Abstract.
Bothrops asper, a highly venomous pit viper distributed from Colombia and northwestern Peru in South America to southern Mexico, is responsible for most snake bites in Central America, affecting especially young agricultural workers. A 17-year-old male from a rural area in northern Honduras was admitted at San Francisco Hospital after a B. asper bite that had occurred 3 days earlier. The puncture wounds were located on the first toe of the right foot. On the second day of admission, the patient developed dyspnea. A physical examination revealed hypoventilation of the left lung with dullness on 75% of the left lung. Left pleural effusion, approximately 90%, was observed on the chest X-ray. The patient was diagnosed with hemothorax, and a thoracostomy drained 1,350 mL of serosanguineous fluid, followed by the installation of a wet suction control system (Pleur-evac®). After 10 days, the patient was discharged. This case illustrates the diversity of hemorrhagic manifestations in envenomations by B. asper.
CASE REPORT
Snakebites are environmental and occupational health hazards that mainly affect rural populations worldwide.1 Bothrops asper inflicts most snake bites in Central America and northern South America, mostly affecting young agricultural workers.2 This species inhabits mainly tropical rainforests and tropical evergreen forests, but is also found in drier regions of tropical deciduous forests, thorn forests, and pine savanna in near proximity to rivers, streams, or lakes. It is also found in agricultural areas and plantations. As a result of deforestation and increasing agricultural activities, this venomous snake is forced to closer contact with humans, hence increasing the risk of bites. Adult B. asper specimens reach a large size and are abundant in agricultural settings, hence explaining the high incidence of cases inflicted by this species.3 Bothrops asper is capable of provoking severe envenomation associated with local and systemic clinical manifestations.2 Honduras reports nearly 700 snake bite cases per year, mostly inflicted by B. asper, predominantly in the northeastern regions of the country.4 We report an unusual case of envenomation by B. asper with the development of hemothorax as a hemorrhagic complication.
A 17-year-old male (1.71 m and 61 kg) from Gualaco, a rural area in the department of Olancho in northeastern Honduras, was driving a motorcycle, wearing sandals. When crossing a shallow river, his vehicle stopped and, when he stepped down, a snake bit him in the right foot. He was admitted to the emergency department at San Francisco Hospital, with a 3-day evolution of a B. asper snake bite, confirmed by personnel at the hospital, with experience in the identification of snakes, on the basis of a photograph of the snake found on the patient’s cellphone. From the photo, the size of the specimen was estimated as being between 70 and 90 cm. Two puncture wounds were located on the first toe of the right foot. A plant-based poultice had been applied to the site of the bite by a traditional healer. The history revealed that 1 hour after the bite, the patient began to experience headache, dizziness, generalized weakness, epistaxis, and paresthesia of the right lower limb. On admission, the patient was pale and sweaty, with distal coldness, a prolonged 5-second capillary filling, and dry mucous membranes. On arrival, blood pressure was 90/60 mmHg, heart rate 159 beats per minute, respiratory rate 32 breaths per minute, temperature 37°C, and oxygen saturation 98%. On physical examination, he had weak peripheral pulses and moderate edema in the lower right leg, which was most prominent in the anterolateral region of the foot. There were no signs of necrosis or neurological findings. Following the national guidelines for the management of snake bites, five vials of polyvalent antivenom (Suero antiofídico polivalente BIOL, Instituto Biológico Argentino, Buenos Aires, Argentina) were administered (diluted in 500 mL dextrose solution) and prophylactic antibiotic coverage with intravenous (IV) ceftriaxone (1 g i.v. every 12 hours) was initiated. No early adverse reactions to antivenom administration were observed. On admission, the abnormal laboratory findings were as follows: prothrombin time (PT), 43 seconds (normal range: 11–14 seconds); partial thromboplastin time (PTT), 125 seconds (normal range: 35–45 seconds); and hemoglobin, 9.7 g/dL (normal range: 11–16 g/dL). The patient received a transfusion with one unit of packed red blood cells and another unit of fresh frozen plasma. A significant improvement in PT and PTT controls was subsequently observed (13 seconds and 38 seconds, respectively).
