Abstract.
Cystic echinococcosis (CE) is a zoonosis caused by the larval stage of the tapeworm Echinococcus granulosus. In humans, the infection induces the formation of parasitic cysts mostly in the liver and lungs, but virtually any organ can be affected. CE of the bone is one of the rarest forms of the disease, yet it is also extremely debilitating for patients and hard to manage for clinicians. Unlike abdominal CE, there is currently no expert consensus on the management of bone CE. In this study, we conducted a survey of the clinical records of seven European referral centers for the management of patients with CE and retrieved data on the clinical management of 32 patients with a diagnosis of bone CE. Our survey confirmed that the patients endured chronic debilitating disease with a high rate of complications (84%). We also found that diagnostic approaches were highly heterogeneous. Surgery was extensively used to treat these patients, as well as albendazole, occasionally combined with praziquantel or nitaxozanide. Treatment was curative only for two patients, with one requiring amputation of the involved bone. Our survey highlights the need to conduct systematic studies on bone CE, both retrospectively and prospectively.
INTRODUCTION
Cystic echinococcosis (CE), a zoonosis caused by the larval stage (metacestode) of the tapeworm Echinococcus granulosus sensu lato complex,1 is a chronic, complex, and neglected parasitic disease, with a worldwide distribution. It is highly endemic in livestock-raising areas.1,2 The adults of E. granulosus reside in the small bowel of the definitive hosts (dogs and other canids) and releases eggs in their feces. After ingestion by the intermediate hosts (ungulates such as sheep, goats, or swines), the larvae released from the eggs penetrate the intestinal wall and migrate to various organs, especially the liver and lungs, where they develop into cysts.1 When the definitive host ingests the infected viscera of the intermediate host, the cycle is completed. Humans may become dead-end intermediate hosts by ingesting parasite eggs.1 Cysts can develop in any part of the body, although the liver and lungs are most frequently involved.3
Osseous CE is very rare; it is reported in the literature that cysts develop in bones in < 1% to 4% of cases reaching medical attention.4–9 Although uncommon, osseous CE is severely disabling.6,10 The destructive growth causes a high morbidity, similar to that of locally malignant bone lesions.4,5,7,8,11–13 CE of the bone is less noted in the medical community because of its rarity. To increase awareness of this important disease we collected the clinical histories of patients suffering from bone CE seen in several referral centers across Europe.
MATERIALS AND METHODS
Patients and centers.
Physical or electronic archives of eight centers in four European countries (Table 1) were searched for clinical records of patients with bone CE from the start of activity of each center to May 2015 for most of the patients. Nine patients, however, had data available until 2018. For each patient, demographic variables (gender, age at last follow-up visit, and country of birth) and clinical variables were collected. Clinical variables included duration of disease, calculated as the time span between the first diagnosis and the last follow-up visit; location of CE lesions; diagnostic modality; pre- or postoperative etiological diagnosis; symptoms at diagnosis; and disease- and treatment-related complications. The diagnosis was defined as certain if it was histologically confirmed, or probable if it was not histologically confirmed but based on the patient’s history, clinical features and laboratory evaluation. The use of albendazole (ABZ) or other anthelmintic drugs, continuous or discontinuous administration of ABZ, medical or surgical treatment, number of surgical interventions, use of polymethylmethacrylate for reconstruction, and use of scolicidal agents to protect the operation field were also recorded. The total number of months of ABZ treatment received by each patient between the first diagnosis and the last follow-up visit was calculated. Finally, the treatment outcome at the time of the last follow-up visit was recorded and defined as “disease persistence” in the case of stable presence of parasite material as the result of an incomplete response of CE lesions to medical treatment or persistence of parasite material after surgical treatment; as “relapse” in the case of parasite reactivation with extension of CE lesions after surgical or medical treatment; and as “freedom from disease” in the absence of CE lesions after treatment.
Table 1.
