Abstract
Background.
Spontaneous heparin-induced thrombocytopenia (HIT) is a rare but serious prothrombotic syndrome characterized by thrombosis, thrombocytopenia and strong platelet-activating HIT antibodies in the absence of heparin exposure, and is frequently characterized by a suboptimal response to standard therapies. Here, we present the first report of intravenous immunoglobulin G (IVIg) use in a patient with Spontaneous HIT.
Study Design and Methods.
Patient information including demographic, clinical and laboratory results were obtained from the electronic medical record. Laboratory testing was performed in the Serotonin Release Assay (SRA), PF4-dependent P-Selectin Expression Assay (PEA) and PF4/Polyvinylsulfonate ELISA (PF4 ELISA) to study the impact of IVIg on HIT antibody-mediated platelet activation. The patient was also genotyped for a polymorphisms in the IgG receptor on platelets, FcγRIIa, at amino acid position 131.
Results.
A 30-year-old male presented with thrombotic stroke and thrombocytopenia and strong HIT serologies in the absence of proximate heparin use. Direct thrombin inhibitor therapy was not associated with a prompt response. Due to severity and extent of thrombosis, and persistent thrombocytopenia, he was treated with high-dose Intravenous Immunoglobulin G (IVIg). This treatment was associated with rapid and sustained normalization of platelet counts and a gradual improvement in thrombotic complications. Platelet activation induced by HIT antibodies in the PEA (Low PF4) was significantly lower after IVIg treatment, correlating well with platelet rise. Consistent with the severity of thrombosis, the patient was found to possess the 131HR polymorphism in FcγRIIa.
Conclusion.
These results suggest that IVIg may be a useful adjunctive therapy in spontaneous HIT.
Keywords: Heparin, Heparin-induced thrombocytopenia, HIT, Spontaneous HIT, Intravenous Immunoglobulin G
Introduction
Heparin-induced thrombocytopenia (HIT) is a serious prothrombotic disorder caused by antibodies to platelet factor 4 (PF4): polyanion complexes that develop after heparin exposure1. Several reports indicate that a particularly severe form of HIT, Spontaneous HIT, occurs in individuals with no prior heparin exposure and is characterized by thrombotic complications, strong HIT serologic test results2–12 and suboptimal response to treatment with direct thrombin inhibitors (DTIs)2. There are less than 20 reported cases worldwide2 and while the triggers for its development are not known, it is believed that HIT antibodies are directed to PF4 bound to the platelet surface likely via endogenous glycosaminoglycans13. Intravenous immunoglobulin G (IVIg) can be strikingly effective in patients with persistent HIT refractory to non-heparin anticoagulant treatment, rapidly and durably counteracting HIT antibody-mediated platelet activation14–18 and this treatment may even prevent HIT from developing in the face of heparin exposure in subacute HIT19. Here, we describe a severely affected patient with spontaneous HIT who was treated successfully with IVIg.
Materials and Methods
The patient consented to research testing under an approved Institutional Review Board protocol (Medical College of Wisconsin, Protocol PRO00023318). The PF4-dependent P-selectin expression assay (PEA) for detection of platelet-activating HIT antibodies was performed as previously described14,20–22. Briefly, pooled washed O blood group normal platelets (1 × 106) were treated with PF4 (37.5 μg/mL-PEA or 3.75μg/mL-PEA, Low PF4) for 20 min followed by patient serum for 1 h. After addition of labeled anti-P-selectin (Monoclonal antibody 424.2, BloodCenter of Wisconsin) and anti-GPIIb (Monoclonal antibody 290.5, BloodCenter of Wisconsin) antibodies, platelet events were gated by GPIIb positivity, and P-selectin expression (median fluorescence intensity, MFI) was recorded. In addition to a normal sample “calibrator,” known positive and negative patient samples were included in each run. Maximum P-selectin expression (100%) was measured by treating platelets with thrombin receptor-activating peptide (TRAP; 25 mg/mL). Results were expressed as the percentage of maximum P-selectin expression corrected for background signal obtained with normal serum as follows:
PEA (%)= (Sample MFI - Normal Serum MFI)/ (TRAP MFI - Normal Serum MFI) x 100 Serotonin release assay (SRA) was performed as previously described (BloodCenter of Wisconsin, Milwaukee)23 and PF4 ELISA testing utilized the LIFECODES PF4 IgG diagnostic kit (Immucor, Norcross, GA).
