Abstract.
The pig is the natural intermediate host of Taenia solium, a parasite causing significant burden of disease in both humans and pigs. Porcine cysticercosis is traditionally detected via tongue palpation and slaughterhouse meat inspection, both with limited sensitivity. Serum antibody detection has a better performance; however, it does not discriminate past from present infection. Serum antigen detection can demonstrate viable infection and gives a good estimate of parasitic load. This study evaluated a sandwich antigen-detection ELISA using monoclonal antibodies (MoAbs) 158C11 and 60H8 for the diagnosis of viable cysticercosis in pigs. Serum samples were used from 35 naturally T. solium cysticerci–infected pigs, 31 cysticercosis-negative pigs, and 22 pigs with Taenia hydatigena infection (to assess cross-reactions). Positive cysticercosis samples were subcategorized at necropsy according to parasitic burden as mild (1–10 viable cysts, n = 10), moderate (11–100 cysts, n = 5), or severe infection (more than 100 cysts, n = 20). This Ag-ELISA showed a sensitivity of 82.9% and a specificity of 96.8% when not considering cross-reactions with T. hydatigena. Hundred percentage of severely infected, 80% of moderately infected, and 50% of mildly T. solium–infected pigs tested positive. Twenty of 22 pigs with only T. hydatigena infections were positive, with 13 reaching saturating levels in the ELISA. The Ag-ELISA revealed the presence of live cysts and is, thus, a fairly reliable test to monitor experimental infection, response to treatment, and follow-up in animal models of cysticercosis. It should, however, be carefully interpreted when used in regions where T. hydatigena is endemic in pigs.
INTRODUCTION
Cysticercosis, an infection caused by the larval stage of Taenia solium, is a challenging public health problem affecting human and porcine populations in endemic areas.1,2 This parasite frequently infects the human central nervous system, causing neurocysticercosis (NCC), the most prominent cause of acquired epilepsy in developing countries.3,4 In addition, NCC is an increasing problem in developed countries due to migrant carriers from endemic areas.5–7
Pigs are intermediate hosts of this parasite, and in endemic areas, the diagnosis of porcine cysticercosis is mainly performed by postmortem inspection of carcasses at the slaughterhouse or via tongue palpation at the point of sale. Meat inspection examines selected parts of the carcasses, and mild infections can, therefore, go undetected. The reported sensitivity of pork inspection to detect infected pigs is less than 40%.8–10 Premortem diagnosis by tongue examination, a diagnostic method consisting of palpation or visual inspection of the tongue for nodules, is only sensitive in highly infected pigs.8,11,12 Serum antibody detection in pigs has similar restrictions as in humans, whereby the presence of antibodies does not imply patency or active infection.
Antigen-detection tests, mainly ELISA (Ag-ELISA), can demonstrate active infection and antigen levels that are associated with parasite burden.13–16 Harrison et al.16 initially developed an ELISA based on the monoclonal antibody HP10 to detect parasite glycoproteins secreted by and present on the surface of Taenia saginata cysticerci. Following this, Brandt et al.13 produced IgM monoclonal antibodies (MoAbs 12G5/2H8) against excretory and secretory (ES) products of T. saginata cysticerci and applied these MoAbs in a double antibody sandwich ELISA. The performance of this ELISA was improved by Van Kerckhoven,17 who produced IgG isotypes (158C11/60H8) and pretreated the sera by heating. This pretreatment of sera was further optimized by Dorny et al. This double sandwich ELISA can efficiently detect circulating T. saginata cysticercus antigen in cattle and T. solium cysticercus antigen in humans and pigs.13,18–20
Diagnosis of porcine cysticercosis is needed to assess the effect of interventions for controlling transmission.21 Currently, no studies have been conducted on diagnostic sandwich Ag-ELISA testing on pigs in a controlled, experimental condition. Many diagnostic studies of porcine cysticercosis to date have been performed in rural community settings where anecdotal evidence has suggested the presence of cysticercosis, but without a confirmation of cysts observed in pig carcass dissection,22 or without enzyme-linked immunoelectrotransfer blot (EITB) antibody assessments.23 In Latin America, there has been no prior in-depth study testing B158/B60 Ag-ELISA. Therefore, the aim of the present study was to assess the sensitivity, specificity, and Taenia hydatigena cross-reactivity of the B158/B60 Ag-ELISA diagnostic test in pigs in a controlled environment by using MoAb IgG (158C11/60H8) to capture ES antigens in well-defined porcine serum samples.