Centers and number of patients per center involved in the study
| Country | Center | City | Years of activity (range) | Last bone CE patient visit included in our cohort | Total number of patients with CE observed during center activity | No. of patients with osseous CE |
|---|---|---|---|---|---|---|
| Italy | University of Pavia | Pavia | 31 (1988–2018) | 2018 | 888* | 9 |
| Romania | Carol Davila University | Bucharest | 24 (1995–2018) | 2016 | 1,000* | 6 |
| Italy | San Bortolo Hospital | Vicenza | 22 (1997–2018) | 2018 | 50* | 2 |
| Italy | Hospital Sacro Cuore | Negrar (Verona) | 31 (1988–2018) | 2016 | 45* | 1 |
| Italy | Careggi University Hospital | Florence | 19 (2000–2018) | 2018 | 27* | 2 |
| Germany | University Hospital Heidelberg | Heidelberg | 21 (1998–2018) | 2016 | 800* | 5 |
| Germany | University Hospital | Düsseldorf | 17 (1999–2016) | 2016 | 106 | 3 |
| United Kingdom | Hospital for Tropical Diseases | London | 12 (2006–2018) | 2016 | 155 | 4 |
CE = cystic echinococcosis.
* Estimated data.
RESULTS
Demographics.
Data from 32 patients coming from 11 countries (one patient each from Afghanistan, Algeria, Bulgaria, and United Kingdom; two patients each from Albania and Iraq; three patients from Turkey; seven patients from Romania; eight patients from Italy; five patients from Morocco) are presented in Table 2.
Table 2.
Demographic variables, signs and symptoms, and diagnostic modality of bone CE
| n | 32 |
| Median age at last follow-up (range) | 52.5 (17–84) |
| Male, n (%) | 20 (63%) |
| Female, n (%) | 12 (37%) |
| Signs and symptoms | |
| Pain, n (%) | 18 (56%) |
| Neurological deficit, n (%) | 12 (37%) |
| Swelling of the involved segment, n (%) | 3 (9%) |
| Diagnostic tools | |
| Ultrasound, n (%) | 21 (66%) |
| CT, n (%) | 25 (78%) |
| MRI, n (%) | 26 (81%) |
| Scintigraphy, n (%) | 8 (25%) |
| X-ray, n (%) | 26 (81%) |
| Serology, n (%) | 26 (81%) |
| Biopsy, n (%) | 12 (37%) |
| Presurgical anatomopathological confirmation* | 10 (31%) |
| Postsurgical anatomopathological confirmation* | 21 (66%) |
| No anatomopathological confirmation* | 6 (18%) |
| Clinical features | |
| Median age at diagnosis in years (range) | 30.5 (10–63) |
| Median disease duration in years (range) | 17.5 (6–62) |
| Patients with confirmed diagnosis | 28 (88%) |
| Patients with disease persistence | 28 (88%) |
| Patients with disease relapse | 23 (71%) |
| Patients free from disease | 2 (6%) |
* The sum of histopathological investigations is higher than the number of patients having a definitive histopathological confirmation as patients received both pre- and postsurgical histopathological confirmation.
Symptoms at diagnosis and diagnostic modalities.
Presenting symptoms were pain (56%), neurological deficits (motor or sensory) (37%), and swelling of the involved bone segment (9%). Two (7%) patients were asymptomatic, and lesions were found during examinations performed for other reasons.
During the diagnostic process, 21 (66%) patients underwent an ultrasound scan, 25 (78%) a CT scan, 26 (81%) a magnetic resonance imaging (MRI) scan, and 26 (81%) a plain X-ray examination; 15 (46%) patients underwent all four types of radiological examinations. In two (7%) patients, scintigraphy was carried out. Serological tests were used in 27 (84%) patients; however, the heterogeneity of the tests used in different centers and throughout the investigated period within centers prevented any data analysis. In nine (28%) patients, bioptic samples were also examined.
Twenty-six (81%) patients had a histopathological diagnosis of bone CE. For three patients (10%), the diagnosis was made by evaluation of bioptic samples obtained before surgery, and in the remaining patients from pathological analysis of surgical specimens. In eight patients (25%), CE was mentioned as confirmed or possible etiology of the lesions before surgery. Twenty-six patients (81%) had a definite diagnosis of CE of the bone, whereas in all other patients the diagnosis was classified as probable. The median disease duration was 17.5 years (range 0.5–57 years).
