Abstract
BACKGROUND:
Prior studies have suggested an association between platelet transfusions (PTX) and worse outcomes among infants with necrotizing enterocolitis (NEC), potentially mediated by pro-inflammatory factors released by platelets. However, the effects of storage on platelet pro-inflammatory factor release and the confounding role of illness severity on NEC outcomes have not been determined.
STUDY DESIGN AND METHODS:
First, Neuropeptide Y (NPY, a potent splanchnic vasoconstrictor released by platelets) was measured by ELISA in fresh frozen plasma (FFP) and in the supernatant of leukoreduced apheresis-derived PLTs (LR-A-PLTs) at different times during storage. Next, we evaluated the relationship between PTX rates and death in a multicenter cohort of very low birth weight (VLBW) infants with NEC, adjusting for illness severity.
RESULTS:
NPY levels increased over time in the supernatant of LR-A-PLTs, and were 4.4-fold and 8.9-fold higher than in FFP on days 2 and 3 of storage, respectively (P<0.001). Among 598 VLBW infants, 44 developed NEC. In unadjusted analysis, PTX rate was 30.3 (95% CI 11.5–80.1) per 100 infant-days among infants who died, compared to 6.0 (95% CI 3.2–11.2) among survivors (incidence rate ratio [IRR] 5.1; 95% CI 1.6–16.2; P=0.006). In multivariable analysis, there was no association between PTX rate and mortality (IRR 3.0; 95% CI 0.6–15.0; P=0.18), although estimation was imprecise.
CONCLUSION:
Pro-inflammatory mediators accumulate in platelet suspensions during storage. Although PTX rates were not associated with increased mortality among infants with NEC in our study, our estimates suggest the potential for such an association that needs evaluation in larger studies.
Keywords: Chemokine, inflammation, necrotizing enterocolitis, neonate, platelet
INTRODUCTION
Necrotizing enterocolitis (NEC) is a devastating disease that predominantly affects premature infants. Although the incidence of NEC is decreasing1, concomitantly with improvements in neonatal intensive care, its mortality has not changed substantially2. Risk factors such as prematurity, formula feeding, and bacterial colonization, among others, are thought to trigger the activation of pro-inflammatory signaling, ultimately leading to bowel necrosis3. More recently, severe anemia has been identified as a significant risk factor for NEC, although the underlying mechanisms remain unclear4.
NEC is one of the main causes of severe neonatal thrombocytopenia5,6. Although there is no correlation between the severity of the thrombocytopenia and the incidence of major bleeding5,6, infants with NEC-associated severe thrombocytopenia (platelet counts <60 × 109/L) had 2-times more bleeding events per day than infants with severe thrombocytopenia not-related to NEC7. In a prospective observational study conducted in the UK, NEC and gestational age were the strongest predictors for an increased number of bleeding events7, although the majority of those were minor. For these reasons, despite the complete absence of data from prospective randomized trials, infants with NEC-associated moderate to severe thrombocytopenia are frequently treated with platelet transfusions in an attempt to diminish the occurrence or the severity of hemorrhages8,9.
Paradoxically, the number of platelet transfusions (PTX) in infants with NEC has been shown to correlate with increased morbidity (short bowel syndrome and/or cholestasis) but not mortality10. These investigators hypothesized that substances such as cytokines, chemokines or bioactive lipids generated during platelet storage may amplify the inflammatory bowel injury10,11. Given that platelets transfused to neonates are typically leukoreduced and therefore WBC-poor, only the platelet-derived bioactive substances likely play a role in NEC.
Pro-inflammatory cytokines involved in the pathogenesis of NEC (TNF-α, IL-1, IL-6, IL-8)11 show increasing levels during platelet storage, but this finding has been predominantly attributed to WBCs12–15, which are present in very low numbers in the leukoreduced products transfused to neonates. Platelets, however, also contain multiple cytokines, including CD40 ligand, regulated on activation normal T expressed and secreted (RANTES), TGF-beta 1 and PF4, among others. These chemokines are released from platelets during storage, and leukoreduction does not prevent their accumulation12,16–18. Platelet activating factor (PAF) has also been implicated as a key mediator of intestinal inflammation and necrosis in models of NEC19,20. Neuropeptide Y (NPY) is a potent vasoconstrictor21,22 responsible for regional splanchnic vasoconstriction during stress. Importantly, NPY is synthesized in megakaryocytes and stored in platelets, with increased platelet levels found under different stress conditions23–25. Although an increase in NPY levels during platelet storage could potentially have an aggravating influence on NEC, and could contribute to the association between PTX and NEC-related morbidity, NPY levels have never been evaluated in stored leukoreduced apheresis-derived PLTs (LR-A-PLTs).
In the present study, we first determined whether NPY, a potential novel contributing factor to the pathophysiology of NEC, accumulates in LR-A-PLTs during storage. Next, we evaluated the frequency and characteristics of PTX administered to a cohort of neonates with NEC Bell Stage 2 or greater, who were part of a large prospective multicenter observational cohort of VLBW infants. In this cohort, we tested the hypothesis that PTX rates after onset of NEC would be higher among infants who died compared to those who survived, after accounting for two major confounding factors, birth weight and illness severity.