MATERIALS AND METHODS
Study facilities and serum samples.
This study was performed at the School of Veterinary Medicine at the National University of San Marcos in Lima, Peru. Defined serum samples were obtained from infected and noninfected pigs, centrifuged, and stored at −20°C. Positive samples were obtained from 35 well-documented naturally T. solium cysticercus–infected pigs. These pigs were acquired from a highly endemic area in Peru (Huancayo, Junín) and transported to the veterinary facilities in Lima. All pigs were positive on tongue palpation and EITB.24 Their burden of infection was subsequently determined from a detailed necropsy25 during which the entire carcass was dissected and carefully sliced12 to determine the presence of cysticerci. The veterinary team examined all tissues, including the brain, heart, and tongue, and determined the burden of cysticercosis by the number and stage of cysts. Only the number of viable cysts was registered for this analysis; degenerating and calcified cysts were not considered because they are unlikely to release antigens to the circulation.26 Lungs, liver, and the gastrointestinal tract were also examined to rule out coinfection with other parasites. Negative samples were obtained from 62 pigs raised on an industrial farm in Lima, shown to be negative for cysticercosis by necropsy and EITB test, including 31 pigs negative to other helminths and 31 with other helminthic infections. We chose not to include a field pig control group because of the uncertainty related to exposure or infection with other endemic cestodes that could result in specific cross-reactions that we would not be aware of.
Antigen detection by sandwich ELISA.
A monoclonal antibody (158C11/60H8)–based sandwich Ag-ELISA was performed using polystyrene ELISA plates Nunc-Immuno™ Modules Loose (469949) F8 MaxiSorp (Thermo Scientific Nunc, Dublin, Ireland). Two IgG MoAbs against ES products of T. saginata cysts, B158C11A10 and biotinylated B60H8A4, were developed at the Institute of Tropical Medicine in Antwerp, Belgium, and were provided to be tested in this study.10 Serum samples were pretreated with 5% trichloroacetic acid (5% TCA) to separate immune complexes and improve assay sensitivity as described by De Jonge et al.27 in 1987 and Draelants et al.28 in 1995. Sera and 5% TCA were combined at the same volume in vials for 20 minutes at room temperature and then centrifuged at 12,000 rpm for 9 minutes, after which the supernatant was neutralized using sodium bicarbonate/bicarbonate buffer (pH = 9.6, 60 mM v/v).
Polystyrene ELISA plates were sensitized with 100 µL of the MoAb B158C11A10 (5 µg/mL in carbonate (CO3) buffer, pH = 9.6 M 0.06) and then incubated in a shaker for 30 minutes at room temperature. The plates were washed once with phosphate-buffered saline 0.05% Tween 20 (PBS-T20), blocked with 100 µL of PBS-T20 plus 1% newborn calf serum (PBS-T20/NCBS), and incubated under gentle shaking for 15 minutes at 37°C. After this incubation, the blocking solution was removed (without washing) and 100 µL of serum samples, pretreated with 5% TCA as described previously, was added to each well, and the plates were incubated at 37°C for 15 minutes. After washing (five times), 100 µL of the biotinylated MoAb B60H8A4 (0.66 mg/mL diluted in PBS-T20/NCBS) was added as detector antibody and incubated for 15 minutes at 37°C on a shaker. After another washing (five times), 100 µL of horseradish peroxidase streptavidin (Jackson Immunoresearch Lucron Bioproducts 016-030-084) diluted in blocking solution (0.1 µg/mL in PBS-T20/NCBS) was added and incubated for 15 minutes at 37°C with gentle shaking. The plates were washed again five times, the substrate/chromogen solution of 100 µL of ortho-phenylenediamine dihydrochloride (DAKO [Glostrup, Denmark] #S2045 at 4°C) and H2O2 diluted in citrate buffer was added, and the plates incubated in the dark for another 15 minutes at room temperature. Finally, 50 µL of H2SO4 (4N) was added to end the reaction. The plates were read at 490 nm and 650 nm with a Bio-Rad 3550 microplate reader (BIO-RAD, Hercules, CA).