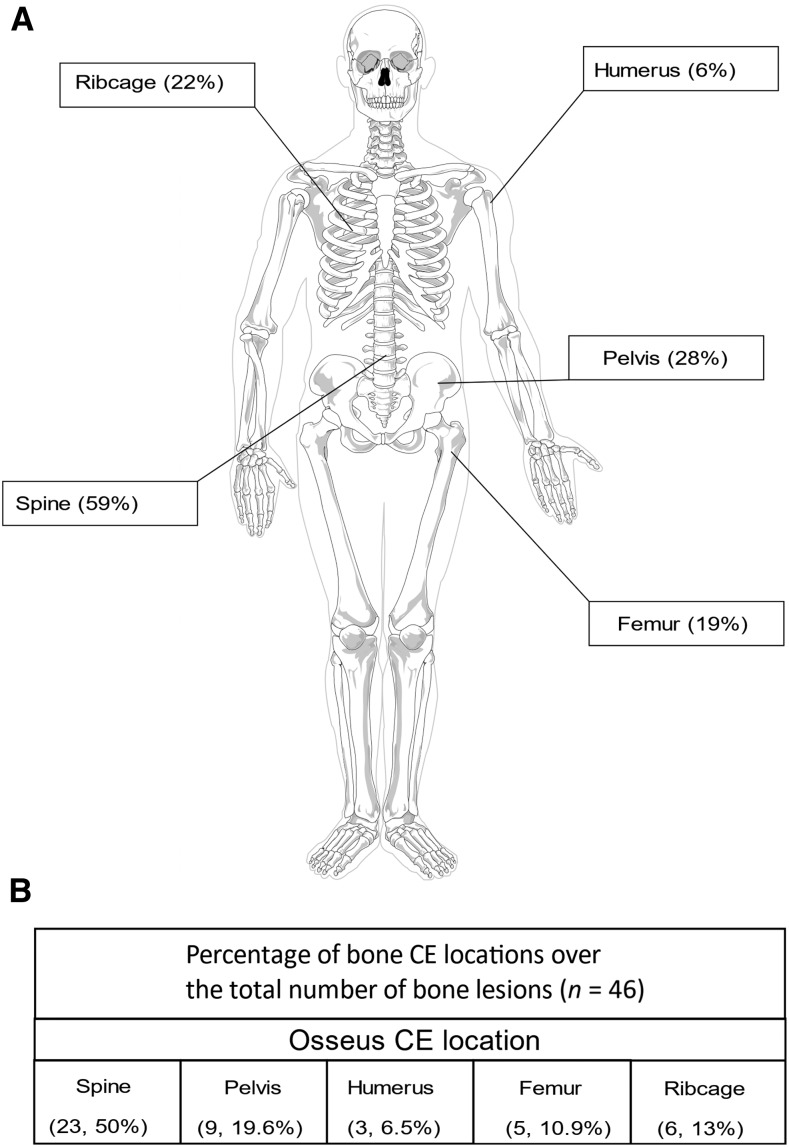
Bone CE topography in study patients.
Patients in our study had 46 CE lesions in bone. Most patients (n = 22; 69%) had CE at only one bony site; six (19%) had two distinct lesions; and four (13%) had three lesions. A total of nine (28%) patients had CE in the pelvic bones, seven (22%) in the ribcage, five (19%) in the femur, and two (6%) in the humerus. A total of 19 (59%) patients had lesions in the spine, which was the most common location (Figure 1). Finally, 20 (63%) patients showed involvement of adjacent soft tissue.
Figure 1.
Distribution of bone cystic echinococcosis (CE) lesions in our cohort of 32 patients. (A) Percentage of patients with bone involvement of the indicated bone segments. (B) Percentage of each bone segment involvement over the total number of bone lesions.
In 12 patients (60%), the diagnosis of bone CE was made after the diagnosis of visceral CE; in seven (35%) patients, it was made before the patient was known to have visceral CE. For one (5%) patient, clinical records were not clear enough to clarify this aspect. A total of 20 (63%) had visceral CE, too.