MATERIAL AND METHODS
Preparation of platelet components for analysis
Leukoreduced apheresis-derived PLTs (LR-A-PLTs) were obtained from the American Red Cross Blood Services, Southern Region (Douglasville, GA, USA); the storage container used for platelets was Baxter PL2410 (Fenwal, Inc., Lake Zurich, IL, USA). No donor collection information was available. The LR-A-PLTs were placed on an agitator (Helmer, Inc., Noblesville, IN) and stored at 22 ± 2ºC. Samples were obtained through sterile couplers from LR-A-PLT units right before being released for transfusion, on day 2 (n=5), day 3 (n=11) and day 4/5 (n=4) of storage. Two individual LR-A-PLTs were additionally serially sampled on days 3, 4 and 5. At each time point, 3 ml were aseptically removed from each unit and processed as described. Samples were centrifuged to pellet the cells, after which the supernatants were removed and stored in aliquots at 70ºC until analysis. Fresh frozen plasma (FFP) samples from healthy donors were used as controls (n=10).
Platelet Assays
Levels of NPY were measured using a competitive enzyme immunoassay (EIA)-extraction-free absorbance assay from Peninsula Laboratories (San Carlos, CA, USA), according to manufacturer’s instructions. As internal control for the degree of platelet activation and degranulation during storage, we measured the levels of another platelet-derived chemokine, platelet factor 4 (PF4), which is known to increase steadily during storage26. PF4 was measured by a commercially available EIA following the manufacturer instructions (Stago Systems, Parsippany, NJ, USA).
Cohort design
To estimate PTX rates among infants with NEC, we performed a secondary, retrospective analysis of a prospective, multi-center, observational birth cohort study that investigated the transfusion-transmission of cytomegalovirus 4,27,28. This cohort consisted of infants cared for in 5 hospitals in Atlanta, Georgia, with initial enrollment at three birthing hospitals and follow-up of any infants referred to two free-standing Children’s hospital NICUs. The inclusion criteria for the parent study included: 1) Birth weight ≤ 1500 grams; and 2) Postnatal age ≤ 5 days. Exclusion criteria included: 1) Not expected to survive for more than 5–7 days; 2) Severe congenital anomaly; 3) Transfusion prior to admission; or 4) Maternal refusal. This study was approved by the Institutional Review Boards and/or Research Oversight Committees at all participating centers. For the present NEC study, enrolled infants who developed NEC Bell Stage 2 or greater were identified and their records reviewed.
Definitions
Data, including all blood products administered and all diagnoses of NEC, were collected in standardized case report forms. NEC was defined as Bell Stage 2 or greater according to established criteria, as previously described4. Onset of NEC was defined as the time of the first clinical signs of NEC (e.g. emesis, bloody stool, etc.). Surgical NEC was defined as the receipt of exploratory laparotomy or peritoneal drain placement. Illness severity at the onset of NEC was quantitatively assessed using the SNAP-II score, a validated measure of neonatal severity of illness29.
Statistical analysis
One-way analysis of variance was used to compare NPY (natural log, base e) and PF4 by storage day. A test for linear trend across storage time was performed using a linear orthogonal polynomial contrast30. Pairwise comparisons between FFP and LR-A-PLTs at each storage day were made by t-tests. After one-way analysis of variance, log NPY mean and its 95% confidence interval (CI) were back transformed to the original scale and reported as the geometric mean with 95% CI. Similarly, back transformation of the difference between the mean of log transformed NPY for FFP and LR-A-PLTs yielded the ratio of the geometric means. Confidence intervals for the geometric mean ratio were computed by back transforming the 95% confidence bounds for the mean difference. A geometric mean ratio of 3.0 suggests the NPY in one group (LR-A-PLTs) is 3 times that of another group (FFP). NPY levels on days 3 and 4/5 from the same LR-A-PLT unit (serial sampling from 2 units, A and B) were analyzed with a repeated-measures model and reported as the mean ± the standard error of the mean. Prior to implementation of simple linear regression to quantify the relationship between NPY (outcome) and PF4 levels (predictor) in platelet supernatants, all assumptions were assessed.
For epidemiologic analyses, PTX rates per 100 infants-days for surviving and non-surviving infants with NEC were estimated and compared by performing a generalized estimating equations (GEE) Poisson regression analysis of the transfusion counts implemented with the SAS GENMOD procedure (version 9.4; SAS Institute, Cary, NC). To calculate PTX rates, the number of platelet transfusions after onset of NEC were included in the numerator and days of hospitalization after onset of NEC (through the study follow-up period) were included in the denominator. For reference, transfusion rates for red cells, FFP, and cryoprecipitate in the same cohort were also reported and calculated using a similar approach. The incidence rate ratio (IRR) was used to compare the incidence rate in non-survivors to that of survivors. Univariable results are presented as the IRR and its 95% confidence interval (CI). The model-based estimates are unbiased with unbalanced and missing data, so long as the missing data are non-informative (missing completely at random, MCAR). To address confounding by indication for platelet transfusion, the regression model for the primary analysis was adjusted for both birth weight and illness severity at the onset of NEC (SNAP-II score). Deaths on the day of NEC onset were excluded, as transfusion rates could not be estimated (< 1 d at risk). Multivariable results are reported as the adjusted IRR and the 95% CI for non-survivors relative to survivors after adjustment for other factors in the final model. The same method described above for the univariable analysis of surviving and non-surviving infants with NEC was used to estimate and compare transfusion rates of blood components after onset of surgical NEC or medical NEC.