Data analysis.
The cutoff value for a positive ELISA result was calculated using a serum pool composed of samples of well-defined negative pigs and set as the mean optical density (OD) value plus three standard deviations. Sensitivity and specificity were calculated comparing the OD result with confirmatory necropsy as definitive diagnosis of active cysticercosis. In addition, receiver operating characteristic (ROC) analysis was performed to evaluate alternative cutoff values. Mean OD values categorized by parasite burden in cysticercotic pigs and pigs with T. hydatigena were analyzed using a linear regression model. Pigs with parasitic infections other than T. solium were evaluated for cross-reactions. The calculations and graphics were performed using Stata software (StataCorp, College Station, TX).
RESULTS
Thirty-five positive pigs (defined by positive serology on EITB, positive tongue examination, and detailed necropsy) were identified and subcategorized as highly infected (n = 20 with more than 100 viable cysts), moderately infected (n = 5 pigs with 11–100 viable cysts), and mildly infected (n = 10 pigs with 1–10 viable cysts). No other helminths were identified at macroscopic examination in these 35 pigs. Thirty-one of 62 pigs that were seronegative for cysticercosis on EITB and had no evidence of cysticercosis or other helminthic infections in detailed necropsy were used as negative controls. In addition, samples from 26 cysticercosis-negative pigs infected with only one helminth (22 with T. hydatigena, two with Echinococcus granulosus, and two with Fasciola hepatica) and five pigs infected with more than one helminth (four with both T. hydatigena and E. granulosus, one with T. hydatigena and F. hepatica) were used to assess potential cross-reactions.
Test sensitivity and specificity.
The analysis was performed using serum samples from positive pigs without coinfections and negative pigs at necropsy; thus, 35 positive and 31 negative serum samples were considered. Samples from pigs with concomitant parasitic infections were not analyzed here but included in a separate analysis for cross-reactions. With the calculated cutoff OD value of 0.422, this Ag-ELISA had a sensitivity of 82.9% (29/35, 95% CI: 69.7–96.0) and a specificity of 96.8% (30/31, 95% CI: 90.2–100.0) (Table 1). The ideal cutoff as defined by ROC analysis was 0.393, where 90.9% of the results were correctly classified, and the sensitivity and specificity were 85.7% and 96.8%, respectively. The area under the ROC curve obtained with these 66 observations was 0.93 (95% CI: 0.87–0.99).
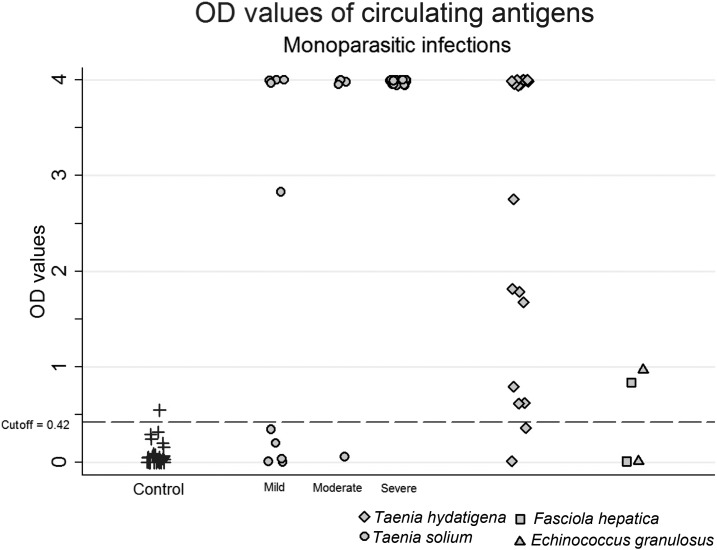
Six samples of infected pigs were not identified by B158/B60 Ag-ELISA; these six pigs had mild (5/10) and moderate (1/5) infections. All highly infected pigs (20/20) were positive with saturating levels of antigen (OD 4.0) (Figure 1).
