Abstract
Osteoarthritis (OA), the most prevalent age-related joint disorder, is characterized by chronic inflammation, progressive articular cartilage destruction, and subchondral bone sclerosis. Accumulating evidences indicate that circular RNAs (circRNAs) play a critical role in various diseases, but the function of circRNAs in OA remains largely unknown. Here we showed that circRNA.33186 was significantly upregulated in IL-1β)-treated chondrocytes and in cartilage tissues of a destabilized medial meniscus (DMM)-induced OA mouse model. Knockdown of circRNA.33186 increased anabolic factor (type II collagen) expression and decreased catabolic factor (MMP-13) expression. Knockdown of circRNA.33186 also promoted proliferation and inhibited apoptosis in IL-1β-treated chondrocytes. Silencing of circRNA.33186 in vivo markedly alleviated DMM-induced OA. Mechanistic study showed that circRNA.33186 directly binds to and inhibits miR-127-5p, thereby increasing MMP-13 expression, and contributes to OA pathogenesis. Taken together, our findings demonstrated a fundamental role of circRNA.33186 in OA progression and provide a potential drug target in OA therapy.
Keywords: circular RNAs, circRNA.33186, miR-127-5p, osteoarthritis
Zhu and colleagues demonstrate that circRNA.33186 regulates chondrocyte functions, including ECM catabolism, proliferation, and apoptosis. Silencing of circRNA.33186 alleviated OA by acting as a sponge of miR-127-5p. These findings reveal a fundamental role of circRNA.33186 in OA progression and provide a potential drug target in OA therapy.
Introduction
Osteoarthritis (OA) is the most prevalent age-related joint disorder in the elderly, leading to chronic pain, stiffness, and disability.1 Chronic inflammation, progressive destruction of articular cartilage, and subchondral bone sclerosis are primarily characteristics of OA.2 Currently, there are no effective disease-modifying therapies available for OA because of limited understanding of its pathogenesis. Thus, joint replacement remains the primary treatment for patients with advanced OA.3 Chondrocytes are the only resident cells in the articular system and are critical for maintaining the dynamic equilibrium between anabolism and catabolism in the extracellular matrix (ECM). Several risk factors such as abnormal mechanical stress and pro-inflammatory cytokines have been shown to reduce chondrocytes and degrade the ECM in cartilage.4, 5 Although increasing efforts have been dedicated to revealing the pathological process of OA, the molecular mechanisms remain elusive. Thus, there is an unmet medical need to find novel drug targets to develop more effective therapeutics.
Circular RNAs (circRNAs) are a class of non-coding RNAs characterized by covalently closed loop structures with neither 5′ to 3′ polarity nor a polyadenylated tail.6 They are produced by precursor mRNA back-splicing and widely expressed in mammals with highly conserved, stable, and tissue-specific patterns.7 There has been accumulating evidence suggesting that circRNAs are critically involved in various diseases, such as diabetes,8 cardiac fibrosis,9 and carcinomas.10, 11, 12 However, their potential roles in OA pathogenesis are poorly understood.
MicroRNAs (miRNAs) are non-coding, single-stranded RNAs that are 19–25 nt long, which suppress protein expression by directly binding to the 3′ UTR of the target mRNAs.13 Recent studies have shown that miRNAs are also involved in the development and progression of OA.14, 15 Interestingly, circRNAs could function as miRNA sponges by competitively interacting and suppressing their downstream functions.16, 17 Thus, revealing the roles of circRNAs and their potential miRNA regulators is critical for understanding the molecular mechanisms of OA and identifying new biomarkers or therapeutic targets for OA.
Our previous study found that circRNAs are differentially expressed in chondrocytes after interleukin-1β (IL-1β) treatment, suggesting that they might be potential regulators of OA.18 In this study, we identified a circRNA derived from the Umad1 gene, circRNA.33186, which is significantly upregulated in IL-1β-treated chondrocytes and cartilage tissues of the destabilized medial meniscus (DMM)-induced OA mouse model. circRNA.33186 regulates chondrocyte functions, including ECM catabolism, proliferation, and apoptosis. Silencing of circRNA.33186 in vivo markedly alleviated OA by acting as a sponge of miR-127-5p and promoted its cartilage-protecting function. Taken together, our findings reveal a fundamental role of circRNA.33186 in the progression of OA and provide a potential drug target in OA therapy.
Results
Characterization and Expression Analysis of circRNA.33186
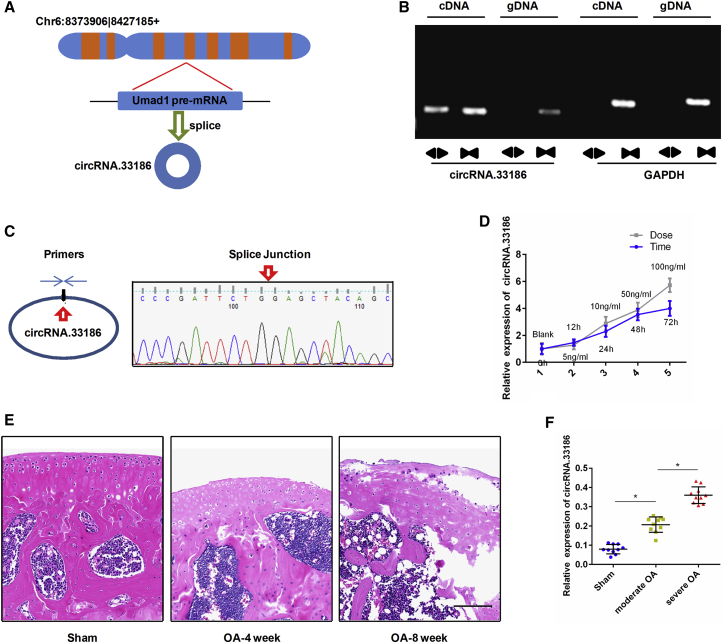
Based on our previous study,18 we focused on a significantly upregulated circRNA, circRNA.33186, which was 4.83-fold upregulated in IL-1β-treated chondrocytes. We found that circRNA.33186 is spliced from the Umad1 gene on chr6:8373906|8427185(+), and the ultimate length of circRNA.33186 is 536 nt (Figure 1A) (Table S1). We designed a set of specific divergent primers for circRNA.33186 (Table S2), and selected the circRNA as the template to perform PCR. PCR analysis yielded no products when using genomic DNA (gDNA) as the template (Figure 1B). Sanger sequencing of the PCR products amplified by divergent primers further confirmed the back-splice junction of circRNA.33186 (Figure 1C). These results showed that the circRNA could be specifically amplified by RT-PCR and confirmed the existence of circRNA.33186 in chondrocytes.