RESULTS
Platelet assays
NPY and PF4 levels during storage.
As hypothesized, these studies demonstrated that NPY levels increased significantly during the storage of platelet concentrates under standard blood banking conditions. NPY levels were 4.4-fold (geometric mean (GM): 60 pg/mL; geometric mean ratio (GMR): 4.4; 95% CI: 2.4–8.1), 8.9-fold (GM 120 pg/mL and GMR 8.9; 95% CI: 5.5–14.4) and 7.1-fold (GM 96 pg/mL and GMR 7.1; 95% CI:3.7–13.7) higher in Day 2, Day 3 and Day 4/5 LR-A-PLTs, respectively, compared with control plasma (GM 13.5 pg/mL: 95% CI: 9.4–19.4) (p<0.001 for all comparisons) (Fig 1A). Similarly, serial sampling in two individual LR-A-PLT on days 3 and 4/5 confirmed that NPY levels increased over time in each individual unit (Fig 1B).
Figure 1. NPY levels increase in LR-A-PLTs during storage.
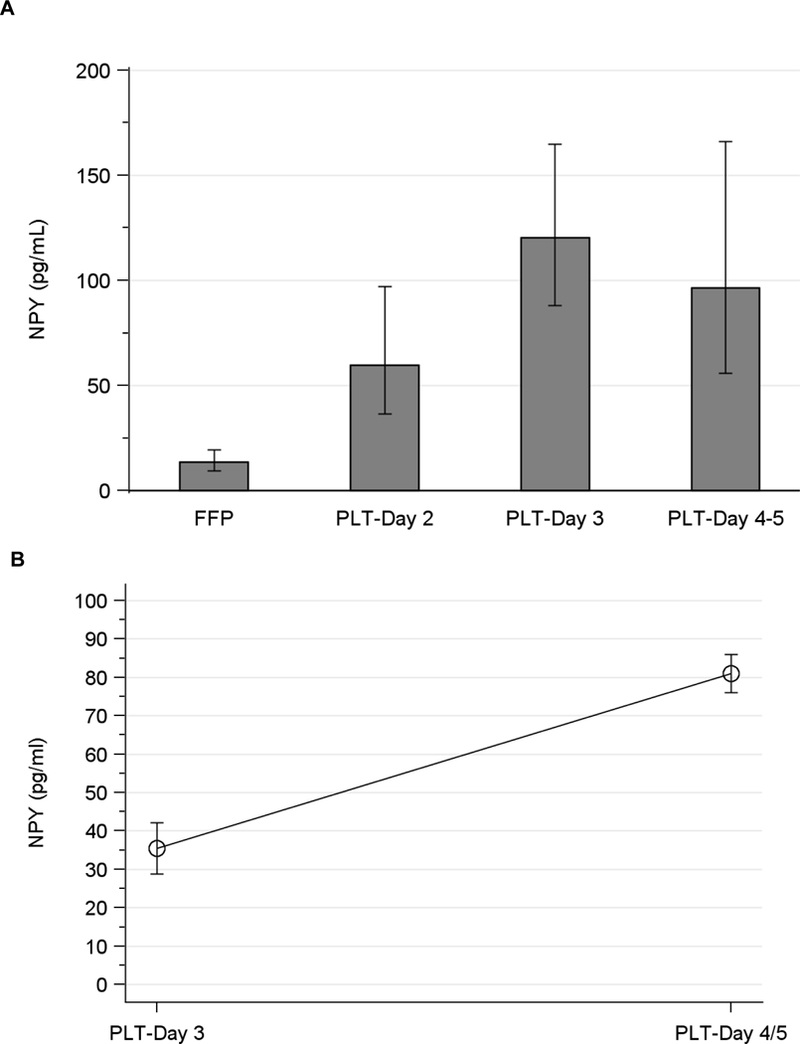
A. NPY levels were measured in individual LR-A-PLTs right before release for transfusion. Columns and error bars represent the geometric mean and 95% confidence interval of 5 (Day 2), 11 (Day 3) and 4 (Day 4/5) samples per time point; test for linear trend, p < 0.001. B. NPY levels were serially measured in two LR-A-PLT units on days 3 and 4/5 of storage; p = 0.02 for increase over time).
We also found a significant time-dependent accumulation of PF4 during platelet storage, compared with the control plasma units (Fig 2A). Consistent with the hypothesis that both chemokines (NPY and PF4) are released due to platelet degranulation during storage, we found a positive correlation between NPY and PF4 levels in platelet supernatants (p<0.001) (Fig 2B). In fact, the NPY pattern of accumulation was very similar to that of PF4 (Figs 1A and 2A, test for linear trend P <0.001). The fact that NPY and PF4, the two cytokines selected in our study, are derived exclusively from platelets excluded the possibility of contaminating leukocytes as a source.
