Abstract.
Extended-spectrum β-lactamases (ESβLs) pose a serious problem in the treatment of urinary tract infections (UTIs). The ESβL-producing organism is an expanding global health problem. Therefore, screening for ESβL, detection of their drug-resistance pattern, and molecular characterization should be a continuous process. The present study was performed to determine the antibiotic resistance profile and the genetic characterization of ESβL isolates from hospital- and community-acquired UTIs. Two hundred fifty Enterobacteriaceae isolates were obtained from urine samples of outpatient clinic attendants and hospitalized patients at Kasr Al-Aini Hospital. By phenotypic screening tests, 100 ESβL isolates were detected among the studied groups. Furthermore, detection of beta-lactamase (bla) cefotaxime (CTX)-M, sulfhydryl variable, and temoneira ESβL genes was investigated by polymerase chain reaction. A subset of 25 CTX-M–positive isolates was further identified by gene sequencing technology. Among the 100 ESβL isolates, 66% were Escherichia coli and 34% were Klebsiella spp. There was no statistical difference in the prevalence of ESβL Enterobacteriaceae in community-acquired versus hospital-acquired UTIs. The susceptibility of all ESβL isolates to carbapenems was the most prevalent finding. In addition, all ESβL E. coli isolates were susceptible to fosfomycin, whereas all community-acquired ESβL isolates were susceptible to nitrofurantoin. A total of 98% of the ESβL isolates harbored bla-CTX-M genes, with CTX-M-15 being the most prevalent. It could be concluded that ESβL production is present at a high rate among Egyptian patients with hospital- and community-acquired UTI. The high prevalence of bla-CTX-M may suggest it as a candidate for molecular screening of ESβL.
INTRODUCTION
Urinary tract infection (UTI) presented the second most common cause of community- and hospital-acquired infections, with Gram-negative bacteria, particularly Escherichia coli, being the main causative organism.1 Although antimicrobial resistance is highly spread among health-care settings and community, the geographical regions showed a heterogeneous pattern of resistance and mutant bacterial strains will acquire drug-resistant genes.2 This will force diversion to more potent antibiotics; therefore, the interest to understand the underlying molecular mechanism of bacterial drug resistance will increase with time.3
The spread of extended-spectrum β-lactamase (ESβL) Enterobacteriaceae UTI is increasing worldwide.4,5 Although most of the ESβL producers are isolated from hospital-acquired UTIs,6,7 a higher rate was screened by Nisha et al.8 and Djuikoue et al.9 from community-acquired UTIs. Escherichia coli and Klebsiella pneumoniae are the most predominant ESβL Enterobacteriaceae in the clinical setting; this has contributed to their beneficial value in estimating the prevalence of ESβL producers among different infections.10–12
Although TEM and SHV are the most common ESβL genotypes, it has been reported that beta-lactamase (bla)-cefotaxime (CTX)-M is the most prevalent ESβL in UTIs worldwide.10,13–15 Presently, CTX-M-15 is the most widely disseminated genotype.15 Early detection and isolation of ESβL will allow proper infection control and targeted management15; therefore, continuous screening and genotyping of Enterobacteriaceae is necessary. The present study was aimed to screen the prevalence rate of ESβL Enterobacteriaceae among Egyptian community- and hospital-acquired UTI patients, to study the antibiotic susceptibility profile, and to determine the most prevalent ESβL genotypes among bacterial isolates.
MATERIALS and METHODS
This cross-sectional study involved 250 Enterobacteriaceae isolates, which were collected in 6 months duration from December 2016 to May 2017. Urine samples were collected in sterile containers from hospitalized patients and outpatient clinic attendants at different departments of Kasr Al-Aini Hospital. Written informed consent was obtained from all participants before the study, and confidentiality of collected data was guaranteed. The research study was approved by the Institutional Review Board of the Kasr Al-Aini Hospital and the Research Ethics Committee, Faculty of Medicine, Cairo University.
Sample collection.
Urine samples were collected in sterile, labeled containers. Catheterized patients or patients who received antibiotics at least 2 days before sample collection were excluded, and samples were transferred to the microbiology laboratory to be processed immediately. Urine samples were cultivated directly onto MacConkey agar plates by using a standard sterile calibrated loop and incubated at 37°C for 24–48 hours. Bacterial growth was examined after 24–48 hours of incubation. Urinary tract infection is confirmed when the bacterial colony count exceeds 105 per mL urine.16 Only one non-duplicated Enterobacteriaceae isolate per culture was considered. Enterobacteriaceae isolates were identified by colonial morphology, Gram-negative staining, and conventional biochemical tests, including motility indole ornithine, urease, citrate, and lysine decarboxylase tests.16 Infections wherein Enterobacteriaceae was isolated from patients 48 hours after admission were considered as hospital-acquired infections, whereas other positive Enterobacteriaceae infections were considered as community-acquired infections.
Screening of ESβL by disc diffusion.
The potentiality of Enterobacteriaceae to produce ESβL was assessed in bacterial isolates using the disc diffusion method. The test was carried out by inoculating a bacterial suspension with a turbidity equivalent to 0.5 McFarland standard on Mueller–Hinton agar; then, ceftazidime (CAZ; 30 µg), ceftriaxone (CRO; 30 µg), CTX (30 µg), and aztreonam (ATM; 30 µg) discs (Oxoid, Basingstoke, Hampshire, United Kingdom) were placed; and after overnight incubation, the test results were interpreted according to Clinical and Laboratory Standards Institute breakpoints.17
Phenotypic ESβL confirmatory tests.
Double-disc synergy test (DDST).