DNA was extracted from whole blood using QIAamp DNA Mini Kit (Qiagen). Polymerase chain reaction was performed using primers F 5’-CTTTCAGAATGGCTGGTGCT-3’ and R 5’- TTTGCTGCTATGGGCTTTCT-3’ specific for the FcγRIIa gene (Integrated DNA Technologies). Polymerase chain reaction products (that included the FcγRIIa H/R 131 polymorphism site) were sequenced in a 3130xl Genetic Analyzer (Applied Biosystems) using standard procedures.
Case Presentation/Results
A previously healthy 30 yr. old man presented with left hemiparesis due to an acute right middle cerebral artery thrombotic stroke. Thrombolysis was not performed because of time delay from the onset of stroke symptoms (~48 hrs.). Platelet count on admission was 84 × 103/μL. Enoxaparin was administered for thrombosis prophylaxis on day +1 (of admission). Antiphospholipid antibodies were negative. On day +2 the patient complained of left calf pain and developed a cool left foot. Imaging revealed complete thrombotic occlusion of the left popliteal artery as well as occlusive thrombosis of the right anterior tibial and peroneal arteries. An emergent left lower extremity thrombectomy was performed and unfractionated heparin (UFH) was started the same day (Fig 1).
Figure 1. IVIg treatment led to prompt recovery of platelet counts.
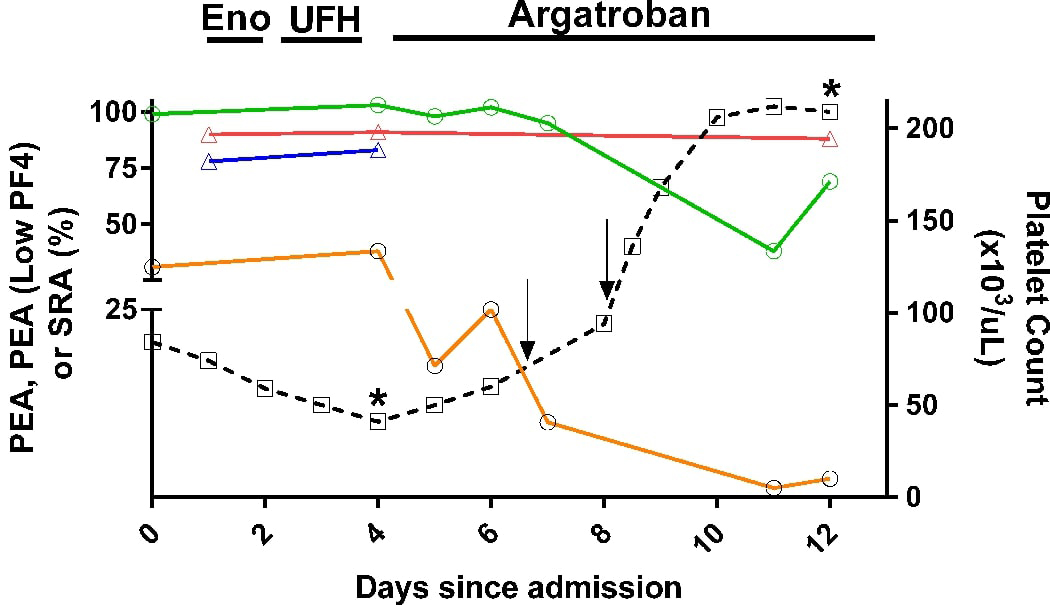
Left ordinate denotes PEA (green), PEA (Low PF4; orange), SRA (red) and zero-heparin SRA (blue) test results. Right ordinate depicts platelet count (dashed black line and open boxes). PEA and PEA, Low PF4 results shown are the average of duplicate measurements, while SRA and platelet counts shown are single determinations. Solid arrows denote IVIg treatment (1g/kg body weight). The assay cut-offs for the PEA (35%) and PEA, Low PF4 (10%) have high specificity for HIT (96% and 98%, respectively, manuscript in preparation). Serotonin release of 20% was used as the positive cut-off for the SRA. The asterisks denote PF4 ELISA OD of 2.782 (day 4) and 2.023 (day 12). Arrows indicate IVIg treatment at a dose of 1gm/Kg. Eno, Enoxaparin; UFH- Unfractionated porcine heparin.