Figure 2. PF4 levels increase in LR-A-PLTs during storage.
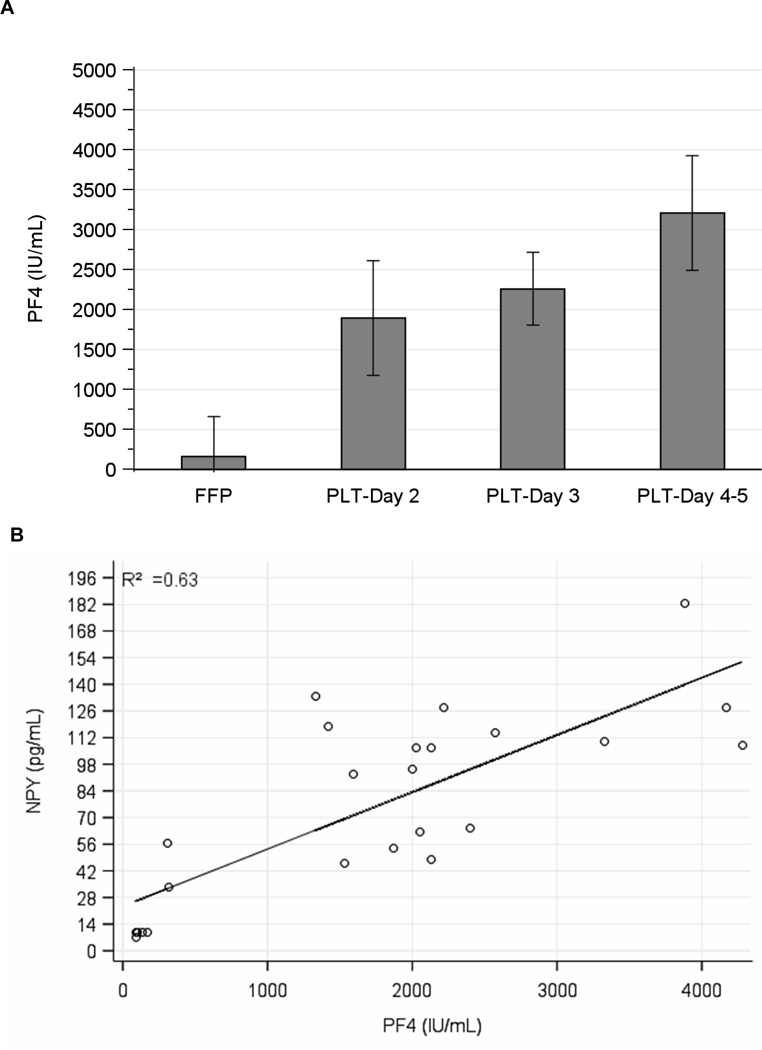
A. Columns and error bars represent the mean and 95% confidence interval of 5 (Day 2), 11 (Day 3) and 4 (Day 4/5) samples per time point; test for linear trend, p < 0.001. B. Association between NPY (y-axis) and PF4 (x-axis) protein levels from 8 samples of fresh frozen plasma (FFP) and 17 samples of platelet (PLT) with both NPY and PF4 levels (p < 0.001 by linear regression, R2 = 0.63).
Platelet transfusion rates among infants with NEC
We identified 44 infants who developed NEC among a cohort of 598 VLBW infants enrolled in the parent study. Among the 44 infants with NEC, the mean (SD) gestational age was 26.6 (2.3) weeks and mean birth weight was 820 (250) grams (Table 1). In the 24 and 48 hours after onset of NEC symptoms, 12% and 26% of affected infants, respectively, received one or more PTX (Table 2). In contrast, 79% of infants with NEC received an RBC transfusion within the 48 hours following the onset of NEC symptoms. Transfusion rates of all blood products were higher among infants with surgical NEC compared to medical NEC, with PTX rates being 12 times higher (incidence rate ratio (IRR) 12; 95% CI 4.1–34) among the 14 (36%) infants with surgical NEC compared to 25 infants with medical NEC (Figure 3 and Supplemental Table 1). Consistent with transfusion threshold data from a prior survey of PTX practices among North American neonatologists8, PTX in our study were given to neonates with a mean platelet count of 70 × 109/L (95% CI: 53–87 × 109/L; range: 6–210 × 109/L) (Figure 4).
Table 1.
Characteristics of infants with NEC
| Characteristics | N=44 |
|---|---|
| Gestational age in weeks, mean ± SD | 26.6 ± 2.3 |
| Birth weight in grams, mean ± SD | 820 ± 250 |
| Male gender | 20 (46%) |
| Race | |
| Black | 31 (71%) |
| White | 11 (25%) |
| Other | 2 (5%) |
| Singleton birth | 34 (77%) |
| Small for gestational age | 14 (32%) |
| Receipt of antenatal steroids | 33 (75%) |
| Death | 13 (30%) |
| Patent ductus arteriosus | 17 (39%) |
| Intraventricular hemorrhage (grade 2 or greater) | 9 (21%) |
| Ever fed breast milk | 43 (98%) |
Data are presented as n, (%) unless indicated otherwise.
