Abstract
Background
Biliary tract cancers (BTC) are rare, aggressive neoplasms. Most patients present with advanced/unresectable or metastatic disease at diagnosis, with no second-line regimen demonstrating clinical benefit. This phase II study evaluated efficacy and safety of regorafenib in patients with advanced/unresectable or metastatic disease after standard therapy.
Patients and Methods
In this single arm study, patients with advanced/unresectable or metastatic BTC who failed at least one line of systemic chemotherapy were given regorafenib once daily, 21-day on; 7-day off in a 28-day cycle. A standard 160mg dose was given initially. After toxicity assessments in the first three patients, the dose was reduced to 120mg for subsequent patients as pre-planned. The primary endpoint is progression-free survival (PFS). Secondary objectives include overall survival (OS), objective response rate, and disease control.
Results
Forty-three patients received at least one dose of regorafenib; 34 patients who had at least 1 cycle of treatment were evaluable for tumor response. The mPFS was 15.6 weeks (90% CI=12.9–24.7)), and mOS was 31.8 weeks (90% CI=13.3–74.3) with survival rate 40% at 12 months and 32% at 18 months. Partial response was achieved in 5 patients (11%), and stable disease in 19 (44%) with disease control rate of 56%. The toxicity profile was as expected, with grade 3/4 adverse events in 40% of patients. The most common toxicities were hypophosphatemia (40%), Hyperbilirubinemia (26%), hypertension (23%), and hand-foot skin reaction (7%).
Conclusions:
This study suggested promising efficacy of regorafenib in chemotherapy refractory advanced/metastatic BTC, warranting further studies to confirm the clinical efficacy.
Keywords: Biliary tract cancer (BTC), cholangiocarcinoma, gallbladder cancer, chemotherapy refractory, regorafenib
Precis for use in the Table of Contents:
There is a clear unmet need for effective therapies in patients with advanced and metastatic biliary tract cancer (BTC). This phase II study showed encouraging data of regorafenib as a single agent in patients with previously treated advanced unresectable and metastatic BTC.
INTRODUCTION
Biliary tract cancer (BTC), including intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma (ECC) and gallbladder carcinoma (GBC), are relatively rare but aggressive neoplasms. There are about 7,000–10,000 new cases diagnosed per year in the United States (1), and biliary tract cancer accounts for 3% of all gastrointestinal malignances (2). Cholangiocarcinoma is the second most common primary hepatic tumor, but is more lethal than hepatocellular carcinoma (2). The incidence of intrahepatic cholangiocarcinoma is rising globally, and this rising rate has not been associated with an increase in the proportion of early stage disease (3,4). Based on a recent study of National Center for Health Statistics (NCHS) data, the mortality rates of cholangiocarcinoma have increased substantially in the past decade (5). The majority of patients have unresectable or metastatic disease at the time of diagnosis. The five-year overall survival for patients with advanced disease is only about 5% (6). While successful new treatments have been developed for many other cancers, there has been relatively limited improvement in treatment outcome and survival for biliary tract adenocarcinoma over the past decades. Since 2010, the combination of gemcitabine and cisplatin has been the first-line and standard systemic chemotherapy option for patients with unresectable and metastatic biliary tract adenocarcinomas (7). Despite a reported tumor control rate of 80%, the median overall survival remains at 11.7 months (7). Other gemcitabine-based chemotherapy regimens have also been tested (8,9); however, no systemic treatment agents or regimens for patients who have failed first line therapy have been approved. There are reports of fluoropyrimidine-based chemotherapy in selected patients with median progression-free survival (mPFS) or time-to-disease-progression (TTP) around 1.6–2.3 months and median overall survival (mOS) varying between 4–6 months (10–12). There is a clear unmet need for more effective therapies in patients with unresectable and metastatic biliary tract adenocarcinomas.
