Abstract.
This study aimed to assess baseline knowledge, attitudes, and practices about severe fever with thrombocytopenia syndrome (SFTS) and identify the target population for health education programs in endemic areas of Anhui, China. This cross-sectional study was conducted from May to June 2017. Of 752 participants, 383 (50.9%) were from Nanqiao District, 397 (52.8%) were female, and 430 (57.2%) were farmers; 37.4% had heard about SFTS, but knowledge of symptoms and signs including fever (34.2%), leukopenia (8.0%), and thrombocytopenia (10.1%) was low. Only 12.1% knew that SFTS virus is transmitted by ticks, 9.4% realized that the blood and body fluid of SFTS are infectious, and only 38.2% thought that the tick should be paralyzed using medical alcohol or iodine. Meanwhile, 61.3% wore long-sleeve clothes, whereas 20.2% used repellents. Median scores for knowledge, attitudes, and practices, and the total score were 4.0, 6.0, 5.0, and 16.0, respectively. Knowledge was influenced by region (OR = 0.632, 95% CI: 0.399–0.999), education (OR = 0.516, 95% CI: 0.434–0.612), gender (OR = 1.865, 95% CI: 1.165–2.987), and age (OR = 3.406, 95% CI: 2.345–4.947). Education was a predictor of lack of appreciation of infection risk (OR = 0.519, 95% CI: 0.449–0.599) and practice (OR = 0.481, 95% CI: 0.396–0.584). Our findings indicate that SFTS-related health education programs are required for females; participants from Qianshan Prefecture; those with an occupation of farmer, retiree, houseworker, or unemployed; elderly participants; and those with low education. Large-scale sustainable health education programs focusing on the target populations are urgently needed in endemic areas.
INTRODUCTION
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a novel virus of the genus Phlebovirus, family Bunyaviridae, which was first isolated from blood specimens of Chinese patients in 2010.1 Subsequently, cases of severe fever with thrombocytopenia syndrome (SFTS) were reported in Japan and South Korea.2,3 The early clinical symptoms of SFTSV infection include fever, weakness, diarrhea, nausea, and vomiting; and biochemical indicators have revealed leukopenia, thrombocytopenia, and increases in aspartic acid, alanine aminotransferase, creatine kinase, lactate dehydrogenase, urea nitrogen, and plasma-thrombin time.4–6 Aggravated thrombocytopenia and plasma-thrombin time prolongation have caused bleeding symptoms in patients, including bleeding gums, epistaxis, skin ecchymosis, hematuria, and gastrointestinal tract hemorrhage.7 Although timely treatment results in good prognosis in most SFTS patients, some severely affected patients die of shock, respiratory failure, disseminated intravascular coagulation, and multiple organ failures.
Severe fever with thrombocytopenia syndrome incidence has rapidly increased in China since the presence of the virus was confirmed, and according to the China Information System for Disease Control and Prevention (CISDCP), the reported cases during 2010–2016 were distributed across 23 provinces.8,9 The cases were mainly reported in rural areas of the eastern, central, and northeastern China, with a fatality rate of 10.0–16.0%.6,9,10 In previous studies, various domesticated animals, including sheep, cattle, goats, and pigs, tested seropositive for SFTSV.11–14 Furthermore, ticks, especially Haemaphysalis longicornis ticks, were the major transmission vectors of SFTSV, based on homology sequence analysis of virus nucleic acids and epidemiological studies.15,16 Although reported cases were mainly sporadic, some clusters have also been reported, and it has been proven that the blood, body fluid, and vomitus of patients with SFTS are infectious, and the possibility of nosocomial infection should not be neglected.17–20
The Guidelines and Recommendations for Preventing and Controlling SFTSV Infection (http://www.moh.gov.cn/mohwsyjbgs/s8348/201010/49272.shtml) for endemic areas were developed in 2010. They attempt to provide public health measures for farmers, loggers, tea pickers, and other field-workers in endemic areas. The main important measures are as follows: 1) proper infection control measures should be strengthened for SFTS patients to reduce the risk of person-to-person transmission; 2) improvement in the early detection, identification, and treatment of SFTS by medical workers and public health officers; 3) gradual increase in SFTSV detection ability, actioned by the Centers for Disease Control and Prevention (CDC) in prevalent districts; 4) improvement in self-protection awareness through tick-bite health education programs; and 5) implementation of transmission vector control measures in endemic areas to reduce tick density and infection risk.
The targeted antiviral drugs and vaccines against SFTSV infection are still under development. For this reason, health education is considered the most effective measure for preventing SFTSV infection. There are numerous studies on SFTS, but studies about community knowledge and perception are lacking. In this study, we investigated the knowledge, attitudes, and practices (KAP) of community members in endemic areas in Anhui Province, eastern China. The objective was to assess baseline KAP levels and determine the target population of SFTS-related health education in endemic areas. Our findings could provide useful information for developing public health strategies for SFTS prevention and control, especially for determining health education content and the target population.