Disease complications, treatment, and outcome.
Twenty-five (78%) patients developed complications. Neurological symptoms due to nerve compression occurred in 15 (47%) cases, either for the first time during follow-up or worsened if already present at diagnosis. In 10 (31%) cases, CE infiltration was complicated by bacterial superinfection. Ten (31%) patients developed pathological fractures. One (3%) patient had a bleeding episode, one (3%) had deep vein thrombosis, and one (3%) underwent amputation of the right arm. This last case was the only truly radical surgical procedure described for the entire cohort.
Twenty-nine (90%) patients underwent surgery. Of these, 25 (86%) patients did so more than once, with eight (27%) undergoing more than three surgical interventions. The total number of operations identified in the medical records of these patients was at least 66 (uncertainty arose from the records of three patients reporting “multiple surgeries” without further details). Scolicidal agents were used in six surgical interventions (hypertonic saline in five cases and hydrogen peroxide in one case). Polymethylmethacrylate was used in two (3.3%) surgical interventions, whereas prosthetic devices were used in three patients (10%), but had to be removed eventually because of superinfection or involvement of the prosthesis by the parasite. Eight (25%) patients also underwent percutaneous procedures, details of which could not be retrieved, for cysts located in the thigh, psoas, or paraspinal muscles.
Albendazole was used in the postoperative management of all patients and was also administered to the two patients who did not undergo surgery. Four (13%) patients were treated with ABZ administered in cycles of various durations (1, 3, or 6 months) each year (on/off protocol) before moving to continuous administration. Fifteen (46%) patients were treated only with an on/off protocol, and 12 (38%) patients were treated with lifelong administration from the start of medical treatment. Only one (3%) patient was treated with nitazoxanide in association with ABZ. Four (13%) patients were treated with praziquantel in combination with ABZ. Data on the duration of medical treatment were available for 27 patients (84%). For these patients, the median cumulative duration was 72 months (range 5–360 months). Records were not clear in the case of one (3%) patient.
Twenty-eight (88%) patients showed signs of disease persistence after treatment, including the three patients managed solely by medical therapy. Four (9%) surgical patients were initially declared disease-free. Overall, 23 (75%) patients developed relapses, including the four patients initially considered to be free from disease. Information concerning relapses was lacking in the records of eight (27%) patients, which included both cases treated with ABZ only. Relapses occurred in 9 patients treated with lifelong administration of ABZ (9/12, 75%) and in 13 patients treated with on/off protocols (13/16, 81%). Four (12%) patients died during the follow-up period, but from the available information it was not possible to determine whether the patients died because of CE-related complications or other, unrelated reasons. Of 32 patients, only two (6%) could be considered disease-free after follow-up.
DISCUSSION
Previous studies report a varied distribution of cysts within the skeleton: the most common sites are the spine (35–50%), the pelvis (21%), and long bones, including the femur (16%) and tibia (10%).6,11,13,14 Involvement of the ribs, skull, scapula, humerus, and fibula appears less common (2–6%).6,13,14 The disease is usually diagnosed between the fifth and the sixth decade10,13 and is rarely encountered in childhood. Growth of the parasite in the bone tissue is a very slow process. The dynamics of bone involvement in CE are poorly understood. Whether bone is a site of primary parasite implantation or is always secondary to infection in other organs remains unclear. However, not all patients with bone CE also have cysts in other organs.6,12 While visceral CE cysts expand at a slow pace and grow mostly concentrically, the rigid structure of the bone prevents this pattern from becoming established here as the cyst is not able to induce the development of an adventitial layer as in the case of other organs,10,13 and the metacestode development leads to the formation of exogenous vesicles containing protoscoleces along bone canals, conferring a branched, polycystic appearance to the lesion.10,13 Bone destruction is likely the consequence of three factors: compression exerted by the growing parasite on surrounding tissues; ischemic damage due to compression of blood vessels; and demineralization due to osteoclastic proliferation around the compressed bone tissue.13 The localization of bone CE in our series is similar to that reported in previous publications.12,15 In our cohort, the spine was the most frequent site, followed by the pelvis and ribcage, with the humerus and femur being