It is essential to understand this disease and its treatments better at the molecular level. Recent studies uncovered spectra of genetic alterations of BTC that included potential therapy targets (13,14). Studies indicated different mutation signatures spectra of ICC, ECC and GBC (e.g. FGFR2 fusion; IDH1/2, EPHAE, and BAP1mutations are more prevalent in ICC; PRKACA or PRKACB fusion, ELF3, and ARID1B mutations in ECC; EGFR, ERBB3, PTEN, ARID2, MLL2, MLL3, and TERT promoter mutatiosn in GBC). Studies found nearly 40% of BTC cases harbored genetic alterations in potential therapeutic targets, and the repertoire of driver genes diverged across anatomical locations. Among these significantly altered genes, KRAS, TP53 and ARID2 mutations are high in BTCs and their mutation status of them was significantly associated with poorer patient prognosis (13). In addition, VEGF has been found to be overexpressed in a majority of patients with cholangiocarcinoma, including both ICC and ECC (15). Attempts of molecular targeted agents in BTC showed limited efficacy by absence of molecular selection, and limited agents for heterogeneous molecular alternations (16, 17).
Regorafenib is a diphenylurea oral multikinase inhibitor that potently inhibits angiogenic (VEGFR1–3, TIE2), and stromal (PDGFR-β, FGFR1) factors that promote tumor neovascularization, vessel stabilization and lymphatic vessel formation, which play significant roles in the tumor microenvironment and metastases development (18,19). Regorafenib also suppresses several oncogenic kinases (KIT, RET, RAF) (20) It inhibits mutant oncogenic kinases KIT and RET, which play key roles in human gastrointestinal stromal and thyroid cancers, respectively; it also targets B-RAF, a member of the RAS/RAF/MEK/ERK signaling pathway (21). The anti-cancer efficacy of regorafenib has been well-established in different types of malignances in several large randomized phase III studies (22–24). Based on these data, the FDA has approved regorafenib for the treatment of refractory colorectal cancer, gastrointestinal stromal tumor (GIST), and hepatocellular carcinoma.
Regorafenib holds promise as a new treatment for biliary tract adenocarcinomas, as it is a multikinase inhibitor targeting multiple tumor pathways involved in biliary tumorigenesis, including EGFR, RAS, RAF, VEGFR, FGFR, and PDGFR; there is evidence that overexpression of these proteins is associated with tumor stage, prognosis, and response to therapy. Regorafenib has also demonstrated efficacy and a manageable toxicity profile in patients with various solid tumors, even after progression on prior systemic treatments. In this study, we evaluated the efficacy and safety of regorafenib in patients with advanced unresectable or metastatic biliary tract adenocarcinoma after standard front-line therapy.
METHODS
Study design
Adult patients (≥ 18 years old) with distant metastatic or advanced unresectable biliary tract adenocarcinoma (including both primary intrahepatic and extrahepatic cholangiocarcinomas and gallbladder adenocarcinoma) that were histologically or cytologically confirmed (either at a primary or a metastatic site) and had failed at least one line of systemic chemotherapy. Patients had an Eastern Cooperative Oncology Group (ECOG) performance-status (PS) score of 0 or 1 and had life expectancy of greater than 3 months. Adequate liver, kidney, and bone marrow function were assessed by the following laboratory requirements: total bilirubin ≤ 3.0 x the upper limit of normal (ULN) (biliary stenting or percutaneous biliary drainage were allowed for cancer-related biliary obstruction), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤ 5.0 x ULN, and international normalized ratio (INR)/partial thromboplastin time (PTT) ≤ 1.5 x ULN; serum creatinine ≤ 1.5 x ULN; serum amylase and lipase ≤ 1.5 x ULN; platelet count ≥ 75,000 /mm3; hemoglobin (Hb) ≥ 9 g/dL; absolute neutrophil count (ANC) ≥ 1500/mm3. Patients with uncontrolled hypertension (systolic pressure >140 mm Hg or diastolic pressure > 90 mm Hg on repeated measurement) despite optimal medical management, unstable/new onset angina or myocardial infarction, evidence or history of bleeding diathesis or coagulopathy, or any hemorrhage or bleeding event ≥ CTCAE grade 3 within 4 weeks prior to registration were excluded from the study. All patients signed the written informed consent form; the study was conducted with the approval of the University of Pittsburgh Institutional Review Board (IRB) and was registered with clinicaltrials.gov (NCT02053376).