Heparin exposure further decreased the platelet count to 41 × 103/μL on day +4 (Fig 1). Due to extensive thrombosis and the presence of thrombocytopenia at the time of presentation (prior to in-hospital heparin exposure), spontaneous HIT was suspected and UFH was discontinued. HIT testing revealed strongly positive IgG-specific Polyvinylsulfonate/PF4 ELISA with an Optical Density of 2.782, and 90% serotonin release (with 0.1U/mL UFH; Fig 1). Serotonin release assay (SRA) performed without UFH on samples from days +1 and +4 were also strongly positive at 77% and 83% release, respectively (Fig 1). Despite discontinuation of heparin and two days of argatroban therapy, platelet counts remained low (60 × 103/μL) and toes in both lower extremities became more cyanotic raising concern for impending gangrene. At this juncture, IVIg was administered at 1g/kg/day on days +7 and +8 (total 2gm/kg) and was followed by a doubling of the platelet count (to 135 × 103/μL on day +8; Fig 1).
Laboratory testing revealed that IVIg therapy significantly inhibited platelet activation by the patient’s HIT antibody in the PF4-dependent P-selectin expression assay (PEA) when low concentrations of PF4 were used (PEA, Low PF4, 3mcg/mL) 14 but had little effect on SRA and HIT ELISA test results, consistent with the presence of strong, platelet-activating HIT antibodies, as noted previously 14 (Fig 1). The PEA also showed a transient decline correlating well with the beginning of platelet rebound. PEA and SRA reactivities were completely inhibited in the presence of 100U/mL UFH confirming that platelet activation was mediated by HIT antibodies (data not shown). Previous work has shown that patients with arginine (R) at amino acid position 131 in the activating IgG receptor on platelets, FcγRIIa are more likely to experience more severe HIT (thrombosis) due to the inability of normal IgG2 present in plasma to compete with HIT antibodies for binding to the receptor24. Patients with histidine (H) at aa131 in FcγRIIa, on the other hand, are competent to be bound by both normal IgG2 and IgG1 leading to greater protection from HIT antibody-mediated platelet activation. The patient was heterozygous for Histidine (H) and Arginine (R), a genotype associated with limited ability (relative to HH131) of the patient’s own plasma immunoglobulins to inhibit HIT antibody-mediated platelet activation14.
Despite the prompt response to IVIg treatment, the patient’s hospital course was complicated by compartment syndrome in both lower extremities, a consequence of extensive lower extremity thrombosis at presentation. The patient recovered, however, with no requirement for digit/limb amputation. He was placed on warfarin with an INR target of 2.5–3.5 after his platelet count had normalized and he continues to do well receiving rehabilitation for his neurological symptoms (spastic hemiparesis). He has had no recurrence of thrombosis or thrombocytopenia at most recent follow up, seven months after initial presentation.
Discussion
Spontaneous HIT can be a major treatment challenge, and carries high risk of morbidity and mortality. IVIg has been used in challenging HIT treatment settings, including severe persistent HIT refractory to standard therapies14–18,25 and in acute/subacute HIT requiring emergent heparin exposure19. This limited published experience suggests that this treatment can induce rapid and sustained platelet recovery. To the best of our knowledge, this is the first reported case of spontaneous HIT treated with IVIg. The lack of proximate heparin exposure, significant thrombocytopenia, extensive thrombosis and strong HIT serologies (including in the SRA performed without addition of heparin in the assay26) support this diagnosis. The absence of a quick response to DTI therapy is consistent with other reports of DTI-refractoriness in patients with spontaneous HIT 2. Treatment with IVIg may be considered in this difficult treatment setting. In addition to IVIg use in severe HIT presentations such as spontaneous HIT and delayed-onset HIT, we believe a strong case can be made for its use in HIT patients whose disease course is characterized by severe thrombocytopenia or thrombosis and strong platelet-activating HIT antibodies. This approach may also be helpful in decreasing bleeding risks seen with alternative anticoagulant use in the setting of severe thrombocytopenia as noted in a recent bleeding-related HIT fatality27. However, this potentially prothrombotic drug should be used with caution after a careful risk-benefit analysis.
Acknowledgments:
We would like to thank Shannon Pechauer, BS and Curtis Jones, BS for assistance in the performance of research testing on patient blood samples.