rarer locations. Most of our patients were symptomatic (94%). Symptoms and imaging features of bone CE are nonspecific, making CE an unlikely early consideration. CE is unsuspected clinically due to its low regional prevalence and an overall neglect of CE as a disease.16 Furthermore, the absence of pathognomonic radiologic findings in bone CE makes it less distinguished from neoplastic and inflammatory processes.13 For this reason, patients commonly undergo multiple radiological examinations, and a definitive diagnosis is often reached only postoperatively5,13 as shown in our cohort. Even in the case of clinical suspicion, serology may be negative, although data on this subject are scant.17–19 Unfortunately, the clinical records of our cohort regarding serology were also generally unclear or lacking altogether, so we can only provide information on the use of serological tests but not a positivity rate. Furthermore, interpretation of such information would be hampered by the use of different tests across different centers and within centers over time, as one of the problems in CE serology is the lack of standardized tests and diagnostic algorithms.20,21
Treatment options for bone CE have not been defined in a systematic way and currently include surgery which is used with or without administration of ABZ. In some cases, the use of other drugs such as praziquantel or nitazoxanide in combination with ABZ has also been reported in murine studies and in human case reports,7,22,23 but their efficacy remains unproven. Currently, no uniform protocol for the use of ABZ has ever been implemented.24
Unfortunately, the retrospective nature of the study, the small number of cases, and the many variables, including different ABZ penetration in bone tissues do not allow us to draw conclusions on the difference in treatment outcome, when ABZ was given continuously or in interrupted cycles.
Some authors are even skeptical about the actual role of medical treatment,4,11,12,25,26 supporting the opinion that only radical surgery is able to cure the disease.13,26 In our cohort, all patients received prolonged courses of ABZ, which was not only longer than the current standard recognized by regulatory agencies (a maximum of three cycles each of 28 days, with a pause of 14 days separating each cycle), but also longer than what is usually done for CE in other organs, where continuous administration of ABZ for 3 to 6 months is generally used.27 Overall, prolonged administration was well tolerated and no patient had to stop the administration of ABZ. Our results suggest that continuous ABZ administrations should be implemented before and after surgery in all patients able to tolerate the treatment, as it is highly plausible that ABZ may halt the progression of the parasitic growth. This is supported by the fairly high survival rate of patients in our study over a long period of time.
With regard to surgery, radical treatment has been advocated as the sole treatment option able to cure the disease, but full removal of the parasitic tissue without harming the patient can be impossible.6,8,12 Moreover, the persistence of parasitic material because of partial removal has a high potential for future reactivation of parasitic growth.4,7,8 In our cohort, 97% of the patients were operated on, but a radical intervention was never possible with the exception of one case where the whole upper arm was amputated, and other cases where patients experienced a high number of relapses. If not managed correctly, bone CE has a very poor prognosis in terms of long-term morbidity, comparable with that of cancer.4,5,7,8,11–13 In fact, vertebral CE was called le cancer blanc by Devé.7,10,13 Cystic echinococcosis of the bone is a highly destructive process, capable of spreading from bone segments to the surrounding soft tissues and vice versa6–8: In our cohort, 27% of patients had more than one localization of bone CE and soft tissue involvement was extremely common.
Overall, our results suggest that radical surgery should only be attempted if there is a high degree of confidence in the possibility of removing all parasitic material, and palliative surgery should be attempted only in cases where a good degree of functionality can be restored. It should also be noted that the use of prosthetics for reconstructive surgery has been discouraged, as the parasite is able to stably attach to the materials used for their construction.8,10 Bone allograft has also been considered, but it is susceptible to parasite invasion if relapses occur.7,10 Polymethylmethacrylate has been reported to be effective in the prevention of relapses,7,10,26 and irrigation of the operational field with hypertonic saline or other scolicidal agents has been shown to reduce the rate of recurrence with a concentration and time-dependent effect.6,7 Traditional radiotherapy has proven to be completely ineffective,6,12,24 although other cutting-edge radioterapeutical approaches have not been applied so far.