Under the original protocol, the starting dose of regorafenib was 160 mg once daily, administered on a 3 weeks on and 1 week off schedule. The medication was taken in the morning with approximately 240 mL of water after a low-fat (<30% fat) breakfast. Due to CTCAE Grade 3 toxicity (fatigue/weakness) experienced by the first 3 patients within the first week of administration, the protocol was amended so that the starting dose of regorafenib was 120 mg once daily, with the same administration schedule. Treatment was continued until any of the following events occurred: disease progression, intercurrent illness that prevented further administration of treatment, unacceptable adverse event(s), patient decision to withdraw from the study or general or specific changes in the patient’s condition rendering patient unacceptable for further treatment in the judgment of the investigators. Doses were delayed or reduced for clinically significant hematologic or non-hematologic toxicities according to NCI-CTCAE v4.0 via the protocol.
Statistical analysis
All patients were re-evaluated for response every two cycles (up to three days before Day 1 of the planned subsequent cycle). In addition to a baseline scan, confirmatory scans were obtained following initial documentation of objective response. Response and progression were evaluated by the criteria proposed by the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.1. The primary endpoint of the per-protocol analysis was progression-free survival (PFS), estimated by the Kaplan-Meier method, with a 90% confidence region. In the primary analysis, the null hypothesis that median PFS was less than two months was to be tested by fitting a two-sided 80% confidence interval around the median PFS; if the interval did not include two months, the null hypothesis was to be rejected (α=0.10). The study as designed (n=37) had 83% power to reject the null hypothesis if the true median PFS was 3.5 months or greater. Secondary endpoints included overall survival (OS), overall response (OR), including complete (CR) and partial response (PR), and disease control (DCR), consisting of OR plus stable disease (SD). These analyses were descriptive and specified appropriate 90% confidence intervals. Toxicities were to be tabulated by grade and type.
RESULTS
Patient characteristics
From March 2014 to March 2017, a total of 43 patients were enrolled who received at least one dose of regorafenib, of whom 34 patients, who had received at least 1 cycle of treatment, were evaluable for tumor response.
The mean age was 62.7 years (range: 34.5–82.8) and 18 patients (42%) were females (Table 1). Seven percent of participants were African-American and the remainder European-American. Twenty-three participants (53%) had an ECOG PS of 0. Twenty-seven (62%) participants had primary intrahepatic cholangiocarcinoma (IHCC); eleven (26%) had primary extrahepatic cholangiocarcinoma (EHCC) and five (12%) had primary gallbladder cancer. All patients had received first-line combination systemic chemotherapy, primarily gemcitabine, with either cisplatin or oxaliplatin. Among them, 41 patients (95%) had the standard first line regimen of gemcitabine and cisplatin combination. Fifteen patients (35%) had received more than two lines of systemic treatments before entering the study. The other chemotherapy agents that the patients had received included carboplatin, 5-FU, capecitabine, irinotecan, nab-paclitaxel, trastuzumab, and cetuximab. Five patients had received prior radiation therapy, and two patients had undergone trans-hepatic artery chemoembolization (TACE) prior to their previous systemic chemotherapy.
Table 1.
Patient Characteristics.
| Patient Characteristics | (N=43) |
|---|---|
| Age | 62.7 (34.5–82.8) |
| Sex | |
| Male | 25 (58%) |
| Female | 18 (42%) |
| Race | |
| White | 40 (93%) |
| African-American | 3 (7%) |
| ECOG PS | |
| 0 | 23 (53%) |
| 1 | 20 (47%) |
| Location of Lesion | |
| IHCC | 27 (62%) |
| EHCC | 11(26%) |
| GB | 5 (12%) |
| Previous Chemo | |
| 1 line | 28 (65%) |
| 2 lines | 15 (35 %) |
| Gemcitabine | 42 |
| Cisplatin | 40 |
| Oxaliplatin | 13 |
| Carboplatin | 5 |
| 5-FU | 13 |
| Capecitabine | 2 |
| Irinotecan | 2 |
| Neb-Paclitaxel | 1 |
| Trastuzumab | 1 |
| Cetuximab | 1 |
| XRT | 6 |
| TACE | 2 |
Efficacy
Five out of 34 evaluable patients achieved partial response with an overall response rate (ORR) of 11%. Nineteen patients (44%) had stable disease, resulting in an overall disease control rate (DCR) of 56%. Ten patients (29%) progressed at the first disease evaluation. Overall response was not related to the number of treatments previously received (Fisher’s Exact Test P=0.94). For evaluable patients, the median progression-free survival (mPFS) was 15.6 weeks (90% CI; 12.9–24.7) (Figure 1) and median overall survival (mOS) was 31.8 weeks (90% CI; 23.3–74.3); 40% of patients survived to 12 months and 32% to 18 months (Figure 2).
