Abstract.
Adults who have not grown up in a malaria-endemic area may experience severe malaria soon after entering a malarious area. Such mortality is usually limited to a short period of time (months), after which they are thought to be “immune.” Such anti-disease immunity may be more accurately considered as tolerance. Malaria rates of British soldiers during the Second World War reflected their time with suppressed infections and the transmission levels. Black workers from non-endemic areas on the Panama Canal experienced higher initial mortality and infection rates than co-located white workers for Plasmodium falciparum, whereas the known genetic resistance of blacks to Plasmodium vivax reversed these rates. The ethnic differences observed in malaria rates may have more to do with acquired tolerance than genetic resistance. Long-term (years) sub-patent infections may maintain host tolerance, and elimination of malaria infections may place these adults at subsequent risk of severe malaria.
Immunity to malaria cannot be measured in the laboratory, so it is functionally defined often as living in a malaria-endemic area with asymptomatic parasitemia. Adults with sub-patent parasitemias represent a large hidden reservoir that has to be removed if one is to eliminate malaria from an endemic area, the risk being that once the parasites are eliminated, immunity is lost and the person could then develop severe malaria on reinfection.1,2 These chronic infections may represent a state of tolerance where the host and the parasite are in an armed truce that permits both to coexist without great loss to either.3 Acquired immunity allows the host to resist infections and lower parasitemias to manageable levels that do not threaten the host’s existence. Tolerance, however, consists of the host’s adaptation to continuing infections (e.g., anti-disease immunity) primarily by downregulating inflammation generated by the innate immune system to avoid tissue damage.3,4 Old epidemiological records can aid our understanding of a population’s tolerance to malaria and help distinguish how fragile the host’s ability is to maintain a relatively asymptomatic state. Malaria records from military units during the Second World War and the building of the Panama Canal with groups of varying ethnicity and disease experience were examined to look for evidence of host tolerance to malaria.
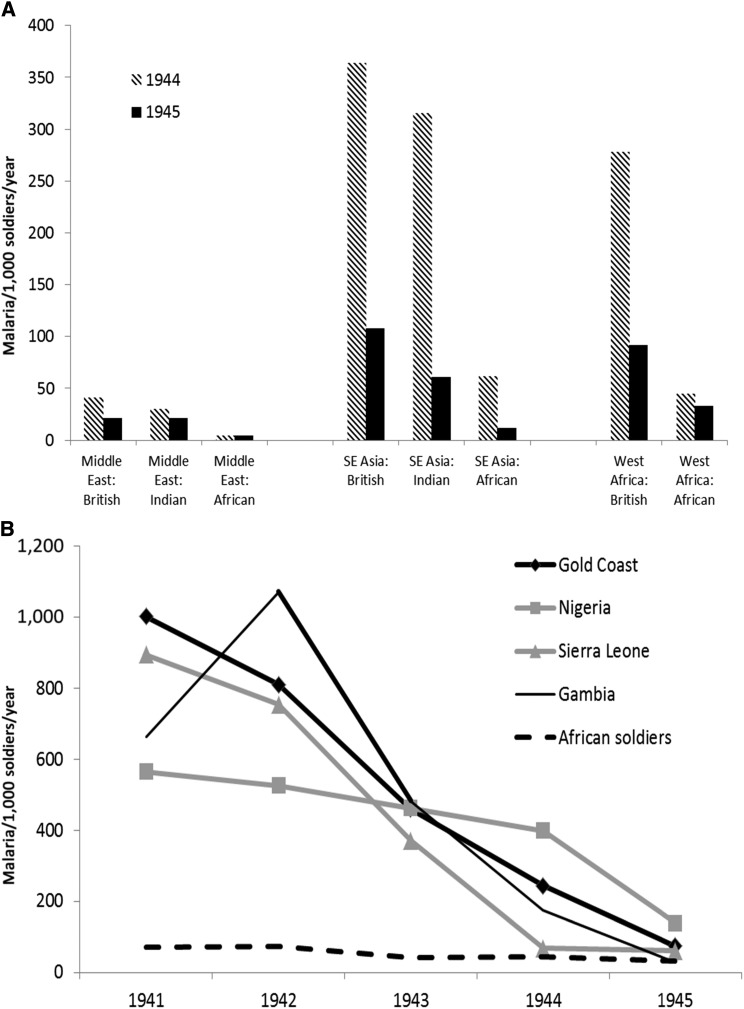
During the Second World War, British Commonwealth forces consisting of British, Indian, and African soldiers were deployed in areas of low (Middle East), moderate (SE Asia), or intense (West Africa) malaria transmission. Figure 1A compares malaria rates in the various ethnic groups with no (British), moderate (Indian), or intense (West Africa) previous malaria experience during 1944–1945.5 Malaria rates decreased between 1944 and 1945, but the pattern of British > Indian > West African soldiers was maintained despite varying transmission in the Middle East, SE Asia, and West Africa. This might seem to superficially confirm the colonial stereotypes of racial immunity to malaria, but malaria was defined as being symptomatic and subsequently having a blood smear that is positive for parasites. Asymptomatic parasitemia would likely have been very high in West African soldiers who would not have had their blood examined.6 Infection pressure in West Africa remained very high, so the differences between British and West African soldiers’ malaria rates (shown in Figure 1B) would not have been due to lack of exposure.7 Chemoprophylaxis was given only to the British soldiers, suppressing the blood infections with varying degrees of success, which improved over time with better use of atabrine/quinacrine.8 It is possible that the dramatic decreases in British soldiers’ malaria rates shown across four West African countries during the Second World War are actually due to accelerated acquisition of tolerance. By 1945, constant infection of British soldiers with variable drug suppression would have gradually produced a state of tolerance with sub-patent parasitemias that approximated that seen in West African soldiers whose low-level parasitemias were controlled without chemoprophylaxis.