Figure 1.
Characterization and Expression Analysis of circRNA.33186
(A) Schematic diagram of circRNA.33186 formed by back-splicing from the mouse Umad1 gene at chromosome 6. (B) Divergent primers detected circular RNAs in complementary DNA (cDNA), but not in genomic DNA (gDNA). (C) Sanger sequencing showed the back-splice junction (arrow) of circRNA.33186. (D) qRT-PCR analysis of circRNA.33186 expression in primary chondrocytes stimulated with 10 ng/mL IL-1β for 0, 12, 24, 48, or 72 h and at 0, 5, 10, 50, or 100 ng/mL for 24 h. (E) Representative pictures of articular cartilage stained by H&E. Scale bar, 100 μm. (F) qRT-PCR analysis of circRNA.33186 expression in DMM-induced OA cartilage tissues (n = 10). *p < 0.01.
Next, we examined the expression pattern of circRNA.33186 in IL-1β-induced chondrocytes and OA cartilage tissues by qRT-PCR assay. Compared with the control group, the expression level of circRNA.33186 was significantly upregulated in IL-1β-treated chondrocytes in a time- and dose-dependent manner (Figure 1D). DMM surgery was used to induce OA in mice, and H&E staining was used to observe morphological structure of the cartilage tissues at the distal femur metaphysis. Whereas cartilage tissues in the control group showed normal morphological structure, the cartilage tissues in the OA group showed moderate and severe destruction at 4 and 8 weeks after surgery (Figure 1E). The expression level of circRNA.33186 in OA cartilage tissues was significantly higher as compared with the control group, particularly in severe OA (Figure 1F). Collectively, these data suggest that abnormal circRNA.33186 expression may be related to OA progression.
Effects of circRNA.33186 on Type II Collagen and MMP-13 Expression in IL-1β-Treated Chondrocytes
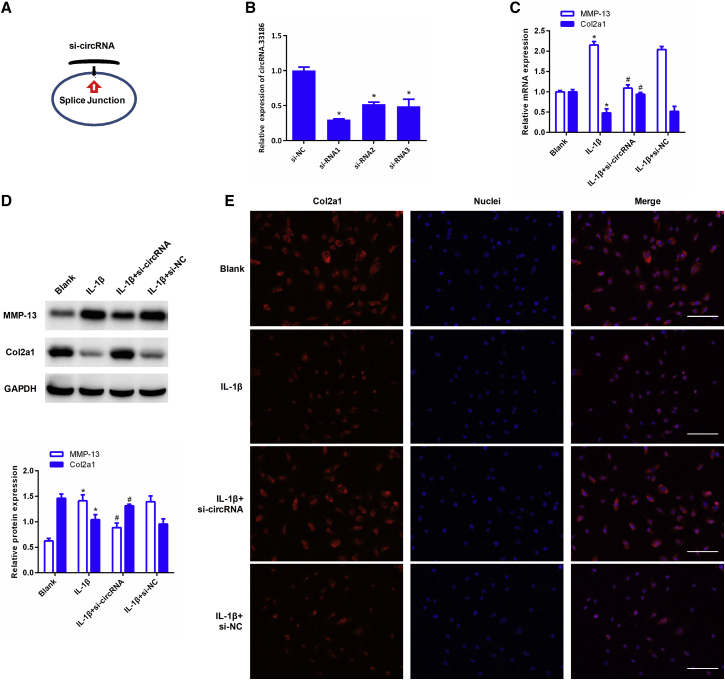
We then examined the biological functions of circRNA.33186 in chondrocytes. Small interfering RNA (siRNA) oligos were designed to knock down circRNA.33186 expression in chondrocytes (Figure 2A). The knockdown efficiency of three independent siRNAs (Table S3) was verified by real-time qRT-PCR (Figure 2B). We selected siRNA1 for subsequent experiments because it was the most effective siRNA. Chondrocytes were transfected with siRNA1 and then treated with 10 ng/mL IL-1β for 24 h. qRT-PCR and western blot analyses showed that the expression level of matrix metalloproteinase 13 (MMP-13), a key metalloproteinase that degrades cartilage matrix, was significantly increased, whereas the expression level of Col2a1, the major collagen component in the cartilage matrix, was downregulated in chondrocytes after IL-1β treatment. Knockdown of circRNA.33186 significantly decreased MMP-13 expression both at the mRNA and protein levels, whereas the expression of Col2a1 was significantly increased as compared with the IL-1β-treated chondrocytes that are transfected with a control siRNA (Figures 2C and 2D). The effects of circRNA.33186 on Col2a1 protein levels were further confirmed by immunofluorescence staining (Figure 2E). Together, these data indicate that knockdown of circRNA.33186 protects against IL-1β-induced OA in vitro.
Figure 2.
Effects of circRNA.33186 on Type II Collagen and MMP-13 Expression in IL-1β-Induced Chondrocytes
Chondrocytes were transfected with si-circRNA or si-NC, and then treated with 10 ng/mL IL-1β for 24 h. (A and B) Schematic illustration of si-circRNA target sites (A) and expression analyses of circRNA.33186 knockdown efficiency by three different si-circRNAs in chondrocytes (B). (C) Effects of circRNA.33186 inhibition on Col2a1 and MMP-13 mRNA levels were determined by qRT-PCR. (D) Effects of circRNA.33186 inhibition on Col2a1 and MMP-13 protein levels were determined by western blot. (E) Effects of circRNA.33186 inhibition on Col2a1 protein levels were determined by immunofluorescence staining. Images were acquired by confocal microscope. Scale bars, 200 μm. *p < 0.01 compared with blank; #p < 0.01 compared with IL-1β group.