Figure 1:
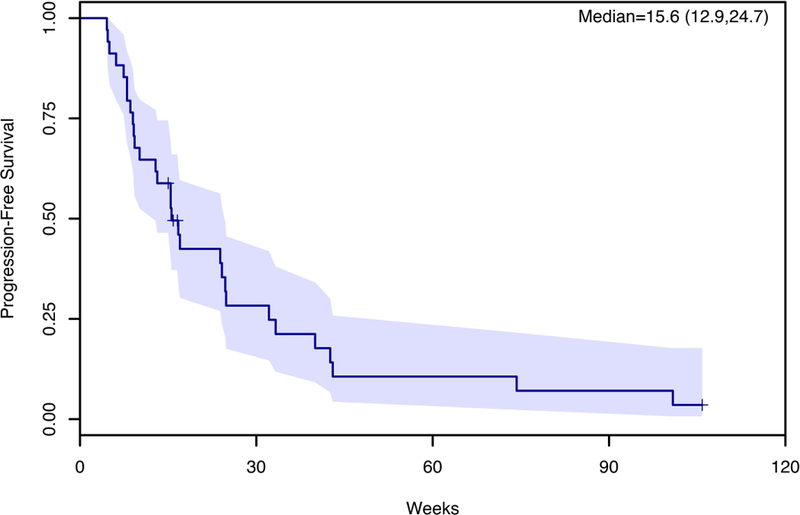
Effect of Regorafenib on Progression-Free Survival (PFS)
Figure 2:
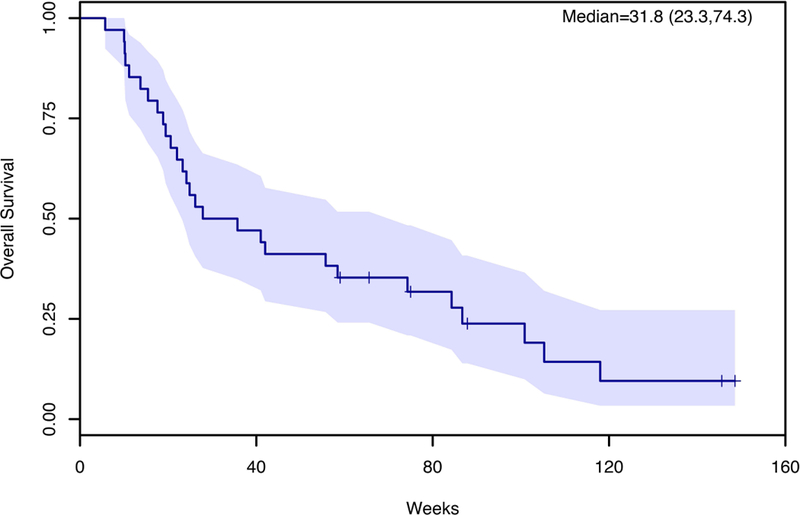
Effect of Regorafenib on Overall Survival (OS).
Toxicity
The overall toxicity profile was as expected, with overall Grade 3 or 4 hematologic or non-hematologic toxicities (according to NCI-CTCAE v4.0) seen in 40% of patients (Table 2). The most common Grade 3 or 4 toxicities were hypophosphatemia (21%), hypertension (18%), and hand-foot skin reaction (7%). There were no treatment-related deaths. Dose modification was required in twelve patients (28%) after initial dose amendment. Overall, the reasons patients stopped regorafenib were disease progression in 70% (30/43), drug toxicity in 18 % (16% 7/43, including the first 3 patients with dose of 160 mg), physicians’ decision in 9% (4/43), and others in 4% (2/43).
Table 2.