Figure 1.
Malaria illness rates in soldiers of the British Commonwealth as reported by their ethnicity and (A) as divided by area of operations in the Middle East, Southeast Asia, or West Africa (1944–1945) or (B) in four countries of West Africa (1941–1945).
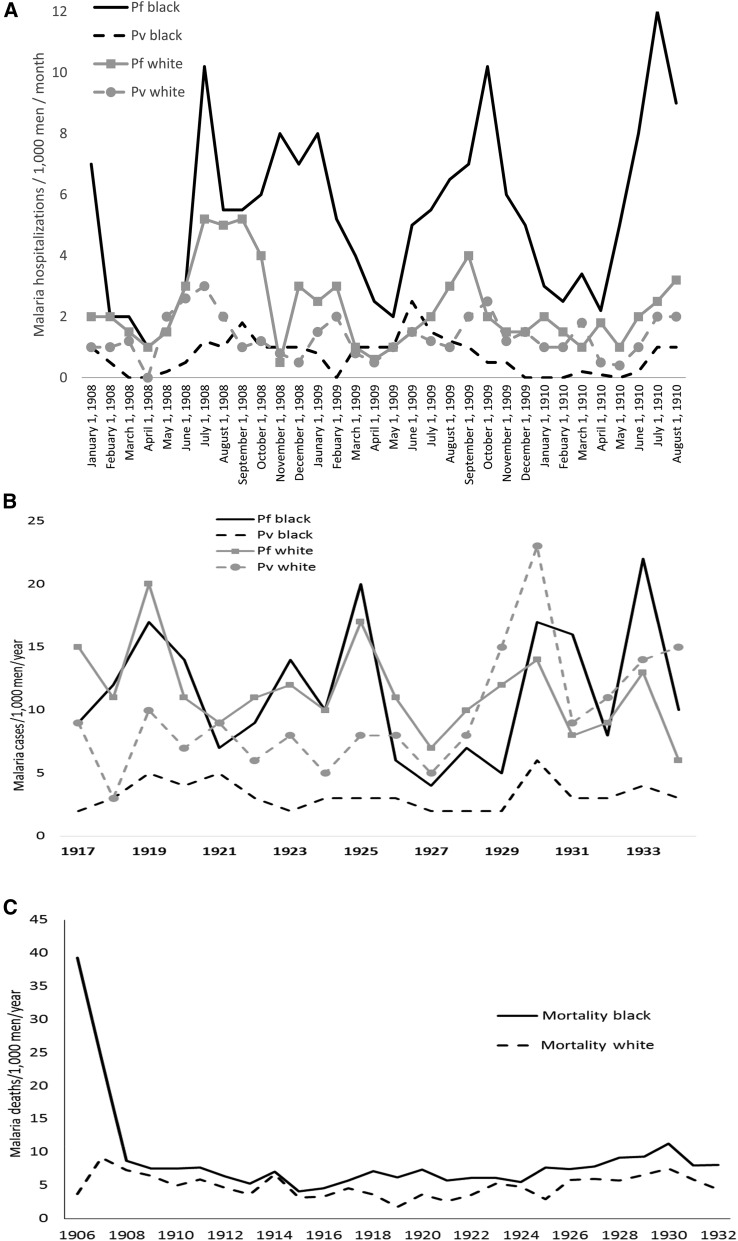
Not all adult blacks grew up in malaria-endemic areas. Many of the workers recruited to construct the Panama Canal came from the Caribbean Islands with no or little malaria endemicity, such as Barbados. Hospitalizations due to malaria during the construction phase of the Panama Canal (1908–1910, shown in Figure 2A) show the highest falciparum malaria rates in black rather than white workers over 3 years.9 The opposite situation (whites > blacks) was seen for vivax malaria likely not due to differential infection rates, but rather to the known genetic resistance of blacks without the Duffy blood group antigen. Caribbean blacks who were relatively few generations removed from West Africa by the slave trade were not intrinsically resistant to falciparum malaria. These differential black > white falciparum rates and white > black vivax rates continued even years (1917–1934) after the construction phase of the Panama Canal (Figure 2B).10 Malaria mortality was initially much higher in black Panama Canal workers (Figure 2C) despite similar access as white workers to hospital treatment for malaria with quinine. The mortality difference between black and white workers decreased over time but remained black > white many years after the completion of the Panama Canal. It is possible that white workers’ lower falciparum rates were because of their greater access to quinine chemo-suppression as enormous amounts of the drug were distributed in the Canal Zone.11 Adult workers could tolerate malaria infections when parasites were suppressed with quinine, but blacks were not intrinsically better able to suppress falciparum unlike vivax malaria.11 Additional social factors may have affected malaria rates, but in both West Africa and Panama, exposure to infected mosquitoes was so common that all would have developed some level of infection within a few months.