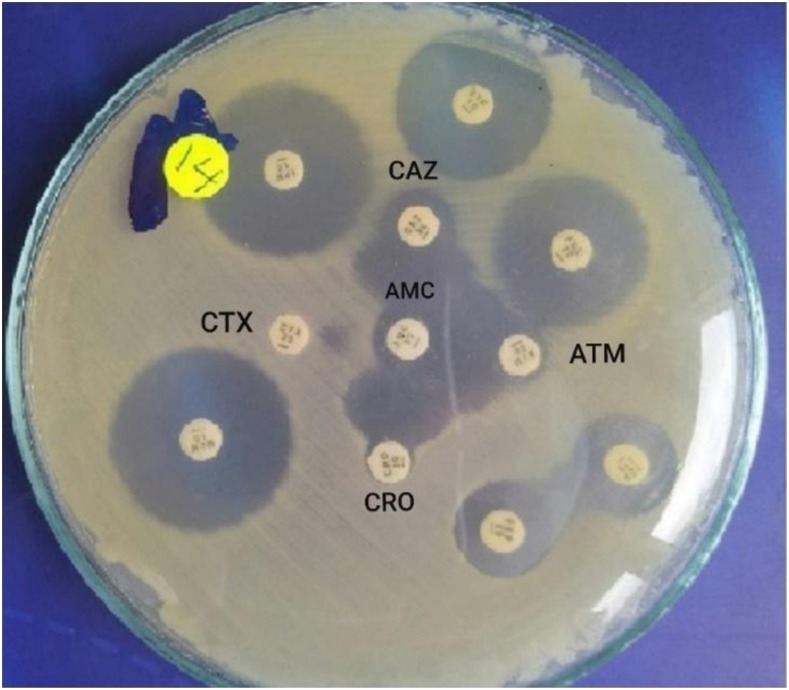
The positive ESβL isolates obtained by the screening test were spread on Mueller–Hinton agar plates; then CTX (30 µg), CRO (30 µg), CAZ (30 µg), and ATM (30 µg) discs were placed 20 mm apart from a clavulanic acid (amoxicillin + clavulanic acid) (20/10 µg) disc (Oxoid). An inhibition zone around any of the cephalosporin discs enhanced on the side of the clavulanic acid disc inferred positive ESβL isolates17,18 (Figure 1).
Figure 1.
Double-disc synergy test for screening of extended-spectrum β-lactamase (ESβL). The inhibition zones around the ceftazidime (CAZ), ceftriaxone (CRO), cefotaxime (CTX), and aztreonam (ATM) discs were augmented in the direction of the disc containing clavulanic acid, amoxicillin/clavulanic acid (AMC); this was interpreted as synergy, indicating positive ESβL. This figure appears in color at www.ajtmh.org.
Combination disc test (CDT).
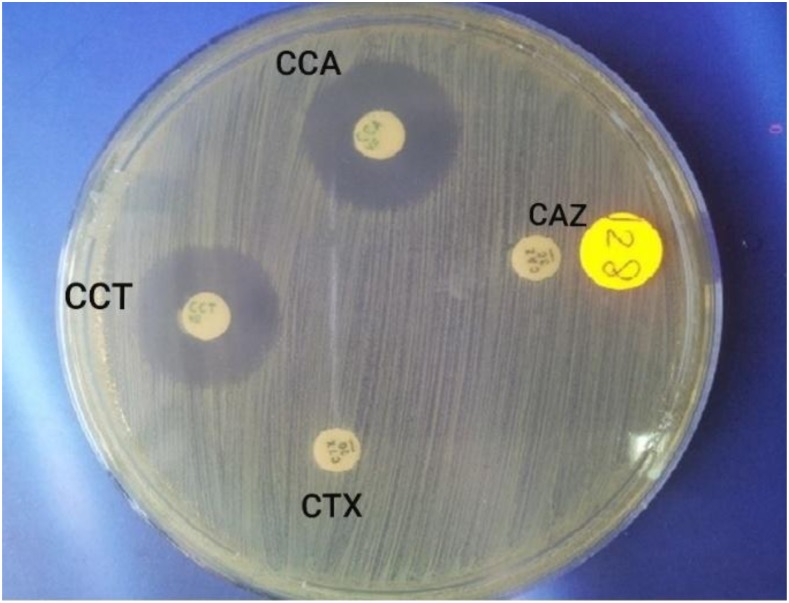
To assess the potential production of ESβL in bacterial isolates, a CDT was performed, in which a combination of antibiotic discs including CAZ disc (30 µg)/combined disc “CAZ + clavulanate (CCA) (30 µg/10 µg)” and CTX disc (30 µg)/combined disc “CTX + CCT (30 µg/10 µg)” was placed on Mueller–Hinton agar plates. Isolates were considered as ESβL positive when the inhibition zone diameter around one of the combination disc was > 5 mm than the inhibition zone of the corresponding cephalosporin disc. Unfortunately, the DDST has some limitations: in case of coexistence of ambler class-C β lactamases and ESβL, false-negative results can be obtained. Therefore, a combination of both DDST and CDT is required to confirm ESβL positivity17 (Figure 2). Klebsiella pneumoniae “ATCC 700603” and E. coli “ATCC 25922” were used as positive and negative control strains for ESβL production, respectively.
Figure 2.
Combination disc test for confirmation of extended-spectrum β-lactamase (ESβL). Ceftazidime (CAZ) disc (30 µg) and combined disc, ceftazidime (CCA)/clavulanate (CCA) (30 µg/10 µg), were placed 20 mm apart. Cefotaxime (CTX) disc (30 µg) and combined disc, cefotaxime (CCT)/CCA (30 µg/10 µg), were placed 20 mm apart. An increase of 5 mm or more in the zone diameter for either antimicrobial agent tested in combination with CCA vs. the zone diameter of the agent when tested alone confirmed the production of ESβL. This figure appears in color at www.ajtmh.org.