Grade 3/4 Adverse Effects.
| Grade 3/4 Adverse Effects | ||
|---|---|---|
| Non-Hematological | N | % |
| Hypertension | 10 | 23 |
| Fatigue | 4 | 9 |
| Abdominal pain | 4 | 9 |
| Generalized Pain | 4 | 9 |
| Rash/ maculo-papular | 3 | 7 |
| Nausea | 3 | 7 |
| Hematological | ||
| Hypophosphatemia | 17 | 40 |
| Hyperbilirubinemia | 11 | 26 |
| Hyponatremia | 9 | 21 |
| Lymphocytopenia | 8 | 19 |
| Thrombocytopenia | 6 | 14 |
| Aspartate aminotransferase increased | 6 | 14 |
| Alkaline phosphatase increased | 6 | 14 |
| Hypokalemia | 5 | 12 |
| Anemia | 5 | 12 |
| Alanine aminotransferase increased | 5 | 12 |
| Lipase increased | 3 | 7 |
| Hypoalbuminemia | 3 | 7 |
DISCUSSION
This study demonstrated the promising clinical activity of regorafenib as an active agent in advanced and metastatic biliary tract adenocarcinoma following progression on standard 1st –line gemcitabine/cisplatin therapy. Thirty-five percent of patients had received more than two lines of systemic treatments before study. The study achieved its primary objective, with an mPFS of 15.6 weeks (90% CI: 12.9–24.7). Moreover, it showed encouraging long-term survival, with mOS of 31.8 weeks (90% CI; 23.3–74.3), beyond the current expectations in this poor prognosis population of previously treated and chemotherapy refractory patients. This promising efficacy reinforces the hypothesis that regorafenib may exert activity in biliary tract adenocarcinomas through simultaneous inhibition of oncogenic (KIT, RET, RAF) kinase, stromal (PDGFR-β, FGFR1), and multiple angiogenic (VEGFR1–3, TIE2) pathways, and that this approach may result in improved outcomes for patients with advanced disease, even after progression after cytotoxic chemotherapy. A recent whole genome and epigenetic molecular study of cholangiocarcinoma demonstrated that the molecular landscape in biliary tract adenocarcinomas is rich and diverse, and may differ radically by etiology of the disease, and location of the disease (ICC, ECC, GBC) (13). These advances in comprehensive whole-exome and transcriptome sequencing have defined the genetic landscape of each cholangiocarcinoma subtype, and potential promising molecular targets for precision medicine are being evaluated in clinical trials (25), including those exploring immunotherapy (26). However, outside of limited case series where therapy was tailored to druggable targets found in individual patients via whole genomic sequencing (27,28), we are generally still unable to personalize therapy based on the molecular characteristics of any individual’s biliary tract tumor. Therefore, efforts must continue to focus on targeting key signaling pathways in the disease to achieving improved outcomes.
The significance and negative prognostic role of the aforementioned angiogenic factors (VEGFR1–3, TIE2) and EGFR family members in biliary tract adenocarcinoma are well known. These mechanisms have been explored and anti-tumor activity in cholangiocarcinoma has been described through inhibition of these pathways in in vitro and in vivo preclinical studies, as well as in clinical case reports (15, 29); however, to date, there has been no confirmation of the potential role of anti-angiogenic or anti-EGFR agents in this disease (30–32), except a phase II study with bevacizumab and elotinib combined as a first line in the advanced biliary cancer with TTP (time to disease progression of 4.4 month (33). This may be due to the multifaceted nature of biliary tract tumors, highlighting the need to attack multiple pathways together, which is suggested by the limited or moderate efficacy of those TKIs with relative narrow spectra of TKs as a single agent, e.g. sorafenib and cediranib. Sorafenib, a kinase inhibitor of VEGFR2/3, PEGFR, and RAF had been tested in the treatment of advanced and metastatic biliary tract adenocarcinoma as the first-line and the second-line settings (34–36). SWOG 0514 was designed to testing the efficacy of sorafenib in the treatment naïve advanced and metastatic biliary cancers (34). The study was terminated after the first stage of accrual due to failure to meet the primary objective with 0% response rate in 31 eligible treated patients. The study showed that DCR of 39%, mPFS of 3 months and mOS of 9 moths in these previous untreated population. The other single institute study showed results of sorafenib as single agent with DCR of 73.3% and mOS of 5.7 months in 15 patients with no prior therapy for metastatic or unresectable diseases (35). The study of sorafenib as the second-line therapy showed the results of DCR of 15.9%, mPFS of 3.2 months and mOS of 5.7 months (36). A randomized placebo controlled phase II study, ABC-03 was unable to demonstrate the efficacy of Cediranib, an oral TKI of VEGFR1/2/3 with additional activity against PDGFR and c-KIT, in combination of gemcitabine and cisplatin as the first-line therapy (mPFS 8.0 months vs. 7.4 months, p= 0.74)(37). It has been suggested that broader