Effects of circRNA.33186 on Proliferation and Apoptosis in IL-1β-Treated Chondrocytes
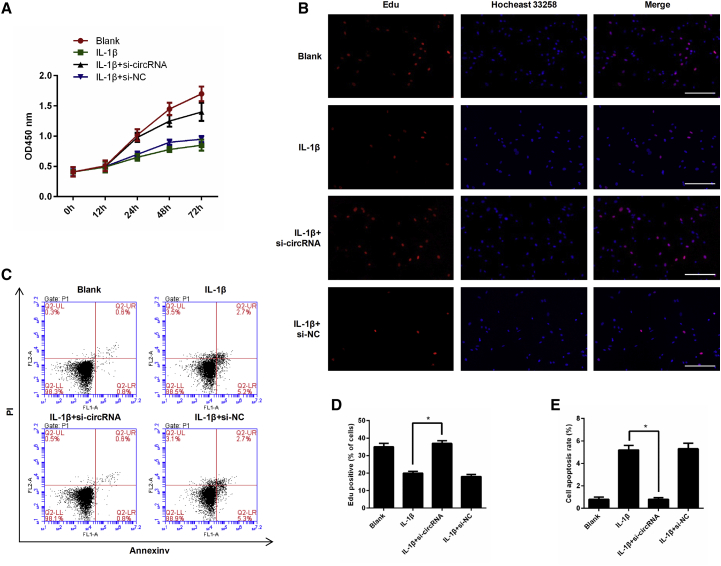
To test whether circRNA.33186 regulates chondrocyte proliferation, we performed Cell Counting Kit-8 (CCK8) and 5-ethynyl-20-deoxyuridine (EdU) incorporation assays. The proliferation of chondrocytes was significantly decreased after IL-1β treatment, and the effect was partly reversed by circRNA.33186 knockdown (Figure 3A). Similar results were obtained by the EdU incorporation assay (Figures 3B and 3D). Next, we determined whether circRNA.33186 had an effect on chondrocyte apoptosis by flow cytometry analysis. Induction of cell apoptosis was observed after IL-1β treatment in chondrocytes, whereas silencing of circRNA.33186 significantly decreased the percentage of chondrocytes that underwent apoptosis as compared with the control siRNA group (Figures 3C and 3E). These data demonstrate that knockdown of circRNA.33186 promotes proliferation and suppressed apoptosis in IL-1β-treated chondrocytes.
Figure 3.
Effects of circRNA.33186 on Proliferation and Apoptosis in IL-1β-Induced Chondrocytes
Chondrocytes were transfected with si-circRNA or si-NC, and then treated with 10 ng/mL IL-1β for 24 h. (A) The effect of circRNA.33186 on cell proliferation in vitro was determined by CCK8 assay. (B and D) Representative photomicrographs of EdU staining (B) and quantitative data showing the percentage of EdU-positive cells in different treatment groups (D). Blue: Hochest labeling of cell nuclei; Red: EdU labeling of nuclei of proliferative cells. Scale bars, 100 μm. (C and E) The effect of circRNA.33186 on cell apoptosis was measured by flow cytometric analysis (C), and the results of flow cytometric analysis are presented as percentages of positive mean values ± SD. *p < 0.01.
Silencing of circRNA.33186 In Vivo Alleviates OA In Vivo
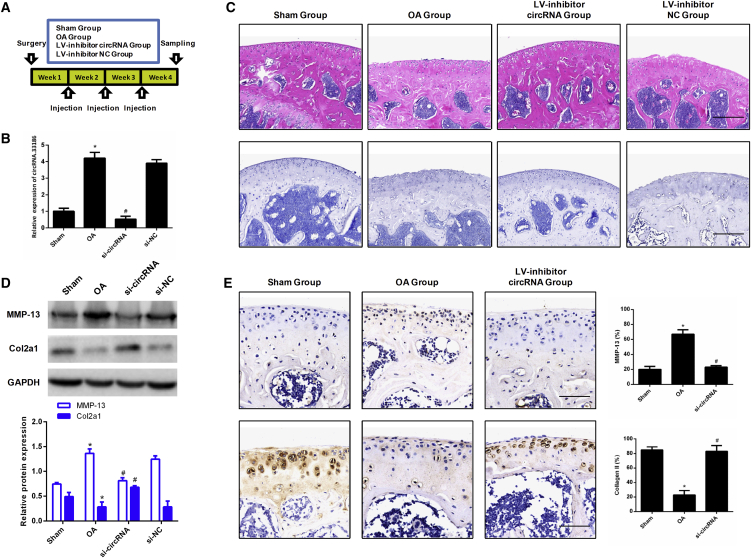
To investigate the in vivo role of circRNA.33186 during OA pathogenesis, we performed intra-articular (IA) lentivirus injection in DMM-operated mice with circRNA.33186 or control siRNA weekly for a total of 3 weeks (Figure 4A). qRT-PCR analysis confirmed the efficiency of circRNA.33186 knockdown after lentivirus injection (Figure 4B). The destruction of articular cartilage at the distal femur metaphysis was induced by DMM in mouse knee joints, and the severity of cartilage destruction was significantly alleviated by silencing of circRNA.33186 (Figure 4C). Western blot analysis showed increased expression of MMP-13 and decreased expression of Col2a1 in the knee articular cartilage of OA mice, which was significantly alleviated by inhibition of circRNA.33186 in vivo (Figure 4D). Similar results were also obtained by immunohistochemistry analysis (Figure 4E). Taken together, these data demonstrated that silencing of circRNA.33186 in vivo alleviates DMM-induced OA in mice.
Figure 4.
Silencing of circRNA.33186 In Vivo Alleviates DMM-Induced OA
(A) Schematic diagram illustrating the design of the OA therapeutic experiment targeting circRNA.33186. (B) qRT-PCR analysis of circRNA.33186 expression in knee articular cartilage from OA mice in different groups after surgery (n = 6). (C) The articular cartilage at the distal femur metaphysis was stained with H&E and toluidine blue. Scale bars, 200 μm. (D) Western blot analysis of Col2a1 and MMP13 expression in knee articular cartilage from OA mice in different groups. (E) Expression of Col2a1 and MMP13 were observed by immunohistochemistry staining in a DMM-induced OA mice model. Scale bars, 100 μm. *p < 0.01 compared with Sham group; #p < 0.01 compared with OA group.