Antimicrobial susceptibility testing of ESβL isolates.
Kirby–Bauer disc diffusion technique was conducted to determine antimicrobial susceptibility. A panel of antibiotics was used for phenotypically confirming ESβL. They include Oxoid: amikacin (30 µg), ciprofloxacin (5 µg), levofloxacin (5 µg), nitrofurantoin (300 µg), trimethoprim–sulfamethoxazole (1.25/23.75 µg), gentamycin (10 µg), doxycycline (5 µg), ofloxacin (5 µg), amoxicillin–CCA (20/10 µg), piperacillin–tazobactam (100/10 µg), cefepime (30 µg), cefoxitin (30 µg), cefoperazone–sulbactam (75/30 µg), imipenem (10 µg), meropenem (10 µg), and ertapenem (10 µg). However, fosfomycin (200 µg) was used for E. coli only.17
Molecular detection of ESβL-coding genes.
Extended-spectrum β-lactamase producers were confirmed by PCR-based detection.18,19 DNA was extracted and purified from phenotypically confirmed ESβL isolates by using spin column technology (DNA Mini Kit; QIAamp®; Qiagen; Germantown, MD). All phenotypically confirmed ESβL producers were screened by multiplex PCR using the specific primers for TEM, SHV, and CTX-M ESβL genes (Table 1).19 Targeted genes were amplified using TaqPCR master mix (a ready-to-use PCR reagent optimized for better PCR amplification) (QIAGEN). The master mix contains 250 units of Taq DNA polymerase, 2× PCR buffer, 3 mM of MgCl2, and 400 mM of each nucleoside triphosphate. The amplified products were visualized by agarose gel electrophoresis; DNA bands of 590 bp, 440 bp, and 767 bp “expected molecular mass” were interpreted as positive specimen for bla-CTX-M, bla TEM, and bla SHV genes, respectively.19,20
Table 1.
Primers used for the detection of bla-CTX-M, TEM, and SHV ESβL genes20
| Target genes | Primer | Sequence (5′-3′) | Amplicon size (bp) |
|---|---|---|---|
| bla-CTX-M | Forward | TTT GCG ATG TGC AGT ACC AGT AA | 590 |
| Reverse | CGA TAT CGT TGG TGG TGC CAT A | ||
| bla TEM | Forward | CCGCATACACTATTCTCAGAATG | 440 |
| Reverse | CTCACCGGCTCCAGATTTATC | ||
| bla SHV | Forward | TGTATTATCTCCCTGTTAGCCACC | 767 |
| Reverse | GTATCCCGCAGATAAATCACCA |
CTX = cefotaxime; ESβL = extended-spectrum β-lactamase.
Sequencing of CTX-M–positive ESβL.
A subset of 25 CTX-M–positive isolates was further identified by gene sequencing technology; the amplified PCR product was purified using the GeneJET™ PCR purification kit (Thermo K0701; ThermoFisher Scientific, Waltham, MA) and sequenced with the Sanger ABI 3730 XL automated DNA sequencer (GATC Biotech, Konstanz, Germany) using primers listed in Table 1. Results obtained were in the form of a series of peaks in fluorescence intensity. The chromatogram peaks were used to identify the DNA sequence, and then the obtained sequences were analyzed using the online GenBank BLAST program, which is available at the National Center for Biotechnology Information.
STATISTICS
Data were analyzed using the Statistical Package for the Social Sciences version 24. Parametric data are presented by mean and standard deviation, whereas nonparametric parameters are presented by median and interquartile ratio. Frequency (count) and relative frequency (percentage) are used to present categorical data. The nonparametric Mann–Whitney test was used to compare quantitative skewed variables, whereas the Chi-square (χ2) test was used to compare categorical data. The Fisher exact test was used instead when the expected frequency was less than 5. P-values less than 0.05 were considered as statistically significant.21,22
RESULTS
Identification of isolates.
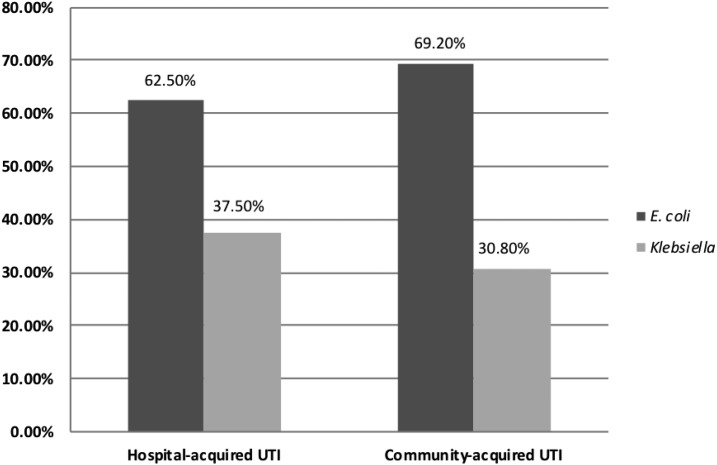
Two hundred and fifty Enterobacteriaceae isolates were isolated from urinary tract–infected patients; the majority was outpatient attendants (n = 134), whereas 116 patients were hospitalized. Females constituted 72% of the studied patients; their mean age was 42 ± 24.5 years in contrast to 46.12 ± 26.13 years for the male gender. Among the studied isolates, the prevalence of ESβL-producing strains was 40% (n = 100). Of them, 48 (48%) were hospital acquired and 52 (52%) were community acquired. There was no significant difference in the prevalence of ESβL hospital-acquired (48/116 [41.4%]) and ESβL community-acquired UTIs (52/134 [38.8%]), (P-value = 0.9). Comparing the distribution of bacterial isolates among the studied groups, all isolates were E. coli and Klebsiella spp.; E. coli isolates were significantly more frequent than Klebsiella spp. isolates among hospital- and community-acquired ESβL isolates (P-value = 0.007 and 0.001, respectively) (Figure 3).