inhibition of different pathways may be benefit. However, a phase II study combining sorafenib and erlotinib, an EGRF inhibitor, was attempted and failed in this setting (38). That study was terminated after the first stage of accrual, owing to failure to meet the predetermined number of patients who were alive and progression-free, with an mPFS of 2 months (95% CI: 2–3) and an mOS of 6 months (95% CI: 3–8 months). One of the main reasons for the study failure was compromised dose intensity due to adverse events from the overlapping toxicity profiles of these two oral tyrosine kinase inhibitors (TKIs). Dose reduction or interruption secondary to adverse events occurred in 17 of 34 patients (50%) in cycle 1 and 14 of 26 patients (54%) in cycle 2, with a patient refusal rate of 24% (8 of 34 patients).
Because of its characteristics of efficient inhibition of multiple key angiogenic (VEGFR1–3, TIE2), tumorigenic kinase pathways (KIT, RET, RAF) and stromal factors (PDGFR-β, FGFR1) (19–21), regorafenib has demonstrated effective control of various cancers, at refractory states in treatment, as a single agent; this has the advantage of minimizing reductions in relative dose intensity caused by the cumulative toxicities seen when combining TKIs. This approach is attractive in biliary tract adenocarcinomas, both due to the need to target multiple molecular pathways and the necessity to avoid adverse events in a patient population prone to morbidities from their disease. The ORR of 11% and DCR of 56% seen in this study are consistent with the response rate of regorafenib in other diseases, e.g. the randomized phase III study of regorafenib for patients with refractory HCC after sorafenib demonstrated ORR of 11% and DCR of 65% (24). Since the stable disease was assigned at the first scheduled scan, the possibility of the DCR being over-estimated may exist. However, there were 10 patients (29%) had substantial stable disease of 6–13 months. The greater benefit of long-term disease control is demonstrated with prolonged survival associated with regorafenib when compared to historical standards.
With the collective experience of regorafenib (and other TKIs) in clinical practice, dose interruption, pulsation, and patient refusal due to intolerability were central concerns coming into this study. The phase III study in treatment of metastatic colorectal cancer patients who failed standard therapies demonstrated significantly effects of regorafenib monotherapy with the standard dose of 160 mg which led the approval of the agent by regulators of US, Europe and Japan. However, the grade 3 or greater adverse events (AEs) are common, e g. there were 54% of grade 3 or 4 AEs reported from the phase III metastatic colorectal cancer study (22). The toxicity of standard 160 mg regorafenib regime has resulted in the clinical use of various dose modifications including dose reduction or interruption. A Japanese retrospective study showed that there is no clear reduction in clinical efficacy when the regorafenib dose modified to intensity of 59%, compared to the standard dose (39). The other study analyzed data in mCRC patients with different starting doses: 54 % of patients at 160 mg, 40% at 120 mg, and 6% at 80 mg. The study showed various starting doses have no association with survival in univariate or multivariate analysis. (40) The recently presented ReDOS (Regorafenib dose optimization study) data demonstrated that a strategy with weekly dose escalation of regorafenib from 80 mg to 160 mg/day was found to be superior to a starting ‘the standard’ dose of 160 mg/day in patients with metastatic colorectal cancer (41). The regorafenib dose of 160 mg, the standard dose for refractory colorectal cancer, was initially given. However, a ‘step down’ dose of 120 mg was planned in case of difficulties found on toxicity/tolerability assessment in the first three enrolled patients. Because Grade 3 adverse events of fatigue or weakness were seen within the first week of administration in all of these patients, the starting dose of 120 mg was administered to all subsequent patients enrolled in the study. Drug compliance and interruption due to toxicity were not significant issues through the study, which may certainly have benefit for maintaining efficacy in more general clinical practice. The overall toxicity profile was as expected and consistent with those seen in the regorafenib phase III studies with less required dose modification (23, 25). Hypophosphatemia is one of the most common toxicities of regorafenib, with reported 5–9 % of grade 3 and greater lever toxicities in phase III studies (22–24). Nearly 40% in this study had grade 3 hypophosphatemia from their scheduled laboratory tests but did not demonstrate clinical symptoms, and most patients were corrected with just oral supplement of phosphates. The likely reason of higher hypophosphatemia is because of biliary drainage in many of these patients.