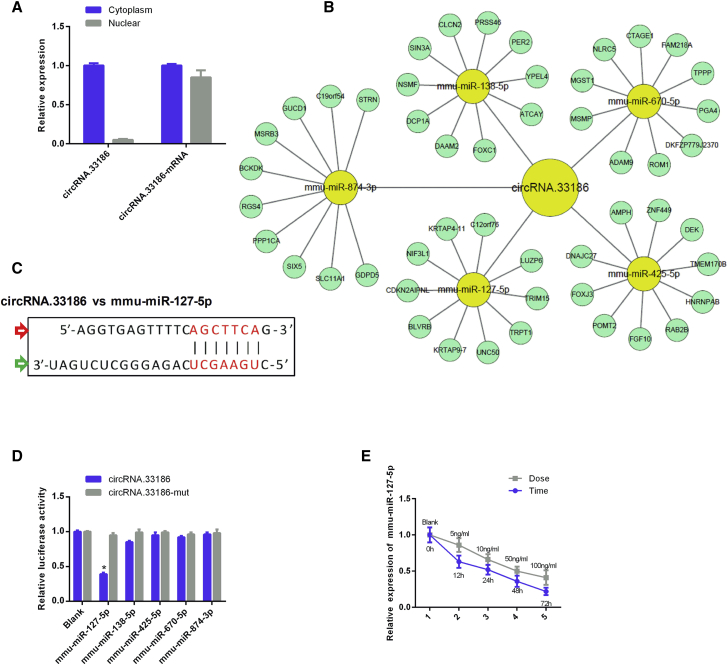
circRNA.33186 Is a Sponge of miR-127-5p
We performed qRT-PCR to determine the intracellular localization of circRNA.33186. The results revealed that the circular form of circRNA.33186 was mainly localized in the cytoplasm (Figure 5A). Previous studies showed that circRNAs in the cytoplasm may competitively bind to miRNAs and subsequently regulate their target genes by acting as miRNA sponges,16, 17 so we speculated that circRNA.33186 could target miRNAs to modulate its downstream function. We profiled the public databases, TargetScan (http://www.targetscan.org/vert_72/) and miRanda (http://sanderlab.org/tools/micrornas.html), to identify potential miRNAs targeted by circRNA.33186. The results showed that miR-138-5p, miR-670-5p, miR-874-3p, miR-127-5p, and miR-425-5p had a binding site for circRNA.33186 (Figure 5B). Next, we performed a luciferase screening assay to verify which miRNA binds to circRNA.33186. Each miRNA mimic was co-transfected with the luciferase reporters containing circRNA.33186 or circRNA.33186 mutant vectors into HEK293T cells. Only miR-127-5p was found to reduce the luciferase reporter activity by over 50% (Figures 5C and 5D). In addition, we found that the expression level of miR-127-5p was significantly downregulated in IL-1β-treated chondrocytes in a time- and dose-dependent manner (Figure 5E), which was negatively correlated with circRNA.33186 expression in chondrocytes. Taken together, these results suggest that circRNA.33186 might function as sponge of miR-127-5p.
Figure 5.
circRNA.33186 Is a Sponge of miR-127-5p
(A) Relative expression of circRNA.33186 and circRNA.33186 mRNA in the cytoplasm and nucleus of chondrocytes were determined by qRT-PCR. (B) Targeted microRNAs matching the circRNA.33186 UTRs predicted by both TargetScan and miRanda. (C) Putative miR-127-5p binding site in the 3′ UTR of circRNA.33186 predicted by TargetScan and miRanda. The red arrow indicates circRNA.33186, and the green arrow indicates mmu-miR-127-5p. (D) Luciferase reporter assay for circRNA.33186 or circRNA.33186 mutant in HEK293T cells co-transfected with five miRNA mimics. (E) Time- (10 ng/mL IL-1β for 0, 12, 24, 48, or 72 h) and dose (0, 5, 10, 50, or 100 ng/mL for 24 h)-dependent downregulation of miR-127-5p expression in primary chondrocytes. *p < 0.01.
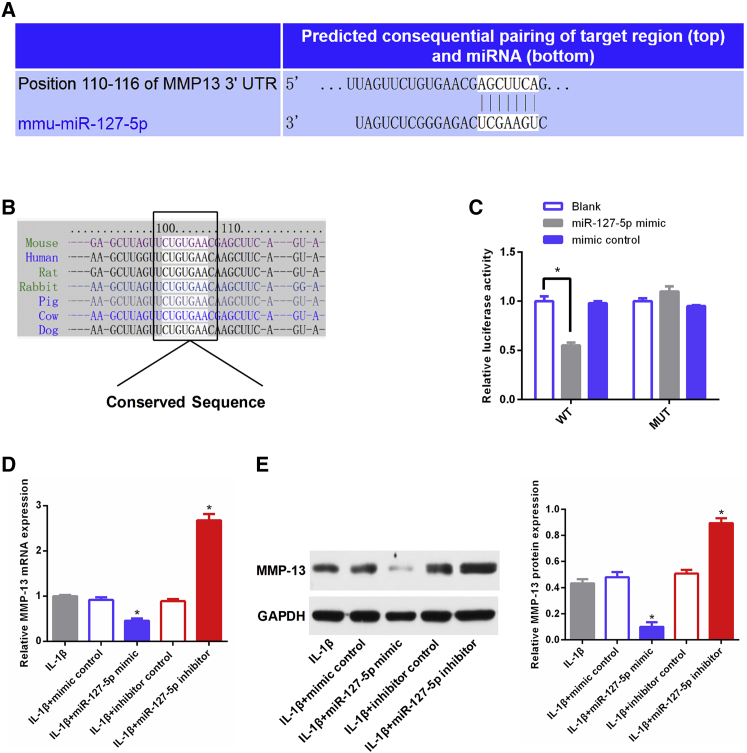
miR-127-5p Is Directly Targeted by MMP-13
It has been reported that miR-127-5p regulates IL-1β-treated catabolic effects in human chondrocytes by targeting MMP-13.19 Thus, we hypothesized that miR-127-5p might exert its functions via regulating MMP-13 in mouse chondrocytes. The putative miR-127-5p binding site in the 3′ UTR of MMP-13 mRNA was predicted by TargetScan, and sequence alignment of the putative binding site within the 3′ UTR of MMP-13 mRNA shows a high level of sequence conservation and complementarity with miR-127-5p among mammals (Figures 6A and 6B). Furthermore, the targeting relationship between miR-127-5p and MMP-13 was investigated by luciferase activity assay. miR-127-5p mimic significantly downregulated the luciferase activity of the reporter gene in wild-type constructs, but not in mutant constructs (Figure 6C). Overexpression of miR-127-5p reduced the expression of MMP-13, whereas the miR-127-5p inhibitor increased the expression of MMP-13 at both mRNA and protein levels (Figures 6D and 6E). These results confirmed that miR-127-5p is directly targeted by MMP-13 in mouse chondrocytes.
Figure 6.
MMP-13 Is a Direct Target of miR-127-5p
(A) Putative miR-127-5p binding site in the 3′ UTR of MMP-13 mRNA. (B) Sequence alignment of a putative miR-127-5p binding site within the 3′ UTR of MMP-13 mRNA shows a high level of sequence conservation and complementarity with miR-127-5p. (C) Interaction between miR-127-5p and MMP-13 was verified by luciferase report assay. (D and E) The effects of miR-127-5p on MMP-13 expression in chondrocytes were analyzed by qPCR (D) and western blot (E). *p < 0.01.