METHODS
Study design.
This is a community-based cross-sectional study conducted in endemic areas of Anhui Province from May to June 2017.
Study sample size.
The sample size for cross-sectional studies was used to calculate the sample size, where Z = 1.96 (the standard normal score at the 95% CI), P = 0.351 (the expected knowledge rate was evaluated as 35.1% from a pilot survey involving 66 participants in a village of Qianshan Prefecture), and d = 0.1 × P (an acceptance error). The sample size was increased by 5% to compensate for inevitable information loss during data collection due to missing, incorrect, or incomplete questionnaires. Consequently, the minimum required sample size was 746 participants.
Setting and participants.
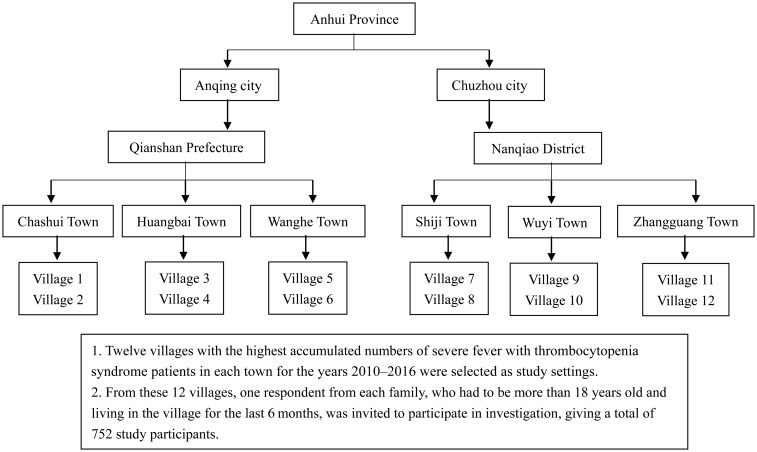
A four-stage sampling technique was used to select the settings for the study. First, we selected the two cities with the highest incidences of endemic SFTSV infection for the years 2010–2016 according to the CISDCP (http://www.cdpc.chinacdc.cn). Second, we selected the prefecture with the highest incidence of endemic SFTSV infection for each of the two selected cities. Third, we selected the three towns with the highest rates of endemic SFTSV infection in each of the selected prefectures. Finally, we selected the two villages with the highest accumulated numbers of SFTSV infection cases in each of the selected towns. Therefore, a total number of 12 villages were selected as the settings for this study (Figure 1). From these 12 villages, one respondent from each family, who had to be more than 18 years old and living in the village for the last 6 months, was invited to participate in our investigation, giving a total of 752 study participants.
Figure 1.
Sampling diagram for the cross-sectional knowledge, attitudes, and practices study.
Questionnaire and data collection.
To collect data, we used a structured questionnaire that was formulated and revised based on a validated questionnaire of epidemiological investigation and on “The Diagnosis and Treatment Programs of SFTSV Syndrome” (http://www.moh.gov.cn/mohwsyjbgs/s8348/201010/49272.shtml). The questionnaire contained four parts, namely, questions concerning 1) demographic characteristics such as age, gender, occupation, and educational level; 2) the participant’s level of SFTS knowledge, including knowledge of its symptoms and signs, and transmission routes; 3) the participant’s attitudes toward SFTS, such as the willingness to participate in screening, if provided free of charge; and 4) the participant’s practices in relation to SFTS. The validity of the second, third, and fourth parts was confirmed by a Cronbach’s alpha (α, internal consistency coefficient) of 0.79 from the pilot survey involving 66 participants in a village of Qianshan Prefecture.
Following the results of the pilot survey and discussion among our research team, we decided to complete the questionnaire using interviews rather than in the form of a self-administered questionnaire. This was because some questions required explanation to the participants by trained investigators and because the participants had low educational levels. Two types of locations were used for the interviews in our study. First, most participants, including farmers, commercial service personnel, retirees, and houseworkers or those who were unemployed, were interviewed at their house. Second, participants who were medical staff, teacher, community leaders, and workers were interviewed at their workplace. The participants were given the right to refuse to participate in the study or to withdraw from it at any time. All investigators were trainees of the Anhui Provincial Field Epidemiology Training Program and participated in a systematic training course before starting the investigation. Furthermore, senior researchers were selected to monitor the process and examine the quality of investigation during data collection.
Scoring.
Correct or incorrect responses to the questions were scored as 1 or 0, respectively. Responses of “No,” “Maybe,” and “Yes” were scored as 0, 1, and 2, respectively. The maximum scores for KAP per participant were 14, 8, and 8, respectively. P90 values of KAP scores were calculated, and scores equal to and greater than the P90 value were defined as good, whereas those less than the P90 value were defined as poor.
Statistical analysis.
