1989. Immunoglobulin M in the cerebrospinal fluid: an indicator of recent immunological stimulation. J Neurol Neurosurg Psychiatry 52: 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarman RG, Nisalak A, Anderson KB, Klungthong C, Thaisomboonsuk B, Kaneechit W, Kalayanarooj S, Gibbons RV, 2011. Factors influencing dengue virus isolation by C6/36 cell culture and mosquito inoculation of nested PCR-positive clinical samples. Am J Trop Med Hyg 84: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra UK, Kalita J, Syam UK, Dhole TN, 2006. Neurological manifestations of dengue virus infection. J Neurol Sci 244: 117–122. [DOI] [PubMed] [Google Scholar]
- 23.Bordignon J, Strottmann DM, Mosimann AL, Probst CM, Stella V, Noronha L, Zanata SM, Dos Santos CN, 2007. Dengue neurovirulence in mice: identification of molecular signatures in the E and NS3 helicase domains. J Med Virol 79: 1506–1517. [DOI] [PubMed] [Google Scholar]
- 24.Solomon T, 2004. Flavivirus encephalitis. N Engl J Med 351: 370–378. [DOI] [PubMed] [Google Scholar]