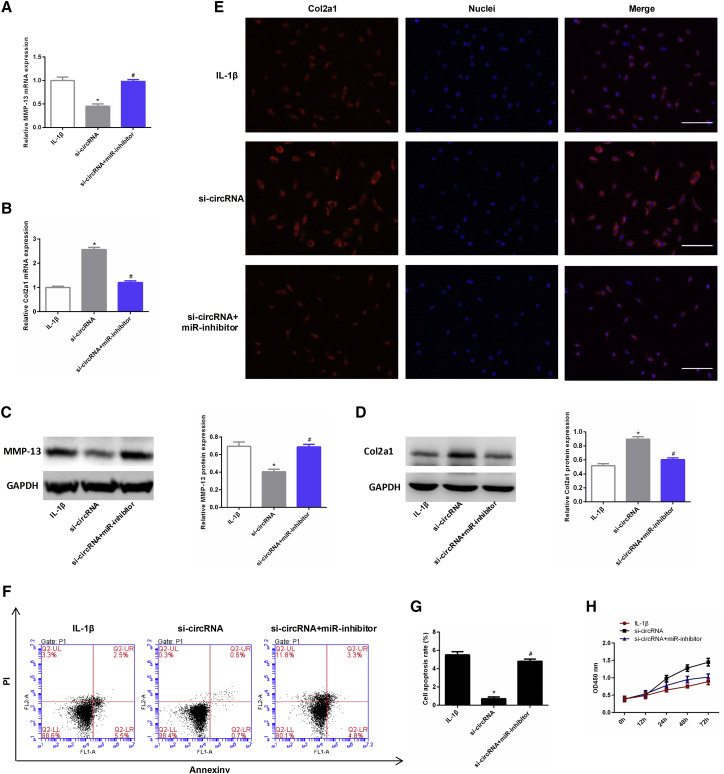
circRNA.33186 Exerts Biological Functions in Chondrocytes via Sponging miR-127-5p
Next, we co-transfected si-circRNA and miR-127-5p inhibitor into chondrocytes and then treated cells with 10 ng/mL IL-1β for 24 h. qRT-PCR and western blot analysis showed that the reduction of MMP-13 expression caused by silencing of circRNA.33186 was significantly restored after co-transfecting with miR-127-5p inhibitor into chondrocytes (Figures 7A and 7C). In contrast, the increase of Col2a1 expression that resulted from circRNA.33186 knockdown was eliminated by co-transfection of si-circRNA and the miR-127-5p inhibitor (Figures 7B and 7D). The effect on Col2a1 protein levels was also confirmed by immunofluorescence staining (Figure 7E). For cell apoptosis and proliferation, knockdown of circRNA.33186 promoted the proliferation of chondrocytes, but silencing miR-127-5p reversed the effect of circRNA.33186 (Figures 7F and 7G). Moreover, the induction of cell apoptosis observed after silencing of circRNA.33186 was also suppressed by inhibiting miR-127-5p (Figure 7H). Together, these results demonstrate that circRNA.33186 promotes OA pathogenesis by functioning as a sponge for miR-127-5p.
Figure 7.
circRNA.33186 Exerts Biological Functions in Chondrocytes via Sponging miR-127-5p
Chondrocytes were transfected with si-circRNA or a combination of si-circRNA and miR-127-5p inhibitor, and then treated with 10 ng/mL IL-1β for 24 h. (A and B) The mRNA levels of MMP-13 (A) and Col2a1 (B) were determined by qRT-PCR. (C and D) The protein levels of MMP-13 (C) and Col2a1 (D) were determined by western blot. (E) The protein levels of Col2a1 were determined by immunofluorescent staining. Scale bars, 20 μm. (F and G) Cell apoptosis was measured by flow cytometric analysis (F), and the results of flow cytometric analysis are presented as percentages of positive mean values ± SD. (H) Cell proliferation in vitro was determined by CCK8 assay. *p < 0.01, compared with IL-1β group; #p < 0.01, compared with si-circRNA group.
Discussion
Although considerable amounts of studies have been designed to unravel the pathology of OA, the medical treatment of OA still focuses on relieving symptomatic synovial joint pain.3 Therefore, it is urgent to explore the molecular mechanisms underlying OA progression and identify novel drug targets for OA therapy.
circRNAs are a class of newly discovered non-coding RNAs, and increasing evidence shows they could be used as diagnostic biomarkers and therapeutic targets for various diseases. For example, Li et al.20 found that hsa_circ_0004277 could be used as a biomarker for acute myeloid leukemia. Zhu et al.21 reported that a circRNA, hsa_circ_0013958, might be a potential novel biomarker for lung adenocarcinoma. With respect to therapeutic functions, Wang et al.9 revealed that a heart-related circRNA acts as a positive regulator to inhibit cardiac hypertrophy and heart failure. Cheng et al.22 showed circRNA VMA21 protects against intervertebral disc degeneration through targeting miR-200c and X-linked inhibitor-of-apoptosis protein. However, few reports describe the role of circRNAs in OA. In this study, we found that circRNA.33186 was upregulated in IL-1β-treated chondrocytes and cartilage tissues of the DMM-induced OA model, and the expression level was positively correlated with cartilage degeneration, indicating that circRNA.33186 may be associated with the development and progression of OA. More importantly, upregulation of circRNA.33186 was determined at an early stage during OA development, suggesting the possibility that circRNA.33186 could be used as a diagnostic biomarker for this disorder. Further experiments showed that knockdown of circRNA.33186 corrected the imbalance between anabolic and catabolic factors (e.g., type II collagen and MMP-13), promoted cell growth, and inhibited cell apoptosis in IL-1β-treated chondrocytes, demonstrating that circRNA.33186 plays a vital role in OA progression and may be a therapeutic target.
It is widely accepted that IA injection of drugs, such as corticosteroids and hyaluronic acid, are effective means for the clinical treatment of OA. IA injection of miRNAs as a therapeutic method for OA in animal models has been also described in recent years.23 IA injection of miR-140 alleviates OA progression by modulating ECM homeostasis in rats.24 Silencing of miR-101 via IA injection of adenovirus prevents cartilage degradation by regulating ECM-related genes in a rat model of OA.25 Here we found that IA injection of lentivirus-incorporated si-circRNAs against circRNA.33186 successfully decreased the expression of circRNA.33186 in cartilage tissues of the DMM-induced OA model, and silencing of circRNA.33186 in vivo significantly alleviated DMM-induced cartilage destruction in mice. Moreover, the effect of DMM surgery on the expression of type II collagen and MMP-13 was also reversed by knockdown of circRNA.33186. These results further confirmed the therapeutic potential of circRNA.33186 in OA.