Abbreviations: NEC, necrotizing enterocolitis; SD, standard deviation.
Table 2.
Frequency of transfusions in the immediate post-NEC period
| Type of blood product | Receipt within 24 hours after NEC onset, number (%) of infants |
Receipt within 48 hours after NEC onset, number (%) of infants |
|---|---|---|
| Red blood cell | 28 (67%) | 33 (79%) |
| Platelet | 5 (12%) | 11 (26%) |
| Fresh frozen plasma | 8 (19%) | 11 (26%) |
| Cryoprecipitate | 0 | 1 (2%) |
| Any of above | 31 (74%) | 34 (81%) |
Figure 3. Transfusion rates after NEC.
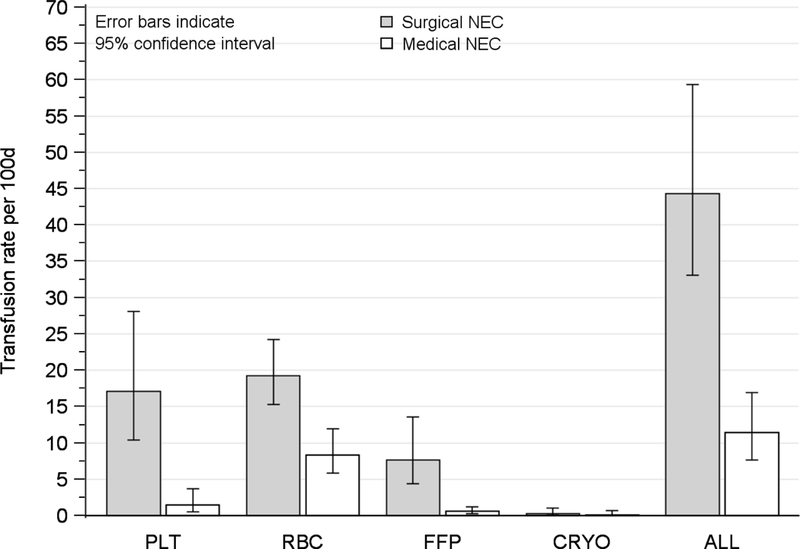
Figure depicts the rates of transfusion of blood components after onset of medical and surgical NEC. Platelet transfusion rates were higher among 14 infants with surgical NEC, compared to 25 infants with medical NEC (IRR 12; 95% CI 4.1–34). Infants who died on the day of NEC onset not included. Abbreviations: NEC, necrotizing enterocolitis; IRR, incidence rate ratio; CI, confidence interval; RBC, Red blood cell; PLT, platelet; FFP, fresh frozen plasma; CRYO, cryoprecipitate; ALL, any blood component.
Figure 4. Mean platelet count at first transfusion.
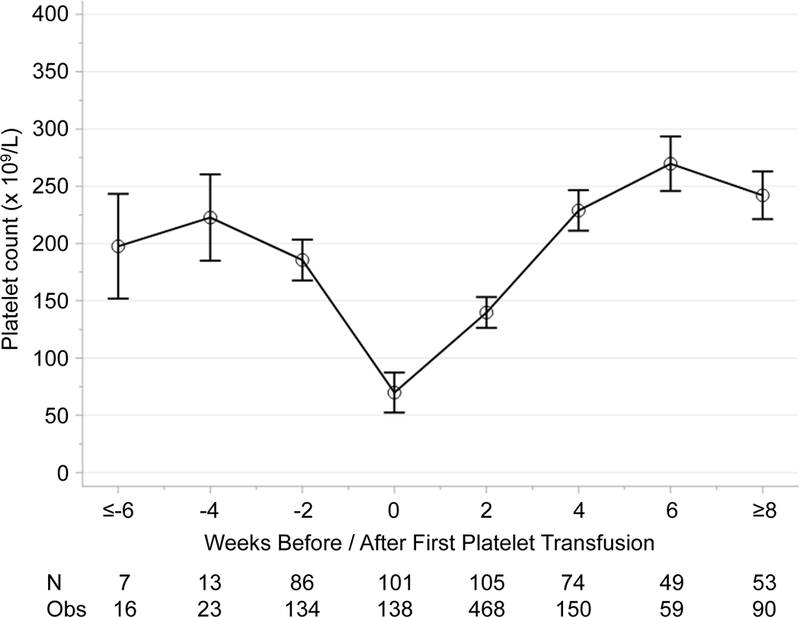
Figure shows platelet trajectory and count at timing of first platelet transfusion to reflect general platelet transfusion practices at centers involved in this study (108 infants and 1078 platelet count measurements). Model-based means and 95% confidence intervals were estimated from repeated measures model.