EpiData software, version 3.1 (a freeware distributed by EpiData Association, Odense, Denmark, available for download at: http://www.epidata.dk/download.php) was used. Data were entered twice independently, and a consistency check was performed to eliminate any incorrect entry. Statistical analysis was performed using SPSS software, version 11.0 (SPSS, Chicago, IL). Descriptive analyses were performed on demographic features and KAP responses, which were described as frequencies and percentages. Abnormally distributed data including KAP scores were expressed as medians and interquartile ranges. The nonparametric methods, namely, the Mann–Whitney U-test and the Kruskal–Wallis H-test, were used to compare KAP scores and categories of demographic characteristics. Spearman’s correlation was used to analyze correlations between KAP scores and demographic characteristics, knowledge–attitude, knowledge–practice, and attitude–practice. Moreover, the binary logistic regression method was used to analyze predictors of poor KAP scores, followed by the “Enter” method of univariate analysis. Predictors with P < 0.1 were further recruited and analyzed using forward stepwise multivariate analysis. The significance level (α) was set at 0.05.
Ethical statement.
Ethical approval was obtained from the Ethics Committee of Anhui Provincial CDC, and written informed consent was obtained from all participants before data collection. Confidentiality and privacy were maintained throughout the study.
RESULTS
Demographic features.
Table 1 shows the demographic features of the study participants. Of the 752 study participants, 383 (50.9%) were from Nanqiao District, 397 (52.8%) were female, and 430 (57.2%) were farmers. The median age was 55.0 years, and 45.5% of participants were 50–69 years old. Regarding educational levels, 28.6% of participants were illiterate, more than 50.0% had primary and junior high school education, and nearly 10.0% had college/university education.
Table 1.
Demographic features of the study participants
| Demographic features | Participants (N = 752) | |
|---|---|---|
| n | % | |
| Region | ||
| Qianshan Prefecture | 369 | 49.1 |
| Nanqiao District | 383 | 50.9 |
| Gender | ||
| Male | 355 | 47.2 |
| Female | 397 | 52.8 |
| Age group (years) | ||
| 19–49 | 278 | 37.0 |
| 50–69 | 342 | 45.5 |
| ≥ 70 | 132 | 17.5 |
| Occupation | ||
| Farmer | 430 | 57.2 |
| Commercial service personnel | 66 | 8.7 |
| Medical staff, teacher, community leader, and worker | 134 | 17.8 |
| Retiree | 20 | 2.7 |
| Houseworker or unemployed | 102 | 13.6 |
| Educational level | ||
| No formal education | 215 | 28.6 |
| 1st–6th grade | 193 | 25.7 |
| 7th–9th grade | 196 | 26.1 |
| 10th–12th grade | 81 | 10.7 |
| College/university | 67 | 8.9 |
| Frequency of fieldwork during the last month | ||
| Never | 238 | 31.6 |
| Occasionally (1–9 days) | 196 | 26.1 |
| Often (10–19 days) | 140 | 18.6 |
| Usually (≥ 20 days) | 178 | 23.7 |
Knowledge, attitudes, and practices responses for SFTS.
Knowledge, attitudes, and practices responses toward SFTS are shown in Table 2. Although 37.4% of participants responded that they had heard about SFTS, knowledge of symptoms and signs, treatment, vaccines, and transmission routes was low. Knowledge of transmission routes was especially low (5.7–12.1%), whereas less than 35.0% of participants provided correct answers regarding SFTS symptoms and signs, including fever (34.2%), leukopenia (8.0%), and thrombocytopenia (10.1%). Approximately 20% of participants knew that SFTS is curable, whereas only 12.0% knew that it is infectious. Regarding attitudes, nearly 40.0% of participants thought that ticks should be paralyzed with medical alcohol or iodine, whereas almost 30.0% thought of directly crushing the tick to death. Most participants (72.3%) held the attitude of visiting a doctor immediately, whereas 6.9% held an attitude of self-medicating following a tick bite and the development of symptoms and signs. Although more than 80.0% of participants said that they would participate in a consultation, the willingness they expressed to participate in a screening, if provided free of charge (69.7%), was lower. More than 60.0% of participants wore long-sleeve clothes, whereas only 20.2% used repellents when working in the field. Furthermore, approximately 80.0% of respondents regularly cleared weeds around the house to reduce tick habitats, 41.5% took protective measures when coming into contact with livestock, and nearly 60.0% removed ticks from livestock.
Table 2.