The results of this study are encouraging, particularly in a disease in dire need of greater active agents and novel approaches to treatment. Confirmatory phase III studies are warranted and recommended. Given the evolving terrain of therapies currently being studied in biliary tract adenocarcinomas, randomized studies of regorafenib as a single agent versus combination regimens with cytotoxic regimens or other biologic/immunotherapeutic agents with molecular alternation profile analysis should be considered.
Acknowledgments
The study is supported by Bayer Healthcare Pharmaceuticals
DN was supported by the Biostatistics Shared Resource Facility of the UPMC Hillman Cancer Center that is funded in part by award NIH/NCI P30CA047904.
The study was registered at www.clinicaltrials.gov with registration number of NCT02053376.
AP reports grants from Analysis Group, Inc., personal fees from Eisai Co., Ltd., outside the submitted work.
NB reports and Consultant for Exelixis and Celgene outside the submitted work.
WS reports grands from Bayer Healthcare Pharmaceuticals, during the conduct of the study; personal fees from Bayer outside the submitted work.
Footnotes
Conflict of interest statement:
REFERENCES
- 1.Siegel R, Miller KD and Jemal A. Cancer Statistics, 2016, CA Cancer J Clin 2016; 66(1):7–30 [DOI] [PubMed] [Google Scholar]
- 2.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013: 145(6):1215–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Njei B Changing pattern of epidemiology in intrahepatic cholangiocarcinoma. Hepatology 2014;60(3):1107–8. [DOI] [PubMed] [Google Scholar]
- 4.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist 2016;21(5):594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao KJ , Salma Jabbour S, Parekh N , Lin Y and Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database BMC Gastroenterology (2016) 16:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet 2005;366(9493):1303–1314. [DOI] [PubMed] [Google Scholar]
- 7.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. NEJM 2010;362(14):1273–1281. [DOI] [PubMed] [Google Scholar]
- 8.Jang JS, Lim HY, Hwang IG, Song HS, Yoo N, Yoon S, Gemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group phase II trial. Cancer Chemother Pharmacol 2010. March;65(4):641–7. [DOI] [PubMed] [Google Scholar]
- 9.Lassen U, Jensen LH, Sorensen M, Rohrberg KS, Ujmajuridze Z, Jakobsen A. A Phase I-II dose escalation study of fixed-dose rate gemcitabine, oxaliplatin and capecitabine every two weeks in advanced cholangiocarcinomas. Acta Oncol. 2011. Apr;50(3):448–54. [DOI] [PubMed] [Google Scholar]
- 10.Oh SY, Lee GW, Kim HG, et al. Phase II trial of S-1 in combination with oxaliplatin in previously untreated patients with recurrent or inoperable biliary tract cancer. Chemotherapy 2008;54(6):479–84. [DOI] [PubMed] [Google Scholar]
- 11.Lee S1, Oh SY, Kim BG, et al. Second-line treatment with a combination of continuous 5-fluorouracil, doxorubicin, and mitomycin-C (conti-FAM) in gemcitabine-pretreated pancreatic and biliary tract cancer. Am J Clin Oncol 2009. August;32(4):348–52. [DOI] [PubMed] [Google Scholar]
- 12.Rogers JE, Law L, Nguyen VD, et al. Second-line systemic treatment for advanced cholangiocarcinoma. Journal of Gastrointestinal Oncology 2014;5(6):408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nature genetics 2015; 47: 1003–1010; [DOI] [PubMed] [Google Scholar]
- 14.Li M, Zhang Z, Li X, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway; 2014 Nature genetics 46: 872–876 [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 2008;98(2):418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moeini A, Sia D, Bardeesy N, et al. Molecular pathogenesis and targeted therapies for intrahepatic cholangiocarcinoma. Clin Cancer Res 2016; 22:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New horizons for precision medicine in biliary tract cancer. Cancer Discov 2017; 7:943–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–55. [DOI] [PubMed] [Google Scholar]