Recently, circRNAs have been shown to act as miRNA sponge to regulate gene expression.6, 16 Han et al.11 found that circRNA MTO1 directly binds to miR-9 and inhibits miR-9 activity to suppress hepatocellular cancer progression. Zheng et al.17 reported that circHIPK3 acts as the sponge of miR-124 to function as a cell-growth modulator. In the present study, we found that circRNA.33186 was mainly localized in the cytoplasm, suggesting it might function as an miRNA sponge. We then identified a group of miRNAs that might interact with circRNA.33186 by means of bioinformatics analyses, and validated the interacting relationship between circRNA.33186 and miR-127-5p using luciferase activity assays. We also found that the expression level of miR-127-5p was negatively correlated with circRNA.33186. Therefore, these data suggest that circRNA.33186 could directly target miR-127-5p by functioning as a sponge, and that miR-127-5p may play an important role in chondrocytes.
To date, more and more miRNAs have been found to play central roles in the pathogenesis and progression of OA by regulating ECM anabolism and catabolism of chondrocytes.26, 27, 28, 29 It has been showed that silencing of miR-34a prevents IL-1β-induced ECM degradation in chondrocytes.30 miR-145 was reported to attenuate tumor necrosis factor alpha (TNF-α-driven cartilage matrix degradation in OA via direct suppression of MKK4.31 Previous study revealed that miR-127-5p is an important regulator of MMP-13 in human chondrocytes and may contribute to the development of OA.19 MMP-13 is a pivotal catabolic factor of OA that degrades type II collagen, a major component of cartilage ECM in chondrocytes.32, 33, 34 Here, our results further confirmed that miR-127-5p could directly target MMP-13 and regulate type II collagen expression in IL-1β-treated mouse chondrocytes. Several lines of evidence indicate that circRNA.33186 functions as a sponge of miR-127-5p to regulate OA progression. First, bioinformatics analyses showed that the 3′ UTR of MMP-13 and circRNA.33186 contains binding sites for miR-127-5p. Second, luciferase reporter assays verified this prediction. Third, knockdown of circRNA.33186 reduced MMP-13 expression. Finally, inhibition of miR-127-5p reversed the effect of circRNA.33186 knockdown.
Taken together, our study indicates that the expression level of circRNA.33186 is upregulated in OA. Silencing of circRNA.33186 markedly alleviates OA progression both in vitro and in vivo by regulating catabolic and anabolic factors, promoting cell growth, and inhibiting cell apoptosis of chondrocytes. Also, circRNA.33186 may exert these biological functions by acting as a sponge for miR-127-5p. Our findings demonstrate that circRNA.33186 can be used as a diagnostic biomarker and potential target in OA therapy. Although our study shed light on a new option for OA treatment, further investigations are still necessary. For example, more studies are needed to determine whether circRNA.33186 contributes to OA through other molecules and pathways. Because IA injection of lentivirus infects not only articular cartilage but also the synovium, which plays an important role in OA development, further studies are required to identify the role of circRNA.33186 in synovium tissue, articular chondrocytes, or other cell types. Finally, more effective vectors should be identified for circRNA delivery via joint injection. Finding the answers to these questions will be critical for a better understanding of the functions of circRNAs in OA pathogenesis and for offering feasible therapeutic targets for clinical use.
Materials and Methods
Animal Experiments
Adult male C57BL/6 mice were used to induce an OA model by DMM surgery as previously described.35 In brief, after anesthetized with chloral hydrate (400 mg/kg) intraperitoneally, the right knee joint of mice (n = 10 per group; 8 weeks old; mean body weight = 25 g) was exposed through a medial capsular incision. Then the medial meniscotibial ligament was transected, and the medial meniscus was displaced medially. Finally, the incision was sutured, and the skin was closed. Sham operation was done in parallel, and only the skin of the right knee joint was resected. Mice were sacrificed at 4 or 8 weeks after surgery, and the right knee joints were harvested for histological and biochemical analyses. For the therapeutic experiment, 8-week-old C57BL/6 male mice were randomly assigned into four groups (n = 6 per group): Sham group, OA group, lentivirus vector (LV)-inhibitor circRNA group, and LV-inhibitor negative control (NC) group. One week after DMM surgery, mice were treated with IA injection of lentivirus-incorporated si-circRNA against circRNA.33186, or negative control lentivirus (1 × 109 plaque-forming units [pfu] in a total volume of 5 μL) once per week for 3 weeks. Mice were sacrificed 3 weeks after the first injection, and the knee joints were harvested for histological analyses. All animal experiments were approved by the Animal Ethics Committee of the Second Military Medical University on the Use and Care of Animals and were performed in accordance with the committee’s guidelines.
Primary Chondrocyte Isolation and Culture, circRNA, and miRNA Transfection
Primary chondrocytes were obtained from the knee joints of newborn mice as previously described36 and then cultured in maintenance medium consisting of DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO, Rockville, MD, USA) and 1% penicillin-streptomycin (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a 5% CO2 incubator. The siRNA specifically targeting circRNA.33186 and lentiviruses that expressed knockdown constructs of circRNA.33186, and the vectors that upregulate and downregulate miRNA expression (miRNA mimic and miRNA inhibitor) were designed and constructed by Geneseed (Guangzhou, China). Chondrocytes were transfected with certain vectors (si-circRNA, miRNA inhibitor [50 nM], miRNA mimic [100 nM]) using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s instructions and then were used for further experiments.
qRT-PCR
Quantification of circRNA, mRNA, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was performed using SYBR Premix Ex Taq (Takara Bio) in a real-time detection system (ABI7500). miRNA and U6 gene concentrations were determined using the Bulge-Loop miRNA qRT-PCR Starter Kit (RiboBio, Guangzhou, China) in accordance with the manufacturer’s instructions. Three independent experiments were conducted for each sample. The expression levels of circRNA.33186 and mRNA were normalized to GAPDH, and miR-127-5p expression was normalized to U6. Data were analyzed by calculating the 2−ΔΔCt relative fold change. The primers used for real-time PCR are shown in Table S2.