Figure 3.
Distribution of extended-spectrum β-lactamase (ESβL)–producing Enterobacteriaceae among hospital- and community-acquired urinary tract infections (UTIs). All isolates were identified as Escherichia coli and Klebsiella spp.; E. coli was more frequently isolated than Klebsiella spp. among both hospital (P-value = 0.007) and community-acquired ESβL isolates (P-value = 0.001).
Antimicrobial susceptibility.
All ESβL isolates were tested against 21 antibiotics included in the study (Table 2). Extended-spectrum β-lactamase –producing Enterobacteriaceae isolates were highly resistant to cefepime and amoxicillin–clavulanic acid, and susceptible to carbapenems. All ESβL E. coli isolates were susceptible to fosfomycin. However, the resistance rate of ESβL E. coli isolates to fluoroquinolones was significantly higher than that of Klebsiella. Meanwhile, ESβL Enterobacteriaceae isolates from community-acquired infections were significantly more susceptible to nitrofurantoin than the ESβL isolates from hospital-acquired UTIs (Table 2).
Table 2.
Antimicrobial susceptibility pattern of the ESβL Enterobacteriaceae isolates
| Antibiotics | Total Enterobacteriaceae (n = 100), % | Escherichia coli (n = 66), % | Klebsiella spp. (n = 34), % | P- value | Hospital-acquired ESβL UTI (n = 48), % | Community- acquired ESβL UTI (n = 52), % | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | I + R | S | I + R | S | I + R | S | I + R | S | I + R | |||
| Imipenem | 100 | 0 | 100 | 0 | 100 | 0 | – | 100 | 0 | 100 | 0 | – |
| Meropenem | 100 | 0 | 100 | 0 | 100 | 0 | – | 100 | 0 | 100 | 0 | – |
| Ertapenem | 100 | 0 | 100 | 0 | 100 | 0 | – | 100 | 0 | 100 | 0 | – |
| Nitrofurantoin | 80 | 20 | 84.8 | 15.2 | 70.6 | 29.4 | 0.1 | 58.3 | 41.7 | 100 | 0 | < 0.001 |
| Cefoxitin | 76 | 24 | 69.7 | 30.3 | 88.2 | 11.8 | 0.1 | 72.9 | 27.1 | 78.8 | 21.2 | 0.5 |
| Piperacillin–tazobactam | 68 | 32 | 72.7 | 27.3 | 58.8 | 41.2 | 0.3 | 68.8 | 31.2 | 67.3 | 32.7 | 0.9 |
| Amikacin | 62 | 38 | 59.1 | 40.9 | 67.6 | 32.4 | 0.4 | 52.1 | 47.9 | 61.2 | 38.8 | 0.1 |
| Gentamicin | 50 | 50 | 50 | 50 | 50 | 50 | 0.9 | 45.8 | 54.2 | 53.8 | 46.2 | 0.1 |
| Cefoperazone–sulbactam | 45 | 55 | 37.9 | 62.1 | 58.8 | 41.2 | 0.1 | 50 | 50 | 40.4 | 59.6 | 0.5 |
| Levofloxacin | 39 | 61 | 27.3 | 72.7 | 61.8 | 38.2 | 0.001 | 39.6 | 60.4 | 38.5 | 61.5 | 1 |
| Ofloxacin | 37 | 63 | 25.8 | 74.2 | 58.8 | 41.2 | 0.001 | 35.4 | 64.6 | 38.5 | 61.5 | 0.6 |
| Ciprofloxacin | 32 | 68 | 25.8 | 74.2 | 44.1 | 55.9 | < 0.001 | 33.3 | 66.7 | 30.8 | 69.2 | 0.9 |
| Doxycycline | 31 | 69 | 33.3 | 66.7 | 26.5 | 73.5 | 0.4 | 33.3 | 66.7 | 28.8 | 71.2 | 0.7 |
| Trimethoprim– sulfamethoxazole | 26 | 74 | 24.2 | 75.8 | 29.4 | 70.6 | 0.7 | 31.2 | 68.8 | 21.2 | 78.8 | 0.3 |
| Amoxicillin–clavulanate | 7 | 93 | 9.1 | 91.9 | 2.9 | 97.1 | 0.5 | 8.3 | 91.7 | 5.8 | 94.2 | 0.5 |
| Cefepime | 3 | 97 | 4.5 | 95.5 | 0 | 100 | 0.7 | 0.5 | 99.5 | 3.8 | 96.2 | 0.5 |
| Cefotaxime | 0 | 100 | 0 | 100 | 0 | 100 | – | 0 | 100 | 0 | 100 | – |
| Ceftazidime | 0 | 100 | 0 | 100 | 0 | 100 | – | 0 | 100 | 0 | 100 | – |
| Ceftriaxone | 0 | 100 | 0 | 100 | 0 | 100 | – | 0 | 100 | 0 | 100 | – |
| Aztreonam | 0 | 100 | 0 | 100 | 0 | 100 | – | 0 | 100 | 0 | 100 | – |
| Fosfomycin | – | – | 100 | 0 | – | – | – | – | – | – | – | – |
ESβL = extended-spectrum β-lactamase; I = intermediate; R = resistant; S = susceptible; UTI = urinary tract infection. Extended-spectrum β-lactamase isolates were extremely resistant to cefepime and amoxicillin–clavulanic acid, and susceptible to carbapenems. All ESβL E. coli isolates19 were sensitive to fosfomycin. The resistance rate of E. coli isolates to fluoroquinolones (levofloxacin, ofloxacin, and ciprofloxacin) was significantly higher than that of Klebsiella (P-value = 0.001, 0.001, and < 0.001, respectively). Extended-spectrum β-lactamase isolates from community-acquired UTIs were significantly more susceptible to nitrofurantoin than the ESβL isolates from hospital-acquired UTIs (P-value < 0.001).