- 19.Schmieder R, Hoffmann J, Becker M, et al. Regorafenib (BAY 73–4506): antitumor and antimetastatic activities in preclinical models of colorectal cancer. Int J Cancer 2014. September 15;135(6):1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ettrich TJ, Seufferlein T. Regorafenib. Recent Results Cancer Res 2018;211:45–56. [DOI] [PubMed] [Google Scholar]
- 21.Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther 2013;12:1322–31. [DOI] [PubMed] [Google Scholar]
- 22.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. [DOI] [PubMed] [Google Scholar]
- 23.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66 [DOI] [PubMed] [Google Scholar]
- 25.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma — evolving concepts and therapeutic strategies Nat Rev Clin Oncol 2018. February;15(2):95–111. doi: 10.1038/nrclinonc.2017.157. Epub 2017 Oct 10. Review.PMID: 28994423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015. June 25;372(26):2509–20. doi: 10.1056/NEJMoa1500596. Epub 2015 May 30. PMID: 26028255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borad MJ, Champion MD, Egan JB, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. See comment in PubMed Commons belowPLoS Genet 2014. February 13;10(2):e1004135. doi: 10.1371/journal.pgen.1004135. eCollection 2014. February [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verlingue L, Malka D, Allorant A, et al. Presicion medicine for patients with advanced biliary tract cancer: An effective strategy within the prospective MOSCATO-01 trial. Eur J Cancer 2017;87:122–130 [DOI] [PubMed] [Google Scholar]
- 29.Yoshikawa D, Ojima H, Kokubu A et al. Vandetanib (ZD6474), an inhibitor of VEGFR and EGFR signaling, as a novel molecular-targeted therapy against cholangiocarcinoma. BJC 2009; 100:1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Khoueiry AB, Rankin CJ, Ben-Josef E, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs 2012; 30:1646–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bengala C, Bertolini F, Malavasi N, et al. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer 2010; 102:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi JH, Thongprasert S, Lee J, et al. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur J Cancer 2012; 48:196–201. [DOI] [PubMed] [Google Scholar]
- 33.Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 2010. July 20;28(21):3491–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Khoueiry AB, Rankin CJ, Ben-Josef E, et al. SWOG 0514: A phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and Cholangiocarcinoma. Invest New Drugs 2012. 30(4): 1646–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan T-T, Wang W, Jia W- D, Xu G- L. A single-center experience of sorafenib monotherapy in patients with advanced intrahepatic cholangiocarcinoma. Onco Letter 2017, 13: 2957–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo X, Jia W, Huang Z, et al. Effectiveness and safety of sorafenib in the treatment of unresectable and advanced intrahepatic cholangiocarcinoma: a pilot study Oncotarget 2017, 8:17246–17257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valle JW, Wasan H, Lopes A, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol 2015; 16: 967–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Khoueiry AB, Rankin C, Siegel AB, et al. S0941: a phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br J Cancer 2014; 110:882–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirano G, Makiyama A, Makiyama C, et al. Reduced dose of salvage-line regorafenib monotherapy for metastatic colorectal cancer in Japan. Anticancer Res 2015. January;35(1):371–7. [PubMed] [Google Scholar]
- 40.Gotfrit J, Vickers M, Sud S, Asmis T, et al. Real-life treatment of metastatic colorectal cancer with regorafenib: a single-centre review; Curr Oncol 2017. August;24(4):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bekaii-Saab TS, Ou F-S, Anderson DM, et al. Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC)—An ACCRU Network study. J Clin Oncol 36, 2018 suppl 4S; abstr 611 [Google Scholar]