CONCLUSION
Bone CE is a challenge for clinicians and, given its relative rarity, available data are scarce. International collaboration helps to unveil previously unconsidered cases.
Our study is different from the majority of the current literature on bone CE in that it is not a signle case report but rather a larger cohort analysis that helps provide a spotlight for this neglected disease to the international community. We also show that patients diagnosed with bone CE present serious, sometimes life-threatening and often disabling, complications, in accordance with previous analyses presented by other authors.7,8 Whereas for liver CE a stage-specific approach has been at least partially agreed on that allows a rational choice among different treatment options,27 no such thing exists for bone CE. As exemplified also in our study, the decision on the general management of patients with bone CE is largely left to the individual physician.
The only possibility of true advancement in the knowledge of this rare yet extremely disabling form of CE will necessarily come only from broad, ideally prospective, multicentric studies, with common protocols for the management of the disease. The increased complexity of bone CE, in comparison to hepatic CE, warrants the organization of a specific international database able to capture its very peculiar clinical features. Such a database would complement the existing ones (such as the European Registry of Cystic Echinococcosis) and provide a basis for further research and thus better diagnosis and treatment.
Acknowledgments:
This study has been partially supported by an FP7-HEALTH-2013-INNOVATION-1 grant «Human Cystic Echinococcosis in Central and Eastern Societies—HERACLES» (to E. B.). The authors thank David M. Abbott, medical student, for proofreading the final version of the paper.
REFERENCES
- 1.Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, Wassermann M, Takahashi K, de la Rue M, 2017. Ecology and life cycle patterns of Echinococcus species. Adv Parasitol 95: 213–314. [DOI] [PubMed] [Google Scholar]
- 2.Budke CM, Deplazes P, Torgerson PR, 2006. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis 12: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A, 2003. Hydatid disease from head to toe. Radiographics 23: 475–494; quiz 536–537. [DOI] [PubMed] [Google Scholar]
- 4.Zlitni M, Ezzaouia K, Lebib H, Karray M, Kooli M, Mestiri M, 2001. Hydatid cyst of bone: diagnosis and treatment. World J Surg 25: 75–82. [DOI] [PubMed] [Google Scholar]
- 5.Togral G, Arkan S, Ekiz T, Kekec A, Eksioglu M, 2016. Musculoskeletal hydatid cysts resembling tumors: a report of five cases. Orthop Surg 8: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumayr A, Tamarozzi F, Goblirsch S, Blum J, Brunetti E, 2013. Spinal cystic echinococcosis–a systematic analysis and review of the literature: part 1. Epidemiology and anatomy. PLoS Negl Trop Dis 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monge-Maillo B, Chamorro Tojeiro S, López-Vélez R, 2017. Management of osseous cystic echinococcosis. Expert Rev Anti Infect Ther 15: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 8.Steinmetz S, Racloz G, Stern R, Dominguez D, Al-mayahi M, Schibler M, Lew D, Hoffmeyer P, Uçkay I, 2014. Treatment challenges associated with bone echinococcosis. J Antimicrob Chemother 69: 821–826. [DOI] [PubMed] [Google Scholar]
- 9.Kireşi DA, Karabacakoğlu A, Odev K, Karaköse S, 2003. Uncommon locations of hydatid cysts. Acta Radiol 44: 622–636. [DOI] [PubMed] [Google Scholar]
- 10.Neumayr A, Tamarozzi F, Goblirsch S, Blum J, Brunetti E, 2013. Spinal cystic echinococcosis–a systematic analysis and review of the literature: part 2. Treatment, follow-up and outcome. PLoS Negl Trop Dis 7: e2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karray S, Zlitni M, Fowles JV, Slimane N, Kassab T, Rosset P, 1990. Vertebral hydatidosis and paraplegia. J Bone Joint Surg Br 72: 84–88. [DOI] [PubMed] [Google Scholar]