Figure 2.
Malaria morbidity and mortality rates in Panama Canal workers by ethnicity. (A) Falciparum and vivax malaria rates during the construction phase (1908–1910). (B) Falciparum and vivax malaria rates in post-construction canal workers (1917–1934). (C) Malaria mortality rates (1906–1932).
The evidence that these data are explained by tolerance to the disease rather than immunity to infection is largely circumstantial.3 Induced falciparum infections for syphilis–malaria therapy showed that in most cases infections could be controlled after several paroxysms over 1 month, perhaps with some help from the use of subtherapeutic quinine. In a series from two US syphilis treatment centers, 42% of 270 patients did not require any chemotherapy, thus self-curing their falciparum infection.12 When parasitemia that is necessary to induce fever (pyrogenic threshold) was examined from induced falciparum infections without chemotherapy, the second febrile paroxysm required a greater concentration of parasites, indicating that the host had downregulated the inflammatory response. This increased tolerance as measured by the pyrogenic threshold was maintained when second infections were induced later irrespective of the duration of the first infection or interval between infections.12
Early versions of the killed, whole-cell typhoid vaccine were essentially injections of bacterial endotoxin causing considerable adverse events by nonspecifically activating the innate immune system. During the first typhoid vaccine trials by Almoth Wright in nineteenth-century India, soldiers noted that their malaria attacks decreased. Even as Wright recorded these unexpected observations, he noted their lack of biological plausibility that typhoid vaccine would protect against malaria.13 Similar results with typhoid vaccine were also obtained by Mark Boyd in the Southern United States in the twentieth century despite the fact that any antigens that might induce immunity are unlikely to be shared between typhoid and malaria.14 Tolerance may be a better explanation. Repeated injections of bacterial endotoxin would greatly stimulate one’s innate immune receptors (e.g., toll-like receptors) with the subsequent downregulation of inflammation.15 Although the actual immunological circumstances must remain a matter of speculation, typhoid vaccine–induced tolerance to malaria is a more plausible mechanism than any cross-reactive immunity to explain the interaction between typhoid vaccine and symptomatic malaria.16
Malaria is rarely the only infection occurring in persons fully exposed to the pathogen-rich environment of developing countries. Malaria-associated mortality is known to be at least as high as deaths strictly assigned to the malaria parasite, but why tuberculosis or pneumonia should be worse in a malaria-infected person remains obscure.17 Malaria-induced tolerance could inhibit an early inflammatory response to new infectious agents, thus allowing the second infection an advantage against the host. An example of this possibility may have occurred during the influenza pandemic of 1918 among British soldiers in Macedonia.18 Nearly all had partially quinine-suppressed falciparum infections which would have induced tolerance to the parasite. Although the malaria rates did not increase in late 1918 in Macedonia, the mortality rates ascribed to malaria markedly increased when influenza arrived, which was largely due to subsequent bacterial pneumonia (see Figure 1 in ref. 19). Synergistically increased mortality may have occurred because the innate immune system of chronically malaria-infected soldiers could not mobilize rapidly against influenza and secondary bacterial pneumonia because of malaria-induced tolerance.
When a malaria-immune/tolerant adult leaves an endemic area and clears any residual plasmodium parasites, reversion to the previous sensitive state occurs fairly quickly, usually in a matter of months; the specific time interval involved is unknown and likely to be highly variable. Examples include New Guinea miners recruited from endemic low-land areas who then work in a malaria-free area of the highlands and later develop symptomatic malaria on returning to their home areas during Christmas holidays.2 The rapid loss of protection could be more likely explained by the loss of tolerance than immunity as the innate immune system has fewer means of establishing lasting memory than the acquired immune system.