Figure 1.
Circulating antigen levels in well-defined pig serum samples.
Pigs with moderate or severe infections had higher OD values than pigs with mild or negative infections. The mean OD value for mild infection was 1.96, and for moderate and heavy infections, considered as a composite group, the mean OD was 3.84 (P < 0.01).
Twenty samples from 22 pigs (90.9%) with larval T. hydatigena mono-infection had positive results in the Ag-ELISA, and 13 (59.1%) had saturating levels of circulating antigens (OD 4.0) (Figure 1). The mean OD value for these 22 T. hydatigena–infected pigs was 2.84, significantly higher than that for noninfected pigs (0.08, P < 0.01). One of two pigs with F. hepatica mono-infection and one of two pigs with hydatid cysts mono-infection had positive OD values but with low values (0.85 and 0.98, respectively).
DISCUSSION
The assessed B158/B60 Ag-ELISA was more than 80% sensitive in detecting ES T. solium products in necropsy-confirmed, naturally infected cysticercotic pigs, and almost 97% specific in pigs with no helminth infections at necropsy. The detection capacity of this assay increased in relation to the cyst burden of the pig, from 50.0% in pigs with 10 cysts or less, to reach 100.0% (20/20) in pigs infected with more than 100 cysts. A large proportion of positive pigs (29/35) had saturating levels of antigens, including all moderately and highly infected pigs, as well as four of the five antigen-positive pigs with mild infections. This supports a previous study which also used the B158/B60 Ag-ELISA, where obviously infected pigs had an OD to cutoff ratio of greater than 10.29 As expected, this assay cross-reacted with T. hydatigena. Cross-reaction with T. hydatigena has been previously described by Dorny et al.30 and demonstrated in assays with HP10 Ag-ELISA31 and B158/B60 Ag-ELISA.23 The present study confirms the cross-reactivity, with 20 of the 22 T. hydatigena–infected pigs (90.9%) testing positive in 158C11/60H8 Ag-ELISA, 13 of which had saturating OD results (59.1%).
The low sensitivity of the Ag-ELISA in detecting low-burden cysticercosis infection, as demonstrated in this study, has been described before. The minimum number of cysts reported by Brandt et al.13 for detecting viable T. saginata cysticerci in cattle was 88, and Van Kerckhoven et al.17 reported that only six of 47 cattle with less than 50 viable T. saginata cysts were detected. An optimized version of this assay has been shown to have a very high analytical sensitivity in pigs, detecting antigen concentrations as low as 3.9 ng/mL,14 and may have better diagnostic sensitivity. Recently, a study documented that this Ag-ELISA has 67% specificity and 68% sensitivity to detect T. solium–infected pigs, with the sensitivity increasing to 91% when detecting pigs with one or more viable cysts, and to 100% in pigs with more than 10 viable cysts.23 The suboptimal sensitivity in pigs with mild cyst burden reported in these studies limits its use as a general screening tool for individual pig diagnosis or for control purposes. It may also affect its use in human diagnosis, where individuals with one or a few intraparenchymal brain cysts are frequently antigen negative.32 Leaving half of the population of mildly infected pigs untreated will likely sustain the endemicity of taeniasis and cysticercosis. Similar limitations have also been pointed out for antigen-detection ELISAs using the MoAb HP10.16
All but one of the pigs negative to other cestode infections (30/31) were antigen negative. However, the assay did cross-react with T. hydatigena, which causes cysticercosis in ruminants and pigs (Cysticercus tenuicollis), which was also to be expected because the MoAbs used in the assay were raised against T. saginata antigens. Most pigs harboring T. hydatigena cysts were antigen positive (20/22, 90.9%), and more than half had high levels of circulating antigen to the point of saturating the test response. This crossed response has previously been described in this10 and other assays.33–35 This series confirms the cross-reaction and provides important data on the antigen levels detected by the test in these animals.