- 12.Loudiye H, Aktaou S, Hassikou H, El Bardouni A, El Manouar M, Fizazi M, Tazi A, Hajjaj-Hassouni N, 2003. Hydatid disease of bone: review of 11 cases. Joint Bone Spine 70: 352–355. [DOI] [PubMed] [Google Scholar]
- 13.Papanikolaou A, 2008. Osseous hydatid disease. Iran J Parasitol 3: 60–64. [Google Scholar]
- 14.Kalinova K, Proichev V, Stefanova P, Tokmakova K, Poriazova E, 2005. Hydatid bone disease: a case report and review of the literature. J Orthop Surg (Hong Kong) 13: 323–325. [DOI] [PubMed] [Google Scholar]
- 15.Bracanovic D, Djuric M, Sopta J, Djonic D, Lujic N, 2013. Skeletal manifestations of hydatid disease in Serbia: demographic distribution, site involvement, radiological findings, and complications. Korean J Parasitol 51: 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunetti E, Garcia HH, Junghanss T, 2011. Cystic echinococcosis: chronic, complex, and still neglected. PLoS Negl Trop Dis 5: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbieri M, Fernandez V, Gonzalez G, Luaces VM, Nieto A, 1998. Diagnostic evaluation of a synthetic peptide derived from a novel antigen B subunit as related to other available peptides and native antigens used for serology of cystic hydatidosis. Parasite Immunol 20: 51–61. [DOI] [PubMed] [Google Scholar]
- 18.Delunardo F, Ortona E, Margutti P, Perdicchio M, Vacirca D, Teggi A, Sorice M, Siracusano A, 2010. Identification of a novel 19 kDa Echinococcus granulosus antigen. Acta Trop 113: 42–47. [DOI] [PubMed] [Google Scholar]
- 19.Schweiger A, Grimm F, Tanner I, Müllhaupt B, Bertogg K, Müller N, Deplazes P, 2012. Serological diagnosis of echinococcosis: the diagnostic potential of native antigens. Infection 40: 139–152. [DOI] [PubMed] [Google Scholar]
- 20.Lissandrin R, Tamarozzi F, Piccoli L, Tinelli C, De Silvestri A, Mariconti M, Meroni V, Genco F, Brunetti E, 2016. Factors influencing the serological response in hepatic Echinococcus granulosus infection. Am J Trop Med Hyg 94: 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmena D, Benito A, Eraso E, 2006. Antigens for the immunodiagnosis of Echinococcus granulosus infection: an update. Acta Trop 98: 74–86. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Molina JA, Diaz-Menendez M, Gallego JI, Norman F, Monge-Maillo B, Ayala AP, Lopez-Velez R, 2011. Evaluation of nitazoxanide for the treatment of disseminated cystic echinococcosis: report of five cases and literature review. Am J Trop Med Hyg 84: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stettler M, et al. 2004. Secondary and primary murine alveolar echinococcosis : combined albendazole/nitazoxanide chemotherapy exhibits profound anti-parasitic activity. Int J Parasitol 34: 615–624. [DOI] [PubMed] [Google Scholar]
- 24.Prabhakar MM, Acharya AJ, Modi DR, Jadav B, 2005. Spinal hydatid disease: a case series. J Spinal Cord Med 28: 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam KS, Faraj A, Mulholland RC, Finch RG, 1997. Medical decompression of vertebral hydatidosis. Spine (Phila Pa 1976) 22: 2050–2055. [DOI] [PubMed] [Google Scholar]
- 26.Yildiz Y, Bayrakci K, Altay M, Saglik Y, 2001. The use of polymethylmethacrylate in the management of hydatid disease of bone. J Bone Joint Surg Br 83: 1005–1008. [DOI] [PubMed] [Google Scholar]
- 27.Brunetti E, Kern P, Vuitton DA, 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 114: 1–16. [DOI] [PubMed] [Google Scholar]
- 28.Rossi P, Tamarozzi F, Galati F, Pozio E, Akhan O, Cretu CM, Vutova K, Siles-Lucas M, Brunetti E, Casulli A; HERACLES extended network , 2016. The first meeting of the European Register of Cystic Echinococcosis (ERCE). Parasit Vectors 9: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]