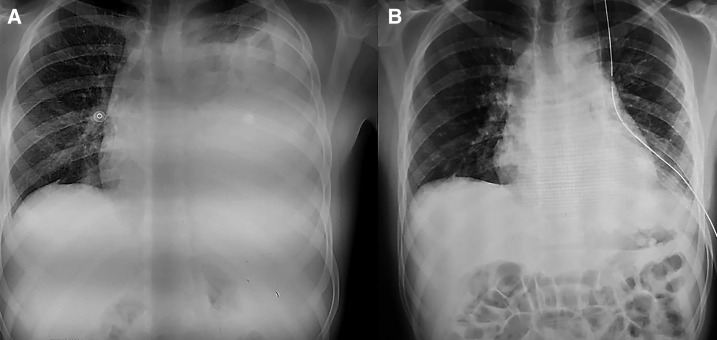
On the second day of admission, the patient developed dyspnea. A physical examination revealed hypoventilation of the left lung with percussive dullness of 75% of the pulmonary surface. The chest X-ray, with the patient in the upright position, showed effusion (approximately 90%) of the left pleural cavity (Figure 1). A complete blood count revealed a rapid fall in the hemoglobin level from 9.7 g/dL to 6.3 g /dL. The patient was diagnosed with a hemothorax. A minimal thoracostomy drained 1,350 mL of serosanguineous fluid, followed by the installation of a Pleur-evac wet suction control system (Wayne, PA). In the subsequent 48 hours, another 250 mL was drained. Because of blood loss, two more units of packed red blood cells were transfused, improving the patient’s hemodynamic state. On the fifth day after admission, the patient showed a marked clinical improvement, and a complete resolution of the pleural effusion was observed in a chest radiograph. By the end of the sixth day, only 55 mL of serous fluid was drained and the wet suction control system was removed. The patient’s general condition continued to improve, and he was discharged after 10 days. Table 1 summarizes the timeline of this case.
Figure 1.
(A) Posteroanterior chest radiograph at admission. (B) Chest radiograph showing complete resolution of the hemothorax.
Table 1.
Timeline of the case
| April 6, 2018 |
|
| April 7, 2018 |
| 08:00 hours: The patient is attended by a traditional healer who applies a plant-based poultice over the bitten area |
| April 8, 2018 |
| The patient refers moderate edema in the bitten region and abdominal pain, dizziness, generalized weakness, and paresthesia in the bitten limb |
| April 9, 2018 |
| 15:00 hours: The patient is admitted to the Emergency Service of Hospital San Francisco, where clinical management starts. Physical examination and laboratory tests are carried out. Five vials of antivenom are administered |
| 22:00 hours: Deterioration of the general condition is observed, together with a mild respiratory difficulty |
| April 10, 2018 |
| 10:50 hours: On physical examination, the patient shows decreased vesicular breath sounds and dullness on percussion |
| 12:00 hours: X-ray analysis is carried out with the patient in the upright position, revealing pleural effusion in the left lung. A thoracic tube (Pleur-evac) is placed |
| 13:00 hours: 1,350 mL of a serosanguineous fluid is collected |
| April 11, 2018–April 12, 2018 |
| An additional volume of 250 mL is drained by Pleur-evac. Two units of packed red blood cells are transfused because of the blood loss |
| April 13, 2018 |
|
| April 14, 2018 |
|
| April 19, 2018 |
|
DISCUSSION
Snakebites are a serious public health concern in Central America. An estimated 5,500 victims present at Central American hospitals annually, but these figures are likely underreported. In Central and other parts of Latin America, as well as in Asia and Africa, an unknown number of snakebite victims are never treated in medical facilities (being instead managed with traditional medicine) or die without examination and/or official reporting.5
Envenomations by B. asper in Central America can be severe, with local and systemic manifestations, including tissue necrosis, edema, blistering, coagulopathies, systemic hemorrhage leading to hemodynamic disturbances, and acute kidney injury.6 The identification of the snake responsible for the bite is often difficult, and a syndromic approach is instead used for diagnosis and management in Central America, whereby patients presenting the described local and systemic manifestations are treated with polyvalent antivenom, which is effective against the venoms of viperid snakes in the region.7 In turn, the severity of these envenomations depends on several factors, such as the amount of venom injected, the anatomical site of the bite, the size and physiological condition of the victim, and the delay in antivenom administration.7 In the case described in this report, a significant delay occurred between the bite and the arrival to the hospital, thus complicating the evolution of the case.
The patient presented a severe systemic hemorrhage, evidenced by the development of hemothorax and by a drastic drop in the hemoglobin concentration in blood, justifying the transfusions of packed red blood cells. Systemic hemorrhage induced by viperid venoms is caused by the action of hemorrhagic metalloproteinases, which degrade the basement membrane of capillary vessels, causing extravasation.6,8,9 In addition, the hemostatic alterations induced by procoagulant enzymes in the venom contribute to the widespread bleeding observed in these cases, by generating defibrinogenation.8 The clinical manifestations of such hemorrhagic activity vary between cases; in the patient studied in this report, the main hemorrhagic effect was hemothorax. The systemic bleeding acts in concert with other manifestations of envenomations, such as increment in vascular permeability, myocardial damage, and the action of other hypotensive components, all of which may cause hemodynamic alterations leading to cardiovascular collapse.8
A hemothorax, as a complication after a snake bite by B. asper, has only been reported once, by Luzardo in 1962, from a series of six autopsies, including three B. asper bite victims. He reported hemorrhages in multiple organs, including the lungs.10 A similar case of hemothorax was reported in a girl in India bitten by another viperid species, Echis carinatus.11 A similar effect was observed in an experimental study by Escalante et al. in 2003. The intravenous injection of a purified hemorrhagic metalloproteinase from the venom of a closely related viperid snake, Bothrops jararaca, induced a rapid and prominent hemorrhage in the lungs. Conspicuous ultrastructural alterations in the cells of the alveolocapillary unit—that is, capillary endothelial cells and type I pneumocytes—showed a characteristic pattern of “regional alveolar damage,” associated with extravasation. These pathological effects were observed while the whole-blood bleeding and clotting time, as well as the fibrinogen levels, were not affected, implying that the disruption of microvessels induced by hemorrhagic toxins is sufficient to cause systemic bleeding.12 In the present case, the lack of prominent local tissue pathology (i.e., only edema was observed), together with systemic manifestations associated with bleeding and coagulopathies, suggests that a portion of the venom injected may have been absorbed by the intravenous route.