Source of Support
This study was supported in part by funds from National Institutes of Health grants HL133479 (A.P), and HL013629 (R.H.A).
Abbreviations:
- HIT
Heparin-induced thrombocytopenia
- IVIg
Intravenous Immunoglobulin G
- PF4 ELISA
PF4/Polyvinylsulfonate Enzyme-linked Immunosorbent Assay
- SRA
Serotonin release assay
- PEA
PF4-dependent P-selectin Expression Assay
- DTI
Direct Thrombin Inhibitors
Footnotes
Conflict of Interest
A.P. discloses the following conflicts: A patent application has been filed related to diagnostic testing in HIT (Method of Detecting Platelet-Activating Antibodies That Cause Heparin-Induced Thrombocytopenia/Thrombosis; PCT/US14/62591). AP has equity ownership in Retham Technologies LLC, a company that seeks to develop HITDx, an in vitro diagnostic assay for HIT.
R.H.A. discloses the following conflicts: A patent application has been filed related to diagnostic testing in HIT (Method of Detecting Platelet-Activating Antibodies That Cause Heparin-Induced Thrombocytopenia/Thrombosis; PCT/US14/62591). RHA serves on the advisory board of Retham Technologies LLC. The other authors have no relevant conflicts of interest to declare.
References
- 1.Cuker A. Clinical and laboratory diagnosis of heparin-induced thrombocytopenia: an integrated approach. Semin Thromb Hemost 2014;40: 106–14. [DOI] [PubMed] [Google Scholar]
- 2.Poudel DR, Ghimire S, Dhital R, Forman DA, Warkentin TE. Spontaneous HIT syndrome post-knee replacement surgery with delayed recovery of thrombocytopenia: a case report and literature review. Platelets 2017;28: 614–20. [DOI] [PubMed] [Google Scholar]
- 3.Okata T, Miyata S, Miyashita F, Maeda T, Toyoda K. Spontaneous heparin-induced thrombocytopenia syndrome without any proximate heparin exposure, infection, or inflammatory condition: Atypical clinical features with heparin-dependent platelet activating antibodies. Platelets 2015;26: 602–7. [DOI] [PubMed] [Google Scholar]
- 4.Mallik A, Carlson KB, DeSancho MT. A patient with ‘spontaneous’ heparin-induced thrombocytopenia and thrombosis after undergoing knee replacement. Blood Coagul Fibrinolysis 2011;22: 73–5. [DOI] [PubMed] [Google Scholar]
- 5.Ketha S, Smithedajkul P, Vella A, Pruthi R, Wysokinski W, McBane R. Adrenal haemorrhage due to heparin-induced thrombocytopenia. Thromb Haemost 2013;109: 669–75. [DOI] [PubMed] [Google Scholar]
- 6.Warkentin TE, Safyan EL, Linkins LA. Heparin-induced thrombocytopenia presenting as bilateral adrenal hemorrhages. N Engl J Med 2015;372: 492–4. [DOI] [PubMed] [Google Scholar]
- 7.Elshoury A, Khedr M, Abousayed MM, Mehdi S. Spontaneous heparin-induced thrombocytopenia presenting as bilateral adrenal hemorrhages and pulmonary embolism after total knee arthroplasty. Arthroplast Today 2015;1: 69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker K, Lim MY. Spontaneous Heparin-Induced Thrombocytopenia and Venous Thromboembolism following Total Knee Arthroplasty. Case Rep Hematol 2017;2017: 4918623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warkentin TE, Makris M, Jay RM, Kelton JG. A spontaneous prothrombotic disorder resembling heparin-induced thrombocytopenia. Am J Med 2008;121: 632–6. [DOI] [PubMed] [Google Scholar]
- 10.Jay RM, Warkentin TE. Fatal heparin-induced thrombocytopenia (HIT) during warfarin thromboprophylaxis following orthopedic surgery: another example of ‘spontaneous’ HIT? J Thromb Haemost 2008;6: 1598–600. [DOI] [PubMed] [Google Scholar]
- 11.Pruthi RK, Daniels PR, Nambudiri GS, Warkentin TE. Heparin-induced thrombocytopenia (HIT) during postoperative warfarin thromboprophylaxis: a second example of postorthopedic surgery ‘spontaneous’ HIT. J Thromb Haemost 2009;7: 499–501. [DOI] [PubMed] [Google Scholar]