Molecular characteristics of the ESβL isolates.
PCR analysis of the 100 ESβL Enterobacteriaceae isolates revealed that bla-CTX-M is the most frequent ESβL genotype, whereas bla TEM gene is the least frequent one. Moreover, 10% of the studied ESβL isolates coproduced CTX-M and SHV genes. No significant difference was found in the distribution of CTX-M and TEM genes among E. coli and Klebsiella spp. for hospital- and community-acquired ESβL isolates. A high level of SHV gene expression was significantly associated with Klebsiella spp.–induced hospital-acquired UTI (Table 3).
Table 3.
Distribution of bla-CTX-M, TEM, and SHV genes among ESβL isolates
| ESβL (n = 100) | Escherichia coli (n = 66), N (%) | Klebsiella spp. (n = 34), N (%) | P- value | Hospital-acquired (n = 48), N (%) | Community- acquired (n = 52), N (%) | P-value |
|---|---|---|---|---|---|---|
| CTX-M | 64 (97) | 34 (100) | 0.547 | 47 (97.9) | 51 (98.1) | 1.00 |
| SHV | 3 (4.5) | 7 (20.6) | 0.029 | 8 (16.7) | 2 (3.8) | 0.045 |
| TEM | 2 (3) | 0 (0) | 0.547 | 1 (2.1) | 1 (1.9) | 1.00 |
CTX = cefotaxime; ESβL = extended-spectrum β-lactamase. There was no significant difference in the distribution of CTX-M and TEM genes among E. coli and Klebsiella spp. nor hospital- and community-acquired ESβL isolates. SHV gene was significantly higher among Klebsiella spp. (P-value = 0.029) and hospital-acquired ESβL isolates (P-value = 0.045).
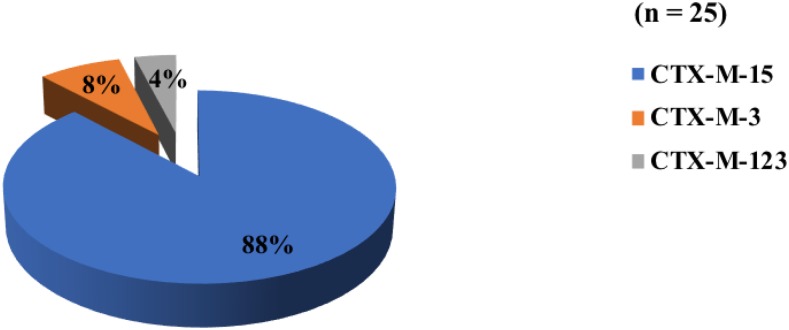
Among the bla-CTX-M gene–positive ESβL isolates, 25 isolates were further sequenced; CTX-M-15 was the most prevalent member, although there was no significant difference in its distribution among the hospital- and the community-acquired ESβL isolates. Cefotaxime-M-3 was solely detected in hospital-acquired isolates, whereas CTX-M-123 was only expressed in the community-acquired ESβL isolates (Figure 4).
Figure 4.
Result of bla cefotaxime (CTX)-M gene sequencing analysis. Within the investigated bla-CTX-M gene positive extended-spectrum β-lactamase (ESβL) isolates, CTX-M-15 was the most prevalent (22/25, 88%). However, there was no significant difference among the hospital-acquired (10/22; 45.5%) and community-acquired (12/22; 54.5%) CTX-M-15 ESβL isolates. Cefotaxime-M-3 (2/25, 8%) was detected only in hospital-acquired and CTX-M-123 (1/25, 4%) was detected only in community-acquired ESβL isolates. This figure appears in color at www.ajtmh.org.
DISCUSSION
One hundred ESβL Enterobacteriaceae isolates were enrolled in the current study. Urine samples were collected from outpatient clinic attendants and hospitalized patients who were suspicious for UTI, and patients were selected from different departments of Kasr Al-Aini Hospital. Majority of the ESβL UTI patients were females (72%), and the mean age was 42 years. A similar pattern was observed in previous studies on ESβL UTI in Australian and Korean tertiary referral hospitals in which the majority of patients were females (72% and 78.6%, respectively), although the mean age was higher than that in our study, 61 and 64 years, respectively.23,24
The present study showed a high prevalence of ESβL UTI (40%). Although ESβL UTI is known to be a hospital-based health problem, it was very concerning that there is no significant difference in ESβL prevalence among hospital- and community-acquired UTIs. This was in agreement with several studies conducted on Egyptian patients in which a high prevalence of ESβL-induced UTI was detected at many hospitals in Egypt: Al-Azhar, Tanata, and the pediatric department at Benha University public hospitals (42.7%, 32.6%, and 31.4%, respectively).11,12,25 Moreover, a higher rate (60%) of ESβL UTI was detected in Assiut University Hospital in Egypt.26 In contrast to our study, a lower ESβL UTI prevalence was detected in other studies, in Dar es Salaam hospital, Tanzania (over 6 months period), and Marrakech University Hospital, Morocco (27% and 4.5%, respectively), between 2010 and 2012.6,27 In Saudi Arabia and Tanzania, a higher frequency of ESβL-induced UTIs was found to be hospital-acquired compared with community-acquired infections (67% and 64%, respectively).6,28 The high frequency of ESβL, especially among community-acquired UTIs, in our study is alarming, and can be attributed to the misuse of broad-spectrum antibiotics and absence of an antibiotic policy.