The difference between malaria immunity and tolerance is not academic. One of the greatest unsolved challenges of malaria elimination is how to quickly get rid of residual parasites in large populations of asymptomatic adults.20 How much this places these adults at risk for future episodes of severe malaria should elimination be less than complete is unknown. But it is not a theoretical concern as evident from earlier failed eradication efforts.21 Tolerance caused by downregulation of the innate immune system appears to be lost much faster than that caused by acquired immunity, such as antibody production or lymphocyte proliferation. This is already seen in some areas of Africa where the age group affected by severe malaria has increased beyond young children as the intensity of malaria exposure decreases. If tolerance explains much of malaria-associated mortality, then understanding it may be critical to further mortality reduction, especially in sub-Saharan Africa. Regardless, distinguishing tolerance from acquired immunity to malaria will become increasingly important as geographic regions approach malaria elimination.
Acknowledgments:
I thank the many unnamed historians, medical librarians, and archivists who have unselfishly provided references, data, and ideas for this publication.
REFERENCES
- 1.Bousema T, Okell L, Felger I, Drakeley C, 2014. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. [DOI] [PubMed] [Google Scholar]
- 2.Shanks GD, Edstein MD, Kereu RK, Spicer PE, Rieckmann KH, 1993. Postexposure administration of halofantrine for the prevention of malaria. Clin Infect Dis 17: 628–631. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Schneider DS, Soares MP, 2012. Disease tolerance as a defense strategy. Science 335: 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen TN, et al. 2018. The persistence and oscillations of submicroscopic Plasmodium falciparum and Plasmodium vivax infections over time in Vietnam: an open cohort study. Lancet Infect Dis 18: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce-Chwatt LJ, 1985. John Hull Grundy lecture. Mosquitoes, malaria and war; then and now. J R Army Med Corps 131: 85–99. [DOI] [PubMed] [Google Scholar]
- 6.Findlay GM, 1949. Blackwater fever in West Africa, 1941-45; blackwater fever in African military personnel. Ann Trop Med Parasitol 43: 213–224. [DOI] [PubMed] [Google Scholar]
- 7.Hogben L, Johnstone MM, Mullings D, 1947. The medical ethnography of the second World War. Br J Soc Med 1: 251–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Findlay GM, 1949. Blackwater fever in West Africa, 1941-45; blackwater fever in European military personnel. Ann Trop Med Parasitol 43: 140–154. [DOI] [PubMed] [Google Scholar]
- 9.Deeks WE, James WM, 1911. A Report on Hemoglobinuric Fever in the Canal Zone: A Study of Its Etiology and Treatment. Department of Sanitation. Mount Hope, Canal Zone: ICC Press. [Google Scholar]
- 10.Simmons JS, 1939. Malaria in Panama. Baltimore, MD: Johns Hopkins Press. [Google Scholar]
- 11.Gorgas WC, 1915. Sanitation in Panama. Baltmore, MD: Appleton. [Google Scholar]
- 12.Gatton ML, Cheng Q, 2002. Evaluation of the pyrogenic threshold for Plasmodium falciparum malaria in naive individuals. Am J Trop Med Hyg 66: 467–473. [DOI] [PubMed] [Google Scholar]
- 13.Wright AE, Leishman WB, 1900. Remarks on the results which have been obtained by the antityphoid inoculations and on the methods which have been employed in the preparation of the vaccine. BMJ 1: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd MF, Kitchen S, 1943. On attempts to hyperimmunize convalescents from vivax malaria. Am J Trop Med Hyg 1: 209–225. [Google Scholar]
- 15.Perkins DJ, Patel MC, Blanco JC, Vogel SN, 2016. Epigenetic mechanisms governing innate inflammatory responses. J Interferon Cytokine Res 36: 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandala W, Msefula C, Gondwe E, Drayson M, Molyneux ME, MacLennan C, 2016. Monocyte activation and cytokine production in Malawian children presenting with P. falciparum malaria. Parasite Immunol 38: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanks GD, Hay SI, Bradley DJ, 2008. Malaria’s indirect contribution to all-cause mortality in the Andaman Islands during the colonial era. Lancet Infect Dis 8: 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wenyon C, Anderson A, McLay K, Hele T, Waterston J, 1921. Malaria in Macedonia, 1915–1919. J Roy Army Med Corps 37: 83–108. [Google Scholar]
- 19.Shanks GD, White NJ, 2013. The activation of vivax malaria hypnozoites by infectious diseases. Lancet Infect Dis 13: 900–906. [DOI] [PubMed] [Google Scholar]
- 20.Peto TJ, et al. 2016. History of malaria treatment as a predictor of subsequent subclinical parasitaemia: a cross-sectional survey and malaria case records from three villages in Pailin, western Cambodia. Malar J 15: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B, 2012. Malaria resurgence: a systematic review and assessment of its causes. Malar J 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]