Assessment of relationship between platelet transfusion and mortality
A total of 13 (30%) infants with NEC died before hospital discharge (Table 1). The PTX rate among infants with NEC who died was higher than among those who survived: 30.3 (95% CI 11.5–80.1) vs. 6.0 (95% CI 3.2–11.2) transfusions per 100 infant days; IRR 5.1; 95% CI 1.6–16.2; P=0.006. After adjusting for birth weight and illness severity using the SNAP II score, however, the PTX rates were no longer significantly different (16.8 vs. 5.6 transfusions per 100 infant days; adjusted IRR 3.0; 95% CI 0.6–15; P=0.18). As birth weight was not significantly associated with mortality after onset of NEC in the multivariable model, we also performed a model only adjusting for illness severity. In this model with adjustment for illness severity at onset of NEC using the SNAP II score (P=0.0004 for association with mortality), the PTX rates were significantly different among infants who died vs. survived (22.5 vs. 5.7 transfusions per 100 infant days; adjusted IRR 3.9; 95% CI 1.1–15.0).
DISCUSSION
In this study, we showed for the first time a substantial time-dependent increase in NPY levels in the supernatant of LR-A-PLTs, stored under normal blood banking conditions. NPY, found in human platelets24,25, is a linear peptide containing 36 amino acid residues, closely related to peptide YY and pancreatic polypeptide (PP)31. In addition to its well-known vasoconstrictor effects22, NPY enhances neutrophil adhesion to endothelial cells, induces histamine release from mast cells, and stimulates macrophage adhesion, chemotaxis, phagocytosis and superoxide anion production32. NPY (as well as peptide YY) also has a profound inhibitory effect on gastric emptying and acid secretion, gut motility, and exocrine pancreatic secretion33. Thus, at least theoretically, a rise in the NPY plasma levels of preterm neonates with NEC could have an aggravating influence on intestinal ischemia and inflammation.
NPY levels have not been measured in preterm infants. However, in full-term cord blood and in the blood of infants and children, NPY levels have been previously reported to be below the limit of detection (50 pg/mL) in the majority of patients, with a few subjects having concentrations slightly above that34. Since our apheresis derived PLTs are usually transfused on day 3 of storage to preterm neonates with a circulating plasma volume of approximately 80 ml/Kg, a 15 ml/Kg PTX containing ~120 pg/ml of NPY would provide an NPY “load” of 1,800 pg/Kg, which would be predicted to increase the plasma NPY levels by approximately 23 pg/mL. Given the low plasma levels found in healthy infants, this might be a significant increase, particularly if multiple platelet transfusions are given to the same patient, as is often the case in neonates with NEC. However, little is known about the in vivo effects of transfused chemokines35 and, therefore, the clinical significance of the transfused NPY in NEC is unclear. Nevertheless, our study provides proof of principle that platelet-derived active substances, such as NPY and PF4, are released and accumulate in platelet concentrates during storage, which may contribute to or exacerbate the inflammatory gut injury among infants with NEC.
To further test this hypothesis, we then evaluated the relationship between PTXs and mortality in a cohort of infants with NEC. As hypothesized, our epidemiological analyses showed that PTX rates were higher among infants with NEC who died, compared to those who survived. However, the association between PTX rate and death diminished after adjustment for illness severity at the onset of NEC, and was no longer significant. Therefore, our findings suggest that higher PTX rates among infants who died could be confounded by an increased severity of NEC. However, given that the point estimate for the PTX rate ratio was 3 times greater among infants who died compared to survivors, even after adjustment for illness severity and birth weight, it is also possible that we were underpowered to detect a difference in mortality among groups. Thus, further studies are needed to understand if platelet transfusions causally contribute to poorer outcomes among infants with NEC, or are simply a marker of illness severity. A previous single center cohort study10 found that infants who died with NEC, compared to those who survived, received a larger number (8 vs. 2) and total volume (170 ml vs. 65 ml) of platelet transfusions, although neither difference was statistically significant. Previous studies have shown that infants with NEC are among the highest users of PTXs in the neonatal intensive care unit 36. Given that NEC is an inflammatory condition37 and PAF has been shown to be an important factor in the pathogenesis19,38, we believe that infants with NEC are an important cohort to study in order to understand the potential inflammatory effects of PTXs.
We should acknowledge the strengths and limitations of our study. We were able to estimate the onset of NEC with good accuracy and were able to follow infants who were transferred for surgery, which allowed us to estimate the transfusion burden through discharge without censoring of outcomes. In addition, we ascertained PTX rates, rather than just number of transfusions, which better accounts for the time at risk and allows for a more robust measure of the intensity of PTXs. Without accounting for time at risk, as was performed in this study, patients who survive could have a relatively longer follow-up period to observe PTX compared to patients who died and this could bias the relationship between PTX and mortality among infants with NEC. Although we had a relatively large multicenter cohort to study, the low incidence of NEC may have yielded too few patients to detect a significant association between PTX rate and mortality. In addition, we were unable to evaluate the relationship between PTX and short-gut syndrome among survivors given our follow-up ending at 90 postnatal days, which is too soon to confidently ascertain the diagnosis of short-gut syndrome in some infants with NEC. Finally, we recognize the potential confounding effects of other unmeasured variables, such as the presence of cytokines and bioactive substances in red cell transfusions,39,40 which nearly all infants in our cohort received.