- 12.Perrin J, Barraud D, Toussaint-Hacquard M, Bollaert PE, Lecompte T. Rapid onset heparin-induced thrombocytopenia (HIT) without history of heparin exposure: a new case of so-called ‘spontaneous’ HIT. Thromb Haemost 2012;107: 795–7. [DOI] [PubMed] [Google Scholar]
- 13.Padmanabhan A, Jones CG, Bougie DW, Curtis BR, McFarland JG, Wang D, Aster RH. Heparin-independent, PF4-dependent binding of HIT antibodies to platelets: implications for HIT pathogenesis. Blood 2015;125: 155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padmanabhan A, Jones CG, Pechauer SM, Curtis BR, Bougie DW, Irani MS, Bryant BJ, Alperin JB, Deloughery TG, Mulvey KP, Dhakal B, Wen R, Wang D, Aster RH. IVIg for Treatment of Severe Refractory Heparin-Induced Thrombocytopenia. Chest 2017;152: 478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tvito A, Bakchoul T, Rowe JM, Greinacher A, Ganzel C. Severe and persistent heparin-induced thrombocytopenia despite fondaparinux treatment. Am J Hematol 2015;90: 675–8. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim IF, Rice L. Intravenous Immunoglobulin for Heparin-Induced Thrombocytopenia. Chest 2017;152: 906–7. [DOI] [PubMed] [Google Scholar]
- 17.Azimov MB, Slater ED. Persistent Heparin-Induced Thrombocytopenia Treated With IVIg. Chest 2017;152: 679–80. [DOI] [PubMed] [Google Scholar]
- 18.Doucette K, DeStefano CB, Jain NA, Cruz AL, Malkovska V, Fitzpatrick K. Treatment of refractory delayed onset heparin-induced thrombocytopenia after thoracic endovascular aortic repair with intravenous immunoglobulin (IVIG). Res Pract Thromb Haemost 2017;1: 134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warkentin TE, Climans TH, Morin PA. Intravenous Immune Globulin to Prevent Heparin-Induced Thrombocytopenia. N Engl J Med 2018;378: 1845–8. [DOI] [PubMed] [Google Scholar]
- 20.Padmanabhan A, Jones CG, Curtis BR, Bougie DW, Sullivan MJ, Peswani N, McFarland JG, Eastwood D, Wang D, Aster RH. A Novel PF4-Dependent Platelet Activation Assay Identifies Patients Likely to Have Heparin-Induced Thrombocytopenia/Thrombosis. Chest 2016;150: 506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones CG, Pechauer SM, Curtis BR, Bougie DW, Irani MS, Dhakal B, Pierce B, Aster RH, Padmanabhan A. A Platelet Factor 4-Dependent Platelet Activation Assay Facilitates Early Detection of Pathogenic Heparin-Induced Thrombocytopenia Antibodies. Chest 2017;152: e77–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones CG, Pechauer SM, Curtis BR, Bougie DW, Aster RH, Padmanabhan A. Normal plasma IgG inhibits HIT antibody-mediated platelet activation: implications for therapeutic plasma exchange. Blood 2018;131: 703–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McFarland J, Lochowicz A, Aster R, Chappell B, Curtis B. Improving the specificity of the PF4 ELISA in diagnosing heparin-induced thrombocytopenia. Am J Hematol 2012;87: 776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rollin J, Pouplard C, Sung HC, Leroux D, Saada A, Gouilleux-Gruart V, Thibault G, Gruel Y. Increased risk of thrombosis in FcgammaRIIA 131RR patients with HIT due to defective control of platelet activation by plasma IgG2. Blood 2015;125: 2397–404. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie DS, Anuforo J, Morgan J, Neculiseanu E. Successful Use of Intravenous Immunoglobulin G to Treat Refractory Heparin-Induced Thrombocytopenia With Thrombosis Complicating Peripheral Blood Stem Cell Harvest. J Investig Med High Impact Case Rep 2018;6: 2324709618755414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost 2017. [DOI] [PubMed] [Google Scholar]
- 27.Kuitunen A, Sinisalo M, Vahtera A, Hiltunen L, Javela K, Laine O. Autoimmune heparin-induced thrombocytopenia of delayed onset: a clinical challenge. Transfusion 2018. [DOI] [PubMed] [Google Scholar]