Severe fever with thrombocytopenia syndrome knowledge, attitudes, and practices responses of the study participants
| Response | Participants (N = 752) | |
|---|---|---|
| n* | % | |
| Knowledge | ||
| Did you hear about SFTS? | 281 | 37.4 |
| Symptoms and signs of SFTS | ||
| Fever | 257 | 34.2 |
| Weakness, headache, and muscle soreness | 160 | 21.3 |
| Nausea, vomiting, anorexia, and diarrhea | 83 | 11.0 |
| Cough and pharyngalgia | 64 | 8.5 |
| Leukopenia | 60 | 8.0 |
| Thrombocytopenia | 76 | 10.1 |
| Can SFTS patients be cured? | 135 | 18.0 |
| Are there vaccines against severe fever with thrombocytopenia syndrome virus? | 104 | 13.8 |
| Can SFTS patients infect others? | 86 | 11.4 |
| Route of transmission | ||
| Living with an SFTS patient | 43 | 5.7 |
| Contact with or talking to a SFTS patient | 45 | 6.0 |
| Direct contact with blood and/or body fluid directly | 71 | 9.4 |
| Tick bite | 91 | 12.1 |
| Attitude | ||
| What do you think someone should do if he or she is bitten by a tick? | ||
| Paralyze the tick with medical alcohol or iodine and remove it | 287 | 38.2 |
| Crush the tick to death and remove it | 209 | 27.8 |
| What would your advice be if someone was bitten by a tick and developed symptoms (fever, headache, vomiting, diarrhea, etc.)? | ||
| Visit a doctor immediately | 544 | 72.3 |
| Self-medication | 52 | 6.9 |
| Would you consult SFTS-related information provided in lectures or informational campaigns conducted in your community? | 621 | 82.6 |
| Would you participate in SFTS-related free detection offered in your community? | 524 | 69.7 |
| Practice | ||
| Which protective measures have you taken when working in the field? | ||
| Wearing long-sleeve clothes and fastening cuffs and collars | 461 | 61.3 |
| Using repellents | 152 | 20.2 |
| Which have you performed when coming into contact with livestock (sheep, pigs, cattle, etc.)? | ||
| Taking protective measures (gloves, hat, etc.) | 312 | 41.5 |
| Removing ticks from livestock | 449 | 59.7 |
| Regularly cleared weeds around the house to reduce tick habitats | 588 | 78.2 |
SFTS = severe fever with thrombocytopenia syndrome.
* The participants responded with “Yes.”
Knowledge, attitudes, and practices scores for SFTS.
Knowledge, attitudes, and practices scores and comparisons between demographic features are given in Table 3. The median scores for knowledge, attitudes, and practices, and the total score of the study participants were 4.0, 6.0, 5.0, and 16.0, respectively. Compared with other groups, the following groups had the lowest scores for knowledge: female participants (Z = −2.214, P = 0.027); participants from the Qianshan Prefecture (Z = −3.147, P = 0.002); farmer, retiree, houseworker, or unemployed participants (x2 = 73.231, P < 0.01); participants aged older than 70 years (x2 = 61.823, P < 0.01); and participants with no formal education (x2 = 91.322, P < 0.01). Knowledge score showed an upward trend with increasing educational level (rs = 0.342, P < 0.01) and a downtrend with increasing age (rs = −0.307, P < 0.01). The results were similar for attitude score (rs = 0.406, P < 0.01; rs = −0.329, P < 0.01) and practice score (rs = 0.336, P < 0.01; = −0.316, P < 0.01). Moreover, positive correlations for knowledge–attitude (rs = 0.426, P < 0.01), attitude–practice (rs = 0.444, P < 0.01), and knowledge–practice ( = 0.336, P < 0.01) were observed.
Table 3.
Comparison of knowledge, attitudes, and practices scores between groups as defined by the categories of demographic characteristics
| Description | n | Knowledge score, median (IQR) | P-value | Attitude score, median (IQR) | P-value | Practice score, median (IQR) | P-value |
|---|---|---|---|---|---|---|---|
| Region | 0.002* | 0.004* | 0.481* | ||||
| Qianshan Prefecture | 369 | 4.0 (3.0–6.0) | 6.0 (4.0–8.0) | 5.0 (3.0–7.0) | |||
| Nanqiao District | 383 | 4.0 (3.0–6.0) | 6.0 (5.0–7.0) | 5.0 (3.0–7.0) | |||
| Gender | 0.027* | 0.220* | 0.113* | ||||
| Male | 355 | 4.0 (3.0–6.0) | 6.0 (5.0–8.0) | 5.0 (3.0–7.0) | |||
| Female | 397 | 4.0 (3.0–6.0) | 6.0 (5.0–7.0) | 5.0 (4.0–7.0) | |||
| Age group (years) | P < 0.01† | P < 0.01† | P < 0.01† | ||||
| 19–49 | 278 | 5.0 (3.0–7.0) | 7.0 (6.0–8.0) | 6.0 (4.0–7.0) | |||
| 50–69 | 342 | 3.5 (3.0–5.0) | 6.0 (5.0–7.0) | 5.0 (3.0–7.0) | |||
| ≥ 70 | 132 | 3.0 (3.0–5.0) | 5.0 (4.0–6.0) | 4.0 (2.3–5.0) | |||