In the present study, E. coli was the predominant (66%) isolated bacteria, followed by Klebsiella spp. Similar results were reported by previous Egyptian studies conducted at Al-Azhar and Benha University hospitals, with E. coli at 61% and 55.1% followed by Klebsiella spp. at 23.3% and 21.2%, respectively.11,12
The results of the antimicrobial resistance testing in the current study were very concerning; no significant difference was obtained in the antimicrobial susceptibility pattern of community- and hospital-acquired ESβL UTIs. We observed a high susceptibility of the ESβL-UTI isolates to carbapenems. A similar pattern was detected by previous studies conducted at Minia University Hospitals in Egypt, Riyadh, and Texas.29–31 On the contrary, high resistance of ESβL isolates to carbapenem was demonstrated in previous Egyptian studies; they reported a degree of resistance that reach up to 20%.11 This could be attributed to the wide use of carbapenems as an empirical treatment for ESβL infection.
With the emergence of the carbapenem-resistant Enterobacteriaceae, some old antibiotics were recruited for treatment of ESβL infections. In our study, all E. coli ESβL isolates were susceptible to fosfomycin. In concordance, most of the ESβL E. coli isolates were reported to be susceptible to fosfomycin in Cameron and Hong Kong.9,32
A relatively high ESβL susceptibility (80%) to nitrofurantoin was detected, with a significantly higher susceptibility (100%) among community-acquired isolates. Similar results were obtained in Australia and Riyadh (84% and 79%, respectively).23,30 Therefore, nitrofurantoin is listed as the best choice for initial therapy of UTI according to the Australian Antimicrobial guidelines.33
Indeed, in the present study, the ESβL isolates belonged to either hospital- or community-acquired infections, and showed high resistance to fluoroquinolones—the resistance was higher in E. coli isolates than Klebsiella spp. An association between ESβL and resistance to fluoroquinolones has been reported. However, the usage of fluoroquinolones in UTI treatment may raise the emergence of fluoroquinolone-resistant ESβL producers.26
Nowadays, molecular identification of ESβL infections is an essential step for reliable epidemiological screening and comprehensive antimicrobial testing. In the present study, we observed a high expression of CTX-M gene among community-acquired compared with hospital-acquired ESβL isolates. This finding increased the attention to the potential of ESβL, which represents a major problem in Egypt. In concordance with our results, a high prevalence of CTX-M ESβL UTI was detected in other studies in a tertiary care hospital in Tanzania (90.6%), in Minia University Hospital in Egypt (78.6%), and in USA (74%) and Sweden (87%).6,29,31,34 Contrarily, a lower CTX-M and a higher TEM ESβL were observed among Iranians (28% and 49%, respectively) and in an Indian tertiary care hospital (7.6% and 48.7%, respectively).35,36 This conflict may be attributed to the variation in the frequency and predominance of ESβL genes among different geographical regions and even between institutions within the same country.
In the present study, subsequent genotyping of 25 positive CTX-M isolates was performed; bla-CTX-M-15 was the most abundant genotype, with no significant difference in isolates recovered from hospital- and community-acquired UTIs. Similar observations were reported by studies conducted in Saudi Arabia and Lebanon and in the United Kingdom, Spain, and Venezuela.30,37–40 These results are in agreement with another study conducted on Egyptian population; they demonstrated that CTX-M-15 was predominant in ESβL isolates obtained from both hospital- and community-acquired UTIs at Al-Azhar University Hospital.1x1 The high prevalence of CTX-M-15 worldwide could be attributed to the wide dissemination and clonal expansion of what is called pandemic uropathogenic E. coli.41
Regarding the other detected CTX-M genotypes, CTX-M-3 was found to be predominant in hospital-acquired isolates, whereas CTX-M-123 was found to be predominant in community-acquired isolates. A lower rate (2%) of CTX-M-3 UTI was detected in community-acquired isolates at a French tertiary hospital and (7%) in a Greece tertiary care hospital, although a higher detection rate (28%) was reported in 24 Taiwan hospitals over a 2-year duration.42–44
In conclusion, the present study documents the emerging threat of the high prevalence of ESβL Enterobacteriaceae in hospital- and community-acquired UTIs among Egyptian patients. Unfortunately, most of the drugs, such as fluoroquinolones and sulfamethoxazole, are not totally effective for empirical treatment of UTI. Fosfomycin may be recommended as a reliable empirical treatment for UTI. Cefotaxime-M is an appropriate candidate that can be used for screening of ESβL isolates. In addition, the high frequency of CTX-M-15–producing uropathogenic E. coli recorded in Egypt suggested that it may be involved in what is called “CTX-M-15 pandemic.” Finally, we recommend continuous antibiotic susceptibility surveillance in every health institution to ensure the usefulness of the antibiotics used.