In this series, we found apparent cross-reactions in one pig with F. hepatica and one pig with E. granulosus, both with low antigen levels, quite close to the cutoff point. However, we cannot rule out mild T. hydatigena infections missed at necropsy in these pigs, and the low numbers of animals tested preclude further assessment. In humans, the test did not show cross-reactions with sera from patients with confirmed infection with Schistosoma, hydatid cysts, Ascaris, Trichuris, filaria, Entamoeba, Plasmodium, or Trypanosoma.18
Our study tends to overestimate the positive predictive value by making it unlikely that the control animals test positive because of less exposure to potentially cross-reactive infections in field conditions. Besides T. hydatigena, pigs could be exposed to other cestode parasites that could potentially cross-react with the used MoAbs. This drawback mostly affects cross-reactions more than the test’s true specificity and does not undermine the high degree of cross-reactivity with T. hydatigena described in our results.
Diagnosis of porcine cysticercosis has major limitations in that tongue examination and meat inspection grossly underestimate the actual prevalence of the parasitic infection in animals. The sensitivity of tongue examination, the most practical method to detect infected pigs in rural areas, is poor (43–70%, compared with detailed carcass inspection). It primarily detects heavily infected pigs12,36,37 and very rarely detects pigs with fewer than 100 viable cysts.10 Antibody-detecting assays have also demonstrated poor sensitivity and specificity.12,38
This Ag-ELISA assay has been used to assess burden of infection in epidemiological studies of porcine cysticercosis.10,36 Our results show that the assessed B158-B60 MoAb-based Ag-ELISA has reasonable sensitivity and specificity values for the diagnosis of porcine cysticercosis; however, its use as a screening tool or primary diagnosis in slaughterhouses or epidemiological interventions in pigs should take into account its suboptimal sensitivity in pigs with low parasitic burdens, or a possible overdiagnosis due to cross-reaction in areas endemic for T. hydatigena. Nevertheless, its capacity to detect the presence of living cysts makes this Ag-ELISA a unique test in animal models. It shows promise in monitoring experimental infection or response to treatment.39 In humans, its use as a monitoring tool in patients who receive antiparasitic treatment has shown promising results in chronic and refractory types of NCC, such as subarachnoid or ventricular NCC. In addition, its use as a complementary test could be critical in endemic areas where neuroimaging evaluations are often scarce and expensive.40
Acknowledgments:
We wish to thank Natalie Elkheir for her assistance in reviewing and editing an earlier version of this manuscript.
REFERENCES
- 1.Maurice J, 2014. Of pigs and people—WHO prepares to battle cysticercosis. Lancet 384: 571–572. [PubMed] [Google Scholar]
- 2.García HH, Nash TE, Del Brutto OH, 2014. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 13: 1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, Dickey M, Reynolds S, Stoner JA, 2010. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Neglect Trop Dis 4: e870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García HH, et al. 2014. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis 14: 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schantz PM, Wilkins PP, Tsang VCW, 1998. Immigrants, imaging and immunoblots: the emergence of neurocysticercosis as a significant public health problem. Scheld WM, Craig WA, Hughes JM, ed. Emerging Infection 2. Washington, DC: ASM Press, 213–241. [Google Scholar]
- 6.Wallin MT, Kurtzke JF, 2004. Neurocysticercosis in the United States: review of an important emerging infection. Neurology 63: 1559–1564. [DOI] [PubMed] [Google Scholar]