| Occupation | P < 0.01† | P < 0.01† | P < 0.01† | ||||
| Farmer | 430 | 3.0 (3.0–5.0) | 6.0 (4.0–7.0) | 5.0 (3.0–7.0) | |||
| Commercial service personnel | 66 | 5.0 (4.0–7.0) | 7.0 (5.8–8.0) | 5.0 (4.0–7.0) | |||
| Medical staff, teacher, community leader, and worker | 134 | 6.0 (3.0–8.0) | 7.0 (6.0–8.0) | 7.0 (5.0–7.0) | |||
| Retiree | 20 | 4.0 (3.0–5.0) | 6.0 (6.0–8.0) | 4.5 (3.0–6.8) | |||
| Houseworker or unemployed | 102 | 3.0 (3.0–5.0) | 6.0 (4.0–7.0) | 5.0 (4.0–7.0) | |||
| Educational level | P < 0.01† | P < 0.01† | P < 0.01† | ||||
| No formal education | 215 | 3.0 (3.0–5.0) | 5.0 (4.0–6.0) | 4.0 (3.0–6.0) | |||
| 1st–6th grade | 193 | 4.0 (3.0–5.0) | 6.0 (4.0–7.0) | 5.0 (3.0–7.0) | |||
| 7th–9th grade | 196 | 5.0 (3.0–6.0) | 7.0 (5.3–8.0) | 5.0 (4.0–7.0) | |||
| 10th–12th grade | 81 | 6.0 (4.0–8.0) | 7.0 (6.0–8.0) | 7.0 (4.0–7.0) | |||
| College/university | 67 | 7.0 (3.0–9.0) | 8.0 (7.0–8.0) | 7.0 (6.0–8.0) | |||
| Frequency of fieldwork during the last month | 0.006† | 0.430† | 0.120† | ||||
| Never | 238 | 4.0 (3.0–6.0) | 6.0 (4.0–7.0) | 5.0 (3.0–7.0) | |||
| Occasionally (1–9 days) | 196 | 5.0 (3.0–7.0) | 6.0 (5.0–8.0) | 5.0 (4.0–7.0) | |||
| Often (10–19 days) | 140 | 4.0 (3.0–6.0) | 6.0 (5.0–8.0) | 5.0 (3.0–7.0) | |||
| Usually (≥ 20 days) | 178 | 4.0 (3.0–5.0) | 6.0 (5.0–7.0) | 5.0 (3.8–7.0) |
IQR = interquartile range.
* Mann–Whitney U-test.
† Kruskal–Wallis H-test.
Knowledge, attitudes, and practices predictors for SFTS.
Results of binary logistic regression analysis are presented in Table 4. Region, gender, age group, and educational level were determined as predictors of poor knowledge score. Participants from Nanqiao District (OR = 0.632, 95% CI: 0.399–0.999) and those with a higher educational level (OR = 0.516, 95% CI: 0.434–0.612) were more knowledgeable, whereas female participants (OR = 1.865, 95% CI: 1.165–2.987) and older respondents (OR = 3.406, 95% CI: 2.345–4.947) were less knowledgeable. Educational level (OR = 0.519, 95% CI: 0.449–0.599) was associated with attitudes, as respondents with higher educational levels always had better attitude scores toward SFTS, whereas age (OR = 1.456, 95% CI: 1.079–1.965) was a risk predictor for SFTS. Regarding practices, only educational level (OR = 0.481, 95% CI: 0.396–0.584) was a predictor of poor practice scores, and participants with a higher educational level always had good practice scores related to SFTSV infection.
Table 4.
Predictors of poor knowledge, attitudes, and practices scores about severe fever with thrombocytopenia syndrome by the binary logistic regression analysis
| Description | Good, n (%) | Poor, n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Knowledge* | ||||
| Region | ||||
| Qianshan Prefecture | 41 (39.4) | 328 (88.9) | 1.0 | 1.0 |
| Nanqiao District | 63 (16.4) | 320 (83.6) | 0.635 (0.416–0.969) | 0.632 (0.399–0.999) |
| Gender | ||||
| Male | 61 (17.2) | 294 (82.8) | 1.0 | 1.0 |
| Female | 43 (10.8) | 354 (89.2) | 1.708 (1.123–2.599) | 1.865 (1.165–2.987) |
| Age group (years) | ||||
| 19–49 | 67 (24.1) | 211 (75.9) | 1.0 | 1.0 |
| 50–69 | 36 (10.5) | 306 (89.5) | 2.699 (1.736–4.197) | 1.928 (1.154–3.222) |
| ≥ 70 | 1 (0.8) | 131 (99.2) | 41.597 (5.706–303.253) | 26.2 (3.464–198.159) |
| Educational level | ||||
| No formal education | 12 (5.6) | 203 (94.4) | 1.0 | 1.0 |
| 1st–6th grade | 16 (8.3) | 177 (91.7) | 0.654 (0.301–1.420) | 0.977 (0.434–2.197) |
| 7th–9th grade | 25 (12.8) | 171 (87.2) | 0.404 (0.197–0.829) | 0.966 (0.437–2.137) |
| 10th–12th grade | 23 (28.4) | 58 (71.6) | 0.149 (0.070–0.318) | 0.333 (0.147–0.753) |
| College/university | 28 (41.8) | 39 (58.2) | 0.082 (0.039–0.176) | 0.216 (0.092–0.507) |
| Attitude† | ||||
| Age group (years) | ||||
| 19–49 | 102 (36.7) | 176 (63.3) | 1.0 | 1.0 |
| 50–69 | 65 (19.0) | 277 (81.0) | 2.470 (1.716–3.554) | 1.444 (0.953–2.186) |
| ≥ 70 | 13 (9.8) | 119 (90.2) | 5.305 (2.847–9.885) | 2.329 (1.174–4.618) |
| Educational level | ||||
| No formal education | 20 (9.3) | 195 (90.7) | 1.0 | 1.0 |
| 1st–6th grade | 32 (16.6) | 161 (83.4) | 0.516 (0.284–0.937) | 0.566 (0.310–1.034) |
| 7th–9th grade | 53 (27.0) | 143 (73.0) | 0.277 (0.158–0.483) | 0.361 (0.199–0.652) |
| 10th–12th grade | 37 (45.7) | 44 (54.3) | 0.122 (0.065–0.230) | 0.156 (0.081–0.301) |
| College/university | 38 (56.7) | 29 (43.3) | 0.078 (0.040–0.153) | 0.118 (0.057–0.247) |
| Practice‡ | ||||