Based on the findings of the PlaNeT-2 trial in which neonates (including 16% with NEC) randomized to a higher platelet transfusion threshold had a significantly higher rate of death or major bleeding,41 we recommend the use of restrictive platelet thresholds of 25 × 109/L for transfusion to patients with NEC. In addition, larger, well-powered cohort studies are needed to better understand the role of PTX on relevant outcomes among infants with NEC, and particularly the link between donor platelet cytokines, storage time, and neonatal outcomes.
Supplementary Material
Acknowledgments
Sources of support: This work was supported by the National Institutes of Health under the following mechanisms: K23 HL128942 (R.M.P), RO1 HL69990 and P01 HL046925 (M.S-V.), and by a grant from the Health Research Fund, Ministry of Health, Spain (BAE-90058) to F.F-M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflicts of interest: The authors have no relevant conflicts of interest.
Disclaimers: None
REFERENCES
- 1.Horbar JD, Edwards EM, Greenberg LT, Morrow KA, Soll RF, Buus-Frank ME, Buzas JS. Variation in Performance of Neonatal Intensive Care Units in the United States. JAMA Pediatr 2017;171: e164396. [DOI] [PubMed] [Google Scholar]
- 2.Petrosyan M, Guner YS, Williams M, Grishin A, Ford HR. Current concepts regarding the pathogenesis of necrotizing enterocolitis. Pediatr Surg Int 2009;25: 309–18. [DOI] [PubMed] [Google Scholar]
- 3.Frost BL, Jilling T, Caplan MS. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin Perinatol 2008;32: 100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, Easley KA, Josephson CD. Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants. JAMA 2016;315: 889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baer VL, Lambert DK, Henry E, Christensen RD. Severe Thrombocytopenia in the NICU. Pediatrics 2009;124: e1095–100. [DOI] [PubMed] [Google Scholar]
- 6.Stanworth SJ, Clarke P, Watts T, Ballard S, Choo L, Morris T, Murphy MF, Roberts I, Platelets, Neonatal Transfusion Study G. Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics 2009;124: e826–34. [DOI] [PubMed] [Google Scholar]
- 7.Muthukumar P, Venkatesh V, Curley A, Kahan BC, Choo L, Ballard S, Clarke P, Watts T, Roberts I, Stanworth S, Platelets Neonatal Transfusion Study G. Severe thrombocytopenia and patterns of bleeding in neonates: results from a prospective observational study and implications for use of platelet transfusions. Transfus Med 2012;22: 338–43. [DOI] [PubMed] [Google Scholar]
- 8.Josephson CD, Su LL, Christensen RD, Hillyer CD, Castillejo MI, Emory MR, Lin Y, Hume H, Easley K, Poterjoy B, Sola-Visner M. Platelet transfusion practices among neonatologists in the United States and Canada: results of a survey. Pediatrics 2009;123: 278–85. [DOI] [PubMed] [Google Scholar]
- 9.Cremer M, Sola-Visner M, Roll S, Josephson CD, Yilmaz Z, Buhrer C, Dame C. Platelet transfusions in neonates: practices in the United States vary significantly from those in Austria, Germany, and Switzerland. Transfusion 2011;51: 2634–41. [DOI] [PubMed] [Google Scholar]
- 10.Kenton AB, Hegemier S, Smith EO, O’Donovan DJ, Brandt ML, Cass DL, Helmrath MA, Washburn K, Weihe EK, Fernandes CJ. Platelet transfusions in infants with necrotizing enterocolitis do not lower mortality but may increase morbidity. J Perinatol 2005;25: 173–7. [DOI] [PubMed] [Google Scholar]
- 11.Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock 2006;25: 329–37. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer F, Rivera J, Corral J, Gonzalez-Conejero R, Lozano ML, Vicente V. Evaluation of pooled platelet concentrates using prestorage versus poststorage WBC reduction: impact of filtration timing. Transfusion 2000;40: 781–8. [DOI] [PubMed] [Google Scholar]
- 13.Heddle NM. Cytokines in Platelet Concentrates. Hematology 1997;2: 473–84. [DOI] [PubMed] [Google Scholar]
- 14.Heddle NM, Klama L, Singer J, Richards C, Fedak P, Walker I, Kelton JG. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med 1994;331: 625–8. [DOI] [PubMed] [Google Scholar]
- 15.Aloui C, Chakroun T, Prigent A, Jemni-Yacoub S, Cognasse F, Laradi S, Garraud O. Leucocyte cytokines dominate platelet cytokines overtime in non-leucoreduced platelet components. Blood Transfus 2018;16: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood 2006;108: 2455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujihara M, Ikebuchi K, Wakamoto S, Sekiguchi S. Effects of filtration and gamma radiation on the accumulation of RANTES and transforming growth factor-beta1 in apheresis platelet concentrates during storage. Transfusion 1999;39: 498–505. [DOI] [PubMed] [Google Scholar]