- 7.Cantey PT, Coyle CM, Sorvillo FJ, Wilkins PP, Starr MC, Nash TE, 2014. Neglected parasitic infections in the United States: cysticercosis. Am J Trop Med Hyg 90: 805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phiri IK, Dorny P, Gabriël S, Willingham AL, 3rd, Sikasunge C, Siziya S, Vercruysse J, 2006. Assessment of routine inspection methods for porcine cysticercosis in Zambian village pigs. J Helminthol 80: 69–72. [DOI] [PubMed] [Google Scholar]
- 9.Ngowi HA, Kassuku AA, Maeda GE, Boa ME, Willingham AL, 2004. A slaughter slab survey for extra-intestinal porcine helminth infections in northern Tanzania. Trop Anim Health Pro 36: 335–340. [DOI] [PubMed] [Google Scholar]
- 10.Dorny P, Phiri IK, Vercruysse J, Gabriël S, Willingham AL, 3rd, Brandt J, Victor B, Speybroeck N, Berkvens D, 2004. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int J Parasitol 34: 569–576. [DOI] [PubMed] [Google Scholar]
- 11.Sato MO, et al. 2003. Evaluation of tongue inspection and serology for diagnosis of Taenia solium cysticercosis in swine: usefulness of ELISA using purified glycoproteins and recombinant antigen. Vet Parasitol 111: 309–322. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez AE, et al. 1990. Prevalence and comparison of serologic assays, necropsy, and tongue examination for the diagnosis of porcine cysticercosis in Peru. Am J Trop Med Hyg 43: 194–199. [DOI] [PubMed] [Google Scholar]
- 13.Brandt JR, Geerts S, De Deken R, Kumar V, Ceulemans F, Brijs L, Falla N, 1992. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol 22: 471–477. [DOI] [PubMed] [Google Scholar]
- 14.Deckers N, Kanobana K, Silva M, Gonzalez AE, García HH, Gilman RH, Dorny P, 2008. Serological responses in porcine cysticercosis: a link with the parasitological outcome of infection. Int J Parasitol 38: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 15.Nguekam A, Zoli AP, Vondou L, Pouedet SM, Assana E, Dorny P, Brandt J, Losson B, Geerts S, 2003. Kinetics of circulating antigens in pigs experimentally infected with Taenia solium eggs. Vet Parasitol 111: 323–332. [DOI] [PubMed] [Google Scholar]
- 16.Harrison LJ, Joshua GW, Wright SH, Parkhouse RM, 1989. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol 11: 351–370. [DOI] [PubMed] [Google Scholar]
- 17.Van Kerckhoven I, Vansteenkiste W, Claes M, Geerts S, Brandt J, 1998. Improved detection of circulating antigen in cattle infected with Taenia saginata metacestodes. Vet Parasitol 76: 269–274. [DOI] [PubMed] [Google Scholar]
- 18.Erhart A, et al. 2002. Taenia solium cysticercosis in a village in northern Viet Nam: seroprevalence study using an ELISA for detecting circulating antigen. Trans R Soc Trop Med Hyg 96: 270–272. [DOI] [PubMed] [Google Scholar]
- 19.Nguekam JP, Zoli AP, Ongolo-Zogo P, Dorny P, Brandt J, Geerts S, 2003. Follow-up of neurocysticercosis patients after treatment using an antigen detection ELISA. Parasite 10: 65–68. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez S, Dorny P, Tsang VC, Pretell EJ, Brandt J, Lescano AG, Gonzalez AE, Gilman RH, García HH, 2009. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis 199: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García HH, O’Neal SE, Gilman RH, 2016. Elimination of Taenia solium transmission in Peru. New Eng J Med 375: 1196–1197. [DOI] [PubMed] [Google Scholar]
- 22.Rojas RG, Patino F, Perez J, Medina C, Lares M, Mendez C, Aular J, Parkhouse RME, Cortez MM, 2019. Transmission of porcine cysticercosis in the Portuguesa state of Venezuela. Trop Anim Health Prod [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23.Chembensofu M, et al. 2017. Re-visiting the detection of porcine cysticercosis based on full carcass dissections of naturally Taenia solium infected pigs. Parasite Vector 10: 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsang VC, Brand JA, Boyer AE, 1989. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 159: 50–59. [DOI] [PubMed] [Google Scholar]