Western Blot Analysis
Total proteins were extracted from chondrocytes or cartilage tissues with radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, China), and the concentration was measured using the bicinchoninic acid (BCA) protein assay (Beyotime, China). Then proteins were electrophoresed on SDS-polyacrylamide gel and transferred to nitrocellulose membranes (Millipore, Carrigtwohill, Ireland). After blocking with nonfat milk, the membranes were incubated with a primary antibody overnight at 4°C, washed, and then incubated with a secondary antibody for 1 h each at room temperature. The antibodies used were anti-Collagen II (dilution 1:5,000; Abcam, UK), anti-MMP-13 (dilution 1:3,000; Abcam, UK), GAPDH (dilution 1:1,000; Abcam, UK), and goat anti-rabbit secondary antibody (dilution 1:5,000; Abcam, UK). The results were quantified, and the images were processed using ImageJ software. GAPDH was used as an internal loading control.
Immunofluorescence Staining
Chondrocytes were seeded on coverslips in 24-well plates and cultured for 48 h. After being fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and blocked by serum as previously described, cells were incubated with a primary antibody at 4°C overnight and then with a fluorescent Cy3-conjugated secondary antibody at 37°C for 1 h. The antibodies used were anti-Collagen II (dilution 1:100; Abcam, UK), anti-MMP-13 (dilution 1:100; Abcam, UK), and fluorescent Cy3-conjugated secondary goat anti-rabbit antibodies (dilution 1:100; Abcam, UK). Fluorescence images were acquired using a confocal microscope (FV 1000 Olympus IX-81) and were analyzed using Image-Pro Plus 6.0 software (Media Cybernetics).
Nuclear-Cytoplasmic Fractionation
Cytoplasmic and nuclear RNA isolation were performed with PARIS Kit (Invitrogen, USA) according to the manufacturer’s instruction. In brief, chondrocytes were lysed with cell fractionation buffer; then the nuclear and cytoplasmic cell fractions were separated by centrifuge. After transferring the supernatant into fresh RNase-free tubes, the remaining lysate was washed with cell fractionation buffer and centrifuged. The nuclei were lysed with cell disruption buffer. The above lysate and the supernatant were mixed with a 2 × lysis binding solution, and an equal volume of ethanol was added through a filter cartridge. The sample was then washed with wash solution. Finally, the RNA of cytoplasm and nucleus was eluted with elution solution.
Luciferase Reporter Assay
Luciferase reporter vector with the full length of the 3′ UTR of circRNA.33186 or MMP-13 and the mutant version were constructed and then co-transfected with the reporter vector and 50 nM miRNA mimic or their control into HEK293T cells using Lipofectamine 2000 (Life Technologies, Grand Island, NY, USA). The five miRNA mimics (miR-138-5p, miR-670-5p, miR-874-3p, miR-127-5p, miR-425-5p) were obtained from RiboBio. After 48 h of incubation, the firefly and Renilla luciferase activities were measured with a Dual-Luciferase Reporter System (Promega, Madison, WI, USA).
Cell Proliferation Assay
CCK8 and EdU assays were performed to measure the effect of circRNA.33186 on chondrocytes proliferation. For CCK8 assay, cells were transfected with siRNA for 48 h and then seeded into 96-well plates at a density of 2 × 103 cells per well and cultured for 12, 24, 48, or 72 h. CCK8 was used to detect cell proliferation, and the absorbance at 450 nM was measured using Spectra Max250 spectrophotometer (Molecular Devices, USA). All experiments were performed in triplicate. For the EdU assays, cells were transfected with siRNA for 48 h and then seeded into 24-well plates at a density of 2 × 105 cells per well. Cells were then incubated with EdU for 2 h at a concentration of 10 μM. After being fixed with 4% formaldehyde solution for 15 min and permeabilized with 0.5% Triton X-100 for 20 min at room temperature, cells were washed three times with PBS and incubated with 1X Apollo reaction cocktail for 30 min at room temperature. Finally, the cells were stained with Hoechst 33258. The EdU incorporation rate was expressed as the ratio of EdU-positive cells (red cells) to total Hoechst33342-positive cells (blue cells).
Cell Apoptosis Assay
Chondrocyte apoptosis was measured by flow cytometry. Cells were prepared as previously reported. Apoptotic cells were evaluated by Annexin V-FITC (fluorescein isothiocyanate) staining, and dead cells were detected by the propidium iodine (PI) exclusion method. Then cells were analyzed by flow cytometry FACSCalibur instrument (BD Biosciences) according to the manufacturer’s instructions.
Histology and Immunohistochemistry
Right knee joints were fixed in 4% paraformaldehyde for 24 h, decalcified in 12.5% EDTA (pH 7.4) for 4 weeks, and then embedded in paraffin. The whole medial compartment of the joints was cut on the sagittal sections with a thickness of 5 μm and then stained with H&E and toluidine blue. For immunohistochemistry, sections were deparaffinized and rehydrated. After antigen retrieval, sections were blocked with 5% goat serum and then incubated with the following primary antibodies overnight at 4°C and finally stained with horseradish peroxidase (HRP)-conjugated secondary antibodies. The primary antibodies used were anti-Collagen II (dilution 1:100; Abcam, UK) and anti-MMP-13 (dilution 1:100; Abcam, UK). Images were obtained using aFluoView FV1000 confocal microscope (Olympus). The number of cells positive for the marker was expressed relative to the total number of cells.
Statistical Analysis
Data are expressed as the mean ± SD, and statistical analysis was performed by one-way ANOVA for multiple comparisons, or by Student’s t test. p < 0.05 (two-sided) was considered statistically significant for all of the statistical calculations. All statistical analyses were performed with PRISM 6.0 (GraphPad Software, San Diego, CA, USA) and SPSS 19.0 (IBM, Armonk, NY, USA).
Author Contributions
Z.-b.Z. and G.-x.H. contributed equally to this work. Z.-b.Z. and G.-x.H. conducted the experiments. Q.F., B.H., and J.-j.L. helped analyze the data. Z.-b.Z., A.-m.C., and L.Z. designed the experiments. Z.-b.Z., G.-x.H., and L.Z. wrote the paper.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grants 81571204, 81701215, and 81874003) and the Shanghai Sailing Program (grant 17YF1425300). The funders had no role in the study design, data collection, data analysis, decision to publish, or manuscript preparation.
Footnotes
Supplemental Information includes three tables and can be found with this article online at https://doi.org/10.1016/j.ymthe.2019.01.006.