| Educational level | ||||
| No formal education | 6 (2.8) | 209 (97.2) | 1.0 | 1.0 |
| 1st–6th grade | 13 (6.7) | 180 (93.3) | 0.397 (0.148–1.067) | 0.397 (0.148–1.067) |
| 7th–9th grade | 20 (10.2) | 176 (89.8) | 0.253 (0.099–0.643) | 0.253 (0.099–0.643) |
| 10th–12th grade | 16 (19.8) | 65 (80.2) | 0.117 (0.044–0.310) | 0.117 (0.044–0.310) |
| College/university | 25 (37.3) | 42 (62.7) | 0.048 (0.019–0.125) | 0.048 (0.019–0.125) |
CI = confidence interval.
* The adjusted variables included region, gender, age group, occupation, educational level, and frequency of fieldwork during the last month.
† The adjusted variables included gender, age group, occupation, and educational level.
‡ The adjusted variables included age group, occupation, educational level, and frequency of fieldwork during the last month.
DISCUSSION
The potential contribution of KAP studies to SFTS research has not received much attention for endemic areas of China. We conducted a cross-sectional study to determine the baseline KAP level and confirmed the SFTS-related target population of health education in Anhui Province, eastern China.
The SFTS knowledge level reported in this study was 37.4%, which is lower than the 56.2% reported in another KAP study conducted in Lu’an city, another area of Anhui Province in which SFTS is prevalent.21 This low SFTS knowledge level may be due to the lack of regular health education programs in our study settings and limited information sources regarding SFTS. In our study, we further demonstrated that even though participants had heard about SFTS, knowledge of the symptoms and signs was lacking. Presumably, the participants in this study could not state symptoms and signs of SFTS because they had not experienced the disease nor received relevant knowledge in their communities.22 This low knowledge about SFTS, especially with transmission routes, might increase the risk of SFTSV infection. Therefore, systematic health education programs about SFTS are urgently needed for community members in endemic areas.
Timely treatment results in good prognosis in most SFTS patients, but some severely affected patients die of disseminated intravascular coagulation, multiple organ failures, and other severe syndromes.4,9,10 However, only 18.0% of participants in this study knew that SFTS is curable, whereas only 13.8% knew that there is no vaccine against SFTSV. These low results might be due to poor dissemination of SFTS information. Regarding transmission routes, few respondents correctly identified that SFTSV infection could not be transmitted by living with or talking to SFTS patients. Liu et al.16 demonstrated that ticks are the vectors and main cause of SFTSV infection. Yet only approximately 12.0% the participants in this study were aware of this fact. Previous studies on person-to-person transmission have shown that the blood, body fluids, and vomitus are infectious.17–19 However, in this study, less than 10.0% of respondents knew that blood and body fluid contact could cause infection. Most participants in the present study were unaware of facts regarding SFTS symptoms and signs, treatment, vaccination, and transmission routes, even if they had heard about SFTS. However, to reduce the risk of infection and improve awareness on the need for early treatment of patients in endemic areas, this information should be spread when implementing health education programs.
Regarding attitudes toward SFTS, few participants were classified as having the good attitude scores. This may show that only a minority perceived the risk of SFTS, or even if they recognized the risk, they still held attitudes that could put them at risk of SFTS. Most respondents expressed the appropriate attitude of visiting a doctor immediately when bitten by a tick, a finding which is similar to that of a previous KAP study on zoonotic disease.21 Participants with lower knowledge levels have been shown to be more likely to have a right attitude toward the disease.23 In this study, more than 80.0% of participants desired consultation and nearly 70.0% were willing to attend the free detection. These findings suggest that most participants cared about preventing the disease or considered the possibility of SFTSV infection. These findings also reveal a demand for these health services in endemic areas among most of the participants.