- 25.Straw BJ, Meuten DJ, 1992. Physical Examination. Straw BJ, Mengeling WL, D´Allaire S, Taylor DJ, Ames IA, ed. Ames, IA: Iowa State University Press, 793–807. [Google Scholar]
- 26.Zea-Vera A, et al. 2013. Parasite antigen in serum predicts the presence of viable brain parasites in patients with apparently calcified cysticercosis only. Clin Infect Dis 57: e154–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Jonge N, Fillie YE, Deelder AM, 1987. A simple and rapid treatment (trichloroacetic acid precipitation) of serum samples to prevent non-specific reactions in the immunoassay of a proteoglycan. J Immunol Methods 99: 195–197. [DOI] [PubMed] [Google Scholar]
- 28.Draelants E, Brandt JR, Kumar V, Geerts S, 1995. Characterization of epitopes on excretory-secretory antigens of Taenia saginata metacestodes recognized by monoclonal antibodies with immunodiagnostic potential. Parasite Immunol 17: 119–126. [DOI] [PubMed] [Google Scholar]
- 29.Dermauw V, Ganaba R, Cisse A, Ouedraogo B, Millogo A, Tarnagda Z, Van Hul A, Gabriël S, Carabin H, Dorny P, 2016. Taenia hydatigena in pigs in Burkina Faso: a cross-sectional abattoir study. Vet Parasitol 230: 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorny P, Brandt J, Geerts S, 2004. Immunodiagnostic approaches for detecting Taenia solium. Trends Parasitol 20: 259–260; author reply 260–251. [DOI] [PubMed] [Google Scholar]
- 31.Cortez MM, Rojas GC, Parkhouse RME, 2018. The HP10 Taenia monoclonal antibody-based ELISA detects a similar protein in the vesicular fluid of Taenia hydatigena. Trop Anim Health Prod 50: 697–700. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez S, Wilkins P, Dorny P, 2012. Immunological and molecular diagnosis of cysticercosis. Pathog Glob Health 106: 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar D, Gaur SN, 1987. Serodiagnosis of porcine cysticercosis by enzyme-linked immunosorbent assay (ELISA) using fractionated antigens. Vet Parasitol 24: 195–202. [DOI] [PubMed] [Google Scholar]
- 34.Cheng RW, Ko RC, 1991. Cross-reactions between crude antigens of larval Taenia solium (Cysticercus cellulosae) and other helminths of pigs. Vet Parasitol 39: 161–170. [DOI] [PubMed] [Google Scholar]
- 35.Ko RC, Ng TF, 1998. Evaluation of excretory/secretory products of larval Taenia solium as diagnostic antigens for porcine and human cysticercosis. J Helminthol 72: 147–154. [DOI] [PubMed] [Google Scholar]
- 36.Phiri IK, Dorny P, Gabriël S, Willingham AL, 3rd, Speybroeck N, Vercruysse J, 2002. The prevalence of porcine cysticercosis in eastern and southern provinces of Zambia. Vet Parasitol 108: 31–39. [DOI] [PubMed] [Google Scholar]
- 37.Boa ME, Kassuku AA, Willingham AL, 3rd, Keyyu JD, Phiri IK, Nansen P, 2002. Distribution and density of cysticerci of Taenia solium by muscle groups and organs in naturally infected local finished pigs in Tanzania. Vet Parasitol 106: 155–164. [DOI] [PubMed] [Google Scholar]
- 38.Sciutto E, et al. 1998. Limitations of current diagnostic procedures for the diagnosis of Taenia solium cysticercosis in rural pigs. Vet Parasitol 79: 299–313. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez AE, Bustos JA, García HH, Rodriguez S, Zimic M, Castillo Y, Praet N, Gabriël S, Gilman RH, Dorny P, 2015. Successful antiparasitic treatment for cysticercosis is associated with a fast and marked reduction of circulating antigen levels in a naturally infected pig model. Am J Trop Med Hyg 93: 1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabriël S, Blocher J, Dorny P, Abatih EN, Schmutzhard E, Ombay M, Mathias B, Winkler AS, 2012. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Neglect Trop Dis 6: e1851. [DOI] [PMC free article] [PubMed] [Google Scholar]