REFERENCES
- 1.Gupta K, et al. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52: e103–e120. [DOI] [PubMed] [Google Scholar]
- 2.Carlet J, Pittet D, 2013. Access to antibiotics: a safety and equity challenge for the next decade. Antimicrob Resist Infect Control 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masud MR, Afroz H, Fakruddin M, 2014. Prevalence of extended-spectrum β-lactamase positive bacteria in radiologically positive urinary tract infection. Springerplus 3: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biehl LM, Schmidt-Hieber M, Liss B, Cornely OA, 2014. Colonization and infection with extended-spectrum beta-lactamase producing-Enterobacteriaceae in high-risk patients-review of the literature from a clinical perspective. Crit Rev Microbiol 42: 1–16. [DOI] [PubMed] [Google Scholar]
- 5.Morosini MI, García-Castillo M, Coque TM, Valverde A, Novais A, Loza E, Baquero F, Cantón R, 2006. Antibiotic co-resistance in extended-spectrum-beta-lactamase-producing Enterobacteriaceae and invitro activity of tigecycline. Antimicrob Agents Chemother 50: 2695–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manyahi J, Moyo SJ, Tellevik MG, Ndugulile F, Urassa W, Blomberg B, Langeland N, 2017. Detection of CTX-M-15 beta-lactamases in Enterobacteriaceae causing hospital-and community-acquired urinary tract infections as early as 2004, in Dar es Salaam, Tanzania. BMC Infect Dis 17: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moghaddam MN, Beidokhti MH, Jamadar SA, Ghahraman M, 2014. Genetic properties of bla CTX-M and bla PER–beta-lactamase genes in clinical isolates of Enterobacteriaceae by polymerase chain reaction. Iran J Basic Med Sci 17: 378–383. [PMC free article] [PubMed] [Google Scholar]
- 8.Nisha KV, Veena SA, Rathika SD, Vijaya SM, Avinash SK, 2017. Antimicrobial susceptibility, risk factors, and prevalence of bla cefotaximase, temoneira, and sulfhydryl variable genes among Escherichia coli in community-acquired pediatric urinary tract infection. J Lab Physicians 9: 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djuikoue IC, Njajou O, Gonsu KH, Fokunang C, Bongo A, Bruno EO, Tanjung P, Linjouom A, Kakam C, Ngogang J, 2017. Prevalence of CTX-M beta-lactamases in Escherichia coli from community-acquired urinary tract infections and associated risk factors among women in Cameroon. J Epidemiol Res 3: 51–56. [Google Scholar]
- 10.De Oliveira CF, Salla A, Lara VM, Rieger A, Horta JA, Alves SH, 2010. Prevalence of extended-spectrum beta-lactamases-producing microorganisms in nosocomial patients and molecular characterization of the SHV-type isolates. Braz J Microbiol 41: 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salah M, Azab M, Halaby H, Hanora A, 2016. Mutations in β-lactamases detected in multidrug-resistant gram-negative bacteria isolated from community-acquired urinary tract infections in Assiut, Egypt. Afr J Microbiol Res 10: 1938–1943. [Google Scholar]
- 12.Ramadan DS, Bassyoni EA, Amer MM, Emam SM, 2016. Detection of ESBL producing bacteria in cases of urinary tract infection in pediatric department at Benha University Hospital. Egypt J Med Microbiol 25: 77–84. [Google Scholar]
- 13.Valverde A, Coque TM, Sanchez-Moreno MP, Rollán A, Baquero F, Cantón R, 2004. The dramatic increase in the prevalence of fecal carriage of extended-spectrum-beta-lactamase-producing-Enterobacteriaceae during non-outbreak situations in Spain. J Clin Microbiol 42: 4769–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Bano J, Lopez-Cerero L, Navarro MD, Pascual A, 2008. Faecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J Antimicrob Chemother 62: 1142–1149. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan R, Schaus D, John M, Delport JA, 2015. Extended spectrum beta-lactamases: a mini review of clinical relevant groups. J Med Microb Diagn 4: 203. [Google Scholar]
- 16.Cheesbrough M, 2006. Examination of urine. District Laboratory Practice in Tropical Countries , Part 2, 2nd edition. Cambridge University Press, 105–115. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute , 2014. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fourth Informational Supplement. Wayne, PA: CLSI M100-S24. [Google Scholar]
- 18.Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC, 1997. Color Atlas and Textbook of Diagnostic Microbiology, 5th edition Philadelphia, PA: J.B. Lippincott Company Press, 110–145. [Google Scholar]
- 19.Heffernan H, Pope C, Carter P, 2007. Identification of Extended Spectrum Β-Lactamase Types, Plasmid-Mediated AmpC Β-Lactamases and Strains Among Urinary Escherichia coli and Klebsiella in New Zeland in 2006. Communicable Disease Group, Environmental Science and Research, FW07103. [Google Scholar]
- 20.Paterson DL, Bonomo RA, 2005. Extended spectrum β-lactamases: a clinical update. Clin Microbiol Rev 18: 657–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan YH, 2003a. Biostatistics102: quantitative data–parametric & non-parametric tests. Singapore Med J 44: 391–396. [PubMed] [Google Scholar]
- 22.Chan YH, 2003b. Biostatistics 103: qualitative data–tests of independence. Singapore Med J 44: 498–503. [PubMed] [Google Scholar]
- 23.Osthoff M, McGuinness SL, Wagen AZ, Eisen DP, 2015. Urinary tract infections due to extended-spectrum beta-lactamase-producing Gram-negative bacteria: identification of risk factors and outcome predictors in an Australian tertiary referral hospital. Int J Infect Dis 34: 79–83. [DOI] [PubMed] [Google Scholar]