Previous studies had shown that practices are an independent factor that influence the risk of zoonotic disease infection.24,25 Our study revealed that more than 60.0% of participants wore long-sleeve clothes and fastened cuffs and collars when working in the field, a practice which reduces the probability of insect and tick bite. The use of repellents is recommended as another self-protection method by “The Guidelines and Recommendations for Preventing and Controlling SFTSV Infection” (http://www.moh.gov.cn/mohwsyjbgs/s8348/201010/49272.shtml). Approximately 20.0% of respondents in this study reported using repellents when working in the field. This low rate could be because of two reasons: participants were lacking knowledge about use repellents or they lacked access to such repellents. Although a proportion of participants reported adaptation practices against SFTSV infection, the percentage of reported practices that were translated into actual practices was not confirmed, and this finding was also noted in previous studies.22,26,27
Adequate knowledge can lead to appropriate attitudes resulting in good practices.28 Positive knowledge–attitude, attitude–practice, and knowledge–practice correlations that reveal relationships between SFTS knowledge, attitudes, and practices have been reported by Haq et al.29 In our study, participants with good knowledge scores about SFTS had better attitudes and practices than those with poor knowledge scores. For successful implementation of health education programs, the target population should have an adequate understanding of each program.30 Respondents from Qianshan Prefecture were less knowledgeable than those from Nanqiao District, which may be because of the health education programs implemented in Nanqiao District after SFTS cases and cluster infections occurred. On the other hand, a previous study has reported gender as a predictive factor of positive KAP scores.31 In our study, we found that female participants were less knowledgeable than male participants, which might explain the female-to-male ratio of SFTSV infection of 1.16:1 reported in a previous study.8 Moreover, in our study, the KAP scores of participants aged 70 years were significantly lower than that of younger participants. Educational level was also reported as an independent factor associated with KAP scores, consistent with the findings of our study.29 Respondents with higher education levels were more knowledgeable and had appropriate attitudes and good practices. According to these results, the target population for SFTS-related health education as reported by our study would be defined as female participants; participants from Qianshan Prefecture; elderly participants; farmer, retiree, houseworker, and unemployed participants; and participants with low educational level.
In conclusion, our findings suggest that the assessment of baseline KAP level must be the first step in implementing SFTS-related health education programs, to determine the target population and to formulate appropriate health education strategies. In addition, we recommend the implementation of large-scale sustainable health education programs to enhance the knowledge level of community members in endemic areas, focusing on symptoms and signs, transmission routes, and context-specific preventive measures. Furthermore, with a focus on the target population, programs should be implemented to promote appropriate practices, such as the use of personal protective equipment and repellents when working in the field. Furthermore, the cost, availability, reutilization, and comfort of the personal protective equipment should be considered. These might influence the choice of appropriate practices among the people.
Limitations.
This study has some limitations. First, the criteria for selecting the study settings and participants could introduce selection bias, as random sampling was not used. Second, using interview to complete the questionnaire could result in interviewer bias, and interviewing participants (medical staff, teachers, community leaders, and workers) at their workplace might have influenced the disclosure of information. Third, although our sample size was calculated using a formula for cross-sectional studies, some categories, such as age group, might need more participants to accurately explore the risk factors of KAP scores and obtain significant intervals. Despite these limitations, our findings regarding KAP levels of SFTSV in endemic areas of Anhui Province show a clear need for improved health education and promotion, and the result of our study can provide support for development of effective public health strategies. Further research should be conducted to determine intervention effectiveness using the data of the present study as a baseline.
Acknowledgments:
We thank the physicians and staff at the Qianshan Prefectural Center for Disease Control and Prevention, Nanqiao District Center for Disease Control.