- 18.Wadhwa M, Krailadsiri P, Dilger P, Gaines Das R, Seghatchian MJ, Thorpe R. Cytokine levels as performance indicators for white blood cell reduction of platelet concentrates. Vox Sang 2002;83: 125–36. [DOI] [PubMed] [Google Scholar]
- 19.Caplan MS, Sun XM, Hsueh W. Hypoxia, PAF, and necrotizing enterocolitis. Lipids 1991;26: 1340–3. [DOI] [PubMed] [Google Scholar]
- 20.Cho SX, Berger PJ, Nold-Petry CA, Nold MF. The immunological landscape in necrotising enterocolitis. Expert Rev Mol Med 2016;18: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen SH, Han QD. Thrombin-induced neuropeptide Y secretion from rat platelets. Zhongguo Yao Li Xue Bao 1995;16: 360–5. [PubMed] [Google Scholar]
- 22.Clarke JG, Davies GJ, Kerwin R, Hackett D, Larkin S, Dawbarn D, Lee Y, Bloom SR, Yacoub M, Maseri A. Coronary artery infusion of neuropeptide Y in patients with angina pectoris. Lancet 1987;1: 1057–9. [DOI] [PubMed] [Google Scholar]
- 23.Tilan JU, Everhart LM, Abe K, Kuo-Bonde L, Chalothorn D, Kitlinska J, Burnett MS, Epstein SE, Faber JE, Zukowska Z. Platelet neuropeptide Y is critical for ischemic revascularization in mice. FASEB J 2013;27: 2244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers AK, Torres Duarte AP, Zukowska-Grojec Z. Immunoreactive neuropeptide Y (NPY) in plasma and platelets of rat and mouse strains and human volunteers. Regul Pept 1993;47: 239–45. [DOI] [PubMed] [Google Scholar]
- 25.Paiva SP, Veloso CA, Campos FF, Carneiro MM, Tilan JU, Wang H, Umans JG, Zukowska Z, Kitlinska J. Elevated levels of neuropeptide Y in preeclampsia: A pilot study implicating a role for stress in pathogenesis of the disease. Neuropeptides 2016;55: 127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bubel S, Wilhelm D, Entelmann M, Kirchner H, Kluter H. Chemokines in stored platelet concentrates. Transfusion 1996;36: 445–9. [DOI] [PubMed] [Google Scholar]
- 27.Josephson CD, Caliendo AM, Easley KA, Knezevic A, Shenvi N, Hinkes MT, Patel RM, Hillyer CD, Roback JD. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr 2014;168: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josephson CD, Castillejo MI, Caliendo AM, Waller EK, Zimring J, Easley KA, Kutner M, Hillyer CD, Roback JD. Prevention of transfusion-transmitted cytomegalovirus in low-birth weight infants (</=1500 g) using cytomegalovirus-seronegative and leukoreduced transfusions. Transfus Med Rev 2011;25: 125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr 2001;138: 92–100. [DOI] [PubMed] [Google Scholar]
- 30.Freund RJ, Wilson WJ. Statistical methods. Boston: Academic Press, 1993. [Google Scholar]
- 31.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 1982;296: 659–60. [DOI] [PubMed] [Google Scholar]
- 32.Fagerstam JP, Whiss PA, Strom M, Andersson RG. Expression of platelet P-selectin and detection of soluble P-selectin, NPY and RANTES in patients with inflammatory bowel disease. Inflamm Res 2000;49: 466–72. [DOI] [PubMed] [Google Scholar]
- 33.Yang H Central and peripheral regulation of gastric acid secretion by peptide YY. Peptides 2002;23: 349–58. [DOI] [PubMed] [Google Scholar]
- 34.O’Dorisio MS, Hauger M, O’Dorisio TM. Age-dependent levels of plasma neuropeptides in normal children. Regul Pept 2002;109: 189–92. [DOI] [PubMed] [Google Scholar]
- 35.Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med 2001;11: 403–17. [DOI] [PubMed] [Google Scholar]
- 36.Dohner ML, Wiedmeier SE, Stoddard RA, Null D Jr., Lambert DK, Burnett J, Baer VL, Hunt JC, Henry E, Christensen RD Very high users of platelet transfusions in the neonatal intensive care unit. Transfusion 2009;49: 869–72. [DOI] [PubMed] [Google Scholar]
- 37.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364: 255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 2016;13: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sut C, Tariket S, Chou ML, Garraud O, Laradi S, Hamzeh-Cognasse H, Seghatchian J, Burnouf T, Cognasse F. Duration of red blood cell storage and inflammatory marker generation. Blood Transfus 2017;15: 145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karsten E, Breen E, Herbert BR. Red blood cells are dynamic reservoirs of cytokines. Sci Rep 2018;8: 3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, Deary A, Hodge R, Hopkins V, Lopez Santamaria B, Mora A, Llewelyn C, D’Amore A, Khan R, Onland W, Lopriore E, Fijnvandraat K, New H, Clarke P, Watts T, PlaNeT2 MATISSE Collaborators. Randomized Trial of Platelet-Transfusion Thresholds in Neonates. N Engl J Med 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