Intravenous antivenom administration is the mainstay in the therapy of snakebite envenomation.13 The polyvalent antivenom used in Central America for the treatment of viperid snakebite envenomations is highly effective in the neutralization of hemorrhagic activity of B. asper venom because hemorrhagic metalloproteinases, especially those responsible for systemic bleeding, are highly immunogenic.14 In this particular case, however, the patient arrived to the hospital 3 days after the bite; by then, it is likely that systemic bleeding had occurred before the administration of the antivenom, as evidenced by the hemothorax and the drop in hemoglobin concentration. In these circumstances, in addition to antivenom administration, to neutralize circulating toxins, it was necessary to compensate with transfusions for the pronounced blood loss. This case is an example of the broad and sometimes unexpected clinical spectrum exhibited by victims of snakebite envenomation, especially in species such as B. asper, which are capable of injecting a large volume of venom and inflicting severe systemic envenomation. There is a global need for improving snakebite surveillance and for the allocation of resources to prevent and to treat people exposed to this important neglected public health problem. In rural areas where snakebites are endemic, it is important to promote education campaigns in the population to avoid unnecessary delays in the transportation of patients to health facilities where antivenom and other therapeutic resources are provided, hence preventing complications such as the one described in this patient.
Acknowledgements:
We thank our colleague Dr. Fernando Lozano from Hospital Universitario Virgen del Valme in Sevilla, who provided insight and expertise that greatly assisted us with the research and consequently improved the manuscript. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.Chaves LF, Chuang GW, Sasa M, Gutiérrez JM, 2015. Snakebites are associated with poverty, weather fluctuations, and El Niño. Sci Adv 1: e1500249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otero-Patiño R, 2009. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon 54: 998–1011. [DOI] [PubMed] [Google Scholar]
- 3.Sasa M, Wasko DK, Lamar WW, 2009. Natural history of the terciopelo Bothrops asper (Serpentes: Viperidae) in Costa Rica. Toxicon 54: 904–922. [DOI] [PubMed] [Google Scholar]
- 4.Solórzano JO, Aguilar NC, Banegas E, 2016. Mordedura de Serpiente. Boletín Epidemiológico Años 2014–2015. Tegucigalpa, Honduras: Secretaria de Salud. [Google Scholar]
- 5.Gutiérrez JM, 2014. Current challenges for confronting the public health problem of snakebite envenoming in Central America. J Venom Anim Toxins Incl Trop Dis 20: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutiérrez JM, Rucavado A, Chaves F, Díaz C, Escalante T, 2009. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon 54: 958–975. [DOI] [PubMed] [Google Scholar]
- 7.Gutiérrez JM, 2010. Snakebite envenomation in Central America. Mackessy SP, ed. Handbook of Venoms and Toxins of Reptiles. Boca Raton, FL: CRC Press, 491–507. [Google Scholar]
- 8.Gutiérrez JM, Escalante T, Rucavado A, 2009. Experimental pathophysiology of systemic alterations induced by Bothrops asper snake venom. Toxicon 54: 976–987. [DOI] [PubMed] [Google Scholar]
- 9.Escalante T, Rucavado A, Fox JW, Gutiérrez JM, 2011. Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J Proteomics 74: 1781–1794. [DOI] [PubMed] [Google Scholar]
- 10.Luzardo ML, 1962. Aspectos hemorrágicos de las mordeduras de serpientes. Hipofibrinogenemia como hallazgo importante. Investigación Clínica 3: 29–30. [Google Scholar]
- 11.Singh V, Digra SK, Slathia SS, Kakkar T, 2012. Hemothorax following snakebite. Indian Pediatr 49: 242–243. [PubMed] [Google Scholar]
- 12.Escalante T, Núñez J, Moura-da-Silva AM, Rucavado A, Theakston RDG, Gutiérrez JM, 2003. Pulmonary hemorrhage induced by jararhagin, a metalloproteinase from Bothrops jararaca snake venom. Toxicol Appl Pharmacol 193: 17–28. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA, 2017. Snakebite envenoming. Nat Rev Dis Primers 3: 17079. [DOI] [PubMed] [Google Scholar]
- 14.Gutiérrez JM, Lomonte B, Sanz L, Calvete JJ, 2014. Immunological profile of antivenoms: preclinical analysis of the efficacy of a polyspecific antivenom through antivenomics and neutralization assays. J Proteomics 105: 340–350. [DOI] [PubMed] [Google Scholar]