Contributor Information
Ai-min Chen, Email: aiminchen@smmu.edu.cn.
Lei Zhu, Email: hailangzhulei@smmu.edu.cn.
Supplemental Information
References
- 1.Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., Bridgett L., Williams S., Guillemin F., Hill C.L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 2.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijlsma J.W., Berenbaum F., Lafeber F.P. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 4.Goldring M.B. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract. Res. Clin. Rheumatol. 2006;20:1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Liu-Bryan R., Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 7.Szabo L., Salzman J. Detecting circular RNAs: bioinformatic and experimental challenges. Nat. Rev. Genet. 2016;17:679–692. doi: 10.1038/nrg.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H., Guo S., Li W., Yu P. The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells. Sci. Rep. 2015;5:12453. doi: 10.1038/srep12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K., Long B., Liu F., Wang J.X., Liu C.Y., Zhao B., Zhou L.Y., Sun T., Wang M., Yu T. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 2016;37:2602–2611. doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 10.Chen L., Zhang S., Wu J., Cui J., Zhong L., Zeng L., Ge S. circRNA_100290 plays a role in oral cancer by functioning as a sponge of the miR-29 family. Oncogene. 2017;36:4551–4561. doi: 10.1038/onc.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Han D., Li J., Wang H., Su X., Hou J., Gu Y., Qian C., Lin Y., Liu X., Huang M. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66:1151–1164. doi: 10.1002/hep.29270. [DOI] [PubMed] [Google Scholar]
- 12.Zhong Z., Huang M., Lv M., He Y., Duan C., Zhang L., Chen J. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017;403:305–317. doi: 10.1016/j.canlet.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Wang C., Zhao J., Xu J., Geng Y., Dai L., Huang Y., Fu S.C., Dai K., Zhang X. miR-146a facilitates osteoarthritis by regulating cartilage homeostasis via targeting Camk2d and Ppp3r2. Cell Death Dis. 2017;8:e2734. doi: 10.1038/cddis.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu J., Ji M.L., Zhang X.J., Shi P.L., Wu H., Wang C., Im H.J. MicroRNA-218-5p as a potential target for the treatment of human osteoarthritis. Mol. Ther. 2017;25:2676–2688. doi: 10.1016/j.ymthe.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Q., Bao C., Guo W., Li S., Chen J., Chen B., Luo Y., Lyu D., Li Y., Shi G. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z., Du D., Chen A., Zhu L. Circular RNA expression profile of articular chondrocytes in an IL-1β-induced mouse model of osteoarthritis. Gene. 2018;644:20–26. doi: 10.1016/j.gene.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Park S.J., Cheon E.J., Lee M.H., Kim H.A. MicroRNA-127-5p regulates matrix metalloproteinase 13 expression and interleukin-1β-induced catabolic effects in human chondrocytes. Arthritis Rheum. 2013;65:3141–3152. doi: 10.1002/art.38188. [DOI] [PubMed] [Google Scholar]
- 20.Li W., Zhong C., Jiao J., Li P., Cui B., Ji C., Ma D. Characterization of hsa_circ_0004277 as a new biomarker for acute myeloid leukemia via circular RNA profile and bioinformatics analysis. Int. J. Mol. Sci. 2017;18:E597. doi: 10.3390/ijms18030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X., Wang X., Wei S., Chen Y., Chen Y., Fan X., Han S., Wu G. hsa_circ_0013958: a circular RNA and potential novel biomarker for lung adenocarcinoma. FEBS J. 2017;284:2170–2182. doi: 10.1111/febs.14132. [DOI] [PubMed] [Google Scholar]
- 22.Cheng X., Zhang L., Zhang K., Zhang G., Hu Y., Sun X., Zhao C., Li H., Li Y.M., Zhao J. Circular RNA VMA21 protects against intervertebral disc degeneration through targeting miR-200c and X linked inhibitor-of-apoptosis protein. Ann. Rheum. Dis. 2018;77:770–779. doi: 10.1136/annrheumdis-2017-212056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith M.M., Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol. Int. 1987;7:113–122. doi: 10.1007/BF00270463. [DOI] [PubMed] [Google Scholar]
- 24.Si H.B., Zeng Y., Liu S.Y., Zhou Z.K., Chen Y.N., Cheng J.Q., Lu Y.R., Shen B. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthritis Cartilage. 2017;25:1698–1707. doi: 10.1016/j.joca.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Dai L., Zhang X., Hu X., Liu Q., Man Z., Huang H., Meng Q., Zhou C., Ao Y. Silencing of miR-101 prevents cartilage degradation by regulating extracellular matrix-related genes in a rat model of osteoarthritis. Mol. Ther. 2015;23:1331–1340. doi: 10.1038/mt.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akhtar N., Rasheed Z., Ramamurthy S., Anbazhagan A.N., Voss F.R., Haqqi T.M. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62:1361–1371. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang B., Guo H., Zhang Y., Chen L., Ying D., Dong S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS ONE. 2011;6:e21679. doi: 10.1371/journal.pone.0021679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai R., Ahmed S.A. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl. Res. 2011;157:163–179. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tili E., Michaille J.J., Costinean S., Croce C.M. MicroRNAs, the immune system and rheumatic disease. Nat. Clin. Pract. Rheumatol. 2008;4:534–541. doi: 10.1038/ncprheum0885. [DOI] [PubMed] [Google Scholar]
- 30.Abouheif M.M., Nakasa T., Shibuya H., Niimoto T., Kongcharoensombat W., Ochi M. Silencing microRNA-34a inhibits chondrocyte apoptosis in a rat osteoarthritis model in vitro. Rheumatology (Oxford) 2010;49:2054–2060. doi: 10.1093/rheumatology/keq247. [DOI] [PubMed] [Google Scholar]
- 31.Hu G., Zhao X., Wang C., Geng Y., Zhao J., Xu J., Zuo B., Zhao C., Wang C., Zhang X. MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017;8:e3140. doi: 10.1038/cddis.2017.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gargiulo S., Gamba P., Poli G., Leonarduzzi G. Metalloproteinases and metalloproteinase inhibitors in age-related diseases. Curr. Pharm. Des. 2014;20:2993–3018. doi: 10.2174/13816128113196660701. [DOI] [PubMed] [Google Scholar]
- 33.Fosang A.J., Last K., Knäuper V., Murphy G., Neame P.J. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 34.Goldring M.B., Marcu K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glasson S.S., Blanchet T.J., Morris E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Gosset M., Berenbaum F., Thirion S., Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat. Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.