- 24.Lee DS, Lee CB, Lee SJ, 2010. Prevalence and risk factors for extended spectrum beta-lactamase-producing uropathogens in patients with urinary tract infection. Korean J Urol 51: 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labah EA, Afifi IK, Ahmed LM, 2009. Community-acquired urinary tract infections in Tanta, Egypt: aetiology and antibiotics resistance pattern. Egypt J Med Microbiol 18: 179–190. [Google Scholar]
- 26.Ibrahim MA, Agban MN, Thabit AG, El-Khamissy TR, Attia AE, 2014. Prevalence of extended-spectrum B-lactamase producing Klebsiella pneumoniae by phenotypic and genotypic methods in Assiut University Hospital. Egypt J Med Microbiol 23: 61–70. [Google Scholar]
- 27.El Bouamri MC, Arsalane L, Zerouali K, Kathy K, El Kamouni Y, Zouhair S, 2015. Molecular characterization of extended spectrum B-lactamase-producing Escherichia coli in a university hospital in Morocco, North Africa. Afr J Urol 21: 161–166. [Google Scholar]
- 28.Kandeel A, 2014. Prevalence and risk factors of extended-spectrum β-lactamases producing Enterobacteriaceae in a general hospital in Saudi Arabia. J Microbiol Infect Dis 4: 50–54. [Google Scholar]
- 29.Fadil AA, Hakeem MA, Abdelraheem AR, 2017. Esβl-producing E. coli and Klebsiella among patients treated at Minia University Hospitals. J Infect Dis Preve Med 5: 156–163. [Google Scholar]
- 30.Al-Agamy MH, Shibil AM, Hafez MM, Al-Ahdal MN, Memish ZA, Khubnani H, 2014. Molecular characteristics of extended-spectrum-beta-lactamase-producing Escherichia coli in Riyadh: the emergence of CTX-M-15-producing E. coli ST131. Ann Clin Microbiol Antimicrob 13: 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandramohan L, Revell PA, 2012. Prevalence and molecular characterization of extended-spectrum—beta-lactamase—producing Enterobacteriaceae in a pediatric patient population. Antimicrob Agents Chemother 56: 4765–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho PL, Yip KS, Chow KH, Lo JYC, Que TL, Yuen KY, 2010. Antimicrobial resistance among uropathogens that cause acute uncomplicated cystitis in women in Hong Kong: a prospective multicenter study from 2006 to 2008. Diagn Microbiol Infect Dis 66: 87–93. [DOI] [PubMed] [Google Scholar]
- 33.Antibiotic Expert Group , 2010. Therapeutic Guidelines: Antibiotic. Version 14 Melbourne, Australia: Therapeutic Guidelines Limited. [Google Scholar]
- 34.Onnberg A, Mölling P, Zimmermann J, Söderquist B, 2011. Molecular and phenotypic characterization of Escherichia coli and Klebsiella pneumoniae producing extended-spectrum β-lactamases with focus on CTX-M in a low-endemic area in Sweden. APMIS 119: 287–295. [DOI] [PubMed] [Google Scholar]
- 35.Rezai MS, Salehifar E, Rafiei A, Langaee T, Rafati M, Shafahi K, 2015. Characterization of multidrug-resistant extended-spectrum beta-lactamase-producing Escherichia coli among uropathogens of pediatrics in north of Iran. Biomed Res Int 2015: 309478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bajpai T, Pandey M, Varma M, Bhatambare GS, 2017. Prevalence of TEM, SHV, and CTX-M Beta-Lactamase genes in the urinary isolates of a tertiary care hospital. Avicenna J Med 7: 12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moubareck C, Daoud Z, Hakime NI, Hamze M, Mangeney N, Matta H, Mokhbat JE, Rohban R, Sarkis DK, Populaire FD, 2005. Countrywide spread of community-and the hospital-acquired extended-spectrum beta-lactamase (CTX-M-15)-producing Enterobacteriaceae in Lebanon. J Clin Microbiol 43: 3309–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livermore DM, et al. 2007. CTX-M; changing the face of ESβLs in Europe. J Antimicrob Chemother 59: 165–174. [DOI] [PubMed] [Google Scholar]
- 39.Rubio-Perez I, Martin-Perez E, Garcia DD, Calvo ML, Barrera EL, 2012. Extended-spectrum β lactamase producing bacteria in a tertiary care hospital in Madrid: epidemiology, risk factors, and antimicrobial susceptibility patterns. Emerg Health Threats J 5: 11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez E, Araque M, Millan Y, Millan B, Vielmas S, 2014. Prevalence of beta-lactamase CTX-M-15 in phylogenetic groups of uropathogenic Escherichia coli isolated from patients in the community of Merida, Venezuela. Invest Clin 55: 32–43. [PubMed] [Google Scholar]
- 41.Rogers BA, Sidjabat HE, Paterson DL, 2011. Escherichia coli O25b-ST131: a pandemic, multi-resistant, community-associated strain. J Antimicrob Chemother 66: 1–14. [DOI] [PubMed] [Google Scholar]
- 42.Dutour C, Bonnet R, Marchandin H, Boyer M, Chanal C, Sirot D, Sirot J, 2002. CTX-M-1, CTX-M-3, and CTX-M-14β-lactamases from Enterobacteriaceae isolated in France. Antimicrob Agents Chemother 46: 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mavroidi A, Tzelepi E, Miriagou V, Gianneli D, Legakis NJ, Tzouvelekis LS, 2002. CTX-M-3 β-lactamase—producing Escherichia coli from Greece. Microb Drug Resist 8: 35–37. [DOI] [PubMed] [Google Scholar]
- 44.Yu WL, Winokur PL, Von Stein DL, Pfaller MA, Wang JH, Jones RN, 2002. The first description of Klebsiella pneumoniae harboring CTX-M β-lactamases (CTX-M-14 and CTX-M-3) in Taiwan. Antimicrob Agents Chemother 46: 1098–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]