REFERENCES
- 1.Yu XJ, et al. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi T, et al. 2014. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis 209: 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yun SM, Lee WG, Ryou J, Yang SC, Park SW, Roh JY, Lee YJ, Chan P, Han MG, 2014. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis 20: 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng B, et al. 2013. Clinical features and factors associated with severity and fatality among patients with severe fever with thrombocytopenia syndrome bunyavirus infection in northeast China. PLoS One 8: e80802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin C, et al. 2012. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc Natl Acad Sci U S A 109: 10053–10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, et al. 2011. Sporadic case infected by severe fever with thrombocytopenia syndrome bunyavirus in a non-epidemic region of China. Biosci Trends 5: 273–276. [DOI] [PubMed] [Google Scholar]
- 7.Gai ZT, et al. 2012. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis 206: 1095–1102. [DOI] [PubMed] [Google Scholar]
- 8.Zhan J, Wang Q, Cheng J, Hu B, Li J, Zhan F, Song Y, Guo D, 2017. Current status of severe fever with thrombocytopenia syndrome in China. Virol Sin 32: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Chai C, Wang C, Amer S, Lv H, He H, Sun J, Lin J, 2014. Systematic review of severe fever with thrombocytopenia syndrome: virology, epidemiology, and clinical characteristics. Rev Med Virol 24: 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, et al. 2015. SFTS virus in ticks in an endemic area of China. Am J Trop Med Hyg 92: 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang WS, Zeng XY, Zhou MH, Jiao YJ, Wen T, Guo XL, 2011. Seroepidemiology of severe fever with thrombocytopenia syndrome virus in Jiangsu province. Dis Surveill 26: 676–678. [Google Scholar]
- 12.Liang S, et al. 2014. Seroprevalence and risk factors for severe fever with thrombocytopenia syndrome virus infection in Jiangsu province, China, 2011. Am J Trop Med Hyg 90: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyu Y, et al. 2016. Seroprevalence and risk factors of severe fever with thrombocytopenia syndrome virus infection in endemic areas. Infect Dis (Lond) 48: 544–549. [DOI] [PubMed] [Google Scholar]
- 14.Niu G, et al. 2013. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg Infect Dis 19: 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo LM, et al. 2015. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis 21: 1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, He B, Huang SY, Wei F, Zhu XQ, 2014. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis 14: 763–772. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Hu K, Zou J, Xiao J, 2013. A cluster of cases of human-to-human transmission caused by severe fever with thrombocytopenia syndrome bunyavirus. Int J Infect Dis 17: e206. [DOI] [PubMed] [Google Scholar]
- 18.Bao C, et al. 2011. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin Infect Dis 53: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 19.Gong L, et al. 2018. Human-to-human transmissions of severe fever with thrombocytopenia syndrome virus in Anhui province, 2010–2017. Clin Microbiol Infect 24: 920–922. [DOI] [PubMed] [Google Scholar]
- 20.Gai Z, et al. 2012. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin Infect Dis 54: 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyu Y, et al. 2018. Impact of an intervention programme on knowledge, attitudes and practices of population regarding severe fever with thrombocytopenia syndrome in endemic areas of Lu’an, China. Epidemiol Infect 146: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhimal M, Aryal KK, Dhimal ML, Gautam I, Singh SP, Bhusal CL, Kuch U, 2014. Knowledge, attitude and practice regarding dengue fever among the healthy population of highland and lowland communities in central Nepal. PLoS One 9: e102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negash Y, Gebre B, Benti D, Bejiga M, 2003. A community based study on knowledge attitude and practice (KAP) on HIV/AIDS in Gambella town western Ethiopia. Ethiop J Health Dev 17: 234–235. [Google Scholar]
- 24.Aldubai SA, Ganasegeran K, Mohanad RA, Alshagga MA, Saifali R, 2013. Factors affecting dengue fever knowledge, attitudes and practices among selected urban, semi-urban and rural communities in Malaysia. Southeast Asian J Trop Med Public Health 44: 37–49. [PubMed] [Google Scholar]
- 25.Li PW, Shakir SMM, Atefi N, Abubakar S, 2015. Factors affecting dengue prevention practices: nationwide survey of the Malaysian public. PLoS One 10: e0122890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayyamani UD, Gan CY, San OG, 1986. A knowledge attitude and practice (KAP) study on dengue/dengue haemorrhagic fever and the Aedes mosquitoes. Med J Malaysia 41: 108–115. [PubMed] [Google Scholar]
- 27.Hairi F, Ong CH, Suhaimi A, Tsung TW, Ma BAA, Sundaraj C, Soe MM, 2003. A knowledge, attitude and practices (KAP) study on dengue among selected rural communities in the Kuala Kangsar district. Asia Pac J Public Health 15: 37–43. [DOI] [PubMed] [Google Scholar]
- 28.Singh A, Purohit BM, Bhambal A, Saxena S, Singh A, Gupta A, 2011. Knowledge, attitudes, and practice regarding infection control measures among dental students in central India. J Dent Educ 75: 421–427. [PubMed] [Google Scholar]
- 29.Haq NU, Hassali MA, Shafie AA, Saleem F, Farooqui M, Haseeb A, Aljadhey H, 2013. A cross-sectional assessment of knowledge, attitude and practice among hepatitis-B patients in Quetta, Pakistan. BMC Public Health 13: 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkari B, Qasem A, Shafaf MR, 2014. Knowledge, attitude, and practices related to cutaneous leishmaniasis in an endemic focus of cutaneous leishmaniasis, southern Iran. Asian Pac J Trop Biomed 4: 566–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijayakumar KN, Gunasekaran K, Sahu SS, Jambulingam P, 2009. Knowledge, attitude and practice on malaria: a study in a tribal belt of Orissa state, India with reference to use of long lasting treated mosquito nets. Acta Trop 112: 137–142. [DOI] [PubMed] [Google Scholar]